Abstract
A collection of fungal isolates was obtained from a complex microbial mat, which occupied an iron-rich freshwater spring that feeds into Clear Creek, Golden, Colorado, USA. Two of the fungal isolates, a Glomeromycete (possible Entrophospora sp.) and a Dothideomycete (possible Phaeosphaeria sp.), were investigated for bioactive secondary metabolites. In total, six new compounds consisting of clearanols A–E (5, 6, 10–12) and disulochrin (7) were purified and their structures were determined. Disulochrin exhibited modest antibacterial activity against methicillin-resistant Staphylococcus aureus, whereas clearanol C showed weak inhibitory activity against Candida albicans biofilm formation.
Keywords: Biofilm, Fungi, Microbial mat, Secondary metabolites
Polymicrobial biofilms and mats are found in many natural settings at the interfaces between liquids and solid substrates.1–5 Recognizing that these types of environments may provide ideal conditions for promoting interspecies metabolic exchange within and between all three domains of life,6 we have sampled a limited number of naturally-occurring microbial mats for new secondary-metabolite-producing fungi. During the summer of 2010, one of the many springs that feed into Clear Creek near Golden, CO, USA (39°45′15.0″ North, 105°13′39.9″ West, elevation 1729 meters) was host to a luxuriant microbial mat that appeared bright green due to an abundance of photosynthetic algae and cyanobacteria. However, by early autumn, the mat had developed an intense orange hue as iron-oxidizing Gallionella7 became dominant in the iron-rich waters of the spring (4–7 mg/L iron in the spring versus 0.01–1.4 mg/L within the creek). Closer inspection of the mat revealed that a biodiverse mixture of other bacteria, as well as Archaea and Eukarya were present and a sample was collected for the purpose of procuring fungal isolates. In this report, we describe the structure assignments and bioassay screening results for metabolites purified from two fungal isolates obtained from the Clear Creak microbial mat. The results from this investigation provide compelling evidence that naturally-occurring microbial communities offer unique opportunities for obtaining fungi that generate new bioactive compounds.
The Clear Creek mat sample was mechanically disrupted and aliquots of the microbial suspension were placed on Petri plates containing either Czapek or soil-extract agar with chloramphenicol. After two weeks of incubation at room temperature, fungal colonies were transferred to Czapek agar plates. This afforded 32 fungal isolates (20 from Czapek agar and 12 from soil-extract agar) that appeared distinct based on gross morphological features. Small-scale shake-flask cultures of the isolates were prepared and extracted with ethyl acetate. Upon removal of the solvent, the remaining organic residues were tested in our assay panel for antibacterial, antifungal, cancer cell cytotoxicity, and inhibition of Candida biofilm formation. The fungal isolates were also subjected to sequencing of their nuclear ribosomal internal transcribed spacer (ITS) regions. BLAST searches performed on the sequence data revealed that the majority of the fungi were taxonomically affiliated with isolates that were scantily studied or had not been subjected to prior analyses of their secondary metabolites (based on queries of the SciFinder® and Dictionary of Natural Products® databases). Two of the relatively uncommon isolates, a Glomeromycete (possible Entrophospora sp., GenBank accession no. JQ958304) and a Dothideomycete (possible Phaeosphaeria sp., GenBank accession no. JQ958305), were chosen for scale-up chemical investigations.
An examination of different media formulations led to the observation that the Glomeromycete isolate grew particularly well under static conditions on a medium composed of Cheerios® breakfast cereal supplemented with a 3% sucrose solution. The fungal cultures were extracted with MeOH to give a dark reddish-rust colored extract that was subjected to a combination of silica gel chromatography and C18 HPLC. This provided several known compounds in substantial quantities including sulochrin (1) (218 mg), hydroxysulochrin (2) (12 mg), questinol (3) (57 mg), questin (4) (11 mg), and three new compounds (5–7) (Fig. 1).
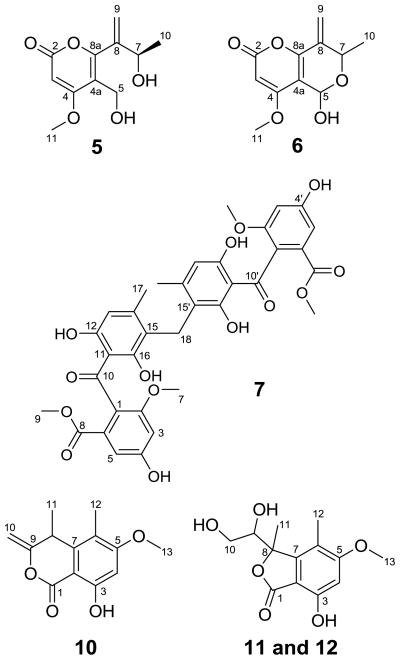
Figure 1.
Structures of clearanols A–E (5, 6, 10–12) and disulochrin (7).
Compound 5 (30 mg)8 (Fig. 1) was assigned the molecular formula C11H14O5 based on HRESIMS (m/z 227.0909 [M+H]+, calcd for C11H15O5 227.0914), which corresponded to five degrees of unsaturation. Inspection of the1H NMR, 13C NMR, and 1H-13C HSQC data provided evidence for five quaternary carbons, two methines, two methylenes, and two methyl groups. The 1H-13C HMBC correlations (Fig. 2) were instrumental in piecing together compound 5 as the only reasonable planar structure for this metabolite. The structure of 5 was later confirmed by single crystal X-ray diffraction,9 which also supported an 11R absolute configuration for the metabolite (Fig. 3) (determined by Hooft parameter, refer to the supplementary information for additional details). Examination of the SciFinder database revealed that although the planar version of this compound had been previously assigned a CAS registry number (1235630-76-8), no references were available concerning its spectroscopic properties or synthetic/biosynthetic origins. We have given compound 5 the trivial name clearanol A.
Figure 2.
Key 2–3JH-C 1H-13C HMBC correlations used to determine the structures of 5, 6, 10–12.
Figure 3.

Structure of 5 determined by single crystal X-ray diffraction experiment.
Compound 6 (0.7 mg)10 (Fig. 1) exhibited 1H and 13C NMR data that were similar to those acquired for 5; however, the HRESIMS data revealed that this metabolite possessed one additional degree of unsaturation (m/z 247.0573 [M+Na]+, calcd for C11H12O5Na 247.0582). The downfield shift of C-5 from δ 55.9 in 5 toδ 87.7 in 6 indicated that the C-7 hydroxyl group formed a new ether linkage to C-5 resulting in a dioxygenated carbon atom. This was readily confirmed with the appearance of two new 3JH-C HMBC correlations extending from H-5 to C-7 (Fig. 2). Compound 6 has been given the trivial name clearanol B. The metabolite degraded upon storage and consequently its relative/absolute configuration was not investigated.
A molecular formula of C35H32O14 was established for compound 7 (7 mg)11 (Fig. 1) based on HRESIMS (m/z 699.1685 [M+Na]+, calcd for C35H32014Na 699.1689), which accounted for 20 degrees of unsaturation. Surprisingly, the 13C NMR data for 7 exhibited only 18 resonances and 17 of the resonances had nearly identical chemical shifts to co-occurring metabolite 1 (C17H16O7). Therefore, we suspected that 7 was a symmetric dimeric compound. After accounting for 17 carbon resonances attributable to 1, a single new methylene signal remained (C-18) that we deduced would serve as a bridge between two sulochrin moieties. 1H-13C HMBC correlations were observed from the new H-18 methylene protons to C-14/14′, C-15/15′, and C-16/16′, which confirmed that the methylene served as a link between C-15 and C-15′. Compound 7 is structurally similar to acremonidin D, which is the dimerized product of the demethoxy sulochrin analog, acremonidin E 12. Metabolite 7 has been assigned the trivial name disulochrin.
The Dothideomycete isolate was grown on Cheerios® supplemented with a 3% sucrose solution and the cultures were extracted with ethyl acetate to afford a brown gummy extract. The extract was subjected to Sephadex LH-20 chromatography and C18 HPLC. This provided five metabolites, two of which were identified by dereplication. Compound 8 (5 mg) was determined to be (R)-4,8-dihydroxy-6-methoxy-4,5-dimethyl-3-methyleneisochroman-1-one 13,14 and 9 (20 mg) was identified as (R)-7-hydroxy-3-((S)-1-hydroxyethyl)-5-methoxy-3,4-dimethylisobenzofuran-1(3H)-one.14 The absolute configurations of the metabolites were confirmed by comparisons of their circular dichroism data to those reported by Tayone et al.14.
Compound 10 (3 mg)15 was assigned the molecular formula C13H14O4 by HRESIMS (m/z 233.0815 [M-H]−, calcd for C13H13O4, 233.0814), which accounted for seven degrees of unsaturation. The considerable similarities of the 1H and 13C NMR data for 10 and 8 suggested that these metabolites were structurally related. However, a notable difference between the two metabolites concerned C-8 which was shifted upfield from δ 72.1 in 8 to δ 36.0 in 10. In addition, the 1H-13C HSQC experiment demonstrated that the C-8 quaternary carbon in 8 was a methine in 10. These data indicated that 10 was the C-8 de-hydroxy analog of metabolite 8. Subsequent analysis of all the 1H-13C HMBC correlation data led to the assignment of the planar structure of 10 (Fig. 2). We have given compound 10 the name clearanol C.
Compounds 1116 and 1217 were obtained as a 1:1 mixture (39 mg) of structurally-related metabolites that resisted chromatographic separation by C18 HPLC with aqueous MeOH and acetonitrile. However, examination of the mixture by 1H and 13C NMR led us to conclude that the structures of both metabolites could be deduced in their combined form. HRESIMS performed on the mixture yielded a single peak at m/z 291.0839 [M+Na]+, which represented a molecular formula of C13H16O6, (calcd for C13H16O6Na, 291.0845) for both compounds. In view of the identical molecular formulae and very similar NMR data for these metabolites, it was deduced that 11 and 12 represented a diastereomeric mixture. We also noted that 11 and 12 showed considerable similarity to 9 with the following exceptions: the highfield C-10 methyl doublet protons found in 9 δ 0.86, J = 6.3 Hz) were missing and instead were replaced by downfield diastereotopic methylene double doublets in 11 δ 3.83, J = 11.3, 3.5 Hz and δ 3.53, J = 11.3, 8.2 Hz) and 12 δ 3.34, J = 11.3, 8.6 Hz and δ 3.14, J = 11.3, 2.7 Hz). This suggested that the additional oxygen atoms in the molecular formulae of 11 and 12 were accounted for by new C-10 hydroxyl groups. 1H-13C HMBC correlations from the C-10 protons of both compounds to their respective C-8 and C-9 carbon atoms (Fig. 2) confirmed these assignments. Further analysis of the 1H-13C HMBC correlation data for both metabolites provided verification of the planar structures of 11 and 12 and we have given these compounds the trivial names clearanols D and E, respectively.
All compounds were tested in bioassays that were performed as previously described.18,19 Compound 7 exhibited modest activity against methicillin-resistant Staphylococcus aureus (ATCC 700787) by completely inhibiting its growth at a concentration of 100 μg/mL; however, growth was not restricted at 10 μg/mL. None of the other compounds inhibited the growth of S. aureus or Klebsiella pneumoniae (ATCC 51503). Metabolite 10 demonstrated modest growth inhibition effects against polyene-resistant Candida albicans (ATCC 38245) and Aspergillus fumigatus (FGSC A1100) by reducing growth 61% and 62%, respectively, at 100 μg/mL. None of the other metabolites inhibited the growth of fungi or pancreatic cancer cells (MIA-PaCa-2). Compounds 8 and 10 inhibited C. albicans (DAY185) biofilm formation with MIC values of 86 ± 3 and 101 ± 3 μM, respectively. None of the other metabolites demonstrated activity in the biofilm assay. Based on these studies, we surmise that microbial mat communities offer intriguing potential for encountering unusual fungal taxa with the capacity to generate new bioactive natural products.
Supplementary Material
Acknowledgments
Financial support from the National Institutes of Health (1R01GM092219 and 1R01AI085161) is greatly appreciated. The X-ray diffractometer was purchased through a grant from the National Science Foundation (CHE-0130835).
Footnotes
Supplementary data (X-ray diffraction experiment for 5) associated with this article can be found, in the online version, at WEB LINK TO BE INSERTED.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Robertson CE, Spear JR, Harris JK, Pace NR. Appl Environ Microbiol. 2009;75:1801–1810. doi: 10.1128/AEM.01811-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feazel LM, Spear JR, Berger AB, Harris JK, Frank DN, Ley RE, Pace NR. Appl Environ Microbiol. 2008;74:329–332. doi: 10.1128/AEM.01448-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall-Stoodley L, Costerton JW, Stoodley P. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 4.Seckbach J, Oren A. Microbial Mats: Modern and Ancient Microorganisms in Stratefied Systems. Springer; New York: 2010. [Google Scholar]
- 5.Ley RE, Harris JK, Wilcox J, Spear JR, Miller SR, Bebout BM, Maresca JA, Bryant DA, Sogin ML, Pace NR. Appl Environ Microbiol. 2006;72:3685–3695. doi: 10.1128/AEM.72.5.3685-3695.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phelan VV, Liu WT, Pogliano K, Dorrestein PC. Nat Chem Biol. 2012;8:26–35. doi: 10.1038/nchembio.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emerson D, Fleming EJ, McBeth JM. Ann Rev Microbiol. 2010;64:561–583. doi: 10.1146/annurev.micro.112408.134208. [DOI] [PubMed] [Google Scholar]
- 8.Clearanol A (5): colorless crystals from MeOH; mp 138–140 °C; [α]24D -88 (c 0.4, MeOH); UV (MeOH) λmax nm (log ε) 210 (4.35), 290 (3.91); IR (NaCl) νmax 3387, 2972, 2346, 1700, 1629, 1560, 1452, 1406, 1246, 1017 cm−1; 1H NMR (500 MHz, methanol-d4) δ 5.75 (1H, d, J =1.5 Hz, H-9a), 5.67 (1H, s, H-3), 5.63 (1H, d, J =1.5 Hz, H-9b), 4.64 (1H, q, J = 6.6 Hz, H-7), 4.44 (1H, d, J = 11.7 Hz, H-5a), 4.33 (1H, d, J = 11.7 Hz, H-5b), 3.94 (3H, s, H-11), 1.30 (3H, d, J = 6.6 Hz, H-10); 13C NMR (125 MHz, methanol-d4) δ 172.9 (C-4), 166.1 (C-2), 163.1 (C-8a), 146.0 (C-8), 119.8 (C-9), 114.4 (C-4a), 89.5 (C-3), 68.3 (C-7), 57.5 (C-11), 55.9 (C-5), 22.6 (C-10); HRESIMS m/z 227.0909 [M+H]+ (calcd for C11H15O5, 227.0914).
- 9.Cambridge Crystallographic Data Centre no. CCDC 876681; experimental parameters are provided in the online supplementary data accompanying this report.
- 10.Clearanol B (6): amorphous powder; UV (MeOH) λmax nm (log ε) 206 (4.29), 298 (3.84); IR (NaCl) νmax 3345, 2922, 2853, 2347, 1707, 1609, 1564, 1461, 1408, 1260, 1055 cm−1; 1H NMR (400 MHz, methanol-d4) δ 5.97 (1H, d, J = 1.6 Hz, H-9a), 5.83 (1H, s, H-5), 5.70 (1H, s, H-3), 5.48 (1H, d, J = 1.6 Hz, H-9b), 4.92 (1H, m, J = 6.3 Hz, H-7), 3.92 (3H, s, H-11), 1.47 (3H, d, J = 6.3 Hz, H-10); 13C NMR (100 MHz, methanol-d4) δ 170.7 (C-4), 165.6 (C-2), 153.9 (C-8a), 138.7 (C-8), 114.5 (C-9), 111.4 (C-4a), 90.5 (C-3), 87.7 (C-5), 65.4 (C-7), 57.6 (C-11), 18.4 (C-10); HRESIMS m/z 247.0573 [M+Na]+ (calcd for C11H12O5Na 247.0582).
- 11.Disulochrin (7): yellow amorphous powder; UV (MeOH) λmax nm (log ε) 210 (4.64), 288 (3.90); IR (NaCl) νmax 3301, 2953, 2352, 1710, 1609, 1589, 1441, 1344, 1145, 1064, 1004 cm−1; 1H NMR (400 MHz, methanol- 6.95 (1H, d, J = 2.0 Hz, H-5), 6.65 (1H, d, J = d4) δ2.0 Hz, H-3), 6.00 (1H, s, H-13), 3.98 (1H, s, H-18), 3.70 (3H, s, H-7), 3.62 (3H, s, H-9), 2.17 (3H, s, H-17); 13C NMR (75 MHz, methanol-d4) δ 202.4 (C-10,10′), 168.2 (C-8,8′), 162.7 (C-12,12′), 159.8 (C-4,4′), 159.5 (C-16,16′), 158.8 (C-2,2′), 149.3 (C-14,14′), 130.1 (C-6,6′), 128.4 (C-1,1′), 119.0 (C-15,15′), 110.8 (C-11,11′), 109.4 (C-13,13′), 109.0 (C-5,5′), 104.4 (C-3,3′), 56.7 (C-7,7′), 52.7 (C-9,9′), 22.0 (C-18), 21.1 (C-17,17′); HRESIMS m/z 699.1685 [M+Na]+ (calcd for C35H32014Na 699.1689).
- 12.He H, Bigelis R, Solum EH, Greenstein M, Carter GT. J Antibiot. 2003;56:923–930. doi: 10.7164/antibiotics.56.923. [DOI] [PubMed] [Google Scholar]
- 13.Chinworrungsee M, Kittakoop P, Isaka M, Chanphen R, Tanticharoen M, Thebtaranonth Y. J Chem Soc, Perkin Trans. 2002;1:2473–2476. [Google Scholar]
- 14.Tayone WC, Honma M, Kanamaru S, Noguchi S, Tanaka K, Nehira T, Hashimoto M. J Nat Prod. 2011;74:425–429. doi: 10.1021/np100838j. [DOI] [PubMed] [Google Scholar]
- 15.Clearanol C (10): amorphous solid, [α]22D +39 (c 0.06, MeOH); UV (MeOH) λmax nm (log ε) 220 (3.83) 270 (3.47), 312 (3.20); IR (NaCl) νmax 3623, 2949, 2851, 1651, 1018 cm−1; 1H NMR (400 MHz, methanol-d4) δ 6.46 (1H, s, H-4), 4.74 (1H, s, H-10a), 4.68 (1H, s, H-10b), 3.99 (1H, q, J = 7.0 Hz, H-8), 3.88 (3H, s, H-13), 2.09 (3H, s, H-12), 1.34 (3H, d, J = 7.0 Hz, H-11); 13C NMR (100 MHz, methanol-d4) δ 168.4 (C-5), 166.6 (C-1), 164.4 (C-3), 159.1 (C-9), 143.8 (C-7), 115.5 (C-6), 99.5 (C-2), 98.5 (C-4), 96.2 (C-10), 56.6 (C-13), 36.0 (C-8), 22.7 (C-11), 10.2 (C-12); HRESIMS m/z 233.0815 [M-H]− (calcd for C13H13O4, 233.0814).
- 16.Clearanol D (11): amorphous solid (1:1 mixture with 12); UV (MeOH) λmax nm (log ε) 218 (4.22), 260 (3.87), 300 (3.59); IR (NaCl) νmax 3596, 3089, 1737, 1651, 1472, 1373, 1319, 1247, 1220, 1153, 1067 cm−1; 1H NMR (400 MHz, methanol-d4) δ 6.47 (1H, s, H-4), 4.13 (1H, dd, J = 3.7 and 8.0 Hz, H-9), 3.87 (3H, s, H-13), 3.83 (1H, dd, J = 3.5 and 11.3 Hz, H-10a), 3.53 (1H, dd, J = 8.2 and 11.3 Hz, H-10b), 2.18 (3H, s, H-12), 1.67 (3H, s, H-11); 13C NMR (100 MHz, methanol-d4) δ 171.9 (C-1), 166.4 (C-5), 157.8 (C-3), 153.1 (C-7), 113.4 (C-6), 105.4 (C-2), 99.5 (C-4), 90.6 (C-8), 75.7 (C-9), 63.9 (C-10), 56.9 (C-13), 22.08 (C-11), 11.4 (C-12); HRESIMS m/z 291.0839 [M+Na]+ (calcd for C13H16O6Na, 291.0845).
- 17.Clearanol E (12): amorphous solid (1:1 mixture with 11); UV (MeOH) λmax nm (log ε) 218 (4.22), 260 (3.87), 300 (3.59); IR (NaCl) νmax 3596, 3089, 1737, 1651, 1472, 1373, 1319, 1247, 1220, 1153, 1067 cm−1; 1H NMR (400 MHz, methanol-d4) δ 6.49 (1H, s, H-4), 4.11 (1H, dd, J = 2.7 and 8.2 Hz, H-9), 3.88 (3H, s, H-13), 3.34 (1H, dd, J = 8.6 and 11.3 Hz, H-10a), 3.14 (1H, dd, J = 2.7 and 11.3 Hz, H-10b), 2.15 (3H, s, H-12), 1.73 (3H, s, H-11); 13C NMR (100 MHz, methanol-d4) δ 171.5 (C-1), 166.6 (C-5), 158.3 (C-3), 152.4 (C-7), 113.3 (C-6), 104.6 (C-2), 99.9 (C-4), 90.4 (C-8), 76.2 (C-9), 63.3 (C-10), 56.9 (C-13), 22.11 (C-11), 11.7 (C-12); HRESIMS m/z 291.0839 [M+Na]+ (calcd for C13H16O6Na, 291.0845).
- 18.Wang X, You J, King JB, Powell DR, Cichewicz RH. J Nat Prod. 2012;75:707–715. doi: 10.1021/np2009994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henrikson JC, Ellis TK, King JB, Cichewicz RH. J Nat Prod. 2011;74:1959–1964. doi: 10.1021/np200454z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.