Abstract
This article reviews recent advances in our understanding of hemodynamic signals, external/compressive forces, and circulating factors that mediate exercise training-induced vascular adaptations, with particular attention to the roles of these signals in prevention and treatment of endothelial dysfunction and cardiovascular (CV) diseases.
1. Introduction
Regular physical activity is required for maintenance of a healthy cardiovascular (CV) system, prevention of CV and metabolic comorbidities, and premature death due to CV causes [1, 2]. However, only ~60% of the reduction in CV disease risk from physical activity can be explained by effects on traditional and novel risk factors such as inflammatory/hemostatic factors, blood pressure, traditional and novel lipids, body mass index, and HbA1c [3]. Thus, approximately 40% of the mechanisms underlying the beneficial CV effects of exercise remain unknown. Furthermore, it is also known that the protective effects of exercise training on endothelial function are not mediated by traditional risk factors [4, 5].
Here, we review recent advances in our understanding of exercise-induced signals for endothelial adaptation that are postulated to account for some of this unexplained risk reduction. Our discussion begins with a review of the influence of exercise on hemodynamic signals. We then examine the role of external compressive forces associated with exercise, with particular focus on recent data from human and animal studies using external pneumatic compression techniques for possible therapeutic gain. Next we discuss circulating factors postulated to contribute to exercise-induced systemic endothelial adaptations, specifically focusing on insulin, adipose tissue-derived cytokines, and circulating angiogenic cells (CACs). Finally, we end with a discussion of how these different exercise-induced signals may interact with each other, and propose some priorities for future research efforts.
2. Hemodynamic Signals
2.1 Role of Shear Stress in the Regulation of Vascular Endothelial Phenotype
The vascular endothelium receives complex signals from shear forces produced by flowing blood. These signals and their functional sequelae are important mediators of exercise-induced endothelial adaptations. There is considerable evidence from in vitro studies of cultured endothelial cells and isolated vessel preparations to support the concept that increases in unidirectional shear stress favorably influence endothelial phenotype. In cultured endothelial cells, physiologically-relevant shear stress levels (i.e., levels that might be experienced during exercise in humans) have been shown to increase production of nitric oxide (NO), expression of endothelial NO synthase (eNOS), production of the eNOS cofactor tetrahydrobiopterin, all classic hallmarks of a healthy, anti-atherogenic endothelial phenotype [6–9]. These findings were supported by work in our laboratory using isolated vessel preparations, in that porcine coronary arteriole eNOS and copper-zinc superoxide dismutase mRNA levels were responsive to high (~6 dyn/cm2) but not low (~2 dyn/cm2) shear stress [10]. Similarly, eNOS gene expression and endothelium-dependent dilation were responsive to moderate and high shear stress in soleus feed arteries of older rats such that eNOS expression and endothelium-dependent dilation were restored to levels observed in arteries of young rats [11]. In vitro data also indicate that shear stress exerts anti-inflammatory effects on cultured endothelial cells, such as reduced expression of adhesion molecules and protection against insult from inflammatory agents [e.g., tumor necrosis factor and oxidized LDL [12]]. Microarray studies indicate that increased mean shear stress downregulates a number of inflammation-related transcripts (VCAM, IL-8) and upregulates protective genes such as eNOS and KLF-2 [13, 14].
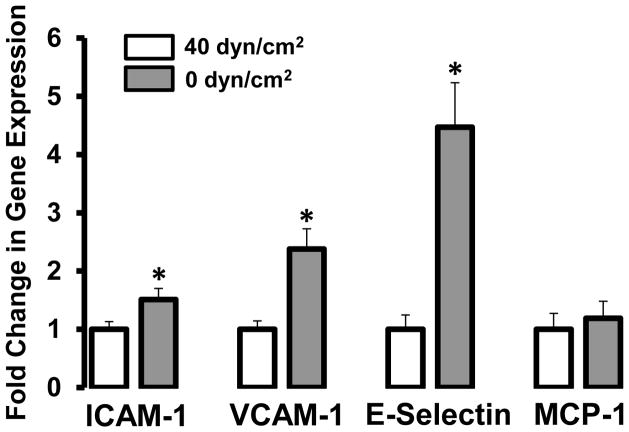
To gain insight into the role of shear stress in the maintenance of a healthy endothelium, an important experimental question might be, “What is the impact of removal of shear on endothelial phenotype?” We recently examined this question by assessing the expression of inflammatory genes (ICAM-1, VCAM-1, E-selectin, and MCP-1) in an isolated, perfused vessel preparation in which rat carotid arteries were either exposed to constant flow (shear stress of 40 dyn/cm2) or no flow (0 dyn/cm2) for 4 hr [15]. The results (Fig 1) indicated that removal of shear significantly induced expression of ICAM-1 (~50%), VCAM-1 (~2.5 fold), and E-Selectin (~4.5 fold). Thus, taken with the evidence discussed above regarding the beneficial effects of shear, these data support the idea that shear signals are critical for the regulation and maintenance of a healthy vascular endothelial phenotype, as even acute removal of shear can augment the expression of inflammatory genes.
Figure 1.
Effect of shear (40 dyn/cm2) vs. no shear (0 dyn/cm2) on expression of intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1), E-selectin, and monocyte chemoattractant protein-1 (MCP-1) gene expression in rat carotid arteries. * P < 0.05 vs. no shear.
2.2 Exercise-induced Shear Stress as an Adaptive Signal to the Endothelium
Endurance exercise induces substantial increases in blood flow through numerous conduit arteries and vascular beds, most notably to contracting skeletal and cardiac muscle to support the increased metabolic demand. Originally proposed in 1992 by Laughlin and McCallister [16], it is now well-accepted that exercise-induced increases in arterial wall shear stress serve as a primary signal driving endothelial adaptations to training [17]. However, the magnitude of training induced endothelial adaptations throughout the vasculature is substantially heterogeneous. For example, among skeletal muscle vascular beds, there is evidence that adaptations are directly related to muscle recruitment patterns, such that greater vascular adaptations occur in vessels perfusing the areas with the greatest increase in contractile activity [18, 19]. However, there is also evidence of systemic vascular adaptations to exercise from both human and animal studies, as arteries supplying non-contracting tissues can experience functional improvements following a period of regular exercise [20, 21]. Numerous studies indicate an increase in shear rates during exercise in vessels of non-contracting tissues (e.g., the conduit arteries of the arm during leg exercise) [22–29]. Recently, experimental evidence has confirmed that these exercise-induced increases in shear stress resulting from hyperemic flow are required for vascular adaptations in conduit arteries that perfuse contracting [30••] and non-contracting [31 ••] muscles. In the first of these investigations [30••], bilateral handgrip exercise training was performed and the exercise-induced increases in shear stress were attenuated in one arm by a pneumatic forearm cuff inflated to 60 mm Hg. Increases in brachial artery FMD and ischemic exercise-induced dilation observed in the non-cuffed arm after 2 wk of training were completely prevented in the cuffed arm. Thus, these data suggest that exercise-induced endothelial adaptations in the conduit artery supplying the working muscle are shear stress-dependent [30••]. More recently, to address the question of whether brachial artery endothelial adaptations to lower limb exercise are also dependent on shear stress, the forearm cuff model was employed to attenuate increases in brachial artery shear stress during an 8-wk cycling exercise training program [31 ••]. Although in this study the 60 mm Hg cuff pressure was not sufficient to completely prevent the increase in brachial artery shear rate during each exercise bout, here again the increase in FMD after 2 wk of training observed in the non-cuffed arm was completely absent in the cuffed arm. Thus, it appears that shear stress in conduit arteries of contracting and non-contracting limbs is a potent and necessary signal for beneficial exercise-induced endothelial adaptations. In addition, the finding that adaptations were prevented despite incomplete removal of the shear stimulus by the 60 mm Hg forearm cuff [31••] suggests the intriguing hypothesis that a threshold of exercise-induced shear stress exists that elicits beneficial training effects on the endothelium. This hypothesis should be addressed in future experiments, as determining the precise exercise-induced shear stimulus required to induce beneficial endothelial effects could have important clinical implications.
2.3 Influence of Shear Rate Patterns on Endothelial Phenotype
Time-average shear stress may not fully capture the intricacies of resting or exercise-induced hemodynamic stimuli, as fluctuations in the profile of the shear waveform throughout the cardiac cycle can also influence endothelial phenotype. During the cardiac cycle, there is not only forward (anterograde) flow, but there is also a brief period of backward (retrograde) flow during diastole in conduit arteries of the limbs. Thus within a given cardiac cycle the endothelium experiences oscillations in the shear stress exerted by flowing blood. Endothelial cells are remarkably capable of sensing directional changes in shear stimulus, as they are equipped with a number of complex mechanotransduction and signaling mechanisms acting in concert to alter gene expression and function [32]. The available data indicate that increased retrograde and oscillatory shear can induce profound pro-atherogenic effects on the endothelium, including increased NADPH oxidase- and mitochondrial-derived ROS production, augmented production of endothelin-1, and enhanced expression of vascular and intercellular adhesion molecules [33–39]. The functional in vivo consequences of these molecular events were recently confirmed in humans, as increases in retrograde and oscillatory shear stress were found to acutely impair endothelium-dependent dilation of peripheral conduit arteries, whereas removal of retrograde and oscillatory shear with increased antegrade shear acutely augmented endothelial function [40••, 41 ••]. Furthermore, it has also been shown that brachial artery FMD is reduced acutely by a hydrostatic challenge produced with arm hanging for 3 hrs. Positioning the arm below heart level for 3 hrs somewhat mimicked the atheroprone hemodynamics of lower limb vasculatures by inducing a reduction in mean and anterograde shear rates and increasing retrograde shear [42•]. Thus, there is solid support for the concept that retrograde and oscillatory shear patterns aredeleterious signals to the endothelium.
Recent evidence indicates that aging is associated with elevations in arm and leg conduit artery retrograde shear rates and oscillatory shear stress [43•−, 45••]. The mechanistic contribution of the NO vasodilator system to the age-related difference in brachial artery shear patterns has been examined [44••]. Arterial administration of L-NMMA in young subjects resulted in reduced forearm vascular conductance accompanied by increased retrograde shear rates that matched those observed in older subjects, while L-NMMA infusions did not further reduce conductance or augment the retrograde shear rate of older subjects [44••]. Thus, the age-associated elevations in pro-atherogenic shear rate patterns were largely dependent on dysfunction of the NO pathway in downstream resistance vessels. Interestingly, both young and older subjects experienced acute exercise-induced increases in mean shear and reductions in retrograde shear rates and the baseline difference between the groups was abolished by exercise [44••]. Thus, exercise acutely attenuates the age-related increase in retrograde shear in the brachial artery, raising the hypothesis, to be examined in future studies, that the removal of pro-atherogenic shear patterns during exercise might serve as a signal for exercise-induced endothelial adaptations. However, as altered shear profiles in the lower limb conduit arteries are thought to contribute to their preferential susceptibility to atherosclerosis [46, 47], it will be important for future studies to investigate whether exercise can alter resting shear patterns in these vessels as well.
3. External Forces
Relevant to a discussion about endothelial adaptations to exercise stimulus are the external pressures encountered by the vasculature. During skeletal muscle contraction, intramuscular pressures increase profoundly to levels that far exceed systolic arterial pressure and can reach upwards of 570 mm Hg [48–50]. As a result, the vasculature embedded within actively contracting tissue is prone to intermittent compression [51]. It has been well established that acute exposure to changes in intravascular pressure and/or collapse elicits smooth muscle cell mediated changes in arterial tone, termed myogenic autoregulation [52–54]. The physical forces that facilitate myogenic autoregulation may be mediated by transduction of physical forces through extracellular matrix proteins, smooth muscle cell cytoskeleton (i.e. integrins) and/or mechanosensitive ion channels ultimately resulting in smooth muscle membrane hyperpolarization and/or calcium efflux [55, 56]. In addition, endothelial derived substances (e.g. NO, prostaglandin I2, endothelial-derived hyperpolarizing factor, etc) are likely to contribute to compression-induced vasodilation as removal of the endothelium reduces, but does not abolish the vasodilatory response to compression in isolated skeletal muscle feed arteries [54, 56]. Of particular importance to this discussion of chronic vascular adaptations to exercise, we will review models of compression, devoid of active skeletal muscle recruitment, to explore the role of intramuscular pressures in the adaptations to exercise training.
3.1 External pneumatic compression – A model for the impact of intramuscular pressure on the vascular adaptation to exercise
External pneumatic compression devices are currently used in clinical practice for the treatment of a number of conditions, particularly vascular disorders, and can provide a model for the evaluation of the effects of compression on vascular biology. Studies that utilize cuff compression to mimic intramuscular pressure oscillations with exercise have demonstrated that, acutely, intermittent compression induces vasodilation [57] and reduces regional arterial stiffness [58]. To address the chronic effects of external compression, we will focus specifically on vascular adaptations following treatments which utilize intermittent external compression cuffs on the lower extremities; enhanced external counterpulsation (EECP) and intermittent pneumatic compression (IPC). Importantly, the intermittent nature of these interventions more closely resembles patterns of muscle contraction observed during exercise. Crenshaw et al. have shown that there is a linear relationship between external and intramuscular pressures with application of external pressure to human cadaver limbs [59]. Furthermore, in a rodent model of intermittent pneumatic compression (IPC), pressure underneath the cuffs closely resembles the pressure programmed on the compression unit [60]. Therefore, vascular adaptations to chronic compression treatment may provide insight into the role of muscle contraction induced vascular compression in the adaptation to exercise. This section will briefly describe the results of recent publications examining the therapeutic efficacy of compressive therapy on CV outcomes. We will then return to the insights gained from these results on compressive forces as exercise-induced signals for endothelial adaptation.
3.2 Enhanced external counterpulsation (EECP)
EECP is a noninvasive, atraumatic, outpatient therapy that consists of three pneumatic compression cuffs applied to the calf, lower thigh, and upper thigh of each leg. These cuffs are sequentially inflated at target inflation pressures of 300 mmHg, from distal to proximal, with compressed air during the diastolic phase of the cardiac cycle and rapidly deflated in early systole. Inflation and deflation of the cuffs is triggered by events in the cardiac cycle via microprocessor interpreted electrocardiogram signals. A standard course of EECP therapy consists of 35 1-hour sessions over 7 weeks. Acutely, EECP increases diastolic augmentation, reduces systolic afterload, and promotes venous return with a subsequent increase in cardiac output [61]. EECP is traditionally used to treat symptomatic coronary aretery disease (CAD) patients who are not readily amendable for interventional procedures. Data from most clinical trials and the International Patient Registry demonstrate that EECP is effective in reducing anginal symptoms and nitroglycerin usage [62–65], increasing exercise tolerance [62, 65–67], and decreasing the need for hospitalization [62, 64, 68]. The clinical benefits of EECP have been sustained for 2, 3, and 5 years after treatment in most patients [69–72].
Most investigations conducted to elucidate the mechanism of action have hypothesized that EECP may promote coronary angiogenesis through robust diastolic pressure augmentation during rapid cuff inflation, analogous to intra-aortic balloon counterpulsation. However, this understanding of EECP is only a theory and remains unconfirmed in clinical trials [66, 73]. In an international trial (7 Centers; 175 chronic stable angina patients), EECP failed to elicit improved cardiac perfusion in 46% of study subjects [74]. However, despite negligible improvements in myocardial perfusion, approximately 85% of patients in EECP clinical trials experience reduction in angina [62, 74]. Although initial theory as to the mechanism of action for angina reduction in CAD patients focused on central angiogenesis, the literature points overwhelmingly to augmentation of peripheral vascular function decreasing cardiac demand [75].
3.3 EECP improves vasoactive balance
There is biochemical evidence that EECP elicits systemic improvements in vascular biology. For example, a standard course of EECP therapy in coronary artery disease patients was shown to increase plasma levels of NOx by 36–62% and decrease endothelin-1 (ET-1) by 25–36% [75, 76]. Importantly, these effects have been shown to persist for up to 3 months following EECP therapy [76]. The benefits of EECP treatment on vasoactive balance are not limited to CAD patients. It has recently been demonstrated that EECP treatment increased plasma NOx levels by 30% in subjects with abnormal glucose tolerance [77••]. NO bioavailability may be improved through an anti-inflammatory effect of EECP, as evidenced by significant reductions in the pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α), C-reactive protein (CRP), and monocyte chemotactic protein-1 (MCP-1) [75, 78]. NO bioavailability may also be favorably influenced through improvement in systemic redox balance, as plasma levels of 8-iso-prostaglandin F2α (8-iso-PGF2α), an established plasma marker of oxidative stress, are significantly decreased following a standard course of EECP in CAD patients [75] and subjects with abnormal glucose tolerance [77••].
3.4 EECP improves vascular reactivity
Functional assessments of vascular reactivity have also demonstrated marked improvements following EECP therapy. In coronary artery disease patients, endothelial-dependent flow mediated dilation (FMD) in the brachial [75, 79] and femoral arteries [75] is significantly improved and peak blood flow in the forearm and calf were also increased [75]. Moreover, improvements in FMD of the brachial and popliteal arteries have also been demonstrated following EECP in patients with abnormal glucose tolerance [77••]. Significant improvements in reactive hyperemia peripheral endothelial function (RH-PAT) assessment following EECP in CAD patients have also been demonstrated with improvements maintained for 1 months following the course of treatment [80]. Arterial stiffness may also be amenable to EECP therapy. Although decreased peripheral arterial stiffness measured using non-invasive tonometry has been demonstrated in a sham controlled study of coronary artery disease patients following EECP [81], another study reported no change [82]. Further support for the functional improvements in the vasculature is demonstrated by the improved acetylcholine sensitivity observed in coronary artery rings of hypercholesterolimc swine following a standard course of EECP therapy [83]. Moreover, further studies in hypercholesterolemic pigs have shown that EECP effectively reduces hypercholesterolemia-induced endothelial damage and increases eNOS expression [84].
3.5 Angiogenesis/Vasculogenesis following External Pneumatic Compression
Despite limited evidence for coronary artery angiogenesis following EECP therapy, some evidence implicates repeated mechanical compression in peripheral vasculogenesis/angiogenesis. It is known that cyclic strain/stretch imposed on cultured endothelial cells and muscle cells can increase the expression of angiogenic factors [60]. Indeed, evidence for vascular remodeling has been demonstrated in models of external pneumatic compression. An acute bout of IPC, which employs lower compressive pressures (~50–120 mmHg) with varying duty cycles that do not coincide with the cardiac cycle, has been shown to significantly upregulate vascular endothelial growth factor (VEGF) and monocyte chemotactic protein-1 (MCP-1) mRNA expression in skeletal muscle in a rodent model of peripheral arterial disease PAD [60]. Furthermore, it has been shown that an acute bout of IPC applied to the hindlimbs of rats increases eNOS expression [85]. Chronically, 2 weeks of IPC treatment in a rodent model of IPC has been shown to improve exercise tolerance, muscle performance, and blood flow to fast-twitch white muscle fibers during skeletal muscle contraction [86]. Further evidence for peripheral angiogenesis in the adaptive response to compression has been demonstrated following 35 1-hour sessions of EECP in patients with abnormal glucose tolerance. In that study, the angiogenic marker VEGF was elevated nearly 2-fold in the serum following 35 weeks of EECP therapy and coincided with improvements in capillary density of the vastus lateralis [77••]. These findings suggest that gene expression profiles and neocapillary formation are altered with compressive therapy.
Thus, external pneumatic compression studies provide proof of concept that mechanical forces improve endothelial and general cardiovascular health. Although the intention of the studies reviewed in this section were to examine compressive therapy efficacy, we believe that these models have been and will continue to be valuable experimental models to gain insight into the role of compressive forces, independent of voluntary muscle contraction, in exercise-induced endothelial adaption.
4. Circulating Factors
We have recently argued [17] that when considering the potential signals for exercise-induced endothelial adaptations, it is important to keep in mind that the typical duration of a single endurance exercise bout ranges from ~30 (minimum to maintain health) to ~120 min (endurance athletes). Thus, exercise-induced alterations in hemodynamic and external compressive forces are present for only ~2–10% of a given day. Circulating factors, however, may be altered far beyond the duration of the exercise bout and could therefore contribute substantially to the exercise stimulus long after cessation of exercise. Consistent with this hypothesis, current evidence indicates that beneficial changes in circulating hormonal, inflammatory, and oxidant stress-related factors may function as signals for exercise-induced vascular endothelial adaptation. We focus this section on three particularly important circulating factors that have recently received considerable attention as putative signals for endothelial adaptations to exercise: insulin, adipose tissue-derived cytokines (i.e. adipokines, specifically leptin and adiponectin), and CACs.
4.1. Insulin
There is substantial evidence that insulin has a number of beneficial effects on endothelial cells, including increased nitric oxide (NO) production, decreased ROS generation, increased antioxidant gene expression, and decreased proinflammatory gene expression [reviewed in [17]]. Beyond these molecular effects, insulin has significant functional effects on the vasculature, including dilation of large and small vessels and recruitment of skeletal muscle capillaries to increase nutritive flow [87]. Recent data indicate that loss of this endothelial-specific insulin signaling reduces whole body and skeletal muscle insulin-stimulated glucose uptake [88], illustrating the broader importance of insulin’s endothelial effects. Furthermore, the vasodilatory effects of insulin are attenuated in subjects with impaired endothelial function and type 2 diabetes, in that insulin-stimulated nutritive flow to skeletal muscle is largely reduced in the insulin resistant state [89–91]. Thus, endothelial cells can effectively become desensitized to insulin, and this endothelial insulin resistance occurs in close association with the development of insulin resistance in downstream tissues (e.g. skeletal muscle).
Additionally, while loss of endothelial insulin signaling is associated with type 2 diabetes and its vascular complications, it is also important to consider the adverse influence of excess insulin on endothelial phenotype. The pathogenesis of the insulin resistant state to overt type 2 diabetes involves well-characterized compensatory increases in pancreatic insulin secretion to maintain euglycemia. In vivo and in vitro data suggest that chronic hyperinsulinemia associated with insulin resistance promotes a dysfunctional, pro-inflammatory, and pro-atherogenic endothelial phenotype [92–95]. Thus, it appears that in health, insulin has beneficial physiological effects on endothelial cells, whereas both hyper- and hypoinsulinemia induce pathophysiologic endothelial signaling. It is of interest, then, to determine strategies for maintenance of appropriate concentrations of insulin and endothelial cell insulin sensitivity for the prevention and treatment of CV disorders associated with metabolic diseases.
It is clear that endurance exercise has potent beneficial effects on glucose tolerance and whole body insulin sensitivity, and a large body of evidence supports the contention that regular physical activity is required for the maintenance of appropriate glucose and insulin metabolism [96–98]. Thus, we propose that regular exercise serves to maintain circulating insulin at “optimal” levels, which could prevent the adverse vascular consequences of too high (i.e., pro-atherogenic hyperinsulinemia, as seen in insulin resistance and pre-diabetic states) or too low (loss of insulin signaling, as seen in hypoinsulinemia of overt type 2 diabetes) levels of insulin (Fig 2).
Figure 2.
Proposed “inverted-U” shaped relationship between circulating insulin concentrations and endothelial function.
4.2. Adipose-derived cytokines (adipokines)
It is becoming increasingly appreciated that impairments in endothelial function and increased risk for CV disease are at least partly attributable to adipose tissue-derived factors. Data from human and animal studies indicate that visceral adipose tissue depots secrete pro-inflammatory adipose-derived cytokines (i.e., adipokines) in response to excessive lipid accumulation. Adipokines are secreted primarily from infiltrated immune cells residing within the adipose tissue, although some adipokines (primarily resistin and adiponectin) are secreted by the adipocytes [99]. Regardless of the specific cellular source of the adipokines, evidence indicates that crosstalk between adipose and vascular tissues exists and that exercise training can favorably influence cytokine production. Here, we summarize the recent advances in our understanding of the role of two adipokines, leptin and adiponectin, in signaling to the endothelium in CV disease as well as in exercise-induced vascular effects. Other adipokines such as tumor necrosis factor-alpha, resistin, interleukin-6, monocyte chemoattractant protein-1, etc. are also of great interest, as these molecules are implicated in the development and progression of atherosclerosis. However, owing to space limitations, we have opted to focus on adiponectin and leptin, as these adipokines have received substantial attention with respect to exercise as prevention and treatment of endothelial dysfunction and CV disease.
Leptin
Leptin was discovered as the product of the mouse obesity gene (Ob) that, when mutated, led to pronounced obesity and type 2 diabetes [100]. Later advances demonstrated that leptin is secreted from adipose tissue [101], is directly related to fat mass and adipocyte size [102], and has potent generalized cardiovascular effects [103]. Specifically, excess circulating leptin is linked to damage and dysfunction of the vascular endothelium through stimulation of neointimal growth and [104] and increased endothelial oxidant stress [105, 106]. Evidence supports the efficacy of regular exercise as one strategy to reduce pathophysiologic levels of adipose-derived circulating leptin, at which leptin has been experimentally demonstrated to impair NO-mediated vasodilation of coronary arterioles [107]. The most consistent circulating leptin-lowering effects of exercise have been observed with dietary co-interventions and/or when weight loss is achieved [108–113]. Several of these studies concluded that exercise without weight loss has no effect on circulating leptin concentrations [108, 111] and the effects of exercise on leptin levels are closely linked to changes in fat mass and/or fat cell size [112, 113]. Nevertheless, although recent studies have repeatedly supported this link between leptin levels and fat mass, it is important to note that two early studies indicated that exercise can affect leptin concentrations independent of changes in body weight or composition [114, 115]. Data from rodents [116–118] and humans [109, 110] indicate that the effects of exercise on adipose tissue leptin gene expression are closely associated with effects on plasma leptin, solidifying the role for leptin as an adipose-derived signal molecule for the vascular adaptation to exercise in obesity-associated CV complications.
Adiponectin
Adiponectin is secreted by adipocytes and is a potent anti-inflammatory and cardioprotective circulating factor. High levels of circulating adiponectin protect against disorders in glucose and insulin metabolism [119] and have beneficial effects on endothelial cells, including NO-mediated vasodilatory effects and inhibition of endothelial inflammatory responses [120, 121]. Low circulating adiponectin concentrations, thought to be the result of reduced adiponectin gene expression in inflamed adipose tissue [122], are also associated with increased circulating levels of soluble adhesion molecules, conduit artery endothelial dysfunction, and risk for CAD [123–125]. The current literature on whether exercise training increases circulating adiponectin is equivocal, as a systematic review on the effects of exercise on adiponectin levels in humans concluded that ~40% of randomized trials found increased adiponectin following exercise of varying intensities, durations, and frequencies [126]. Here again, a critically important distinguishing feature of studies showing positive results is that exercise accompanied by some degree of weight loss produces more consistent increases in circulating adiponectin [127–131]. In contrast, a number of studies have shown that exercise without weight loss does not alter adiponectin levels [132–137], with some notable exceptions [97, 138, 139]. Despite the inconsistent effects of exercise per se on adiponectin levels, it is possible that exercise may alter adiponectin signaling (and hence reduce expression of pro-inflammatory adipokines) through other means. For example, adiponectin receptor expression is increased in human and rodent skeletal muscle [140, 141] as well as human omental and subcutaneous adipose tissue depots [142] following exercise training. Additionally, exercise can acutely increase interstitial concentrations of adiponectin in adipose tissue [143]. Thus, although exercise training per se may not consistently increase circulating adiponectin concentrations, the possibilities arise that exercise may (i) favorably influence the local inflammatory environment of adipose tissue through increased adiponectin secretion and upregulation of receptors; and (ii) increase the sensitivity of peripheral tissues to adiponectin levels, both of which could have implications for beneficial exercise-induced endothelial adaptations.
4.3. Circulating Angiogenic Cells (CACs)
Historically, it was thought that blood vessels lacked intrinsic mechanisms to repair cellular damage that causes endothelial dysfunction, especially damage resulting from inflammatory and oxidative injury that precede atherosclerosis. However, it is now clear that there are several circulating cell types, termed circulating angiogenic cells (CACs), that contribute importantly to vascular endothelial maintenance and repair functions. This section briefly reviews the definitions and functions of CAC subpopulations and recent advances in the understanding of the effects of exercise on CAC number and function.
Definitions and Functions of CAC Types
CACs comprise a number of peripheral blood mononuclear cell (PBMC) subsets that perform a variety of functions in maintaining the endothelial lining of blood vessels. Current theory is that CACs support blood vessel maintenance in at least one of the following ways: by migration to sites of endothelial damage and incorporation into the endothelial monolayer, thus providing a ‘fresh’ endothelial cell to replace a damaged one; contributing to vasculogenesis/angiogenesis by incorporating into new vessels as they sprout/expand into tissues requiring increased perfusion; or via the release angiogenic growth factors and cytokines in a paracrine manner at sites of new vessel growth or endothelial damage, aiding in recruitment and proliferation of endothelial cells or other CACs. While an extensive discussion of these putative mechanisms is beyond the scope of the present review, current evidence favors paracrine/autocrine effects of CACs as the primary mechanism underlying their role in maintenance of a healthy endothelium. There are at least four PBMC subpopulations that can function as CACs under certain conditions: (i) endothelial progenitor cells [144], i.e. cells that co-express a stem/progenitor cell surface marker and an endothelial cell marker; (ii) circulating bone marrow-derived CD34+ progenitor cells [regardless of co-expression of an endothelial antigen [145]]; (iii) pro-angiogenic monocytes [146] and T-cells [147]; and (iv) circulating endothelial cells that originate from the vessel wall [148]. This section discusses the current evidence relative to the effects of endurance exercise on the number and functional properties of these CAC types in an integrative manner, and the generic term “CACs” is used for simplicity.
Effects of Exercise Training on CAC number and function
The first report of exercise training-induced effects on CACs indicated that mice with access to a running wheel had greater circulating levels of bone marrow-derived CACs than sedentary mice [149]. Importantly, these effects of exercise were not observed in mice treated with the NOS inhibitor L-NMMA or in eNOS knockout mice, indicating the necessity of NO signaling in exercise-induced CAC mobilization. This finding was supported recently by data from human subjects, as exercise-induce mobilization of bone marrow progenitor cells was inhibited by systemic L-NMMA infusion [150••]. There is evidence that reactive oxygen species-related homeostatic mechanisms are involved in the regulation of chronic exercise-induced changes in CAC number, as experimental knockdown of catalase activity in mice prevented increases in bone marrow CACs following a 3-wk period of physical activity [151]. Most investigations of the effects of exercise training on CAC numbers in humans have used CV disease patients as study participants and indicate that patients with CV risk factors or overt CV disease demonstrate increases in CAC number with regular exercise [149, 152–154], with the most robust and consistent response being observed in patients with exercise-induced limb ischemia. However, healthy patients can also increase CAC number following exercise training in the absence of ischemia, indicating that while limb ischemia may be a sufficient stimulus, it is not necessary for exercise induced mobilization of CACs [155]. Cross-sectional studies of healthy subjects have been less supportive of chronic exercise-induced effects on CAC numbers [156, 157], although there has been one report of higher CD34+ cells in trained than in sedentary older men [156].
Concentrations of CACs in peripheral blood are an important indicator of endogenous endothelial repair capacity [158, 159], but it is also critical to consider the functional capacity of CACs in examining their role as a putative exercise-induced signal for endothelial adaptation. Substantial data suggest that a number of functional aspects are impaired in the presence of CV disease or risk factors. For example, CACs from subjects with type 2 diabetes, coronary heart disease, and chronic limb ischemia display remarkable impairments in ex vivo reparative capacity and migratory action towards proangiogenic chemokines compared to healthy controls [160–162]. Furthermore, impairments in CAC functional capacity are closely associated with increased CV and metabolic risk [for recent reviews, see [163–165]]. Here again, it is becoming increasingly clear that exercise may serve as an effective strategy for the preservation of CAC function in health as well as for the reversal of CAC dysfunction in CV disease. For example, the pioneering study by Laufs et al. [149] indicated attenuated CAC apoptosis in mice following 28 d of exercise compared to sedentary control mice. In addition, Exercise training in healthy older men enhances the migration of CACs towards angiogenic growth factors [155], increases CAC-mediated formation of capillary-like structures ex vivo in ischemic and sub-ischemic patients with peripheral arterial occlusive disease [166], and increases capillary-like structure formation [167] and migratory capacity in patients with congestive heart failure [168].
Regarding the mechanistic underpinnings of these beneficial effects of regular exercise on CAC function, the available data suggest that exercise improves the redox status of CACs. Specifically, recent work has focused on the regulatory effects of exercise on intracellular NO and ROS dynamics, as several studies in the CAC field have suggested that these pathways are functionally important for CAC actions in a manner similar to that widely observed for vascular endothelial cells [145, 169, 170]. For example, it was recently shown that cultured CACs of exercise-trained young men had ~60% higher levels of basal intracellular NO concentrations than sedentary age and body mass index-matched men. Furthermore, experiments with the NADPH oxidase inhibitor apocynin indicated that ~50% of this difference was explained by increased NADPH oxidase activity and gene expression in CACs from the sedentary group [171•]. The findings that cultured CAC NADPH oxidase activity is reduced and intracellular NO levels are increased by regular endurance exercise were recently replicated in a longitudinal exercise training study in patients with the metabolic syndrome [172]. Similarly, freshly-isolated CD34+ CACs from sedentary individuals were demonstrated to have greater basal levels of intracellular NADPH oxidase-derived superoxide levels compared to endurance-trained subjects [173•]. However, it is important to note that the redox biology of CACs and endothelial cells present some important differences, as CD34+ CACs from these sedentary subjects [173•], surprisingly, also had higher intracellular NO levels. This was associated with higher expression of iNOS mRNA and lower expression of eNOS mRNA, suggesting that CD34+ cell iNOS expression may be upregulated in the sedentary state. Importantly, high levels of iNOS-derived NO are associated with cellular responses to inflammation and result in the production of more reactive deleterious reactive oxygen/nitrogen species such as peroxynitrite. Thus, it can be speculated that regular exercise is associated with a reduced state of cellular nitro-oxidative stress in CD34+ CACs, but the functional implications of this association, and whether the same association exists in other CAC types, requires further research.
Several questions remain regarding the functional importance of CACs as exercise-induced vascular signals. One particularly critical issue is that it is still unknown whether CACs aid directly or indirectly in exercise-induced angiogenesis of skeletal muscle. A recent report suggested for the first time that CD34+ CACs actually incorporated into the skeletal muscle vasculature following a period of exercise training in congestive heart failure patients [168]. The authors interpreted these data as evidence for CD34+ cell-mediated exercise-induced neovascularization. However, it is important to keep in mind that the CD34 antigen is expressed not only on circulating bone-marrow derived progenitor cells but also on microvascular endothelial cells [174, 175]. Thus, it is a possibility that the increased CD34+ protein expression in muscle samples following exercise training in these subjects simply reflected normal exercise-induced angiogenesis independent of CAC involvement. This blood vessel-mediated skeletal muscle angiogenesis is a well-documented phenomenon [176], and in fact the prevailing evidence indicates that most CAC types probably do not incorporate into growing vasculature but exert their proangiogenic effects through paracrine mechanisms [177, 178]. Thus, future research should (i) determine definitively whether CACs contribute to exercise-induced “neovascularization” of muscle, and (ii) if CACs are indeed found to contribute significantly to exercise-induced angiogenesis through incorporation into growing vasculature, then the magnitude of their contribution should be quantified.
5. Possible Interactions among Exercise-Induced Signals for Vascular Adaptations
The exercise-induced signals we discussed thus far could interact with each other in the regulation of endothelial phenotype and function. Rather than exhaustively discuss all possible permutations, we present this section as an overview of documented interactions between shear stress and the other factors discussed. This “shear centric” view stems from our long-standing interest in exercise-induced changes in shear stress as a primary driving force of exercise-induced endothelial adaptations [16], and the substantial evidence from the literature that local hemodynamic forces are key determinants of endothelial cell phenotype, even in the face of fluctuating conditions in the circulating environment [179].
5.1 Interaction between compression and shear signals
An important mechanism responsible for improvements in vascular function and phenotype following compressive therapy is likely related to the intermittent bouts of increased shear stress created with each inflation-deflation cycle of the pneumatic cuffs. In vitro support for shear as a mechanistic mediator of adaptations comes from an experiment in which human umbilical vein endothelial cells were exposed to compression and flow rates similar to those observed from external compression in veins, and it was demonstrated that flow is the major contributor to adaptations in NO production and eNOS protein expression [180]. At present, there is not a model available to study similar outcomes with arterial flow and pressure patterns and evaluating the effect of long term compression in arterioles is problematic. Development of such models would help tease out the causal molecular mechanisms involved in effects of compression per se and of compression-shear interactions on endothelial phenotype. For example, sequential inflation (~300 mm Hg) of the 3 pneumatic EECP cuffs from calf to buttocks during diastole produces a robust retrograde pressure wave in the central aorta that subsequently increases coronary artery perfusion pressure, analogous to intraaortic balloon counterpulsation [181]. Consequently, inflation of the EECP cuffs also produces an increase in retrograde blood flow in the femoral arteries and a simultaneous increase in antegrade flow in the brachial arteries [84, 182]. Using color Doppler imaging in a porcine EECP model, it has been reported that brachial artery wall shear stress increases by > 200% during hindlimb compression with EECP pneumatic cuffs [84]. In humans, during an acute bout of EECP antegrade endothelial shear stress in the brachial artery was increased by 75% and retrograde endothelial shear stress in the popliteal artery was increased by 402% [183].
Despite this large acute increase in retrograde shear, recent evidence from an EECP trial showed substantial increases in FMD of peripheral arteries [77••]. This finding generates an interesting hypothesis about the role of contraction-induced retrograde shear as a possible exercise-induced signal for endothelial adaptations. During exercise, the contraction-induced compression of intramuscular vessels causes a well-documented increase in retrograde flow [51]. In conduit arteries perfusing active muscles, the magnitude of change in the retrograde component depends on a number of factors such as force, velocity, and frequency of muscle contraction [184]. Thus, taken with the evidence discussed above indicating that retrograde shear is a potent pro-oxidant and pro-inflammatory stimulus, it is possible that acute increases in retrograde shear during exercise may provide a stimulus for endothelial adaptations in accordance with the concept of hormesis. Specifically, transient increases in inflammation and oxidant stress might trigger the upregulation of antioxidant and anti-inflammatory pathways as a protective adaptation which results in improved endothelial function following chronic repeated exposure. In support of this idea, the aforementioned EECP trial [77••] also found favorable changes in circulating markers of nitric oxide bioavailability (i.e., increased plasma nitrates and nitrites and reduced asymmetric dimethylarginine) and oxidant stress (reduced 8-isoprostane-PGF-F2α), indicating that muscle compression can alter systemic redox balance in association with increases in endothelial function. However, whether the acute increases in retrograde shear along the endothelium are responsible for this association should be the focus of future studies.
5.2 Interaction between shear and insulin
Some recent studies support the idea that exercise may preserve sensitivity of the endothelium to insulin’s vasodilatory effects. For example, the insulin sensitizing effects of endurance exercise training are linked to increases in insulin-stimulated bulk blood flow to human skeletal muscle [185••], consistent with previous data from rats showing that 14 days of exercise training improves insulin-induced capillary recruitment [186]. Surprisingly, the study of Rattigan et al. [186] is, to our knowledge, the only study to date that has examined the effects of exercise training on insulin-stimulated capillary recruitment. However, recent experiments from our group showed that regular physical activity increased insulin-induced vasodilation of isolated second order skeletal muscle arterioles in a rat model of type 2 diabetes [187••]. Thus, there is emerging evidence that exercise has beneficial effects on endothelial insulin sensitivity, but the mechanisms regulating increased sensitivity to insulin-induced capillary recruitment and arteriolar vasodilation remain to be determined. We propose that shear-induced signals to the endothelium may act to promote a more insulin-sensitive endothelial phenotype [17], which manifests itself in the form of improved insulin-stimulated blood flow to and glucose uptake by skeletal muscle.
5.3 Interaction between shear and CACs
There is support from several lines of evidence that shear stress may be a key physiological signal for the mobilization of some types of CACs, particularly endothelial progenitors, from their bone marrow niche. Endothelial cells within the bone marrow are subjected to increased shear during exercise as a result of increased blood flow, which does occur in some bones in an exercise duration- and partially intensity-dependent manner [188]. Additionally, eNOS knockout in mice [149] and systemic NOS inhibition by L-NMMA infusion in humans [150••] prevents exercise induced mobilization of bone marrow-derived CACs, suggesting a requirement of the eNOS pathway. Further, it is well-documented that NO activity is required for mobilization of bone marrow cells from a variety of stimuli [189]. Thus, we can postulate that exercise-induced increases in shear-induced NO production in the endothelium of bone marrow vasculature results in mobilization of progenitor/angiogenic cell to support systemic maintenance of the vascular endothelium.
6. Conclusions
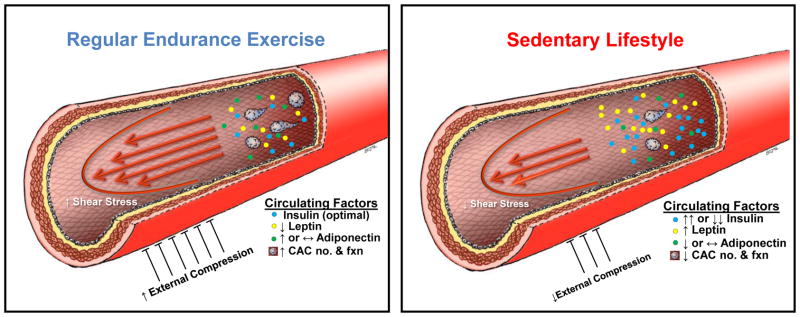
In summary, endothelial adaptations to endurance exercise appear to involve the independent and interactive effects of hemodynamic, external compression, and circulating factors, as summarized in Fig 3. We propose that these factors contribute to the proportion of CV risk reduction through regular exercise that cannot be explained by traditional risk factors. Future studies should focus not only on quantifying the extent to which these factors account for the risk reduction, but should also consider the possibility that the interactions among different signals may be more important than teasing out the roles of single factors in a reductionist fashion. [Figure 3 Here]
Figure 3.
Summary of hemodynamic signals, compressive forces, and circulating factors involved in exercise training-induced endothelial adaptations, illustrated by contrasting the effect of regular endurance exercise (high mean shear stress and external compression, optimal levels of insulin [see Fig 2], low leptin levels, increased adiponectin levels, and increased number and function of CACs; left side) vs. that of a sedentary lifestyle (for simplicity, depicted as opposite of regular endurance exercise; right side).
Acknowledgments
This work was supported by NIH T32AR048523 (to NTJ and JSM), NIH RO1HL036088 (to MHL) and AHA 11POST5080002 (to JP). We thank Stephanie Jenkins for assistance the illustration of Figure 3.
Footnotes
Disclosure: No conflicts of interest relevant to this article were reported.
References
- 1.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 2.Lees SJ, Booth FW. Sedentary death syndrome. Can J Appl Physiol. 2004;29:447. doi: 10.1139/h04-029. [DOI] [PubMed] [Google Scholar]
- 3.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical Activity and Reduced Risk of Cardiovascular Events: Potential Mediating Mechanisms. Circulation. 2007;116:2110–8. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green DJ, Walsh JH, Maiorana A, Best MJ, Taylor RR, O’Driscoll JG. Exercise-induced improvement in endothelial dysfunction is not mediated by changes in CV risk factors: pooled analysis of diverse patient populations. Am J Physiol Heart Circ Physiol. 2003;285:H2679–87. doi: 10.1152/ajpheart.00519.2003. [DOI] [PubMed] [Google Scholar]
- 5.Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol. 2009;587:5551–8. doi: 10.1113/jphysiol.2009.179432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimmeler S, Hermann C, Galle J, Zeiher AM. Upregulation of superoxide dismutase and nitric oxide synthase mediates the apoptosis-suppressive effects of shear stress on endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19:656–64. doi: 10.1161/01.atv.19.3.656. [DOI] [PubMed] [Google Scholar]
- 7.Widder JD, Chen W, Li L, Dikalov S, Thony B, Hatakeyama K, et al. Regulation of tetrahydrobiopterin biosynthesis by shear stress. Circ Res. 2007;101:830–8. doi: 10.1161/CIRCRESAHA.107.153809. [DOI] [PubMed] [Google Scholar]
- 8.Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, Alexander RW, et al. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol. 1995;269:C1371–8. doi: 10.1152/ajpcell.1995.269.6.C1371. [DOI] [PubMed] [Google Scholar]
- 9.Ranjan V, Xiao Z, Diamond SL. Constitutive NOS expression in cultured endothelial cells is elevated by fluid shear stress. Am J Physiol. 1995;269:H550–5. doi: 10.1152/ajpheart.1995.269.2.H550. [DOI] [PubMed] [Google Scholar]
- 10.Woodman CR, Muller JM, Rush JW, Laughlin MH, Price EM. Flow regulation of ecNOS and Cu/Zn SOD mRNA expression in porcine coronary arterioles. Am J Physiol. 1999;276:H1058–63. doi: 10.1152/ajpheart.1999.276.3.H1058. [DOI] [PubMed] [Google Scholar]
- 11.Woodman CR, Price EM, Laughlin MH. Shear stress induces eNOS mRNA expression and improves endothelium-dependent dilation in senescent soleus muscle feed arteries. J Appl Physiol. 2005;98:940–6. doi: 10.1152/japplphysiol.00408.2004. [DOI] [PubMed] [Google Scholar]
- 12.Tsao PS, Buitrago R, Chan JR, Cooke JP. Fluid flow inhibits endothelial adhesiveness. Nitric oxide and transcriptional regulation of VCAM-1. Circulation. 1996;94:1682–9. doi: 10.1161/01.cir.94.7.1682. [DOI] [PubMed] [Google Scholar]
- 13.Himburg HA, Dowd SE, Friedman MH. Frequency-dependent response of the vascular endothelium to pulsatile shear stress. Am J Physiol Heart Circ Physiol. 2007;293:H645–53. doi: 10.1152/ajpheart.01087.2006. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Friedman MH. Adaptive response of vascular endothelial cells to an acute increase in shear stress magnitude. Am J Physiol Heart Circ Physiol. 2012;302:H983–91. doi: 10.1152/ajpheart.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padilla J, Whyte JJ, Newcomer SC, Laughlin MH. Evaluating the impact of retrograde shear stress on expression of pro-inflammatory genes in rat carotid artery. Medicine & Science in Sports & Exercise. 2010;42:302. (Abstract) [Google Scholar]
- 16.Laughlin MH, McAllister RM. Exercise training-induced coronary vascular adaptation. J Appl Physiol. 1992;73:2209–25. doi: 10.1152/jappl.1992.73.6.2209. [DOI] [PubMed] [Google Scholar]
- 17.Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology (Bethesda) 2011;26:132–45. doi: 10.1152/physiol.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laughlin MH, Woodman CR, Schrage WG, Gute D, Price EM. Interval sprint training enhances endothelial function and eNOS content in some arteries that perfuse white gastrocnemius muscle. J Appl Physiol. 2004;96:233–44. doi: 10.1152/japplphysiol.00105.2003. [DOI] [PubMed] [Google Scholar]
- 19.McAllister RM, Jasperse JL, Laughlin MH. Nonuniform effects of endurance exercise training on vasodilation in rat skeletal muscle. J Appl Physiol. 2005;98:753–61. doi: 10.1152/japplphysiol.01263.2003. [DOI] [PubMed] [Google Scholar]
- 20.Lash JM. Exercise training enhances adrenergic constriction and dilation in the rat spinotrapezius muscle. J Appl Physiol. 1998;85:168–74. doi: 10.1152/jappl.1998.85.1.168. [DOI] [PubMed] [Google Scholar]
- 21.Linke A, Schoene N, Gielen S, Hofer J, Erbs S, Schuler G, et al. Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. J Am Coll Cardiol. 2001;37:392–7. doi: 10.1016/s0735-1097(00)01108-6. [DOI] [PubMed] [Google Scholar]
- 22.Padilla J, Simmons GH, Vianna LC, Davis MJ, Laughlin MH, Fadel PJ. Brachial artery vasodilatation during prolonged lower limb exercise: role of shear rate. Exp Physiol. 2011;96:1019–27. doi: 10.1113/expphysiol.2011.059584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmons GH, Padilla J, Young CN, Wong BJ, Lang JA, Davis MJ, et al. Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling: role of thermoregulatory vasodilation. J Appl Physiol. 2011;110:389–97. doi: 10.1152/japplphysiol.00936.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padilla J, Harris RA, Rink LD, Wallace JP. Characterization of the brachial artery shear stress following walking exercise. Vasc Med. 2008;13:105–11. doi: 10.1177/1358863x07086671. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka H, Shimizu S, Ohmori F, Muraoka Y, Kumagai M, Yoshizawa M, et al. Increases in blood flow and shear stress to nonworking limbs during incremental exercise. Med Sci Sports Exerc. 2006;38:81–5. doi: 10.1249/01.mss.0000191166.81789.de. [DOI] [PubMed] [Google Scholar]
- 26.Green DJ, Walsh JH, Maiorana A, Burke V, Taylor RR, O’Driscoll JG. Comparison of resistance and conduit vessel nitric oxide-mediated vascular function in vivo: effects of exercise training. J Appl Physiol. 2004;97:749–55. doi: 10.1152/japplphysiol.00109.2004. discussion 8. [DOI] [PubMed] [Google Scholar]
- 27.Green DJ. Exercise training as vascular medicine: direct impacts on the vasculature in humans. Exerc Sport Sci Rev. 2009;37:196–202. doi: 10.1097/JES.0b013e3181b7b6e3. [DOI] [PubMed] [Google Scholar]
- 28.Green DJ, Spence A, Halliwill JR, Cable NT, Thijssen DH. Exercise and vascular adaptation in asymptomatic humans. Exp Physiol. 2011;96:57–70. doi: 10.1113/expphysiol.2009.048694. [DOI] [PubMed] [Google Scholar]
- 29.Silber D, McLaughlin D, Sinoway L. Leg exercise conditioning increases peak forearm blood flow. J Appl Physiol. 1991;71:1568–73. doi: 10.1152/jappl.1991.71.4.1568. [DOI] [PubMed] [Google Scholar]
- 30••.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55:312–8. doi: 10.1161/HYPERTENSIONAHA.109.146282. By preventing the exercise-induced increase in shear in one arm but not the other during a bilateral handgrip training program, these results demonstrated that exercise-induced changes are required for the adaptation in flow-mediated dilation to exercise training in healthy humans. [DOI] [PubMed] [Google Scholar]
- 31••.Birk GK, Dawson EA, Atkinson C, Haynes A, Cable NT, Thijssen DH, et al. Brachial Artery Adaptation to Lower Limb Exercise Training: Role of Shear Stress. J Appl Physiol. 2012 doi: 10.1152/japplphysiol.01489.2011. in press. This study provides the first experimental evidence that lower limb cycle training induces a transient increase in upper limb vascular function in healthy young humans that is mediated via shear stress. [DOI] [PubMed] [Google Scholar]
- 32.Johnson BD, Mather KJ, Wallace JP. Mechanotransduction of shear in the endothelium: basic studies and clinical implications. Vasc Med. 2011;16:365–77. doi: 10.1177/1358863X11422109. [DOI] [PubMed] [Google Scholar]
- 33.O’Keeffe LM, Muir G, Piterina AV, McGloughlin T. Vascular cell adhesion molecule-1 expression in endothelial cells exposed to physiological coronary wall shear stresses. J Biomech Eng. 2009;131:081003. doi: 10.1115/1.3148191. [DOI] [PubMed] [Google Scholar]
- 34.Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components: low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol. 2010;298:H367–74. doi: 10.1152/ajpheart.00565.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, et al. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101:14871–6. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takabe W, Jen N, Ai L, Hamilton R, Wang S, Holmes K, et al. Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling. Antioxid Redox Signal. 2011;15:1379–88. doi: 10.1089/ars.2010.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willett NJ, Kundu K, Knight SF, Dikalov S, Murthy N, Taylor WR. Redox signaling in an in vivo murine model of low magnitude oscillatory wall shear stress. Antioxid Redox Signal. 2011;15:1369–78. doi: 10.1089/ars.2010.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, et al. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res. 2003;93:1225–32. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, et al. Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003;278:47291–8. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 40••.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension. 2009;53:986–92. doi: 10.1161/HYPERTENSIONAHA.109.131508. These authors demonstrated that retrograde shear rate dose-dependently reduces flow-mediated dilation. These in vivo human data corroborate a substantial body of in vitro research demonstrating that retrograde and oscillatory shear stress are deleterious signals to the endothelium. [DOI] [PubMed] [Google Scholar]
- 41••.Tinken TM, Thijssen DH, Hopkins N, Black MA, Dawson EA, Minson CT, et al. Impact of shear rate modulation on vascular function in humans. Hypertension. 2009;54:278–85. doi: 10.1161/HYPERTENSIONAHA.109.134361. This study demonstrated that experimentally blocking the shear rate stimulus induced by forearm heating, handgrip exercise, and recumbent cycling prevents the acute augmentation of flow-mediated dilation induced by these maneuvers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Padilla J, Sheldon RD, Sitar DM, Newcomer SC. Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: a limb-specific response. Am J Physiol Heart Circ Physiol. 2009;297:H1103–8. doi: 10.1152/ajpheart.00167.2009. In this study, we employed an arm hanging model in this experiment to mimic hemodynamic milieu present in the lower limbs during the seated position. Because making the arm “look like” the leg resulted in blunted endothelium-dependent vasodilation at the brachial artery, this study suggested that, when a “healthy” vessel is abruptly exposed to proatherogenic shear rate patterns, the endothelium becomes compromised. [DOI] [PubMed] [Google Scholar]
- 43•.Young CN, Deo SH, Padilla J, Laughlin MH, Fadel PJ. Pro-atherogenic shear rate patterns in the femoral artery of healthy older adults. Atherosclerosis. 2010;211:390–2. doi: 10.1016/j.atherosclerosis.2010.03.017. In this study our laboratory observed reduced mean and antegrade shear and increased retrograde and oscillatory shear in femoral arteries at rest in older compared to younger adults, suggesting that altered hemodynamic patterns may contribute to the age-associated increase in risk of atherosclerosis in lower limb conduit vessels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Padilla J, Simmons GH, Fadel PJ, Laughlin MH, Joyner MJ, Casey DP. Impact of aging on conduit artery retrograde and oscillatory shear at rest and during exercise: role of nitric oxide. Hypertension. 2011;57:484–9. doi: 10.1161/HYPERTENSIONAHA.110.165365. This study demonstrated that reduced NO bioavailability in the resistance vessels contributes to age-related discrepancies in resting shear patterns, potentially identifying a mechanism for increased risk of atherosclerotic disease in conduit arteries. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Credeur DP, Dobrosielski DA, Arce-Esquivel AA, Welsch MA. Brachial artery retrograde flow increases with age: relationship to physical function. Eur J Appl Physiol. 2009;107:219–25. doi: 10.1007/s00421-009-1117-3. This study was the first in the literature to provide data indicating that aging is associated with increases in conduit artery retrograde flow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu SP, Ringgaard S, Oyre S, Hansen MS, Rasmus S, Pedersen EM. Wall shear rates differ between the normal carotid, femoral, and brachial arteries: an in vivo MRI study. J Magn Reson Imaging. 2004;19:188–93. doi: 10.1002/jmri.10441. [DOI] [PubMed] [Google Scholar]
- 47.Newcomer SC, Sauder CL, Kuipers NT, Laughlin MH, Ray CA. Effects of posture on shear rates in human brachial and superficial femoral arteries. Am J Physiol Heart Circ Physiol. 2008;294:H1833–9. doi: 10.1152/ajpheart.01108.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sejersted OM, Hargens AR, Kardel KR, Blom P, Jensen O, Hermansen L. Intramuscular fluid pressure during isometric contraction of human skeletal muscle. J Appl Physiol. 1984;56:287–95. doi: 10.1152/jappl.1984.56.2.287. [DOI] [PubMed] [Google Scholar]
- 49.Hirche H, Grun D, Waller W. Utilization of carbohydrates and free fatty acids by the gastrocnemius of the dog during long lasting rhythmical exercise. Pflugers Arch. 1970;321:121–32. doi: 10.1007/BF00586367. [DOI] [PubMed] [Google Scholar]
- 50.Radegran G, Saltin B. Muscle blood flow at onset of dynamic exercise in humans. Am J Physiol. 1998;274:H314–22. doi: 10.1152/ajpheart.1998.274.1.H314. [DOI] [PubMed] [Google Scholar]
- 51.Barcroft H, Dornhorst AC. The blood flow through the human calf during rhythmic exercise. J Physiol. 1949;109:402–11. doi: 10.1113/jphysiol.1949.sp004403. pl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson PC. Autoregulation of blood flow. Circ Res. 1986;59:483–95. doi: 10.1161/01.res.59.5.483. [DOI] [PubMed] [Google Scholar]
- 53.Meininger GA, Davis MJ. Cellular mechanisms involved in the vascular myogenic response. Am J Physiol. 1992;263:H647–59. doi: 10.1152/ajpheart.1992.263.3.H647. [DOI] [PubMed] [Google Scholar]
- 54.Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol. 2006;572:561–7. doi: 10.1113/jphysiol.2005.099507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hill MA, Davis MJ, Meininger GA, Potocnik SJ, Murphy TV. Arteriolar myogenic signalling mechanisms: Implications for local vascular function. Clin Hemorheol Microcirc. 2006;34:67–79. [PubMed] [Google Scholar]
- 56.Clifford PS. Local control of blood flow. Adv Physiol Educ. 2011;35:5–15. doi: 10.1152/advan.00074.2010. [DOI] [PubMed] [Google Scholar]
- 57.Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol. 2007;583:861–74. doi: 10.1113/jphysiol.2007.131250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heffernan KS, Edwards DG, Rossow L, Jae SY, Fernhall B. External mechanical compression reduces regional arterial stiffness. Eur J Appl Physiol. 2007;101:735–41. doi: 10.1007/s00421-007-0550-4. [DOI] [PubMed] [Google Scholar]
- 59.Crenshaw AGHA, Gershuni DH, Rydevik B. Wide tourniquet cuffs more effective at lower inflation pressures. Acta Orthop Scand. 1988;59:447–51. doi: 10.3109/17453678809149401. [DOI] [PubMed] [Google Scholar]
- 60.Roseguini BT, Mehmet Soylu S, Whyte JJ, Yang HT, Newcomer S, Laughlin MH. Intermittent pneumatic leg compressions acutely upregulate VEGF and MCP-1 expression in skeletal muscle. Am J Physiol Heart Circ Physiol. 2010;298:H1991–2000. doi: 10.1152/ajpheart.00006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barsness GW. Enhanced External Counterpulsation in Unrevascularizable Patients. Current interventional cardiology reports. 2001;3:37–43. [PubMed] [Google Scholar]
- 62.Arora RR, Chou TM, Jain D, Fleishman B, Crawford L, McKiernan T, et al. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol. 1999;33:1833–40. doi: 10.1016/s0735-1097(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 63.Lawson WE, Hui JC, Zheng ZS, Burgen L, Jiang L, Lillis O, et al. Improved exercise tolerance following enhanced external counterpulsation: cardiac or peripheral effect? Cardiology. 1996;87:271–5. doi: 10.1159/000177103. [DOI] [PubMed] [Google Scholar]
- 64.Lawson WE, Hui JC, Lang G. Treatment benefit in the enhanced external counterpulsation consortium. Cardiology. 2000;94:31–5. doi: 10.1159/000007043. [DOI] [PubMed] [Google Scholar]
- 65.Braith RWCD, Beck DT, Nichols WW, Choi CY, Kuddus MA, Conti CR. Peripheral endothelial function is a primary clinical target for enhanced external counterpulsation in patients with refractory angina. J Am Coll Cardiol. 2009;53:A300. [Google Scholar]
- 66.Urano H, Ikeda H, Ueno T, Matsumoto T, Murohara T, Imaizumi T. Enhanced external counterpulsation improves exercise tolerance, reduces exercise-induced myocardial ischemia and improves left ventricular diastolic filling in patients with coronary artery disease. J Am Coll Cardiol. 2001;37:93–9. doi: 10.1016/s0735-1097(00)01095-0. [DOI] [PubMed] [Google Scholar]
- 67.Michaels AD, Barsness GW, Soran O, Kelsey SF, Kennard ED, Hui JC, et al. Frequency and efficacy of repeat enhanced external counterpulsation for stable angina pectoris (from the International EECP Patient Registry) Am J Cardiol. 2005;95:394–7. doi: 10.1016/j.amjcard.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 68.Stys T, Lawson WE, Hui JC, Lang G, Liuzzo J, Cohn PF. Acute hemodynamic effects and angina improvement with enhanced external counterpulsation. Angiology. 2001;52:653–8. doi: 10.1177/000331970105201001. [DOI] [PubMed] [Google Scholar]
- 69.Lawson WE, Hui JC, Kennard ED, Kelsey SF, Michaels AD, Soran O. Two-year outcomes in patients with mild refractory angina treated with enhanced external counterpulsation. Clinical cardiology. 2006;29:69–73. doi: 10.1002/clc.4960290207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lawson WE, Hui JC, Zheng ZS, Oster Z, Katz JP, Diggs P, et al. Three-year sustained benefit from enhanced external counterpulsation in chronic angina pectoris. Am J Cardiol. 1995;75:840–1. doi: 10.1016/s0002-9149(99)80427-5. [DOI] [PubMed] [Google Scholar]
- 71.Michaels AD, Linnemeier G, Soran O, Kelsey SF, Kennard ED. Two-year outcomes after enhanced external counterpulsation for stable angina pectoris (from the International EECP Patient Registry [IEPR]) Am J Cardiol. 2004;93:461–4. doi: 10.1016/j.amjcard.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 72.Celik T, Iyisoy A, Yuksel UC, Amasyali B. A new treatment modality in management of patients with cardiac syndrome X: Enhanced external counterpulsation. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.11.159. [DOI] [PubMed] [Google Scholar]
- 73.Masuda D, Nohara R, Hirai T, Kataoka K, Chen LG, Hosokawa R, et al. Enhanced external counterpulsation improved myocardial perfusion and coronary flow reserve in patients with chronic stable angina; evaluation by(13)N-ammonia positron emission tomography. Eur Heart J. 2001;22:1451–8. doi: 10.1053/euhj.2000.2545. [DOI] [PubMed] [Google Scholar]
- 74.Stys TP, Lawson WE, Hui JC, Fleishman B, Manzo K, Strobeck JE, et al. Effects of enhanced external counterpulsation on stress radionuclide coronary perfusion and exercise capacity in chronic stable angina pectoris. Am J Cardiol. 2002;89:822–4. doi: 10.1016/s0002-9149(02)02191-4. [DOI] [PubMed] [Google Scholar]
- 75.Braith RW, Conti CR, Nichols WW, Choi CY, Khuddus MA, Beck DT, et al. Enhanced external counterpulsation improves peripheral artery flow-mediated dilation in patients with chronic angina: a randomized sham-controlled study. Circulation. 2010;122:1612–20. doi: 10.1161/CIRCULATIONAHA.109.923482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akhtar M, Wu GF, Du ZM, Zheng ZS, Michaels AD. Effect of external counterpulsation on plasma nitric oxide and endothelin-1 levels. Am J Cardiol. 2006;98:28–30. doi: 10.1016/j.amjcard.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 77••.Martin JS, Beck DT, Aranda JM, Jr, Braith RW. Enhanced external counterpulsation improves peripheral artery function and glucose tolerance in subjects with abnormal glucose tolerance. J Appl Physiol. 2012;112:868–76. doi: 10.1152/japplphysiol.01336.2011. This study found beneficial effects of enhanced external counterpulation (EECP) compressive therapy on peripheral arterial function and glucose tolerance in subjects with abnormal glucose tolerance. [DOI] [PubMed] [Google Scholar]
- 78.Casey DP, Conti CR, Nichols WW, Choi CY, Khuddus MA, Braith RW. Effect of enhanced external counterpulsation on inflammatory cytokines and adhesion molecules in patients with angina pectoris and angiographic coronary artery disease. Am J Cardiol. 2008;101:300–2. doi: 10.1016/j.amjcard.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hashemi M, Hoseinbalam M, Khazaei M. Long-term effect of enhanced external counterpulsation on endothelial function in the patients with intractable angina. Heart Lung Circ. 2008;17:383–7. doi: 10.1016/j.hlc.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 80.Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41:1761–8. doi: 10.1016/s0735-1097(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 81.Casey DP, Beck DT, Nichols WW, Conti CR, Choi CY, Khuddus MA, et al. Effects of enhanced external counterpulsation on arterial stiffness and myocardial oxygen demand in patients with chronic angina pectoris. Am J Cardiol. 2011;107:1466–72. doi: 10.1016/j.amjcard.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dockery F, Rajkumar C, Bulpitt CJ, Hall RJ, Bagger JP. Enhanced external counterpulsation does not alter arterial stiffness in patients with angina. Clinical cardiology. 2004;27:689–92. doi: 10.1002/clc.4960271206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tao J, Tu C, Yang Z, Zhang Y, Chung XL, Ma H, et al. Enhanced external counterpulsation improves endothelium-dependent vasorelaxation in the carotid arteries of hypercholesterolemic pigs. Int J Cardiol. 2006;112:269–74. doi: 10.1016/j.ijcard.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, He X, Chen X, Ma H, Liu D, Luo J, et al. Enhanced external counterpulsation inhibits intimal hyperplasia by modifying shear stress responsive gene expression in hypercholesterolemic pigs. Circulation. 2007;116:526–34. doi: 10.1161/CIRCULATIONAHA.106.647248. [DOI] [PubMed] [Google Scholar]
- 85.Tan X, Qi WN, Gu X, Urbaniak JR, Chen LE. Intermittent pneumatic compression regulates expression of nitric oxide synthases in skeletal muscles. Journal of biomechanics. 2006;39:2430–7. doi: 10.1016/j.jbiomech.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 86.Roseguini BT, Arce-Esquivel AA, Newcomer SC, Yang HT, Terjung RL, Laughlin MH. Intermittent pneumatic leg compressions enhance muscle performance and blood flow in a model of peripheral arterial insufficiency. J Appl Physiol. 2012 doi: 10.1152/japplphysiol.01337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, et al. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab. 2003;284:E241–58. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- 88.Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13:294–307. doi: 10.1016/j.cmet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 89.Baron AD, Laakso M, Brechtel G, Hoit B, Watt C, Edelman SV. Reduced postprandial skeletal muscle blood flow contributes to glucose intolerance in human obesity. J Clin Endocrinol Metab. 1990;70:1525–33. doi: 10.1210/jcem-70-6-1525. [DOI] [PubMed] [Google Scholar]
- 90.Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990;85:1844–52. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clark MG. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab. 2008;295:E732–50. doi: 10.1152/ajpendo.90477.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Han KA, Patel Y, Lteif AA, Chisholm R, Mather KJ. Contributions of dysglycaemia, obesity, and insulin resistance to impaired endothelium-dependent vasodilation in humans. Diabetes Metab Res Rev. 2011;27:354–61. doi: 10.1002/dmrr.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Madonna R, De Caterina R. Prolonged exposure to high insulin impairs the endothelial PI3-kinase/Akt/nitric oxide signalling. Thromb Haemost. 2009;101:345–50. [PubMed] [Google Scholar]
- 94.Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiol. 1999;83:25F–9F. doi: 10.1016/s0002-9149(99)00211-8. [DOI] [PubMed] [Google Scholar]
- 95.Ferri C, Bellini C, Desideri G, De Mattia G, Santucci A. Endogenous insulin modulates circulating endothelin-1 concentrations in humans. Diabetes Care. 1996;19:504–6. doi: 10.2337/diacare.19.5.504. [DOI] [PubMed] [Google Scholar]
- 96.Holloszy JO. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol. 2005;99:338–43. doi: 10.1152/japplphysiol.00123.2005. [DOI] [PubMed] [Google Scholar]
- 97.Kelly KR, Blaszczak A, Haus JM, Patrick-Melin A, Fealy CE, Solomon TP, et al. A 7-d Exercise Program Increases High-Molecular Weight Adiponectin in Obese Adults. Med Sci Sports Exerc. 2012;44:69–74. doi: 10.1249/MSS.0b013e318228bf85. [DOI] [PubMed] [Google Scholar]
- 98.Rogers MA, Yamamoto C, King DS, Hagberg JM, Ehsani AA, Holloszy JO. Improvement in glucose tolerance after 1 wk of exercise in patients with mild NIDDM. Diabetes Care. 1988;11:613–8. doi: 10.2337/diacare.11.8.613. [DOI] [PubMed] [Google Scholar]
- 99.Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators Inflamm. 2010;2010:513948. doi: 10.1155/2010/513948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 101.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–8. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 102.Lee MJ, Fried SK. Integration of hormonal and nutrient signals that regulate leptin synthesis and secretion. Am J Physiol Endocrinol Metab. 2009;296:E1230–8. doi: 10.1152/ajpendo.90927.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Knudson JD, Payne GA, Borbouse L, Tune JD. Leptin and mechanisms of endothelial dysfunction and cardiovascular disease. Curr Hypertens Rep. 2008;10:434–9. doi: 10.1007/s11906-008-0082-2. [DOI] [PubMed] [Google Scholar]
- 104.Bodary PF, Shen Y, Ohman M, Bahrou KL, Vargas FB, Cudney SS, et al. Leptin regulates neointima formation after arterial injury through mechanisms independent of blood pressure and the leptin receptor/STAT3 signaling pathways involved in energy balance. Arterioscler Thromb Vasc Biol. 2007;27:70–6. doi: 10.1161/01.ATV.0000252068.89775.ee. [DOI] [PubMed] [Google Scholar]
- 105.Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276:25096–100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 106.Beltowski J, Wojcicka G, Marciniak A, Jamroz A. Oxidative stress, nitric oxide production, and renal sodium handling in leptin-induced hypertension. Life Sci. 2004;74:2987–3000. doi: 10.1016/j.lfs.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 107.Knudson JD, Dincer UD, Zhang C, Swafford AN, Jr, Koshida R, Picchi A, et al. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H48–56. doi: 10.1152/ajpheart.01159.2004. [DOI] [PubMed] [Google Scholar]
- 108.Lowndes J, Zoeller RF, Caplan JD, Kyriazis GA, Moyna NM, Seip RL, et al. Leptin responses to long-term cardiorespiratory exercise training without concomitant weight loss: a prospective study. J Sports Med Phys Fitness. 2008;48:391–7. [PubMed] [Google Scholar]
- 109.Kraemer RR, Kraemer GR, Acevedo EO, Hebert EP, Temple E, Bates M, et al. Effects of aerobic exercise on serum leptin levels in obese women. Eur J Appl Physiol Occup Physiol. 1999;80:154–8. doi: 10.1007/s004210050572. [DOI] [PubMed] [Google Scholar]
- 110.Polak J, Klimcakova E, Moro C, Viguerie N, Berlan M, Hejnova J, et al. Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor alpha in obese women. Metabolism. 2006;55:1375–81. doi: 10.1016/j.metabol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 111.Thong FS, Hudson R, Ross R, Janssen I, Graham TE. Plasma leptin in moderately obese men: independent effects of weight loss and aerobic exercise. Am J Physiol Endocrinol Metab. 2000;279:E307–13. doi: 10.1152/ajpendo.2000.279.2.E307. [DOI] [PubMed] [Google Scholar]
- 112.Perusse L, Collier G, Gagnon J, Leon AS, Rao DC, Skinner JS, et al. Acute and chronic effects of exercise on leptin levels in humans. J Appl Physiol. 1997;83:5–10. doi: 10.1152/jappl.1997.83.1.5. [DOI] [PubMed] [Google Scholar]
- 113.Kohrt WM, Landt M, Birge SJ., Jr Serum leptin levels are reduced in response to exercise training, but not hormone replacement therapy, in older women. J Clin Endocrinol Metab. 1996;81:3980–5. doi: 10.1210/jcem.81.11.8923847. [DOI] [PubMed] [Google Scholar]
- 114.Pasman WJ, Westerterp-Plantenga MS, Saris WH. The effect of exercise training on leptin levels in obese males. Am J Physiol. 1998;274:E280–6. doi: 10.1152/ajpendo.1998.274.2.E280. [DOI] [PubMed] [Google Scholar]
- 115.Hickey MS, Houmard JA, Considine RV, Tyndall GL, Midgette JB, Gavigan KE, et al. Gender-dependent effects of exercise training on serum leptin levels in humans. Am J Physiol. 1997;272:E562–6. doi: 10.1152/ajpendo.1997.272.4.E562. [DOI] [PubMed] [Google Scholar]
- 116.Miyazaki S, Izawa T, Ogasawara JE, Sakurai T, Nomura S, Kizaki T, et al. Effect of exercise training on adipocyte-size-dependent expression of leptin and adiponectin. Life Sci. 2010;86:691–8. doi: 10.1016/j.lfs.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 117.Zachwieja JJ, Hendry SL, Smith SR, Harris RB. Voluntary wheel running decreases adipose tissue mass and expression of leptin mRNA in Osborne-Mendel rats. Diabetes. 1997;46:1159–66. doi: 10.2337/diab.46.7.1159. [DOI] [PubMed] [Google Scholar]
- 118.Laye MJ, Rector RS, Warner SO, Naples SP, Perretta AL, Uptergrove GM, et al. Changes in visceral adipose tissue mitochondrial content with type 2 diabetes and daily voluntary wheel running in OLETF rats. J Physiol. 2009;587:3729–39. doi: 10.1113/jphysiol.2009.172601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–8. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- 120.Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, et al. Adiponectin is a novel humoral vasodilator. Cardiovasc Res. 2007;75:719–27. doi: 10.1016/j.cardiores.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 121.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–6. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 122.You T, Yang R, Lyles MF, Gong D, Nicklas BJ. Abdominal adipose tissue cytokine gene expression: relationship to obesity and metabolic risk factors. Am J Physiol Endocrinol Metab. 2005;288:E741–7. doi: 10.1152/ajpendo.00419.2004. [DOI] [PubMed] [Google Scholar]
- 123.Fargnoli JL, Sun Q, Olenczuk D, Qi L, Zhu Y, Hu FB, et al. Resistin is associated with biomarkers of inflammation while total and high-molecular weight adiponectin are associated with biomarkers of inflammation, insulin resistance, and endothelial function. Eur J Endocrinol. 2010;162:281–8. doi: 10.1530/EJE-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–6. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 125.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–9. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 126.Simpson KA, Singh MA. Effects of exercise on adiponectin: a systematic review. Obesity (Silver Spring) 2008;16:241–56. doi: 10.1038/oby.2007.53. [DOI] [PubMed] [Google Scholar]
- 127.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290:E961–7. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 128.Ring-Dimitriou S, Paulweber B, von Duvillard SP, Stadlmann M, LeMura LM, Lang J, et al. The effect of physical activity and physical fitness on plasma adiponectin in adults with predisposition to metabolic syndrome. Eur J Appl Physiol. 2006;98:472–81. doi: 10.1007/s00421-006-0291-9. [DOI] [PubMed] [Google Scholar]
- 129.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–42. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Monzillo LU, Hamdy O, Horton ES, Ledbury S, Mullooly C, Jarema C, et al. Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res. 2003;11:1048–54. doi: 10.1038/oby.2003.144. [DOI] [PubMed] [Google Scholar]
- 131.Kondo T, Kobayashi I, Murakami M. Effect of exercise on circulating adipokine levels in obese young women. Endocr J. 2006;53:189–95. doi: 10.1507/endocrj.53.189. [DOI] [PubMed] [Google Scholar]
- 132.Jacobs DR, Jr, Sluik D, Rokling-Andersen MH, Anderssen SA, Drevon CA. Association of 1-y changes in diet pattern with cardiovascular disease risk factors and adipokines: results from the 1-y randomized Oslo Diet and Exercise Study. Am J Clin Nutr. 2009;89:509–17. doi: 10.3945/ajcn.2008.26371. [DOI] [PubMed] [Google Scholar]
- 133.Kelly AS, Steinberger J, Olson TP, Dengel DR. In the absence of weight loss, exercise training does not improve adipokines or oxidative stress in overweight children. Metabolism. 2007;56:1005–9. doi: 10.1016/j.metabol.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 134.Hulver MW, Zheng D, Tanner CJ, Houmard JA, Kraus WE, Slentz CA, et al. Adiponectin is not altered with exercise training despite enhanced insulin action. Am J Physiol Endocrinol Metab. 2002;283:E861–5. doi: 10.1152/ajpendo.00150.2002. [DOI] [PubMed] [Google Scholar]
- 135.Ryan AS, Nicklas BJ, Berman DM, Elahi D. Adiponectin levels do not change with moderate dietary induced weight loss and exercise in obese postmenopausal women. Int J Obes Relat Metab Disord. 2003;27:1066–71. doi: 10.1038/sj.ijo.0802387. [DOI] [PubMed] [Google Scholar]
- 136.Marcell TJ, McAuley KA, Traustadottir T, Reaven PD. Exercise training is not associated with improved levels of C-reactive protein or adiponectin. Metabolism. 2005;54:533–41. doi: 10.1016/j.metabol.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 137.Hara T, Fujiwara H, Nakao H, Mimura T, Yoshikawa T, Fujimoto S. Body composition is related to increase in plasma adiponectin levels rather than training in young obese men. Eur J Appl Physiol. 2005;94:520–6. doi: 10.1007/s00421-005-1374-8. [DOI] [PubMed] [Google Scholar]
- 138.Lim S, Choi SH, Jeong IK, Kim JH, Moon MK, Park KS, et al. Insulin-sensitizing effects of exercise on adiponectin and retinol-binding protein-4 concentrations in young and middle-aged women. J Clin Endocrinol Metab. 2008;93:2263–8. doi: 10.1210/jc.2007-2028. [DOI] [PubMed] [Google Scholar]
- 139.Balducci S, Zanuso S, Nicolucci A, Fernando F, Cavallo S, Cardelli P, et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis. 2010;20:608–17. doi: 10.1016/j.numecd.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 140.Zeng Q, Isobe K, Fu L, Ohkoshi N, Ohmori H, Takekoshi K, et al. Effects of exercise on adiponectin and adiponectin receptor levels in rats. Life Sci. 2007;80:454–9. doi: 10.1016/j.lfs.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 141.Van Berendoncks AM, Garnier A, Beckers P, Hoymans VY, Possemiers N, Fortin D, et al. Exercise training reverses adiponectin resistance in skeletal muscle of patients with chronic heart failure. Heart. 2011;97:1403–9. doi: 10.1136/hrt.2011.226373. [DOI] [PubMed] [Google Scholar]
- 142.Bluher M, Williams CJ, Kloting N, Hsi A, Ruschke K, Oberbach A, et al. Gene expression of adiponectin receptors in human visceral and subcutaneous adipose tissue is related to insulin resistance and metabolic parameters and is altered in response to physical training. Diabetes Care. 2007;30:3110–5. doi: 10.2337/dc07-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hojbjerre L, Rosenzweig M, Dela F, Bruun JM, Stallknecht B. Acute exercise increases adipose tissue interstitial adiponectin concentration in healthy overweight and lean subjects. Eur J Endocrinol. 2007;157:613–23. doi: 10.1530/EJE-07-0213. [DOI] [PubMed] [Google Scholar]
- 144.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science. 1997;275:964–6. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 145.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. The Journal of Clinical Investigation. 2001;108:391–7. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Harraz M, Jiao C, Hanlon HD, Hartley RS, Schatteman GC. CD34- blood-derived human endothelial cell progenitors. Stem Cells. 2001;19:304–12. doi: 10.1634/stemcells.19-4-304. [DOI] [PubMed] [Google Scholar]
- 147.Kushner EJ, MacEneaney OJ, Morgan RG, Van Engelenburg AM, Van Guilder GP, DeSouza CA. CD31+ T cells represent a functionally distinct vascular T cell phenotype. Blood Cells Mol Dis. 2010;44:74–8. doi: 10.1016/j.bcmd.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 148.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–6. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 149.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, et al. Physical Training Increases Endothelial Progenitor Cells, Inhibits Neointima Formation, and Enhances Angiogenesis. Circulation. 2004;109:220–6. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 150••.Cubbon RM, Murgatroyd SR, Ferguson C, Bowen TS, Rakobowchuk M, Baliga V, et al. Human exercise-induced circulating progenitor cell mobilization is nitric oxide-dependent and is blunted in South Asian men. Arterioscler Thromb Vasc Biol. 2010;30:878–84. doi: 10.1161/ATVBAHA.109.201012. Exercise-induced increases in bone marrow-derived circulating angiogenic cells were completely prevented by systemic infusion of L-NMMA, indicating that NO signaling is necessary for mobilization of these vasoprotective cells from the bone marrow. [DOI] [PubMed] [Google Scholar]
- 151.Suvorava T, Kumpf S, Rauch BH, Dao VT, Adams V, Kojda G. Hydrogen peroxide inhibits exercise-induced increase of circulating stem cells with endothelial progenitor capacity. Free Radic Res. 2010;44:199–207. doi: 10.3109/10715760903402898. [DOI] [PubMed] [Google Scholar]
- 152.Schlager O, Giurgea A, Schuhfried O, Seidinger D, Hammer A, Groger M, et al. Exercise training increases endothelial progenitor cells and decreases asymmetric dimethylarginine in peripheral arterial disease: A randomized controlled trial. Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 153.Adams V, Lenk K, Linke A, Lenz D, Erbs S, Sandri M, et al. Increase of Circulating Endothelial Progenitor Cells in Patients with Coronary Artery Disease After Exercise-Induced Ischemia. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:684–90. doi: 10.1161/01.ATV.0000124104.23702.a0. [DOI] [PubMed] [Google Scholar]
- 154.Sarto P, Balducci E, Balconi G, Fiordaliso F, Merlo L, Tuzzato G, et al. Effects of exercise training on endothelial progenitor cells in patients with chronic heart failure. J Card Fail. 2007;13:701–8. doi: 10.1016/j.cardfail.2007.06.722. [DOI] [PubMed] [Google Scholar]
- 155.Hoetzer GL, Van Guilder GP, Irmiger HM, Keith RS, Stauffer BL, DeSouza CA. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. Journal of Applied Physiology. 2007;102:847–52. doi: 10.1152/japplphysiol.01183.2006. [DOI] [PubMed] [Google Scholar]
- 156.Thijssen DHJ, Vos JB, Verseyden C, van Zonneveld AJ, Smits P, Sweep FCGJ, et al. Haematopoietic stem cells and endothelial progenitor cells in healthy men: effect of aging and training. Aging Cell. 2006;5:495–503. doi: 10.1111/j.1474-9726.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- 157.Witkowski S, Lockard MM, Jenkins NT, Obisesan TO, Spangenburg EE, Hagberg JM. Relationship between circulating progenitor cells, vascular function and oxidative stress with long-term training and short-term detraining in older men. Clin Sci (Lond) 2010;118:303–11. doi: 10.1042/CS20090253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating Endothelial Progenitor Cells and Cardiovascular Outcomes. The New England Journal Of Medicine. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 159.Fadini GP, de Kreutzenberg SV, Coracina A, Baesso I, Agostini C, Tiengo A, et al. Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. European Heart Journal. 2006;27:2247–55. doi: 10.1093/eurheartj/ehl198. [DOI] [PubMed] [Google Scholar]
- 160.Caballero S, Sengupta N, Afzal A, Chang KH, Li Calzi S, Guberski DL, et al. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes. 2007;56:960–7. doi: 10.2337/db06-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circulation Research. 2001;89:e1–e7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 162.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–22. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 163.Bautch VL. Stem cells and the vasculature. Nat Med. 2011;17:1437–43. doi: 10.1038/nm.2539. [DOI] [PubMed] [Google Scholar]
- 164.Dimmeler S. Regulation of bone marrow-derived vascular progenitor cell mobilization and maintenance. Arterioscler Thromb Vasc Biol. 2010;30:1088–93. doi: 10.1161/ATVBAHA.109.191668. [DOI] [PubMed] [Google Scholar]
- 165.Witkowski S, Jenkins NT, Hagberg JM. Enhancing treatment for cardiovascular disease: exercise and circulating angiogenic cells. Exerc Sport Sci Rev. 2011;39:93–101. doi: 10.1097/JES.0b013e31820a595e. [DOI] [PubMed] [Google Scholar]
- 166.Sandri M, Adams V, Gielen S, Linke A, Lenk K, Krankel N, et al. Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes: results of 3 randomized studies. Circulation. 2005;111:3391–9. doi: 10.1161/CIRCULATIONAHA.104.527135. [DOI] [PubMed] [Google Scholar]
- 167.Van Craenenbroeck EM, Hoymans VY, Beckers PJ, Possemiers NM, Wuyts K, Paelinck BP, et al. Exercise training improves function of circulating angiogenic cells in patients with chronic heart failure. Basic Res Cardiol. 2010;105:665–76. doi: 10.1007/s00395-010-0105-4. [DOI] [PubMed] [Google Scholar]
- 168.Erbs S, Hollriegel R, Linke A, Beck EB, Adams V, Gielen S, et al. Exercise training in patients with advanced chronic heart failure (NYHA IIIb) promotes restoration of peripheral vasomotor function, induction of endogenous regeneration, and improvement of left ventricular function. Circ Heart Fail. 2010;3:486–94. doi: 10.1161/CIRCHEARTFAILURE.109.868992. [DOI] [PubMed] [Google Scholar]
- 169.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, et al. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666–74. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 170.Heiss C, Schanz A, Amabile N, Jahn S, Chen Q, Wong ML, et al. Nitric oxide synthase expression and functional response to nitric oxide are both important modulators of circulating angiogenic cell response to angiogenic stimuli. Arterioscler Thromb Vasc Biol. 2010;30:2212–8. doi: 10.1161/ATVBAHA.110.211581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171•.Jenkins NT, Witkowski S, Spangenburg EE, Hagberg JM. Effects of acute and chronic endurance exercise on intracellular nitric oxide in putative endothelial progenitor cells: role of NAPDH oxidase. AJP - Heart and Circulatory Physiology. 2009;297:H1798–H805. doi: 10.1152/ajpheart.00347.2009. This paper reported that intracellular NO levels were lower in cultured CACs of sedentary compared to trained young men, and the mechanism underlying this difference was ~50% attributable to excess NADPH oxidase enzyme activity in CACs of the sedentary group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sonnenschein K, Horvath T, Mueller M, Markowski A, Siegmund T, Jacob C, et al. Exercise training improves in vivo endothelial repair capacity of early endothelial progenitor cells in subjects with metabolic syndrome. Eur J Cardiovasc Prev Rehabil. 2011;18:406. doi: 10.1177/1741826710389373. [DOI] [PubMed] [Google Scholar]
- 173•.Jenkins NT, Landers RQ, Prior SJ, Soni N, Spangenburg EE, Hagberg JM. Effects of acute and chronic endurance exercise on intracellular nitric oxide and superoxide in circulating CD34 and CD34 cells. J Appl Physiol. 2011;111:929–37. doi: 10.1152/japplphysiol.00541.2011. In this study, basal NADPH oxidased-derived intracellelar superoxide levels were significantly higher in CD34+ cells of sedentary men compared to trained men, providing the first evidence of impaired redox balance in from sedentary individuals in freshly-isolated CACs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vici M, Pasquinelli G, Preda P, Martinelli GN, Gibellini D, Freyrie A, et al. Electron microscopic and immunocytochemical profiles of human subcutaneous fat tissue microvascular endothelial cells. Ann Vasc Surg. 1993;7:541–8. doi: 10.1007/BF02000148. [DOI] [PubMed] [Google Scholar]
- 175.Nor JE, Peters MC, Christensen JB, Sutorik MM, Linn S, Khan MK, et al. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81:453–63. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 176.Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training? J Appl Physiol. 2004;97:1119–28. doi: 10.1152/japplphysiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- 177.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, et al. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–8. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 178.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 179.Kliche K, Jeggle P, Pavenstadt H, Oberleithner H. Role of cellular mechanics in the function and life span of vascular endothelium. Pflugers Arch. 2011;462:209–17. doi: 10.1007/s00424-011-0929-2. [DOI] [PubMed] [Google Scholar]
- 180.Dai G, Tsukurov O, Chen M, Gertler JP, Kamm RD. Endothelial nitric oxide production during in vitro simulation of external limb compression. Am J Physiol Heart Circ Physiol. 2002;282:H2066–75. doi: 10.1152/ajpheart.00288.2001. [DOI] [PubMed] [Google Scholar]
- 181.Michaels AD, Accad M, Ports TA, Grossman W. Left ventricular systolic unloading and augmentation of intracoronary pressure and Doppler flow during enhanced external counterpulsation. Circulation. 2002;106:1237–42. doi: 10.1161/01.cir.0000028336.95629.b0. [DOI] [PubMed] [Google Scholar]
- 182.Ozawa ET, Bottom KE, Xiao X, Kamm RD. Numerical simulation of enhanced external counterpulsation. Annals of biomedical engineering. 2001;29:284–97. doi: 10.1114/1.1359448. [DOI] [PubMed] [Google Scholar]
- 183.Gurovich AB, RW Three-dimension blood flow classification scheme better describes NO-mediated arterial vasodilation (Abstract) Med Sci Sports Exerc. 2011;42:7. [Google Scholar]
- 184.Gonzales JU, Thompson BC, Thistlethwaite JR, Scheuermann BW. Role of retrograde flow in the shear stimulus associated with exercise blood flow. Clin Physiol Funct Imaging. 2008;28:318–25. doi: 10.1111/j.1475-097X.2008.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185••.Mikus CR, Fairfax ST, Libla JL, Boyle LJ, Vianna LC, Oberlin DJ, et al. Seven days of aerobic exercise training improves conduit artery blood flow following glucose ingestion in patients with type 2 diabetes. J Appl Physiol. 2011;111:657–64. doi: 10.1152/japplphysiol.00489.2011. This study showed that 7 days of aerobic exercise training improves conduit artery blood flow during an OGTT in individuals with type 2 diabetes, possibly suggesting that this short-term exercise training protocol enhanced senstivity of the endothelium to insulin’s vasodilatory effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rattigan S, Wallis MG, Youd JM, Clark MG. Exercise training improves insulin-mediated capillary recruitment in association with glucose uptake in rat hindlimb. Diabetes. 2001;50:2659–65. doi: 10.2337/diabetes.50.12.2659. [DOI] [PubMed] [Google Scholar]
- 187••.Mikus CR, Rector RS, Arce-Esquivel AA, Libla JL, Booth FW, Ibdah JA, et al. Daily physical activity enhances reactivity to insulin in skeletal muscle arterioles of hyperphagic Otsuka Long-Evans Tokushima Fatty rats. J Appl Physiol. 2010;109:1203–10. doi: 10.1152/japplphysiol.00064.2010. This recent study from our laboratory demonstrated that wheel running prevents obesity and type 2 diabetes-associated impairments in insulin-stimulated vasodilation in skeletal muscle arterioles through mechanisms that were (i) NO-mediated and (ii) independent of attenuating excess adiposity in hyperphagic OLETF rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, et al. Comprehensive Physiology. John Wiley & Sons, Inc; 2011. Peripheral Circulation. [DOI] [PubMed] [Google Scholar]
- 189.Aicher A, Heeschen C, Dimmeler S. The role of NOS3 in stem cell mobilization. Trends Mol Med. 2004;10:421–5. doi: 10.1016/j.molmed.2004.07.007. [DOI] [PubMed] [Google Scholar]