Abstract
Increased hepatocyte apoptosis is a hallmark of nonalcoholic fatty liver disease (NAFLD) and contributes to the profibrogenic state responsible for the progression to nonalcoholic steatohepatitis (NASH). Strategies aimed at reducing apoptosis may result in better outcomes for individuals with NAFLD. We therefore examined the effect of a short-term exercise program on markers of apoptosis—plasma cytokeratin 18 (CK18) fragments, alanine aminotransferase (ALT), aspartate aminotransferase (AST), soluble Fas (sFas), and sFas ligand (sFasL)—in 13 obese individuals with NAFLD [body mass index 35.2 ± 1.2 kg/m2, >5% intrahepatic lipid (IHL) assessed by 1H-MR spectroscopy]. Exercise consisted of treadmill walking for 60 min/day on 7 consecutive days at ∼85% of maximal heart rate. Additionally, subjects underwent an oral glucose tolerance test and a maximal oxygen consumption (V̇o2max) test before and after the exercise intervention. The Matsuda index was used to assess insulin sensitivity. We observed significant decreases in CK18 fragments (558.4 ± 106.8 vs. 323.4 ± 72.5 U/l, P < 0.01) and ALT (30.2 ± 5.1 vs. 24.3 ± 4.8 U/l, P < 0.05), and an increase in whole body fat oxidation (49.3 ± 6.1 vs. 69.4 ± 7.1 mg/min, P < 0.05), while decreases in circulating sFasL approached statistical significance (66.5 ± 6.0 vs. 63.0 ± 5.7 pg/ml, P = 0.06), as did the relationship between percent change in circulating CK18 fragments and ALT (r = 0.55, P = 0.05). We also observed a significant correlation between changes in fat oxidation and circulating sFasL (rho = −0.65, P < 0.05). There was no change in IHL following the intervention (18.2 ± 2.5 vs. 17.5 ± 2.1%, NS). We conclude that short-term exercise reduces a circulatory marker of hepatocyte apoptosis in obese individuals with NAFLD and propose that changes in the proapoptotic environment may be mediated through improved insulin sensitivity and increased oxidative capacity.
Keywords: nonalcoholic steatohepatitis, cytokeratin 18, Fas, insulin resistance, obesity
nonalcoholic fatty liver disease (NAFLD) is among the most common forms of chronic liver disease today. Current estimates suggest that approximately 20–30% of the population are affected by the condition (3). The pathogenesis of NAFLD is marked by excessive accumulation of intrahepatic lipid (IHL), increases in insulin resistance, and an elevation in profibrogenic activities such as inflammation, oxidative stress (12) and activation of hepatic stellate cells (7). Recently it has been shown that hepatocyte apoptosis contributes to this profibrogenic state (41). Apoptosis, a form of programmed cell death, is an active ATP-dependent process that under normal physiological conditions contributes to the maintenance of tissue homeostasis. However, in certain pathophysiological conditions, such as NAFLD, apoptosis is upregulated, overwhelming the normal phagocytic engulfment of apoptotic cells, triggering a proinflammatory and profibrogenic response from hepatocytes (22).
Activation of apoptosis in hepatocytes initiates the caspase cleavage of the intermediate filament cytokeratin 18 (CK18) at two sites, Asp238 and Asp326 (8, 33). This cleavage is highly specific to apoptosis, and fragments (M30) can be detected by antibody specific ELISA (16). In a recent study, Wieckowska et al. (48) demonstrated that M30 fragments were elevated in NAFLD patients compared with controls and that the levels of these fragments were correlated with the presence of liver fibrosis. This finding has been confirmed in subsequent studies (49, 50), and it is thought that caspase-cleaved CK18 fragments may be a highly sensitive, noninvasive biomarker for determining the severity of NAFLD (46). Current evidence suggests that activation of the Fas apoptotic pathway may be involved in initiating hepatocyte apoptosis in NAFLD. Fas is a glycosylated protein that is widely expressed in the liver (2). It is activated by the binding of Fas ligand (FasL), leading to receptor trimerization and formation of the death-inducing signaling complex (DISC) (37). Data from Feldstein et al. (14) demonstrate elevations in Fas protein expression in liver samples from nonalcoholic steatohepatitis (NASH) patients. Additionally, Fas expression is upregulated in human HepG2 cells exposed to free fatty acids (FFA), and in a mouse model of NAFLD, resulting in increased sensitivity to Fas-mediated apoptosis (15).
Given its central role in the pathogenesis of NAFLD, identifying therapeutic treatments that minimize apoptosis in these patients may result in better outcomes for individuals with NAFLD. Due to its association with obesity, lifestyle interventions are recommended to patients presenting with NAFLD. As early as 1970, Drenick et al. (13) showed that diet-induced weight loss over 5 mo reduced hepatic steatosis, and when weight loss was maintained for an average of 17 mo, liver histology was either completely or almost completely normalized. More recently, Palmer and Schaffer (34) observed an improvement in alanine aminotransferase (ALT) and aspartate aminotransferase (AST), surrogate markers of liver damage, following 10% weight loss over an average of 16 mo. Lifestyle interventions utilizing a combination of diet and exercise have also been shown to result in reductions in serum levels of ALT and AST, both in the presence and absence of weight loss (44). Furthermore, data from animal studies indicate that exercise training prevents the development of steatosis (4, 40), even in the presence of a high-fat diet (9). However, no study to date has investigated the effect of exercise training, independent of weight loss, on markers of apoptosis in individuals with NAFLD. We therefore examined the effect of a 7-day exercise program on plasma CK18 fragments in obese individuals with NAFLD. We hypothesized that exercise training would provide an antiapoptotic stimulus, resulting in reductions in CK18 fragments, and that this would be mediated via reductions in the Fas signaling pathway.
MATERIALS AND METHODS
Participants.
Thirteen obese, previously sedentary (individuals exercising for 20 min or more at least 2 times per week were excluded) adults (age 58 ± 3 yr; body mass index 35.2 ± 1.2 kg/m2; means ± SE) with NAFLD (>5% IHL assessed by 1H MR spectroscopy) were recruited from the local population to undergo a 7-day exercise training intervention. All volunteers underwent a medical history, physical exam, oral glucose tolerance test (OGTT), and complete blood profile (lipid profile, and hepatic/renal/hematological function tests). Medical screening excluded individuals with any disease, and/or taking medications known to affect our outcome variables. In addition, individuals consuming more than 5 units of alcohol per week were excluded. Screening also excluded those with any contraindications to physical activity that was detected during a resting 12-lead electrocardiogram and a submaximal exercise stress test. Female subjects were either postmenopausal and not using any hormone replacement therapy, or premenopausal and in the follicular phase of the menstrual cycle during the study period. The study was approved by the Institutional Review Board, and all subjects provided signed informed consent in accordance with our guidelines for the protection of human subjects.
Aerobic fitness.
Each participant performed an incremental graded treadmill exercise test to determine his or her maximal oxygen consumption (V̇o2max), as previously described (32). Expired air was continuously sampled online with the use of an automated system (Jaeger Oxycon Pro; Viasys, Yorba Linda, CA). V̇o2max testing was conducted on the pre- and posttesting days following an OGTT.
Intervention.
All participants undertook 60 min of supervised aerobic exercise at ∼80–85% of maximum heart rate each day for 7 consecutive days. Compliance to exercise intensity was monitored using a heart rate monitor (Polar Electro, Woodbury, NY). Participants were instructed to consume their normal diet and not make any dietary changes during the 7-day period of the intervention. Furthermore, subjects were instructed to avoid caffeine consumption for 12 h prior to and alcohol for 48 h prior to testing and to consume the same diet containing 250 g of carbohydrate on the day prior to the pre- and poststudy testing days. Posttesting occurred the morning of the day following the final exercise bout.
Body composition.
Height and body weight were measured by standard techniques. Whole body adiposity was measured by dual-energy X-ray absorptiometry (model iDXA; Lunar, Madison, WI).
IHL.
Hepatic triglyceride content was measured by 1H MR spectroscopy using a 3T MR system (Siemens Sonata, Erlangen, Germany). Subjects arrived at the laboratory at 0600 following an overnight (8 h) fast. Briefly, a body array MRI coil was affixed to each subject's back with velcro straps. The center of the body array coil was aligned with the subject's spine and shoulders for accurate repositioning during longitudinal studies. Each subject was positioned face down and head first in the Siemens Verio 3T MRI scanner on a memory foam mattress to further minimize respiratory motion. After the completion of localizer scans for positioning, an 8-cm3 voxel was positioned within the right posterior lobe of the subject's liver with guidance from the high-resolution localization images. Manual shimming was performed to a line width of ∼40 Hz to ensure the high-quality spectra required to delineate water and the various lipid species. MR spectra with and without water suppression were acquired with a single-voxel PRESS acquisition with a long repetition time (TR = 5,000 ms), and a short echo time (TE = 30 ms) to limit the effects of magnetic relaxation (20). The acquisition was acquired with 32 averages to obtain sufficient signal to accurately assess lipid components. The data were Fourier-transformed, filtered, baseline corrected, and phased. All NAFLD patients were confirmed to have greater than 5% IHL, which is the diagnostic criteria for hepatic steatosis (45).
Insulin sensitivity and substrate metabolism.
Subjects arrived at the Clinical Research Unit following an overnight fast and lay supine in bed for 30 min followed by assessment of whole body fat oxidation (FOX) by indirect calorimetry using the following equation: FOX = 1.695V̇o2 − 1.701V̇co2, where V̇o2 is oxygen consumption and V̇co2 is carbon dioxide production. (35). Subsequently, a 75-g OGTT was administered. Baseline blood draws were obtained from an antecubital vein prior to ingestion of the glucose drink. Blood samples were drawn in EDTA tubes containing aprotonin at 30, 60, 90, 120, and 180 min after ingestion. Plasma glucose was determined using the YSI 2300 STAT Plus analyzer (Yellow Springs, OH), plasma insulin was determined via radioimmunoassay (Millipore, Billerica, MA) and plasma FFA were measured by colorimetric assay (NEFA C; Wako Pure Chemical Industries, Richmond, VA). Insulin sensitivity during the OGTT (ISIOGTT) was calculated using the Matsuda index (30). We also calculated adipose insulin resistance (adipose-IR = fasting plasma FFA × fasting plasma insulin) (28).
Plasma analyses.
Plasma analyses were performed on plasma which was stored at −80°C immediately following postdraw processing. Plasma caspase-3-generated CK18 fragments were quantified by M30 apoptosense ELISA (PEVIVA; Alexis, Grunwald, Germany). The Human Fas/TNFRSF6 Quantikine ELISA Kit and the Human Fas Ligand/TNFSF6 Quantikine ELISA Kit (R&D systems, Minneapolis, MN) were used for quantitative measurement of soluble Fas (sFas) and soluble Fas ligand (sFasL), respectively. ALT and AST were assessed using the Cobra Integra Alanine Aminotransferase (ALTL) test, test ID 0–495, and the Aspartate Aminotransferase (ASTL) test, test ID 0–494 (Roche Diagnostics, Indianapolis, IN), respectively.
Statistical analyses.
Values were tested for normality using the D'Agostino and Pearson omnibus normality test on GraphPad Prism 4.0 (Graphpad Software, San Diego CA). Pre to post intervention changes were assessed using a repeated-measures ANOVA for normally distributed samples. Pre to post changes that were not normally distributed were log transformed [area under the curve (AUC) insulin]. Data that were not normalized by log transformation [fasting plasma insulin (FPI) and ISIOGTT] were assessed using a Wilcoxon signed rank test. Linear regression analysis was used to determine associations between normally distributed data. In addition, Spearman's rank correlation analyses were used to identify relationships between variables that failed the test for normality (ΔFOX and ΔsFasL). Statistical significance was accepted when P < 0.05. These analyses were carried out using StatView for Windows 5.0.1 (SAS Institute, NC), and all data are expressed as means ± SE.
RESULTS
Participant characteristics.
Anthropometric data for the group are summarized in Table 1. Seven of the participants presented at screening with elevated ALT and AST values. Seven days of exercise did not alter body weight or body composition; however, aerobic fitness, as measured by V̇o2max, did increase following exercise training. In addition, total IHL assessed by 1H MR Spectroscopy remained unchanged following the intervention (Table 1).
Table 1.
Summary of subject characteristics before and after the exercise training
| Variable | Pre | Post | P Value |
|---|---|---|---|
| Age, yr | 58 ± 3 | ||
| Body weight, kg | 100.3 ± 3.8 | 100.4 ± 3.7 | 0.727 |
| BMI, kg/m2 | 35.2 ± 1.2 | 35.3 ± 1.1 | 0.532 |
| FM, kg | 45.8 ± 2.4 | 45.6 ± 2.3 | 0.298 |
| FFM, kg | 54.4 ± 3.1 | 54.9 ± 3.1 | 0.065 |
| IHL, % | 18.2 ± 2.5 | 17.5 ± 2.1 | 0.285 |
| V̇o2max, ml · kg−1 · min−1 | 22.0 ± 1.4 | 23.6 ± 1.3 | <0.05 |
| Fasting glucose, mg/dl | 112.9 ± 5.7 | 108.5 ± 3.7 | 0.285 |
| Fasting insulin, μU/ml | 25.3 ± 3.1 | 22.8 ± 2.7 | 0.249 |
| Glucose AUC, mg · dl−1 · 3 h | 13,663.6 ± 1,423.4 | 11,528.1 ± 1,517.1 | <0.01 |
| Log insulin AUC, log pg · ml−1 · 3 h | 4.3 ± 0.1 | 4.16 ± 0.1 | <0.01 |
| Fasting FFA, μmol/l | 598 ± 51 | 605 ± 39 | 0.885 |
| Adipose-IR, AU | 14.6 ± 1.4 | 13.2 ± 1.4 | 0.190 |
| Resting FOX, mg/min | 49.3 ± 6.1 | 69.4 ± 7.1 | <0.05 |
Data are presented as means ± SE. Pre, before exercise training; Post, after exercise training; BMI, body mass index; FM, fat mass; FFM, fat-free mass; IHL, intrahepatic lipid; V̇o2max, maximal oxygen consumption; AUC, incremental area under the curve; FFA, free fatty acids; IR, insulin resistance; AU, arbitrary units; FOX, fat oxidation.
Blood glucose and insulin sensitivity.
The plasma glucose and insulin responses to glucose ingestion were significantly reduced (Table 1), and insulin sensitivity, measured by the Matsuda index, increased following exercise (Fig. 1A). Fasting glucose, FFA, and insulin did not change after the 7-day program; neither was adipose-IR altered by the intervention (Table 1).
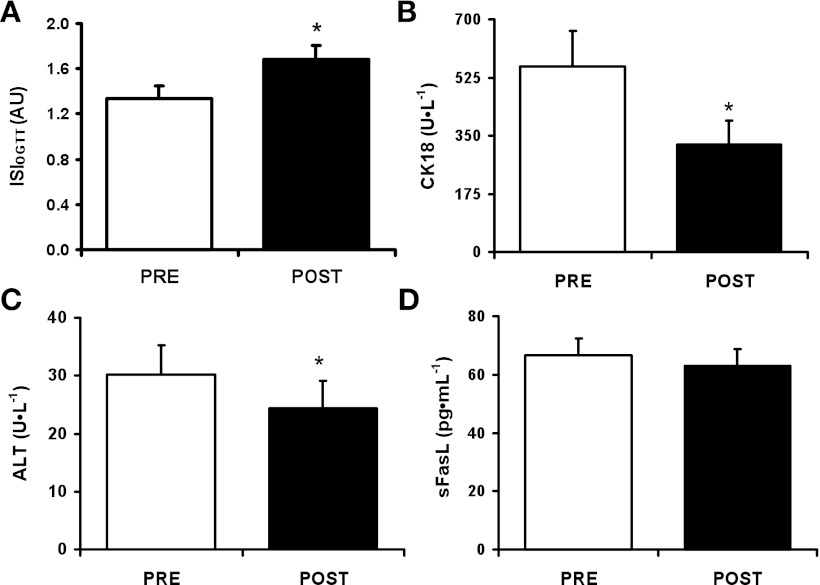
Fig. 1.
Short-term aerobic exercise training increases insulin sensitivity (A) and reduces circulating cytokeratin 18 (CK18) fragments (B), alanine aminotransferase (ALT) (C), and circulating soluble Fas ligand (sFasL) (D). Data are presented as means ± SE. *P < 0.05. OGTT, oral glucose tolerance test; ISIOGTT, insulin sensitivity (Matsuda) index; AU, arbitrary units.
Apoptotic biomarkers.
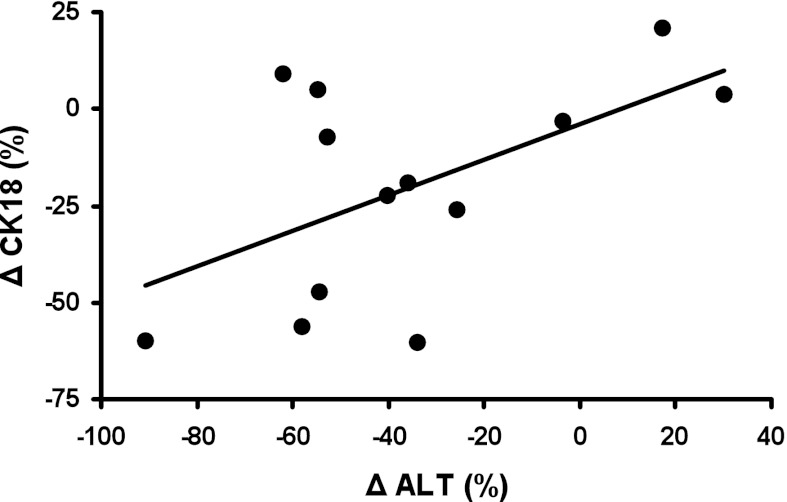
Plasma CK18 (Fig. 1B; 558.4 ± 106.8 vs. 323.4 ± 72.5 U/l, P < 0.01) and ALT (Fig. 1C; 30.2 ± 5.1 vs. 24.3 ± 4.8 U/l, P < 0.05) were significantly reduced by the intervention, while the reduction in sFasL approached significance (Fig. 1D; 66.5 ± 6.0 vs. 63.0 ± 5.7 pg/ml, P = 0.06). However, there was no change in plasma AST (35.1 ± 6.2 vs. 34.5 ± 5.8 U/l, NS) or sFas (6,483.2 ± 358.0 vs. 6,284.9 ± 315.7 pg/ml, NS). There was a trend toward an association between the intervention-induced change in CK18 and ALT (Fig. 2; r = 0.55, P = 0.05). We found no correlation between the change in insulin sensitivity or adipose insulin resistance and markers of cell death.
Fig. 2.
Association between exercise training-induced percent changes in circulating CK18 and ALT in obese individuals with nonalcoholic fatty liver disease (NAFLD) (r = 0.55, P = 0.05). Δ, change.
Substrate oxidation.
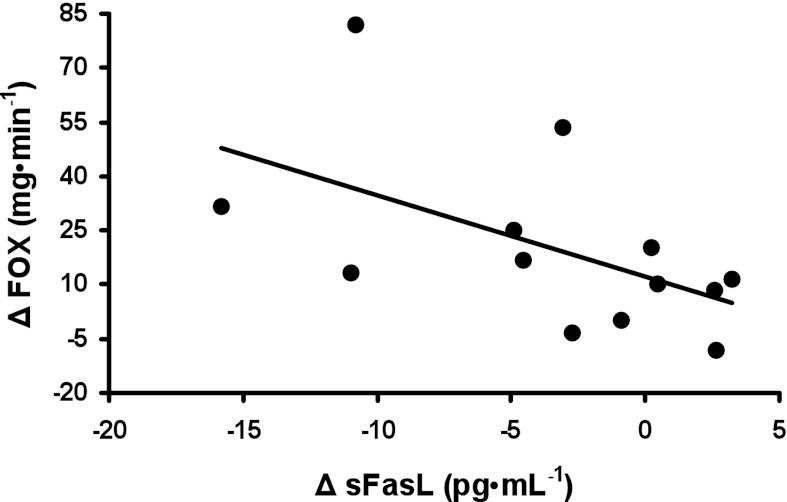
Resting FOX was increased following the exercise intervention (Table 1). In addition, changes in FOX were significantly correlated with changes in sFasL (Fig. 3; rho = −0.65, P < 0.05).
Fig. 3.
Association between exercise training-induced changes in basal fat oxidation (FOX) and changes in circulating sFasL (rho = −0.65, P < 0.05).
DISCUSSION
Exercise currently forms a major component of the treatment recommended by the American Gastroenterological Association (1) for NAFLD, despite a lack of published evidence on the effectiveness of exercise in treating this disease (26). Here we show, for the first time, that exercise in the absence of weight loss significantly reduces plasma levels of the apoptotic marker, caspase-cleaved CK18 fragments, in previously sedentary obese individuals with NAFLD. These data collectively indicate a reduction in the profibrogenic apoptotic state present in NAFLD. This finding is important as hepatocyte apoptosis contributes significantly to the pathogenesis of NAFLD and progression to NASH and liver fibrosis (41). Recently Kistler et al. (26) examined the relationship between self-reported physical activity and the severity of liver fibrosis. They reported that individuals who met the vigorous activity levels recommended by the US Department of Health and Human Services had significantly lower odds of having advanced fibrosis. Our data support the view that exercise serves a protective function against the pathogenesis of NAFLD.
The changes in plasma CK18 fragments in our data were mirrored by changes in ALT levels, suggesting that the reductions we observed in plasma CK18 indeed reflect decreases in hepatocyte apoptosis. CK18 is expressed in epithelial cells, and therefore the reductions could theoretically be derived from decreased apoptosis in almost any tissue. However, ALT is an enzyme of the alanine cycle and is predominantly expressed in the liver. For this reason, it is used as a surrogate biomarker for liver injury. Several previous studies have demonstrated a positive effect of longer term exercise on ALT levels in serum (10–11, 44). It has also recently been reported that administration of a caspase inhibitor may reduce ALT and CK18 in NAFLD patients in a dose-dependent manner (39). Here, we confirm the beneficial effect of exercise on ALT levels and show that these effects become apparent within 1 wk of moderate- to high-intensity exercise. Furthermore, the positive correlation between percent changes in plasma CK18 levels and ALT suggests that improvements in CK18 may be a result of reduced hepatocyte apoptosis.
The exact mechanism by which exercise may reduce apoptosis in NAFLD remains to be elucidated. Insulin resistance is a key component in the pathogenesis of NAFLD (12). Our exercise program resulted in an increase in insulin sensitivity of ∼25% in this subject population. Exercise-mediated improvements in insulin sensitivity are well documented (5, 18, 19, 24, 25). Exercise elicits favorable alterations in lipid uptake and metabolism in skeletal muscle (31), and it has been proposed that exercise-induced improvements in insulin sensitivity may decrease de novo lipogenesis and hepatic triglyceride synthesis (38, 40). We did observe an increase in whole body fat oxidation. Moreover, the alterations in FOX were negatively correlated with changes in sFasL. Collectively, these data support the hypothesis that improved whole body insulin sensitivity and greater fat oxidation may act to reduce lipotoxicity in the liver and thereby reduce the proapoptotic stimulus to hepatocytes.
Apoptosis is mediated via two pathways, the extrinsic and intrinsic pathway. In this study we also investigated the effect of exercise on biomarkers of the Fas death-inducing pathway, a component of the extrinsic pathway. We found that exercise resulted in a modest reduction in sFasL but not sFas. Previous reports have linked exercise training to reductions in oxidative stress (42). Induction of high levels of reactive oxygen species (ROS) subjects cells to oxidative stress, which is believed to play a pivotal role in the pathogenesis of NAFLD (27, 29, 47). While hepatocytes express Fas in large numbers, they do not normally express large numbers of Fas ligand. However, increased ROS production in hepatocytes can cause increased Fas ligand expression, resulting in fratricidal apoptosis (36). Mitochondrial dysfunction is believed to be a major contributor to ROS production in NAFLD (17). Exercise increases mitochondrial function and content principally through high-molecular weight (HMW) adiponectin (21). We have previously demonstrated increases in HMW adiponectin in insulin-resistant obese individuals following 7 days of moderate- to high-intensity exercise. Furthermore, the changes in HMW adiponectin were positively correlated with changes in FOX (23). Therefore, improved oxidative capacity and reduced oxidative stress following exercise training may cause a reduction in the generation of Fas ligand within hepatocytes and consequently attenuate the apoptotic signal. However, the relationship between improvements in FOX and ROS production in a NAFLD population following exercise remains to be confirmed.
There is also evidence that ROS increases TNF-α production, which interacts with and activates caspase-8, resulting in permeabilization of the mitochondrial membrane via truncated Bid and induction of cytochrome c release (36). Therefore exercise may also reduce apoptosis via reductions in intrinsic pathway signaling. Upregulation of the antioxidant defense system can also reduce oxidative stress-mediated apoptosis via the intrinsic pathway. Indeed, Sinha-Hikim et al. (43) recently demonstrated that a high-fat diet upregulated proapoptotic and downregulated antiapoptotic enzymes BAX and BCL-2, respectively, in a mouse model of NAFLD. However, supplementation with a glutathione precursor significantly reduced the high-fat-feeding upregulation of BAX and suppressed activation of caspase 3 in hepatocytes. Cakir et al. (6) recently demonstrated that exercise protects against glutathione depletion in hepatocytes of rats with alcoholic liver disease; however, data from human or NAFLD studies are currently lacking. The possibility that reductions in apoptosis are mediated through downregulation of the intrinsic pathway, on the basis of these data, cannot be excluded.
Conclusions.
Empirical data on the effect of exercise on hepatocyte apoptosis in NAFLD are currently lacking; however, here for the first time we demonstrate that short-term exercise, in the absence of weight loss and changes in intrahepatic lipid, reduces a circulating marker of hepatocyte apoptosis in previously sedentary obese individuals with NAFLD. The exact mechanism by which this occurs is currently under investigation and for now our interpretation of the data is limited by the use of whole body indexes. Nonetheless, we provide evidence that reductions in apoptosis may be related to reductions in FasL, possibly resulting from improvements in oxidative capacity. These findings have significant clinical implications for the prevention of disease progression in NAFLD patients and support the use of exercise as an effective treatment for NAFLD.
GRANTS
This research was supported by National Institute of Health (NIH) Grants R01-AG-12834 (J. P. Kirwan) and T32-DK-0661917 (M. Pagadala) and was supported in part by the NIH National Center for Research Resources, CTSA-1UL1-RR-024989, and the Case Center for Imaging Research, Case Western Reserve University, Cleveland, OH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.E.F., J.M.H., T.P.S., M.P., and C.A.F. performed experiments; C.E.F., J.M.H., C.A.F., and J.P.K. analyzed data; C.E.F., J.M.H., M.P., C.A.F., and J.P.K. interpreted results of experiments; C.E.F. prepared figures; C.E.F. drafted manuscript; C.E.F., J.M.H., T.P.S., M.P., C.A.F., A.J.M., and J.P.K. edited and revised manuscript; J.M.H., T.P.S., C.A.F., A.J.M., and J.P.K. conception and design of research; J.P.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the research volunteers for their outstanding dedication and effort, and the staff of the Clinical Research Unit and the technical staff and students who helped with the implementation of the study and assisted with data collection. We also acknowledge our clinical research coordinator, Julianne Filion, for her excellent nursing and organizational assistance.
REFERENCES
- 1. American Gastroenterological Association American Gastroenterological medical position statement: nonalcoholic fatty liver disease. Gastroenterology 123: 1702–1704, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Akazawa Y, Gores GJ. Death receptor-mediated liver injury. Semin Liver Dis 27: 327–338, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 346: 1221–1231, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Borengasser SJ, Rector RS, Uptergrove GM, Morris EM, Perfield JW, 2nd, Booth FW, Fritsche KL, Ibdah JA, Thyfault JP. Exercise and omega-3 polyunsaturated fatty acid supplementation for the treatment of hepatic steatosis in hyperphagic OLETF rats. J Nutr Metab 2012: 268680, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bourey RE, Kohrt WM, Kirwan JP, Staten MA, King DS, Holloszy JO. Relationship between glucose tolerance and glucose-stimulated insulin response in 65-year olds. J Gerontol 48: M122–M127, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Cakir B, Kasimay O, Kolgazi M, Ersoy Y, Ercan F, Yegen BC. Stress-induced multiple organ damage in rats is ameliorated by the antioxidant and anxiolytic effects of regular exercise. Cell Biochem Funct 28: 469–479, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest 83: 655–663, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Caulin C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol 138: 1379–1394, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chapados NA, Lavoie JM. Exercise training increases hepatic endoplasmic reticulum (er) stress protein expression in MTP-inhibited high-fat fed rats. Cell Biochem Funct 28: 202–210, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Chen SM, Liu CY, Li SR, Huang HT, Tsai CY, Jou HJ. Effects of therapeutic lifestyle program on ultrasound-diagnosed nonalcoholic fatty liver disease. J Chin Med Assoc 71: 551–558, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Church TS, Kuk JL, Ross R, Priest EL, Biltoft E, Blair SN. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology 130: 2023–2030, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Day CP, James OFW. Steatohepatitis: a tale of two “hits”? Gastroenterology 114: 842–845, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Drenick EJ, Simmons F, Murphy JF. Effect on hepatic morphology of treatment of obesity by fasting, reducing diets and small-bowel bypass. N Engl J Med 282: 829–834, 1970 [DOI] [PubMed] [Google Scholar]
- 14. Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 125: 437–443, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Feldstein AE, Canbay A, Guicciardi ME, Higuchi H, Bronk SF, Gores GJ. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol 39: 978–983, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology 50: 1072–1078, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fromenty B, Robin MA, Igoudjil A, Mansouri A, Pessayre D. The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab 30: 121–138, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Haus JM, Solomon TP, Marchetti CM, Edmison JM, Gonzalez F, Kirwan JP. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance. J Clin Endocrinol Metab 95: 323–327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hughes VA, Fiatarone MA, Fielding RA, Kahn BB, Ferrara CM, Shepherd P, Fisher EC, Wolfe RR, Elahi D, Evans WJ. Exercise increases muscle GLUT 4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol Endocrinol Metab 264: E855–E862, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Irwan R, Edens MA, Sijens PE. Assessment of the variations in fat content in normal liver using a fast MR imaging method compared with results obtained by spectroscopic imaging. Eur Radiol 18: 806–813, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca2+ and AMPK/SIRT1. Nature 464: 1313–1319, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis 28: 370–379, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Kelly KR, Blaszczak A, Haus JM, Patrick-Melin A, Fealy CE, Solomon TP, Kalinski MI, Kirwan JP. A 7-d exercise program increases high-molecular weight adiponectin in obese adults. Med Sci Sports Exerc 44: 69–74, 2012 [DOI] [PubMed] [Google Scholar]
- 24. Kirwan JP, Kohrt WM, Wojta DM, Bourey RE, Holloszy JO. Endurance exercise training reduces glucose-stimulated insulin levels in 60- to 70-year old men and women. J Gerontol 48: M84–M90, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 297: E151–E156, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol 106: 460–468; quiz 469, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kojima H, Sakurai S, Uemura M, Fukui H, Morimoto H, Tamagawa Y. Mitochondrial abnormality and oxidative stress in nonalcoholic steatohepatitis. Alcohol Clin Exp Res 31: S61–S66, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F, Cusi K. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 55: 1389–1397, 2012 [DOI] [PubMed] [Google Scholar]
- 29. Mantena SK, King AL, Andringa KK, Eccleston HB, Bailey SM. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic Biol Med 44: 1259–1272, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matsuda M, DeFronzo R. Insulin sensitivity indices obtained from oral glucose tolerance testing. Diabetes Care 22: 1462–1470, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Newsom SA, Schenk S, Thomas KM, Harber MP, Knuth ND, Goldenberg N, Horowitz JF. Energy deficit after exercise augments lipid mobilization but does not contribute to the exercise-induced increase in insulin sensitivity. J Appl Physiol 108: 554–560, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O'Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol 100: 1584–1589, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Omary MB, Ku NO, Liao J. Apoptosis generates stable fragments of human type I keratins. J Biol Chem 272: 33197–33203, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Palmer M, Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology 99: 1408–1413, 1990 [DOI] [PubMed] [Google Scholar]
- 35. Peronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci 16: 23–29, 1991 [PubMed] [Google Scholar]
- 36. Pessayre D, Mansouri A, Haouzi D, Fromenty B. Hepatotoxicity due to mitochondrial dysfunction. Cell Biol Toxicol 15: 367–373, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ 10: 26–35, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Rabol R, Petersen KF, Dufour S, Flannery C, Shulman GI. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals. Proc Natl Acad Sci USA 108: 13705–13709, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ratziu V, Sheikh MY, Sanyal AJ, Lim JK, Conjeevaram H, Chalasani N, Abdelmalek M, Bakken A, Renou C, Palmer M, Levine RA, Bhandari BR, Cornpropst M, Liang W, King B, Mondou E, Rousseau FS, McHutchison J, Chojkier M. A phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology 55: 419–428, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rector RS, Uptergrove GM, Morris EM, Borengasser SJ, Laughlin MH, Booth FW, Thyfault JP, Ibdah JA. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol 300: G874–G883, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ribeiro PS, Cortez-Pinto H, Sola S, Castro RE, Ramalho RM, Baptista A, Moura MC, Camilo ME, Rodrigues CM. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol 99: 1708–1717, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Roberts CK, Won D, Pruthi S, Kurtovic S, Sindhu RK, Vaziri ND, Barnard RJ. Effect of a short-term diet and exercise intervention on oxidative stress, inflammation, MMP-9, and monocyte chemotactic activity in men with metabolic syndrome factors. J Appl Physiol 100: 1657–1665, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Sinha-Hikim I, Sinha-Hikim AP, Shen R, Kim H, French SW, Vaziri ND, Crum A, Rajavashisth TB, Norris KC. A novel cystine based antioxidant attenuates oxidative stress and hepatic steatosis in diet-induced obese mice. Exp Mol Pathol 91: 419–428, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sreenivasa Baba C, Alexander G, Kalyani B, Pandey R, Rastogi S, Pandey A, Choudhuri G. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J Gastroenterol Hepatol 21: 191–198, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 288: E462–E468, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Tamimi TI, Elgouhari HM, Alkhouri N, Yerian LM, Berk MP, Lopez R, Schauer PR, Zein NN, Feldstein AE. An apoptosis panel for nonalcoholic steatohepatitis diagnosis. J Hepatol 54: 1224–1229, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trauner M, Arrese M, Wagner M. Fatty liver and lipotoxicity. Biochim Biophys Acta 1801: 299–310, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology 44: 27–33, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Yilmaz Y, Dolar E, Ulukaya E, Akgoz S, Keskin M, Kiyici M, Aker S, Yilmaztepe A, Gurel S, Gulten M, Nak SG. Soluble forms of extracellular cytokeratin 18 may differentiate simple steatosis from nonalcoholic steatohepatitis. World J Gastroenterol 13: 837–844, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Younossi ZM, Jarrar M, Nugent C, Randhawa M, Afendy M, Stepanova M, Rafiq N, Goodman Z, Chandhoke V, Baranova A. A novel diagnostic biomarker panel for obesity-related nonalcoholic steatohepatitis (NASH). Obes Surg 18: 1430–1437, 2008 [DOI] [PubMed] [Google Scholar]