Abstract
HIV-1 Nef protein is an approximately 27-kDa myristoylated protein that is a virulence factor essential for efficient viral replication and infection in CD4+ T cells. The functions of CD4+ T cells are directly impeded after HIV infection. HIV-1 Nef plays a crucial role in manipulating host cellular machinery and in HIV pathogenesis by reducing the ability of infected lymphocytes to form immunological synapses by promoting virological synapses with APCs, and by affecting T-cell stimulation. This article reviews the current status of the efficient Nef-mediated spread of virus in the unreceptive environment of the immune system by altering CD4+ T-lymphocyte signaling, intracellular trafficking, cell migration and apoptotic pathways.
Keywords: activation-induced cell death, CD4+ cell, endocytic recycling compartment, HIV-1, immunological synapse, Nef, T-cell activation, virological synapse
The continuous battle between the host immune system and HIV results in AIDS. HIV-1 is a lentivirus belonging to the Retroviridae family. The major receptor that facilitates binding of HIV to human cells is the CD4 cell surface receptor. The chemokine receptors CCR5 and CXCR4 were identified as being the predominantly important coreceptors for HIV-1. Owing to the presence of these coreceptors, HIV-1 infects not only CD4+ T cells, but also other APCs, such as macrophages and dendritic cells [1,2]. One of the main cellular targets for HIV replication and infection are the host CD4+ T cells, and the rate of infection depends on the activation profile of these, which is usually observed to be higher in activated memory T cells – as these cells support viral replication – when compared with resting helper T cells, which are quite resistant to infection [3,4]. Few in vitro and ex vivo studies have shown that viral infection does not require full T-cell activation. Varying degrees of T-lymphocyte activation can be mediated by the T-cell receptor (TCR) or other stimuli that are modulated by HIV. Virus particles set the equilibrium between the apoptosis-prone activation state and the replication-adverse environment of resting cells in order to achieve efficient multiplication [5,6]. In HIV-infected cells, intracellular trafficking, transcriptional activation, signaling and apoptotic pathways are altered, mainly by Nef and Tat proteins, along with the host proteins, resulting in a higher viral load in the infected cells, although the exact mechanism is still unclear [7–13]. Viral and host immune factors are required for efficient viral multiplications. In order to achieve this, the virus impairs immunological synapses (IS) and promotes virological synapses (VS) with the help of the Nef protein [14–17]. VS refers to close contacts between virus and T lymphocytes and IS refers to close contacts between APCs and T lymphocytes, which are closely related in organization and polarization to VS. Several studies have reported Nef to be an important player in regulating T-cell activation and IS formation between infected lymphocytes and APCs [18,19]. Therefore, we tried to elucidate the role played by the Nef protein in cellular and molecular mechanisms of T-cell activation, T-cell migration, T-cell signaling alterations and its relationship with apoptosis. Emphasis has been given on the interaction of Nef with host cellular mechanisms and its key role in pathogenesis.
HIV-1 Nef: a crucial player in viral infectivity & replication
AIDS is caused by HIV-1, affecting millions of lives globally (~34 million at the end of 2010) over the last 28 years but there is still no effective vaccine available [20]. Nef is an accessory factor of HIV, and is considered to be one of the most important factors in HIV disease progression, which has been extensively studied. Attempts have been made to understand the complete mechanism of Nef’s role in HIV/AIDS disease progression. Nef is a pathogenic factor, and acts to preserve high levels of HIV-1 replication as a consequence of the synergism of its several roles [21]. Nef is a small, approximately 27-kDa flexible myristoylated protein, consisting of 206 amino acids, and is expressed early in the viral replicative cycle with a key role in viral replication and pathogenesis [22–25]. Its early expression in viral replication ensures T-cell activation and the establishment of a constant state of infection. The Nef protein can be divided into four discrete domains: a flexible myristoylated N-terminal anchor domain; a loop containing a proline-rich region; a conserved globular core structure; and a C-terminal flexible loop [23]. The myristoylation domain regulates many of the functions associated with Nef.
Previously, Nef was reported as a negative factor for infection because it was believed to serve in a negative role (transcriptional silencer) in viral pathogenesis. However, consensus on its function has changed from gene silencing to the escalation of viral pathogenesis [26]. HIV-1 Nef plays a vital role in CD4 downregulation, MHC class I downregulation, CD28 downregulation, T-cell activation and CD8αβ downregulation, which eventually contributes to disease progression [27,28]. Nef-mediated CD4 downregulation occurs by the interaction of Nef complexes with adapter protein 2 and the cytoplasmic tail of CD4 [29]. Nef also downregulates MHC class I by interacting with adapter protein 1 and the cytoplasmic tail of MHC class I. HIV-1 Nef triggers downregulation of cell-surface MHC class I by initiating its binding with sorting protein PACS-2, which facilitates the assembly of the Src family kinase (SFK)-ZAP-70/Syk-PI3K cascade [30]. Nef also binds to PAK2 and other cellular proteins including a GTPase Rac, CDC42 and a guanine nucleotide exchange factor that may be required for upregulation of HIV transcription, remodeling of the actin cytoskeleton, prevention of apoptosis and enhancement of virion infectivity [31].
In Nef-infected cells, it is expected that ternary or higher order complexes of cellular proteins bound to Nef are important to its activity [24,25]. Nef is involved in disease progression, which was shown by studies on Rhesus macaques in which those subjects infected with nef-deleted SIVmac showed slow disease progression [32]. Similarly, individuals infected with nef-defective strains showed slow progression and remained healthy postinfection, highlighting the crucial role of nef in disease induction [33]. Studies on infected primates carrying a 12-bp deleted nef or nef mutant showed inefficient PAK binding, leading to slow disease progression. Defective Nef protein and 12-bp nef deletion was repaired in vivo by sequence duplication, and its evolution was persistent until the repair was approximately indistinguishable from the wild-type sequence [34,35]. In cohort studies of HIV-infected individuals, approximately 5% of individuals were reported to be long-term nonprogressors or slow progressors – that is, infected patients who remain asymptomatic for over 10 years without receiving any anti-HIV treatment, showing constant CD4+ T-cell counts and low viral loads [36]. One possible reason for low viral load was suggested to be a defective nef gene in the virus of those patients [37]. Studies performed with the Sydney Blood Bank cohort showed the uniqueness of infection in different patients even though they were infected by a single source [38–40]. Although Nef protein has no intrinsic enzymatic activity, it plays an important role in preparing a favorable environment for the virus by interacting with endogenous host cell proteins. These interactions alter cell functions and intracellular signaling cascades generating a favorable cellular environment for nef-mediated infection (Figure 1). The multifarious functions of Nef hinder Nef-based drug development, and there is an urgent need for the characterization of the functional diversity of Nef.
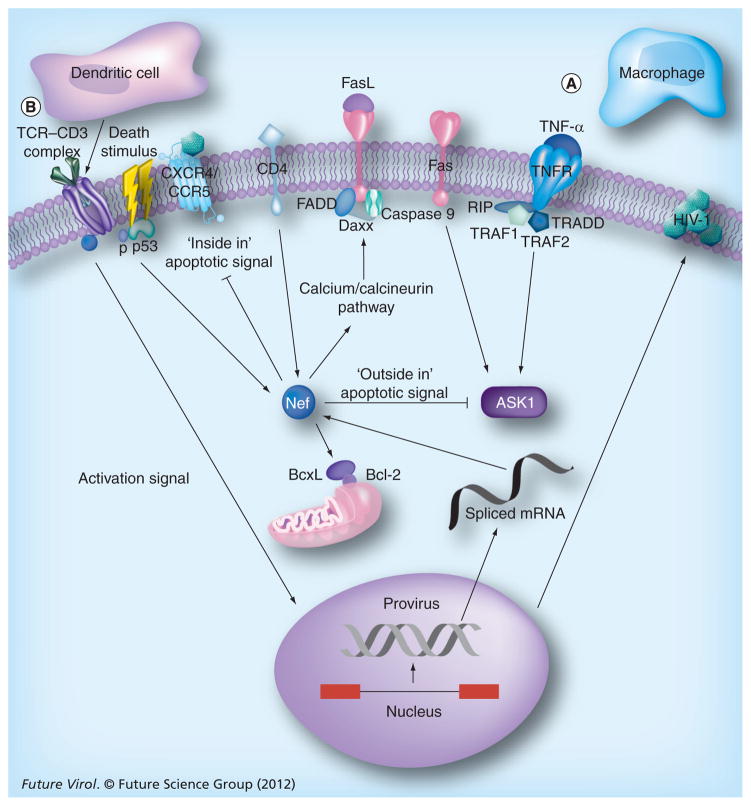
Figure 1. Modulation of host cell signaling by HIV-1 Nef.
HIV-1 Nef promotes viral replication and viral survival by hijacking host cell machinery to achieve immune evasion and anti-apoptosis.
(A) HIV infection activates the two main pathways of cell death in lymphocytes: activation-induced cell death through the TNF family of death receptors; and activated T-cell autonomous death through Bcl-2-related proteins and mitochondria. Host apoptosis signals – ‘inside in’ (HIV replication initiating viral particle attack) and ‘outside in’ (FasL–Fas and membrane-bound TNF-α–TNFR2 ligations) – cause cell death before the viral lifecycle is completed. Both forms of apoptosis signaling are blocked by Nef, and in addition, Nef controls cell death in the host by phosphorylation of p53 and by promoting the upregulation of FasL and the downregulation of MHC class I (not shown) that forces the cytotoxic T lymphocyte into an apoptotic pathway and prevents the recognition of Nef-infected cells.
(B) Activation of the TCR cascade by Nef requires a costimulus activation signal (through dendritic cells) to initiate a signal strong enough to stimulate viral replication.
P: Phosphate; TCR: T-cell receptor.
HIV-1 nef is considered to be crucial for pathogenesis and disease progression, as it supports viral replication by increasing the infectivity of virions. The role of Nef in replication rate and disease progression was demonstrated by studies on nef-positive and nef-defective HIV-1 clones in activated peripheral blood lymphocytes [41]. Single-round infection assays showed that mutations in the nef gene of HIV can reduce the rate of infection by 4–40% compared with the wild-type virus. Nef has a potent role in producing fully infectious viral particles by phosphorylating substrates such as viral matrix by virtue of its associated kinases, and also increasing infection by inducing lymphocyte-stimulating factor production by macrophages resulting in a virus-friendly environment [42,43]. Nef has a potent role in both early and late phases of the viral life-cycle. In the early phase, it modulates the fusion properties of the cell, and in the late phase, it stimulates/enhances viral reverse transcription, which increases the number of virions budding out to infect surrounding cells [44–46]. Virions budding out from the infected cells are supported by interaction of Nef with the GagPol polyprotein transframe portion, AIP1 and cholesterol. Cholesterol also has a major role in enhancing viral infection by increasing the synthesis and integration of cholesterol into progeny virions [47]. Nef plays a major role in the HIV budding and replication process, as HIV buds from lipid rafts and requires cholesterol for its egress from and entry into cells. Nef not only enhances the biosynthesis of lipid rafts and viral particles with newly synthesized cholesterol, but also enriches them. Nef provides the necessary building blocks for the formation of viruses by transporting newly synthesized cholesterol to the site of viral budding. In addition to this, Nef alters the lipid composition of the host cell microdomain, along with the proteome, facilitating viral propagation in vivo [48,49]. Increased incidences of dyslipidemia and cardiovascular disease have been reported to be associated with HIV infection and subsequent antiretroviral therapy. Studies have suggested that Nef represses cholesterol efflux from virus-infected macrophages by downregulating ABCA1. Two mechanisms have been found to be responsible for this phenomenon: first, post-transcriptional downregulation of ABCA by Nef; and second, Nef-mediated redistribution of ABCA1 to the plasma membrane (PM) and inhibited internalization of apolipoprotein A-I [50,51]. In vitro and in vivo studies demonstrated the role of the Nef protein in the enhancement of budding by infection assay, which measures HIV infectivity in different cell lines when compared with the indicator cells that determine normalized infectivity, thus detecting enhanced infection potential for HIV-1 virions [52–55]. Ex vivo cell systems (cocultures of T cells with immature dendritic cells or endothelial cells), as well as cultures of intact human tonsil tissue studies, have also supported these above functions of Nef in HIV and SIV [56–60].
Role of Nef in TCR signaling
In Nef-mediated activation of T cells, a cascade of events occurs in a hierarchical manner with increasing stimulation via TCRs. The major factor is the activation status of targeted T cells, which dictates the viral spread in the host. Nef protein is released in the early phase of infection and it controls all subsequent steps of the virus lifecycle. Nef plays a role in blocking superinfection with another virus, avoiding superinfection-induced hyperactivation-mediated cell death [61–63]. Nef inhibits immunological recognition by downregulating the MHC class I and CD4 of the infected cells, preventing immune recognition by cytotoxic T cells, which have the ability to restrict viral replication. Studies suggest that Nef maintains an equilibrium in terms of cell activation and prevents hyperactivation-mediated apoptosis. It also optimizes the environment for HIV infection by sensitizing T cells for activation by acting as an enhancer of T-cell signaling [12,64].
Modulation at the IS
The contact site between TCR clusters on T cells and the APC is called an IS. This structure plays a major role in the apoptosis of both non-HIV-infected cells and HIV-infected cells. In non-HIV-infected cells, the mechanism of apoptosis occurs through the binding of APCs to the TCRs present on the cell surface, resulting in a series of events including the clustering of TCRs, coreceptors and adhesion molecules, as well as cytoskeleton components forming a supramolecular activation cluster. In HIV-infected cells, the apoptotic pathway is altered/blocked by the Nef protein. Nef causes alterations in IS formation and the TCR activation cascade, thereby preventing apoptosis resulting from repetitive TCR stimulation. This process is further supported by enhanced IL-2 secretion, which might increase the lifespan of infected cells and support viral spread. This process might also favor the development of quiescent, latently infected lymphocytes [65]. In addition, Nef also impairs various apoptotic signals in infected cells, promoting efficient HIV replication. Altogether, these multifarious functions of Nef impart a selective advantage for infected lymphocytes that creates a favorable environment for cell survival [9,66]. APCs make close contact with T lymphocytes at IS. In VS, the infected cells have the capacity to attract or bind to the noninfected cells, which results in cell-to-cell viral transmission [67, 68].
At IS, T-lymphocyte morphology and actin remodeling is affected by Nef interfering with signal transmission as well as with chemotaxis to avoid hyperactivation of infected T lymphocytes [69,70]. The HIV-1 transmission process is influenced by cell polarization, comprising dynamic reorganization of the actin cytoskeleton and recruitment of virion components to cell–cell contacts at VS [71,72]. Nef enhances the infection of neighboring uninfected cells in the initial stages of infection [73]. A recent study suggests that Nef affects the organization and function of T-lymphocyte VS by increasing virion infectivity via cell-to-cell transmission and by interfering with a distinct set of host cell actin remodeling processes [74]. Actin rearrangements and subsequent impairment of IS are associated with the interaction of Nef and Vav in the infected cells. Nef has a role in the formation of active CDC42–GTP and Rac1–GTP proteins by activating the guanine nucleotide exchange factor activity of Vav [75]. Furthermore, NAK are activated by the activity of small GTPases, resulting in a cascade of downstream signaling events comprising cytoskeletal rearrangements and the activation of the JNK/SAPK cascade [76]. By virtue of these functions, Nef plays an important role in replication by optimizing the cellular environment, aided by Vav expression, which activates T cells, resulting in IL-2 production in Jurkat cells. The activation of the JNK/SAPK cascade leads to the enhancement of transcription from the HIV-1 long terminal repeat in T-cell lines [77–79]. The viral transactivator Tat can also be affected by this function of Nef, as its pTEFb is regulated by cellular activation [80,81]. Lck, a TCR-proximal Src family tyrosine kinase, has been shown to play important roles in IS morphology and functions, and it was observed to accumulate in endocytic recycling compartments and not at the synapse [82,83]. Host Lck protein is activated upon TCR ligation with an APC, and then recruits ZAP-70, which binds to ζ chain of TCR, which subsequently undergoes phosphorylation and kinase activity, regulating T-cell activation and recruiting various host cellular factors such as SLP76, Vav and LAT that aid in the formation of IS. ZAP also has another role in the Src kinase cascade, which in turn helps to downregulate MHC class I via binding with class I PI3K in primary CD4+ T cells. This suggests that ZAP-70 is required for cell-to-cell HIV transmission and for the formation of VS [84,85]. NFAT, a transcription factor that plays a central role in coordinating T-cell activation, is induced during lymphocyte signaling in a Nef-dependent manner, resulting in the formation of a permissive state of expression that supports HIV replication [86–89]. This was supported by the overexpression of NFAT target genes IL-2 and FasL and showed the hyper-responsiveness to activation of HIV-infected lymphocytes compared with uninfected cells [90]. In non-HIV-infected cells, the production of IL-2 is increased compared with HIV-infected cells [16].
Alteration in trafficking of signaling molecules (TCR & Lck)
TCRs play an important role in T-cell signaling at IS. A complex intracellular transport system controls the abundance of TCRs at the cell surface. TCRs and Lck molecules in resting T cells constantly traffic through early endosomes during normal physiological conditions. During TCR engagement, endocytosis and degradation of TCRs in lysosomes via the ubiquitin-mediated transport pathway occurs, which leads to downregulation of TCRs at the cell surface, which prevents T-cell hyperactivation [91,92]. At the time of activation of T cells by antigen, TCR and Lck molecules are targeted to the IS through polarized recycling [93]. When the cells are infected with HIV-1 virus, alteration of intracellular trafficking of Lck and TCR–CD3 are observed as intracellular compartmentalization of TCR signaling is induced to regulate the TCR response in a Nef-dependent manner [3]. Consequently, TCRs accumulate in recycling endosomes, and their expression is slightly increased at the cell surface due to slowdown of trafficking at both the recycling and the endocytic steps. Nef provokes intracellular accumulation of Lck, inhibiting Lck translocation from recycling endosomes to the IS to prolong signal transduction (Figure 2) [94]. Lck is redirected away from the PM to the trans-Golgi network by Nef, thereby limiting the signal initiation at the PM [95]. This process is further supported by inducing Lck-dependent activation of trans-Golgi network-associated Ras–Erk signaling to promote production of IL-2 for efficient virus replication [86]. In addition to these functions, Nef controls the function of c-Cbl, that controls TCR degradative transport [96,97]. Overall, Nef creates the suitable environment for HIV-1 replication by altering the T-lymphocyte response to antigenic stimulation and modulating the process of TCR–CD3 and Lck intracellular trafficking in the T-cell signaling cascade.
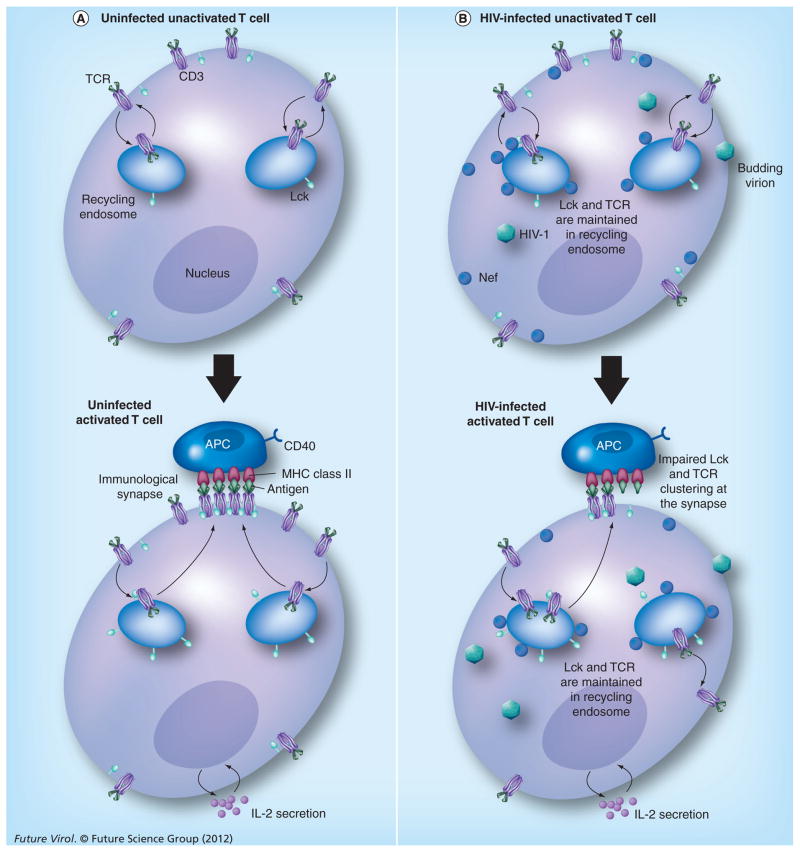
Figure 2. HIV-1 Nef-mediated modulation of Lck and the T-cell receptor pathway.
(A) TCRs and Lck molecules in resting T cells constantly traffic through early endosomes during normal physiological conditions. At the time of activation of T cells by antigen, TCR and Lck molecules are trafficked to the immunological synapse through polarized reprocessing. (B) When the cells are infected with HIV-1, Nef mediates alteration of intracellular trafficking of Lck and TCR–CD3. TCRs accumulate in recycling endosomes and their expression is slightly increased at the cell surface due to the slowing down of trafficking at both the recycling and endocytic steps. Nef stimulates the intracellular accumulation of Lck, inhibiting the process of Lck translocation from recycling endosomes to the immunological synapse to prolong signal transduction. HIV-1 particles represent all stages of the viral replicative cycle.
TCR: T-cell receptor.
T-cell signaling & apoptosis
Fas ligand (FasL or CD95L) is a type-II transmembrane protein that belongs to the TNF family. HIV-1 Nef induces FasL expression on the infected CD4+ T cell, causing bystander cell apoptosis [98–100]. The interaction of FasL with Fas (CD95) displayed on neighboring cytotoxic T lymphocytes leads to bystander cell killing and creates a mechanism of immune evasion [101–103]. FasL is induced through the calcium/calcineurin pathway of NFAT-dependent gene expression in T cells, which supports HIV replication. The binding of Nef with ASK1 (a key signaling intermediate in the Fas and TNF-α death signaling pathways) suppresses the Fas- and TNF-α-mediated apoptosis of HIV-infected cells [11]. The Nef protein protects infected cells from death signaling by the Bad protein through its phosphorylation by PI3K and PAK, resulting in the release of the anti-apoptotic Bcl-XL protein from Bad/Bcl-XL complexes, which enhances cell survival and virus production [9]. Nef has the very interesting ability to interact with the p53 tumor suppressor protein via its N-terminus. This interaction results in the destabilization of p53, leading to decrease in its proapoptotic, transcriptional and DNA-binding activities, and hence protects HIV-1-infected cells from p53-mediated apoptosis [104]. Studies have shown the link between Nef-induced TCR downregulation and the pathogenicity of HIV and SIV. Nef has been found to act differently in a species-dependent manner – that is, SIV and HIV-1 strains – in terms of its function of downregulating the CD3 molecule [105–107]. The pathogenic potential of Nef is correlated with the inability of the viral protein to decrease the cell-surface levels of CD3. By contrast, non-pathogenic Nef variants were able to decrease the cell-surface levels of CD3, thereby efficiently protecting T cells from activation-induced cell death [108]. This study encouraged researchers to examine the multifunctional activity of Nef protein activity in hindering the IS machinery.
T-cell migration
Host factors, such as integrins and chemokine receptors on lymphocytes, adhesion molecules on endothelial cells and chemokines in the local microenvironment, are involved in the complex process of lymphocyte trafficking. HIV-1 Nef inhibits T-cell chemotaxis in response to the physiological ligand SDF-1α without altering CXCR4 expression [109]. A study has shown that transwell and transendothelial migration of T lymphocytes are inhibited by HIV-1 Nef by the downmodulation of LFA-1 expression on T lymphocytes and the diminished adhesion and polarization of T lymphocytes through its myristoylation site and ΔSD domain (SH3 binding domain) [110]. Nef has also been reported to inhibit cell motility by phosphorylating cofilin (conserved actin-depolymerizing factor), and hence altering the function of the cellular kinase PAK2, which usually promotes motility. This indirectly restricts T-lymphocyte migration, serving as a valid viral strategy to invade the human immune system [111]. Nef binds major upstream regulators, DOCK2 and ELMO1, and activates Rac in T cells in the absence of antigen stimulation or chemokines. In this way, Nef inhibits chemotaxis and promotes T-cell activation, resulting in the modulation of the downstream processes by regulating Rac GTPases downstream of chemokine receptors and the antigen-initiated signaling pathways [112]. The points highlighted above demonstrate Nef-mediated T-lymphocyte migration, suggesting that intracellular signaling and membrane localization processes contribute to the inhibitory effects of Nef on T-cell migration. These inhibitory effects enhance the pathogenicity of the HIV-1 Nef protein. As this process obstructs the movement of the infected T cells and thus reduces interactions of the infected T cells with other immune cells, it is highly reasonable that it can increase the viral load in the bloodstream, and also protect these cells from immunological attack.
Conclusion
Nef has a potent role in virus replication and HIV pathogenesis in the early and late stages of viral infection. The function of Nef is far more complex than the simple amplifier of the HIV replication that it had previously been considered to be. It influences cellular functions of the host cell, such as intracellular trafficking, transcriptional activation, signaling and the apoptotic pathway, among others. In T cells, Nef participates in many protein signaling cascades and serves as a TCR-associated adapter protein (a viral TR AP) and supports viral replication [113]. In this way, Nef handles the complicated mission of keeping the cell alive until the next virus generation is ready to be released.
The complex multifarious roles played by Nef in coordination with host cellular proteins makes it an ideal candidate for the development of drugs against HIV. These drugs could be used in combination with currently available antiretroviral drugs, which may be of immense help during HIV infection. The emergence of drug-resistant strains or the adaptive nature of the HIV poses a great challenge to antiviral drug development against HIV [114,115]. In this scenario the drugs that can target the interaction of viral proteins with the host protein may be of great importance. Targeting Nef along with other viral proteins (such as Tat or Env) may offer a superior approach to control HIV infection and disease progression. Drugs that can block the interaction of immunogenic epitopes of Nef with host cellular proteins may be better future strategies for controlling HIV/AIDS. Our recent studies suggest the presence of various novel epitopes of Nef, most of which are conserved and interacting, which might serve as potential drug targets [Saxena SK et al., Unpublished Data]. However, alternative strategies to control mechanisms in Nef-mediated pathways should be devised.
Future perspective
There is a persistent need to increase our understanding of Nef. Studies involving the visualization, localization and dynamics of Nef protein interaction with host cellular factors will be of great importance. The underlying molecular mechanism of Nef-mediated formation of VS in T-cell signaling and MHC class I downregulation via ZAP-70 must be further characterized in order to understand its role in controlling HIV infection. Abrogation of the interaction with Nef and Vav may serve as an excellent target for the development of therapeutic strategies, as this interaction supports the enhancement of viral transcription and replication. Various domains of Nef have discrete roles in TCR signaling pathways (via CD4 and CD28 downregulation, Lck relocalization, and association with activated PAK2 kinase). Hence, each domain needs to be well-characterized for its specific role in TCR signaling. Further investigations are required to reveal Nef functions during HIV-1 pathogenesis. Nef functions, such as virus transfer via cell–cell transmission, signaling and exosome formation provide new ways of understanding its complex role in HIV pathogenesis [116–120]. The results of these studies can be used for the development of novel therapeutic targets and strategies to prevent HIV infection and pathogenesis. Nef is an identifiable hacker of the host cell [25], and this shortcoming may be used as a target against it. Novel epitopes of Nef and miRNAs produced in HIV-1-infected cells that may suppress both Nef function and HIV virulence through a RNAi pathway may be considered as novel therapeutic targets. Devising alternative pathways for targeting Nef is the need of the hour, and requires the contribution of global efforts. Relevant understanding of each aspect responsible for Nef’s multifarious activities is still elusive. Global cooperation between virologists, molecular biologists, system biologists, biochemists, immunologists, industry experts, medical intellectuals and policy makers may serve as a milestone in the process. In silico methods for the drug candidate discovery may be employed for efficient future drugs. However, our current knowledge of the Nef protein and its interaction with cellular proteins is still under exploration. A great sense of urgency is required to address this matter.
Executive summary.
Viral infectivity & infection
HIV-1 Nef has been shown to play a crucial role in the pathogenesis and development of AIDS in animal as well as human models.
Nef allows the production of fully infectious viral particles by phosphorylating a variety of substrates, including the viral matrix with its associated kinases.
Nef has a role in promoting growth and infection in early as well as late stages of the viral lifecycle.
Nef-mediated modulation at immunological synapses
Nef establishes an equilibrium in cell activation and prevention of activation-induced apoptosis by interacting with numerous host cell factors, thus optimizing the environment for HIV infection.
HIV-1 Nef impairs the formation of immunological synapses by changing the localization of Lck and N-Wasp and influencing actin polymerization.
Nef increases cell-free infection and cell-to-cell HIV-1 transmission by elevating the infectivity of cell-free virions.
Alteration in trafficking of signaling molecule
T-cell receptor (TCR)–CD3 and Lck intracellular trafficking has been identified as a crucial T-cell activation stage for Nef-mediated modulation.
Nef promotes intracellular accumulation of TCR in recycling endosomes and alters Lck signaling pathways for altering the antigenic stimulation response of T lymphocytes.
Nef-mediated apoptosis
Nef inhibits both ‘inside in’ and ‘outside in’ apoptotic signals, protecting infected cells from host-mediated apoptotic signals.
Nef upregulates FasL, creating a means of immune evasion via bystander cell killings.
There is a fair relationship between Nef-mediated apoptosis and T-cell signaling.
T-cell migration
Nef interacts with several host factors to inhibit T-cell migration at several steps, to increase viral loads in the bloodstream and to protect infected cells from immunological attacks.
Future perspective
Better characterization of other viral proteins such as Tat and Rev participating in T-cell activation and virological synapses are needed.
Interaction of Nef with host proteins such as TCR, Vav, ZAP-70 and Lck may also serve as targets for the development of therapeutic strategies, but more work remains to be done in this area.
An important goal of future work is the efficient molecular dissection and effective validation of Nef specificity in T-cell signaling pathways.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The authors are grateful to Council of Scientific and Industrial Research (CSIR-CCMB), India, for the encouragement and support for this work. SK Saxena and MPN Nair are supported by NIH grants R37DA025576 and R01MH085259. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Barré-Sinoussi F, Chermann JC, Rey F, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 2.Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381(6584):667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 3▪▪.Fackler OT, Alcover A, Schwartz O. Modulation of the immunological synapse: a key to HIV-1 pathogenesis? Nat Rev Immunol. 2007;7(4):310–317. doi: 10.1038/nri2041. Demonstrates modulation of lymphocyte signaling, apoptosis and intracellular trafficking that ensures the efficient spread of the virus in the hostile environment of the immune system. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson M. HIV-1 pathogenesis. Nat Med. 2003;9:853–860. doi: 10.1038/nm0703-853. [DOI] [PubMed] [Google Scholar]
- 5.Zack JA, Arrigo SJ, Weitsman SR, Haislip A, Chen IS. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 6.Bukrinsky MI, Stanwick TL, Dempsey MP, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baur AS, Sawai ET, Dazin P, Fantl WJ, Cheng-Mayer C, Peterlin BM. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity. 1994;1(5):373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 8.Simmons A, Aluvihare V, McMichael A. Nef triggers a transcriptional program in T cells imitating single-signal T cell activation and inducing HIV virulence mediators. Immunity. 2001;14(6):763–777. doi: 10.1016/s1074-7613(01)00158-3. [DOI] [PubMed] [Google Scholar]
- 9▪▪.Wolf D, Witte V, Laffert B, et al. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat Med. 2001;7(11):1217–1224. doi: 10.1038/nm1101-1217. Discusses the Nef-mediated protection of HIV-infected host cells by the induction of anti-apoptotic signals. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Marsh JW. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science. 2001;293(5534):1503–1506. doi: 10.1126/science.1061548. [DOI] [PubMed] [Google Scholar]
- 11.Geleziunas R, Xu W, Takeda K, Ichijo H, Greene WC. HIV-1 Nef inhibits ASK1-dependent death signaling providing a potential mechanism for protecting the infected host cell. Nature. 2001;410(6830):834–838. doi: 10.1038/35071111. [DOI] [PubMed] [Google Scholar]
- 12.Keppler OT, Tibroni N, Venzke S, Rauch S, Fackler OT. Modulation of specific surface receptors and activation sensitization in primary resting CD4+ T lymphocytes by the Nef protein of HIV-1. J Leukoc Biol. 2006;79(3):616–627. doi: 10.1189/jlb.0805461. [DOI] [PubMed] [Google Scholar]
- 13.Ott M, Emiliani S, Van Lint C, et al. Immune hyperactivation of HIV-1-infected T cells mediated by Tat and the CD28 pathway. Science. 1997;275(5305):1481–1485. doi: 10.1126/science.275.5305.1481. [DOI] [PubMed] [Google Scholar]
- 14.Kirchhoff F. Immune evasion and counteraction of restriction factors by HIV-1 and other primate lentiviruses. Cell Host Microbe. 2010;8(1):55–67. doi: 10.1016/j.chom.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 15▪.Fenard D, Yonemoto W, de Noronha C, Cavrois M, Williams SA, Greene WC. Nef is physically recruited into the immunological synapse and potentiates T cell activation early after TCR engagement. J Immunol. 2005;175(9):6050–6057. doi: 10.4049/jimmunol.175.9.6050. Discusses the physical recruitment of Nef into the immunological synapse and the induction of T-cell activation for impairing the immunological synapse. [DOI] [PubMed] [Google Scholar]
- 16▪▪.Thoulouze MI, Sol-Foulon N, Blanchet F, Dautry-Varsat A, Schwartz O, Alcover A. Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity. 2006;24(5):547–561. doi: 10.1016/j.immuni.2006.02.016. Demonstrated the Nef-mediated alteration of Lck and T-cell receptor endosomal trafficking in synapse formation and early T-cell signaling that likely impacts the function and fate of HIV-1-infected cells. [DOI] [PubMed] [Google Scholar]
- 17.Coiras M, López-Huertas MR, Sánchez del Cojo M, Mateos E, Alcamí J. Dual role of host cell factors in HIV-1 replication. restriction and enhancement of the viral cycle. AIDS Rev. 2010;12(2):103–112. [PubMed] [Google Scholar]
- 18.Haller C, Rauch S, Fackler OT, et al. HIV-1 Nef employs two distinct mechanisms to modulate Lck subcellular localization and TCR induced actin remodeling. PLoS One. 2007;2(11):e1212. doi: 10.1371/journal.pone.0001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arhel N, Lehmann M, Clauss K, Nienhaus GU, Piguet V, Kirchhoff F. The inability to disrupt the immunological synapse between infected human T cells and APCs distinguishes HIV-1 from most other primate lentiviruses. J Clin Invest. 2009;119(10):2965–2975. doi: 10.1172/JCI38994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diskin R, Scheid JF, Marcovecchio PM, et al. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science. 2011;334(6060):1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster JL, Garcia JV. HIV-1 Nef: at the crossroads. Retrovirology. 2008;5:84. doi: 10.1186/1742-4690-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirchhoff F. Silencing HIV-1 in vivo. Cell. 2008;134(4):566–568. doi: 10.1016/j.cell.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Geyer M, Fackler OT, Peterlin BM. Structure–function relationships in HIV-1. Nef EMBO Rep. 2001;2(7):580–585. doi: 10.1093/embo-reports/kve141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briggs SD, Scholtz B, Jacque JM, Swingler S, Stevenson M, Smithgall TE. HIV-1 Nef promotes survival of myeloid cells by a Stat3-dependent pathway. J Biol Chem. 2001;276(27):25605–25611. doi: 10.1074/jbc.M103244200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saxena SK, Shrivastava G, Tiwari S, Nair MPN. HIV-1 Nef: hacker of the host cell. Future Virol. 2012;7(2):117–120. doi: 10.2217/fvl.11.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad N, Venkatesan S. Nef protein of HIV-1 is a transcriptional repressor of HIV-1 LTR. Science. 1988;241(4872):1481–1485. doi: 10.1126/science.3262235. [DOI] [PubMed] [Google Scholar]
- 27.O’Neill E, Kuo LS, Krisko JF, Tomchick DR, Garcia JV, Foster JL. Dynamic evolution of the human immunodeficiency virus type 1 pathogenic factor, Nef. J Virol. 2006;80(3):1311–1320. doi: 10.1128/JVI.80.3.1311-1320.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heigele A, Schindler M, Gnanadurai CW, Leonard JA, Collins KL, Kirchhoff F. Down-modulation of CD8αβ is a fundamental activity of primate lentiviral Nef proteins. J Virol. 2012;86(1):36–48. doi: 10.1128/JVI.00717-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudhuri R, Mattera R, Lindwasser OW, Robinson MS, Bonifacino JS. A basic patch on alpha-adaptin is required for binding of human immunodeficiency virus type 1 Nef and cooperative assembly of a CD4–Nef–AP-2 complex. J Virol. 2009;83(6):2518–2530. doi: 10.1128/JVI.02227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dikeakos JD, Atkins KM, Thomas L, et al. Small molecule inhibition of HIV-1-induced MHC-I down-regulation identifies a temporally regulated switch in Nef action. Mol Biol Cell. 2010;21(19):3279–3292. doi: 10.1091/mbc.E10-05-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rauch S, Pulkkinen K, Saksela K, Fackler OT. Human immunodeficiency virus type 1 Nef recruits the guanine exchange factor Vav1 via an unexpected interface into plasma membrane microdomains for association with p21-activated kinase 2 activity. J Virol. 2008;82(6):2918–2929. doi: 10.1128/JVI.02185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kestler HW, III, Ringler DJ, Mori K, et al. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65(4):651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 33.Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332(4):228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 34.Whatmore AM, Cook N, Hall GA, Sharpe S, Rud EW, Cranage MP. Repair and evolution of nef in vivo modulates simian immunodeficiency virus virulence. J Virol. 1995;69(8):5117–5123. doi: 10.1128/jvi.69.8.5117-5123.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawai ET, Khan IH, Montbriand PM, Peterlin BM, Cheng-Mayer C, Luciw PA. Activation of PAK by HIV and SIV Nef: importance for AIDS in rhesus macaques. Curr Biol. 1996;6(11):1519–1527. doi: 10.1016/s0960-9822(96)00757-9. [DOI] [PubMed] [Google Scholar]
- 36.Rodés B, Toro C, Paxinos E, et al. Differences in disease progression in a cohort of long-term non-progressors after more than 16 years of HIV-1 infection. AIDS. 2004;18(8):1109–1116. doi: 10.1097/00002030-200405210-00004. [DOI] [PubMed] [Google Scholar]
- 37.Deacon NJ, Tsykin A, Solomon A, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270(5238):988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 38.Gorry PR, McPhee DA, Verity E, et al. Pathogenicity and immunogenicity of attenuated, nef-deleted HIV-1 strains in vivo. Retrovirology. 2007;4:66. doi: 10.1186/1742-4690-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birch MR, Learmont JC, Dyer WB, et al. An examination of signs of disease progression in survivors of the Sydney Blood Bank Cohort (SBBC) J Clin Virol. 2001;22(3):263–270. doi: 10.1016/s1386-6532(01)00198-6. [DOI] [PubMed] [Google Scholar]
- 40.Churchill MJ, Rhodes DI, Learmont JC, et al. Longitudinal analysis of human immunodeficiency virus type 1 nef/long terminal repeat sequences in a cohort of long-term survivors infected from a single source. J Virol. 2006;80(2):1047–1052. doi: 10.1128/JVI.80.2.1047-1052.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chowers MY, Spina CA, Kwoh TJ, Fitch NJ, Richman DD, Guatelli JC. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swingler S, Gallay P, Camaur D, Song J, Abo A, Trono D. The Nef protein of human immunodeficiency virus type 1 enhances serine phosphorylation of the viral matrix. J Virol. 1997;71(6):4372–4377. doi: 10.1128/jvi.71.6.4372-4377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43▪.Swingler S, Mann A, Jacque J. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat Med. 1999;5:97–103. doi: 10.1038/12433. Demonstrates the role of Nef in inducing chemokines in macrophages, thereby stimulating both the chemotaxis and activation of resting T lymphocytes, permitting productive HIV-1 infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aiken C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J Virol. 1997;71(8):5871–5877. doi: 10.1128/jvi.71.8.5871-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaeffer E, Geleziunas R, Greene WC. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J Virol. 2001;75:2993–3000. doi: 10.1128/JVI.75.6.2993-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costa LJ, Chen N, Lopes A, et al. Interactions between Nef and AIP1 proliferate multivesicular bodies and facilitate egress of HIV-1. Retrovirology. 2006;3:33. doi: 10.1186/1742-4690-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brügger B, Krautkrämer E, Tibroni N. Human immunodeficiency virus type 1 Nef protein modulates the lipid composition of virions and host cell membrane microdomains. Retrovirology. 2007;4:70. doi: 10.1186/1742-4690-4-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng YH, Plemenitas A, Fielding CJ, Peterlin BM. Nef increases the synthesis of and transports cholesterol to lipid rafts and HIV-1 progeny virions. Proc Natl Acad Sci USA. 2003;100(14):8460–8465. doi: 10.1073/pnas.1437453100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mujawar Z, Rose H, Morrow MP. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol. 2006;4(11):e365. doi: 10.1371/journal.pbio.0040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asztalos BF, Mujawar Z, Morrow MP. Circulating Nef induces dyslipidemia in simian immunodeficiency virus-infected macaques by suppressing cholesterol efflux. J Infect Dis. 2010;202(4):614–623. doi: 10.1086/654817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pizzato M, Helander A, Popova E, et al. Dynamin 2 is required for the enhancement of HIV-1 infectivity by Nef. Proc Natl Acad Sci USA. 2007;104:6812–6817. doi: 10.1073/pnas.0607622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pizzato M, Popova E, Göttlinger HG. Nef can enhance the infectivity of receptor-pseudotyped human immunodeficiency virus type 1 particles. J Virol. 2008;82(21):10811–10819. doi: 10.1128/JVI.01150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qi M, Aiken C. Nef enhances HIV-1 infectivity via association with the virus assembly complex. Virology. 2008;373:287–297. doi: 10.1016/j.virol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fackler OT, Wolf D, Weber HO, Laffert B, et al. A natural variability in the proline-rich motif of Nef modulates HIV-1 replication in primary T cells. Curr Biol. 2001;11(16):1294–1299. doi: 10.1016/s0960-9822(01)00373-6. [DOI] [PubMed] [Google Scholar]
- 56.Messmer D, Ignatius R, Santisteban C, Steinman RM, Pope M. The decreased replicative capacity of simian immunodeficiency virus SIVmac239Δnef is manifest in cultures of immature dendritic cells and T cells. J Virol. 2000;74:2406–2413. doi: 10.1128/jvi.74.5.2406-2413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petit C, Buseyne F, Boccaccio C, Abastado JP, Heard JM, Schwartz O. Nef is required for efficient HIV-1 replication in cocultures of dendritic cells and lymphocytes. Virology. 2001;286(1):225–236. doi: 10.1006/viro.2001.0984. [DOI] [PubMed] [Google Scholar]
- 58.Choi J, Walker J, Boichuk S, et al. Human endothelial cells enhance human immunodeficiency virus type 1replication in CD4+ T cells in a Nef-dependent manner in vitro and in vivo. J Virol. 2005;79(1):264–276. doi: 10.1128/JVI.79.1.264-276.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi J, Walker J, Talbert-Slagle K, Wright P. Endothelial cells promote human immunodeficiency virus replication in nondividing memory T cells via Nef-, Vpr-, and T-cell receptor-dependent activation of NFAT. J Virol. 2005;79(17):11194–11204. doi: 10.1128/JVI.79.17.11194-11204.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glushakova S, Grivel JC, Suryanarayana K, et al. Nef enhances human immunodeficiency virus replication and responsiveness to interleukin-2 in human lymphoid tissue ex vivo. J Virol. 1999;73(5):3968–3974. doi: 10.1128/jvi.73.5.3968-3974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swingler S, Brichacek B, Jacque JM, Ulich C, Zhou J, Stevenson M. HIV-1 Nef intersects the macrophage CD40L signaling pathway to promote resting-cell infection. Nature. 2003;424(6945):213–219. doi: 10.1038/nature01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahlknecht U, Deng C, Lu MC, et al. Resistance to apoptosis in HIV-infected CD4+ T lymphocytes is mediated by macrophages: role for Nef and immune activation in viral persistence. J Immunol. 2000;165(11):6437–6446. doi: 10.4049/jimmunol.165.11.6437. [DOI] [PubMed] [Google Scholar]
- 63.Stevenson M, Stanwick TL, Dempsey MP, Lamonica CA. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9(5):1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schrager JA, Marsh JW. HIV-1 Nef increases T cell activation in a stimulus-dependent manner. Proc Natl Acad Sci USA. 1999;96(14):8167–8172. doi: 10.1073/pnas.96.14.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams SA, Greene WC. Host factors regulating post-integration latency of HIV. Trends Microbiol. 2005;13(4):137–139. doi: 10.1016/j.tim.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 66.Nyakeriga AM, Fichtenbaum CJ, Goebel J, Nicolaou SA, Conforti L, Chougnet CA. Engagement of the CD4 receptor affects the redistribution of Lck to the immunological synapse in primary T cells: implications for T-cell activation during human immunodeficiency virus type 1 infection. J Virol. 2009;83(3):1193–1200. doi: 10.1128/JVI.01023-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piguet V, Sattentau Q. Dangerous liaisons at the virological synapse. J Clin Invest. 2004;114(5):605–610. doi: 10.1172/JCI22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68▪▪.Haller C, Fackler OT. HIV-1 at the immunological and T-lymphocytic virological synapse. Biol Chem. 2008;389(10):1253–1260. doi: 10.1515/BC.2008.143. Demonstrates the interaction of Nef with several host cellular molecules to prevent immunological synapse formation and promote T-cell activation. [DOI] [PubMed] [Google Scholar]
- 69.Arhel NJ, Kirchhoff F. Implications of Nef: host cell interactions in viral persistence and progression to AIDS. Curr Top Microbiol Immunol. 2009;339:147–175. doi: 10.1007/978-3-642-02175-6_8. [DOI] [PubMed] [Google Scholar]
- 70.Haller C, Rauch S, Michel N, et al. The HIV-1 pathogenicity factor Nef interferes with maturation of stimulatory T-lymphocyte contacts by modulation of N-Wasp activity. J Biol Chem. 2006;281(28):19618–19630. doi: 10.1074/jbc.M513802200. [DOI] [PubMed] [Google Scholar]
- 71.Hübner W, McNerney GP, Chen P, et al. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science. 2009;323(5922):1743–1747. doi: 10.1126/science.1167525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jolly C, Mitar I, Sattentau QJ. Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T cells. J Virol. 2007;81(24):13916–13621. doi: 10.1128/JVI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alimonti JB, Ball TB, Fowke KR. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J Gen Virol. 2003;84(Pt 7):1649–1661. doi: 10.1099/vir.0.19110-0. [DOI] [PubMed] [Google Scholar]
- 74.Haller C, Tibroni N, Rudolph JM, Grosse R, Fackler OT. Nef does not inhibit F-actin remodelling and HIV-1 cell–cell transmission at the T lymphocyte virological synapse. Eur J Cell Biol. 2011;(11):913–921. doi: 10.1016/j.ejcb.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 75.Lu X, Wu X, Plemenitas A, et al. CDC42 and Rac1 are implicated in the activation of the Nef-associated kinase and replication of HIV-1. Curr Biol. 1996;6(12):1677–1684. doi: 10.1016/s0960-9822(02)70792-6. [DOI] [PubMed] [Google Scholar]
- 76.Fackler OT, Luo W, Geyer M, Alberts AM, Peterlin MB. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol Cell. 1999;(6):729–739. doi: 10.1016/s1097-2765(01)80005-8. [DOI] [PubMed] [Google Scholar]
- 77.Wu J, Motto DG, Koretzky GA, Weiss A. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity. 1996;4(6):593–602. doi: 10.1016/s1074-7613(00)80485-9. [DOI] [PubMed] [Google Scholar]
- 78.Tuosto L, Marinari B, Andreotti M, Federico M, Piccolella E. Vav exchange factor counteracts the HIV-1 Nef-mediated decrease of plasma membrane GM1 and NF-AT activity in T cells. Eur J Immunol. 2003;33(8):2186–2196. doi: 10.1002/eji.200323682. [DOI] [PubMed] [Google Scholar]
- 79.Quaranta MG, Mattioli B, Spadaro F, et al. HIV-1 Nef triggers Vav-mediated signaling pathway leading to functional and morphological differentiation of dendritic cells. FASEB J. 2003;17(14):2025–2036. doi: 10.1096/fj.03-0272com. [DOI] [PubMed] [Google Scholar]
- 80.Cheng H, Tarnok J, Parks WP. Human immunodeficiency virus type 1 genome activation induced by human T-cell leukemia virus type 1 Tax protein is through cooperation of NF-kappaB and Tat. J Virol. 1998;72(8):6911–6916. doi: 10.1128/jvi.72.8.6911-6916.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Herrmann CH, Carroll RG, Wei P, Jones KA, Rice KP. Tat-associated kinase, TAK, activity is regulated by distinct mechanisms in peripheral blood lymphocytes and promonocytic cell lines. J Virol. 1998;72(12):9881–9888. doi: 10.1128/jvi.72.12.9881-9888.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holdorf AD, Lee KH, Burack WR, Allen PM, Shaw AS. Regulation of Lck activity by CD4 and CD28 in the immunological synapse. Nat Immunol. 2002;3(3):259–264. doi: 10.1038/ni761. [DOI] [PubMed] [Google Scholar]
- 83.Li QJ, Dinner AR, Qi S, et al. CD4 enhances T cell sensitivity to antigen by coordinating Lck accumulation at the immunological synapse. Nat Immunol. 2004;5(8):791–799. doi: 10.1038/ni1095. [DOI] [PubMed] [Google Scholar]
- 84.Hung CH, Thomas L, Ruby CE, et al. HIV-1 Nef assembles a Src family kinase-ZAP-70/Syk-PI3K cascade to downregulate cell-surface MHC-I. Cell Host Microbe. 2007;1(2):121–133. doi: 10.1016/j.chom.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 85.Sol-Foulon N, Sourisseau M, Porrot F, et al. ZAP-70 kinase regulates HIV cell-to-cell spread and virological synapse formation. EMBO J. 2007;26(2):516–526. doi: 10.1038/sj.emboj.7601509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fortin JF, Barat C, Beauséjour Y, Barbeau B, Tremblay MJ. Hyper-responsiveness to stimulation of human immunodeficiency virus-infected CD4+T cells requires Nef and Tat virus gene products and results from higher NFAT, NF-kappaB, and AP-1 induction. J Biol Chem. 2004;279(38):39520–39531. doi: 10.1074/jbc.M407477200. [DOI] [PubMed] [Google Scholar]
- 87.Manninen A, Huotari P, Hiipakka M, Rankema GH, Saksela K. Activation of NFAT-dependent gene expression by Nef: conservation among divergent Nef alleles, dependence on SH3 binding and membrane association, and cooperation with protein kinase C-theta. J Virol. 2001;75(6):3034–3037. doi: 10.1128/JVI.75.6.3034-3037.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schrager JA, Der Minassian V, Marsh JW. HIV Nef increases T cell ERK MAP kinase activity. J Biol Chem. 2002;277(8):6137–6142. doi: 10.1074/jbc.M107322200. [DOI] [PubMed] [Google Scholar]
- 89.Manninen A, Renkema GH, Saksela K. Synergistic activation of NFAT by HIV-1 nef and the Ras/MAPK pathway. J Biol Chem. 2000;275(22):16513–16517. doi: 10.1074/jbc.M910032199. [DOI] [PubMed] [Google Scholar]
- 90.Kinoshita S, Chen BK, Kaneshima H, Nolan GP. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell. 1998;95(5):595–604. doi: 10.1016/s0092-8674(00)81630-x. [DOI] [PubMed] [Google Scholar]
- 91.Naramura M, Jang IK, Kole H, Huang F, Haines D, Gu H. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat Immunol. 2002;3(12):1192–1199. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
- 92.Valitutti S, Müller S, Salio M, Lanzavecchia A. Degradation of T cell receptor (TCR)-CD3-zeta complexes after antigenic stimulation. J Exp Med. 1997;185(10):1859–1864. doi: 10.1084/jem.185.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Das V, Nal B, Dujeancourt A, et al. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity. 2004;20(5):577–588. doi: 10.1016/s1074-7613(04)00106-2. [DOI] [PubMed] [Google Scholar]
- 94.Ehrlich LI, Ebert PJ, Krummel MF, Weiss A, Davis MM. Dynamics of p56Lck translocation to the T cell immunological synapse following agonist and antagonist stimulation. Immunity. 2002;17(6):809–822. doi: 10.1016/s1074-7613(02)00481-8. [DOI] [PubMed] [Google Scholar]
- 95.Pan X, Rudolph JM, Abraham L, et al. HIV-1 Nef compensates for disorganization of the immunological synapse by inducing trans-Golgi network-associated Lck signaling. Blood. 2012;119(3):786–797. doi: 10.1182/blood-2011-08-373209. [DOI] [PubMed] [Google Scholar]
- 96.Simmons A, Gangadharan B, Hodges A, et al. Nef-mediated lipid raft exclusion of UbcH7 inhibits Cbl activity in T cells to positively regulate signaling. Immunity. 2005;23(6):621–634. doi: 10.1016/j.immuni.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 97.Yang P, Henderson AJ. Nef enhances c-Cbl phosphorylation in HIV-infected CD4+ T lymphocytes. Virology. 2005;336(2):219–228. doi: 10.1016/j.virol.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 98.Xu XN, Screaton GR, Gotch FM, et al. Evasion of cytotoxic T lymphocyte (CTL) responses by nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J Exp Med. 1997;186(1):7–16. doi: 10.1084/jem.186.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu XN, Laffert B, Screaton GR, et al. Induction of Fas ligand expression by HIV involves the interaction of Nef with the T cell receptor zeta chain. J Exp Med. 1999;189(9):1489–1496. doi: 10.1084/jem.189.9.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hodge S, Novembre FJ, Whetter L, Gelbard HA, Dewhurst S. Induction of Fas ligand expression by an acutely lethal simian immunodeficiency virus, SIVsmmPBj14. Virology. 1998;252(2):354–363. doi: 10.1006/viro.1998.9477. [DOI] [PubMed] [Google Scholar]
- 101.Debatin KM, Fahrig-Faissner A, Enenkel-Stoodt S, Kreuz W, Benner A, Krammer PH. High expression of APO-1 (CD95) on T lymphocytes from human immunodeficiency virus-1-infected children. Blood. 1994;83(10):3101–3103. [PubMed] [Google Scholar]
- 102.Katsikis PD, Wunderlich ES, Smith CA, Herzenberg LA, Herzenberg LA. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med. 1995;181(6):2029–2036. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sloand EM, Young NS, Kumar P, Weichold FF, Sato T, Maciejewski JP. Role of Fas ligand and receptor in the mechanism of T-cell depletion in acquired immunodeficiency syndrome: effect on CD4+ lymphocyte depletion and human immunodeficiency virus replication. Blood. 1997;89(4):1357–1363. [PubMed] [Google Scholar]
- 104.Greenway AL, McPhee DA, Allen K, et al. Human immunodeficiency virus type 1 Nef binds to tumor suppressor p53 and protects cells against p53-mediated apoptosis. J Virol. 2002;76(6):2692–2702. doi: 10.1128/JVI.76.6.2692-2702.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bell I, Ashman C, Maughan J, Hooker E, Cook F, Reinhart TA. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) zeta chain leads to TCR down-modulation. J Gen Virol. 1998;79(Pt 11):2717–2727. doi: 10.1099/0022-1317-79-11-2717. [DOI] [PubMed] [Google Scholar]
- 106.Howe AY, Jung JU, Desrosiers RC. Zeta chain of the T-cell receptor interacts with nef of simian immunodeficiency virus and human immunodeficiency virus type 2. J Virol. 1998;72(12):9827–9834. doi: 10.1128/jvi.72.12.9827-9834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schindler M, Schmökel J, Specht A, et al. Inefficient Nef-mediated downmodulation of CD3 and MHC-I correlates with loss of CD4+ T cells in natural SIV infection. PLoS Pathog. 2008;4(7):e1000107. doi: 10.1371/journal.ppat.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schindler M, Münch J, Kutsch O, et al. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell. 2006;125(6):1055–1067. doi: 10.1016/j.cell.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 109▪.Park IW, He JJ. HIV-1 Nef-mediated inhibition of T cell migration and its molecular determinants. J Leukoc Biol. 2009;86(5):1171–1178. doi: 10.1189/jlb.0409261. Demonstrates Nef-mediated inhibition of chemotaxis in response to SDF-1α in both Jurkat T cells and primary peripheral CD4+ T lymphocytes, suggesting that Nef may blunt the T-cell response to chemokines, contributing to the pathogenesis of AIDS. [DOI] [PubMed] [Google Scholar]
- 110▪.Choe EY, Schoenberger ES, Groopman JE, Park IW. HIV Nef inhibits T cell migration. J Biol Chem. 2002;277(48):46079–46084. doi: 10.1074/jbc.M204698200. Demonstrates Nef-mediated inhibition of chemotaxis in response to SDF-1α in both Jurkat T cells and primary peripheral CD4+ T lymphocytes, suggesting that Nef may blunt the T-cell response to chemokines, contributing to the pathogenesis of AIDS. [DOI] [PubMed] [Google Scholar]
- 111.Stolp B, Reichman-Fried M, Abraham L, et al. HIV-1 Nef interferes with host cell motility by deregulation of Cofilin. Cell Host Microbe. 2009;6(2):174–186. doi: 10.1016/j.chom.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 112.Janardhan A, Swigut T, Hill B, Myers MP, Skowronski J. HIV-1 Nef binds the DOCK2–ELMO1 complex to activate rac and inhibit lymphocyte chemotaxis. PLoS Biol. 2004;2(1):E6. doi: 10.1371/journal.pbio.0020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fackler OT, Baur AS. Live and let die: Nef functions beyond HIV replication. Immunity. 2002;16(4):493–497. doi: 10.1016/s1074-7613(02)00307-2. [DOI] [PubMed] [Google Scholar]
- 114.Saxena SK, Gupta A, Bhagyashree K, et al. Targeting strategies for human immunodeficiency virus: a combinatorial approach. Mini Rev Med Chem. 2012;12(3):236–254. doi: 10.2174/1389557511209030236. [DOI] [PubMed] [Google Scholar]
- 115.Clavel F, Hance AJ. HIV drug resistance. N Engl J Med. 2004;350(10):1023–1035. doi: 10.1056/NEJMra025195. [DOI] [PubMed] [Google Scholar]
- 116.Xu W, Santini PA, Sullivan JS, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol. 2009;10(9):1008–1017. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Muratori C, Cavallin LE, Krätzel K, et al. Massive secretion by T cells is caused by HIV Nef in infected cells and by Nef transfer to bystander cells. Cell Host Microbe. 2009;6(3):218–230. doi: 10.1016/j.chom.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 118.Lenassi M, Cagney G, Liao M, et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11(1):110–222. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119▪▪.Baur AS. HIV-Nef and AIDS pathogenesis: are we barking up the wrong tree? Trends Microbiol. 2011;19(9):435–440. doi: 10.1016/j.tim.2011.06.002. Emphasizes the role of Nef in exocytosis and provides evidence that Nef-induced secretion is probably the key factor of pathogenesis behind this elusive viral effector. [DOI] [PubMed] [Google Scholar]
- 120.Percario ZA, Mangino G, Gallo V, et al. HIV-1 Nef transfer and intracellular signaling in uninfected cells. In: Chang TL, editor. HIV-Host Interactions. Tech Europe Publisher; Croatia: 2011. [Google Scholar]