Abstract
The activity of p53 as a tumor suppressor primarily depends on its ability to transactivate specific target genes in response to genotoxic and other potentially mutagenic stresses. Several histone acetyl transferases (HATs), including p300, CBP, PCAF and GCN5 have been implicated in the activation of p53-dependent transcription of the cyclin-dependent kinase (cdk) inhibitor p21 as well as other target genes. Here we show that PCAF, but not CBP or p300, is a critical regulator of p53-dependent p21 expression in response to multiple p53-activating stresses. PCAF was required for the transcriptional activation of p21 in response to exogenous p53 in p53-null cells, nutlin-3, DNA damaging agents and p14ARF expression, suggesting a broad requirement for PCAF in p53 signaling to p21 after stress. Importantly, cells lacking PCAF failed to undergo cell cycle arrest in response to nutlin-3 treatment or p14ARF expression, consistent with a physiologically important role for PCAF in this p53 function. Surprisingly, the role for PCAF in induction of p21 was independent of p53 lysine 320 acetylation, a previously suggested target of PCAF-mediated acetylation. Though p21 promoter occupancy by p53 was not altered by PCAF knockdown, activation of p21 transcription required an intact PCAF HAT domain, and induction of chromatin marks acetyl-H3K9 and acetyl-H3K14 at the p21 promoter by p53 was dependent upon physiologic levels of PCAF. Together, our experiments indicate that PCAF is required for stress-responsive histone 3 acetylation at the p21 promoter, p53-directed transcription of p21 and the resultant growth arrest.
Keywords: ARF, DNA damage, PCAF, histone acetylation, p21, p53
Introduction
The tumor suppressor p53 is mutated in about half of all cancers and is believed to be functionally antagonized in the majority of cancers retaining wild-type p53 (see ref. 1 for review). Tumor suppressive activities of p53 are achieved through both transcription-dependent and -independent coordination of an array of cellular responses to various genotoxic stresses. Among the better-characterized transcriptional targets of p53 is the CDK-inhibitory protein p21, which is upregulated, often in a p53-dependent manner, in response to many physiologic stresses and results in a transient or sustained cell cycle arrest. More recently, the p53-p21 signaling circuit has been shown to act as a barrier to generation of induced pluripotent stem (iPS) cells2-4 and tissue regeneration in mice.5 Another recent report has also identified p21 itself as a positive regulator of p53 activity, which acts by promoting ATM-dependent p53 phosphorylation, nuclear localization of p53, and subsequent transcription of p53 target genes,6 suggesting a positive feedback loop in p21 regulation and highlighting the importance of developing a complete understanding of p21 regulation by p53.
Proper coordination of p53-dependent cell cycle arrest requires tight regulation of its transcriptional activity. Histone/protein acetyltransferases (HATs) play a vital role in regulating transcription by acetylating histone tails, which is believed to enhance accessibility of chromatin to other transcription factors and adaptors. Additionally, many HATs directly regulate stability or activity of transcription factors, such as p53, through acetylation.7 Perhaps unsurprisingly then, some histone acetyltransferases (HATs) also harbor tumor suppressor activity and represent a promising class of therapeutic targets.8,9
Among HATs known to act on p53 and enhance its transcriptional activity are p300, CREB-binding protein (CBP), p300/CBP-associated factor (PCAF), Tip60 and GCN5.10-17 The biological relevance of each of these HATs in p53 function, and whether one or more HATs serve as p53 co-activators in specific contexts, remains to be fully resolved. The p300 protein and, to a lesser extent, its paralog CBP are the best characterized HATs involved in p53 function and are important for growth arrest and apoptotic responses to DNA damage.14-17 Both HATs are known to acetylate p53 in its C-terminal regulatory domain.12,18 However, the interplay of p300 and CBP with other HATs in non-genotoxic p53 responses, such as to ARF expression or Nutlin treatment, has not been explored.
PCAF is a member of the GNAT (GCN5-related N-acetyltransferase) family of protein acetyltransferases.12,19 PCAF was originally identified as a factor displaced from p300/CBP-containing complexes by expression of the adenoviral oncoprotein E1A.20 The PCAF complex is known to acetylate histones and transcriptional regulators21 and has been shown to modulate activities of several tumor suppressors and oncogenes. Functional and structural characterization of PCAF acetyltransferase activity has indicated that lysines 9 and 14 of histone H3 are the preferred substrates for this activity, and this residue appears to play a critical role in promoting the association of histone H3 with PCAF.22,23
A role for PCAF in p53 signaling has been previously suggested but is incompletely understood. Several reports using in vitro and overexpression analyses suggest that PCAF is capable of acetylating p53 at lysine 320 and enhancing site-specific p53 DNA-binding activity, and acetylation of this site increases after UV irradiation.12,24 In addition, PCAF is recruited to the p21 promoter during neuronal differentiation in a manner dependent upon the p53 family member p73,25 though in vivo data demonstrating the physiologic significance of PCAF in p53 acetylation and activity are lacking.
Here we show that under a variety of p53-activating stress conditions and cell types, PCAF is critical for p21 expression and p53-dependent cell cycle arrest. This role of PCAF in p21 expression was transcriptional, as shown by PCAF-dependent expression of p21 transcripts in p53-transfected cells. Depletion of PCAF by siRNA was associated with a reduction in histone H3 acetylation marks at K9 and K14, both previously described PCAF-directed modifications commonly associated with transcriptional activation, at the distal p53 response element of the p21 promoter. Rescue of p21 expression by exogenous PCAF in PCAF-depleted cells required an intact HAT domain, suggesting PCAF’s role in p53/p21 signaling was HAT-dependent. These data indicate that PCAF regulates p53-dependent p21 activation through canonical histone acetylation at the p21 promoter, creating an environment permissive for p21 transcription.
Results
Role of co-activators in p21 induction after p53-activating stress.
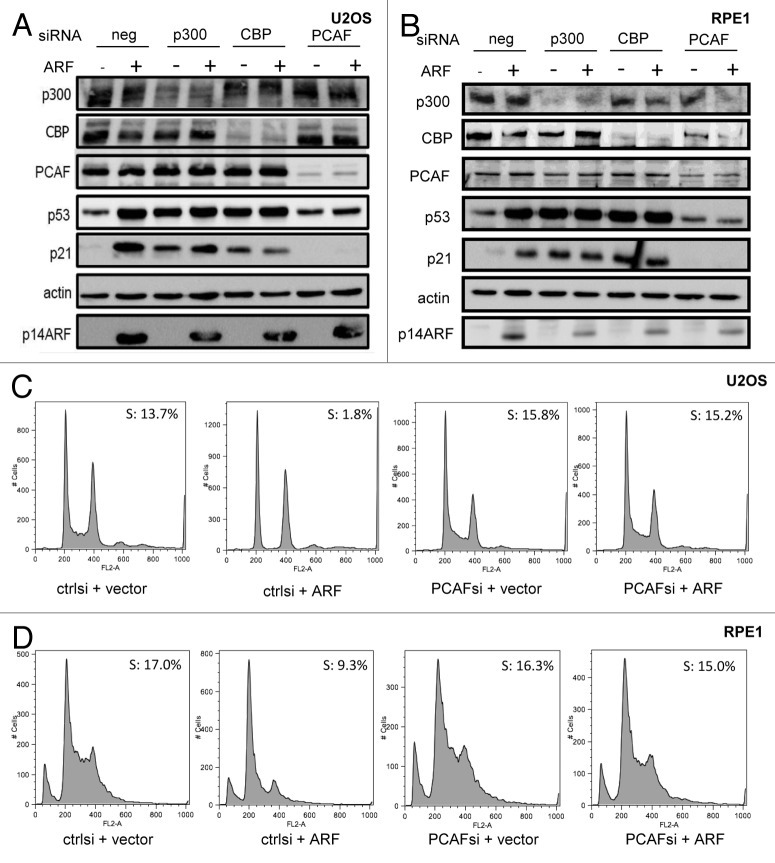
To initially examine the role of known p53 co-activators in non-genotoxic p53 acetylation and activation, we surveyed the effects of p300, CBP or PCAF depletion by siRNA on p14ARF/p53-induced p21 expression and growth arrest in U2OS cells (Fig. 1A). In line with previous reports,26,27 p300 and CBP appeared to negatively regulate p53 stability under basal conditions, with increased p53 abundance observed after their depletion, presumably through their roles as E4 ubiquitin ligases, which recognize already monoubiquitinated proteins as substrates. Strikingly, PCAF depletion prevented stabilization of p53 and induction of p21 in response to p14ARF, while p300 or CBP depletion exhibited neither effect (Fig. 1A). To rule out the possibility that the role of PCAF might be unique to transformed cells, we repeated the experiment in hTERT-immortalized, diploid RPE1 cells; again, PCAF but not p300 or CBP, was necessary for p14ARF-directed p53 stabilization and p21 induction (Fig. 1B). Populations of both cell types were stained with propidium iodide and analyzed by flow cytometry (Fig. 1C and D). As expected, p14ARF transfection induced a potent cell cycle arrest in both U2OS and RPE1 cells. However, in PCAF-depleted populations, p14ARF failed to induce a cell cycle arrest. Importantly, transfection of p16INK4A, which induces an Rb-dependent cell cycle arrest, retained the ability to promote cell cycle arrest even in the absence of PCAF (Fig. S1), suggesting that PCAF is not uniformly required for cells to undergo a cell cycle arrest.
Figure 1. PCAF regulates p21 levels and cell cycle arrest in response to ARF. U2OS (A and C) or RPE1 (B and D) cells were transfected with siRNA at a final concentration of 10 nM, as well as 1 µg pcDNA3-p14ARF or pcDNA3 vector and harvested 48 h post-transfection for western blotting with the indicated antibodies (A and B), or fixed for propidium iodide staining and flow cytometric cell cycle analysis (C and D).
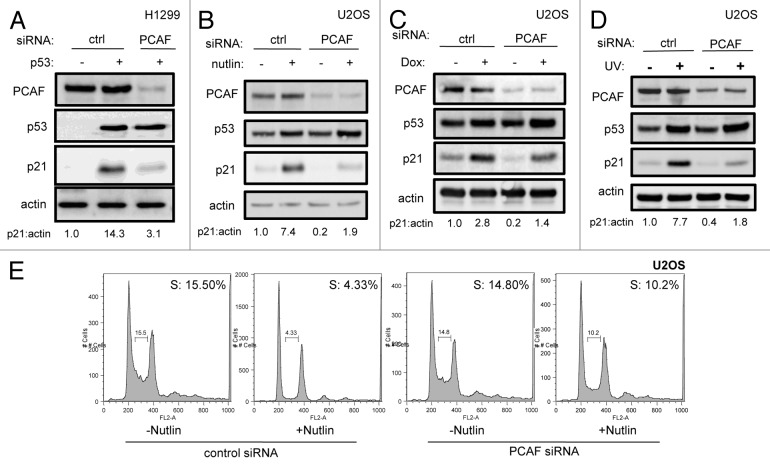
To determine whether the involvement of PCAF in p53-induced p21 expression was limited to the p14ARF response, we examined p21 levels following activation of the p53 pathway after other stress stimuli. Depletion of PCAF by siRNA in p53-transfected H1299 cells resulted in the elimination of p21 induction by exogenous transfected p53 (Fig. 2A). Nutlin-3, a nanomolar competitive inhibitor of the MDM2-p53 interaction, stabilizes endogenous p53 in U2OS cells, resulting in the induction of p21 and growth arrest in most cells with wild-type p53.28 As expected, both p53 and p21 levels increased in response to Nutlin-3. However, while Nutlin-3 also resulted in p53 stabilization after silencing of PCAF, a corresponding increase in p21 levels was not observed (Fig. 2B). This deficiency in p21 induction was functionally important, as PCAF-depleted cells also failed to undergo cell cycle arrest in response to Nutlin-3 treatment (Fig. 2E).
Figure 2. PCAF is required for p53-dependent p21 expression. U2OS (B–E) or H1299 (A) cells were transfected with p53 (+) or vector (-) cDNA (A) along with indicated siRNA (A–E) and harvested for immunoblot (A–D) or stained with propidium iodide and subjected to flow cytometric cell cycle profile analysis (E). Nutlin 3a-treated U2OS cells (B and E) were exposed to 5 µM Nutlin-3 for 16 h prior to lysis or fixation. Doxorubicin-treated U2OS cells (C) were exposed to 1 µM doxorubicin for 16 h prior to lysis. UV-irradiated U2OS (D) cells were treated with 20 J/m2 UV-C 24 h prior to lysis.
To further characterize PCAF function in diverse p53 stress responses, PCAF-depleted cells were treated with the topoisomerase II inhibitor doxorubicin or UV radiation. After exposure to 100 nM doxorubicin for 24 h (Fig. 2C) or 20 J/m2 UV (Fig. 2D), p21 levels were evaluated. Again, PCAF was necessary for full induction of p21 in response to doxorubicin and UV irradiation. Partial p21 induction after PCAF silencing was still observed following doxorubicin or UV irradiation, likely a consequence of NFκB-mediated transactivation of the p21 promoter.29-31
Regulation of MDM2 stability does not account for PCAF’s role in p21 regulation.
Functions of PCAF in p53/p21 signaling are presumably related to its known function as a transcriptional co-activator, which operates through its ability to acetylate both histone and non-histone factors. However, PCAF has also been reported to exhibit intrinsic E3 ligase activity toward MDM2,32 raising the possibility that PCAF exerts its effects on p21 levels indirectly by inhibition of MDM2 and consequent upregulation of p53 levels. An examination of steady-state levels of MDM2 and p53 in PCAF-depleted U2OS cells reproducibly revealed no appreciable change in the levels of p53 or MDM2 proteins (Fig. S2), suggesting that modulation of MDM2 abundance does not account for the importance of PCAF in p21 expression.
PCAF regulates p21 mRNA levels.
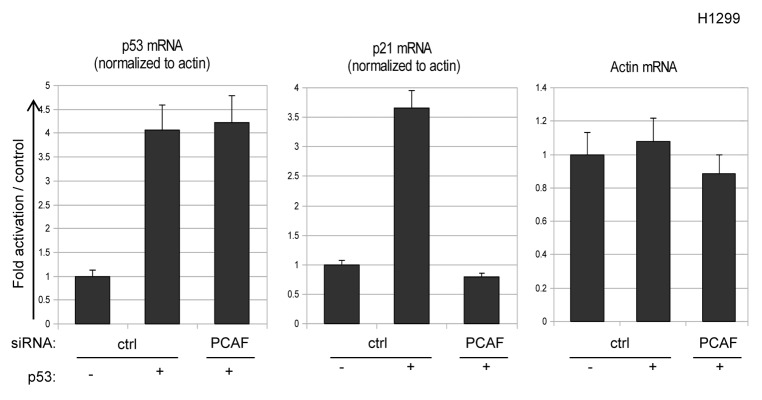
To investigate whether p21 expression levels are directly regulated by PCAF at the level of transcription, mRNA from H1299 cells transfected with a control vector or wild-type p53 in combination with either control or PCAF siRNA was analyzed for p21 transcripts by Q-PCR. As expected, p21 mRNA increased following transfection of p53, while induction of p21 mRNA was blocked by silencing of PCAF (Fig. 3). Importantly, exogenous p53 and endogenous β-actin mRNA levels were unaffected following depletion of PCAF (Fig. 3), indicating that the impact of PCAF knockdown on p21 expression is not a consequence of global effects of PCAF depletion on transcription.
Figure 3. PCAF is a transcriptional regulator of p21 expression. H1299 cells were transfected with empty vector or p53 expression plasmid along with the indicated siRNA and cellular RNA was analyzed by RQ-PCR for β-actin, p21 and p53 mRNA levels. Relative transcript levels were measured by normalizing p53 and p21 levels to β-actin using the Δ ΔCt method.
p53-K320 acetylation is not required for PCAF-dependent induction of p21.
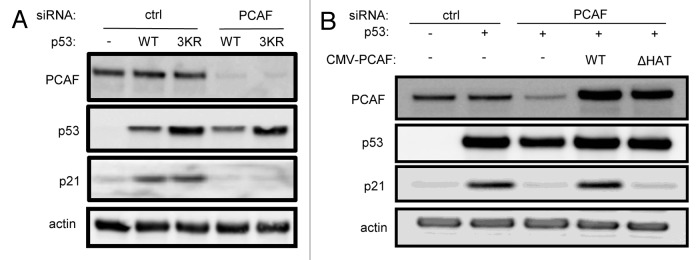
Because PCAF has been previously ascribed a role in acetylation of p53 at K320 in its regulatory domain, we tested whether a p53 K319-321R (lysines 319, 320 and 321 mutated to arginine) mutant is competent for transactivation of p21, and whether PCAF is also critical for p21 expression in this context. We found that p53 K319-321R stimulated p21 expression to a level comparable to wild-type p53, and that PCAF was indeed critical for p21 expression driven by p53 K319-321R (Fig. 4A). Identical results were found with the single K320R mutation (data not shown). These results indicate that PCAF plays an additional and critical role in p53-dependent stimulation of p21 transcription independent of K320 posttranslational modification.
Figure 4. PCAF regulates p21 expression independently of p53 K320 acetylation through its HAT domain. (A) H1299 cells were transfected with wild-type p53 or a p53 K319–321R triple mutant in combination with either a control or PCAF-directed siRNA. PCAF, p53 and p21 levels were then measured by western blot. (B) H1299 cells were transfected with p53 in combination with either control or PCAF siRNA, as well as siRNA-resistant cDNAs encoding wild-type PCAF or PCAF bearing a deletion in the HAT domain (ΔHAT). PCAF, p53 and p21 levels were then measured by western blot.
Transactivation of p21 by p53 requires an intact PCAF HAT domain.
We further reasoned that if PCAF acts independently of p53 acetylation and modulation of MDM2 stability to modulate p21 expression, that the p53-dependent induction of p21 may be directly dependent upon PCAF enzymatic HAT activity. Using a small internal deletion mutant cDNA of PCAF lacking residues 608–628 and deficient in HAT activity,33 we introduced silent mutations into the siRNA-hybridizing region of the PCAF reading frame and rescued PCAF knockdown with either a wild-type or HAT-deficient PCAF allele. In H1299 cells expressing exogenous p53, co-expression of wild-type PCAF cDNA with PCAF siRNA restored p21 expression, whereas co-expression of PCAF siRNA with the HAT mutated allele did not (Fig. 4B). This experiment therefore indicates that PCAF HAT activity is necessary for p53-directed p21 expression.
p53 or ARF-induced histone H3 acetylation at the p21 locus is dependent on PCAF.
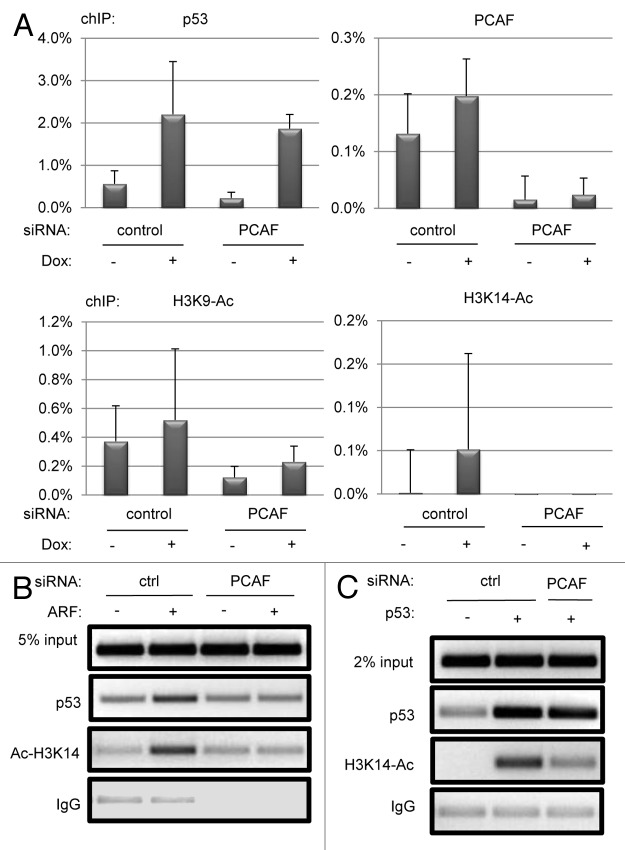
Based on our results that PCAF knockdown modulates p21 transcription by both wild-type and K319-321R p53 in a manner dependent upon its HAT domain, we reasoned that PCAF may be functioning through histone acetylation to regulate p21 transcription. PCAF exhibits HAT activity toward several lysines of histone 3, most potently lysine 14 (H3K14)34 as well as lysine 9.35 We first utilized chromatin immunoprecipitation (ChIP) in cells exposed to doxorubicin to determine whether PCAF could be localized directly to the p21 promoter, and whether induction of H3 acetylation marks at the p21 promoter after doxorubicin treatment required PCAF (Fig. 5A). A p53 ChIP was performed in parallel to be sure that PCAF depletion did not cause the loss of p53 from the p21 promoter (Fig. 5A). Additional acetyl-H3K14 and p53 ChIPs were then performed on chromatin from cells transfected with p53 or expressing ARF to confirm that results with doxorubicin treatment were applicable across different settings for p53 activity (Fig. 5B and C).
Figure 5. PCAF regulates histone H3 acetylation at the p21 promoter. U2OS (A) or H1299 (B) cells were transfected with the indicated siRNA and plasmids, or treated with or without 1 μM doxorubicin for 12 h (A). Real-time chIP signal was normalized to 5% input controls, and background IgG signal (less than 0.1% binding in all cases) was subtracted in each condition. ChIPs were performed using anti-p53 (FL-393, Santa Cruz), anti-acetyl-H3K14 (Active Motif), rabbit IgG (Jackson Immunoresearch), anti-acetyl-H3K9 (Abcam) and a mixture of PCAF antibodies [E-8, H-369 (Santa Cruz) and rabbit α-PCAF, a gift from Dr. Dietmar Spengler]. Immunoprecipitated DNA was amplified with GoTaq Polymerase (Promega) for 33 cycles and visualized by agarose gel electrophoresis and ethidium bromide staining (B and C), or with Power SYBR Green (Life Technologies) in an Eppendorf Realplex2 thermocycler (A).
Both PCAF and p53 could be localized to the distal p53 response element of the p21 promoter after doxorubicin treatment, with p53 showing clear induction of promoter occupancy, while PCAF demonstrated constitutive occupancy with a further modest induction after doxorubicin exposure (Fig. 5A). PCAF depletion resulted in the near complete loss of PCAF ChIP signal, whereas the p53 ChIP signal was unaffected (Fig. 5A). Both H3K9 and H3K14 acetylation marks were stimulated (2-fold for H3K9, 10-fold for H3K14) in relation to untreated cells. However, cells expressing PCAF siRNA exhibited only basal levels of H3K14 and H3K9 acetylation after doxorubicin treatment, consistent with a requirement for PCAF in stimulating histone H3 acetylation at the p21 promoter after genotoxic p53 activation (Fig. 5A).
The acetylation mark at H3K14 was also upregulated in ARF-transfected U2OS cells and p53-transfected H1299 cells (Fig. 5B and C), and this increase was also dependent upon PCAF. The reduction in promoter-bound p53 in ARF-expressing U2OS cells is likely a consequence of the low p53 levels unique to ARF-expressing, PCAF-depleted cells (Fig. 1A), as p53 occupancy at the p21 promoter was unaffected by PCAF knockdown in doxorubicin-treated U2OS cells (Fig. 5A) and p53-transfected H1299 cells (Fig. 5C). Thus, PCAF stimulates H3 acetylation at the p21 promoter after both genotoxic and non-genotoxic p53 activation.
Discussion
We have shown that PCAF is necessary to facilitate the stress-induced activation of the p21 promoter by creating a chromatin environment permissive for transcriptional activation. PCAF was necessary for p53-directed p21 transcription and cell cycle arrest in several cell types and in response to several stimuli, including p14ARF, p53 overexpression, Nutlin-3a-stimulated p53 activation and DNA damage induced by UV and doxorubicin. PCAF, however, was not required for p16INK4a-driven cell cycle arrest in U2OS cells, indicating its specific role in p53/p21 induced cell cycle arrest. Stress-induced induction of H3 acetylation marks acetyl-H3K9 and/or acetyl-H3K14 at the p21 promoter also required physiologic levels of PCAF in settings linked to p21 activation, including the introduction of p53 into H1299 cells or of ARF into U2OS cells as well as after doxorubicin-treatment of U2OS cells. The lack of histone acetylation in the absence of PCAF correlated tightly with p21 protein abundance and mRNA levels in all contexts. PCAF regulation of p21 expression required an intact PCAF HAT domain, suggesting that PCAF acetylase activity is directly required for p53-mediated transactivation of p21, and that PCAF may be acting directly as a key HAT at the p21 promoter.
In contrast to the significant contribution of PCAF to stress-induced p21 activation, p300 and CBP were dispensable for p21 induction by p14ARF. Depletion of CBP and p300 in U2OS or RPE1 cells resulted in spontaneous upregulation of basal p21 expression, suggesting that neither CBP nor p300 is absolutely necessary for p21 expression (Fig. 1A and B). In further support of our data, a recent report indicates that p300/CBP double-null MEFs are competent for p21 and MDM2 expression in response to the DNA damaging agents etoposide or doxorubicin.36 Furthermore, the same report also indicates that recruitment of CBP or p300 to target genes does not correlate well with the transcriptional status of these loci, suggesting that the presence of a putative co-activator protein at a locus is not itself predictive of target gene transcription. The roles of p300 and CBP in target gene activation by p53 therefore remain under investigation.
PCAF has previously been linked to p53 signaling via two separate mechanisms: an intrinsic MDM2-directed E3 ubiquitin ligase activity32 and direct acetylation of the p53 regulatory domain at lysine 320. Under conditions utilized in this study, we were unable to detect an increase in steady-state MDM2 in PCAF-depleted U2OS cells (Fig. S2). The lack of an effect of PCAF depletion on MDM2 abundance in our studies therefore raised the possibility that the effect on p21 expression was due to PCAF’s well-characterized HAT activity, either toward p53 or toward histones. However, a K320R p53 mutant that cannot be acetylated at K320 or a triple lysine to arginine mutation of the adjacent potentially compensating lysines (K319-321R) was still dependent on PCAF for p21 transactivation. This does not rule out the possibility that under certain stress conditions or in specific cell contexts, K320 is a physiologically relevant acetylation target of PCAF or another HAT. Previous experiments have shown that acetylation of the p53 K320 and K382 residues is associated with occupancy of a unique set of p53-responsive promoters, interaction with a unique set of p53 co-activators and differential roles in p53 localization and differential biologic outcomes.37 Furthermore, mp53 K317R (the corresponding murine residue to human p53 K320) knock-in MEFs exhibit increased apoptosis after genotoxic insult, suggesting that posttranslational modifications of mp53 K317 may contribute to p53 cell-fate decisions.38
A recent report indicates that PCAF acetylation of Rb plays a critical role in keratinocyte differentiation and cell cycle exit.39 However, we show that PCAF is dispensable for p16INK4a-induced (and presumably Rb-dependent) cell cycle arrest in U2OS cells (Fig. S1B), arguing against a general role for PCAF in cell cycle arrest downstream of p21 or p16INK4a expression. This difference in PCAF dependence of a presumed Rb-dependent cell cycle arrest between our system and the keratinocyte system may reflect a developmental or tissue-specific role for PCAF in Rb acetylation and localization.
While PCAF was critical for full induction of p21 in the stress conditions tested, p21 was still partially induced under DNA damaging conditions, even after PCAF silencing (Fig. 2C and D). In contrast, the p21 response to ARF expression (Fig. 1A and B) and Nutlin-3 treatment (Fig. 2B) was critically dependent upon the presence of physiologic PCAF levels. This difference is likely due to transactivation of the p21 promoter under DNA damage conditions through p53-independent mechanisms, a number of which have been described. In response to doxorubicin alone, a variety of factors are known to regulate p21 expression in a p53-independent manner, including the NFκB subunit p65,29-31 sphingosine kinase 2,40 and the transcriptional cofactor FHL2.41 This raises the intriguing possibility that NFκB and other factors may recruit acetyltransferase activities other than PCAF to the p21 promoter. The interplay between PCAF and p53-dependent and independent responses is clearly complex, and a full understanding of the interactions between PCAF and p53 transactivation will require further study.
The role of PCAF in direct acetylation of histones is similarly complex; the well-described correlation between acetylation of nucleosomes at promoters and transcription activity is still poorly understood,42-44 and the difficulty in engineering mutant histone knock-ins in mammalian cells precludes the definitive assessment of the importance of independent histone marks in transcription. Additionally, many of lines of evidence suggest multiple context-specific roles for GNAT acetyltransferases in histone acetylation. Analysis of the crystal structure of tetrahymena GCN5 in complex with acetyl-CoA and a histone H3 peptide indicate that lysine 14 and five surrounding residues of histone H3 mediate the interaction with GCN5.45 Importantly, the GCN5 residues involved in these interactions are evolutionarily conserved throughout the GNAT family.46 While in vitro analyses of GCN5 acetyltransferase activity indicate that H3K14 serves as the primary substrate for this activity,47 analysis of native SAGA complexes point to a broader specificity of these acetyltransferases than originally believed.48 It is possible that components of the native SAGA or PCAF complexes stabilize the weaker interaction of GCN5/PCAF with other histone H3 residues, such as those surrounding lysine 9.
Our data best support a model in which recruitment of PCAF HAT activity to the p21 locus by a transcriptional activator, in this case p53, allow for the occurrence of subsequent downstream events that ultimately lead to elongation by the RNA polymerase II complex. Previous analyses of the kinetics of p21 transcriptional activation suggest that histone acetylation is induced following a multitude of genotoxic stresses, but that histone acetylation alone is not sufficient to promote transcriptional elongation by poised Pol II.49-51 This suggests that acetylation of nucleosomes at the p21 promoter may allow the recruitment of additional regulatory factors required for the activation of poised Pol II, steps which are likely also regulated and represent an additional level of transcriptional control over p21 expression.
The mechanism of PCAF recruitment to the p21 promoter remains unclear. Several studies indicate that phosphorylated histone H3 peptides serve as preferred substrates for Gcn5-mediated binding and acetylation, and that phosphorylation and acetylation of histone H3 are coupled in vivo at the EGF-responsive c-fos promoter in response to stimulation by EGF.52,53 More recently, CDK8, which has been defined as a factor necessary for p21 transcriptional elongation in human cells,49 has been identified as a component of the Mediator complex responsible for phosphorylation of serine 10 of H3 at the p21 locus, priming H3 for subsequent acetylation of H3.54 Although the mode of recruitment of CDK8 and Mediator to the p21 promoter is not fully understood, histone phosphorylation by this complex could serve as the signal for the recruitment of PCAF HAT activity.
In addition to furthering the importance of PCAF in p53-dependent transcription, our experiments also provide a plausible explanation for previously described tumor suppressive functions of PCAF. Despite the normal development of PCAF-null mice,55 likely due to compensation by GCN5, PCAF is downregulated through methylation or allelic loss in a variety of cancers.56,57 Notably, PCAF induces G1 arrest and inhibits soft agar growth and tumorigenesis upon its reintroduction into cell lines established from these tumors. While the levels of several key cell cycle regulators, including p21, were evaluated in these studies, the mechanistic role of PCAF in this activity was not explored further. Our data suggest that expression of p21 in response to a panel of stresses that occur during oncogenesis specifically requires PCAF HAT activity and could provide at least a partial explanation for PCAF-mediated tumor suppression.
Taken together, this work defines a critical and specific role for PCAF in p53-p21 signaling and further highlights the importance of histone acetylation as opposed to non-histone (i.e., p53) acetylation, in facilitating transcriptional activation by p53. This also suggests a mechanism through which PCAF may exert tumor suppressor activity by regulating p53-dependent transcription. Of immediate interest will be to determine which, if any, broader subset of p53-responsive genes is regulated by PCAF. Recent work examining the p21 and PUMA promoters indicates a broad heterogeneity in chromatin structure and regulator recruitment between the two genes,51,58 raising the question of how HAT recruitment and activity are regulated at different p53 target loci. Recent evidence suggests that differential kinetics of transcriptional activation between these genes may be a consequence of the core promoter element (CPE) organization unique to each gene.59,60 Specifically, Nutlin-3-induced histone 4 acetylation levels at p21 or the pro-apoptotic Fas promoters exhibited highly contrasting rates of decay following Nutlin-3 removal, suggesting that the dynamics of histone acetylation differ between p53 target promoters. The interplay between PCAF and other regulatory factors present at the p21 promoter during p53-dependent stress responses10,19,49,61 will provide further insight into the complex regulation governing p21 transcriptional activation. Our data raise the possibility that PCAF could serve to impact p53-dependent cell-fate decisions; especially the critical arrest/senescence/apoptosis decision point that is a feature of all p53 responses.
Methods and Materials
Cell culture, plasmids and transfections.
U2OS cells were grown in DMEM supplemented with 10% fetal calf serum (Mediatech) and Penicillin/Streptomycin (Gibco). RPE1 and H1299 cells were grown under conditions recommended by ATCC. For transfections, 3.7 x 105 U2OS or RPE1, or 5 x 105 H1299 cells were plated in 6 cm dishes (BD-Falcon). Twenty-four h after plating, media was replaced with antibiotic-free media, and cells were transfected with 10 nM indicated siRNA and 1 µg pcDNA3-p14ARF or pcDNA3 vector using Lipofectamine 2000 (Life Technologies). Cells were harvested after transfection or treatment as indicated in figure legends. Lysates to be used in ChIP assays were harvested as described below. All other cell pellets were lysed in SDS buffer (2% SDS, 50 mM Tris pH 6.8). siRNA sequences: p300-CAG AGC AGU CCU GGA UUA Ctt; CBP-AAU CCA CAG UAC CGA GAA AUG UU; PCAF-UCG CCG UGA AGA AAG CGC Att.
Western blotting.
Protein concentrations were determined using the BCA assay kit (Pierce), 20 µg protein loaded in each well, separated by SDS-PAGE and transferred onto PVDF membranes. Blots were blocked using 5% milk in PBST for at least one hour prior to blotting. Antibodies: p53 DO-1 (Santa Cruz), PCAF E-8 (Santa Cruz), p21 Ab-1 (Oncogene Research), actin A2066 (Sigma Aldrich), p14ARF DCS-241 (Santa Cruz), p300 N-15 (Santa Cruz), CBP A-22 (Santa Cruz). When used, image brightness and contrast processing (ImageJ) was applied to the entire blot, maintaining linear exposures.
Flow cytometry.
Following transfection, three fourths of each cell pellet was fixed in ice-cold 95% EtOH for 24–48 h and stained with propidium iodide for flow cytometric analysis (Fig. 1C and D). Data analysis was performed using FlowJo (Tree Star, Inc.). S phase populations were determined by gating the same (250–350) FL2-A signal in each sample.
rtPCR.
Total cellular RNA was purified using an RNeasy kit according to manufacturer’s suggested protocol (Qiagen). Total cDNA was generated using AffinityScript reverse transcriptase (Agilent Technologies). One µL resulting cDNA was amplified in triplicate using SYBR green and primers specific to β-actin, p21 or p53.
Chromatin immunoprecipitation.
1 x 107 U2OS or H1299 cells were plated and transfected as described above. Prior to lysis, cells were crosslinked with 1.5 mM ethylene glycol-bis(succinimidylsuccinate) for 20 min in PBS, then treated with 1% formaldehyde in media for an additional 10 min. Crosslinking was quenched with 50 mM glycine in PBS for 10 min.62 Chromatin immunoprecipitations were performed using 100 µg (or 200 µg for PCAF chIPs) sonicated DNA as described.63 PCR was performed by amplifying the distal p53 response element of the p21 promoter with GoTaq polymerase (Promega) for 33 cycles for all samples. Primer sequences used to amplify the distal p53-RE of the p21 promoter by GoTaq were: 5'-TGA GCC TCC CTC CAT CCC TA and 5'-ACC ATC CCC TTC CTC ACC TG. All real-time ChIP analyses were performed for using Power SYBR Green Master Mix (Life Technologies) in an Eppendorf Realplex thermocycler and analyzed by the ΔΔCt method. Primer sequences used to amplify the distal p53-RE of the p21 promoter by real-time PCR were: 5'-AGC AGG CTG TGG CTC TGA TT and 5'-CAA AAT AGC CAC CAG CCT CTT CT. Ct values were determined by identifying the cycle at which signal reached 10 standard deviations above the mean signal of cycles 3–15. Antibodies: p53 goat FL-393 (Santa Cruz), acetylated H3K14 #39697 (ActiveMotif), acetylated H3K9 (Abcam), total histone H3 (Abcam), rabbit IgG (Jackson Immunoresearch), PCAF E-8 (Santa Cruz) and PCAF antisera raised in rabbit, which was a kind gift from Dr. Dietmar Spengler.
Supplementary Material
Acknowledgements
The authors wish to thank members of the Androphy and Grossman labs for helpful discussions and K. Ozato for PCAF cDNA. Rabbit PCAF antisera used in chIPs was a kind gift from Dr. Dietmar Spengler. E.A. was supported by R01-CA107394, and S.R.G. was supported by R01-CA107532. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here:
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/20864
References
- 1.Junttila MR, Evan GI. p53--a Jack of all trades but master of none. Nat Rev Cancer. 2009;9:821–9. doi: 10.1038/nrc2728. [DOI] [PubMed] [Google Scholar]
- 2.Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009;23:2134–9. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–5. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, Collado M, Villasante A, Strati K, Ortega S, Cañamero M, et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460:1136–9. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedelbaeva K, Snyder A, Gourevitch D, Clark L, Zhang XM, Leferovich J, et al. Lack of p21 expression links cell cycle control and appendage regeneration in mice. Proc Natl Acad Sci U S A. 2010;107:5845–50. doi: 10.1073/pnas.1000830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang LY, Scott M, Hayward RL, Mohammed H, Whitelaw CB, Smith GC, et al. p21(WAF1) is component of a positive feedback loop that maintains the p53 transcriptional program. Cell Cycle. 2011;10:932–50. doi: 10.4161/cc.10.6.15012. [DOI] [PubMed] [Google Scholar]
- 7.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–14. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiltz RL, Nakatani Y. The PCAF acetylase complex as a potential tumor suppressor. Biochim Biophys Acta. 2000;1470:M37–53. doi: 10.1016/s0304-419x(99)00037-2. [DOI] [PubMed] [Google Scholar]
- 9.Grossman SR. p300/CBP/p53 interaction and regulation of the p53 response. Eur J Biochem. 2001;268:2773–8. doi: 10.1046/j.1432-1327.2001.02226.x. [DOI] [PubMed] [Google Scholar]
- 10.Gamper AM, Roeder RG. Multivalent binding of p53 to the STAGA complex mediates coactivator recruitment after UV damage. Mol Cell Biol. 2008;28:2517–27. doi: 10.1128/MCB.01461-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barlev NA, Liu L, Chehab NH, Mansfield K, Harris KG, Halazonetis TD, et al. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol Cell. 2001;8:1243–54. doi: 10.1016/S1097-2765(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Scolnick DM, Trievel RC, Zhang HB, Marmorstein R, Halazonetis TD, et al. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol Cell Biol. 1999;19:1202–9. doi: 10.1128/mcb.19.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi K, Herrera JE, Saito S, Miki T, Bustin M, Vassilev A, et al. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 1998;12:2831–41. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lill NL, Grossman SR, Ginsberg D, DeCaprio J, Livingston DM. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–7. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 15.Scolnick DM, Chehab NH, Stavridi ES, Lien MC, Caruso L, Moran E, et al. CREB-binding protein and p300/CBP-associated factor are transcriptional coactivators of the p53 tumor suppressor protein. Cancer Res. 1997;57:3693–6. [PubMed] [Google Scholar]
- 16.Avantaggiati ML, Ogryzko V, Gardner K, Giordano A, Levine AS, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–84. doi: 10.1016/S0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 17.Gu W, Shi XL, Roeder RG. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–23. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 18.Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/S0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 19.Di Stefano V, Soddu S, Sacchi A, D’Orazi G. HIPK2 contributes to PCAF-mediated p53 acetylation and selective transactivation of p21Waf1 after nonapoptotic DNA damage. Oncogene. 2005;24:5431–42. doi: 10.1038/sj.onc.1208717. [DOI] [PubMed] [Google Scholar]
- 20.Yang XJ, Ogryzko VV, Nishikawa J, Howard BH, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–24. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 21.Ogryzko VV, Kotani T, Zhang X, Schiltz RL, Howard T, Yang XJ, et al. Histone-like TAFs within the PCAF histone acetylase complex. Cell. 1998;94:35–44. doi: 10.1016/S0092-8674(00)81219-2. [DOI] [PubMed] [Google Scholar]
- 22.Poux AN, Marmorstein R. Molecular basis for Gcn5/PCAF histone acetyltransferase selectivity for histone and nonhistone substrates. Biochemistry. 2003;42:14366–74. doi: 10.1021/bi035632n. [DOI] [PubMed] [Google Scholar]
- 23.Schiltz RL, Mizzen CA, Vassilev A, Cook RG, Allis CD, Nakatani Y. Overlapping but distinct patterns of histone acetylation by the human coactivators p300 and PCAF within nucleosomal substrates. J Biol Chem. 1999;274:1189–92. doi: 10.1074/jbc.274.3.1189. [DOI] [PubMed] [Google Scholar]
- 24.Le Cam L, Linares LK, Paul C, Julien E, Lacroix M, Hatchi E, et al. E4F1 is an atypical ubiquitin ligase that modulates p53 effector functions independently of degradation. Cell. 2006;127:775–88. doi: 10.1016/j.cell.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann A, Spengler D. A new coactivator function for Zac1’s C2H2 zinc finger DNA-binding domain in selectively controlling PCAF activity. Mol Cell Biol. 2008;28:6078–93. doi: 10.1128/MCB.00842-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossman SR, Deato ME, Brignone C, Chan HM, Kung AL, Tagami H, et al. Polyubiquitination of p53 by a ubiquitin ligase activity of p300. Science. 2003;300:342–4. doi: 10.1126/science.1080386. [DOI] [PubMed] [Google Scholar]
- 27.Shi D, Pop MS, Kulikov R, Love IM, Kung AL, Grossman SR. CBP and p300 are cytoplasmic E4 polyubiquitin ligases for p53. Proc Natl Acad Sci U S A. 2009;106:16275–80. doi: 10.1073/pnas.0904305106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 29.Basile JR, Eichten A, Zacny V, Münger K. NF-kappaB-mediated induction of p21(Cip1/Waf1) by tumor necrosis factor alpha induces growth arrest and cytoprotection in normal human keratinocytes. Mol Cancer Res. 2003;1:262–70. [PubMed] [Google Scholar]
- 30.Ma S, Tang J, Feng J, Xu Y, Yu X, Deng Q, et al. Induction of p21 by p65 in p53 null cells treated with Doxorubicin. Biochim Biophys Acta. 2008;1783:935–40. doi: 10.1016/j.bbamcr.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Li G, Ji Y, Liu C, Zhu J, Lu Y. Cytoplasmic p21 induced by p65 prevents doxorubicin-induced cell death in pancreatic carcinoma cell line. J Biomed Sci. 2012;19:15. doi: 10.1186/1423-0127-19-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linares LK, Kiernan R, Triboulet R, Chable-Bessia C, Latreille D, Cuvier O, et al. Intrinsic ubiquitination activity of PCAF controls the stability of the oncoprotein Hdm2. Nat Cell Biol. 2007;9:331–8. doi: 10.1038/ncb1545. [DOI] [PubMed] [Google Scholar]
- 33.Jiang H, Lu H, Schiltz RL, Pise-Masison CA, Ogryzko VV, Nakatani Y, et al. PCAF interacts with tax and stimulates tax transactivation in a histone acetyltransferase-independent manner. Mol Cell Biol. 1999;19:8136–45. doi: 10.1128/mcb.19.12.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marmorstein R. Structure of histone acetyltransferases. J Mol Biol. 2001;311:433–44. doi: 10.1006/jmbi.2001.4859. [DOI] [PubMed] [Google Scholar]
- 35.Jin Q, Yu LR, Wang L, Zhang Z, Kasper LH, Lee JE, et al. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 2011;30:249–62. doi: 10.1038/emboj.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasper LH, Thomas MC, Zambetti GP, Brindle PK. Double null cells reveal that CBP and p300 are dispensable for p53 targets p21 and Mdm2 but variably required for target genes of other signaling pathways. Cell Cycle. 2011;10:212–21. doi: 10.4161/cc.10.2.14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knights CD, Catania J, Di Giovanni S, Muratoglu S, Perez R, Swartzbeck A, et al. Distinct p53 acetylation cassettes differentially influence gene-expression patterns and cell fate. J Cell Biol. 2006;173:533–44. doi: 10.1083/jcb.200512059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chao C, Wu Z, Mazur SJ, Borges H, Rossi M, Lin T, et al. Acetylation of mouse p53 at lysine 317 negatively regulates p53 apoptotic activities after DNA damage. Mol Cell Biol. 2006;26:6859–69. doi: 10.1128/MCB.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickard A, Wong PP, McCance DJ. Acetylation of Rb by PCAF is required for nuclear localization and keratinocyte differentiation. J Cell Sci. 2010;123:3718–26. doi: 10.1242/jcs.068924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sankala HM, Hait NC, Paugh SW, Shida D, Lépine S, Elmore LW, et al. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67:10466–74. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- 41.Martin BT, Kleiber K, Wixler V, Raab M, Zimmer B, Kaufmann M, et al. FHL2 regulates cell cycle-dependent and doxorubicin-induced p21Cip1/Waf1 expression in breast cancer cells. Cell Cycle. 2007;6:1779–88. doi: 10.4161/cc.6.14.4448. [DOI] [PubMed] [Google Scholar]
- 42.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 43.Pogo BG, Allfrey VG, Mirsky AE. RNA synthesis and histone acetylation during the course of gene activation in lymphocytes. Proc Natl Acad Sci U S A. 1966;55:805–12. doi: 10.1073/pnas.55.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of rna synthesis. Proc Natl Acad Sci U S A. 1964;51:786–94. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rojas JR, Trievel RC, Zhou J, Mo Y, Li X, Berger SL, et al. Structure of Tetrahymena GCN5 bound to coenzyme A and a histone H3 peptide. Nature. 1999;401:93–8. doi: 10.1038/43487. [DOI] [PubMed] [Google Scholar]
- 46.Clements A, Rojas JR, Trievel RC, Wang L, Berger SL, Marmorstein R. Crystal structure of the histone acetyltransferase domain of the human PCAF transcriptional regulator bound to coenzyme A. EMBO J. 1999;18:3521–32. doi: 10.1093/emboj/18.13.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuo MH, Brownell JE, Sobel RE, Ranalli TA, Cook RG, Edmondson DG, et al. Transcription-linked acetylation by Gcn5p of histones H3 and H4 at specific lysines. Nature. 1996;383:269–72. doi: 10.1038/383269a0. [DOI] [PubMed] [Google Scholar]
- 48.Grant PA, Eberharter A, John S, Cook RG, Turner BM, Workman JL. Expanded lysine acetylation specificity of Gcn5 in native complexes. J Biol Chem. 1999;274:5895–900. doi: 10.1074/jbc.274.9.5895. [DOI] [PubMed] [Google Scholar]
- 49.Donner AJ, Szostek S, Hoover JM, Espinosa JM. CDK8 is a stimulus-specific positive coregulator of p53 target genes. Mol Cell. 2007;27:121–33. doi: 10.1016/j.molcel.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Espinosa JM, Verdun RE, Emerson BM. p53 functions through stress- and promoter-specific recruitment of transcription initiation components before and after DNA damage. Mol Cell. 2003;12:1015–27. doi: 10.1016/S1097-2765(03)00359-9. [DOI] [PubMed] [Google Scholar]
- 51.Gomes NP, Espinosa JM. Gene-specific repression of the p53 target gene PUMA via intragenic CTCF-Cohesin binding. Genes Dev. 2010;24:1022–34. doi: 10.1101/gad.1881010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell. 2000;5:905–15. doi: 10.1016/S1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- 53.Lo WS, Trievel RC, Rojas JR, Duggan L, Hsu JY, Allis CD, et al. Phosphorylation of serine 10 in histone H3 is functionally linked in vitro and in vivo to Gcn5-mediated acetylation at lysine 14. Mol Cell. 2000;5:917–26. doi: 10.1016/S1097-2765(00)80257-9. [DOI] [PubMed] [Google Scholar]
- 54.Meyer KD, Donner AJ, Knuesel MT, York AG, Espinosa JM, Taatjes DJ. Cooperative activity of cdk8 and GCN5L within Mediator directs tandem phosphoacetylation of histone H3. EMBO J. 2008;27:1447–57. doi: 10.1038/emboj.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamauchi T, Yamauchi J, Kuwata T, Tamura T, Yamashita T, Bae N, et al. Distinct but overlapping roles of histone acetylase PCAF and of the closely related PCAF-B/GCN5 in mouse embryogenesis. Proc Natl Acad Sci U S A. 2000;97:11303–6. doi: 10.1073/pnas.97.21.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez RE, Knights CD, Sahu G, Catania J, Kolukula VK, Stoler D, et al. Restoration of DNA-binding and growth-suppressive activity of mutant forms of p53 via a PCAF-mediated acetylation pathway. J Cell Physiol. 2010;225:394–405. doi: 10.1002/jcp.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu C, Qin YR, Xie D, Chua DT, Fung JM, Chen L, et al. Characterization of tumor suppressive function of P300/CBP-associated factor at frequently deleted region 3p24 in esophageal squamous cell carcinoma. Oncogene. 2009;28:2821–8. doi: 10.1038/onc.2009.137. [DOI] [PubMed] [Google Scholar]
- 58.Beckerman R, Donner AJ, Mattia M, Peart MJ, Manley JL, Espinosa JM, et al. A role for Chk1 in blocking transcriptional elongation of p21 RNA during the S-phase checkpoint. Genes Dev. 2009;23:1364–77. doi: 10.1101/gad.1795709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morachis JM, Murawsky CM, Emerson BM. Regulation of the p53 transcriptional response by structurally diverse core promoters. Genes Dev. 2010;24:135–47. doi: 10.1101/gad.1856710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gomes NP, Espinosa JM. Disparate chromatin landscapes and kinetics of inactivation impact differential regulation of p53 target genes. Cell Cycle. 2010;9:3428–37. doi: 10.4161/cc.9.17.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill R, Madureira PA, Waisman DM, Lee PW. DNA-PKCS binding to p53 on the p21WAF1/CIP1 promoter blocks transcription resulting in cell death. Oncotarget. 2011;2:1094–108. doi: 10.18632/oncotarget.378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Zeng PY, Vakoc CR, Chen ZC, Blobel GA, Berger SL. In vivo dual cross-linking for identification of indirect DNA-associated proteins by chromatin immunoprecipitation. Biotechniques. 2006;41:694–, 696, 698. doi: 10.2144/000112297. [DOI] [PubMed] [Google Scholar]
- 63.Carey MF, Peterson CL, Smale ST. Chromatin immunoprecipitation (ChIP) Cold Spring Harb Protoc. 2009;2009:t5279. doi: 10.1101/pdb.prot5279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.