Abstract
Comment on: Du J, et al. Blood 2012; 119:2789-98.
Hematopoietic stem cells (HSCs) are defined by their capacity of self-renewal and giving rise to multi-lineage blood cells constantly. In adults, the HSC population is believed to reside in the hypoxic bone marrow microenvironment and remains relatively quiescent. Although many cell-intrinsic and/or -extrinsic factors have been shown to regulate HSC quiescence, the exact mechanism(s) is still not well understood.
We have shown previously that Cited2 is required for murine fetal liver hematopoiesis.1 Using a conditional knockout mouse model, we recently demonstrated that Cited2 is crucial for the maintenance of HSC quiescence in part by modulating Hypoxia-Inducible Factor (HIF)-1 activity.2 We found that Cited2 deficiency in adult mice results in the loss of HSC quiescence and impaired HSC reconstitution capacity, while additional deletion of HIF-1α partially rescues these defects. Cited2 negatively regulates HIF-1 activity by competing with HIF-1α for binding with CBP/p300. Precise regulation of HIF-1 activity has been demonstrated to be essential for the maintenance of HSC functions. Conditional deletion of HIF-1α reduces HSC quiescence, while elevated HIF-1 activity by deletion of von Hippel-Lindau (VHL) gene impairs HSC homing and reconstitution capacity.3 Therefore, the defects in Cited2 knockout HSCs are likely due in part to the perturbation of HIF-1 activity.
Given that a large number of HIF-1 target genes have been identified, it will be important to determine the major effectors in controlling HSC cell cycle status when HIF-1 activity is disturbed. These effectors could be direct or indirect target genes of HIF-1. In HIF-1α-knockout mice, the defects of HSCs are in part due to the upregulation of p16Ink4a and p19Arf.3 In our Cited2-knockout mice, where HIF-1 activity is elevated, the loss of HSC quiescence is partially mediated by the decreased expression of p57kip2, Egr1 and Hes12 (Fig. 1). Our results are in agreement with previous findings that p57kip2 is downregulated in HSCs when cultured under hypoxia.4 Vegf is a conventional HIF-1 target gene and has been shown to be important for HSC functions using a knock-in mouse model with the mutation of hypoxia response element in the Vegf promoter.5 However, the expression of Vegf is unaffected in HIF-1α- or Cited2-knockout HSCs, probably due to a compensatory effect from other regulators.2,3
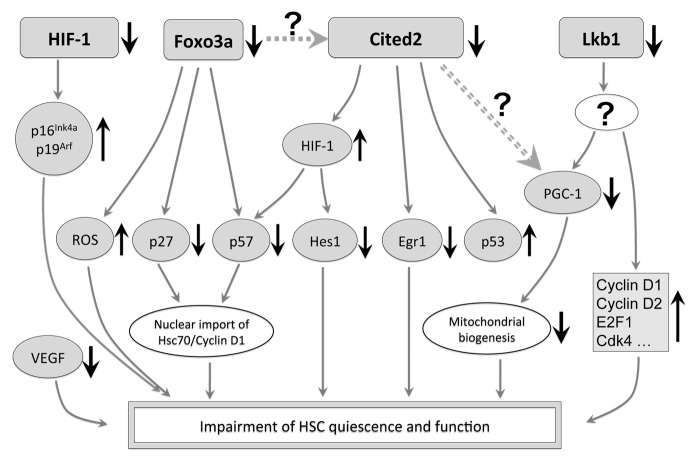
Figure 1. Molecular network of Cited2 and its associated players in the regulation of HSC quiescence. HIF-1 maintains the quiescence of HSCs partly via the repression of p16Ink4a and p19Arf. Although the deletion of HIF-1α does not affect the expression of Vegf, the lack of VEGF results in impaired HSC reconstitution capacity. The deficiency of Cited2 results in downregulation of multiple HSC quiescence related genes, including Egr1, p57kip2 and Hes1. The downregulation of p57kip2 and Hes1 is most likely mediated by the elevated activity of HIF-1, as additional deletion of HIF-1α in Cited2-knockout mice largely resumes the expression of p57kip2 and Hes1 to normal levels. Previous reports also showed increased activity of p53 in Cited2-knockout HSCs. Foxo3a regulates HSC quiescence mainly by repression of reactive oxygen species (ROS) and activation of p27kip1 and p57kip2. Although Foxo3a has been shown to induce the transcription of the Cited2 gene in fibroblasts (dashed line), whether Cited2 is downstream of Foxo3a in HSCs requires further investigation. Lkb1 regulates HSC cell cycle progression and mitochondrial biogenesis partly by the regulation of PGC-1 in an AMPK- and mTOR-independent manner, but the underlying mechanism remains unknown. Cited2 positively regulates the activity of PGC-1α in hepatic cells (double dashed line), and the significance of this regulation in HSC metabolism needs to be further pursued. Upward and downward arrows stand for increased and decreased activities, respectively.
Others have shown that p57kip2 cooperates with p27kip1 to control HSC quiescence through the regulation of cytoplasmic localization of the Hsc70/cyclin D1 complex.6 Importantly, downregulation of p57kip2 has been observed in many knockout mouse models where HSCs lose quiescence, such as in Foxo3a-, Cxcr4-, JunB-, thrombopoietin-, STAT5- and Cited2-knockout mice. Therefore, it is tempting to speculate that p57kip2 which is preferentially expressed in long-term HSCs, serves as a common downstream mediator of the network controlling HSC quiescence. Furthermore, it has been shown that in fibroblasts Foxo3a can be activated by HIF-1 in response to hypoxic stress, and the transcription of Cited2 can be induced by Foxo3a, thus forming a feedback loop to fine tune the activity of HIF-1. It will be interesting to decipher whether this regulatory loop also exists in HSCs.
The key role of HIF-1 in regulating both glycolysis and mitochondrial respiration has been widely recognized for decades. Recently, Cripto/GRP78 signaling, which causes glycolysis-biased metabolism in HSCs, was identified as one of the key intermediates in HIF-1 mediated pathways under hypoxia.7 Simsek et al. also showed that long-term HSCs utilize glycolysis instead of mitochondrial oxidative phosphorylation, and Meis1 regulates HSC metabolism via transcriptional activation of HIF-1α.8 These studies underscore the essential role of HIF-1 mediated metabolic regulation in the maintenance of HSCs.
Several recent studies have implicated that metabolic regulation is intricately coordinated with cell cycle progression in HSCs. For instance, the lack of Lkb1 leads to increased HSC division and rapid HSC depletion.9 This adverse impact of Lkb1 deletion on HSCs is AMPK and mTOR-independent and involves mitochondrial impairment possibly through downregulation of PGC-1 coactivators.9 The role of Cited2 in metabolic regulation of HSCs is currently unknown. Our unpublished microarray data showed that conditional deletion of Cited2 results in deregulation of genes related to glutathione metabolism and mitochondrial functions in HSCs. In addition, Mashito et al. showed that Cited2 regulates hepatic gluconeogenesis through the modulation of GCN5-dependent PGC-1α acetylation and loss of hepatic Cited2 functions suppresses gluconeogenesis in diabetic mice.10 It will be of interest to determine the role of Cited2 in HSC metabolism and cell cycle control, particularly in the adaptation of HSCs to the hypoxic microenvironment.
In summary, we demonstrated that Cited2 maintains HSC quiescence partly through the regulation of HIF-1 activity. In the future, it will be interesting to investigate whether Cited2 is required for the self-renewal of leukemic stem cells, since elevated activity of HIF-1 has been implicated in certain types of leukemia. Metabolic regulation by Cited2 and its associated players in controlling HSC quiescence should also be interrogated through generation of compound animal models and metabolite/gene expression profiling. In addition, given that Cited2 is a negative regulator of HIF-1 via competitive binding to CBP/p300, it is tempting to explore the therapeutic potential of small compounds mimicking Cited2/p300 interactions.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/20803
References
- 1.Chen Y, et al. Blood. 2007;110:2889–98. doi: 10.1182/blood-2007-01-066316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du J, et al. Blood. 2012;119:2789–98. doi: 10.1182/blood-2011-10-387902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takubo K, et al. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Eliasson P, et al. Exp Hematol. 2010;38:301–10, e2. doi: 10.1016/j.exphem.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Rehn M, et al. Blood. 2011;118:1534–43. doi: 10.1182/blood-2011-01-332890. [DOI] [PubMed] [Google Scholar]
- 6.Zou P, et al. Cell Stem Cell. 2011;9:247–61. doi: 10.1016/j.stem.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Miharada K, et al. Cell Stem Cell. 2011;9:330–44. doi: 10.1016/j.stem.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Simsek T, et al. Cell Stem Cell. 2010;7:380–90. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan B, et al. Nature. 2010;468:701–4. doi: 10.1038/nature09595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakai M, et al. Nat Med. 2012;18:612–7. doi: 10.1038/nm.2691. [DOI] [PubMed] [Google Scholar]