Abstract
Although central to the susceptibility of adult diseases characterized by abnormal rhythmogenesis, characterizing the genes involved is a challenge. We took advantage of the C57BL/6J (B6) trait of hypoxia-induced periodic breathing and its absence in the C57BL/6J-Chr 1A/J/NaJ chromosome substitution strain to test the feasibility of gene discovery for this abnormality. Beginning with a genetic and phenotypic analysis of an intercross study between these strains, we discovered three quantitative trait loci (QTLs) on mouse chromosome 1, with phenotypic effects. Fine-mapping reduced the genomic intervals and gene content, and the introgression of one QTL region back onto the C57BL/6J-Chr 1A/J/NaJ restored the trait. mRNA expression of non-synonymous genes in the introgressed region in the medulla and pons found evidence for differential expression of three genes, the highest of which was apolipoprotein A2, a lipase regulator; the apo a2 peptide fragment (THEQLTPLVR), highly expressed in the liver, was expressed in low amounts in the medulla but did not correlate with trait expression. This work directly demonstrates the impact of elements on mouse chromosome 1 in respiratory rhythmogenesis.
Keywords: respiratory control, apnea, genetics, quantitative trait linkage, hypoxia
respiratory rhythmogenesis is a complex physiological process and is abnormal in a number of clinical disorders, including RETT syndrome, stroke, Shy-Drager syndrome, and sleep apnea (31). An inherited basis for respiratory instability is shown from studies in humans and genetically engineered mice (10); however, physical demonstration of naturally occurring genetic variations has been elusive (31). The C57BL/6J (B6) strain of mice exhibits spontaneous pauses (25, 32) and posthypoxic recurrent apneas, whereas the A/J strain of mice do not (12), indicating an inherent resistance to respiratory instability in the A/J genome. Furthermore, although in both strains breathing is slowed, apnea length is prolonged in the B6 following administration of an inhibitor of neural nitric oxide synthase, and apnea is not produced by the drug in the A/J strain (23). Finally, use of buspirone as a 5-HT agonist (29), inhibitors of hydrogen sulfide production (6), and acetazolamide (30) reduce or eliminate the apnea in the B6.
Our laboratory previously reported, using mouse chromosomal substitution strains (19), that post-sigh apneas in the B6 could be reversed by introducing chromosome 1 from the A/J on the B6 background (the C57BL/6J-Chr 1A/J/NaJ or B6a1 mouse) (32). The B6a1 strain demonstrated a reduced number of spontaneous pauses and resistance to exhibiting posthypoxic pauses and periodic breathing. This suggests that the B6 genome has biological elements resulting in alterations in ventilatory rate and rhythm.
Such studies in inbred mice address genetic and environmental issues, and in the comparison on the B6 and A/J strain an extraordinary amount of variance in rhythmogenesis can be attributed primarily to genetic variation. This assumption makes quantitative trait loci (QTL) analysis an attractive approach for identifying gene regions and potentially genes within the QTL region regulating physiological traits. In regard to respiratory control, this approach was used to examine differences between strains in hypoxic and hypercapnic ventilatory responses (27), but no physical evidence was found either in identification of a QTL or in a gene to prove causality and set the stage for translational studies in clinical populations with disturbances in respiratory patterning.
In the present study, the hypothesis is that genes encoding components of the NOS, 5-HT, and/or H2S systems would be implicated in a candidate region on chromosome 1. We identified a QTL using an intercross breeding strategy and confirmed its importance with selective breeding of B6 alleles in this QTL region back on to the B6a1 background to restore the abnormal trait. To address gene or pathway identification, we utilized bioinformatic tools to identify candidate gene(s) within the QTL (26) likely to have amino acid changes between B6 and A/J strains. Gene expression in the medulla and pons from the parental mouse inbred strains was utilized to identify differentially expressed genes in the single-QTL region. With this approach, we demonstrate an ability to locate gene regions that regulate control of respiratory rhythmogenesis in the adult mammal.
METHODS
Animals.
C57BL/6J (B6) andC57BL/6J-Chr 1A/J/NaJ (B6a1) mice were obtained at ∼6 wk of age from the Jackson Laboratory (Bar Harbor, ME). All purchased animals were housed for at least 1 wk before investigation in the Louis Stokes Cleveland VA Animal Research Facility with food and water ad libitum with a consistent 12:12-h light-dark cycle. Animals were studied at 8–10 wk. The experimental protocols were approved by the Louis Stokes VA Institutional Animal Care and Use Committee (IACUC).
The F1 mice were generated both from B6 females mated to B6a1 males as well as from B6 males mated to B6a1 females. These F1 mice were subsequently intercrossed at random to generate 462 F2 animals, all of which were tested for posthypoxic spontaneous apnea phenotypes. After a preliminary analysis identified a broad but highly significant QTL, a B6a1.B6-(rs30880354-rs6157620) strain was generated from selected F2 animals and refined using breeding directed by genetic markers.
Measurement of ventilatory behavior.
Ventilatory behavior was measured by placing animals in a 600-ml Lucite cylindrical plethysmography chamber, and this apparatus has been described previously (11, 12). Briefly, we measured breathing data via pressure change across a pressure transducer (Validyne DP45, Validyne Engineering, Northridge, CA), which was also attached to a reference chamber. Gas mixtures of various concentrations of O2 and N2 were introduced to the chamber (∼10 LPM) for 20 s to flush the gas mixture into the chamber and then returned to the low-flow flush. The variations of pressure in the chamber were expressed as voltage swings by the transducer output and were recorded using Lab View 7.1 (National Instruments, Austin, TX). The output was then analyzed and scored using a program custom written for our laboratory (Breath Detect). This program scores each individual breath according to the crest and trough of the transducer's voltage output. Frequency (f) is determined and used as the major metric.
On the day before testing, animals were allowed 60 min to acclimate at normoxia to the testing apparatus. On the day of testing, the animals were allowed to acclimate for 60 min before testing. Following this acclimatization period, 5 min of resting breathing data were recorded. After this, the animals were challenged with 5 min of poikilocapnic hypoxia (8% O2, bal-N2), followed immediately by 5 min of reoxygenation with 100% O2. At the conclusion of this protocol, animals were returned to room air.
Breathing analysis.
Frequency (f) was measured under normoxic conditions at rest and after reoxygenation (100% O2) recovery from poikilocapnic hypoxia (8% O2, 0% CO2). The number of pauses, not counting post-sigh pauses, in the first 2 min of reoxygenation was counted (spontaneous apnea, defined as cessations of breathing of ≥2.0 respiratory cycles), and the number of pauses in the first 2 min of recovery was the quantitative trait since the majority of events occur in this time period (12).
Genetic analysis.
DNA was isolated from the tail of each F2 mouse. Genotyping was performed after identifying 285 single nucleotide polymorphism (SNP) markers chosen for an ability to distinguish between A/J and B6 alleles on chromosome 1 and creating a custom genotyping panel by the Genomics Core Facility in the Genetics Department at Case Western Reserve University. The physical and genomic positions of the SNPs were obtained from http://cgd.jax.org/mousemapconverter/, using the new standard genetic map for the laboratory mouse (5). QTL analysis was performed using the R/qtl software package designed for mapping QTL in experimental crosses. Interval mapping was performed for the number of spontaneous apneas after brief hypoxic exposure. In addition to a single-QTL analysis, which is a standard approach, we performed a two-dimensional, two-QTL scan to allow for the possibility that a second gene locus contributes to the presentation of the observed phenotype value (1, 17, 18, 22). A full two-QTL model considers the possibility that two genetic loci interact epistatically (i.e., show interaction), whereas an additive two-QTL model considers the possibility that two genetic loci contribute to phenotype independently of one another. Significance thresholds for both two-QTL models were determined by 10,000 permutation tests. Log-likelihood odds ratio (LOD) were calculated for each interval, and the LOD number represents the statistical level of association, with higher values being greater differences among the effects of different alleles.
Bioinformatics approach.
Genes listed under the single-QTL were downloaded from www.jax.org. A gene was qualified as candidate if the gene was 1) located under the interval that restored the trait, 2) differed in the haplotype of the parental strains, and 3) carried non-synonymous, protein-coding differences between A/J and B6.
Tissue collection for gene expression.
Three animals from each parental strain were chosen on the basis of having a representative posthypoxic ventilatory phenotype. All animals were euthanized via 2 min of exposure to carbon dioxide in a designated necropsy chamber. The animal was decapitated with a guillotine at the cervical-thoracic vertebral junction. The medulla and pons were identified and dissected from one another, informed by the prepontine cistern and transverse pontine vein. Total time of dissection did not exceed 5 min, and samples were immediately labeled and stored in a −80°C freezer.
mRNA extraction.
RNA extraction was performed using the Qiagen (Valencia, CA) RNEasy Mini Kit (catalog no. 74104), following the protocol handbook. The final RNA samples were stored in a −80°C freezer.
RNA purification, hybridization, and microarray.
The extracted total RNA was further purified, and 1 μl of each RNA sample was used for quality estimation with Experion (Bio-Rad); the qualified sample with RQI ≥ 7.0 was used for Affymetrix Gene Chip Whole Transcript Sense Target Labeling protocol. The labeled cDNA with biotin was hybridized with mouse Gene Expression Array 1.0 array.
Data analysis: robust multichip analysis, Bayesian analysis of microarray data, and quantitative real-time RT-PCR.
In compliance with MIAME standards, Affymetrix data files (CHP and CEL) have been deposited in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus repository. Raw Affymetrix data files (CEL) were imported into Affymetrix Expression Console (EC), and a robust multichip analysis (RMA) was performed (14). A variety of assessments for quality and variability of intensity were performed using the EC platform. Chip intensity data files were then exported as text files (TXT) from EC and imported as TXT into the Bayesian analysis of microarray (BAM) statistical package (14). BAM performed the following comparative analyses: 1) B6a1 medulla vs. B6 medulla and 2) B6a1 pons vs. B6 pons. BAM determined both the fold changes between each comparison for each gene and performed a diagnostic analysis for false discovery rate. All differentially expressed genes identified herein met the following two criteria: 1) numerical fold change was found to be >2, and 2) false detection rate was found to be <0.01.
After total RNA extraction, real-time PCR (qRT-PCR) was carried out with a 7900HT RT-PCR system (Applied Biosystems, Foster City, CA). The PCR primers and TaqMan MGB probe (FAM dye-labeled) used in the detection and quantification of gene sequences were obtained in an assays-on-demand gene expression kit (Applied Biosystems, p/n 4331182). The Taqman Universal PCR master mix (Applied Biosystems, p/n 4304437) was run in accordance with the protocol accompanying the kit. The thermal cycling conditions include 25°C for 10 min, followed by 37°C for 120 min, and finally 4°C. Three endogenous controls were selected: mouse ACTB (beta actin), mouse GAPDH (glyceraldehyde-3-phosphate dehydrogenase), and mouse TBP (TATA box binding protein). SDS 2.3 software package was utilized for data analysis.
Proteomics for apo a2 peptides.
The presence and levels of apoa2 peptide in the brain stem was detected via multiple-reaction monitoring (MRM) analysis using a selected peptide for lysates of brain stem vs. liver tissue using standard proteomic approaches (20, 24). Initial data-dependent discovery was based on data from Peptide Atlas (http://www.peptideatlas.org/), where apo a2 peptides were reported from the liver (8). Since there was no report of peptide fragments in brain or neural tissues, THEQLTPLVR was selected since it has been detected in a variety of human non-brain tissues such as the liver. An in silica digest of this proposed target was identified using Pinpoint software by ThermoFinnigan (San Jose, CA). Standard peptide was ordered and purchased from New England Peptide (Gardner, MA). A standard peptide concentration curve range from 0.10 fmole/μl to 50 fmole/μl was generated using 5 pmol/μl glucagon in 0.1% formic acid as solvent.
Sample preparation from brain (medulla and pons) and liver samples and optimization was established based on results from trial samples. Isolation of the appropriate parent ions was followed by fragmentation, which produces the transition(s). A total of four transitions was monitored for targeting protein peptide THEQLTPLVR in brain and liver tissues. Linearity was determined for the selected peptide by spiking standard isotopically labeled peptides into an appropriate matrix. Four transitions (597.3 m/z → 484.3 m/z, 597.3 m/z → 698.4 m/z, 597.3 m/z → 826.5 m/z, and 597.3 m/z → 955.5 m/z) were selected to be monitored and used for peptide quantification. All four SRM transitions for this peptide exhibited linear responses, with R2 of 0.993, 0.994, 0.990, and 0.993, respectively. The assay limit of detection (LOD) was 50 amole/μl. The limit of quantification was 100 amole/μl. The apoa2 protein peptide THEQLTPLVR in brain and liver tissue was successfully detected and quantified. The peptide in tissue sample was plotted against the standard curve to determine concentration per 1 μl of processed tissue. Absolute quantification of peptide was performed using Thermo Pinpoint software.
RESULTS
Phenotype data.
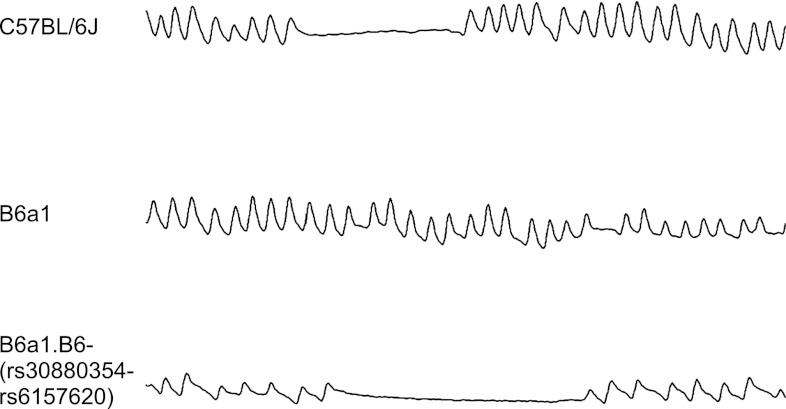
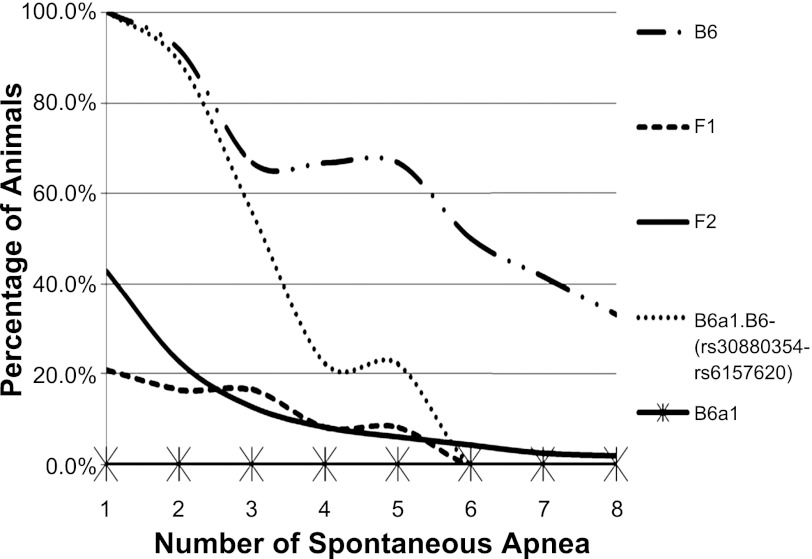
During recovery from hypoxia, spontaneous apneas were present in ventilatory tracings from the B6 or the subcongenic strain, but not the B6a1 parental strain (Fig. 1). Specifically, in the first 2 min of reoxygenation, all B6 animals (n = 12) displayed irregular breathing (number of apnea ranged from 1–18), as shown in Fig. 2. In contrast, none of the B6a1 animals (n = 12) exhibited a spontaneous pause. In the F1 generation, 5 of 24 animals displayed spontaneous apnea (number of apneas ranged from 1 to 5). In the F2 generation, 198 of 462 animals displayed spontaneous apnea (number of apneas ranged from 1 to 16). The subcongenic B6a1.B6-(rs30880354-rs6157620) animals (n = 15) displayed irregular breathing (number of events ranged from 1 to 5), less than that seen in the B6.
Fig. 1.
Shown are recordings from plethysmography records of the three major strains. Each strip represents a screen shot from the analysis program and is 8 s long. The C57BL/6J and the congenic strain [B6a1.B6-(rs30880354-rs6157620)] show traits of posthypoxic recurrent apnea, whereas the B6a1 chromosomal substitution strain does not. The breathing patterns in the posthypoxic period for the A/J are similar to that of the B6a1 (data not shown).
Fig. 2.
Shown are the pause phenotype distribution, as represented by trend lines, in the mouse strains and the first (F1) and second (F2) populations of mice reported in this study. Each line represents a different group of animals. The B6a1 animals did not exhibit apneas. The percentages of animals vs. number of apneas are displayed. There are significant differences among all curves (P < 0.032), and post hoc analysis revealed that F1 and F2 curves are similar (P = 0.35), but curves for B6a1, B6, and B6a1.B6-(rs30880354-rs6157620) congenic strains are different (P < 0.04).
Standard QTL analysis for the number of spontaneous apnea after brief hypoxic exposure.
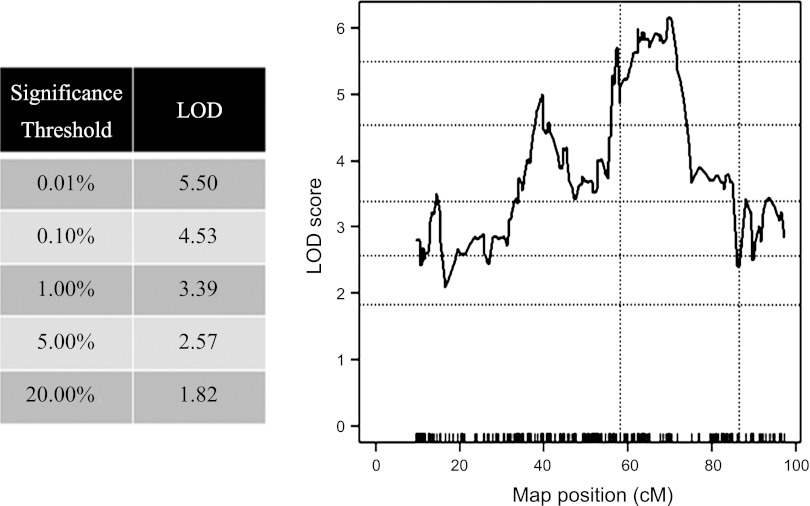
A one-dimensional, single QTL analysis was performed, linking the number of posthypoxic spontaneous apnea among F2 offspring to mouse chromosome 1. Significance thresholds for this analysis were determined via 100,000 permutation tests. As displayed in Fig. 3, a highly significant peak LOD score of 6.15 was located at ∼69.9 cM (marker rs6239070).
Fig. 3.
Shown are the results of a single-QTL analysis for pause trait expressed as ≤1 of >0 in the 2-min reoxygenation period immediately after the hypoxic exposure. Log-likelihood odds ratio (LOD) plots linking pauses to markers mapped to positions on chromosome 1 among F2 offspring. Significance thresholds (Table) were determined by 100,000 permutation test. Peak LOD (6.15) is located at 69.9 cM (marker rs6239070). Dotted vertical lines indicate region introgressed from the B6 onto the B6a1 genetic background to generate the B6a1.B6-(rs30880354-rs6157620) congenic strain.
Two-QTL scan for the number of spontaneous apneas after brief hypoxic exposure.
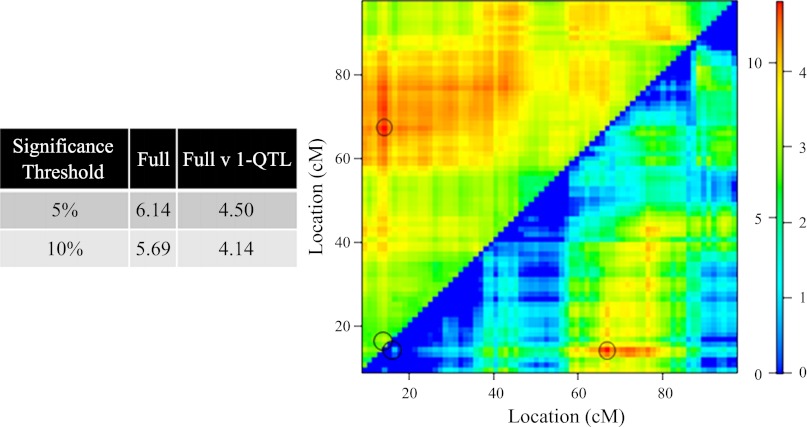
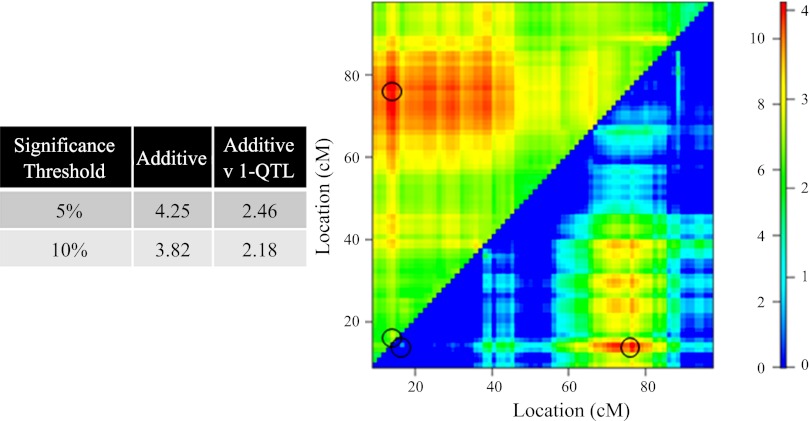
Significance thresholds of 5.00 and 10.00% for the full two-QTL model in which interactions are permitted were found to be LOD values of 6.14 and 5.69, respectively, whereas 5.00 and 10.00% thresholds for the full two-QTL vs. one-QTL were found to be 4.50 and 4.14, respectively. Maximum LODfull score of 11.9 and LODfullv. single-QTL score of 4.92 identify both 14.3 and 67.0 cM on mouse chromosome 1 as loci of interest. Figure 4 displays these results, where the LODfull is plotted in the top left triangle and LODfull v. single-QTL is plotted in the bottom right triangle. See legend of Fig. 4 for more details.
Fig. 4.
This is a heatmap representation of a full two-QTL models, in which epistasis (interaction) is allowed. LOD associated with the full model (LODfull) are plotted in the top left triangle. LODfull v. 1-QTL, plotted in the bottom right triangle, demonstrates the improvement of the full model over all possible single-QTL models. Numbers on the left and right of color scale correspond to LODfull and LODfull v. 1-QTL significance levels, respectively. Significance thresholds were determined by performing a 10,000 permutation test. Noted by the circles are peak LODfull of 11.9 and LODfv1 of 4.92 located QTL at 14.3 and 67.0 cM, respectively. For more information, see text.
For the additive two-QTL model, significance thresholds of 5.00 and 10.00% were found to be LOD values of 4.25 and 3.82. For the additive two-QTL vs. one-QTL, the respective 5.00 and 10.00% thresholds were found to be 2.46 and 2.18. Maximum LODadditive score of 11.1 and LODadditivev. single-QTL score of 4.08 identify both 14.3 and 76.7 cM on mouse chromosome 1 as loci of interest. Figure 5 displays these results in the form of a heatmap, in which LODadditive is plotted in the top left triangle and LODadditive v. single-QTL is plotted in the bottom right triangle. See legend of Fig. 5 for more details.
Fig. 5.
The figure is set up similarly to Fig. 4. It illustrates the impact by which two loci independently contribute to phenotype compared with a conventional QTL. Log-likelihood associated with the additive model (LODadditive) are plotted in the top left triangle. LODadditive v. 1-QTL is plotted in the bottom right triangle, and the figure demonstrates the improvement of the additive model over all possible single-QTL models. Numbers on the left and right of color scale correspond to LODadditive and LODadditive v. 1-QTL, respectively. Significance thresholds were determined by performing a 10,000 permutation test. Indicated by a circle are the maximum LODadditive score of 11.1 and LODadditive v. single-QTL score of 4.08, identifying 14.3 and 76.7 cM on mouse chromosome 1 as loci of interest. For more information, see text.
Bioinformatics.
In this QTL region (rs30880354-rs6157620), there are estimated to be 431 proteins expressed. In regard to non-synonymous SNPs, those that would confer protein coding differences, there were ∼80 genes or suspected genes in the introgressed region (see supplemental Table 1).
Table 1.
List of genes with nonsynonymous SNPs between the B6 and A/J encoded between 136.1–185.4 Mb on chromosome 1
| Position (NCBI Build 37) | Gene ID |
|---|---|
| 153276077 | 1190005F20Rik |
| 153259952 | 1200016B10Rik |
| 158327657 | 9430070O13Rik |
| 158572386 | Abl2 |
| 173181032 | Adamts4 |
| 182109286 | Adck3 |
| 181678774 | Ahctf1 |
| 175657892 | AI607873 |
| 173155827 | Apoa2 |
| 173338561 | Arhgap30 |
| 141353333 | Aspm |
| 172771841 | Atf6 |
| 177908889 | B020018G12Rik |
| 182785994 | BC031781 |
| 173509255 | Cd244 |
| 173625989 | Cd48 |
| 173781966 | Cd84 |
| 178712385 | Cep170 |
| 157709499 | Cep350 |
| 141710019 | Cfhr2 |
| 181539934 | Cnst |
| 174049099 | Copa |
| 141131356 | Crb1 |
| 174628880 | Crp |
| 171917235 | Ddr2 |
| 172815300 | Dusp12 |
| 137152660 | Elf3 |
| 180248678 | Fam36a |
| 175152709 | Fcer1a |
| 172893464 | Fcgr2b |
| 172987924 | Fcgr3 |
| 172848377 | Fcrla |
| 172837666 | Fcrlb |
| 176432274 | Fmn2 |
| 181639505 | Gm1305 |
| 179945329 | Gm16432 |
| 174408203 | Gm2685 |
| 136543332 | Gm3834 |
| 137026195 | Gm3842 |
| 141635809 | Gm4788 |
| 183004804 | Gm6606 |
| 175894107 | Ifi202b |
| 175864020 | Ifi203 |
| 174423745 | Igsf9 |
| 137298827 | Ipo9 |
| 142251124 | Kcnt2 |
| 180845359 | Kif26b |
| 173290282 | Klhdc9 |
| 182865244 | Lefty1 |
| 136884586 | Lgr6 |
| 173524038 | Ly9 |
| 175805797 | Mndal |
| 173080700 | Mpz |
| 158256231 | Nphs2 |
| 175113764 | Olfr1406 |
| 176180120 | Olfr419 |
| 176082455 | Olfr421 |
| 175980650 | Olfr432 |
| 174058854 | Pex19 |
| 174307447 | Pigm |
| 151951626 | Ptgs2 |
| 137015837 | Ptprv |
| 157626578 | Qsox1 |
| 158762876 | Ralgps2 |
| 171620658 | Rgs5 |
| 137180206 | Rnpep |
| 178756333 | Sdccag8 |
| 173075936 | Sdhc |
| 173568800 | Slamf7 |
| 174407561 | Slamf9 |
| 154706566 | Smg7 |
| 180973992 | Smyd3 |
| 158371497 | Soat1 |
| 158200776 | Tdrd5 |
| 161788479 | Tnr |
| 157869448 | Tor1aip1 |
| 157912175 | Tor1aip2 |
| 158603717 | Tor3a |
| 145607144 | Trove2 |
| 154233693 | Tsen15 |
Gene expression.
Expression array data identified hundreds of differentially expressed genes across the entire genome as a result of substitution of A/J Chromosome 1onto the B6 background. Table 2 presents the list for numbers of medullary and pontine genes, illustrating the numerical differences in regard to fold change between the B6a1 and the B6. However, in regard to genes that were present in the single QTL region, there were three genes with non-synonymous codon changes and greater than twofold differences in expression. These genes along with confirmatory quantitative PCR (qPCR) expression measurement data are listed in Table 3.
Table 2.
Descriptive metrics of medullary and pontine microarray data
| Fold Change Threshold | Total Number of Differentially Expressed Genes in B6a1 v B6 | Number of Genes in Which Expression in the B6a1 > B6 | Number of Genes in Which Expression in the B6 > B6a1 |
|---|---|---|---|
| Medullary microarray data | |||
| 2 | 1042 | 195 | 847 |
| 3 | 545 | 91 | 454 |
| 5 | 132 | 14 | 118 |
| 10 | 12 | 2 | 10 |
| 15 | 2 | 1 | 1 |
| Pontine microarray data | |||
| 2 | 684 | 248 | 436 |
| 3 | 404 | 130 | 274 |
| 5 | 109 | 32 | 77 |
| 10 | 7 | 1 | 6 |
| 15 | 1 | 1 | 0 |
Table 3.
mRNA expression (fold difference) of B6 vs. B6a1 with brain stem issue
| Gene | Method of Quantification | Pons B6a1 vs. B6 | Medulla B6a1 vs. B6 |
|---|---|---|---|
| Apoa2 | qPCR fold change | −74.43 | −62.15 |
| Microarray fold change | −9.86 | −6.86 | |
| Cd84 | qPCR fold change | −1.07 | * |
| Microarray fold change | −3.14 | * | |
| Sdhc | qPCR fold change | 1.28 | 1.56 |
| Microarray fold change | 2.81 | 6.43 |
Affimetric GEO 1.0 array was used. qPCR is quantitative PCR on separate samples.
Not detected.
Proteomic investigation of apoa2 protein in the brain.
In all strains, the levels of the apoa2 peptide fragment THEQLTPLVR were several fold greater in the liver samples (as previously known) than in the brain (Table 4). In the brain, the values for THEQLTPLVR in medullary samples from the A/J were generally lower than those from the B6 or B6a1; the order might be B6a1 > B6 > A/J and does not track with trait expression. Moreover, the trend does not appear to be directly related to the mRNA expression profiles, where the B6 with the pause trait showed a very high expression compared with the B6a1 without the pause trait (see Table 4).
Table 4.
Apoa2 peptide concentration in tissue samples
| Tissue | Peptide Concentration in B6a1, fmole/μl | Peptide Concentration in B6, fmole/μl | Peptide Concentration in A/J, fmole/μl |
|---|---|---|---|
| Medulla | 2.2 | 2.3 | 1.1 |
| Medulla | 3 | 2.1 | 1.2 |
| Medulla | 3.1 | 2 | 1.5 |
| Pons | 2.6 | 0 | 0 |
| Pons | 2.7 | 1.4 | 1.3 |
| Pons | 4.2 | 2 | 1.6 |
| Liver | 115.7 | 70.7 | 136.2 |
| Liver | 158 | 98.1 | 106.7 |
| Liver | 157.9 | 100.9 | 79.5 |
DISCUSSION
In this study, we identified a region on mouse chromosome 1 and introgressed B6 alleles onto the B6a1 background to restore the trait of posthypoxic pauses. Hence, we provide physical evidence for genes that confer resistance to, or alternatively promote, posthypoxic apneas and spontaneous pauses at rest. We then utilized bioinformatics to identify genes that potentially could confer the differential response between the two strains. Differential mRNA expression in the pons and medulla between the parental strains highlighted expression differences in three genes within this introgressed region (discussed below), arguably causal to the trait. Using this combination of genomic tools, none of the candidate genes in this region directly relate to known 5-HT, HS, or NOS neurotransmitter receptor proteins or neurotransmittion activation pathways. Another more proximal location on chromosome 1 might also contribute to the number, but not the appearance, of pauses following reoxygenation. Thus, on the basis of this new information, we now suspect the influence of other novel polymorphic genes and pathways, possibly interacting with known “cardiovascular risk” apolipoprotein pathways, to produce this complex trait of unstable breathing.
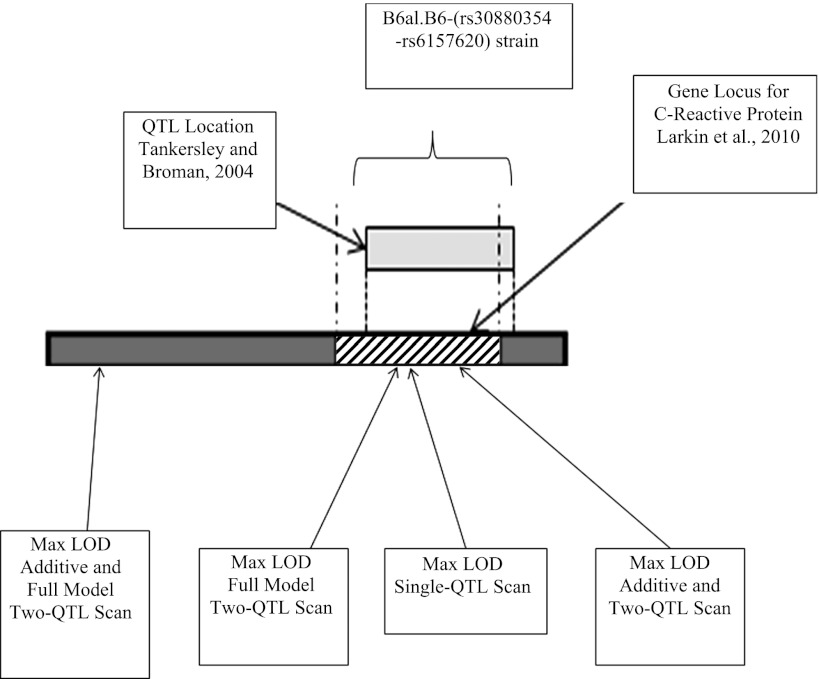
The significance of the single-QTL result was confirmed by recovering the B6 phenotype in the B6a1.B6-(rs30880354-rs6157620) congenic strain. Other studies of ventilatory behavior, carried out in both mice and human models, may give some added confidence that gene(s) in this region contribute to respiratory control. Figure 6 is a rendering of chromosome 1 that illustrates the current published literature on this area. A bar represents the D1Mit14-D1Mit291 region of chromosome 1, a QTL previously significantly linked to variation in hypercapnic hypoxic minute ventilation, tidal volume, and mean inspiratory flow (Fig. 6 in Ref. 27). In a broad sense, ventilatory traits to hypoxia-reoxygenation are probably best understood in regard to the relevance of loop-gain and its role in predisposing one to recurrent sleep apnea (28). The model of recurrent apneas in the B6 is one of abnormal patterning and recurrent apneas, and as a trait potentially relevant to apneas during sleep in adults and neonates or in conditions of inherited or acquired neurological disease (31).
Fig. 6.
Summary of known information on mouse chromosome 1 for genes or QTL loci associated with ventilatory traits. Red indicates A/J alleles, and blue indicates B6 alleles. The gray area represents the region described by Tankersley and Broman and corrected for the new B6 alignment (see discussion for further comments).
In the single-QTL and in the introgressed region, there is present C-reactive protein (CRP). Plausibility is based on a common risk for and interactions among human sleep apnea and cardiovascular disease, particularly in Caucasians, and its presence under a QTL in a genome-wide association study of obstructive sleep apnea linked to the trait of apnea-hypopnea index (15). In existing databases, there are noted 13 informative SNPs present in the B6 and B6a1 CRP gene, but we did not find recurrent posthypoxic apneas to link with expression differences in CRP. This does not negate the potential importance for CRP in the expression of sleep apnea or respiratory control but does lead one away from the CRP gene and protein as a principal target in the present report. Although specific interpretations regarding the significance of these overlapping observations would be purely speculative, the alignment with CRP and with the linkage in the prior report (27) increases the confidence that this is a region in which gene(s) contributing to the dynamic elements of respiratory control and/or for the consequences of sleep disordered breathing might reside.
We used traditional and expanded QTL models for linkage. Since power is diminished by adding a dimension, our use of a two-QTL model is meaningful only if it demonstrates an improvement over all single-QTL models, and it did. In the expanded analyses, there are clues to the existence of three smaller regions on chromosome 1 that might contribute to the trait. Further details as to the number and identification of specific genes is, however, limited. Phenotypically, the difference in the number, but not the appearance, of posthypoxic pauses between the B6 and the subcongenic strain (see Fig. 2) suggests that the more proximal QTL outside the introgressed region is not required for the presence of a pause but contributes to the number of posthypoxic pauses. This proximal region is near a second possible, but not significant, QTL region for O2/CO2 interactions, as described by Tankersley and Broman in a B6/C3H/HeJ intercross (Fig. 6 in Ref. 27).
In the medulla, there was a limited number of differentially expressed genes encoded on chromosome 1 and containing non-synonymous SNPs between the B6 and A/J strains. None are linked directly, i.e., within one degree of freedom, to suspected targets (buspirone, acetazolamide, or hydrogen sulfide inhibitors), which we and others have shown to mitigate posthypoxic pauses in the B6. In regard to NOS, one gene residing in the introgressed region, i.e., NOS interactive protein, fails to show expression differences. As for the differentially expressed non-synonymous candidates, none are known to operate directly in ventilatory control. Apoa2 is the second most abundant protein in HDL; overexpression in the mouse results in amyloidosis in liver, heart, and tongue (9); and in humans, polymorphisms in Apoa2 have been associated with increased body mass index and insulin resistance (4), factors associated with the appearance of obstructive sleep apnea/hypopnea. Polymorphisms of the SDHC gene appear in the human population, associated with multiple endocrine neoplasia syndromes, pheochromocytomas, and paragangliomas in humans (21); the gene is also known to increase expression with intracellular oxidative stress resulting in apoptosis (13), and therefore it is considered relevant to plasticity in respiratory control (31). Furthermore, mutations have been found to upregulate HIF-1-alpha expression (3), potentially relevant to hypoxic consequences of sleep-disordered breathing. Found expressed in pons but not in medulla samples, the gene for Cd84 resides in a region on chromosome 1 associated with neural tube defects in the loop-tail mouse (7). There are, however, no known links between this structural gene and ventilatory responses or sleep-disordered breathing.
There is only one previous report in which apoa2 mRNA expression was reported to be weakly present in neurons of the brain (region not specified) from a congenic strain of mice with amyloidogenicapolipoprotein A-II (Apoa2c) on a genetic background of the amyloidosis-resistant SAM-R/1 strain, called the R1.P1-Apoa2c.strain (8). In this strain, deposition of fibrils occurred only in peripheral tissues. Allen Brain Atlas indicates mRNA expression of apoa2 in the spinal cord but lists as “negative” all others tissues in the brain and brain stem (http://www.brain-map.org/). Thus, if one were to suspect a priori the significance of apoa2 in brain health or disease, or specifically in conferring differences in ventilatory traits among mouse strains, one would not suspect involvement of this gene or its product. One obstacle we encountered was that the commercial antibody currently available did not work in brain tissue, either because antibody was directed at human protein (40% different in amino acid sequences) or the epitopes used for the commercial antibody are not expressed in the brain (2) or because the available antibody is outdated. Thus a proteomic approach was chosen to determine whether the apoa2 mRNA was functional, and the apoa2 fragment THEQLTPLVR was chosen, being expressed at several-fold higher levels in liver, the control tissue. Levels of peptide fragment was easureable in the medulla of all three strains and in some of the pontine samples. This discovery of an apoa2 peptide fragment is a novel finding; however, there was no direct correlation between peptide levels from the brain stem (or liver) samples and the trait of unstable breathing, i.e., the levels in the B6a1 (stable strain) being similar to the B6 (unstable strain). There is precedent for difference in posttranslational modification of proteins that are tissue specific or alternatively mRNA modification of cellular processes (20). Another limitation of the proteomic approach is that it does not localize the site of apoa2 peptide nor its distribution in or around respiratory neurons in the medulla. Because of this lack of correlation between the apoa2 fragment and trait expression, it may be premature to examine apoa2 mRNA expression as well as peptide properties in a more direct manner for its potential relevance to human sleep apnea, given its clinical correlation to dyslipidemia (16).
This report for the first time mechanistically links a specific region on chromosome 1 to respiratory rhythmogenesis. Given the prior art with these models in fields of cardiovascular, neurological, rheumatic, and cancer risk, elements found in mammalian models can give insight into the molecular pathways for human diseases characterized by disturbances in ventilatory patterning.
GRANTS
This work is primarily supported by a Merit Award from the VA Research Service and by National Institute of Neurological Disorders and Stroke Grant NS-052452. Dr. Yamauchi was supported by a grant from Fuji Respironics. The proteomic study was made possible in part by the Case Western Reserve University/Cleveland Clinic CTSA Grant No. UL1 RR-024989 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the VA Research Service, the NINDS, or the NCRR.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.B.G., M.Y., M.D.A., J.D., S.C., and M.W.M. performed experiments; C.B.G., M.Y., M.D.A., M.W.M., L.M.D., and K.P.S. analyzed data; C.B.G. drafted the manuscript; C.B.G. and K.P.S. approved the final version of the manuscript; M.Y., M.D.A., S.C., L.M.D., F.H., and K.P.S. edited and revised the manuscript; M.D.A., F.H., and K.P.S. conception and design of research; S.C., L.M.D., and K.P.S. prepared figures; K.P.S. interpreted results of experiments.
REFERENCES
- 1. Arends D, Prins P, Jansen RC, Broman KW. R/qtl: high-throughput multiple QTL mapping. Bioinformatics 26: 2990–2992, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blanco-Vaca F, Escola-Gil JC, Martin-Campos JM, Julve J. Role of apoA-II in lipid metabolism and atherosclerosis: advances in the study of an enigmatic protein. J Lipid Res 42: 1727–1739, 2001 [PubMed] [Google Scholar]
- 3. Cervera AM, Apostolova N, Crespo FL, Mata M, McCreath KJ. Cells silenced for SDHB expression display characteristic features of the tumor phenotype. Cancer Res 68: 4058–4067, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Corella D, Peloso G, Arnett DK, Demissie S, Cupples LA, Tucker K, Lai CQ, Parnell LD, Coltell O, Lee YC, Ordovas JM. APOA2, dietary fat, and body mass index: replication of a gene-diet interaction in 3 independent populations. Arch Intern Med 169: 1897–1906, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cox A, Ackert-Bicknell CL, Dumont BL, Ding Y, Bell JT, Brockmann GA, Wergedal JE, Bult C, Paigen B, Flint J, Tsaih SW, Churchill GA, Broman KW. A new standard genetic map for the laboratory mouse. Genetics 182: 1335–1344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Donovan LM, Moore MW, Gillombardo CB, Chai S, Strohl KP. Effects of hydrogen sulfide synthesis inhibitors on posthypoxic ventilatory behavior in the C57BL/6J mouse. Respiration 82: 522–529, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doudney K, Murdoch JN, Paternotte C, Bentley L, Gregory S, Copp AJ, Stanier P. Comparative physical and transcript maps of ∼1 Mb around loop-tail, a gene for severe neural tube defects on distal mouse chromosome 1 and human chromosome 1q22-q23. Genomics 72: 180–192, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Fu L, Matsuyama I, Chiba T, Xing Y, Korenaga T, Guo Z, Fu X, Nakayama J, Mori M, Higuchi K. Extrahepatic expression of apolipoprotein A-II in mouse tissues: possible contribution to mouse senile amyloidosis. J Histochem Cytochem 49: 739–748, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Ge F, Yao J, Fu X, Guo Z, Yan J, Zhang B, Zhang H, Tomozawa H, Miyazaki J, Sawashita J, Mori M, Higuchi K. Amyloidosis in transgenic mice expressing murine amyloidogenic apolipoprotein A-II (Apoa2c). Lab Invest 87: 633–643, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Han F, Strohl KP. Inheritance of ventilatory behavior in rodent models. Respir Physiol 121: 247–256, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Han F, Subramanian S, Dick TE, Dreshaj IA, Strohl KP. Ventilatory behavior after hypoxia in C57BL/6J and A/J mice. J Appl Physiol 91: 1962–1970, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Han F, Subramanian S, Price ER, Nadeau J, Strohl KP. Periodic breathing in the mouse. J Appl Physiol 92: 1133–1140, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Ishii T, Yasuda K, Akatsuka A, Hino O, Hartman PS, Ishii N. A mutation in the SDHC gene of complex II increases oxidative stress, resulting in apoptosis and tumorigenesis. Cancer Res 65: 203–209, 2005 [PubMed] [Google Scholar]
- 14. Ishwaran H, Rao JS, Kogalur UB. BAMarraytrade mark: Java software for Bayesian analysis of variance for microarray data. BMC Bioinformatics 7: 59, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Larkin EK, Patel SR, Goodloe RJ, Li Y, Zhu X, Gray-McGuire C, Adams MD, Redline S. A candidate gene study of obstructive sleep apnea in European Americans and African Americans. Am J Respir Crit Care Med 182: 947–953, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lurie A. Metabolic disorders associated with obstructive sleep apnea in adults. Adv Cardiol 46: 67–138, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Manichaikul A, Moon JY, Sen S, Yandell BS, Broman KW. A model selection approach for the identification of quantitative trait loci in experimental crosses, allowing epistasis. Genetics 181: 1077–1086, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manichaikul A, Palmer AA, Sen S, Broman KW. Significance thresholds for quantitative trait locus mapping under selective genotyping. Genetics 177: 1963–1966, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nadeau JH, Singer JB, Matin A, Lander ES. Analysing complex genetic traits with chromosome substitution strains. Nat Genet 24: 221–225, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Nibbe RK, Chance MR. Approaches to biomarkers in human colorectal cancer: looking back, to go forward. Biomark Med 3: 385–396, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Opocher G, Schiavi F. Functional consequences of SDH Mutations. Endocr Pract 1–19, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Peirce JL, Broman KW, Lu L, Chesler EJ, Zhou G, Airey DC, Birmingham AE, Williams RW. Genome reshuffling for advanced intercross permutation (GRAIP): simulation and permutation for advanced intercross population analysis. PLos One 3: e1977, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Price ER, Han F, Dick TE, Strohl KP. 7-Nitroindazole and posthypoxic ventilatory behavior in the A/J and C57BL/6J mouse strains. J Appl Physiol 95: 1097–1104, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Schlatzer DM, Dazard JE, Dharsee M, Ewing RM, Ilchenko S, Stewart I, Christ G, Chance MR. Urinary protein profiles in a rat model for diabetic complications. Mol Cell Proteomics 8: 2145–2158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stettner GM, Zanella S, Huppke P, Gartner J, Hilaire G, Dutschmann M. Spontaneous central apneas occur in the C57BL/6J mouse strain. Respir Physiol Neurobiol 160: 21–27, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Su Z, Wang X, Tsaih SW, Zhang A, Cox A, Sheehan S, Paigen B. Genetic basis of HDL variation in 129/SvImJ and C57BL/6J mice: importance of testing candidate genes in targeted mutant mice. J Lipid Res 50: 116–125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tankersley CG, Broman KW. Interactions in hypoxic and hypercapnic breathing are genetically linked to mouse chromosomes 1 and 5. J Appl Physiol 97: 77–84, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Wellman A, Eckert DJ, Jordan AS, Edwards BA, Passaglia CL, Jackson AC, Gautam S, Owens RL, Malhotra A, White DP. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol 110: 1627–1637, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamauchi M, Dostal J, Kimura H, Strohl KP. Effects of buspirone on posthypoxic ventilatory behavior in the C57BL/6J and A/J mouse strains. J Appl Physiol 105: 518–526, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamauchi M, Dostal J, Strohl KP. Acetazolamide protects against posthypoxic unstable breathing in the C57BL/6J mouse. J Appl Physiol 103: 1263–1268, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Yamauchi M, Kimura H, Strohl KP. Mouse models of apnea: strain differences in apnea expression and its pharmacologic and genetic modification. Adv Exp Med Biol 669: 303–307, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Yamauchi M, Ocak H, Dostal J, Jacono FJ, Loparo KA, Strohl KP. Post-sigh breathing behavior and spontaneous pauses in the C57BL/6J (B6) mouse. Respir Physiol Neurobiol 162: 117–125, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]