Abstract
Chronic pulmonary hypertension (PH) leads to right-ventricular failure (RVF) characterized by RV remodeling. Ventricular remodeling is emerging as an important process during heart failure and recovery. Remodeling in RVF induced by PH is not fully understood. Recently we discovered that estrogen (E2) therapy can rescue severe preexisting PH. Here, we focused on whether E2 (42.5 μg·kg−1·day−1, 10 days) can reverse adverse RV structural and extracellular matrix (ECM) remodeling induced by PH using monocrotaline (MCT, 60 mg/kg). RV fibrosis was evident in RVF males. Intact females developed less severe RV remodeling compared with males and ovariectomized (OVX) females. Novel ECM-degrading disintegrin-metalloproteinases ADAM15 and ADAM17 transcripts were elevated ∼2-fold in all RVF animals. E2 therapy reversed RV remodeling in all groups. In vitro, E2 directly inhibited ANG II-induced expression of fibrosis markers as well as the metalloproteinases in cultured cardiac fibroblasts. Estrogen receptor-β agonist diarylpropionitrile (DPN) but not estrogen receptor-α agonist 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT) was as effective as E2 in inhibiting expression of these genes. Expression of ECM-interacting cardiac fetal-gene osteopontin (OPN) also increased ∼9-fold in RVF males. Intact females were partially protected from OPN upregulation (∼2-fold) but OVX females were not. E2 reversed OPN upregulation in all groups. Upregulation of OPN was also reversed in vitro by E2. Plasma OPN was elevated in RVF (∼1.5-fold) and decreased to control levels in the E2 group. RVF resulted in elevated Akt phosphorylation, but not ERK, in the RV, and E2 therapy restored Akt phosphorylation. In conclusion, E2 therapy reverses adverse RV remodeling associated with PH by reversing fibrosis and upregulation of novel ECM enzymes ADAM15, ADAM17, and OPN. These effects are likely mediated through estrogen receptor-β.
Keywords: pulmonary hypertension, right ventricular failure, ventricular remodeling, estrogen, extracellular matrix
right ventricular failure (RVF) is a common cause of death in patients suffering from long-standing pulmonary arterial pressure overload caused by pulmonary hypertension (PH) (5, 47, 48). Chronic PH first leads to compensated hypertrophy of the right ventricle (RV) that ultimately progresses to severe decompensated RV failure (RVF) (5, 47, 48). This RVF is accompanied by extensive RV structural and extracellular matrix (ECM) remodeling (5, 47, 48). End-stage heart failure has long been regarded as a terminal state of cardiac pathological remodeling that is almost impossible to reverse by any available therapy (17). However, some therapies have been shown recently to be somewhat effective in reversing the adverse remodeling of the left ventricle (LV) induced by pressure and volume overload (44). Unfortunately there is still no ideal therapy that halts or reverses the progression of RV remodeling secondary to PH (5, 47, 48).
RV remodeling is generally characterized by extensive fibrosis and additional changes in the expression of cardiac ECM-associated proteins (5, 47, 48). These expression changes are a key event in promoting the adverse remodeling of the RV (5, 47, 48). Together, these structural and ECM changes affect contractility, signaling, and electrical conduction (25).
The cardiac ECM provides structural support and facilitates mechanical, electrical, and chemical signals during homeostasis and in response to stress or injury (7). Matrix metalloproteinases (MMPs) and the related “a disintegrin and metalloproteinase” (ADAM) family degrade ECM anchoring proteins but are strictly regulated in normal physiological conditions (7). Altering the homeostasis of the ECM plays an important role in various cardiac pathologies, including dilated cardiomyopathy, myocardial infarction, and hypertensive cardiac hypertrophy (46).
Many changes in ECM protein expression in RV hypertrophy and failure have been reported, including altered collagen and metalloproteinase expression (31, 45). Although not all ADAMs show altered regulation during cardiac stress, ADAM15 and ADAM17 are emerging as two metalloproteinases in the heart responsible for pathological remodeling in LV stress and failure (13, 23, 52). However, their expression during RVF induced by PH and recovery has not been characterized. Because these two ADAMs share function and regulation with metalloproteinases well-established as responsible for adverse RV remodeling, they are likely to be involved during RVF as well (8, 22, 37). Another ECM protein well characterized during LV dysfunction is osteopontin (OPN), a matricellular cardiac fetal gene that is reactivated during cardiac stress and plays a role in heart failure. Again, its role in PH-induced RVF has not been characterized (20, 30, 40, 55, 56).
During cardiac stress induced by PH, the cardiac fibroblasts undergo a transition to activated myofibroblasts that are responsible for fibrosis and the secretion of metalloproteinases and other ECM enzymes that contribute to adverse ventricular remodeling (32). Recently we discovered that E2 therapy can rescue severe preexisting PH in male rats primarily by reversing pulmonary vascular remodeling (46). Pedram et al. (32) recently showed that E2 therapy can directly inhibit the transition to myofibroblasts in vitro, preventing the secretion of many of adverse remodeling proteins. Additionally, E2 treatment has been shown to directly mitigate adverse ECM remodeling in left ventricular hypertrophy and failure by attenuating altered collagen, and metalloproteinase expression in vivo (45, 51). Here we explore whether the rapid restoration of RV function by E2 therapy during PH-induced RVF is at least in part due to a direct effect of E2 on the adverse ECM remodeling of the RV.
Using the well-established model of PH-induced RVF using monocrotaline (MCT) in rats (9, 48), we hypothesize that markers of adverse RV remodeling including fibrosis, ADAM15, ADAM17, and OPN are increased during RVF and are directly reversed with E2 therapy. We also explore the effects of E2 on RV remodeling in intact and ovariectomized (OVX) females.
METHODS
Animals
Male and female (intact and OVX) rats (Sprague-Dawley, 350–400 g) were used. All protocols received institutional review and committee approval. The investigation conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85–23, Revised 1996).
Experimental Protocols
In vivo.
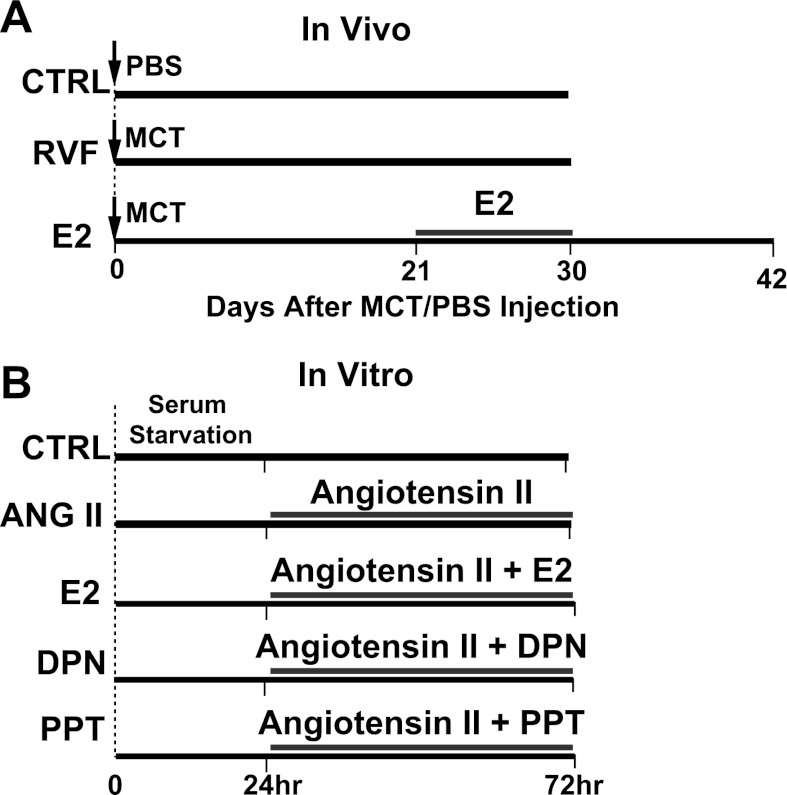
RVF was induced secondary to chronic PH in rats either with a single subcutaneous injection of MCT (60 mg/kg, Sigma) to induce PH or saline (CTRL) at day 0. MCT was dissolved in 1 N HCl; the pH was adjusted to 7.4 and diluted with PBS before injection. Severe PH was evident in MCT-injected rats at day 21. At this time point, the rats were either left untreated to develop right ventricular failure (RVF group) by day 30 or received estrogen therapy using continuous release 17β-estradiol (E2) pellets (42.5 μg·kg−1·day−1, Innovative Research of America) for 10 days. The E2-treated rats were kept for an additional 12 days after E2 withdrawal at day 30 until the point of full recovery previously observed (46) (E2 group, Fig. 1A). For signaling, E2-treated rats were euthanized at day 30 (as opposed to day 42). This time point was chosen because changes in signal transduction such as phosphorylation are transient and the signature in the long run may differ from the immediate change following therapy. All groups had n = 4–10 animals.
Fig. 1.
Experimental protocols. A: in vivo. Three experimental groups: control (CTRL) received no monocrotaline (MCT) injection at day 0. RVF received MCT injection at day 0 and was left untreated to develop severe right ventricular failure. E2 received 10-day estrogen (E2) treatment beginning day 21 post-MCT injection, and left for an additional 12 days after the end of therapy. B: in vitro. Neonatal rat ventricular myocytes (NRVMs) and fibroblasts were cocultured, starved for 24 h, and then treated with ANG II (ANG II group); ANG II with E2, estrogen receptor-β agonist diarylpropionitrile (DPN), or estrogen receptor-α agonist 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT) (ANG II + E2, ANG II + DPN, and ANG II + PPT groups, respectively); or left untreated (CTRL).
In vitro.
Neonatal rat ventricular myocytes (NRVMs) and fibroblasts were isolated as previously described and cocultured (42). Cells were serum starved for 24 h and then were serum replenished and either left untreated (CTRL) or treated with ANG II (100 nM, Sigma A9525) in the absence or presence of E2 (10 nM), selective estrogen receptor-β agonist diarylpropionitrile (DPN, 10 nM), or selective estrogen receptor-α agonist 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT, 10 nM) for 48 h (ANG II, ANG II + E2, DPN, PPT groups) (Fig. 1B). Gene expression was measured by RT-PCR. All in vitro studies were performed in triplicate three independent times.
Cardiac and Pulmonary Hemodynamics
Hemodynamics were measured as previously described (46). Briefly, B-Mode and M-Mode echocardiography was performed under isoflurane anesthesia using a VisualSonics Vevo 770 equipped with a 30-MHz linear transducer. The Vevo770 software calculated RV ejection fraction (RVEF) and RV fractional shortening (RVFS) using RV M-mode echocardiogram. The RV pressure was measured directly by inserting a catheter (1.4F Millar SPR-671) connected to a pressure transducer (Power Lab, ADInstruments) into the RV immediately before the rat was euthanized. The rats were sedated using a mixture of ketamine/xylazine (80 mg/kg ketamine, 8 mg/kg xylazine) administered intraperitoneally. After cardiac catheterization, rats were euthanized by removing the heart while they were under anesthesia.
Real-Time PCR
Total RNA from right ventricle or from cells was isolated using Trizol (Invitrogen). Total RNA (2 μg) was reverse transcribed with gene specific primers using Omniscript RT kit (Qiagen). GAPDH was used as internal reference gene. The sequences of the primers are as follows: OPN forward primer 5′-TGACGGCCGAGGTGA-3′, OPN reverse primer 5′-ATGGCTGGTCTTCCCGTTGCT-3′ (accession no. NM_012881); ADAM15 forward primer 5′-CCCTATGTCCTCTTTGTGTGGA-3′, ADAM15 reverse primer 5′-GCAGAACTCAGGCAAATCGCAA-3′ (accession no. NM_020308); ADAM17 forward primer 5′-GTGCTGACACCGACAACTCGT-3′, ADAM17 reverse primer 5′-CAGCTGGTCAATGAAATCCCAAA-3′ (accession no. NM_020306); transforming growth factor (TGF)-β forward primer 5′-GATACGCCTGAGTGGCTGTCT-3′, TGF-β reverse primer 5′-CTTCTCTGTGGAGCTGAAGCAA-3′ (accession no. NM_020306); collagen type 1 forward primer 5′-GACCGATGGATTCCAGTTCGA-3′, collagen type 1 reverse primer 5′-AAGGTCAGCTGGATAGCGACAT-3′ (accession no. NM_053304.1); lysil oxidase forward primer 5′-CACGCAGCAGCAGAATGGGC-3′, lysil oxidase reverse primer 5′-CGCAGTACCAGCCTCAGCGA-3′ (accession no. NM_017061.2); GAPDH forward primer 5′-CCTGCACCACCAACTGCTTAG-3′, GAPDH reverse primer 5′-ATGACCTTGCCCACAGCCTTG-3′ (accession no. NM_017008).
Single peak was detected from the first derivative of fluorescence (dF/dT) vs. temperature plots (melting curve) indicating amplification of a single product. Agarose gel electrophoresis at the end of the reaction also confirmed the amplification of a single product of the expected size. Controls were 1) reaction cocktail without reverse transcriptase tested in a regular 40 cycle PCR; and 2) H2O instead of cDNA tested in parallel to real-time PCR.
Immunofluorescence Staining
Heart cross sections (6 μm) were fixed in acetone for 15 min at −20°C. The sections were then washed with PBS + 0.1% Triton three times, incubated with 10% normal goat serum in PBS + 0.1% Triton for 30 min to block the background. The sections were incubated with primary antibodies in PBS + 0.1% Triton + 1% normal goat serum at 4°C overnight, washed with PBS + 0.1% Triton three times, and incubated with the appropriate secondary antibodies in PBS + 0.1% Triton + 1% normal goat serum at room temperature for 1 h. After washing the secondary antibodies, the sections were mounted using Prolong gold (Molecular Probes) for imaging.
Trichrome Staining and Histologic Analysis
Standard Masson trichrome staining was performed on RV tissue sections (Sigma-Aldrich). Percent tissue fibrosis in RV sections was determined using the stain for collagen with the use of a grid that divided the field of view into 100 squares; the number of collagenous tissue (blue stain) at the 100 intersection points in the grid was scored as 1 (present) or 0 (absent). Results are expressed as the percentage occupied by fibrosis to the total area examined.
Plasma Osteopontin ELISA
Concentration of OPN in plasma samples was determined by a quantitative sandwich enzyme immunoassay technique by rat OPN ELISA (27360, IBL-America) with a measurement range of 0.07–4.75 ng/ml. Total plasma was diluted to 10 μg/μl of protein and 100 μl of each sample was loaded onto the precoated plate. Absorbance at a wavelength of 450 nm was read with a spectrophotometer and plasma concentrations were calculated using a relative standard curve.
Western Blotting
Right ventricles were isolated and homogenized at 4°C in (in mM) 150 NaCl, 50 Tris·HCl, 1 EGTA. 1 EDTA, 1 NaF, 1 PMSF, 1 Na3VO4, 1% NP-40, 0.1% SDS, and 0.5% sodium deoxycholate (pH 7.4) supplemented with protease and phosphatase inhibitor cocktails (Roche). The samples were centrifuged at 12,000 g for 10 min and the supernatants were collected. Protein concentration was measured and 100 μg of total protein was loaded on a 4–20% gradient Tris·HCl/SDS polyacrylamide gel, electrotransferred to nitrocellulose paper, blocked with 5% nonfat dry milk in 20 mM TBS with 0.1% Tween, and incubated with primary antibodies. Blots were then indirectly labeled using infrared fluorophore conjugated anti-rabbit and anti-mouse secondary antibodies for 1 h, and visualized with the Odyssey Imaging System (Li-Cor). Equal loading of protein onto each lane in the gel was confirmed by probing for vinculin.
Reagents
The primary antibodies used were anti-ADAM17 (1:200, Calbiochem), anti-Akt (1:500, Cell Signaling rabbit polyclonal), anti-phospho-Akt (1:200 Cell Signaling, rabbit polyclonal); anti-ERK1/2 (1:500, Cell Signaling, rabbit polyclonal), anti-phospho ERK1/2 (1:200, Cell Signaling, mouse monoclonal), anti-OPN (Santa Cruz Biotechnology, 1:200 or Abcam, 1:200), and anti-α1C (1:200, Alomone Labs). The secondary antibodies used were goat anti-rabbit Alexa 488 (1:1,000, Invitrogen) and goat anti-mouse Alexa 568 (1:1,000, Invitrogen) for immunocytochemistry, or goat anti-rabbit IgG-Alexa Fluor680 (1:100,000, Invitrogen) and goat anti-mouse IgG-IR Dye800CW (1:100,000, Odyssey, LI-COR) for Western blot.
Statistics
One-way ANOVA tests were used to compare between groups using SPSS13.0 for Windows. When significant differences were detected, individual mean values were compared by post hoc tests that allowed for multiple comparisons. P values <0.05 were considered statistically significant. Values are expressed as means ± SE.
RESULTS
Adverse RV Remodeling and RV Fibrosis Induced by PH is Reversed by E2 Therapy
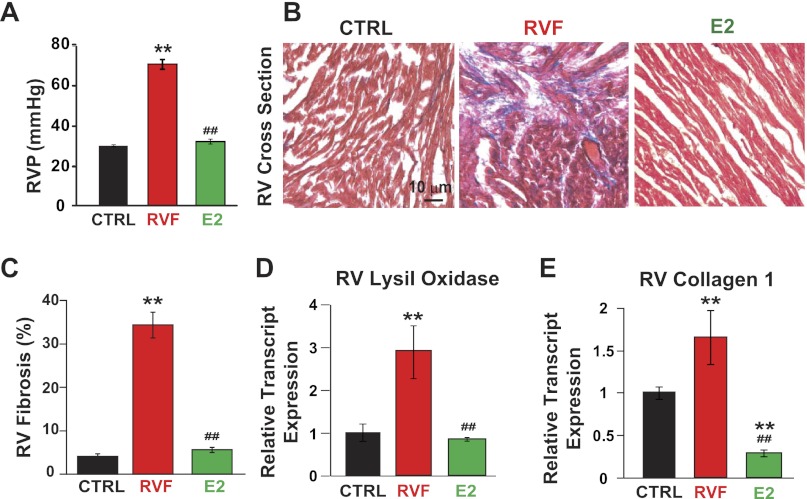
A single injection of MCT induced RV failure in ∼30 days (see Figs. 1A, 2A, and 7A). As we recently described, E2 therapy rescued advanced pulmonary hypertension and restored RV function in male rats by improving RV pressure from 72.0 ± 1.4 to 31.1 ± 0.9 mmHg (Fig. 2A), RV ejection fraction from 30.4 ± 1.7% to 61.5 ± 0.8%, and RV fractional shortening from 13.35 ± 0.47% to 31.81 ± 1.02% (46). In fact, estrogen therapy resulted in 100% survival whereas all untreated rats died by day 32 (46). Here we explored the direct effects of E2 therapy on RV remodeling. E2 treatment was able to completely reverse PH-induced RV fibrosis in males in vivo (RV fibrosis = 5.66 ± 0.33% vs. 33 ± 3.2%, P < 0.05, Fig. 2, B and C). E2 also reversed PH-induced transcript upregulation of profibrotic markers in the RV including lysil oxidase (1.00 ± 0.19 in CTRL, 2.90 ± 0.62 in RVF, 0.83 ± 0.05 in E2, all P < 0.05, Fig. 2D) and collagen 1 (1 ± 0.07 in CTRL, 1.65 ± 0.31 in RVF, 0.29 ± 0.04 in E2, all P < 0.05, Fig. 2E).
Fig. 2.
E2 restores RV function and reverses RV fibrosis associated with pulmonary hypertension (PH). A: RV pressure in CTRL, RVF, and E2. B: trichrome staining of RV cross sections. Red indicates cardiomyocytes and blue shows collagen deposition (fibrosis). C: quantification of %RV fibrosis in CTRL, RVF, and E2. D and E: RV lysil oxidase (D) and collagen I relative transcript expression (E) in CTRL, RVF, and E2. **P < 0.05 vs. CTRL; ##P < 0.05 vs. RVF.
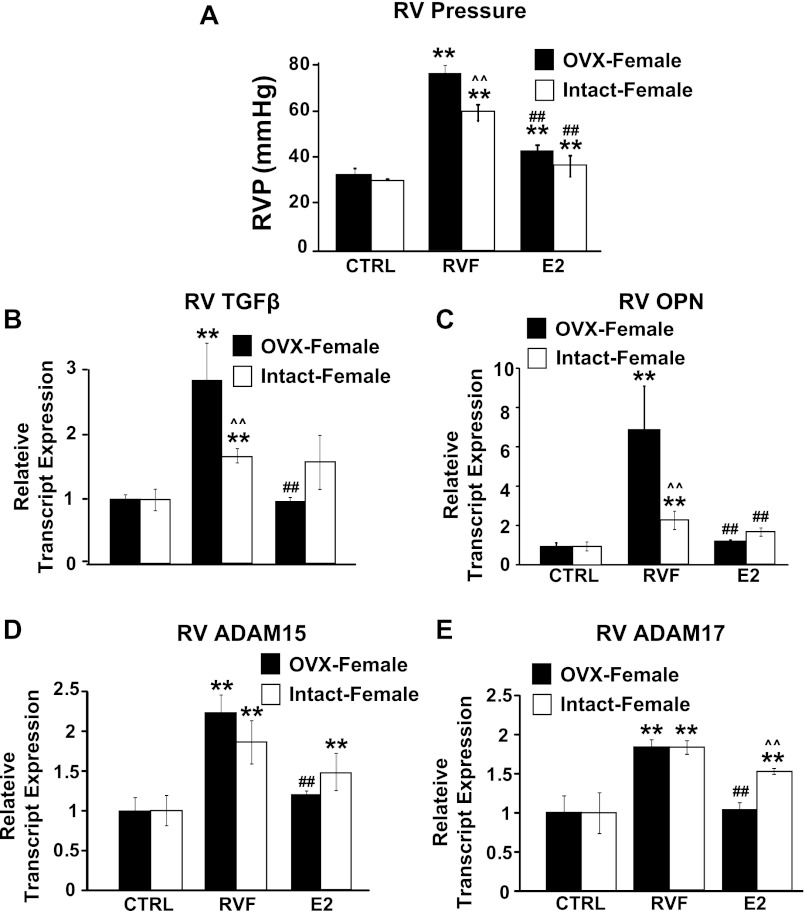
Fig. 7.
Intact females are partially protected from PH-induced RV remodeling while ovariectomized (OVX) females are not. A: RV pressure in OVX and intact females. Relative transcript expression of TGF-β (B), OPN (C), ADAM15 (D), and ADAM17 (E). **P < 0.05 vs. corresponding CTRL; ##P < 0.05 vs. corresponding RVF; ∧∧P < 0.05 vs. corresponding OVX group.
E2 Therapy Reverses PH-Induced Increase in ADAM Expression in the RV
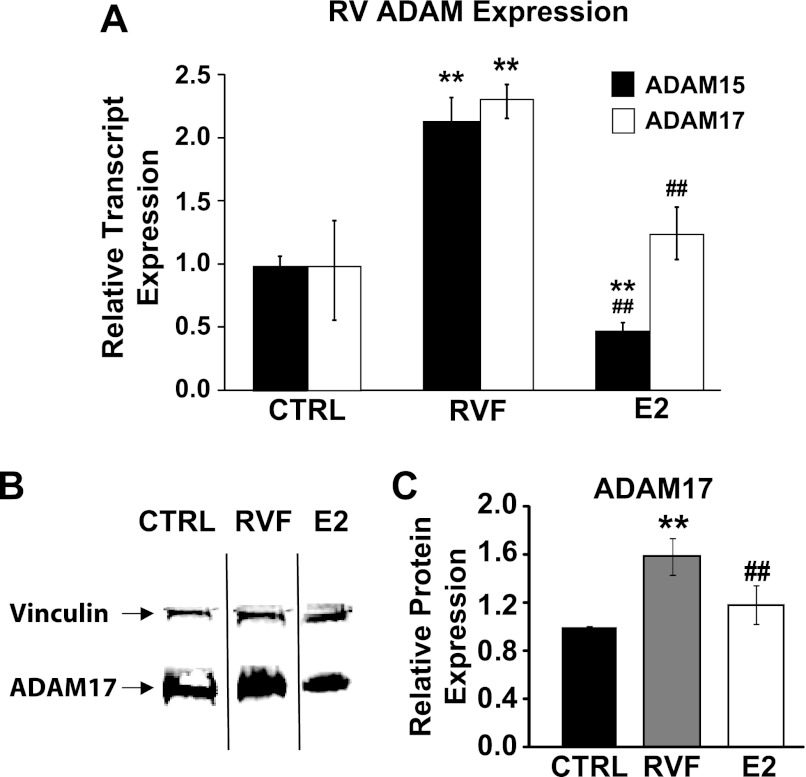
Since ADAM15 and ADAM17 are emerging as two of the most important ADAMs present in the heart during LV dysfunction, we examined their expression in the RV during PH and whether E2 therapy can reverse these changes. RVF was associated with a ∼2 fold upregulation of ADAM15 and ADAM17 transcripts in male rats (2.13 ± 0.19 for ADAM15 and 1.85 ± 0.04 for ADAM17, normalized to their corresponding CTRLs, all P < 0.05 vs. CTRL, Fig. 3A). E2 therapy fully restored ADAM15 and ADAM17 expression to normal levels in vivo (0.47 ± 0.07 for ADAM15, 1.3 ± 0.14 for ADAM17, all P < 0.05 vs. RVF, Fig. 3A). Western blotting also revealed that ADAM17 was upregulated in the RV of RVF animals and reversed with E2 therapy (1.00 ± 0.01 in CTRL, 1.59 ± 0.15 in RVF, 1.18 ± 0.16 in E2, all P < 0.05 Fig. 3, B and C).
Fig. 3.
E2 reverses RV ADAM15 and ADAM17 expression induced by PH. A: ADAM15 and ADAM17 relative transcript expression in the RV in CTRL, RVF, and E2. B: Western blot showing ADAM17 (bottom band) and vinculin (top band) representative samples from CTRL, RVF, and E2 groups. C: quantification of ADAM17 relative protein expression normalized to vinculin and CTRL from Western blot in CTRL, RVF, and E2 groups. **P < 0.05 vs. CTRL; ##P < 0.05 vs. RVF.
Estrogen Therapy Reverses the Expression of Remodeling Enzymes In Vitro Through Estrogen Receptor β
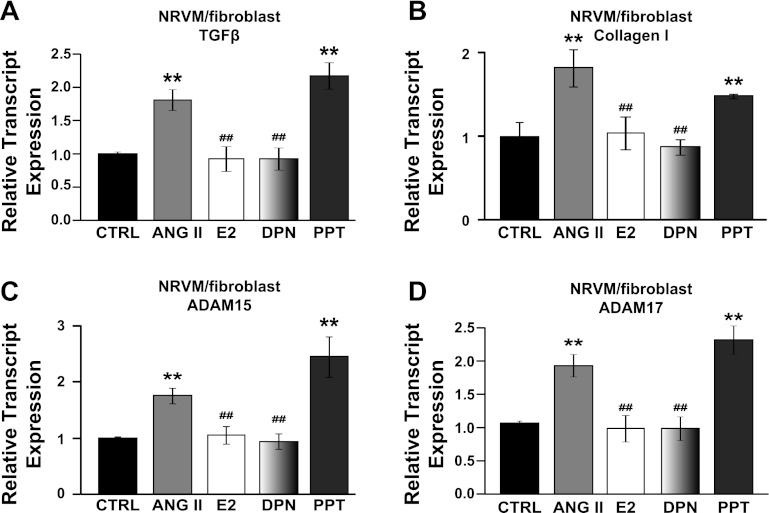
E2 therapy was also able to reverse ANG II-induced upregulation of TGF-β and collagen type 1 in vitro in cocultured cardiac NRVMs and fibroblasts (1.75 ± 0.09 in ANG II, 0.97 ± 0.12 in ANG II + E2 for TGF-β; 1.82 ± 0.22 in ANG II, 1.04 ± 0.20 ANG II + E2 for collagen I, normalized to CTRL, all P < 0.05, Fig. 4, A and B). Estrogen receptor-β agonist DPN was as effective as E2 in reversing ANG II-induced expression of TGF-β and collagen 1 (0.88 ± 0.09 for TGF-β, 87 ± 0.09 for collagen 1, all P < 0.05 vs. ANG II, Fig. 4, A and B), but estrogen receptor-α agonist PPT was not [2.18 ± 0.35 for TGF-β, 1.48 ± 0.02 for collagen 1, all P = not significant (NS) vs. RVF, Fig. 4, A and B]. ANG II treatment of cocultured cardiac NRVMs and fibroblasts also resulted in increased expression of ADAM15 and ADAM17 transcripts (1.76 ± 0.14 and 1.81 ± 0.16-fold, respectively; P < 0.05 vs. CTRL, Fig. 4, C and D). E2 restored upregulation of ADAM15 and ADAM17 in vitro (1.05 ± 0.15 and 0.92 ± 0.19 respectively; P < 0.05 vs. ANG II, Fig. 4, C and D). Angiotensin II-induced upregulation of ADAM15 and ADAM17 was reversed by DPN (0.95 ± 0.14 for ADAM15, 0.92 ± 0.17 for ADAM17, all P < 0.05 vs. RVF, Fig. 4, C and D) but not with PPT (2.45 ± 0.36 for ADAM15, 2.17 ± 0.20 for ADAM17, all P = NS vs. CTRL, Fig. 4, C and D).
Fig. 4.
Estrogen therapy directly inhibits the expression of extracellular matrix (ECM) remodeling enzymes in vitro in cocultured NRVMs and fibroblasts. Relative transcript expression of ECM remodeling enzymes TGF-β (A), collagen I (B), ADAM15 (C), and ADAM17 (D) in cocultured NRVMs and fibroblasts in CTRL, ANG II, ANG II + E2, ANG II + DPN, and ANG II + PPT groups. **P < 0.05 vs. CTRL; ##P < 0.05 vs. ANG II.
Upregulation of PH-Induced RV OPN Expression in RVF is Reversed by E2 Therapy
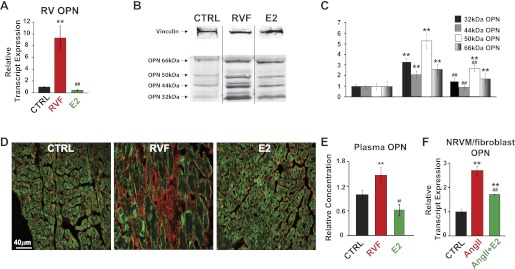
The transcript levels of OPN, a matricellular fetal gene, were upregulated more than ninefold in RVF male rats (1.00 ± 0.10 CTRL to 9.33 ± 2.07 in RVF, P < 0.05, Fig. 5A). Similar to ADAMs, OPN expression returns to control levels following E2 therapy (0.45 ± 0.16, P < 0.05, Fig. 5A). Western blotting revealed four isoforms of OPN, 32 kDa, 44 kDa, 50 kDa, and 66 kDa, which were all upregulated in the RV in RVF group (Fig. 5, B and C). Estrogen was able to reverse the protein expression of the 32-kDa, 44-kDa, and 50-kDa isoforms (Fig. 5, B and C). The full-length OPN is partially downregulated but shows no statistical significance in response to E2 therapy (Fig. 5, B and C). Immunoctyochemistry staining of RV cross sections shows localization of OPN exclusively to the extracellular space (Fig. 5D). The total OPN expression observed is elevated in RVF and reversed with E2 therapy (Fig. 5D).
Fig. 5.
Osteopontin (OPN) expression is elevated in the RV of RVF group and is reversed with E2-therapy. A: OPN relative transcript expression measured in CTRL, RVF, and E2. **P < 0.05 vs. CTRL; ##P < 0.05 vs. RVF. B: Western blot showing 4 different length isoforms of OPN (bottom 4 bands, 32 kDa, 44 kDa, 50 kDa, and 66 kDa) and vinculin (top band) representative samples. C: quantification of OPN protein expression normalized to appropriate CTRL and vinculin protein from Western blot in CTRL, RVF, and E2 groups. **P < 0.05 vs. corresponding CTRL; ##P < 0.05 vs. corresponding RVF. D: staining of RV cross sections double labeled with L-type calcium channel α1c (green) and OPN (red). E: OPN plasma levels measured by sandwich-ELISA, normalized to CTRL in CTRL, RVF, and E2. **P < 0.05 vs. CTRL; ##P < 0.05 vs. RVF. F: OPN transcript expression in NRVM/fibroblast culture in CTRL, ANG II treated, and ANG II + E2 treated. **P < 0.05 vs. CTRL; ##P < 0.05 vs. ANG II.
OPN plasma levels have also shown to be increased in PH, supporting the view that OPN has some potential as a biomarker (26). Here we explored whether E2 therapy could reverse increased plasma OPN levels observed in PH. Plasma OPN levels increased significantly ∼1.5 fold in RVF males (1.47 ± 0.19 normalized to CTRL, P < 0.05, Fig. 5E). Circulating OPN levels were restored to control levels (0.62 ± 0.14, P < 0.05 vs. RVF, Fig. 5E) following E2 therapy. In vitro, OPN expression is elevated in ANG II-treated cocultured cardiac NRVMs and fibroblasts, and reversed in the presence of E2 (2.74 ± 0.19-fold in ANG II, 1.72 ± 0.01-fold in ANG II + E2; all P < 0.05, Fig. 5F).
Akt Signaling is Involved in the Progression of PH-Induced Adverse RV Remodeling and Both Akt and ERK in Estrogen-Mediated Reverse Remodeling
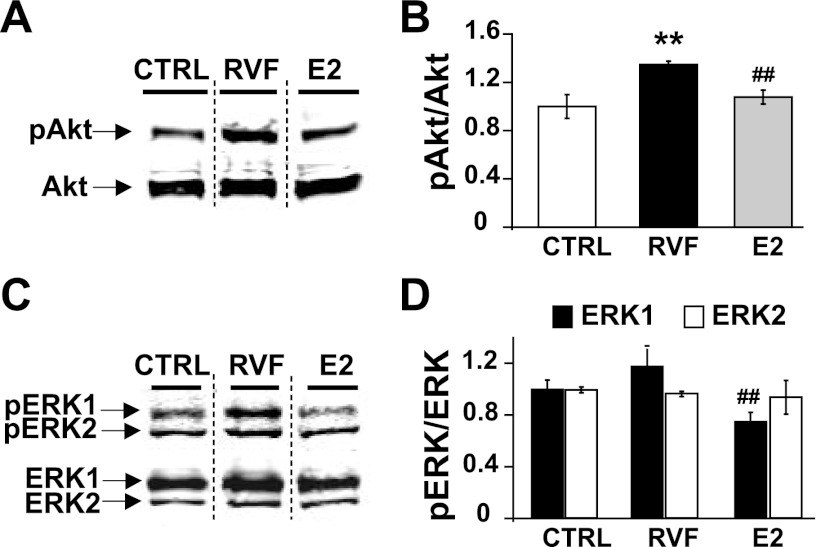
AKT and ERK signaling are known to be regulated by E2 (60). We explored whether these signaling cascades are involved in RV remodeling secondary to PH and are regulated by E2. The progression to RVF was associated with upregulation of Akt phosphorylation (pAkt/Akt =1.00 ± 0.10 in CTRL, 1.35 ± 0.03 in RVF, P < 0.05, normalized to CTRL, Fig. 6, A and B) but not ERK phosphorylation (pERK1/ERK1 = 1.00 ± 0.07 in CTRL, 1.17 ± 0.13 in RVF; pERK2/ERK2 = 1.00 ± 0.02 in CTRL, 0.97 ± 0.02 in RVF; all P = NS, Fig. 6, C and D). With E2 therapy, Akt phosphorylation is significantly decreased (pAkt/Akt = 1.07 ± 0.06, P < 0.05 vs. RVF, Fig. 6, A and B) as is ERK phosphorylation (pERK1/ERK1 = 0.75 ± 0.07, P < 0.05 vs. RVF and CTRL, pERK2/ERK2 = 0.94 ± 0.13, P = NS, Fig. 6, C and D).
Fig. 6.
RV failure is associated with elevated Akt phosphorylation, but not ERK, in the RV. E2 therapy restores RVF-induced Akt phosphorylation. A: Western blot showing phospho-Akt (pAkt; top band) and Akt (bottom band) representative samples from CTRL, RVF, and E2 groups. B: quantification of relative pAkt/Akt and protein expression from Western blots. **P < 0.05 vs. CTRL; ##P < 0.05 vs. RVF. C: Western blot showing phospho-ERK1/2 (pERK1/2) (top two bands) and ERK1/2 (bottom two bands) representative samples from CTRL, RVF, and E2 groups. Quantification of relative pERK1/ERK1 (D) and pERK2/ERK2 protein expression (E) from Western blots. **P < 0.05 vs. CTRL; ##P < 0.05 vs. RVF.
Less Severe PH-Induced RV Remodeling in Intact Female Rats is Abolished by Ovariectomy
Intact female rats developed less severe PH compared with male and OVX rats as the RV pressures was significantly lower (62.53 ± 3.34 mmHg in females vs. 77.61 ± 3.49 mmHg in OVX and 72 ± 1.4 mmHg in male; P < 0.05 for all RVF groups vs. corresponding CTRL; P < 0.05 for RVF females vs. RVF males and RVF OVX-females; P = NS for RVF male vs. RVF OVX, Fig. 7A). E2 therapy was able to reverse elevated RV pressure in both intact and OVX females as in males (RVP = 36.69 ± 4.47 mmHg in intact females, 44.06 ± 2.52 mmHg in OVX females, all P < 0.05 vs. corresponding RVF animals, Fig. 7A). Intact females also appeared to develop less RV fibrosis compared with OVX rats (TGF-β transcript = 1.64 ± 0.11 in intact females, 2.58 ± 0.52 in OVX females, normalized to respective controls; P < 0.05 vs. corresponding control, P < 0.05 between intact and OVX females, Fig. 7B). Interestingly, E2 therapy was able to successfully reverse RV TGF-β expression in OVX females (0.97 ± 0.07, P < 0.05 vs. corresponding RVF, normalized to control, Fig. 7B) but was not as successful in intact females (1.42 ± 0.38, normalized to control, P = NS vs. corresponding RVF, Fig. 7B). OPN expression was upregulated ∼2 fold in intact females, and ∼7 fold in OVX (OPN transcript = 2.30 ± 0.47 for intact females, 7.16 ± 2.26 for OVX females, all P < 0.05 vs. corresponding CTRL; P < 0.05 between intact and OVX females, Fig. 7C). Both intact and OVX females showed elevated ADAM15 (2.22 ± 0.21 and 1.85 ± 0.27, P < 0.05 vs. respective CTRL, P = NS vs. each other, Fig. 7D) and ADAM17 (1.84 ± 0.09 and 1.83 ± 0.08, P < 0.05 vs. respective CTRL, P = NS vs. each other, Fig. 7E). E2 therapy also reversed expression of these enzymes to CTRL levels (P < 0.05 vs. corresponding RVF, P = NS vs. each other).
DISCUSSION
Recently we showed that E2 can rescue severe preexisting PH (46). The main finding of this study is that E2 therapy reverses PH-induced adverse structural and ECM remodeling of the RV induced by PH, including fibrosis and altered expression of ECM proteins OPN, ADAM15, and ADAM17 in male rats. We found that the same dose of MCT induced less severe PH in intact females as RV systolic pressure (RVSP) was significantly lower than in males (Fig. 7). Ovariectomy however exacerbates the disease in females as the RVP was similar to males (see Fig. 2, 7). The adverse RV remodeling induced by MCT in intact females was also not as extensive as in OVX females, e.g., less fibrosis compared with OVX females and males. Exogenous estrogen reversed MCT-induced increased in RVSP and adverse RV remodeling in both intact and OVX females. In vitro, E2 reversed ANG II-induced expression of TGF-β, collagen I, OPN, ADAM15, and ADAM17. Estrogen receptor-β agonist DPN, but not estrogen receptor-α agonist PPT, also reversed ANG II-induced expression of these markers. These in vitro data suggest a possible direct action of E2 on the RV through estrogen receptor-β.
RV Failure Secondary to Chronic PH
PH is a chronic lung disorder, the progression of which causes RV hypertrophy and failure leading to sudden cardiac death (47). Current treatments aim to delay the progression of the disease, but there is still no definitive ideal therapy for preexisting PH (3). Although RV failure secondary to chronic PH is a cause of significant mortality, the specifics of PH-induced RV failure are still being elucidated (10, 19, 47). As a result, improving the lung condition has been the target of current therapies, but there is a lack of treatments targeting the failing RV. As the mechanisms of PH-induced RVF are illuminated, there is potential for developing RV-specific therapies for PH. These therapies have the potential to improve the survival of patients suffering from chronic PH (10).
As PH-induced RV failure progresses and adverse cardiac remodeling begins, the cardiac ECM composition is drastically altered (25, 45). These changes in the ECM can disrupt cardiac function in several ways. For example, deposition of collagen and other ECM proteins can inhibit contractility and electrical signal conduction. The degradation of integrins can have an effect on chemical signaling (35). However, RV failure also leads to the activation of a fetal gene program, a reexpression of several genes normally only present in the developing heart (4). It is thought that the purpose of these reexpressed genes is partially to support cardiac structure and function during failure (11).
E2 Therapy and Cardiac Remodeling
We have recently shown that E2 therapy can rescue severe preexisting pulmonary vascular disease associated with PH (46). E2 has been shown to directly inhibit cardiac fibrosis. Pedram et al. (32) recently showed that E2 can directly prevent cardiac fibrosis by inhibiting the transition of fibroblasts to myofibroblasts through an estrogen receptor-β-mediated mechanism (32). Females have also been shown to be protected from PH-induced RV failure (34). The role of E2 in preventing or reversing the changes in RV structure associated with PH is an area of current interest.
The fact that intact females develop less severe adverse RV remodeling (including RV pressures, fibrosis, and expression of ECM remodeling enzymes) compared with OVX females (Fig. 7) further supports a protective role of endogenous estrogen (Fig. 7). E2 therapy resulted in reversal of RV remodeling associated with RV dysfunction in both males and females (OVX and intact). These data suggest that endogenous estrogen plays a protective role in PH-induced RVF, and that exogenous estrogen therapy could have a positive effect as postmenopausal hormone replacement therapy to mitigate PH and the resulting RV remodeling. Our in vitro studies show that estrogen receptor-β agonist DPN, but not estrogen receptor-α agonist PPT, is almost as effective as E2 in reversing ANG-II induced overexpression of TGF-β and collagen I. Our data support the view that the direct inhibitory effect of E2 on cardiac fibrosis is mainly mediated through estrogen receptor-β and are in agreement with Pedram et al. (32).
E2 Therapy Reverses PH-Induced ADAM15 and ADAM17 Upregulation in the RV
Among the ECM-associated proteins, the MMP family has been well documented for its direct role in causing adverse remodeling associated with PH-induced RV remodeling(45). The role of ADAMs in this remodeling is a much more recently emerging field. The ADAM family, like the related MMP family, is responsible for breaking down ECM anchoring proteins, but in addition to the metalloproteinase function, ADAMs also have a disintegrin domain (7). Integrins play a role in ECM interactions between cardiac myocytes and fibroblasts, and also provide overall structural integrity and mediate cell-cell communication in the heart (7). Among their many functions, integrins have been found to be responsible for cardioprotective signaling in the failing myocardium, as well as in the prevention of fibrosis leading to heart failure, and integrins are known to be downregulated during PH-induced RV remodeling (18, 39, 41). Although ADAM15 and ADAM17 have been less studied, they are emerging as two of the most important ADAMs present in the heart during LV dysfunction, as not all ADAMs show altered regulation during cardiac stress (13, 23, 52). Here we found that both ADAM15 and ADAM17 are upregulated in RVF males but are restored to control levels with E2 treatment. Both ADAMs are also upregulated in both OVX and intact females, Interestingly E2 therapy is not as effective in reversing expression in intact females. We also show that ADAM15 and ADAM17 expression is reversed by E2 therapy through estrogen receptor-β in our in vitro model of cardiac stress.
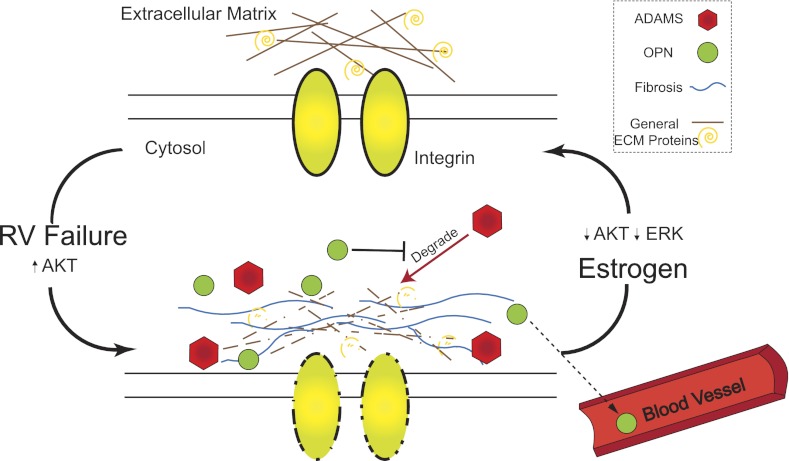
E2 has been shown to inhibit metalloproteinase transcription, specifically MMP-2 via the MAP kinase pathway (28). E2 may have a similar inhibitory effect on other metalloproteinases, including ADAMs. It is possible that E2 therapy reverses the progression to RV failure by directly inhibiting adverse ECM remodeling metalloproteinases in the cardiac ECM (Fig. 8).
Fig. 8.
Hypothetical scheme of PH-induced adverse RV remodeling and its reversal by estrogen. In a healthy right ventricle, the ECM is well-ordered and ECM degradation is tightly controlled. Severe PH leads to an increase in RV Akt signaling, and also elevated RV fibrosis, a degradation of general RV ECM proteins and integrins, and a reexpression of ADAMs and fetal gene OPN in the RV. Some OPN leaks into the plasma where it can be used as a biomarker. E2 therapy reduces Akt and ERK signaling and reverses the changes in the ECM, returning the RV to a healthy state.
Elevated RV OPN Expression in PH is Reversed by E2 Therapy
The ECM interacting matricellular protein OPN, originally described for its role in bone tissue, is expressed in the developing heart but is virtually nonexistent in a healthy adult heart (43). OPN has been shown to reexpress in the heart in response to stress (6, 55, 56). OPN also plays a role in the inflammatory response in the heart, although its exact role in inflammation is still not fully understood (36). We observed elevated OPN expression in the RV of RVF both in males and females, although to a much lesser degree in intact females (∼2-fold in intact females vs. ∼8-fold in male and OVX females). E2 therapy was able to reverse increased OPN expression in all three groups to normal levels in vivo. OPN expression was also elevated in an in vitro model of cardiac stress and reversed with E2 therapy.
OPN is known to have several isoforms (58). Western blotting of RV tissue from males revealed at least four major isoforms of OPN; we observed OPN at 66 kDa, 50 kDa, 44 kDa, and 32 kDa. Full-length OPN is known to be cleaved into 40- and 32-kDa isoforms with thrombin or MMP treatments (15, 61). OPN has been shown to be involved with cell migration, fusion, and motility, and all these isoforms of OPN appear to play a role in cell adhesion and migration, suggesting their role in ECM integrity (1, 14). Others have also observed a 50-kDa OPN that appears to be predominantly secreted from cells (50). Numerous other isoforms are suggested to be formed by cleavage and posttranslation modifications such as glycosylation and phosphorylation (59).
Here we found four main isoforms in the RV, which were all upregulated (∼2- to 5-fold) in RVF male rats. The 32-, 44-, and 50-kDa isoforms were significantly downregulated by E2 therapy. Although full-length OPN appeared partially downregulated by E2 therapy, there was no statistical significance. It is possible that E2 is involved in regulating the cleavage of OPN into its active isoforms but has a less pronounced effect on total OPN expression.
We speculate that OPN plays a role in mitigating adverse ECM remodeling in the failing RV. OPN knockout mice have been shown to demonstrate altered collagen deposition (24). Furthermore, OPN has directly been shown to inhibit metalloproteinases, specifically MMP-2 and MMP-9, through IL1-beta and PKC-zeta (57). MMP-2 and MMP-9 are known to be important proteins in advancing the adverse remodeling of the RV during failure and causing many of the negative structural changes that accompany RV failure (45). It is possible that OPN is reexpressed to support the ECM that is being degraded in a failing heart. Since E2 and OPN both have been observed to inhibit metalloproteinases, it is possible that there is a more direct link between E2 therapy and fetal gene reexpression. Further study on the connection between E2, ADAMs, and fetal genes like OPN is warranted.
OPN as a Potential Plasma Marker for PH
Several cardiac fetal genes, especially the natriuretic peptides brain natriuretic peptide (BNP), NT-proBNP, and atrial natriuretic factor (ANF), are emerging as clinical plasma markers of heart failure (49). The plasma ratio of MMP-1 to its inhibitor TIMP-1 is already a well-established marker of PH-induced RVF (16). Schumann et al. recently showed in human samples that plasma levels of TIMP-4, tenascin-C, MMP-2, and NT-proBNP, all ECM interacting proteins, are elevated in PH and are correlated with disease severity (38). OPN seems to be another promising biomarker for PH-induced RVF (26). Here we observed that OPN plasma levels significantly increase in the RVF group. E2 therapy was associated with complete reversal of increased plasma OPN levels. It is important to note that the plasma OPN observed may also be partially due to elevated OPN expression in the lung during pulmonary hypertension as recently reported in addition to the OPN in the RV we report here (53). Regardless of the origin of the plasma OPN, our data further support the role of OPN as a potential biomarker for PH-induced RVF and recovery.
Akt and ERK Signaling During PH-Induced RV Failure and Estrogen-Mediated Recovery
Akt and ERK signaling are critical mediators of cardiac hypertrophy, survival, and stress. Akt signaling is known to be involved in both cardioprotection as well as in several models of cardiac stress, including ischemia, transaortic constriction, and in human patients with left ventricular assist devices (2, 21, 33). Recently, Drake et al. (12) showed that Akt signaling is increased during right heart failure PH. We also find increased Akt phosphorylation in the RV of RVF males (Fig. 6). With E2 therapy, we observe a significant decline in Akt phosphorylation (Fig. 6). Similarly, ERK is known to play a role in left ventricular pathological heart hypertrophy (29). Interestingly, we saw no increase in ERK1/2 signaling during RVF. However, with E2 therapy there is a decline in ERK1 phosphorylation. These results provide some insight into the molecular signature of PH-induced RVF and E2-mediated recovery.
Limitations
This study is not a global analysis of ECM remodeling enzymes during RVF and their response to E2 therapy. The focus of this study was to instead examine the role of E2 therapy in reversing adverse RV remodeling and to identify some novel enzymes involved in the remodeling process. It would be interesting to pursue a more global analysis of ECM and fetal genes involved in the remodeling process of the RV. Here we observed that OPN plasma levels significantly increase in the RVF group. It would be interesting to explore whether circulating OPN is predominantly of cardiac or pulmonary origin. Some additional studies to measure OPN concentration in blood from the aortic root and from the coronary sinus would provide some insight into the source of circulating OPN. Regardless of the origin of OPN in the plasma, it still serves a valuable role as a circulating biomarker in this model and has also been shown in patients with PH (27, 54).
Conclusions
We show significant RV structural and ECM remodeling in PH-induced RVF in males and to a lesser extent in female rats. Protection from PH-induced RV remodeling in intact female rats is abolished by ovariectomy. Matricellular protein and fetal gene OPN, as well as disintegrin metalloproteinases ADAM15 and ADAM17, show elevated expression in RVF group. E2 therapy reversed adverse RV remodeling in both male and female rats. In vitro, E2 therapy directly inhibits expression of remodeling enzymes most likely through estrogen receptor-β. These in vitro studies support the direct action of E2 on the myocardium in addition to its effects on the pulmonary vasculature. OPN also shows promise as a plasma biomarker of PH that correlates with recovery.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-089876 and HL-089876S1 (Mansoureh Eghbali).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.D.N., S.U., G.W., Mansour Eghbali, and Mansoureh Eghbali conception and design of research; R.D.N., S.U., G.W., Mansour Eghbali, A.I., H.M., and R.P.-N. performed experiments; R.D.N., S.U., G.W., Mansour Eghbali, A.I., H.M., R.P.-N., and Mansoureh Eghbali analyzed data; R.D.N., S.U., G.W., Mansour Eghbali, A.I., H.M., R.P.-N., and Mansoureh Eghbali interpreted results of experiments; R.D.N., S.U., G.W., Mansour Eghbali, and Mansoureh Eghbali prepared figures; R.D.N. drafted manuscript; R.D.N., S.U., G.W., Mansour Eghbali, and Mansoureh Eghbali edited and revised manuscript; R.D.N., S.U., G.W., Mansour Eghbali, A.I., H.M., R.P.-N., and Mansoureh Eghbali approved final version of manuscript.
REFERENCES
- 1. Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J Biol Chem 276: 28261–28267, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Baba HA, Stypmann J, Grabellus F, Kirchhof P, Sokoll A, Schafers M, Takeda A, Wilhelm MJ, Scheld HH, Takeda N, Breithardt G, Levkau B. Dynamic regulation of MEK/Erks and Akt/GSK-3beta in human end-stage heart failure after left ventricular mechanical support: myocardial mechanotransduction-sensitivity as a possible molecular mechanism. Cardiovasc Res 59: 390–399, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 122: 164–172, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Black FM, Packer SE, Parker TG, Michael LH, Roberts R, Schwartz RJ, Schneider MD. The vascular smooth muscle alpha-actin gene is reactivated during cardiac hypertrophy provoked by load. J Clin Invest 88: 1581–1588, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bogaard HJ, Abe K, Vonk NA, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 135: 794–804, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 120: 1951–1960, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Bowers SL, Banerjee I, Baudino TA. The extracellular matrix: at the center of it all. J Mol Cell Cardiol 48: 474–482, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Butler GS, Apte SS, Willenbrock F, Murphy G. Human tissue inhibitor of metalloproteinases 3 interacts with both the N- and C-terminal domains of gelatinases A and B. Regulation by polyanions. J Biol Chem 274: 10846–10851, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Cowan KN, Heilbut A, Humpl T, Lam C, Ito S, Rabinovitch M. Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat Med 6: 698–702, 2000 [DOI] [PubMed] [Google Scholar]
- 10. D′Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 115: 343–349, 1991 [DOI] [PubMed] [Google Scholar]
- 11. Depre C, Park JY, Shen YT, Zhao X, Qiu H, Yan L, Tian B, Vatner SF, Vatner DE. Molecular mechanisms mediating preconditioning following chronic ischemia differ from those in classical second window. Am J Physiol Heart Circ Physiol 299: H752–H762, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drake JI, Bogaard HJ, Mizuno S, Clifton B, Xie B, Gao Y, Dumur CI, Fawcett P, Voelkel NF, Natarajan R. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am J Respir Cell Mol Biol 45: 1239–1247, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fedak PW, Moravec CS, McCarthy PM, Altamentova SM, Wong AP, Skrtic M, Verma S, Weisel RD, Li RK. Altered expression of disintegrin metalloproteinases and their inhibitor in human dilated cardiomyopathy. Circulation 113: 238–245, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Gao YA, Agnihotri R, Vary CP, Liaw L. Expression and characterization of recombinant osteopontin peptides representing matrix metalloproteinase proteolytic fragments. Matrix Biol 23: 457–466, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Gao YA, Agnihotri R, Vary CP, Liaw L. Expression and characterization of recombinant osteopontin peptides representing matrix metalloproteinase proteolytic fragments. Matrix Biol 23: 457–466, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Gonzalez A, Lopez B, Querejeta R, Zubillaga E, Echeverria T, Diez J. Filling pressures and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction. Hypertension 55: 1418–1424, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez A, Ravassa S, Beaumont J, Lopez B, Diez J. New targets to treat the structural remodeling of the myocardium. J Am Coll Cardiol 58: 1833–1843, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Hessel M, Steendijk P, den AB, Schutte C, van der LA. Pressure overload-induced right ventricular failure is associated with re-expression of myocardial tenascin-C and elevated plasma tenascin-C levels. Cell Physiol Biochem 24: 201–210, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jais X, Montani D, Souza R, Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 122: 156–163, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Hwang DM, Dempsey AA, Lee CY, Liew CC. Identification of differentially expressed genes in cardiac hypertrophy by analysis of expressed sequence tags. Genomics 66: 1–14, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Ivanova M, Janega P, Matejikova J, Simoncikova P, Pancza D, Ravingerova T, Barancik M. Activation of Akt kinase accompanies increased cardiac resistance to ischemia/reperfusion in rats after short-term feeding with lard-based high-fat diet and increased sucrose intake. Nutr Res 31: 631–643, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Kwak HI, Mendoza EA, Bayless KJ. ADAM17 co-purifies with TIMP-3 and modulates endothelial invasion responses in three-dimensional collagen matrices. Matrix Biol 28: 470–479, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Li JK, Du WJ, Jiang SL, Tian H. Expression of ADAM-15 in rat myocardial infarction. Int J Exp Pathol 90: 347–354, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1). J Clin Invest 101: 1468–1478, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 104: 545–556, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Lorenzen JM, Nickel N, Kramer R, Golpon H, Westerkamp V, Olsson KM, Haller H, Hoeper MM. Osteopontin in patients with idiopathic pulmonary hypertension. Chest 2010 [DOI] [PubMed] [Google Scholar]
- 27. Lorenzen JM, Nickel N, Kramer R, Golpon H, Westerkamp V, Olsson KM, Haller H, Hoeper MM. Osteopontin in patients with idiopathic pulmonary hypertension. Chest 139: 1010–1017, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Mahmoodzadeh S, Dworatzek E, Fritschka S, Pham TH, Regitz-Zagrosek V. 17Beta-estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts. Cardiovasc Res 85: 719–728, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meng R, Pei Z, Zhang A, Zhou Y, Cai X, Chen B, Liu G, Mai W, Wei J, Dong Y. AMPK activation enhances PPARalpha activity to inhibit cardiac hypertrophy via ERK1/2 MAPK signaling pathway. Arch Biochem Biophys 511: 1–7, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Mirotsou M, Dzau VJ, Pratt RE, Weinberg EO. Physiological genomics of cardiac disease: quantitative relationships between gene expression and left ventricular hypertrophy. Physiol Genomics 27: 86–94, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Okada M, Harada T, Kikuzuki R, Yamawaki H, Hara Y. Effects of telmisartan on right ventricular remodeling induced by monocrotaline in rats. J Pharm Sci 111: 193–200, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Pedram A, Razandi M, O'Mahony F, Lubahn D, Levin ER. Estrogen receptor-beta prevents cardiac fibrosis. Mol Endocrinol 24: 2152–2165, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Previlon M, Pezet M, Dachez C, Mercadier JJ, Rouet-Benzineb P. Sequential alterations in Akt, GSK3beta, and calcineurin signalling in the mouse left ventricle after thoracic aortic constriction. Can J Physiol Pharmacol 88: 1093–1101, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Rabinovitch M, Gamble WJ, Miettinen OS, Reid L. Age and sex influence on pulmonary hypertension of chronic hypoxia and on recovery. Am J Physiol Heart Circ Physiol 240: H62–H72, 1981 [DOI] [PubMed] [Google Scholar]
- 35. Reddy HK, Tjahja IE, Campbell SE, Janicki JS, Hayden MR, Tyagi SC. Expression of matrix metalloproteinase activity in idiopathic dilated cardiomyopathy: a marker of cardiac dilatation. Mol Cell Biochem 264: 183–191, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol 283: H1802–H1810, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol 161: 3340–3346, 1998 [PubMed] [Google Scholar]
- 38. Schumann C, Lepper PM, Frank H, Schneiderbauer R, Wibmer T, Kropf C, Stoiber KM, Rudiger S, Kruska L, Krahn T, Kramer F. Circulating biomarkers of tissue remodelling in pulmonary hypertension. Biomarkers 15: 523–532, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res 90: 458–464, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Stawowy P, Blaschke F, Pfautsch P, Goetze S, Lippek F, Wollert-Wulf B, Fleck E, Graf K. Increased myocardial expression of osteopontin in patients with advanced heart failure. Eur J Heart Fail 4: 139–146, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Suryakumar G, Kasiganesan H, Balasubramanian S, Kuppuswamy D. Lack of beta3 integrin signaling contributes to calpain-mediated myocardial cell loss in pressure-overloaded myocardium. J Cardiovasc Pharmacol 55: 567–573, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Swildens J, de Vries AA, Li Z, Umar S, Atsma DE, Schalij MJ, van der LA. Integrin stimulation favors uptake of macromolecules by cardiomyocytes in vitro. Cell Physiol Biochem 26: 999–1010, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Thayer JM, Giachelli CM, Mirkes PE, Schwartz SM. Expression of osteopontin in the head process late in gastrulation in the rat. J Exp Zool 272: 240–244, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Udelson JE, Konstam MA. Ventricular remodeling fundamental to the progression (and regression) of heart failure. J Am Coll Cardiol 57: 1477–1479, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Umar S, Hessel M, Steendijk P, Bax W, Schutte C, Schalij M, van der WE, Atsma D, van der LA. Activation of signaling molecules and matrix metalloproteinases in right ventricular myocardium of rats with pulmonary hypertension. Pathol Res Pract 203: 863–872, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Umar S, Iorga A, Matori H, Nadadur RD, Li J, Maltese F, van der LA, Eghbali M. Estrogen rescues preexisting severe pulmonary hypertension in rats. Am J Respir Crit Care Med 184: 715–723, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Umar S, Steendijk P, Ypey DL, Atsma DE, van der Wall EE, Schalij MJ, van der LA. Novel approaches to treat experimental pulmonary arterial hypertension: a review. J Biomed Biotechnol 2010: 702836, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Umar S, de Visser YP, Steendijk P, Schutte CI, Laghmani EH, Wagenaar GTM, Bax WH, Mantikou E, Pijnappels DA, Atsma DE, Schalij MJ, van der Wall EE, van der Laarse A. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol 297: H1606–H1616, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Veien KT, Jensen JK, Hildebrandt P, Gotze JP, Nielsen OW, Kober L. [Natriuretic peptides as cardiac markers in clinical practice]. Ugeskr Laeger 172: 2111–2116, 2010 [PubMed] [Google Scholar]
- 50. Vejda S, Piwocka K, McKenna SL, Cotter TG. Autocrine secretion of osteopontin results in degradation of I kappa B in Bcr-Abl-expressing cells. Br J Haematol 128: 711–721, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Voloshenyuk TG, Gardner JD. Estrogen improves TIMP-MMP balance and collagen distribution in volume-overloaded hearts of ovariectomized females. Am J Physiol Regul Integr Comp Physiol 299: R683–R693, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Wang X, Oka T, Chow FL, Cooper SB, Odenbach J, Lopaschuk GD, Kassiri Z, Fernandez-Patron C. Tumor necrosis factor-alpha-converting enzyme is a key regulator of agonist-induced cardiac hypertrophy and fibrosis. Hypertension 54: 575–582, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Wang Y, Han DD, Wang HM, Liu M, Zhang XH, Wang HL. Downregulation of osteopontin is associated with fluoxetine amelioration of monocrotaline-induced pulmonary inflammation and vascular remodelling. Clin Exp Pharmacol Physiol 38: 365–372, 2011 [DOI] [PubMed] [Google Scholar]
- 54. Wang Y, Han DD, Wang HM, Liu M, Zhang XH, Wang HL. Downregulation of osteopontin is associated with fluoxetine amelioration of monocrotaline-induced pulmonary inflammation and vascular remodelling. Clin Exp Pharmacol Physiol 38: 365–372, 2011 [DOI] [PubMed] [Google Scholar]
- 55. Williams EB, Halpert I, Wickline S, Davison G, Parks WC, Rottman JN. Osteopontin expression is increased in the heritable cardiomyopathy of Syrian hamsters. Circulation 92: 705–709, 1995 [DOI] [PubMed] [Google Scholar]
- 56. Xie Z, Singh M, Singh K. Osteopontin modulates myocardial hypertrophy in response to chronic pressure overload in mice. Hypertension 44: 826–831, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Xie Z, Singh M, Siwik DA, Joyner WL, Singh K. Osteopontin inhibits interleukin-1beta-stimulated increases in matrix metalloproteinase activity in adult rat cardiac fibroblasts: role of protein kinase C-zeta. J Biol Chem 278: 48546–48552, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Zhao W, Wang L, Zhang L, Yuan C, Kuo PC, Gao C. Differential expression of intracellular and secreted osteopontin isoforms by murine macrophages in response to toll-like receptor agonists. J Biol Chem 285: 20452–20461, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59. Zhao W, Wang L, Zhang L, Yuan C, Kuo PC, Gao C. Differential expression of intracellular and secreted osteopontin isoforms by murine macrophages in response to toll-like receptor agonists. J Biol Chem 285: 20452–20461, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60. Zhou H, Hou SZ, Luo P, Zeng B, Wang JR, Wong YF, Jiang ZH, Liu L. Ginseng protects rodent hearts from acute myocardial ischemia-reperfusion injury through GR/ER-activated RISK pathway in an endothelial NOS-dependent mechanism. J Ethnopharmacol 135: 287–298, 2011 [DOI] [PubMed] [Google Scholar]
- 61. Zohar R, Suzuki N, Suzuki K, Arora P, Glogauer M, McCulloch CA, Sodek J. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J Cell Physiol 184: 118–130, 2000 [DOI] [PubMed] [Google Scholar]