Abstract
As key effector molecules of jawed vertebrate’s adaptive immune system, immunoglobulins are produced by B lymphocytes, either as a secretory form (antibody) or as a membrane form (B cell receptor). Until recently, teleost fish B cells were thought to express only two classes of immunoglobulins, IgM and IgD. In addition, IgM in these species was thought to be the only immunoglobulin isotype responding to pathogens both in systemic or mucosal compartments. However, the unexpected discovery of IgT, a new teleost immunoglobulin unearthed in 2005, has provided for new opportunities to analyze further roles of teleost immunoglobulins in these two physiologically distinct compartments. The smoke about the potential function of IgT has cleared recently with the finding that this immunoglobulin appears to be specialized in gut mucosal immunity. Significantly, the new capability of measuring not only IgM but also IgT responses will greatly facilitate the evaluation and understanding of fish immune responses as well as the protective effects of fish vaccines. The purpose of this review is to summarize the molecular characterization of new IgT orthologs and subtypes in teleosts, as well as to describe the new findings concerning the protein structure of IgT, the B cells producing it, and its role in mucosal immunity.
Keywords: IgT, mucosal immunoglobulin, fish
1. Introduction
The adaptive immune system (AIS) appears to have emerged in the common ancestor of all vertebrates, more than 500 million years ago [1,2], since a parallel version of jawed vertebrate AIS was discovered recently in jawless vertebrates [3–5]. Antigen recognition in jawless vertebrates is mediated by variable lymphocyte receptors (VLRs) [6] whereas in jawed vertebrates the key molecules involved in antigen recognition are the B and T cell receptors (BCR and TCR respectively).
A typical immunoglobulin (Ig) molecule consists of two heavy (H) and two light (L) chains (H2L2 unit), each of which containing one amino-terminal variable (V) immunoglobulin superfamily (IgSF) domain and one (in the L chain) or more (in the H chain) carboxyl-terminal constant (C) IgSF domain. Generally, the paired V domains provided by each of the H and L chains, are associated non-covalently and confer antigenic specificity, while the H chain C domains define effector functions of the immunoglobulins through binding to their receptors on effector cells or activating other immune mechanisms, such as complement [7]. Immunoglobulins can be classified into different isotypes (classes) and subtypes (subclasses) based on the nature of the C domains of their H chains. In mammals, five Ig isotypes, IgM, IgD, IgG, IgE, and IgA, have been identified, which possess specific effector functions in humoral and cellular immune responses (see reference [2] for a recent review on structural, functional and evolutionary aspects of vertebrate immunoglobulins). Among the antibody isotypes, IgM is the most ancient and the only isotype functionally conserved in all gnathostomes (jawed vertebrates) [8]. IgD has been found in all gnathostomes, except birds, indicating that it is also a primordial antibody class despite its highly plastic structure and unclear function in evolution (see reference [2]).
Immunoglobulin isotypes have evolved to play specialized roles either within mucosal or systemic compartments. In mammals and birds, IgM, IgG and IgY isotypes have major roles in systemic responses, while IgA is the main player in mucosal areas. In amphibians, IgM and IgY play a prevalent role in systemic immunity whereas IgX is an isotype chiefly expressed in the gut [9]. Until recently, teleost fish were thought to contain only two classes of immunoglobulins, IgM and IgD. It was also generally accepted that IgM was the only immunoglobulin class responding to antigenic challenge both in systemic and mucosal compartments, and thus, teleost were believed to be devoid of an immunoglobulin specialized in mucosal surfaces. A new teleost Ig H chain gene, named ighτ in rainbow trout [10] or ighζ in zebrafish [11], was reported in 2005. The assembled immunoglobulin containing the ighτ product was named IgT in trout (or IgZ in zebrafish), and it was suggested to represent the last immunoglobulin class to be found in a vertebrate species [12]. During the last five years ighτ/ighζ has been cloned and characterized at the gene level in a number of teleosts species [13–18]. However, until very recently its protein structure, production, and potential role in immunity were not reported. In that regard, a 2010 study revealed that rainbow trout IgT is an immunoglobulin specialized in gut mucosal immune responses, while IgM appears to be specialized in systemic immunity [19]. These findings have challenged the paradigm that specialization of immunoglobulin isotypes into mucosal and systemic areas arose during tetrapod evolution. The new capability of measuring not only IgM but also IgT responses will greatly facilitate the evaluation and understanding of teleost immune responses as well as the protective effects of fish vaccines. In this review, we summarize the molecular characterization of newly discovered ighτ/ighζ genes in teleosts, as well as recently reported aspects of the protein structure of IgT, its production by a novel lineage of B cells and its role in immunity.
2. Characterization of the genes encoding IgT
2.1. Organization and rearrangement of the igh locus in teleost fish
The immunoglobulin heavy (IgH) and light (IgL) chains are encoded by separate genomic loci, igh and igl, respectively, and their individual V and C domains are each encoded by independent elements: the variable (V), diversity (D, only for H chains), and joining (J) gene segments for the V domain, and individual constant (C) gene segments for the C domains. The V domain of the IgH and IgL chains is functionally divided into three hypervariable sequences termed complementarity-determining regions (CDR) that are located between four relatively stable sequences named framework regions (FR). The diversity of the V domain is provided mostly by the three CDR regions. CDR1 and CDR2 are encoded by the V gene alone, while CDR3 is encoded by the V-J or V-D-J rearrangement junction and thus represents the most diverse CDR [7].
To date, two major organization types of igh and igl loci have been reported: translocons or clusters [2]. In humans, the igh locus is in ‘translocon’ configuration: V-D-J-Cμ-Cδ-Cγ3-Cγ1-Cγ2b-Cγ2a-Cε-Cα. The messenger RNAs for the H chains of IgM (μ) and IgD (δ) are generated by alternatively splicing the recombined VDJ to either Cμ or Cδ in their igh loci, whereas the H chains of IgG (γ), IgE (ε), and IgA (α) are expressed through a process known as class-switch recombination. In cartilaginous fish, the igh genes are in the ‘cluster’ configuration with many sets of V(D)2–3JC clusters [1,2].
Until five years ago, the igh loci in bony fish were thought to be organized only in ‘translocon’ configuration, where multiple V gene segments are upstream of multiple D and J segments, followed by a Cμ and Cδ genes, encoding and generating the μ and δ chains by alternative splicing [20,21]. However, the detailed analyses of the complete igh loci from rainbow trout (Oncorhynchus mykiss) [10] and zebrafish (Danio rerio) [11] revealed a novel genomic architecture that had never been observed in any igh loci: upstream of the known (VH)-DJCμCδ elements for μ and δ IgH chains, another set of VH-DJC elements were found, which encoded for the H chain (τ, for teleost fish) of a previously unknown Ig isotype, IgT, named by Hansen and colleagues [10]. In zebrafish this new immunogobulin was termed IgZ [11]. To avoid a mixed terminology, throughout this review we will mostly use the term ‘IgT’ to refer to IgT/IgZ, and we will use ‘ighτ’ for the gene encoding its H chain. The organization of the above mentioned ighτ-ighμ locus is strikingly similar to that of the mouse Tcrd-Tcra locus encoding T cell receptor δ (TCRδ) and TCRα. In both loci, upstream V segments rearrange either to DJCτ (or DJCδ) to encode τ (or TCRδ) or to DJCμ (or DJCα) to encode μ (or TCRα) [1,11,12]. Based on the aforementioned arquitecture of the ighτ-ighμ locus, and despite of the fact that class switching mechanisms are absent in fish [22,23], B cells of these species were predicted to express either IgT or IgM, which was demonstrated to be the case in rainbow trout at protein level [19] and in zebrafish at gene level [24].
Subsequent reports have showed that IgT orthologs exist in almost all studied species belonging to the main orders of teleost fish (see Table 1), such as fugu (Takifugu rubripes) [13], common carp (Cyprinus carpio) [14,15], zebrafish [25], stickleback fish (Gasterosteus aculeatus) [16,17], grass carp (Ctenopharyngodon della) [18], Atlantic salmon (Salmo salar) [26,27], Chinese perch (Siniperca chuatsi), and orange-spotted grouper (Epinephelus coioides). The only exception where IgT has not been found thus far is in the channel catfish (Ictalurus punctatus) [28]. As the genome sequence of the channel catfish had not been finished at present time, it is possible that IgT may be found once the genome of this species is completed.
Table 1.
Organization of teleost igh loci and the constant regions encoded by ighτ
| Order | Species | Potentially functional IgT Subtypes | Organization of igh locus | Constant region | Referencea |
|---|---|---|---|---|---|
| Salmoniforms | Oncorhynchus mykiss | IgT1 IgT2 |
VHDτJτCτ-VHDμJμCμCδ Data not available |
Cτ1-Cτ2-Cτ3-Cτ4 | [10] [11] |
| Salmo salar | IgT4, IgT5 IgT2 |
locus A: (VHDτJτCτ)5-VHDμJμCμCδ locus B: (VHDτJτCτ)3-VHDμJμCμCδ |
Cτ1-Cτ2-Cτ3-Cτ4 | [26,27] | |
| Cypriniformes | Danio rerio | IgT1 IgT2 |
VHDτJτCτ-DμJμCμCδ Data not available |
Cτ1-Cτ2-Cτ3-Cτ4 | [11] [25] |
| Ctenopharyngodon idella | IgT1 IgT2 |
VHDτJτCτ-DμJμCμCδ Data not available |
Cτ1-Cτ2-Cτ3-Cτ4 | [18] DQ478943 |
|
| Cyprinus carpio | IgT1 IgT2 (chimeric IgM-IgT) |
Data not available | Cτ1-Cτ2-Cτ3-Cτ4 Cμ1------------Cτ4 |
[15] [14,15] |
|
| Tetraodontiformes | Takifugu rubripes | IgT | VHDτJτCτ-DμJμCμCδ | Cτ1------Hb----Cτ4 | [13] |
| Gasterosteiformes | Gasterosteus aculeatus | IgT1, IgT2, IgT3, IgT4 | (VHDτJτCτ-DμJμCμCδ)3-VHDτJτCτ | Cτ1-------Cτ3-Cτ4 | [16] [17] |
| Perciformes | Siniperca chuatsi | IgT | Data not available | Cτ1-Cτ2-Cτ3-Cτ4 | Q016660 |
| Epinephelus coioides | IgT | Data not available | Cτ1-Cτ2-Cτ3-Cτ4 | GU182366 |
When no literature is available the GenBank accession number is showed.
H, the putative hinge region: GPV(KPTV)5.
It is important to highlight that most of the above species possess more than one subclass of IgT, which may be encoded by duplicated ighτ genes in the same locus, as for example in stickleback fish [16,17]). Alternatively, these additional IgT subclasses are found in different igh loci. For instance, in Atlantic salmon, two igh loci were discovered (see Table 1), in each of which, 3 or 5 VHDτJτCτ clusters are upstream of one copy of VHDμJμCμCδ, encoding for three putatively functional IgT subclasses [26,27]. Similarly, two igh loci also exist in rainbow trout [10]. Most of the H chains of IgT contain four C domains (Cτ), whereas stickleback IgTs lack the Cτ2, and in fugu IgT and carp IgT2 they lack Cτ2 and Cτ3. Among the Cτ domains, Cτ1 always maintains its higher similarity to Cμ1, and Cτ4 provides the specificity to the IgT isotype [16]. The reason that only Cτ1 and Cτ4 are always conserved among species may be due to their unique role in binding to IgL chain (through the cysteine residue [Cys] in Cτ1) or to other IgH chains (through the Cys in Cτ4 or secretory tail).
Although the genomic organization and domain numbers of IgT are variable among different species, the phylogenetic studies, based on the sequences of complete C regions or individual C domains, have shown that IgT occupies a separate clade on phylogenetic trees, distinct from IgM or other Ig isotypes. While a possible relationship between the teleost τ and μ chains has been suggested, they most likely represent distinct IgH lineages with common evolutionary origins [10,11]. Besides the recent findings in rainbow trout [19], the biochemical features and functional roles of IgT and its subclasses in different teleost species await to be investigated.
2.2. Features of the V and C domains of the H chain of IgT
Through analysis of the cDNA clones of trout and zebrafish ighτ and ighμ, it was shown that the VH gene segments in their igh loci were shared by both Cτ and Cμ. However, the upstream D and J segments were found to be used exclusively by the nearby Cτ or Cμ to generate their own CDR3 [10,11], a region with crucial function in antigen recognition. Strikingly, the CDR3 of trout τ chain is longer (5–10 amino acid (aa)) compared to that of μ chain (4–5 aa) [10], which suggested that IgT might recognize a more diverse range of pathogenic epitopes because of the more extended CDR3 in its τ chain [10].
Both membrane and secretory forms of τ chains can be encoded by the same genes. The membrane τ chain can be generated by splicing from a cryptic splice site near the very end of Cτ4 exon to the first membrane exon (TM1), similar to the splice pattern in the mammalian membrane μ chain, but unlike the teleost membrane μ chain, where the Cμ3 is spliced directly to TM1. As a result, teleost membrane μ chain has three C domains, while membrane τ chain retains all four C domains [10,11]. The teleost secretory τ chain is usually predicted to be shorter than its secretory μ chain, despite both of them having four C domains. This is because each of the C domains as well as the secretory tail of τ chain is shorter than the corresponding domain of μ chain (see Fig. 1), which leads to a probable smaller mass of τ chain (theoretical Mw: 58.3 kDa) when compared to that of μ chain (theoretical Mw: 62.8 kDa), without taking into consideration the putative glycosylation or other postranslational modifications in the C domains.
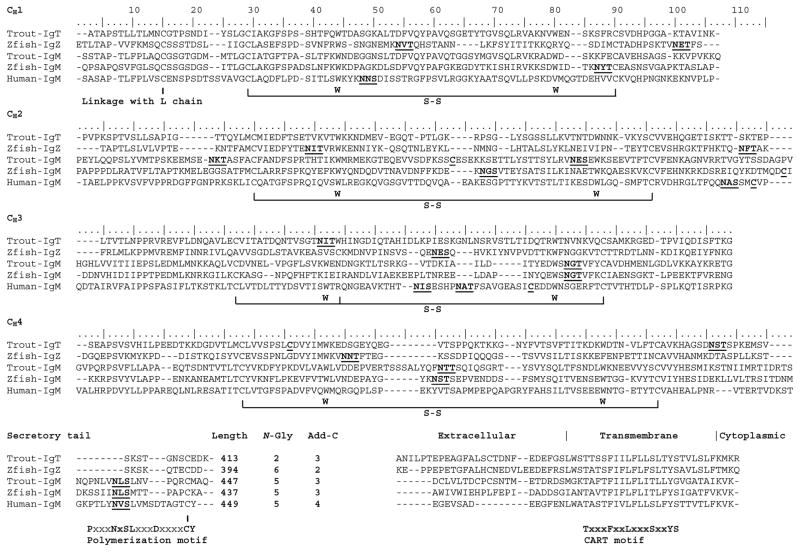
Fig. 1.
Amino acid sequence alignment of the heavy chains of IgT/IgZ and IgM. Gaps are indicated by dashes to optimize the alignment. The conserved (identical and similar) residues are shaded. The conserved cystein (S-S) and relatively conserved tryptophan (W) residues required to form the fold of each IgSF domain are shown below the alignment. The conserved cystein residues involved in the disulfide bond linking with IgL chain (in CH1 domains) and the ones important for Ig polymerization (in secretory tails) are indicated, respectively. The polymerization and CART motifs located in the secretory tail and transmembrane region of human IgM are indicated below the alignment. The N-linked glycosylation sites are in bold and underlined in each sequence. The numbers of N-linked glycosylation sites (N-Gly) and additional cysteins (Add-C) are shown at the end of each sequence. The GenBank accession numbers for the aligned IgH sequences are: rainbow trout τ (Trout-IgT, AY870263 and AY870265), zebrafish ζ (Zfish-IgZ, AY643752 and AY643750); rainbow trout μ (Trout-IgM, AY870258 and U04615), zebrafish μ (Zfish-IgM, AY643753and AY643751), and human μ (Human-IgM, X14940 and X58529).
In the IgSF domain, Cys and Trp (tryptophan) residues are crucial for the folding of this domain [29]. The two Cys residues, involved in intra-domain disulfide linkages that form the core Ig domain loop in each of the Cτ1, Cτ2, and Cτ4 domains of all studied τ chains are conserved in their positions (like those of μ chains), and are separated by approximately 50 residues. Interestingly, in the Cτ3 domains of cyprinid fish, the first Cys residue was found 16–17 aa closer to the second one when compared to Cτ3 domains in other fish. However, it was predicted that the atypical Cys residues of this cyprinid Cτ3 domains would not affect the formation of typical IgSF domains [1]. There are several additional Cys residues that exist in the C domains and secretory tails of teleost τ chains, among which the one within Cτ1 is conserved in all teleost, which is predicted to form a disulfide bridge with a Cys from the IgL chain. In mammals, the secretory tails of polymeric IgM and IgA contain a conserved PX3NXS/TL/VX3E/DX4CY motif, where the penultimate Cys (C) together with the N-linked glycosylation site (NXS/T) were suggested to be typically required for polymer assembly and J chain incorporation [30–33]. Within the shorter secretory tails of teleost τ chains there is also a conserved Cys residue, however, the N-glycosylation site and the polymerization motif are missing, which raised an intriguing question: Does the IgT unit τ2L2 polymerizes into a dimer like mammalian IgA, or a tetramer like teleost IgM, or just behaves as a monomer like IgG? (Studies described below in section 3.1 address this question). The assembly of IgTs with an H chain only having two conserved additional Cys (mentioned above for zebrafish, Chinese perch, common carp, and grass carp IgT2) are even harder to be predicted based only on the primary sequences of their τ chains.
Conserved Antigen Receptor Transmembrane (CART) motifs have been identified in the transmembrane domains of all antigen receptors (including membrane Ig and TCR) of jawed vertebrates. These motifs are known to interact with other proteins that play a role in the assembly and/or the signaling properties of lymphocyte antigen receptors [34]. As shown in Fig. 1, the transmembrane regions and especially the CART motifs of teleost τ chains, are very similar to those of teleost and mammalian μ chains. This suggests that membrane IgT has signaling capacities similar to those found in other BCRs. In order to shed light on the specific roles of IgT and IgM in fish immunity, future studies will have to investigate differences in the signaling properties between membrane IgM and IgT.
2.3. Expression of ight gene
As a first step to evaluate the function of IgT, its expression during development and in specific organs of adult fish was studied in most of the species shown in Table 1. Compared to IgM, detectable expression of IgT1 was observed earlier and increased more rapidly during the early developmental stage of zebrafish [11], while the earlier IgT expression was not seen in other teleost species [10,13,15], which might be due to differences in the time points studied in all the above mentioned species [1].
The expression of IgT1 in adult zebrafish was found almost exclusively localized in primary lymphoid organs (i.e., head kidney and thymus) while IgM expression could be detected in both primary and secondary lymphoid organs [11,25]. However, the expression patterns of trout IgT, fugu IgT, carp IgT1 and IgT2, grass carp IgT1, and Zebrafish IgT2, are quite similar to that of IgM, i.e., they are detectable in both primary and secondary lymphoid organs, or in some cases even non-lymphoid organs (i.e. muscle and brain in carp [15]), although IgM was always the predominant isotype [10,13,15,18,25]. Interestingly, in common carp the relative expression level of IgT2 was higher than that of IgT1 in the mucosal organs (intestine and gills). Combined with the strong expression of IgT2 in goblet cells of intestine and gill epithelium detected by in situ hybridization, this suggests that IgT2 may play important roles in mucosal surfaces of fish [13,15].
In summary, both IgT and IgM can be expressed from the very early developmental stages (as early as 4 days post fertilization) of teleost fish, with the expression of IgT increasing more rapidly when compared to that of IgM, which may suggest that IgT plays a significant role in protecting fish larvae. In most adult fish, IgT and IgM can be expressed constitutively in both primary and secondary lymphoid organs, with IgM always being the dominant isotype and in some cases (i.e. after parasite infection) IgT being more highly expressed in mucosal organs [15,19]. This suggests that IgM may protect adult fish from infection through systemic immune responses and IgT may be specialized in mucosal immunity. As shown below this appears to be the case at least in rainbow trout [19].
3. Biochemical and functional characterization of IgT
The first account for a mucosal immune system in fish dates from 1942, when Duff observed protection in rainbow trout orally immunised withAeromonas salmonicida antigens [35]. In 1969, Fletcher and Grant reported for the first time the presence of immunoglobulin in gut and skin mucus in plaice (Pleuronectes platessa L.) [36] and, since then, the evaluation of mucosal antibody responses in fish has always been limited to IgM [37].
Since IgT was discovered in 2005 [10,11], up until recently nothing was known about the protein structure of this immunoglobulin, the cells producing it, and most importantly, its role in teleost immunity. Below we summarize the latest findings concerning the biochemical and functional characterization of this new teleost immunoglobulin class.
3.1. Biochemical features of IgT
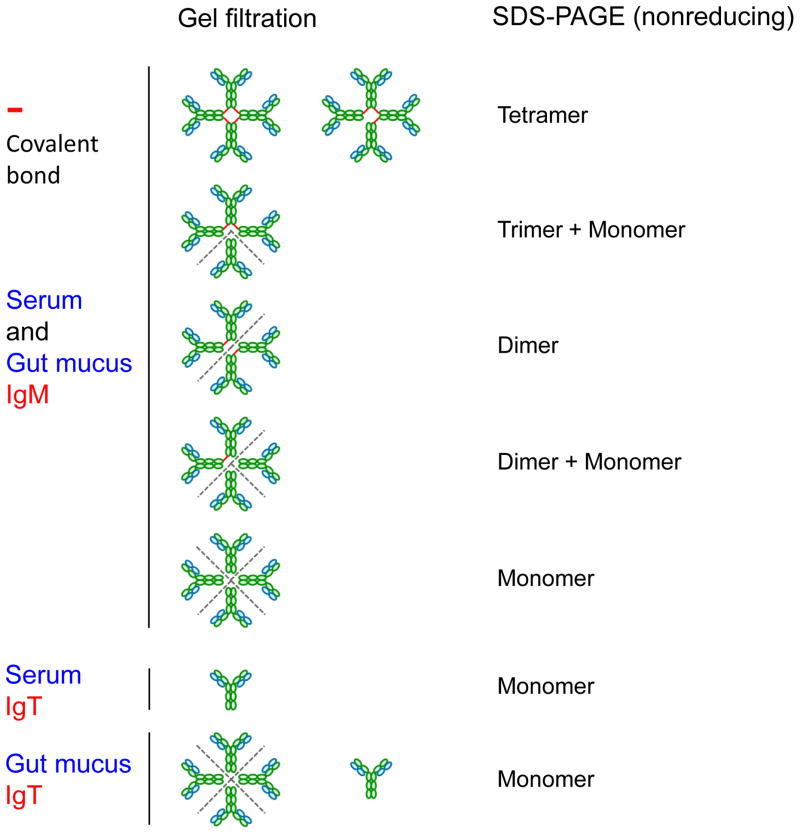
Antibodies raised against rainbow trout IgT have recently enabled its protein identification and biochemical characterization from several body fluids [19]. It was shown that trout IgT has a molecular mass of ~180 kDa under nonreducing conditions, being the heavy and light chain masses of ~70–75 kDa and ~25 kDa respectively. These masses are in close agreement with the theoretical masses obtained from the IgT primary sequences [19]. As predicted from the analysis of its amino acid sequence [12], gel filtration analysis confirmed that serum IgT eluted at the position expected for a monomer (~180 kDa) compared to IgM, which is found in tetramers. When the same study was conducted in trout gut mucus, most of the IgT was eluted as a multimer, in very close proximity to IgM. Surprisingly, immunoblot analysis under nonreducing conditions revealed that the mucus IgT multimers were associated by non-covalent bonds. In contrast, serum and mucus IgM were found in various redox forms that differ in the number of monomers associated through disulfide bonds [38]. The mechanisms that hold these subunits together in mucosal IgT are at this point unknown although they may relate to the presence of a piece of the trout polymeric Ig receptor (pIgR) bound to it (see below section 4).
IgT and IgM concentrations substantially differed in serum and gut mucus. The IgM concentration in serum (~2.5 mg/ml) was ~34 fold higher than that of gut mucus, whilethe IgT levels in gut mucus (~7 μg/ml) were double to those found in serum. In essence, the IgT/IgM ratio was much higher in the gut mucus (9.5%) when compared to that in serum (0.15%), thus suggesting that IgT could play a role in gut mucosal immunity [19]. Overall, the above mentioned biochemical characteristics of IgT greatly resemble those of human IgA, which is found in monomeric form in serum, while in the gut mucosa it forms polymers [39]. In addition, like IgT, human IgA is the prevalent immunoglobulin in the gut [39]. The lack of similar studies on other species limits our understanding of IgT across teleosts and it remains to be investigated whether the trout IgT features are universally present in other fish.
3.2 A new lineage of B cells in teleost fish and its role in innate immunity
The antibodies raised against trout IgT enabled the identification and characterization of a new lineage of B cells uniquely expressing surface IgT. These IgT+ B cells did not express any IgM or IgD transcripts and were not stained by monoclonal antibodies against IgM [19]. While the IgM+ B cell population has been identified in most analyzed teleosts species, thus far, the IgM−/IgD−/IgT+ and the IgM−/IgD+ B cell subsets have been definitively characterized with the corresponding monospecific antibodies only in rainbow trout [19] and catfish [40,41], respectively. Hence, each teleost fish species appears to contain two or more major subsets of B cells, in rainbow trout the IgM+/IgD+/IgT− (IgM+ B cells) and IgM −/IgD−/IgT+ (IgT+ B cells), or in catfish the IgM+/IgD−, IgM+/IgD+ and IgM−/IgD+ subsets. Thus, the presence of the IgT+ and IgD+ B cell subsets needs to be confirmed in other teleost fish species. The rainbow trout IgT+ B cell subset represents ~16–27% of all B cells (including both IgT+ and IgM+ B cells) in the main systemic lymphoid organs, whereas IgM+ B cells comprise about ~72–83% of all B cells. In contrast, in trout gut, the percentage of IgT+ and IgM+ lymphocytes was around 54% and 46% of all B cells, respectively. Combining the higher IgT/IgM ratio in gut mucus than serum, these results were strong indications that constitutive IgT was produced more efficiently in trout gut which may play specific functions in the mucosal immunity. In trout, both flow cytometry and immunofluorescence analyses showed no double stained IgT+/IgM+ cells. These results confirmed the aforementioned prediction that teleost fish exist two mutually exclusive B cell lineages that express either IgT or IgM. Hence, it appears that teleosts use a different mechanism for generating B cell isotype diversity when compared to tetrapod species. As teleost ighτ-ighμ and mammalian Tcrd-Tcra loci have comparable genomic structures, it is possible that the mechanism involved in the commitment of fish B cells to expressing either IgT or IgM resembles that involved in generation of αβ or γδ T cells. From a morphological perspective, sorted IgT+ B cells displayed typical lymphocyte features that make them indistinguishable from IgM+ B cells under both light and electron microscopy.
Hu and colleagues have raised polyclonal antibodies against a novel subtype of zebrafish IgT termed as IgT2 [25]. They showed that double positive IgM+/IgT2+ accounted for about 2% of the blood lymphocytes. Whether this double positive population indeed co-expresses both IgM and IgT2, remains to be demonstrated. At the transcript level, in vivo LPS treatment up-regulated IgT2 after 12h in most lymphoid organs examined, including kidney, spleen, gill, gut, and skin, while IgT1 was only up-regulated in kidney and was detectable in spleen [25]. This is in agreement with gene expression studies in adult zebrafish where IgT1 expression was limited to the primary lymphoid organs [11]. In the future, specific monoclonal antibodies against zebrafish IgM, IgT1 and IgT2 will help to unravel their differential roles in immunity.
Teleost IgM+ B cells displayed innate immune functions such as the ability to phagocytose particles and kill internalized bacteria [42]. Similarly, IgT+ B cells were equally able to engulf latex beads or bacteria and had also an intracellular killing capacity [19]. In addition, both trout IgM+ and IgT+ B cells proliferated in vitro following stimulation with LPS and Vibrio bacterin. Furthermore, after 7 days, stimulated IgT+ B cells differentiated into larger lymphocytes with greater IgT-secreting capacity than the small IgT+ B lymphocytes. The latter suggests that the IgT+ B cell subset was able to respond to pathogen-associated molecular patterns (PAMP) and differentiated into antibody-secreting cells in the same way as IgM+ B cells did.
3.3. Prevalent IgT coating on the surface of intestinal bacteria
Animal gastrointestinal tracts are populated by complex microbial communities whichlive in symbiosis with the host’s mucosal environment. Antibodies are known to play a pivotal role in the maintenance of gut homeostasis in mammals [43,44]. In addition, gut microbial communities modulate the nature of mucosal and systemic immune responses (reviewed in [45]). In mammals, IgA and to a lesser extent, IgM and IgG, coat commensal intestinal bacteria [46], precluding their translocation into the intestinal epithelium by a process known as immune exclusion [43]. Similar to mammals, the gut of fish is filled with high densities of bacteria [47]. Analogously to mammalian IgA, rainbow trout IgT was shown to coat a significantly higher percentage of gut bacteria (48%) when compared to IgM (24%) [19]. The prevalence of IgT over IgM on the coating of intestinal bacteria reinforced the concept that IgT in trout behaves as a mucosal immunoglobulin. In addition, these results revealed for the first time a potential immune exclusion role of immunoglobulins in a non-mammalian species.
The use of a model that combines germ-free mice and non-replicable labelled bacteria has considerably highlighted the importance of antibody-mediated responses against gut bacteria. Using this model, a highly specific anti-commensal IgA response in germ-free mice was observed, although sequential (boosting) doses of commensals lacked the classical prime-boost effect seen in systemic vaccination [48]. Thus, in mammals gut IgA responses to commensals are different when compared to that to pathogens. It will be interesting in the future to analyze whether teleost fish respond to commensals in a similar fashion, as this will have important implications for the application of probiotics in aquaculture.
3.4 IgT responses against pathogens
Up until now, IgM was thought to be the only antibody isotype responsible for specific immunity in teleost fish both in systemic and mucosal compartments. This paradigm was changed recently when rainbow trout IgT was shown to be specifically involved in gut antibody responses against Ceratomyxa shasta, an intestinal parasite [19]. This parasite is endemic to rivers of the Northwest Coast of the United States, and causes severe infections in the gut of rainbow trout [49]. We reported that the gut of fish that survived to C. shasta infections (3 months post-parasite exposure), contained large accumulations of IgT + B cells whereas the number of IgM+ B cells did not change with respect to control fish [19]. The lamina propria of surviving fish was enlarged and IgT+ B lymphocytes even penetrated the intestinal epithelium. At that point, the majority of C. shasta trophozoites were located in the gut lumen, and therefore, they appeared to have been expelled from the host’s intestinal tissue. These observations were coupled to very large increases in the same area of IgT but not IgM, both at the protein and gene levels. More critically, gut mucus from surviving fish contained parasite-specific IgT titres but no specific IgM titres. Conversely, the serum of surviving fish had parasite-specific IgM, but not IgT titres. Whether IgT is responsible for eliciting protection against the parasite will require further work. Overall, this model provided the first evidence in teleost fish or any other non-tetrapod species, for a compartmentalization of immunoglobulin isotypes into mucosal (IgT) and systemic (IgM) areas in response to pathogenic challenge.
Trout vaccination trials against Yersinia ruckeri through two different routes, bath vaccination and intraperitoneal (i.p.) injection, provided interesting results regarding the differential stimulation of IgT and IgM expression [50,51]. Bath vaccination resulted in increased IgT expression levels in the spleen 72h post-vaccination in vaccinated fish compared to controls, but no differences in IgM expression were found [51]. Conversely, IgT expression was not up-regulated neither in the spleen nor in the head-kidney of trout that received the Yersinia vaccine i.p; however IgM transcript levels were significantly higher in the head-kidneybut not the spleen of vaccinated compared to control fish [50]. In a recent study, trout surviving primary infections with Y. ruckeri were re-exposed to the same pathogen to study secondary immune responses [52]. Gene transcription in the spleen revealed no changes in IgM or IgT levels during secondary infection. However, this study was conducted by i.p. injection of Y. ruckeri and therefore, it is possible that no mucosal sites were directly stimulated. As described above, C. shasta has a strong tropism for the gut of trout. Thus, direct stimulation of the mucosal site may be important to induce strong IgT responses. This is indeed the case for mammalian mucosal IgA, which require direct stimulation of the mucosal area [53]. In carp, different infection models were tested in order to investigate the role of the two IgT subtypes, IgT1 and IgT2 present in this species. Expression analyses showed that IgT1 was more abundant in systemic organs while IgT2 was prevalently expressed at mucosalsites. Moreover, infection with the mucosal parasite Lernea induced IgM and IgT2 gene expression but no IgT1 [15]. Future work on different gut and systemic pathogens is needed to understand further the nature of compartmentalized IgT and IgM responses in teleosts.
4. Transport of fish immunoglobulins to mucosal surfaces
In mammalian mucosal tissues, secretion of antibodies to the external mucus lining is accomplished through a unique cooperation between the mucosal B cell system and the polymeric Ig receptor (pIgR), which is expressed basolaterally on epithelial cells [54]. Both mammalian IgA and IgM are transported across mucosal epithelia to the outer mucus layer via pIgR. At the apical pole, pIgR-IgA/IgM is cleaved releasing the secretory form of IgA (sIgA) or IgM (sIgM) which remain bound to a portion of the pIgR, called secretory component (SC) [55].
Teleost pIgR has been cloned in different species such as fugu [56], carp [57], orange-spotted grouper [58] and trout [19]. They all share common molecular characteristics: they are considered type I transmembrane proteins consisting of a ligand-binding extracellular region that includes two immunoglobulin-like domains (ILDs). In mammals, pIgR contains 5 ILDs (for more details about fish pIgR see review by Rombout and colleagues in this issue). As previously stated, the SC of mammalian pIgRis known to be associated to sIgA or sIgM. In the case of rainbow trout, an anti-trout pIgR antibody was used to verify the presence of a trout secretory component-like molecule (tSC) in the gut mucus but not in the serum, with the molecular mass of tSC being of ~38 kDa. More critically, tSC was found associated to IgT and IgM of the gut mucus by co-immunoprecipitation studies using anti-IgT or anti-IgM antibody. Thus, similar to mammalian sIgA and sIgM, trout mucosal IgT and IgM need to associate to pIgR for their transport into the gut lumen. Similarly, IgM binding to pIgR has also been demonstrated in fugu [56] and grouper [58].
In higher vertebrates, the joining chain (J chain) is thought to be critical for the interaction of pIgR with mucosal immunoglobulins. Braathen and colleagues showed that the Jchain from divergent tetrapods including mammals, birds, and amphibians efficiently induced polymerization of human IgA whereas the J chain from nurse shark (a lower vertebrate) did not [59]. Interestingly, in teleost species, a J chain-like molecule has not been identified thus far, despite of the availability of several teleost genomes [19,56]. This probably indicates that teleosts are devoid of a J chain. It should be pointed out that while amphibians have a J chain, this molecule does not associate with IgX (an intestinal amphibian immunoglobulin) [60]. Accordingly, it is possible that in teleost fish and amphibians, the J chain is not required for the interaction of pIgR with their respective mucosal immunoglobulins. In addition, whether pIgR is the sole transport mechanism for fish mucosal antibodies awaits further investigation.
To understand further the presence and transport of IgT in mucosal sites, it is important to understand where IgT antibody-secreting cells (ASCs) are localized within systemic and mucosal lymphoid tissue, as well as potential routes of IgT transport from systemic compartments into mucosal surfaces. IgT-ASCs could be present locally at the mucosal sites (in situ production) or originate ex situ in other organs, or both. With regards to the trafficking routes, while it seems likely that IgT can reach the gut lumen through its transport from locally produced IgT (gut lamina propria or epithelium), it is possible that IgT, or mucosal IgM for that matter, are derived from systemic organs. In that regard, there is evidence that in Antarctic fish, plasma IgM could be transported across the hepatocytes to be secreted into the bile, and through the bile, reach the intestinal epithelium [61]. Analogously, in rats, the liver shows a remarkable capacity fortransport of dimeric IgA from blood into the bile [62,63]. Thus, a similar mechanism may be responsible for IgT (and IgM) transport in fish. Hence, generating a geographical map of both IgT and IgM ASCs during primary and secondary immune responses in teleost, as well as analyzing the transport routes of IgT and IgM, would substantially complete the present picture of teleost antibody responses both in systemic and mucosal sites.
5. Future perspectives
While significant advances have been made on the identification and characterization of ighτ gene orthologs, a considerable amount of intriguing questions remainto be answered. While it is impossible to summarize them all here, we propose some priority areas that need to be addressed: 1) To understand further the role of IgT in immunity, it is necessary to unravel how many subtypes of IgT (including chimeric IgM-IgT molecules) are present in teleosts, to develop monospecific antibodies against each subtype, and to investigate if different B cell subsets express unique IgT subtypes. 2) It will be critical to identify which heavy and light chain genes (since three IgL isotypes have been reported in several teleost species, see the summarized Table 1 in [64]) are involved in the assembly of IgT and IgM, and which are the dominant Ig products expressed in systemic and mucosal sites before and after pathogenic challenge or vaccination. 3) To understand further the functions of teleost immunoglobulin molecules, it will be crucial to compare the immune repertoires of IgT, IgM and IgD in systemic and mucosal sites of naïve and infected animals. 4) Since there is no class switch in teleost fish, it will be critical to elucidate how, where and when B cells commit to expressing either IgT or IgM, and the reason why IgT+ B cells do not express IgD. For example, which transcription factors control such mechanism? Is this commitment decided in early stages of development or can it be modified in adults? To which degree can PAMPs influence this commitment?
While some important biochemical and functional properties of IgT have finally been unearthed, we are far from understanding the specific contributions of IgT in teleost immunity. Below we propose some priority areas that need to be addressed: 1) Trout mucosal IgT is polymeric although no disulfide bonds are involved in holding the monomers together, thus, which are the molecular mechanisms involved in generating polymeric IgT? Is the SC of pIgR required for IgT polymerization? 2) What are the phenotypic differences between IgT+ and IgM+ B cell subsets? It will be fascinating to perform microarray and deep sequencing studies on normal and activated IgT+ and IgM+ B cells to elucidate which genes are differentially expressed in these two B cell lineages. 3) Both IgT+ and IgM+ B cells can phagocytose large particles. Can they present phagocytosed antigen to T cells? 4) IgT has been shown to be the dominant immunoglobulin in the gut mucosa of rainbow trout. Is IgT also the prevalent immunoglobulin system in other mucosal sites, including the skin and gills? 5) If IgT also coats commensal bacteria found in other mucosal sites, including the skin surface, then does IgT play a role in immune exclusion of skin bacteria? Can fish probiotics modulate the expression of IgT in mucosal and systemic sites? Will probiotics affect the capacity of gut IgT to coat commensal bacteria? 6) How do the different B cell subsets home and traffic during the course of an immune response? Which chemokines and chemokine receptors are involved in the homing of IgT+ and IgM+ B cells in systemic and mucosal tissues? 7) What are the specific contributions of IgT and IgM antigen-specific responses in primary and secondary responses? Are there differences in the kinetics and extent of the affinity maturation of IgT and IgM during the course of an immune response? 8) Can IgT activate the classical pathway of the complement system? This point will be really interesting as mammalian IgA appears to be poor activator of the complement system. 9) How are IgT mucosal responses modulated by different routes (especially oral and bath) of vaccination? Can we find specific adjuvants that improve the efficacy of mucosal responses? This research will be critical for the development and design of a new generation of vaccines that not only induce effective systemic response, but also protective mucosal immunity.
The list of new captivating research opportunities with the IgT system is obviously much larger than that proposed above. It is clear that the field of mucosal immunoglobulins in teleost fish is facing a new era. We cannot wait to see how the next review on IgT will look like in a few years from now. We now have the tools for a more integrated understanding of immunoglobulin responses in teleost fish. While discovering new and fascinating roles of immunoglobulinsin fish immunity, this research will be fundamental for the future design of novel immunotherapies for aquaculture.
Fig. 2.
Redox forms of trout IgM and IgT in serum and gut mucus. Distinct chromatographic (gel filtration) and electrophoretic (nonreducing SDS-PAGE) behaviors of trout IgM and IgT are shown diagrammatically. Note the number and location of covalent bonds between the subunits of IgM are different in various redox forms [38]. The dotted lines indicate the areas where the non-covalent bonds are broken by SDS-PAGE under nonreducing conditions. The putative secretory component (SC) of a pIgR is not shown.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Danilova N, Amemiya CT. Going adaptive: the saga of antibodies. Ann N Y Acad Sci. 2009;1168:130–55. doi: 10.1111/j.1749-6632.2009.04881.x. [DOI] [PubMed] [Google Scholar]
- 2.Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat Rev Genet. 2010;11:47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pancer Z, Amemiya CT, Ehrhardt GR, Ceitlin J, Gartland GL, Cooper MD. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–80. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]
- 4.Alder MN, Rogozin IB, Iyer LM, Glazko GV, Cooper MD, Pancer Z. Diversity and function of adaptive immune receptors in a jawless vertebrate. Science. 2005;310:1970–3. doi: 10.1126/science.1119420. [DOI] [PubMed] [Google Scholar]
- 5.Alder MN, Herrin BR, Sadlonova A, Stockard CR, Grizzle WE, Gartland LA, et al. Antibody responses of variable lymphocyte receptors in the lamprey. Nat Immunol. 2008;9:319–27. doi: 10.1038/ni1562. [DOI] [PubMed] [Google Scholar]
- 6.Herrin BR, Cooper MD. Alternative adaptive immunity in jawless vertebrates. J Immunol. 2010;185:1367–74. doi: 10.4049/jimmunol.0903128. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder HW, Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125:S41–52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flajnik MF. Comparative analyses of immunoglobulin genes: surprises and portents. Nat Rev Immunol. 2002;2:688–98. doi: 10.1038/nri889. [DOI] [PubMed] [Google Scholar]
- 9.Flajnik MF. All GOD’s creatures got dedicated mucosal immunity. Nat Immunol. 2010;11:777–9. doi: 10.1038/ni0910-777. [DOI] [PubMed] [Google Scholar]
- 10.Hansen JD, Landis ED, Phillips RB. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: Implications for a distinctive B cell developmental pathway in teleost fish. Proc Natl Acad Sci U S A. 2005;102:6919–24. doi: 10.1073/pnas.0500027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danilova N, Bussmann J, Jekosch K, Steiner LA. The immunoglobulin heavy-chain locus in zebrafish: identification and expression of a previously unknown isotype, immunoglobulin Z. Nat Immunol. 2005;6:295–302. doi: 10.1038/ni1166. [DOI] [PubMed] [Google Scholar]
- 12.Flajnik MF. The last flag unfurled? A new imunoglobulin isotype in fish expressed in early development. Nat Immunol. 2005;6:229–30. doi: 10.1038/ni0305-229. [DOI] [PubMed] [Google Scholar]
- 13.Savan R, Aman A, Sato K, Yamaguchi R, Sakai M. Discovery of a new class of immunoglobulin heavy chain from fugu. Eur J Immunol. 2005;35:3320–31. doi: 10.1002/eji.200535248. [DOI] [PubMed] [Google Scholar]
- 14.Savan R, Aman A, Nakao M, Watanuki H, Sakai M. Discovery of a novel immunoglobulin heavy chain gene chimera from common carp (Cyprinus carpio L. ) Immunogenetics. 2005;57:458–63. doi: 10.1007/s00251-005-0015-z. [DOI] [PubMed] [Google Scholar]
- 15.Ryo S, Wijdeven RH, Tyagi A, Hermsen T, Kono T, Karunasagar I, et al. Common carp have two subclasses of bonyfish specific antibody IgZ showing differential expression in response to infection. Dev Comp Immunol. 2010;34:1183–90. doi: 10.1016/j.dci.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Gambon-Deza F, Sanchez-Espinel C, Magadan-Mompo S. Presence of an unique IgT on the IGH locus in three-spined stickleback fish (Gasterosteus aculeatus) and the very recent generation of a repertoire of VH genes. Dev Comp Immunol. 2010;34:114–22. doi: 10.1016/j.dci.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Bao Y, Wang T, Guo Y, Zhao Z, Li N, Zhao Y. The immunoglobulin gene loci in the teleost Gasterosteus aculeatus. Fish Shellfish Immunol. 2010;28:40–8. doi: 10.1016/j.fsi.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Xiao FS, Wang YP, Yan W, Chang MX, Yao WJ, Xu QQ, et al. Ig heavy chain genes and their locus in grass carp Ctenopharyngodon idella. Fish Shellfish Immunol. 2010;29:594–9. doi: 10.1016/j.fsi.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YA, Salinas I, Li J, Parra D, Bjork S, Xu Z, et al. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat Immunol. 2010;11:827–35. doi: 10.1038/ni.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hordvik I. Identification of a novel immunoglobulin delta transcript and comparative analysis of the genes encoding IgD in Atlantic salmon and Atlantic halibut. Mol Immunol. 2002;39:85–91. doi: 10.1016/s0161-5890(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 21.Bengten E, Quiniou SM, Stuge TB, Katagiri T, Miller NW, Clem LW, et al. The IgH locus of the channel catfish, Ictalurus punctatus, contains multiple constant region gene sequences: different genes encode heavy chains of membrane and secreted IgD. J Immunol. 2002;169:2488–97. doi: 10.4049/jimmunol.169.5.2488. [DOI] [PubMed] [Google Scholar]
- 22.Saunders HL, Magor BG. Cloning and expression of the AID gene in the channel catfish. Dev Comp Immunol. 2004;28:657–63. doi: 10.1016/j.dci.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Cannon JP, Haire RN, Rast JP, Litman GW. The phylogenetic origins of the antigen-binding receptors and somatic diversification mechanisms. Immunol Rev. 2004;200:12–22. doi: 10.1111/j.0105-2896.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- 24.Schorpp M, Bialecki M, Diekhoff D, Walderich B, Odenthal J, Maischein HM, et al. Conserved functions of Ikaros in vertebrate lymphocyte development: genetic evidence for distinct larval and adult phases of T cell development and two lineages of B cells in zebrafish. J Immunol. 2006;177:2463–76. doi: 10.4049/jimmunol.177.4.2463. [DOI] [PubMed] [Google Scholar]
- 25.Hu YL, Xiang LX, Shao JZ. Identification and characterization of a novel immunoglobulin Z isotype in zebrafish: implications for a distinct B cell receptor in lower vertebrates. Mol Immunol. 2010;47:738–46. doi: 10.1016/j.molimm.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Yasuike M, de Boer J, von Schalburg KR, Cooper GA, McKinnel L, Messmer A, et al. Evolution of duplicated IgH loci in Atlantic salmon, Salmo salar. BMC Genomics. 2010;11:486. doi: 10.1186/1471-2164-11-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tadiso TM, Lie KK, Hordvik I. Molecular cloning of IgT from Atlantic salmon, and analysis of the relative expression of tau, mu, and delta in different tissues. Vet Immunol Immunopathol. 2011;139:17–26. doi: 10.1016/j.vetimm.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 28.Bengten E, Quiniou S, Hikima J, Waldbieser G, Warr GW, Miller NW, et al. Structure of the catfish IGH locus: analysis of the region including the single functional IGHM gene. Immunogenetics. 2006;58:831–44. doi: 10.1007/s00251-006-0139-9. [DOI] [PubMed] [Google Scholar]
- 29.Lesk AM, Chothia C. Evolution of proteins formed by beta-sheets. II. The core of the immunoglobulin domains. J Mol Biol. 1982;160:325–42. doi: 10.1016/0022-2836(82)90179-6. [DOI] [PubMed] [Google Scholar]
- 30.O’Connor SE, Imperiali B. Modulation of protein structure and function by asparagine-linked glycosylation. Chem Biol. 1996;3:803–12. doi: 10.1016/s1074-5521(96)90064-2. [DOI] [PubMed] [Google Scholar]
- 31.Cals MM, Guenzi S, Carelli S, Simmen T, Sparvoli A, Sitia R. IgM polymerization inhibits the Golgi-mediated processing of the mu-chain carboxy-terminal glycans. Mol Immunol. 1996;33:15–24. doi: 10.1016/0161-5890(95)00132-8. [DOI] [PubMed] [Google Scholar]
- 32.Wiersma EJ, Chen F, Bazin R, Collins C, Painter RH, Lemieux R, et al. Analysis of IgM structures involved in J chain incorporation. J Immunol. 1997;158:1719–26. [PubMed] [Google Scholar]
- 33.Yoo EM, Coloma MJ, Trinh KR, Nguyen TQ, Vuong LU, Morrison SL, et al. Structural requirements for polymeric immunoglobulin assembly and association with J chain. J Biol Chem. 1999;274:33771–7. doi: 10.1074/jbc.274.47.33771. [DOI] [PubMed] [Google Scholar]
- 34.Campbell KS, Backstrom BT, Tiefenthaler G, Palmer E. CART: a conserved antigen receptor transmembrane motif. Semin Immunol. 1994;6:393–410. doi: 10.1006/smim.1994.1049. [DOI] [PubMed] [Google Scholar]
- 35.Duff D. The oral immunization of trout against Bacterium salmonicida. J Immunol. 1942;44:87–94. [Google Scholar]
- 36.Fletcher TC, Grant PT. Immunoglobulins in the serum and mucus of the plaice (Pleuronectes platessa) Biochem J. 1969;115:65P. doi: 10.1042/bj1150065p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rombout JHWM, Abelli L, Picchietti S, Scapigliati G, Kiron V. Teleost intestinal immunology. Fish Shellfish Immunol. 2010 doi: 10.1016/j.fsi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Kaattari S, Evans D, Klemer J. Varied redox forms of teleost IgM: an alternative to isotypic diversity? Immunol Rev. 1998;166:133–42. doi: 10.1111/j.1600-065x.1998.tb01258.x. [DOI] [PubMed] [Google Scholar]
- 39.Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Pathol. 2006;208:270–82. doi: 10.1002/path.1877. [DOI] [PubMed] [Google Scholar]
- 40.Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10:889–98. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edholm ES, Bengten E, Stafford JL, Sahoo M, Taylor EB, Miller NW, et al. Identification of two IgD+ B cell populations in channel catfish, Ictalurus punctatus. J Immunol. 2010;185:4082–94. doi: 10.4049/jimmunol.1000631. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Barreda DR, Zhang YA, Boshra H, Gelman AE, Lapatra S, et al. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol. 2006;7:1116–24. doi: 10.1038/ni1389. [DOI] [PubMed] [Google Scholar]
- 43.Stokes CR, Soothill JF, Turner MW. Immune exclusion is a function of IgA. Nature. 1975;255:745–6. doi: 10.1038/255745a0. [DOI] [PubMed] [Google Scholar]
- 44.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–50. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 46.van der Waaij LA, Limburg PC, Mesander G, van der Waaij D. In vivo IgA coating of anaerobic bacteria in human faeces. Gut. 1996;38:348–54. doi: 10.1136/gut.38.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim DH, Brunt J, Austin B. Microbial diversity of intestinal contents and mucus in rainbow trout (Oncorhynchus mykiss) J Appl Microbiol. 2007;102:1654–64. doi: 10.1111/j.1365-2672.2006.03185.x. [DOI] [PubMed] [Google Scholar]
- 48.Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–9. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bjork SJ, Bartholomew JL. Invasion of Ceratomyxa shasta (Myxozoa) and comparison of migration to the intestine between susceptible and resistant fish hosts. Int J Parasitol. 2010;40:1087–95. doi: 10.1016/j.ijpara.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Raida MK, Buchmann K. Temperature-dependent expression of immune-relevant genes in rainbow trout following Yersinia ruckeri vaccination. Dis Aquat Organ. 2007;77:41–52. doi: 10.3354/dao01808. [DOI] [PubMed] [Google Scholar]
- 51.Raida MK, Buchmann K. Bath vaccination of rainbow trout (Oncorhynchus mykiss Walbaum) against Yersinia ruckeri: effects of temperature on protection and gene expression. Vaccine. 2008;26:1050–62. doi: 10.1016/j.vaccine.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 52.Raida MK, Buchmann K. Development of adaptive immunity in rainbow trout, Oncorhynchus mykiss (Walbaum) surviving an infection with Yersinia ruckeri. Fish Shellfish Immunol. 2008;25:533–41. doi: 10.1016/j.fsi.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–84. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Kaetzel CS, Robinson JK, Chintalacharuvu KR, Vaerman JP, Lamm ME. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc Natl Acad Sci U S A. 1991;88:8796–800. doi: 10.1073/pnas.88.19.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev. 2005;206:83–99. doi: 10.1111/j.0105-2896.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 56.Hamuro K, Suetake H, Saha NR, Kikuchi K, Suzuki Y. A teleost polymeric Ig receptor exhibiting two Ig-like domains transports tetrameric IgM into the skin. J Immunol. 2007;178:5682–9. doi: 10.4049/jimmunol.178.9.5682. [DOI] [PubMed] [Google Scholar]
- 57.Rombout JH, van der Tuin SJ, Yang G, Schopman N, Mroczek A, Hermsen T, et al. Expression of the polymeric Immunoglobulin Receptor (pIgR) in mucosal tissues of common carp (Cyprinus carpio L. ) Fish Shellfish Immunol. 2008;24:620–8. doi: 10.1016/j.fsi.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 58.Feng LN, Lu DQ, Bei JX, Chen JL, Liu Y, Zhang Y, et al. Molecular cloning and functional analysis of polymeric immunoglobulin receptor gene in orange-spotted grouper (Epinephelus coioides) Comp Biochem Physiol B Biochem Mol Biol. 2009;154:282–9. doi: 10.1016/j.cbpb.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 59.Braathen R, Hohman VS, Brandtzaeg P, Johansen FE. Secretory antibody formation: conserved binding interactions between J chain and polymeric Ig receptor from humans and amphibians. J Immunol. 2007;178:1589–97. doi: 10.4049/jimmunol.178.3.1589. [DOI] [PubMed] [Google Scholar]
- 60.Mussmann R, Du Pasquier L, Hsu E. Is Xenopus IgX an analog of IgA? Eur J Immunol. 1996;26:2823–30. doi: 10.1002/eji.1830261205. [DOI] [PubMed] [Google Scholar]
- 61.Abelli L, Coscia MR, De Santis A, Zeni C, Oreste U. Evidence for hepato-biliary transport of immunoglobulin in the antarctic teleost fish Trematomus bernacchii. Dev Comp Immunol. 2005;29:431–42. doi: 10.1016/j.dci.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Orlans E, Peppard J, Reynolds J, Hall J. Rapid active transport of immunoglobulin A from blood to bile. J Exp Med. 1978;147:588–92. doi: 10.1084/jem.147.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orlans E, Peppard J, Fry JF, Hinton RH, Mullock BM. Secretory component as the receptor for polymeric IgA on rat hepatocytes. J Exp Med. 1979;150:1577–81. doi: 10.1084/jem.150.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coscia MR, Giacomelli S, De Santi C, Varriale S, Oreste U. Immunoglobulin light chain isotypes in the teleost Trematomus bernacchii. Mol Immunol. 2008;45:3096–106. doi: 10.1016/j.molimm.2008.03.006. [DOI] [PubMed] [Google Scholar]