Abstract
FGF signaling inhibits chondrocyte proliferation and requires the function of the p107 and p130 members of the Rb protein family to execute growth arrest. p107 dephosphorylation plays a critical role in the chondrocyte response to FGF, as overexpression of cyclin D1/CDK4 complexes (the major p107 kinase) in rat chondrosarcoma (RCS) cells overcomes FGF-induced p107 dephosphorylation and growth arrest. In cells overexpressing cyclin D1/CDK4, FGF-induced downregulation of cyclin E/CDK2 activity was absent. To examine the role of cyclin E/CDK2 complexes in mediating FGF-induced growth arrest, this kinase was overexpressed in RCS cells. FGF-induced dephosphorylation of either p107 or p130 was not prevented by overexpressing cyclin E/CDK2 complexes. Unexpectedly, however, FGF-treated cells exhibited sustained proliferation even in the presence of hypophosphorylated p107 and p130. Both pocket proteins were able to form repressive complexes with E2F4 and E2F5 but these repressors were not translocated into the nucleus and therefore were unable to occupy their respective target DNA sites. Overexpressed cyclin E/CDK2 molecules were stably associated with p107 and p130 in FGF-treated cells in the context of E2F repressive complexes. Taken together, our data suggest a novel mechanism by which cyclin E/CDK2 complexes can promote cell cycle progression in the presence of dephosphorylated Rb proteins and provide a novel insight into the key Retinoblastoma/E2F/cyclin E pathway. Our data also highlight the importance of E2F4/p130 complexes for FGF-mediated growth arrest in chondrocytes.
Keywords: cyclin E, CDK2, E2F4, p107, p130, chondrocytes
Introduction
Proper regulation of the cell cycle is vital for development, cell growth and differentiation. The family of cyclin-dependent kinases (CDKs) is one of the main players in this well-orchestrated process. CDKs pair with cell cycle-specific regulatory subunits known as cyclins to govern cell cycle progression. CDKs’ activity is regulated at multiple levels, transcriptionally, post-translationally and by CDK inhibitors (CKIs). Abnormal cyclin/CDK expression/activity can lead to deregulation of the cell cycle and ultimately to a malignant phenotype.1-5
Among the key CDKs substrates is the family of Retinoblastoma proteins, or “pocket proteins,” that includes the Retinoblastoma (pRb) and related p107 and p130 proteins. Retinoblastoma proteins regulate the cell cycle by controlling the activities of the E2F family of transcription factors.6 The activity of Rb proteins themselves is modulated by phosphorylation at several Ser/Thr residues: phosphorylation by CDKs inactivates pocket proteins and permits transcriptional activation of genes necessary for cell cycle progression.7,8
Cyclin D/CDK4 complexes and cyclin E or cyclin A/CDK2 complexes control G1 progression as well as S-phase entry and are responsible for inactivation of Retinoblastoma proteins.9 We previously established that p107 and p130, but not pRb, are required for the cell type-specific response of chondrocytes to FGF.10 While in most cell types FGF induces proliferation and protects from apoptosis, in chondrocytes, it causes growth arrest and induces some aspects of differentiation. Consistent with the growth inhibitory response, all Rb proteins become dephosphorylated upon FGF treatment, but while p130 and pRb undergo dephosphorylation long after exposure of the cells to FGF, p107 is dephosphorylated within the first hour. By overexpressing the cyclin D1/CDK4 complex (the major p107 kinase11), we were able to prevent p107 as well as p130 dephosphorylation and the growth suppression exerted by FGF.12 Interestingly, overexpression of cyclin D1/CDK4 also prevented the downregulation of cyclin E/CDK2 activity normally induced by FGF.
In the present study, we have examined the role of cyclin E/CDK2 complexes in mediating FGF-induced growth arrest by overexpressing this kinase. FGF-induced dephosphorylation of either p107 or p130 was not prevented by overexpressing cyclin E/CDK2 complexes. Surprisingly, however, FGF-treated cells exhibited sustained proliferation, even in the presence of hypophosphorylated p107 and p130. We were able to show that, while formed, E2F/p130 or p107 repressive complexes were not translocated into nucleus and therefore were unable to occupy their respective target DNAs. We demonstrate that in FGF-treated cells, overexpressed cyclin E/CDK2 molecules are stably associated with p107 and p130 in the context of E2F repressive complexes that are blocked in nuclear translocation. Taken together our data suggest a novel mechanism by which cyclin E/CDK2 overexpression promotes cell cycle progression in the presence of dephosphorylated Rb proteins and provide novel insights into the key Retinoblastoma/E2F/cyclin E pathway.
Results
Cyclin E/CDK2 complexes prevent FGF-induced cell cycle arrest in chondrocytes but not dephosphorylation of p107 and p130.
In our search for the key effector molecules responsible for the cell type-specific response of chondrocytes to FGF, we have established that FGF-induced growth arrest requires the function of the p107 and p130 members of Rb family, but not of pRb, consistent with the observation that p107/p130-knockout mice die at birth with exaggerated chondrocyte proliferation.13 Overexpression of cyclin D1/CDK4 in rat chondrosarcoma (RCS) cells prevented p107 dephosphorylation and the growth suppression exerted by FGF. These cells were also resistant to FGF-induced dephosphorylation of p130 and did not exhibit FGF-induced downregulation of cyclin E/CDK2 activity.12 This finding raised the question of whether downregulation of cyclin E/CDK2 activity was critical for mediating FGF-induced growth arrest.
We therefore generated RCS cell lines constitutively expressing cyclin E and CDK2 (Fig. 1). RCS cells were chosen for these experiments, as they exhibit most properties of proliferating chondrocytes, including the FGF-inhibitory response. FGF causes accumulation of chondrocytes in G0/G1,10 but overexpression of cyclin E alone resulted in partial resistance of cells to FGF-induced growth inhibition as determined by cell cycle analysis (Fig. 1A). When cyclin E was co-expressed with CDK2, the majority of the cells kept proliferating even after 48 h of FGF treatment (Fig. 1A), indicating that constitutive expression of cyclin E/CDK2 efficiently prevented FGF-induced growth arrest. Similar results were obtained either for nine different clones or for cell pools. Overexpression of CDK2 alone had no effect on FGF-induced growth inhibition.
Figure 1. Overexpression of active cyclin E/CDK2 complexes prevents FGF-induced cell-cycle arrest in the presence of hypo-phosphorylated p107 and p130. Retroviral vectors expressing FLAG-CDK2 or cyclin E were stably introduced into Rat ChondroSarcoma (RCS) cells individually or in combination. RCS cells with empty pBABEpuro vector were used as a control. Cells were treated with FGF1 (5 ng/ml) and heparin (5 μg/ml) for indicated periods of time. (A) FACScan™ analysis of RCS cells overexpressing CDK2, cyclin E or cyclin E/CDK2. Changes in the levels of S-phase cells are indicated. Several clones of each cell type as well as a pool were used with essentially identical results. (B) Cells overexpressing cyclin E/CDK2 proliferate in the presence of hypo-phosphorylated p107 and p130. 50 (upper panel) or 20 (lower panel) μg of total protein from indicated cell lines were analyzed by SDS-PAGE followed by immunoblotting with anti-p130 (upper panel) and anti-p107 (lower panel) antibodies. Hyper-(-P) and hypo-phosphorylated (deP) forms of p107 and p130 are indicated. (C) Expression of the cell cycle regulators in cyclin E/CDK2 cell line. Twenty μg of total protein were analyzed by western blots (WBs) with antibodies against CDK2, cyclin A, cyclin E, p21 and p27. Equal amount of protein loading was confirmed by α-tubulin immunodetection. (D–E) Effect of cyclin E/CDK2 overexpression on cyclin E/CDK2 and cyclin A/CDK2 kinase activities. (D) Kinase activity associated with cyclin E and cyclin A were determined in vitro, using histone H1 as a substrate. Kinase complexes were isolated using anti-cyclin E and anti-cyclin A antibodies from the indicated cell lines at different time points of FGF treatment. Typical autoradiographs of SDS-PAGE are shown. (E) Graphs show relative levels of cyclin E and cyclin A activities upon FGF treatment in different cell lines, as summarized from 5 independent experiments.
The protein levels of cyclin E were always higher when the cyclin was co-expressed with CDK2, likely due to increased cyclin E stability in the kinase complexes (Fig. 1C and data not shown). As cells continued to proliferate in the presence of FGF, we expected that a significant proportion of p107 and p130 proteins would be phosphorylated and therefore inactive. Surprisingly, both proteins were dephosphorylated in the cells overexpressing cyclin E or cyclin E/CDK2 with kinetics similar to parental RCS cells (Fig. 1B). As seen on the upper panel, FGF treatment leads to increased levels of p130, because the dephosphorylated form is more stable than hyperphosphorylated p130, which is targeted for degradation.14 The unexpected dephosphorylation of p107 and p130 in proliferating FGF-treated cyclin E/CDK2 cells, prompted us to determine the kinase activity of overexpressed cyclin E/CDK2 complexes.
We used in vitro kinase assay to evaluate relative levels of cyclin E-associated activity. Cyclin E was immunoprecipitated, and histone H1 was used as a substrate in the presence of γP32ATP. As shown on Figure 1D (upper panel), cyclin E/CDK2 kinase activity is drastically decreased in FGF-treated RCS cells, but cyclin E/CDK2 cells maintained about 20% of initial kinase activity after 24 h of FGF treatment. Interestingly, in these cells, endogenous levels of CDK2 were downregulated by FGF [lower band on CDK2 western blot (Fig. 1C)] similar to the CDK2 decline in parental RCS cells, but no such effect was observed in the case of overexpressed CDK2. CDK2 also binds cyclin A, and cyclin A/CDK2 complexes have been implicated in phosphorylation of pocket proteins. We therefore tested the kinase activity associated with cyclin A. As shown in Figure 1D (lower panel), the level of cyclin A-associated kinase activity remained up to 50% of an untreated control in cyclin E/CDK2 cells, compared with less than 5% in parental RCS cells. The data from all in vitro kinase assays are summarized in the graphs (Fig. 1E). Therefore, cyclin E/CDK2 overexpression modulates the activity of cyclin A/CDK2 complexes, and both kinases retained higher levels of activity throughout FGF treatment compared with parental RCS cells. Both p107 and p130, however, are being dephosphorylated upon FGF treatment. One of the reasons why cyclin E or cyclin A/CDK2 failed to phosphorylate p107 and p130 is perhaps the requirement for active cyclin D1/CDK4 complexes. Cyclin D1/CDK4 kinase is essential for differential phosphorylation of p107 and p130,8,11,12,14 and CDK4 expression is strongly downregulated by FGF in RCS cells. Surprisingly, cells overexpressing cyclin E/CDK2 showed downregulation of CDK4 (data not shown) and dephosphorylation of p107 and p130 in response to FGF, similar to parental RCS cells, yet these cells continued to proliferate. We therefore next investigated if dephosphorylated pocket proteins were capable of forming E2F-repressive complexes.
E2F4/p130 complexes do not occupy their FGF-responsive promoters when cyclin E/CDK2 complexes are overexpressed.
The best-defined role of the pocket proteins is to modulate the activity of E2F transcription factors, which control transcription of many essential components of cell cycle progression.15 Some E2F family members (E2F1–3) act as activators and some (E2F4– 5) as repressors. Pocket proteins associate differentially with these subgroups:16 hypophosphorylated Rb sequesters E2F1–3, preventing activation of cell cycle progression genes, and p107/p130 exclusively target E2F4 and E2F5, forming repressive complexes on corresponding target promoters. E2F4 and E2F5 are dispensable for the cell cycle progression but necessary for pocket protein-mediated G1 arrest.17,18 It is well established that both proteins lack a nuclear localization signal (NLS) and shuttle between the cytoplasm and nucleus mainly by association with their nuclear partners, which themselves possess NLS, such as p107 and p130.19,20
p107/E2F complexes are bound to a number of promoters in asynchronously growing cells,21 but most of these targets are repressed by E2F4/p130 complexes in growth arrested cells,22,23 and E2F4 accounts for the majority of E2F repressive complexes in G0/G1 arrested cells.24 Since we observed accumulation of dephosphorylated p130 in FGF-treated RCS as well as in cyclin E/CDK2 cells (Fig. 1B), one might expect that p130 should be able to execute its repressive functions in the context of E2F4/p130 complexes. To address this question we performed chromatin immunoprecipitation (ChIP) of pocket proteins and E2F4 complexes. The occupancy of several previously identified E2F4 target genes23 was assessed upon FGF treatment. We chose cyclin A, ATF-2 transcription factor and replication factor cdc6 as targets, as these genes are also known to be downregulated by FGF in chondrocytes.25 Starved RCS cells were used as cells differently accumulated in G0/G1. As shown in Figure 2, the occupancy of cdc6 promoter by E2F4 was significantly increased upon FGF treatment in parental RCS cells. Similar effects were observed for the cyclin A and ATF-2 promoters. The same patterns were observed when p130 antibodies were used for ChIP, indicating that both binding partners are present in repressive complexes. p130 was barely detected on the described promoters in untreated cells, likely either because of fluctuations in the p130 protein levels or because p107 can occupy these promoters in asynchronously growing cells. No FGF-induced increase in occupancy of these promoters by either E2F4 or p130 was detected in cyclin E/CDK2-overexpressing cells (Fig. 2), providing an explanation for why FGF-treated cyclin E/CDK2 cells were still proliferating. p107 was detected at cdc6 and cyclin A promoters at early time of FGF treatment but never later (data not shown), when RCS cells accumulated in G1/G0, suggesting that p107/E2F4 complexes are likely to be important intermediates only at early times of FGF treatment.
Figure 2. Analysis of E2F-specific promoter occupancy. RCS and cyclin E/CDK2 cell lines were treated with FGF for indicated periods of time. RCS cells were starved for 15 h in the presence of 0.1% FBS (marked as “S” above the panel). Chromatin and indicated immunoprecipitation were prepared according to the manufacture’s protocol (ChIP-ITTM Express Enzymatic, Active Motif®). Recovered DNA was assayed by PCR, using corresponding primers for cdc6, cyclin A2 and ATF-2 promoters. The resulting PCR products were separated on 2% agarose gels and visualized by ethidium bromide staining (upper row). Bar graphs (bottom row) show input-normalized intensity of each ChIP band: [ChIP volume-mock volume (IgG)]/(input volume-mock volume). Input reaction represents 0.5% of total chromatin for each sample. α-actin promoter was used as a negative control on which no E2F4/p130 complexes were detected (data not shown).
Overexpression of cyclin E/CDK2 prevents translocation of E2F4/p130 inhibitory complexes into nuclei.
As we were unable to detect any increase in promoter occupancy by E2F4 repressive complexes in FGF-treated cyclin E/CDK2 cells, we checked the cellular localization of E2F4. It’s known that E2F4 activity is regulated by its ability to be translocated to the nucleus as a result of its association with specific binding partners.26 E2F4 was mostly detected in nuclei of FGF-treated RCS cells as assayed by immunofluorescence (Fig. 3A). In FGF-treated cyclin E/CDK2 cells, however, E2F4 was mainly localized in the cytoplasm (Fig. 3B), suggesting an inability of the E2F4/p130 complexes to translocate into the nucleus. We therefore assessed whether E2F4 and E2F5 were able to complex with p107 or p130 in cyclin E/CDK2-overexpressing cells.
Figure 3. Overexpression of cyclin E/CDK2 complexes prevents FGF-induced translocation of E2F4 into the nuclei. (A) RCS and (B) cyclin E/CDK2 cell lines were plated on coverslips and treated with FGF for 15 h. Following PFA fixation and permeabilization, cells were processed for indirect immunofluorescence microscopy using anti-E2F4 antibodies (red), as indicated. Cell nuclei were visualized with Hoescht staining (blue). Higher magnification images are shown on the lower panels for both cells lines.
Separation of nuclear and cytoplasmic extracts was monitored by marker proteins specific for nucleus (HDAC1) or cytoplasm (α-tubulin) (Fig. 4A). In RCS cells FGF induced p107 and p130 translocation to the nucleus with kinetics that matched the different dephosphorylation kinetics of each protein. A major increase in the concentration of p107 in the nucleus was detected at 1–3 h of FGF treatment and much later for p130 (Fig. 4A and B). In contrast, no considerable changes in p107 localization were observed in FGF-treated cyclin E/CDK2 cells, and dephosphorylated p130 was found to persist mainly in the cytoplasm. A similar scenario was observed for E2F4, with almost no protein detected in the nuclear fraction of FGF-treated cyclin E/CDK2 cells, while E2F4 was detected in the nucleus of FGF-treated RCS cells. There was no difference in the localization of E2F2 (pRb partner that possesses NLS) (Fig. 4B).
Figure 4. Overexpression of cyclin E and CDK2 prevents translocation of E2F4 repressive complexes into the nuclei. RCS and cyclin E/CDK2 cell lines were treated with FGF for indicated period of times and fractionated using Thermo Scientific* NE-PER Nuclear and Cytoplasmic Extraction Kit, according the manufacture’s protocol. (A, B) 20 (or 50 for p130) μg of total protein from each cytoplasmic fraction and equivalent volume of nuclear extract were separated on SDS-PAGE following inmmunodetection with indicated antibodies. Equal amount of protein loading was confirmed by α-actin immunodetection. Anti-HDAC1 and anti-α-tubulin antibodies were used to monitor equal loading and fractionation of lysates.
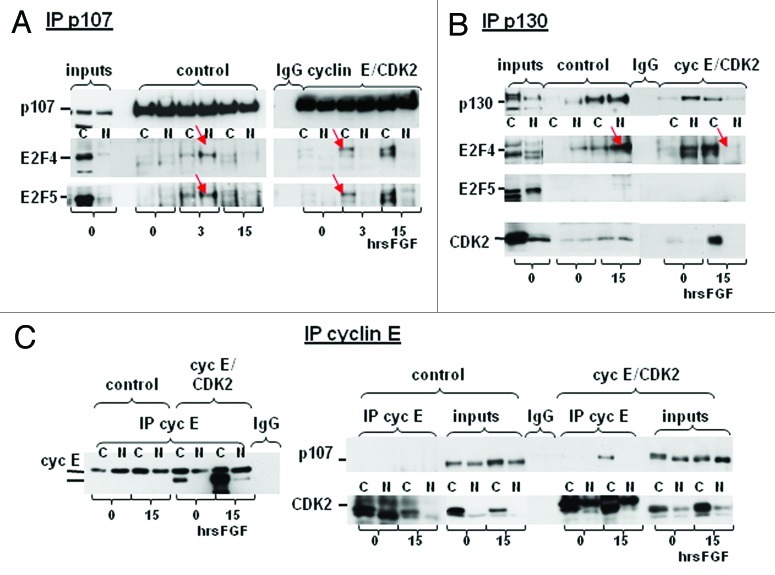
To determine whether hypophosphorylated p107 and p130 were unable to bind E2F in cyclin E/CDK2 cells, and therefore unable to mediate E2F4–5 translocation, we assayed complex formation between p107/p130 and E2F4 and E2F5 in the cytoplasm and nucleus upon FGF treatment. Consistent with our hypothesis that E2F4–5/p107 repressive complexes are transient in nature, we were able to detect FGF-induced association of p107 with E2F4 and E2F5 in RCS cells only at 3 h, but not at later times (Fig. 5A). Importantly, the proportion of E2F4 or E2F5/p107 complexes was significantly higher in nucleus than in the cytoplasm. In cyclin E/CDK2 cells, FGF strongly induced this association even at later times, but the complex was almost entirely cytoplasmic (Fig. 5A), and a similar situation was observed when p130 immunoprecipitates were assayed for the presence of E2F4 and E2F5 (Fig. 5B). While E2F4/p130 complexes were mainly detected in the nucleus of FGF-treated RCS cells, they were primarily restricted to the cytoplasm in FGF-treated cyclin E/CDK2 cells. We were not able to detect any E2F5/p130 complexes, consistent with previously published data, pointing to E2F4/p130 as the main repressive complex in G1/G0.23 Taken together, these data suggest that, while E2F4 or E2F5/p107 and E2F4/p130 repressive complexes are formed upon FGF treatment of cyclin E/CDK2 cells, their translocation to the nucleus is prevented, and, consequently, the cells are resistant to FGF-induced growth arrest.
Figure 5. Overexpression of cyclin E and CDK2 does not interfere with E2F/pocket proteins complex formation but blocks translocation of these repressive complexes into the nuclei. (A–C) RCS and cyclin E/CDK2 cell lines were treated with FGF for indicated period of times and fractionated using Thermo Scientific* NE-PER Nuclear and Cytoplasmic Extraction Kit, according the manufacture’s protocol. The immune complexes (50%) and inputs (3%) were resolved on SDS-PAGE and analyzed by immunoblotting with indicated antibodies. Arrows indicate differences in nuclear localization of repressive E2F4–5 complexes in RCS and cyclin E/CDK2 cell lines. (C) The lower band recognized by cyclin E antibodies on the left panel corresponds to overexpressed human cyclin E which runs lower then endogenous rat cyclin E.
To investigate a possible reason for inability of p107/p130/E2F complexes to translocate to the nucleus, we checked if overexpression of cyclin E/CDK2 affected the composition of E2F4–5/p107/p130 complexes. Association of p107 and cyclin E in either FGF-treated or untreated RCS cells was barely detectable, but this binding was strongly induced by FGF exclusively in the cytoplasm of cyclin E/CDK2 cells (Fig. 5C). The same result was obtained for p130 (data not shown). At the same time, as expected, CDK2 was detected in cyclin E immunoprecipitates in both cell lines (Fig. 5C). To confirm these data, complementary IP was performed, and p130 immunoprecipitates from FGF-treated and untreated cells were checked for the presence of CDK2. As shown in Figure 5B, CDK2 was barely detectable but was observed to be equally distributed in p130 complexes in the nuclear and the cytoplasmic lysates from RCS cells. On the other hand, association of p130 with CDK2 was strongly induced by FGF but only detected in the cytoplasmic fraction of cyclin E/CDK2 cells.
Taken together, our data reveal a novel mechanism by which cells overexpressing cyclin E can overcome growth inhibition mediated by the members of Rb family. When cyclin E/CDK2 levels are elevated, E2F4/p130 complexes, which are vital for suppression of genes during growth arrest, are retained in the cytoplasm and therefore cannot bind to and repress the expression of genes that promote cell cycle progression.
Discussion
The E2F/Rb pathway is one of major determinants of cell cycle progression and therefore is tightly regulated. Cell cycle arrest mediated by Rb proteins is generally dominant, but when overridden can lead to abnormal cell proliferation. We show in this report that overexpression of cyclin E/CDK2 overcomes FGF-induced growth arrest in chondrocytes even in the presence of dephosphorylated/active Rb proteins. The unique FGF-inhibitory response of chondrocytes requires the function of p107 and p130 but not pRb, and both proteins become dephosphorylated upon FGF treatment. Several kinases (cyclin D1/CDK4 and cyclin E or cyclin A/CDK2) are important for inactivation of pocket proteins. We have previously shown that p107 dephosphorylation was a critical step in the FGF-inhibitory response of chondrocytes, as constitutive overexpression of the cyclin D1/CDK4 kinase resulted in the prevention of FGF-induced p107 dephosphorylation as well as of cell cycle arrest. FGF-treated cyclin D1/CDK4 cells also exhibited higher levels of cyclin E/CDK2 activity.
To fully understand the role of cyclin E/CDK2 complexes in FGF-induced growth arrest, we overexpressed them in RCS cells. These cells were able to overcome FGF-induced growth arrest. The basal level of cyclin E/CDK2 activity was only moderately higher in these cells, probably due to elevated levels of CDK inhibitors p21 and p27. The existing level of cyclin E/CDK2 activity was not sufficient to overcome FGF-induced p107 and p130 dephosphorylation (Fig. 1B), but cells proliferated even in the presence of hypophosphorylated p107 and p130. In chondrocytes the FGF-induced p107 dephosphorylation is mediated by PP2A, but it is not yet clear whether dephosphorylation of p130 results from activation of a phosphatase or inactivation of a kinase. There may be several reasons why overexpression of cyclin E/CDK2 does not overcome FGF-induced p107 and p130 dephosphorylation. As pointed out before, cyclin D1/CDK4 kinase is a major p107 kinase, but CDK4 levels were downregulated by FGF in parental RCS cells as well as in cyclin E/CDK2 cells. The cyclin D1/CDK4 kinase was also shown to be involved in phosphorylation of p130; the prominent levels of cyclin E and cyclin A/CDK2 activities present in FGF-treated cyclin E/CDK2 cells may not be sufficient to inactivate p107 and p130 when cyclin D1/CDK4/activity is low. A more trivial explanation could be that the levels of E/CDK2 activity reached are not sufficient to counteract FGF-induced phosphatases. Cyclin A/CDK2 activity is barely inhibited in FGF-treated cyclin E/CDK2 cells in contrast to FGF-treated RCS cells. This resistance to inhibition can be mediated (1) by higher cyclin A protein level; accordingly, E2F4/p130 repressive complexes were barely detectible on the cyclin A promoter in FGF-treated cells or (2) by additional sequestering of CKIs p21 and p27 by overexpressed cyclin E/CDK2 complexes. The latter should be an important factor, as FGF signaling causes a prominent increase in the expression of CDK inhibitors (Fig. 1C).27
The most intriguing finding in our studies is that despite p107 and p130 dephosphorylation, the cells still proliferate. It was reported that overexpression of cyclin E can drive cell cycle progression in the presence of hypo-phosphorylated Rb proteins.28 The authors suggested Rb-independent, cell cycle-related functions of cyclin E. It was shown later that one of the cyclin E/CDK2 substrates, p220NPAT, is important for S-phase progression and mediates transcription of histone genes.29,30 While p220NPAT is indeed a cyclin E/CDK2 substrate, even non-dephosphorylated p220NPAT mutant was able to support histone H4 gene transcription. We demonstrated that, in FGF-treated chondrocytes, dephosphorylated p130 formed functional repressive complexes with E2F4 on target gene promoters, while cyclin E/CDK2 cells failed to show any increase in the promoter occupancy by E2F4/p130 complexes. For example, the occupancy of ATF-2 promoter by E2F4/p130 was strongly increased in FGF-treated RCS cells but not in cyclin E/CDK2 cells. In chondrocytes, ATF-2 is a known activator of cyclin A, and downregulation of both cyclin A and its activator is likely necessary for FGF-induced growth arrest.31
To occupy their targets, E2F4/p130 must be translocated into the nucleus. It is known that E2F4 and E2F5 lack NLS and shuttle between the cytoplasm and nucleus mainly by association with their nuclear partners, which contain NLS themselves.19,20 As we demonstrated, E2F4 was present in nucleus of FGF-treated chondrocytes, but E2F4 was not translocated into the nucleus of FGF-treated cyclin E/CDK2 cells. Comparable results were obtained when these cells were fractionated, and formation of E2F4, E2F5/p107 and p130 complexes was assayed. E2F4 or E2F5/p107 complexes were detected in chondrocytes only at 1–3 h of FGF treatment, supporting our hypothesis that these intermediates are transient in nature and responsible for the induction of transcriptional repression. Indeed, E2F4/p130 complexes are the main repressors at the late times of FGF treatment, when the majority of RCS cells have accumulated in G0/G1, and therefore are important late mediators of the FGF inhibitory response. We were unable to detect any E2F5/p130 complexes in FGF-treated cells, consistent with previously published data pointing to E2F4/p130 as the main repressive complex in G1/ G0.23
Accumulation of E2F4/p130 complexes in the cytoplasm of FGF-treated cyclin E/CDK2 cells correlates with high protein levels of cyclin E and CDK2. We were able to show that E2F4/p130 complexes are distinct in their composition from those observed in parental RCS cells. Cyclin E and CDK2 were found to be stably associated with p107 and p130 exclusively in the cytoplasm, and this interaction was stimulated by FGF. The stable binding of p107 and p130 with CDK2 might be mediated either by p107/p130s cyclin binding domain, which is located in the spacer region or by CDK inhibitory domain.32 p21 and p27 CDK inhibitor levels are increased in FGF-treated cells and likely to effectively compete with p107/p130 for CDK binding, thereby leaving the spacer region available for maintaining p107/p130 and CDK2 interaction. Cyclin E/CDK2 and p107/p130 binding does not interfere with pocket region-mediated interaction of p107 and p130 with E2F4 and E2F5, as we were able to detect these proteins in p107 and p130 immunoprecipitates simultaneously with cyclin E/CDK2.
The bipartite NLS of p130 is located near the C terminus. In addition to the C-terminal NLS, the intact pocket domain of p130 itself was shown to be sufficient for nuclear translocation.33 While direct hindrance with NLSs seems unlikely, the E2F/p107 or p130/cyclin E/CDK2 complexes probably undergo conformational changes that block translocation or interfere with importin binding. There is also a possibility that cyclin E/CDK2 complexes stably associated with p107 and p130 might mediate binding of another unidentified protein, which would directly mask NLS.
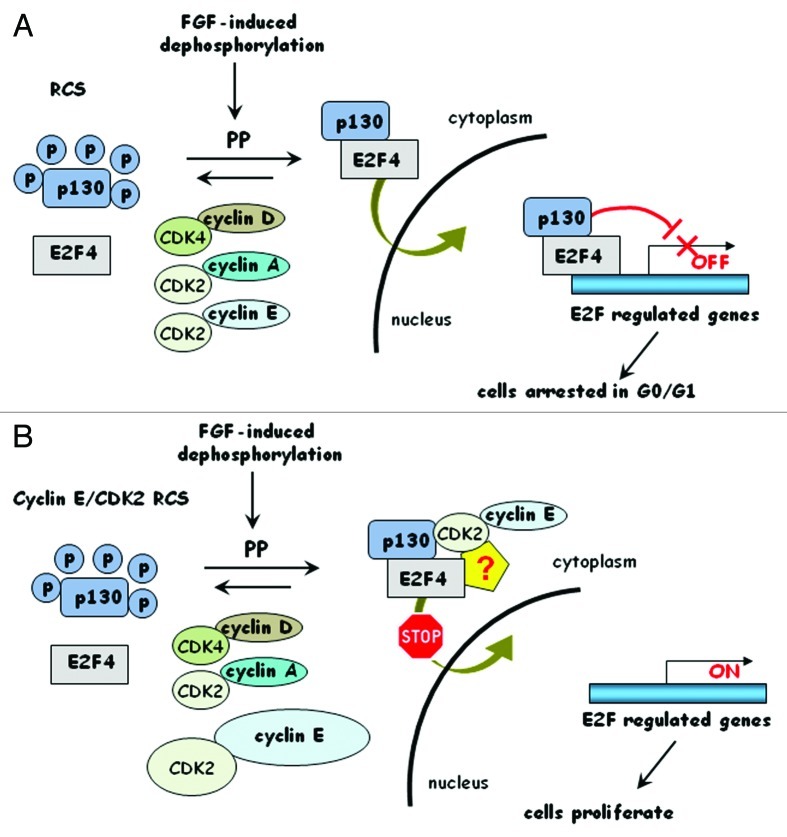
To our knowledge, the ability of cyclin E/CDK2 overexpression to antagonize the translocation of E2F/p107/p130 complexes has not been previously reported and, while the residual kinase activity of cyclin E/CDK2 (and cyclin A/CDK2) might be important for observed proliferation (for p220NPAT or other substrates), it is clear that inability of E2F/p107 and p130 complexes to translocate into the nucleus is a major component of the FGF resistance of cyclin E/CDK2 cells (Fig. 6).
Figure 6. A working model of how constitutive overexpression of cyclin E/CDK2 overcomes the FGF-growth inhibitory response in chondrocytes. (A) FGF signaling causes cell cycle arrest in chondrocytes (RCS cells). It induces dephosphorylation of all Rb proteins and p107 and p130 are important mediators of this unique non-proliferative response. Dephosphorylated p130 as well as p107 (not shown) mediate E2F4 translocation into the nucleus and therefore promotes transcriptional repression of cell cycle progression genes. CDKs implicated in inactivation of Rb proteins are indicated. Protein Phosphatases (PPs) counteract CDK-mediated phosphorylation and mediate the FGF-response, although this has been conclusively shown so far only for PP2A-meditated dephosphorylation of p107. (B) In cells overexpressing cyclin E/CDK2, dephosphorylated p130 forms stable complexes not only with E2F4 but also with the cyclin E/CDK2 kinase. This complex is unable to translocate into the nucleus either because of some structural rearrangements or due to cyclin E/CDK2-mediated binding to another unidentified protein (yellow pentagon). E2F regulated genes are not repressed and these cells continue to proliferate in the presence of dephosphorylated Rb proteins.
Taken together, our data reveal a novel mechanism, by which cells overexpressing cyclin E/CDK2 can overcome growth inhibition mediated by members of the Rb family. Overexpression of cyclin E/CDK2 complexes strongly stabilizes their interaction with dephosphorylated p107 and p130 in the context of E2Fs repressive complexes and blocks their translocation into the nucleus. This mechanism might be particular important in case of disregulated cyclin E expression when E2F4/p130 function is required. In this scenario, often seen in cancers, overexpressed cyclin E would inevitably jeopardize E2F4-repressive functions.
Materials and Methods
Reagents and antibodies.
All chemicals were from Sigma-Aldrich, ATP from New England BioLabs, γP32ATP from PerkinElmer. The following antibodies were used: anti-cyclin E, anti-CDK2, anti-CDK4, anti-p21, anti-p130, anti-p107, anti-E2F4, anti-E2F5, anti-E2F2, agarose-conjugated anti-cyclin E (Santa Cruz Biotechnology); anti-α-tubulin (clone B-5-1-2); anti-actin (Sigma-Aldrich); anti-cyclin A (Zymed), anti-HDAC1 (Upstate) and anti-p27 (Cell Signaling Technology).
Cell culture and FACS analysis.
Rat chondrosarcoma (RCS) cells were maintained in DMEM supplemented with 10% fetal calf serum at 37°C and 9% CO2. Retroviral vectors expressing FLAG-CDK2 (pBABEpuro backbone; FLAG-CDK2 was inserted between EcoR1 and BamH1 sites) or cyclin E (pLXSN backbone; cyclin E was inserted between EcoR1 and BamH1 sites) were stably introduced into rat chondrosarcoma (RCS) cells individually or in combination. Several clones of each cell type as well as a pool were used with essentially identical results. RCS cells with empty pBABEpuro or pLXSN vectors were used as a control. Cells were treated with FGF1 (5 ng/ ml) (a kind gift from M. Mohamadi) and heparin (5 µg/ml), as indicated in figure legends. Flow cytometry was performed using FACScanTM (Becton Dickinson) and analyzed using ModFit LTTM (Verity Software House) software.
Immunoprecipitation, western blot analysis and in vitro kinase assay.
Protein lysates were prepared using buffer A (50 mM TRIS-HCl pH 7.4, 150 mM NaCl, 10 mM KCl, 1% NP-40, 1 mM EDTA) in the presence of phosphatase inhibitors (1 mM Na3VO4, 10 mM NaF, 10 mM Na4P2O7) and protease inhibitors (leupeptin, pepstatin and aprotinin 1 µg/ml each). For immunoprecipitation, 0.5 mg of total protein was pre-cleared by incubating with Protein G-Sepharose® 4B Conjugate (ZYMED) for 30 min at 4°C, and then incubated with 2 µg of antibody overnight at 4°C. Agarose-conjugated anti-p107 (sc-318AC), anti-p130 (sc-317AC) and anti-cyclin E (sc-481AC) antibodies (Santa Cruz Biotechnology) were used. The immune complexes were washed four times with 1 ml of buffer A. Input (3%) from RCS cells containing empty vector and immunoprecipitates (50%) were resolved on 10% SDS-PAGE and analyzed by immunoblotting. The cyclin A/CDK2 and cyclin E/CDK2 activities were determined using histone H1 as a substrate as described previously.25 Anti-mouse IgG was used as a negative control. Kinase reactions were resolved on 10% SDS-PAGE and subjected to autoradiography. To fractionate cellular lysates Thermo Scientific* NE-PER Nuclear and Cytoplasmic Extraction Kit was used according the manufacture’s protocol.
ChIP analysis.
Chromatin was prepared according to the manufacturer’s protocol (ChIP-ITTM Express Enzymatic, Active Motif®). Crude chromatin was incubated with 2 µg of antibodies against E2F4 (sc-1082) or p130 (sc-317) (Santa Cruz). Recovered DNA was assayed by PCR using corresponding primers for cdc6, cyclin A2 and ATF-2 promoters (sequences available upon request). The primer sets were designed similar to the locations of corresponding primers used for ChIP experiments in human cells.23 The resulting PCR products were separated on 2% agarose gel and visualized by ethidium bromide staining. The intensities of the enrichment in ChIP assays were quantitated using ImageJ software (NIH) and normalized to the input. Bar graphs show input-normalized intensity of each ChIP band: (ChIP volume-mock volume)/(input volume-mock volume). Input reaction represents 0.5% of total chromatin for each sample. α-actin promoter was used as a negative control. RCS cells were starved for 15 h in the presence of 0.1% FBS (marked as S in Fig. 3).
Immunofluorescence microscopy.
RCS and cyclin E/CDK2 cell lines were plated on coverslips for 24 h and then treated with FGF for 15 h. Following fixation with 4% PFA and permeabilization, cells were processed for indirect immunofluorescence microscopy34 using anti-E2F4 or anti-p130 antibodies. Cell nuclei were visualized with Hoescht staining (1 µg/ml final).
Acknowledgments
We thank J. Kraynak for his help in some of the experiments, Lisa Dailey, Upal Basu Roy and Alka Mansukhani for critical reading of the manuscript. We are grateful to B. Dynlacht, B. Amati and M. Pagano for provided plasmids. This investigation was supported by PHS Grant DE013745 from the NICDR, and by a postdoctoral fellowship from the NCI Training Grant T32 CA009161 to V.K.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/20944
References
- 1.Müller-Tidow C, Metzger R, Kügler K, Diederichs S, Idos G, Thomas M, et al. Cyclin E is the only cyclin-dependent kinase 2-associated cyclin that predicts metastasis and survival in early stage non-small cell lung cancer. Cancer Res. 2001;61:647–53. [PubMed] [Google Scholar]
- 2.Furihata M, Ohtsuki Y, Sonobe H, Shuin T, Yamamoto A, Terao N, et al. Prognostic significance of cyclin E and p53 protein overexpression in carcinoma of the renal pelvis and ureter. Br J Cancer. 1998;77:783–8. doi: 10.1038/bjc.1998.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eguchi N, Fujii K, Tsuchida A, Yamamoto S, Sasaki T, Kajiyama G. Cyclin E overexpression in human gallbladder carcinomas. Oncol Rep. 1999;6:93–6. doi: 10.3892/or.6.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Dellas A, Schultheiss E, Leivas MR, Moch H, Torhorst J. Association of p27Kip1, cyclin E and c-myc expression with progression and prognosis in HPV-positive cervical neoplasms. Anticancer Res. 1998;18(6A):3991–8. [PubMed] [Google Scholar]
- 5.Scaltriti M, Eichhorn PJ, Cortés J, Prudkin L, Aura C, Jiménez J, et al. Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc Natl Acad Sci USA. 2011;108:3761–6. doi: 10.1073/pnas.1014835108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang HS, Gavin M, Dahiya A, Postigo AA, Ma D, Luo RX, et al. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell. 2000;101:79–89. doi: 10.1016/S0092-8674(00)80625-X. [DOI] [PubMed] [Google Scholar]
- 7.Hinds PW, Mittnacht S, Dulic V, Arnold A, Reed SI, Weinberg RA. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-C. [DOI] [PubMed] [Google Scholar]
- 8.Zarkowska T, Mittnacht S. Differential phosphorylation of the retinoblastoma protein by G1/S cyclin-dependent kinases. J Biol Chem. 1997;272:12738–46. doi: 10.1074/jbc.272.19.12738. [DOI] [PubMed] [Google Scholar]
- 9.Planas-Silva MD, Weinberg RA. The restriction point and control of cell proliferation. Curr Opin Cell Biol. 1997;9:768–72. doi: 10.1016/S0955-0674(97)80076-2. [DOI] [PubMed] [Google Scholar]
- 10.Laplantine E, Rossi F, Sahni M, Basilico C, Cobrinik D. FGF signaling targets the pRb-related p107 and p130 proteins to induce chondrocyte growth arrest. J Cell Biol. 2002;158:741–50. doi: 10.1083/jcb.200205025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leng X, Noble M, Adams PD, Qin J, Harper JW. Reversal of growth suppression by p107 via direct phosphorylation by cyclin D1/cyclin-dependent kinase 4. Mol Cell Biol. 2002;22:2242–54. doi: 10.1128/MCB.22.7.2242-2254.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolupaeva V, Laplantine E, Basilico C. PP2A-mediated dephosphorylation of p107 plays a critical role in chondrocyte cell cycle arrest by FGF. PLoS One. 2008;3:e3447. doi: 10.1371/journal.pone.0003447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cobrinik D, Lee MH, Hannon G, Mulligan G, Bronson RT, Dyson N, et al. Shared role of the pRB-related p130 and p107 proteins in limb development. Genes Dev. 1996;10:1633–44. doi: 10.1101/gad.10.13.1633. [DOI] [PubMed] [Google Scholar]
- 14.Tedesco D, Lukas J, Reed SI. The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCF(Skp2) Genes Dev. 2002;16:2946–57. doi: 10.1101/gad.1011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blais A, Dynlacht BD. E2F-associated chromatin modifiers and cell cycle control. Curr Opin Cell Biol. 2007;19:658–62. doi: 10.1016/j.ceb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beijersbergen RL, Bernards R. Cell cycle regulation by the retinoblastoma family of growth inhibitory proteins. Biochim Biophys Acta. 1996;1287:103–20. doi: 10.1016/0304-419x(96)00002-9. [DOI] [PubMed] [Google Scholar]
- 17.Gaubatz S, Lindeman GJ, Ishida S, Jakoi L, Nevins JR, Livingston DM, et al. E2F4 and E2F5 play an essential role in pocket protein-mediated G1 control. Mol Cell. 2000;6:729–35. doi: 10.1016/S1097-2765(00)00071-X. [DOI] [PubMed] [Google Scholar]
- 18.Beijersbergen RL, Kerkhoven RM, Zhu L, Carlée L, Voorhoeve PM, Bernards R. E2F-4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev. 1994;8:2680–90. doi: 10.1101/gad.8.22.2680. [DOI] [PubMed] [Google Scholar]
- 19.Lindeman GJ, Gaubatz S, Livingston DM, Ginsberg D. The subcellular localization of E2F-4 is cell-cycle dependent. Proc Natl Acad Sci USA. 1997;94:5095–100. doi: 10.1073/pnas.94.10.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaubatz S, Lees JA, Lindeman GJ, Livingston DM. E2F4 is exported from the nucleus in a CRM1-dependent manner. Mol Cell Biol. 2001;21:1384–92. doi: 10.1128/MCB.21.4.1384-1392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balciunaite E, Spektor A, Lents NH, Cam H, Te Riele H, Scime A, et al. Pocket protein complexes are recruited to distinct targets in quiescent and proliferating cells. Mol Cell Biol. 2005;25:8166–78. doi: 10.1128/MCB.25.18.8166-8178.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cam H, Balciunaite E, Blais A, Spektor A, Scarpulla RC, Young R, et al. A common set of gene regulatory networks links metabolism and growth inhibition. Mol Cell. 2004;16:399–411. doi: 10.1016/j.molcel.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi Y, Rayman JB, Dynlacht BD. Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev. 2000;14:804–16. [PMC free article] [PubMed] [Google Scholar]
- 24.Moberg K, Starz MA, Lees JA. E2F-4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol Cell Biol. 1996;16:1436–49. doi: 10.1128/mcb.16.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dailey L, Laplantine E, Priore R, Basilico C. A network of transcriptional and signaling events is activated by FGF to induce chondrocyte growth arrest and differentiation. J Cell Biol. 2003;161:1053–66. doi: 10.1083/jcb.200302075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verona R, Moberg K, Estes S, Starz M, Vernon JP, Lees JA. E2F activity is regulated by cell cycle-dependent changes in subcellular localization. Mol Cell Biol. 1997;17:7268–82. doi: 10.1128/mcb.17.12.7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aikawa T, Segre GV, Lee K. Fibroblast growth factor inhibits chondrocytic growth through induction of p21 and subsequent inactivation of cyclin E-Cdk2. J Biol Chem. 2001;276:29347–52. doi: 10.1074/jbc.M101859200. [DOI] [PubMed] [Google Scholar]
- 28.Alevizopoulos K, Vlach J, Hennecke S, Amati B. Cyclin E and c-Myc promote cell proliferation in the presence of p16INK4a and of hypophosphorylated retinoblastoma family proteins. EMBO J. 1997;16:5322–33. doi: 10.1093/emboj/16.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye X, Wei Y, Nalepa G, Harper JW. The cyclin E/Cdk2 substrate p220(NPAT) is required for S-phase entry, histone gene expression, and Cajal body maintenance in human somatic cells. Mol Cell Biol. 2003;23:8586–600. doi: 10.1128/MCB.23.23.8586-8600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miele A, Braastad CD, Holmes WF, Mitra P, Medina R, Xie R, et al. HiNF-P directly links the cyclin E/CDK2/p220NPAT pathway to histone H4 gene regulation at the G1/S phase cell cycle transition. Mol Cell Biol. 2005;25:6140–53. doi: 10.1128/MCB.25.14.6140-6153.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beier F, Taylor AC, LuValle P. Activating transcription factor 2 is necessary for maximal activity and serum induction of the cyclin A promoter in chondrocytes. J Biol Chem. 2000;275:12948–53. doi: 10.1074/jbc.275.17.12948. [DOI] [PubMed] [Google Scholar]
- 32.Castaño E, Kleyner Y, Dynlacht BD. Dual cyclin-binding domains are required for p107 to function as a kinase inhibitor. Mol Cell Biol. 1998;18:5380–91. doi: 10.1128/mcb.18.9.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chestukhin A, Litovchick L, Rudich K, DeCaprio JA. Nucleocytoplasmic shuttling of p130/RBL2: novel regulatory mechanism. Mol Cell Biol. 2002;22:453–68. doi: 10.1128/MCB.22.2.453-468.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham RE. Indirect immunofluorescent labeling of fixed cells. Methods Mol Biol. 2010;588:335–9. doi: 10.1007/978-1-59745-324-0_34. [DOI] [PubMed] [Google Scholar]