Abstract
Aim
To evaluate the safety of compacted DNA nanoparticles (NPs) in retinal pigment epithelial (RPE) cells.
Materials & Methods
Enhanced GFP expression cassettes controlled by the RPE-specific vitelloform macular dystrophy promoter were constructed with and without a bacterial backbone and compacted into NPs formulated with polyethylene glycol-substituted lysine 30-mers. Single or double subretinal injections were administered in adult BALB/c mice. Expression levels of enhanced GFP, proinflammatory cytokines and neutrophil/macrophage mediators, and retinal function by electroretinogram were evaluated at different time-points postinjection.
Results
Immunohistochemistry and real-time PCR demonstrated that NPs specifically transfect RPE cells at a higher efficiency than naked DNA and similar results were observed after the second injection. At 6 h postinjections, a transient inflammatory response was observed in all cohorts, including saline, indicating an adverse effect to the injection procedure. Subsequently, no inflammation was detected in all experimental groups.
Conclusion
This study demonstrates the safety and efficacy of NP-mediated RPE gene transfer therapy following multiple subretinal administrations.
Keywords: enhanced green fluorescent protein, gene transfer, nanoparticle, nonviral, repeat delivery, retinal pigment epithelial cell, subretinal injection, toxicity
The success of any gene therapy strategy is dependent on the route of administration and expression of the therapeutic gene in the diseased tissues with minimal or no toxicity. Viral-mediated gene delivery is widely used in gene therapy applications. However, safety concerns associated with the use of viruses in humans make nonviral delivery an attractive alternative. While most nonviral systems are less efficient at introducing and maintaining transgene expression, they can be nonpathogenic and nonimmunogenic. Recent advancements on the formulation of nonviral vectors have significantly improved their efficiency in differentiated, postmitotic cells [1–3]. An example of such an enhancement is the development of compacted DNA nanoparticles (NPs) formulated with polyethylene glycol (PEG)-substituted lysine 30-mers (CK30PEG). We and others have used this nonviral system in vivo and demonstrated efficacy in gene transfer to the eye, lung and brain [3–8]. The diameter of acetate formulated rod-like NPs is approximately 8 nm and can compact DNA up to at least 20 kbp without compromising efficiency [6]. If properly adapted, DNA NPs may provide a vehicle for delivery of genes to treat and prevent different forms of ocular diseases. For such indications, tissue-specific expression in various retinal cell types, including retinal pigment epithelial (RPE) cells, may be required.
Limited toxicity studies of CK30PEG NPs have been studied in the lung, brain and retina [3,5–7]. In the lung, where NPs are administered to airway epithelia of mice, the particles elicited only a minimal cytokine response and minor histological findings at the highest dose of 100 μg DNA but no preclinical toxicity [3]. Compaction of the DNA appears to minimize potential CpG dinucleotide-mediated inflammation [3,8]. The system has been successfully used in a Phase I/IIa clinical trial in cystic fibrosis subjects with no adverse events attributable to the NPs and with most patients having improved cystic fibrosis gene function [9]. In the brain, NPs also showed minimal signs of inflammation although transgene expression in neuronal and glial tissues was significantly high [7]. In the eye, subretinal delivery of NPs carrying a reporter gene such as enhanced GFP (eGFP) directed by a cytomegalovirus promoter or a photoreceptor-specific gene, such as RDS directed by interphoto receptor retinoid-binding protein, a tissue-specific promoter, showed no signs of a local inflammatory response or disruption of retinal structure and function in adult and newborn mice [4,10]. Although NP safety studies thus far demonstrate their lack of immunogenicity and ability to induce an inflammatory response, additional safety evaluation in ocular tissues is still warranted. Moreover, potential toxicities were never assessed with repeated administration of NPs in the eye. Since transgene expression with compacted DNA NPs in the eye might have restricted expression duration, repeat injection could be an option for patients to boost gene expression for long-term treatment. Furthermore, the expression plasmids contained in the NPs may induce inflammation in different tissues based on CpG content. Several studies suggest that bacterial backbones when depleted of CpG dinucleotides generate reduced inflammation [11–14]. The bacterial backbone may also influence expression duration [15]. Therefore, in this study we decided to evaluate gene expression and potential induction of inflammation by compacted NPs containing either plasmid DNA with a typical bacterial backbone containing 292 CpG dinucleotides or a linear DNA fragment of the identical expression cassette without a prokaryotic backbone (carrying 75 CpG dinucleotides).
Disorders of RPE cells represent a specific class of genetic diseases for which there are no proven therapies. As conventional treatments are limited, exploring the next generation of therapeutics is justifiably warranted, which may involve the use of target-specific genes and/or gene replacement therapy. Very recently, we have tested the transfection efficiency, distribution and uptake of our RPE-specific NPs in driving transgene expression [16]. The vitelliformmacular dystrophy (VMD2) promoter was used to express eGFP in a circular plasmid (C-eGFP). We reported that this NP injected in eyes retained higher number of intact episomal plasmids than their naked DNA counterparts and was able to achieve expression levels up to 30 days postinjection (PI). Furthermore, unlike naked DNA, which only transfected cells at the site of injection, NPs were able to transfect cells throughout the RPE cell layer. To advance the compacted DNA NP technology for future use as a gene delivery strategy for the treatment of RPE-associated diseases, in the present study we have evaluated expression and focused on safety profiles after single and double subretinal administrations of two RPE-specific NPs and compared these profiles with their uncompacted (naked) DNA counterparts. The VMD2 promoter was used to express eGFP in a C-eGFP or in a linear DNA fragment containing the expression cassette (L-eGFP) and lacking the bacterial backbone. Results presented here demonstrate for the first time that repeated subretinal delivery of NPs produces equivalent gene expression, regardless of whether NPs contain circular or linear DNA. No signs of inflammation, defects in retinal function, or reduction in endogenous gene expression in photoreceptors or RPE cells were detected in eyes receiving single or double injections of either NPs.
Materials & methods
Animal studies
All mice studied here were maintained in the breeding colony under cyclic light (12L:12D) conditions; all experiments were approved by the local Institutional Animal Care and Use Committees. All experiments and animal maintenance were approved by the University of Oklahoma Health Science Center Institutional Animal Care and Use Committee, conformed to the National Institute of Health Guide for the Care and Use of Laboratory Animals and the Association for Research in Vision and Ophthalmology Resolution on the Use of Animals in Research. Balb/c mice that were 1-month-old were obtained from Harland Laboratories (CO, USA) and maintained in our breeding colony under cyclic light (12L:12D) conditions for 2 weeks before their use; cage illumination was approximately 7 footcandles during the light cycle.
Vector construction
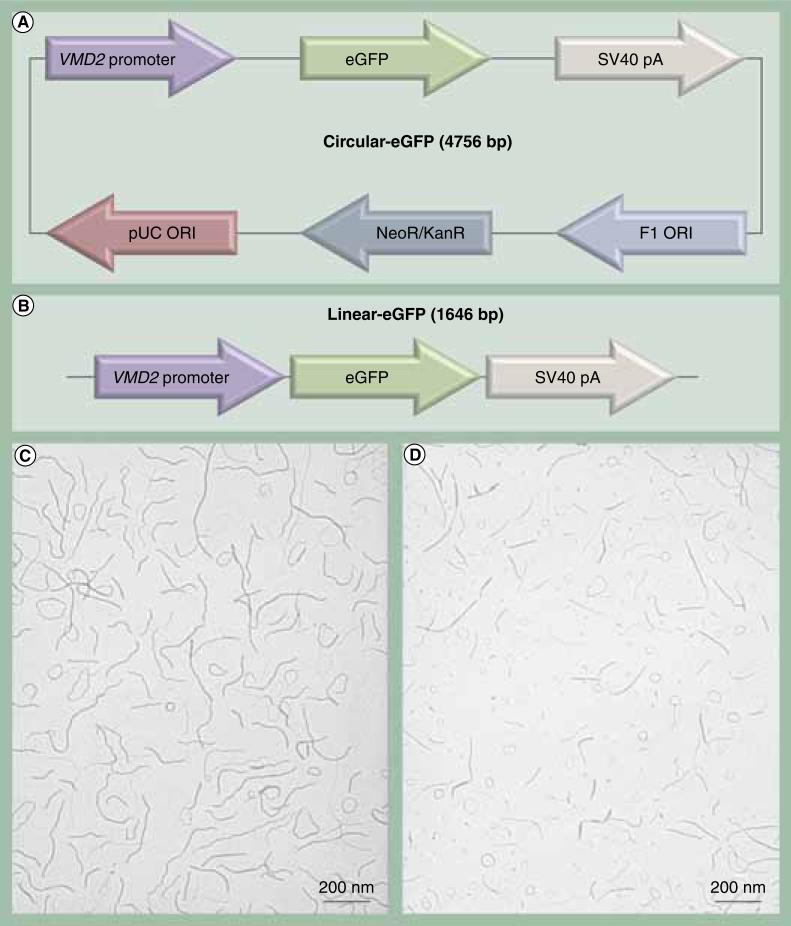
Previously, our laboratory constructed the VMD2-eGFP vector (Figure 1A) and showed RPE-specific eGFP expression in a proof-of-principle study [16]. We chose an approximately 600-bp fragment of the human VMD2 promoter (-585 to +38) based on recently published work demonstrating RPE-specific expression in transgenic mice [17]. VMD2-eGFP plasmid (C-eGFP) was used as a template for PCR amplification of the expression cassette, including the promoter, eGFP cDNA and the SV40 polyA sequences, to produce an L-eGFP. PCR amplification was performed using a high fidelity and high yield DNA polymerase (KOD Hot Start DNA Polymerase from Novagencatalog #71086-4) (Figure 1B). The fidelity and accuracy of the linear construct was confirmed by sequence analysis. PCR products were processed for endotoxin removal by anion exchange chromatography at Aldevron LLC (ND, USA). DNA was concentrated to 4.3 mg/ml. The final endotoxin levels for L-eGFP and C-eGFPwere <5 EU/mg DNA.
Figure 1. Vector design and transmission electron microscopy of dNA nanoparticles formulated with acetate.
(A) Maps of the circular and (B) linear eGFP expression cassettes containing 292 and 75 CpG dinucleotides, respectively. (C) Transmission electron microscopy of circular eGFP and (d) linear eGFP nanoparticles. The presence of acetate as the lysine counterion during the compaction procedure produces rod-shaped nanoparticles with minor diameters ranging from 8 to 11 nm. Magnification is 40,000. eGFP: Enhanced GFP.
DNA NP preparation
DNA NPs were formulated by mixing DNA with CK30PEG10K, a 30-mer lysine peptide with an N-terminal cysteine that is conjugated via a maleimide linkage to 10 kDa PEG, as described previously [2,9]. The polycation has an acetate counterion at the time of DNA mixing that formulates rod-shaped NPs. NPs were concentrated to 4.3 mg/ml of DNA in saline and processed by tangential flow filtration to remove excess CK30PEG10k. DNA NPs were characterized by a panel of quality control tests, including: transmission electron microscopy (NP size and shape) (Figures 1C & 1D); turbidity and saline sedimentation analyses (colloidal stability); serum stability test (protection of DNA from nucleases); endotoxin measurements; and gel analysis (DNA integrity). DNA NPs containing either C-eGFP or L-eGFP that met the standards of all quality control assays were used in this study.
Subretinal injections
Single or double subretinal injections were performed as described previously [18]. The second subretinal injection was administered 1 month after the first injection. Briefly, 6-week-old wild-type Balb/c mice were anesthetized by an intramuscular injection of 80 mg/kg ketamine (Fort Dodge Animal Health; IA, USA) and 14 mg/kg xylazine (The Butler Company; OH, USA). After complete dilation, a sterile 28-gauge needle (BD Biosciences, NJ, USA) was used to carefully puncture the cornea, avoiding any contact with the lens. A 33-gauge blunt-ended needle attached to a 10 μl Nanofil® syringe (World Precision Instruments, FL, USA) was then inserted into the puncture under an operating microscope (Carl Zeiss Surgical, Inc., NY, USA). In total 1 μl of saline containing 4.3 μg of compacted or naked DNA was delivered into the subretinal space. We chose this concentration for two reasons: first, we have shown in prior studies that this dose can achieve high transgene levels in RPE cells [16] and in the retina equivalent to approximately 88% of rhodopsin [5] and second, this is the highest concentration of DNA NPs that can be efficiently manufactured and showed no toxicity in the lung and brain. Saline alone (vehicle) and uninjected animals were used as controls. In all cases, the samples were injected into the nasal-central part of the eye. GonakHypromellose eye drops (2.5%; Akorn, Inc., IL, USA) were applied on the cornea immediately after the procedure. Injected mice were then evaluated at 6, 24 and 72 h PI. Treated mice did not develop any adverse side effects such as shivering, irritability and anxiety at any time points after vector delivery. Animals with complications, such as subretinal bleeding or damage to the lens, were excluded from analysis, which was less than 1%. We routinely achieve approximately 60–70% detachment of the retina from 1 μl injection. We have previously reported the extent of retinal detachment in mice achieved from 1 μl injection and the time course of reattachment, along with functional recovery [18].
Quantitative real-time PCR analysis
Real-time PCR was performed as previously described [5,19]. Briefly, total RNA was extracted from the tissue of a single eye using Trizol reagent (Invitrogen Inc., CA, USA) and then 2 μg of isolated RNA was treated with RNAse-free DNAse I (Promega Inc., CA, USA). Reverse transcription (RT) was performed using an oligo-dT primer and Superscript III reverse transcriptase (Invitrogen Inc., CA, USA). A no-RT (contains everything except the reverse transcriptase) sample was used as a control for any residual compacted DNA or genomic DNA contaminants. Quantitative real-time PCR was performed in triplicate on each cDNA sample using a Bio-Rad C1000 Thermal Cycler and ΔcT values were calculated against the mouse β-actin as a housekeeping gene. Relative gene expression values were determined using Equation 1: relative expression = 2-ΔcT where ΔcT = (Gene cT−β-actin cT). At least four injected (including saline injected controls) and four uninjected eyes from each treatment group at each of the scheduled time-points were analyzed. Three independent quantitative real-time PCR experiments for each set of samples were performed. We did not see any signals in the uninjected or saline injected samples, as well as the no-RT controls. Agarose gel electrophoresis and disassociation curve analysis were also performed on all PCR products to confirm proper amplification. A complete list of primers used in this study is presented in Table 1.
Table 1.
Table of primers used.
| Name | Primer |
|---|---|
| IL-6 | F 5′ ATGAACAACGATGATGCACTTG |
| R 5′ TATC CAGTTTGGTAGCATCCAT | |
| IL-β | F 5′ TTCAAGGGGACATTAGGCAG |
| R 5′ TGTGCTGGTGCTTCATTCAT | |
| TNF-α | F 5′ GGGACAGTGACCTGGACTGT |
| R 5′ CTCCCTTTGCAGAACTCAGG | |
| IFN-γ | F 5′ TGAACGCTACACACTGCATC |
| R 5′ CACTCGGATGAACTCATTGA | |
| Mouse β-actin | F 5′ TGTTACCAACTGGGACGACA |
| R 5′ CTTTTCACGGTTGGCCTTAG | |
| Enhanced GFP | F 5′ GACGTAAACGGCCACAAGTT |
| R 5′ AAGTCGTGCTGCTTCATGTG | |
Cytokine protein assay
All mice were perfused with saline before eyes were enucleated [5,19] for cytokine quantification as described. Eyes were snap frozen in liquid nitrogen and pulverized. A total of 500 μl of 1 × phosphate-buffered saline (PBS) containing a protease inhibitor cocktail was added to the pulverized tissue powder. The samples were homogenized using a pestle pellet homogenizer and freeze-thawed twice followed by centrifugation for 10 min at 13,000 rpm at 4°C. Supernatants were evaluated by protein assay and kept frozen at -80°C overnight. The cytokine assay was performed as previously described [20]. Briefly, tissue homogenates were assayed for cytokines (IL-6, IL-1β, TNF-α and IFN-γ) using a multiplexed magnetic bead-based immunoassay kit from Bio-Rad Hercules (CA, USA; catalog #171-304070). The cytokine assay consisted of eight standards in duplicates, three positive controls (endotoxin-injected tissues) in triplicates, two blank wells and other samples in triplicates. For the actual assay, 25 μg of protein in 50 μl total volume were prepared for each sample. Antibody and bead dilutions were prepared according to the manufacturer's protocol. Samples were immediately processed using the Bio-Plex™ Suspension Array system in a low PhotoMultiplier Tube® setting. Cytokine assay data were analyzed using Bio-PlexManager™ (version 4.0) software (Bio-Rad).
Immunohistochemistry
Tissue fixation and sectioning were performed as described previously [5,19]. Briefly, eyes from mice at 6, 24 and 72 h PI were enucleated and fixed with PBS containing 4% paraformaldehyde at 4°C for 1 h. The cornea and lens were then removed and the eye was returned to fixative for an additional 2 h. The eyecups were immersed in 10 and 20% (w/v) sucrose solutions for at least 1 h each and 30% sucrose solution overnight. Individual eyes were embedded in M1 embedding medium (Thermo Electron Corporation, PA, USA) and frozen on dry ice; frozen sections (10 μm thickness) aligned with the vertical meridian were cut with a cryostat (Leica, Inc., IL, USA) and collected on precleaned Superfrost-plus® microscope slides (Fisher Scientific, Inc., PA, USA). The entire eye was sectioned and every tenth section was collected. For immunohistochemistry, sections were blocked in 5% bovine serum albumin/ PBS containing 0.5% Triton-X for 30 min at room temperature. Slides were washed briefly with PBS and incubated with the rat monoclonal anti-F4/80 (1:500; Abcam, Inc., MA, USA) macrophage marker or rat monoclonal antibody (NIMP-R14) to neutrophils (1:500; Abcam, Inc.) in 1× bovine serum albumin/PBS, 0.5% Triton-X overnight at 4°C, followed by a brief wash in PBS and incubation with the secondary antibody, anti-rat fluorescent dye(1:1000; Alexa 555; Invitrogen Inc., CA, USA), in 1× bovine serum albumin/PBS and 0.5% Triton-X for 40 min at room temperature. After a brief wash in PBS, mounting medium with 4’,6-diamidino-2-phenylindole (Vectashield; Vector Laboratories, CA, USA) was applied, and a coverslip was applied to the slide. Observation and imaging were performed using an epifluorescent microscope (AxiophotZeiss Ltd, Germany) and a spinning disk confocal microscope (BX62 Olympus, Japan). Exposure time of treated eyes was calibrated in fluorescein isothiocyanate channel to eliminate autofluoresce seen throughout the RPE and outer segment layer.
Scotopic & photopic electroretinography
Electroretinography (ERG) measurements were performed as previously described [5,19]. To quantitate rod- and cone-mediated visual function, both scotopic (rod) and photopic (cone) ERGs were recorded for all cohorts from 30 days PI post single and double treatments. Significance was determined using one-way analysis of variance and post hoc tests using Bonferroni's pair-wise comparisons (Prism, ver. 3.02; GraphPad, CA, USA).
Results
Synthesis & characterization of NPs
To further evaluate the efficacy of CK30PEG NPs in delivering genes to RPE cells, we conducted a set of proof-of-principle experiments using NPs containing a VMD2-eGFP expression cassette as either C-eGFP or a L-eGFP lacking a bacterial backbone (Figures 1A & 1B). Highly purified C-eGFP and L-eGFP DNA with endotoxin less than 5 EU/mg were compacted into rod-shaped CK30PEG NPs and concentrated to 4.3 μg DNA/μl. To evaluate the homogeneity of sizes and shapes of the prepared NPs, scanning electron microscopy analyses were performed on both NPs and images are shown in Figures 1C & 1D. Both NPs were uniformly compacted as rod-like structures having diameters between 8 and 11 nm. Both NPs otherwise demonstrated colloidal stability in saline and met all quality control standards [2,3,9].
Quantification of eGFP expression
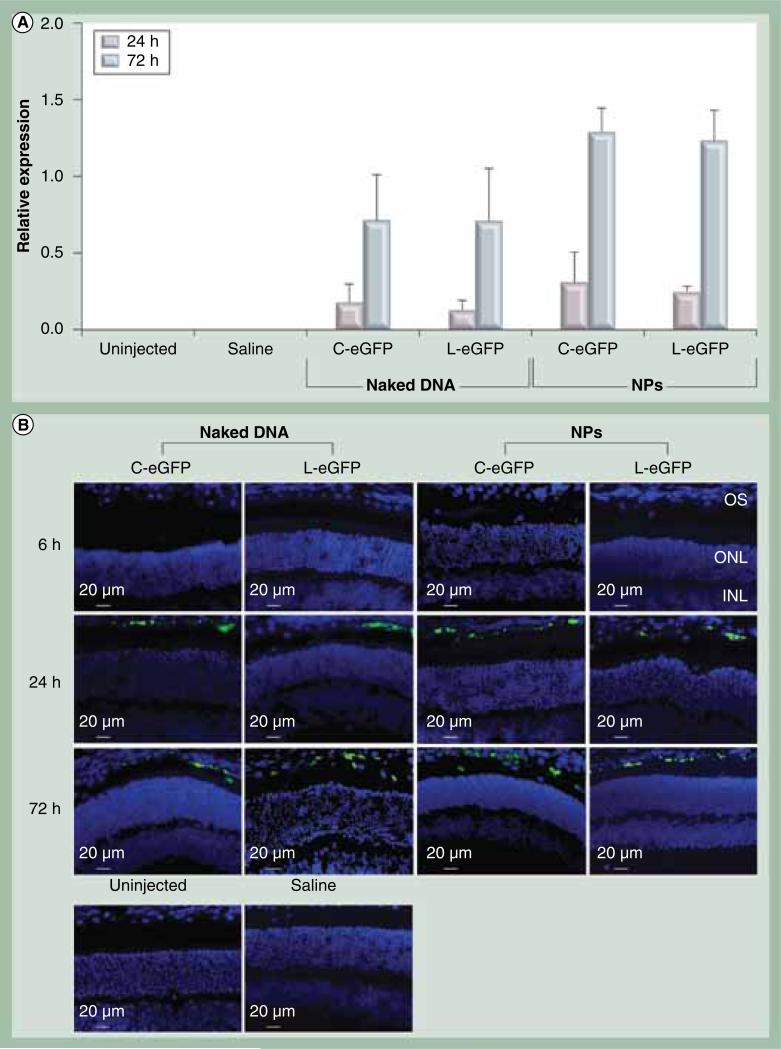
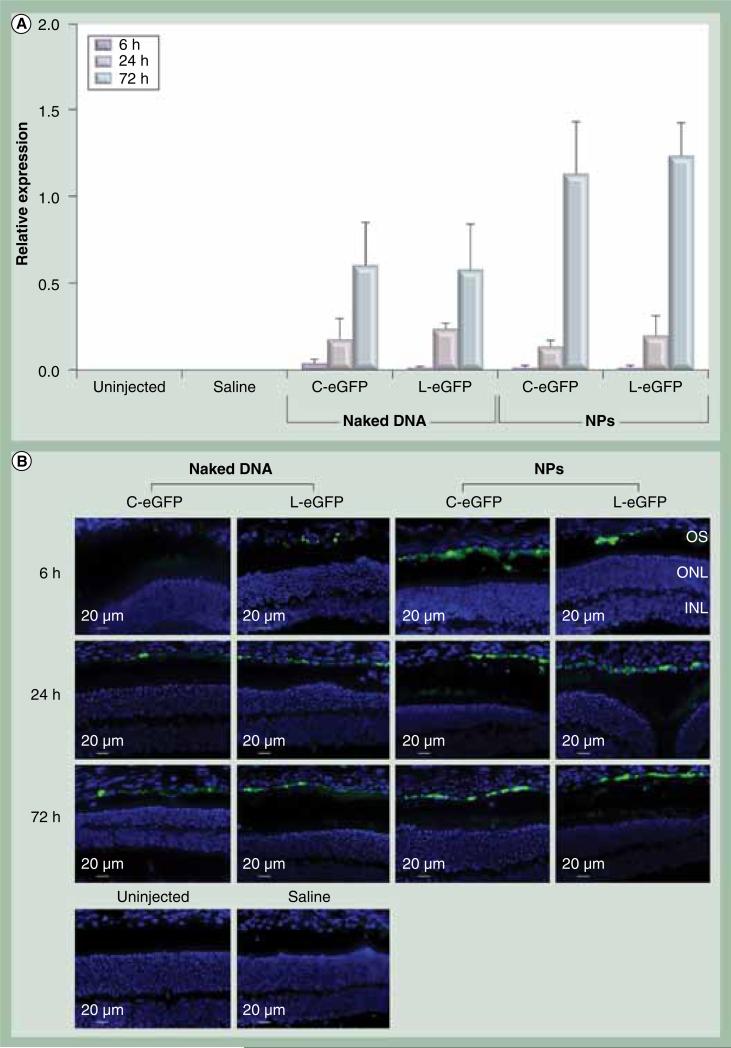
To target the RPE, 1 μl of the NPs at 4.3 μg/μl was delivered to the subretinal space of 6-week-old wild-type Balb/c mice. For every mouse, one eye was injected with NPs, naked DNA or saline while the contralateral eye was left uninjected to serve as a control. eGFP expression was evaluated in the whole eye by quantitative real-time PCR at 6, 24 and 72 h PI. Expression analysis demonstrated that both C-eGFP and L-eGFP NPs were capable of promoting eGFP expression in the eye appearing at 24 h PI and markedly increased at 72 h PI. No eGFP signal was detected on the message level at 6 h PI (Figure 2A). Expression of eGFP was also detected in the eyes subretinally treated with naked C-eGFP and L-eGFP, albeit at a 45% lower level than that observed with their compacted counterparts. The increase in eGFP expression with time (between 24 and 72 h PI) is statistically significant for naked- and NP-treated eyes with both vectors. Eliminating the bacterial backbone did not affect eGFP expression levels in the treated eyes with NPs or naked DNA. No expression was detected in the saline-treated and uninjected eyes.
Figure 2. Ocular expression and enhanced GFP localization post first subretinal injection.
(A) Quanitiative real-time PCR of eGFP mRNA was quantified relative to endogenous β-actin at the indicated time points post first subretinal injection of 1 μl saline containing 4.3 μg C-eGFP or L-GFP as naked DNA or NPs. Saline alone and uninjected eyes were used as negative controls. The increase in eGFP expression levels from 24 to 72 h postinjection was significant for naked-and NP-treated eyes with both vectors. A value of p < 0.05 was considered statistically significant. Error bars show standard deviation from four independent experiments. (B) Direct eGFP fluorescence in rentinal pigment epithelium cell layer at the site of injection and for the same time points presented in (A).
C-eGFP: Circular-enhanced GFP; L-eGFP: Linear-enhanced GFP; NP: Nanoparticle.
To evaluate tissue-specificity of the VMD2 promoter, direct fluorescence microscopy was performed on cryosections of whole eyes taken from animals treated with either NPs or their naked counterparts at 6, 24, and 72 h PI. Saline injected and uninjected eyes were used as controls. The saline and uninjected eyes showed no signs of eGFP signal except for low levels of autofluorescence in the fluorescein isothiocyanate channel that was used to calibrate all images taken from treated eyes (Figure 2B; lower panels). Examination of the eyes injected with C-eGFP and L-eGFP NPs and their naked counterparts showed eGFP fluorescent signals only in the RPE cell layer at 24 and 72 h PI (Figure 2B). It is important to mention that these images were taken at the injection sites and clearly exhibited comparable eGFP signal between NPs and naked DNA. Unlike eyes treated with naked DNA where eGFP-positive RPE cells were restricted to the injection site (Supplementary Figure 1, see online: www.futuremedicine.com/doi/suppl/10.2217/nnm.11.158), the distribution in NP treated eyes from both vectors spread from the injection site to the periphery (Supplementary Figure 1). Furthermore, no eGFP signal was detected in all cohorts at 6 h PI. This result coincides with the appearance of eGFP message.
Subretinal injections but not NPs or naked DNAs lead to transient inflammation
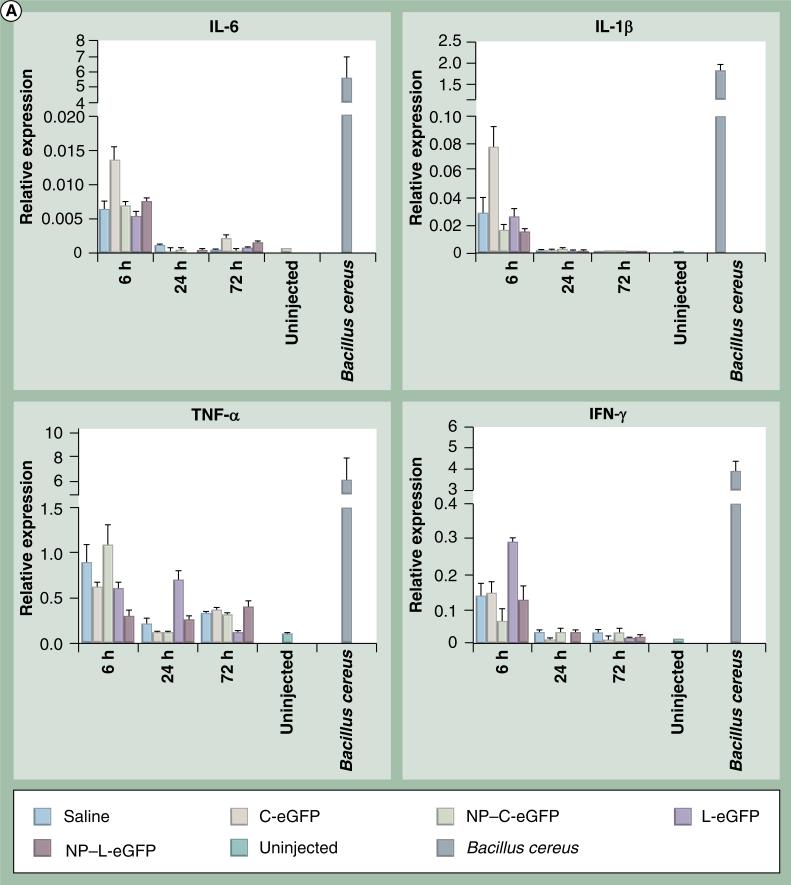
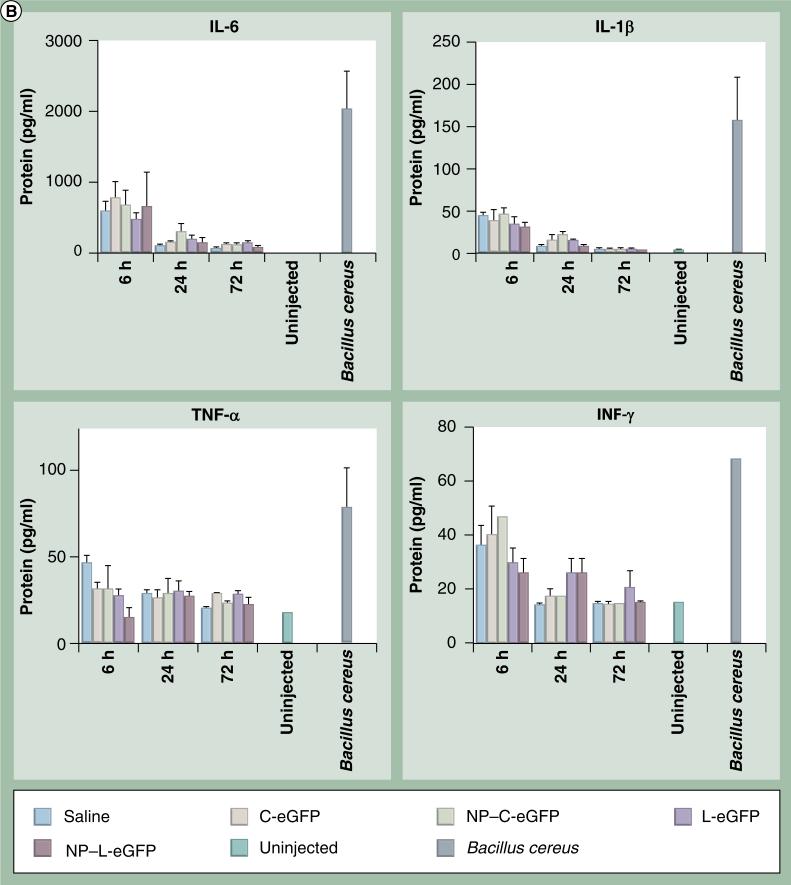
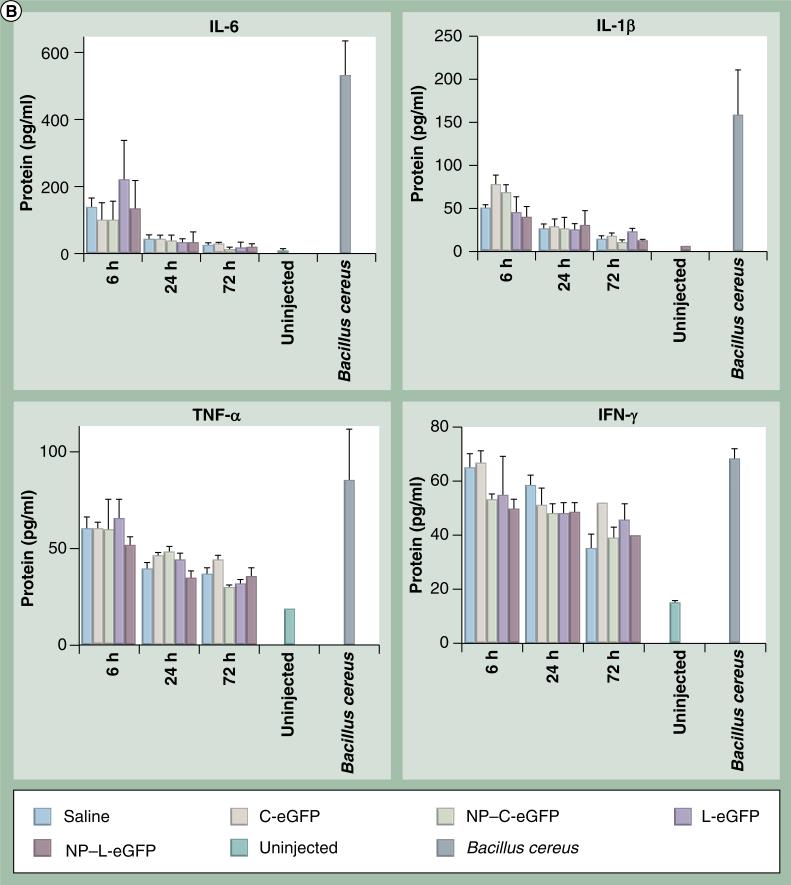
To determine if any acute inflammatory responses were induced by NPs, we evaluated mRNA and protein levels of proinflammatory cytokines in injected eyes from all cohorts at 6, 24 and 72 h PI. The message levels of IL-6, IL-1β, TNF-α and IFN-γ in mouse eyes were assessed by quantitative real-time PCR using primers specific for these cytokines. Mice were dosed with C-eGFP or L-eGFP naked DNA or NPs, saline, or were untreated, and total RNA was isolated from whole eyes. β-actin was used as an internal control for mRNA integrity. Multiplex cytokine assays were performed to quantify protein levels of IL-6, IL-1β, TNF-α and IFN-γ using the Bio-Plex Suspension array system. Upregulation of all cytokines (IL-6, IL-1β, TNF-α and IFN-γ) expression was observed both at the message (Figure 3A) and protein (Figure 3B) levels in all treated eyes, including saline, at 6 h PI. However, the expression declined to near baseline level at 24 and 72 h PI for IL-6 and IL-1β, although TNF-α and IFN-γ protein levels remained elevated in some samples.
Figure 3. Expression of cytokine mRNA and protein post first subretinal injection.
(A) Ocular cytokine mRNA levels post subretinal injection. Quantitative real-time PCR was performed on total RNA isolated from whole eyes at 6, 24 and 72 h postfirst subretinal injection with 1 μl saline containing 4.3 μg C-eGFP or L-eGFP as naked DNA or NPs. Saline alone and uninjected eyes were used as negative controls. Values depict the relative mRNA levels of cytokines normalized to β-actin.
eGFP: Enhanced GFP; C-eGFP: Circular-enhanced GFP; L-eGFP: Linear-enhanced GFP; NP: Nanoparticle.
(B) Bio-Plex cytokine array to evaluate protein levels of ocular cytokines post first subretinal injection. Protein levels of ocular cytokines were evaluated in supernatants isolated from whole eyes treated as indicated in (A). Levels of acute and chronic phase cytokines (IL-6, IL-1β, TNF-α and IFN-γ) are presented as mean ± standard devitaion from three independent experiments that were run in triplicate and measured at the indicated time points post first injection. Eyes from untreated and subretinal injected animals with Bacillus cereus were respectively used as negative and positive controls.
eGFP: Enhanced GFP; C-eGFP: Circular-enhanced GFP; L-eGFP: Linear-enhanced GFP; NP: Nanoparticle.
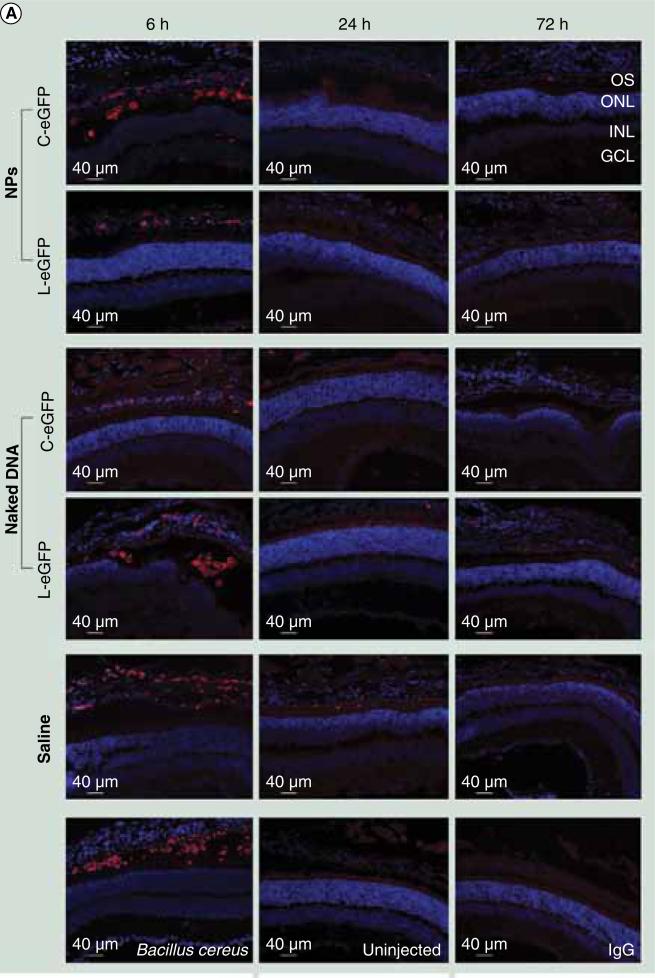
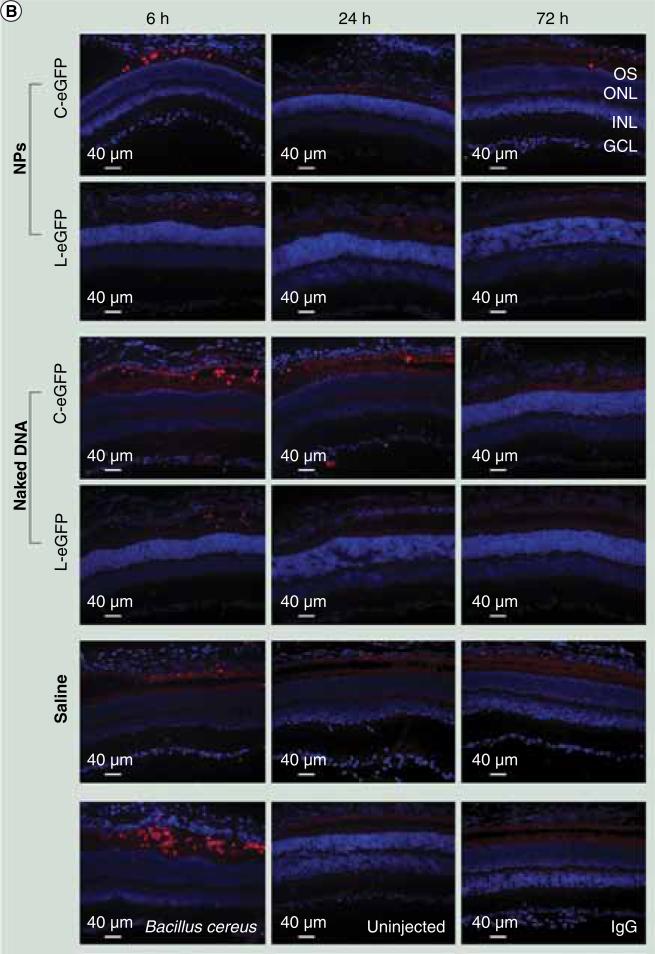
Immunohistochemistry analysis was performed using monoclonal antibodies to neutrophils and macrophages to visualize the infiltration of these inflammatory cells. All injected eyes exhibited positive staining for neutrophil and macrophage markers, including the saline-treated eyes, at 6 h PI but not at 24 and 72 h PI (Figure 4). Cells that exhibited positive staining were mainly located at the periphery of the injection site. There was no staining at later time points. No positive staining was observed in the eyes of age-matched uninjected mice or the tissue sections incubated with secondary antibody only (no primary antibody control). Overall, the time course of cytokine mRNA and protein expression and the appearance of macrophages and neutrophils were very similar, being evident only at 6 h PI in the saline and DNA dosed eyes. These data suggest an inflammatory response to the subretinal injection procedure itself rather than a response to naked or NP DNA.
Figure 4. Immunohistochemical assessment of infiltrating macrophages and neutrophils post first subretinal injection.
Immunohistochemistry was performed on retinal sections reacted with (A) a macrophage-specific marker (F4/80).
C-eGFP: Circular enhanced GFP; GCL: Ganglion cell layer; INL: Inner nuclear layer; L-eGFP: Linear enhanced GFP; NP: Nanoparticle; ONL: Outer nuclear layer; OS: Outer segment.
(B)A neutrophil-specific marker (NIMP-R14). Sections were obtained from mice injected with 1 μl saline containing 4.3 μg C-eGFP or L-eGFP as naked DNA or NPs (upper panels) and from untreated (negative control) and Bacillus cereus (positive control) injected eyes (lower panels). Analyses were performed at 6, 24, and 72 h post first injection. Positive staining was observed in all cohorts with both markers at 6 h PI and vanished at later time points. The positive staining cells are labeled in red.
C-eGFP: Circular enhanced GFP; GCL: Ganglion cell layer; INL: Inner nuclear layer; L-eGFP: Linear enhanced GFP; NP: Nanoparticle; ONL: Outer nuclear layer; OS: Outer segment.
Repeated subretinal NP dosing produces comparable gene expression & the injection procedure produces transient inflammation
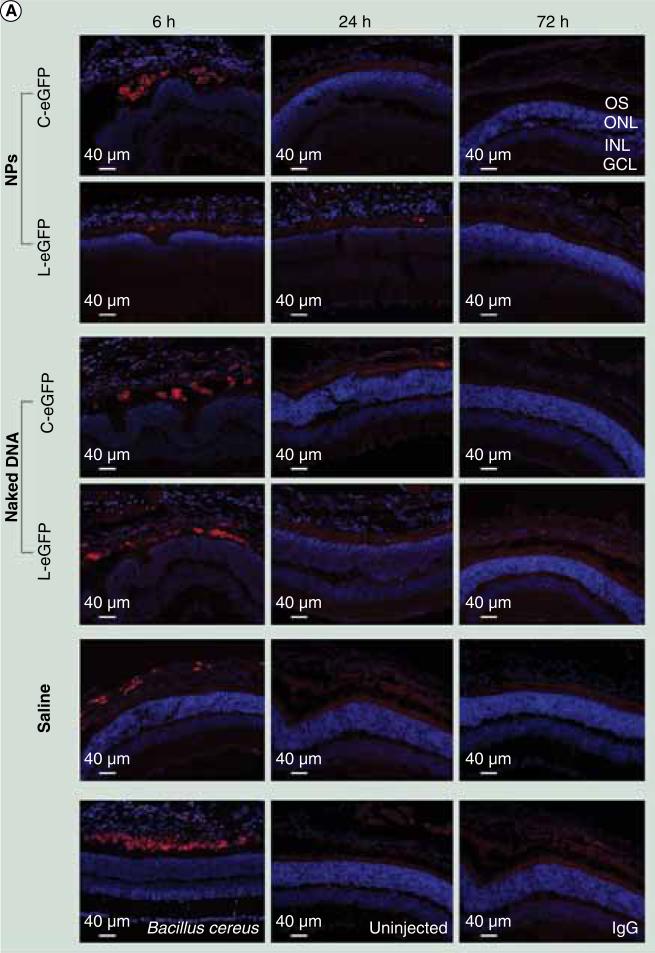
To evaluate whether repeated administration of both NPs into the subretinal space is efficient and safe, wild-type mice received a second subretinal injection of 1 μl saline containing 4.3 μg of either compacted NPs or naked DNA of C-eGFP or L-eGFP at 1 month after the first injection. At 6, 24, and 72 h PI after the second dosing, eGFP mRNA expression was evaluated by quantitative real-time PCR and protein expression in histologic sections by direct fluorescence. Results were similar to those obtained after the first injections (Figure 5) with the exception that eGFP direct fluorescence was detected at 6 h PI from both NPs (but not naked DNA), whereas no eGFP expression was detected at this early time point after the first dosing. This expression is likely a continuation from the first injection. We also evaluated levels of cytokines and infiltration of neutrophils/macrophages at the same time points post second dosing. As shown in Figures 6 & 7, the acute phase production of cytokines and infiltration by neutrophils/macrophages were found at 6 h PI in all eyes dosed with NPs, naked DNA and saline, similar to that seen after the first administration. These responses declined to baseline levels at 24 and 72 h PI, except for TNF-α and IFN-γ protein (Figure 6B).
Figure 5. Ocular expression and enhanced GFP localization post second subretinal injection.
(A) Quantitative real-time PCR of GFP mRNA was quantified relative to endogenous β-actin at 6, 24 and 72 h PI after the second injection of C- or L-eGFP expression DNA as either naked DNA or NPs. Saline and uninjected eyes were used as negative controls. The increase in eGFP expression levels from 24 to 72 h PI was significant for naked and NP-treated eyes with both vectors. A value of p < 0.05 was considered statistically significant. Error bars show standard deviation from four independent experiments and each was run in triplicate. (B) Direct eGFP in rentinal pigmented epithelial cell layer at the site of injection and for the same time points presented in (A).
C-eGFP: Circular enhanced GFP; INL: Inner nuclear layer; L-eGFP: Linear enhanced GFP; NP: Nanoparticle; ONL: Outer nuclear layer; OS: Outer segment.
Figure 6. Expression of cytokines mRNA and protein post second subretinal injection.
(A) Ocular cytokine mRNA levels post subretinal injection. Quantitative real-time PCR was performed on total RNA isolated from whole eyes at 6, 24 and 72 h post second subretinal injection with 1 μl saline containing 4.3 μg C-eGFP or L-eGFP as naked DNA or NPs. Saline alone and uninjected eyes were used as negative controls. Values depict the relative mRNA levels of cytokines normalized to β-actin.
eGFP: Enhanced GFP; C-eGFP: Circular-enhanced GFP; L-eGFP: Linear-enhanced GFP; NP: Nanoparticle. (B) Bio-Plex cytokine array to evaluate protein levels of ocular cytokines post second subretinal injection. Protein levels of ocular cytokines were evaluated in supernatants isolated from whole eyes treated as indicated in (A). Levels of acute and chronic phase cytokines (IL-6, IL-1 b, TNF-α and IFN-γ) were presented as mean ± standard deviation from three independent experiments that were run in triplicate and measured at the indicated time points post second injection. Eyes from untreated and subretinal injected animals with Bacillus cereus were respectively used as negative and positive controls. eGFP: Enhanced GFP; C-eGFP: Circular-enhanced GFP; L-eGFP: Linear-enhanced GFP; NP: Nanoparticle.
Figure 7. Immunohistochemical assessment of infiltrating macrophages and neutrophils post second subretinal injection.
Immunohistochemical labeling of retinal sections obtained from mice injected with 1 μl saline containing 4.3 μg C-eGFP or L-eGFP as naked DNA or NPs (upper panels) and from untreated (negative control) and Bacillus cereus (positive control) injected eyes (lower panels). Analyses were performed at 6, 24, and 72 h post second injection and sections were labeled with (A) F4/80, a macrophage-specific marker.
C-eGFP: Circular enhanced GFP; GCL: Ganglion cell layer; INL: Inner nuclear layer; L-eGFP: Linear enhanced GFP; NP: Nanoparticle; ONL: Outer nuclear layer; OS: Outer segment.
(B)A neutrophil-specific marker (NIMP-14). Positive staining was seen in all cohorts with both markers at 6 h PI and disappeared at later time points. The positive staining cells are labeled in red. C-eGFP: Circular enhanced GFP; GCL: Ganglion cell layer; INL: Inner nuclear layer; L-eGFP: Linear enhanced GFP; NP: Nanoparticle; ONL: Outer nuclear layer; OS: Outer segment.
RPE-specific NPs exert no toxic effect on retinal function
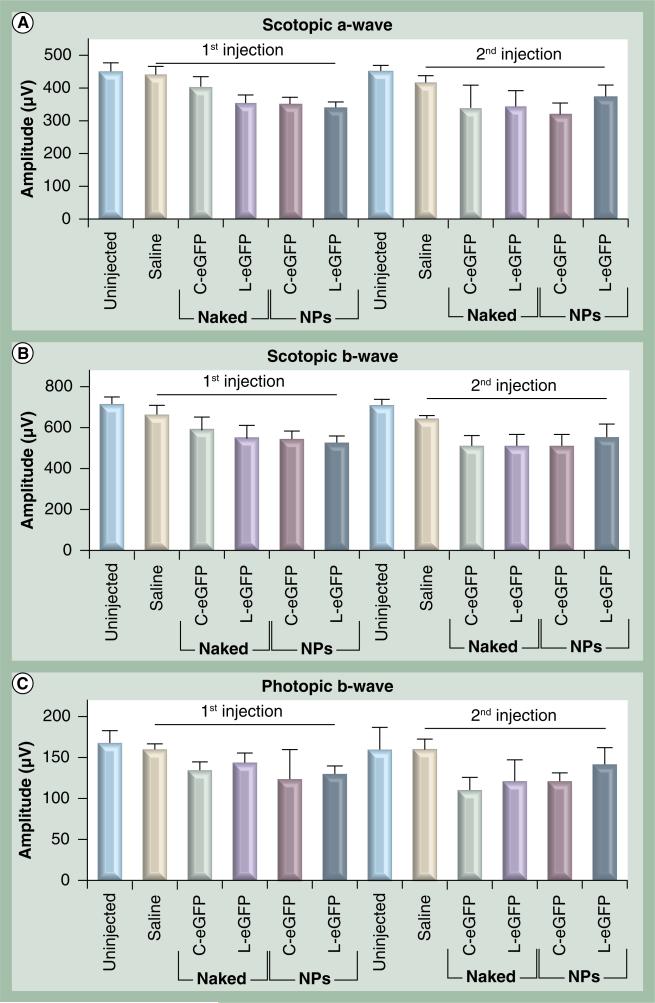
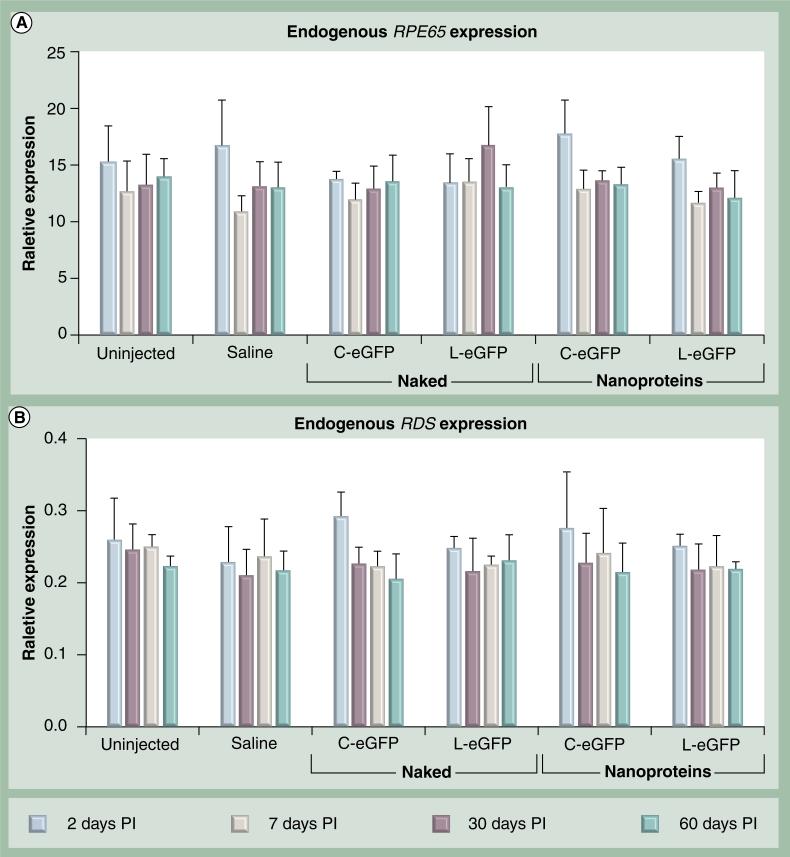
Surgical procedures and exogenous DNA delivery may lead to functional and morphological damage to the RPE and photoreceptors, especially in the setting of inflammation. Since the VMD2 promoter is RPE-specific and the RPE layer serves as a protector and supporter of retinal photoreceptor cells, we evaluated the effect of single and repeated treatments on photoreceptor function. Scotopic and photopic ERGs were first performed on all animals before any treatments, and then at 1 month after the first and at 1 month after the second dose administration. As shown in Figure 8, there were no significant differences in rod or cone ERG amplitudes in all cohorts at 30 days PI after the first and the second treatments when compared with their corresponding age-matched uninjected ERG values. These functional data suggest that neither subretinal delivery of NPs, naked DNAs, or saline resulted in adverse effects on the retinas. To further evaluate the health of the treated retinas, the level of expression of a photoreceptor-specific gene, RDS, and an RPE-specific gene, RPE65, were monitored at multiple time points PI of naked DNA, NP DNA or saline. Untreated animals served as a control. RDS and RPE65 expression levels were evaluated by quantitative real-time PCR in whole eyes isolated at 2, 7, 30 and 60 days PI. As shown in Figure 9, no significant differences were detected in RPE65 and RDS mRNA levels from all treated cohorts when compared with saline injected and uninjected eyes. These results coincide with the functional analyses and strongly support the lack of any significant adverse effect on the health of the RPE and the photoreceptor cells as a consequence of the injection procedure or the delivered material of NPs or naked DNAs.
Figure 8. Electroretinogram analyses of retinal function in wild-type mice post first and second subretinal injection.
Scotopic a-wave (A), scotopic b-wave (B) and photopic b-wave (C) amplitudes were evaluated in animals treated at the indicated time points with 4.3 μg C-eGFP or L-eGFP as naked DNA or NPs. Saline alone and uninjected animals were evaluated as negative controls. Amplitudes from all treated cohorts were comparable with signals from uninjected age-matched wild-type mice (uninjected). Amplitudes are presented as means ± standard deviation (n = 10) and a p < 0.05 is considered statistically significant. There were no significant differences in retinal function observed between treated and untreated mice in each group.
eGFP: Enhanced GFP: C-eGFP: Circular-enhanced GFP; L-eGFP: Linear-enhanced GFP; NP: Nanoparticle.
Figure 9. Evaluation of endogenous rentinal pigment epithelial- and photoreceptor-specific gene expression.
Relative expression levels by quantitative real-time PCR to β-actin of (A) RPE-specific gene (RPE65) and (B) photoreceptor-specific (RDS) gene were evaluated at the indicated time points and from eyes treated with 4.3 μg C-eGFP or L-eGFP as naked DNA or nanoparticles. Saline and uninjected eyes were used as negative controls. Expression levels are presented as means ± standard deviation (n = 4) and p < 0.05 is considered statistically significant. There were no significant differences observed between treated and untreated mice in each group.
C-eGFP: Circular-enhanced GFP; L-eGFP: Linear-enhanced GFP; PI: Post injection.
Discussion
The challenges associated with gene therapy for ocular disorders are numerous. Regardless, the goal is to develop highly targeted functional vectors and delivery strategies with minimal to no toxicity. Furthermore, long-term transgene expression at biologically active levels must be achieved or repetitive dosing must be feasible. In an earlier study we reported that CK30PEG DNA NPs do not induce inflammatory infiltrates or significant levels of cytokines following a single subretinal delivery [10]. In this study, compacted plasmid DNA containing a cytomegaloviruseGFP expression cassette was tested at doses ranging from 0.3 to 3 μg, and only transient expression of eGFP was produced due to cytomegalovirus promoter downregulation. Since our goals include prolonged and targeted transgene expression for ocular tissue, we evaluated NPs carrying an RPE-specific eGFP expression plasmid (C-eGFP). Since repetitive administration of therapeutic genes might be necessary for some chronic ocular disorders, such as age-related macular degeneration, diabetic retinopathy and retinitis pigmentosa, we assessed the effects of single and double subretinal doses of two NPs. However, since it is well known that the presence of prokaryotic bacterial backbone limits transgene expression and induces cytotoxicity [21,22], we prepared NPs carrying the same expression cassette either in a circular plasmid containing bacterial backbone or in a linear DNA fragment lacking it. Importantly, the data clearly demonstrate that single or repetitive dosing of either class of NPs did not induce inflammation, whereas an early, transient inflammatory response was induced by the subretinal injection procedure. In addition, there were no functional consequences of repetitive NP dosing, as assessed by ERGs, and no perturbation of endogenous RPE and photoreceptor gene expression as monitored by RPE65 and RDS mRNA levels, respectively. These findings extend prior benign NP toxicology results in the lung, brain and eye [3,7–10], and further indicate that repetitive ocular doses of DNA NPs may be nontoxic.
It is well known that during acute-phase inflammation, a pathogen will meet infiltrating neutrophils first and macrophages and production of proinflammatory cytokines (IL-6, IL-1β, TNF-α and IFN-γ) will immediately follow [23,24]. These particular inflammatory mediators are very critical signaling molecules for clinical and experimental studies of inflammatory and autoimmune disorders. Additionally, neutrophil/macrophage infiltration and proinflammatory cytokine production can be caused not only by inflammatory compounds but can also be triggered by injury and stress responses. In this study, no detectable (by real-time PCR or BioPlex assay) or visible (by immunohistochemistry) inflammatory response could be observed in the untreated group. However, we found that both of the vector-treated and saline-treated animals showed inflammatory responses at the 6 h time point. Most inflammatory responses were found surrounding the center of the eyecup around the injection bleb. Since the inflammatory response was seen in saline-injected mouse retinas, it appears that these responses were caused by the subretinal injection stress, which in turn may trigger a cellular stress signal and further initiate a rapid transient inflammatory response. It has been well-studied that surgery can elevate inflammatory mediators, such as TNF-α and IL-1β, due to enhanced nociceptive sensitivity [25–29]. Clark et al. recently reported that acute morphine administration can reduce inflammatory cell infiltrates and some cytokine expression after surgical incision, whereas chronic morphine administration can increase wound cytokine levels because of enhanced nociceptive sensitivity [30,31]. Inflammatory mediator expression started as early as 2 h after surgery and was significantly reduced 24 h after surgery [24,30,32]. These findings are consistent with our present work that the neutrophil/macrophage infiltration and cytokine production occurs early after the injection stress and gradually returns to baseline level.
Another interesting finding in the present study is that the protein levels of TNF-α and IFN-γ as tested by the BioPlex assay showed delayed recovery in contrast to the mRNA level (Figure 6). Furthermore, levels of these cytokines appeared somewhat greater, compared with uninjected controls, after the second injection. This phenomenon of uncorrelated expression of mRNA and protein levels is well described [33–36], although why this occurred specifically for TNF-α and IFN-γ in the present study is unclear. Presumably, this could be due to different transcriptional and translational efficiencies or post-translational regulation PI stress. Since the delayed return to the baseline levels of TNF-α and IFN-γ (more than 24 or 72 h) were similar for NPs and saline groups, this perturbation appears to be due to the injection procedure itself rather than the contents of the NPs or the naked DNAs.
Although the retina is known to be immune privileged, potential interruption of this barrier could occur by the subretinal injection procedure, particularly if re-administration is required. Importantly, there was no detectable NP-specific inflammatory response in the mouse retina after secondary administration of the same NPs 1 month later. Apart from the surgical injection procedure, these data indicate that there is no increased risk of toxicity development caused by re-administration of the eGFP NPs in wild-type mice. We appreciate, however, that the encoded transgene may induce specific toxicities independent of the NP structure. Although eGFP protein appeared to be nontoxic in the eye [37], expression of genes that modulate retinal cell biology may require cell-specific expression and careful modulation of gene expression levels to be effective and safe. Hence, each therapeutic NP for a given ocular indication will require a specific toxicology analysis as part of an Investigative New Drug track program.
The function of the retina was evaluated by ERG analysis after the first and second subretinal NP injection. As compared with age-matched uninjected mice, there were no significant differences in the amplitudes of scotopic a- and b-waves, as well as photopic b-waves, 30 days PI. To additionally investigate the biologic status of the retina post-treatments, mRNA levels of endogenous RPE-specific genes such as RPE65 and photoreceptor-specific genes such as RDS were evaluated at 2, 7, 30, and 60 days PI. No perturbations were observed compared with uninjected mice, further supporting the conclusion that DNA NPs are relatively nontoxic after ocular delivery.
Successful gene therapy has two core requirements: first that the therapeutic gene be functionally active; and second, that the timecourse of expression should suit the timecourse of the disease. Most often, for degenerative diseases this means that a vector that generates early-onset, long-term gene expression will yield the best rescue. In this study, eGFP mRNA and protein expression was initiated as early as 24 h PI (Figure 2). Although eGFP mRNA levels generated by both NPs were approximately 45% higher than their comparable naked DNA at 24 h PI, levels from both (NPs and naked) increased to approximately 3.5-fold at 72 h PI. These data concur with our recent study on the same NPs demonstrating a minor increase in eGFP expression from NPs when compared with their naked DNA during the first few days post-treatment, while a significantly higher expression was seen at later time points (approximately threefold) and continued up to 30 days PI [16]. Interestingly, a similar profile of eGFP expression was also seen after the second NP injection, indicating that NPs have biological activity at 30 days after the initial subretinal injection in the same eye. Although there are no validated anti-NP antibodies to permit direct evaluation, the data suggest that DNA NPs do not elicit adverse side effects from multiple injections. By contrast, some studies evaluating ocular re-administration of adeno-associated viral vectors to the same eye have demonstrated neutralizing antibody production after subretinal delivery, whereas others noted antibodies only after repeat intravitreal delivery [38–41]. Hence, an examination of NP gene expression after repeat intravitreal dosing will be an important future study to fully address this immunogenicity question. Otherwise, similar repeat dosing results have been observed in the lung, where three prior intrapulmonary doses of DNA NPs, given at 3-week intervals, did not affect transgene expression after a fourth dose [Cooper MJ, Unpublished Data].
Interestingly, after the second ocular injection, eGFP expression was detectable in RPE cells at 6 h in retinal sections of the NP injected eyes, which was not seen after the first injection at the same time point (Figures 2 & 5), suggesting that eGFP expression persisted at least 1 month after the first injection. This interpretation is supported by quantitative real-time PCR data showing eGFP mRNA expression in NP injected eyes at 30 days PI. This prolonged eGFP expression was observed for both C-eGFP and L-eGFP NPs, but not for naked DNA (Figure 5B). In other systems, Chen et al. reported that an expression cassette lacking the bacterial backbone can enhance long-term transgene expression in vivo [21,22], but this was not evident in our studies since both C-eGFP and L-eGFP NPs generated essentially identical results. Furthermore, compacted eGFP NPs, in comparison to naked DNA, transfect RPE cells at higher efficiencies (Figures 2 & 5) and were more widely distributed, both inside and outside of the injection bleb (Supplementary Figure 1, see online: www.futuremedicine.com/doi/suppl/10.2217/nnm.11.158).
Conclusions
Overall, the findings from these studies demonstrate that repeat subretinal dosing of NPs are efficacious and relatively nontoxic. Taken together, our data showed that no specific toxicity was observed in the experimental groups due to the composition of the NPs or the DNA vectors. Surgical stress triggered by subretinal injection does cause upregulation of acute-phase inflammatory cytokines and induces a neutrophil and macrophage infiltrate, which are mainly resolved by several days PI. Prior studies using CK30PEG NPs have also shown an encouraging safety profile in the retina, particularly in photoreceptor cells [4,5,10,42]. The prospects for using compacted DNA NPs for ocular gene therapy deserves further testing.
Supplementary Material
Executive summary.
■ In a large number of animals (~324 mice), four naked and nanocompacted DNA vectors were systemically evaluated for their safety and efficacy.
■ A set of inflammatory mediators, which strongly associated with the impact of acute inflammation, were systematically investigated both at mRNA and at protein levels.
■ A single and double administration into the eye was performed.
■ Retinal function and structure were further investigated for extended periods for retinal light electrical responses, and retinal pigment epithelial cells and photoreceptors endogenous gene expression.
■ No signs of disruption were noted over a period of 2 months after the first and second injections.
Acknowledgements
The authors thank Shannon Conley for her assistance in the statistical evaluation of the data.
This work was supported by the National Eye Institute (EY10609-MI Naash and EY018656-MI Naash), the Foundation Fighting Blindness (MI Naash), the Oklahoma Center forthe Advancement of Science and Technology (Z Han and MI Naash), the Ohio Third Frontier BRCP program (BRCP 08-055, MJ Cooper) and William W Talley II Research Award (Z Han). MJ Cooper owns stock and stock options in Copernicus Therapeutics, Inc.
Footnotes
supplementary material
For supplementary material see the Future Medicine website (www.futuremedicine.com/doi/suppl/10.2217/nnm.11.158).
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investi gations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
■of interest
■■of considerable interest
- 1.Ziady AG, Gedeon CR, Miller T, et al. Transfection of airway epithelium by stable PEGylated poly-l-lysine DNA nanoparticles in vivo. Mol. Ther. 2003;8(6):936–947. doi: 10.1016/j.ymthe.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Liu G, Li D, Pasumarthy MK, et al. Nanoparticles of compacted DNA transfect postmitotic cells. J. Biolog. Chem. 2003;278(35):32578–32586. doi: 10.1074/jbc.M305776200. [DOI] [PubMed] [Google Scholar]
- 3■.Ziady AG, Gedeon CR, Muhammad O, et al. Minimal toxicity of stabilized compacted DNA nanoparticles in the murine lung. Mol. Ther. 2003;8(6):948–956. doi: 10.1016/j.ymthe.2003.09.002. [In this study nanoparticles formulated with polyethylene glycol-substituted lysine 30-mers (CK30PEG) were utilized as a delivery system for mouse lungs and toxicity of the nanoparticles were evaluated.] [DOI] [PubMed] [Google Scholar]
- 4■■.Cai X, Conley SM, Nash Z, Fliesler SJ, Cooper MJ, Naash MI. Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J. 2010;24(4):1178–1191. doi: 10.1096/fj.09-139147. [In this study a gene replacement therapy was achieved using CK30PEG nanoparticles in a mouse model of retinitis pigmentosa.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5■.Farjo R, Skaggs J, Quiambao AB, Cooper MJ, Naash MI. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS One. 2006;1:e38. doi: 10.1371/journal.pone.0000038. [Efficiency of different type of CK30PEG nanoparticles has been systemically tested in ocular tissues of wild-type mice.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fink TL, Klepcyk PJ, Oette SM, et al. Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using compacted DNA nanoparticles. Gene Ther. 2006;13(13):1048–1051. doi: 10.1038/sj.gt.3302761. [DOI] [PubMed] [Google Scholar]
- 7.Yurek DM, Fletcher AM, Smith GM, et al. Long-term transgene expression in the central nervous system using DNA nanoparticles. Mol. Ther. 2009;17(4):641–650. doi: 10.1038/mt.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziady AG, Davis PB, Konstan MW. Nonviral gene transfer therapy for cystic fibrosis. Exp. Opin. Biol. Ther. 2003;3(3):449–458. doi: 10.1517/14712598.3.3.449. [DOI] [PubMed] [Google Scholar]
- 9■.Konstan MW, Davis PB, Wagener JS, et al. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum. Gene Ther. 2004;15(12):1255–1269. doi: 10.1089/hum.2004.15.1255. [A Phase I/IIa clinical trial has been performed using CK30PEG nanoparticles in cystic fibrosis subjects, showing no adverse events attributable to the nanoparticles and with evidence of biological function of the transferred hCFTR gene.] [DOI] [PubMed] [Google Scholar]
- 10■.Ding XQ, Quiambao AB, Fitzgerald JB, Cooper MJ, Conley SM, Naash MI. Ocular delivery of compacted DNA-nanoparticles does not elicit toxicity in the mouse retina. PLoS One. 2009;4(10):e7410. doi: 10.1371/journal.pone.0007410. [Toxicity of cytomegalovirus-enhanced GFP CK30PEG nanoparticles has been evaluated at doses ranging from 0.3 to 3 μg following a single subretinal injection in wild-type mice.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reyes-Sandoval A, Ertl HC. CpG methylation of a plasmid vector results in extended transgene product expression by circumventing induction of immune responses. Mol. Ther. 2004;9(2):249–261. doi: 10.1016/j.ymthe.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Yew NS, Zhao H, Przybylska M, et al. CpG-depleted plasmid DNA vectors with enhanced safety and long-term gene expression in vivo. Mol. Ther. 2002;5(6):731–738. doi: 10.1006/mthe.2002.0598. [DOI] [PubMed] [Google Scholar]
- 13.Yew NS, Zhao H, Wu IH, et al. Reduced inflammatory response to plasmid DNA vectors by elimination and inhibition of immunostimulatory CpG motifs. Mol. Ther. 2000;1(3):255–262. doi: 10.1006/mthe.2000.0036. [DOI] [PubMed] [Google Scholar]
- 14.Hyde SC, Pringle IA, Abdullah S, et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat. Biotechnol. 2008;26(5):549–551. doi: 10.1038/nbt1399. [DOI] [PubMed] [Google Scholar]
- 15.Przybylska M, Wu IH, Zhao H, et al. Partial correction of the a-galactosidase A deficiency and reduction of glycolipid storage in Fabry mice using synthetic vectors. J. Gene. Med. 2004;6(1):85–92. doi: 10.1002/jgm.468. [DOI] [PubMed] [Google Scholar]
- 16■.Koirala A, Makkia RS, Cooper MJ, Naash MI. Nanoparticle-mediated gene transfer specific to retinal pigment epithelial cells. Biomaterials. 2011;32(35):9483–9493. doi: 10.1016/j.biomaterials.2011.08.062. [Transfection efficiency, distribution and uptake of the retinal pigment epithelial cell-specific vitelliformmacular dystrophy 2-enhanced GFP nanoparticles have been evaluated in vivo and after a single injection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esumi N, Oshima Y, Li Y, Campochiaro PA, Zack DJ. Analysis of the VMD2 promoter and implication of E-box binding factors in its regulation. J. Biolog. Chem. 2004;279(18):19064–19073. doi: 10.1074/jbc.M309881200. [DOI] [PubMed] [Google Scholar]
- 18.Nour M, Quiambao AB, Peterson WM, Al-Ubaidi MR, Naash MI. P2Y (2) receptor agonist INS37217 enhances functional recovery after detachment caused by subretinal injection in normal and rds mice. Invest. Ophthalmol. Vis. Sci. 2003;44(10):4505–4514. doi: 10.1167/iovs.03-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai X, Conley SM, Naash MI. RPE65: role in the visual cycle, human retinal disease, and gene therapy. Ophthalmic Genet. 2009;30(2):57–62. doi: 10.1080/13816810802626399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulse RE, Kunkler PE, Fedynyshyn JP, Kraig RP. Optimization of multiplexed bead-based cytokine immunoassays for rat serum and brain tissue. J. Neurosci. Methods. 2004;136(1):87–98. doi: 10.1016/j.jneumeth.2003.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen ZY, He CY, Meuse L, Kay MA. Silencing of episomal transgene expression by plasmid bacterial DNA elements in vivo. Gene Ther. 2004;11(10):856–864. doi: 10.1038/sj.gt.3302231. [DOI] [PubMed] [Google Scholar]
- 22.Chen ZY, Yant SR, He CY, Meuse L, Shen S, Kay MA. Linear DNAs concatemerize in vivo and result in sustained transgene expression in mouse liver. Mol. Ther. 2001;3(3):403–410. doi: 10.1006/mthe.2001.0278. [DOI] [PubMed] [Google Scholar]
- 23.Butterfield TA, Best TN, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J. Athl. Train. 2006;41(4):457–465. [PMC free article] [PubMed] [Google Scholar]
- 24.Lu CH, Chao PC, Borel CO, et al. Preincisional intravenous pentoxifylline attenuating perioperative cytokine response, reducing morphine consumption, and improving recovery of bowel function in patients undergoing colorectal cancer surgery. Anesth. Analg. 2004;99(5):1465–1471. doi: 10.1213/01.ANE.0000132974.32249.C8. [DOI] [PubMed] [Google Scholar]
- 25.Bryan D, Walker KB, Ferguson M, Thorpe R. Cytokine gene expression in a murine wound healing model. Cytokine. 2005;31(6):429–438. doi: 10.1016/j.cyto.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Sato Y, Ohshima T. The expression of mRNA of proinflammatory cytokines during skin wound healing in mice: a preliminary study for forensic wound age estimation (II). Int. J. Legal Med. 2000;113(3):140–145. doi: 10.1007/s004140050285. [DOI] [PubMed] [Google Scholar]
- 27.Ahn DK, Chae JM, Choi HS, et al. Central cyclooxygenase inhibitors reduced IL-1β-induced hyperalgesia in temporomandibular joint of freely moving rats. Pain. 2005;117(1–2):204–213. doi: 10.1016/j.pain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Inglis JJ, Nissim A, Lees DM, Hunt SP, Chernajovsky Y, Kidd BL. The differential contribution of tumour necrosis factor to thermal and mechanical hyperalgesia during chronic inflammation. Arthr. Res. Ther. 2005;7(4):R807–R816. doi: 10.1186/ar1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vissers KC, De Jongh RF, Hoffmann VL, Meert TF. Exogenous interleukin-6 increases cold allodynia in rats with a mononeuropathy. Cytokine. 2005;30(4):154–159. doi: 10.1016/j.cyto.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Clark JD, Shi X, Li X, et al. Morphine reduces local cytokine expression and neutrophil infiltration after incision. Mol. Pain. 2007;3:28. doi: 10.1186/1744-8069-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang D, Shi X, Qiao Y, Angst MS, Yeomans DC, Clark JD. Chronic morphine administration enhances nociceptive sensitivity and local cytokine production after incision. Mol. Pain. 2008;4:7. doi: 10.1186/1744-8069-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawasaki T, Ogata M, Kawasaki C, Tomihisa T, Okamoto K, Shigematsu A. Surgical stress induces endotoxin hyporesponsiveness and an early decrease of monocyte mCD14 and HLA-DR expression during surgery. Anesth. Analg. 2001;92(5):1322–1326. doi: 10.1097/00000539-200105000-00046. [DOI] [PubMed] [Google Scholar]
- 33.Ebrahimi M, Roudkenar MH, Imani Fooladi AA, et al. Discrepancy between mRNA and protein expression of neutrophil gelatinase-associated lipocalin in bronchial epithelium induced by sulfur mustard. J. Biomed. Biotechnol. 2010;2010:823131. doi: 10.1155/2010/823131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shebl FM, Pinto LA, Garcia-Pineres A, et al. Comparison of mRNA and protein measures of cytokines following vaccination with human papillomavirus-16 L1 virus-like particles. Cancer Epidemiol. Biomarkers Prev. 2010;19(4):978–981. doi: 10.1158/1055-9965.EPI-10-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lichtinghagen R, Musholt PB, Lein M, et al. Different mRNA and protein expression of matrix metalloproteinases 2 and 9 and tissue inhibitor of metalloproteinases 1 in benign and malignant prostate tissue. Eur. Urol. 2002;42(4):398–406. doi: 10.1016/s0302-2838(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 36.Anderson L, Seilhamer J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis. 1997;18(3–4):533–537. doi: 10.1002/elps.1150180333. [DOI] [PubMed] [Google Scholar]
- 37.Nour M, Ding XQ, Stricker H, Fliesler SJ, Naash MI. Modulating expression of peripherin/rds in transgenic mice: critical levels and the effect of overexpression. Invest. Ophthalmol. Vis. Sci. 2004;45(8):2514–2521. doi: 10.1167/iovs.04-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q, Miller R, Han PY, et al. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol. Vis. 2008;14:1760–1769. [PMC free article] [PubMed] [Google Scholar]
- 39.Li W, Kong F, Li X, et al. Gene therapy following subretinal AAV5 vector delivery is not affected by a previous intravitreal AAV5 vector administration in the partner eye. Mol. Vis. 2009;15:267–275. [PMC free article] [PubMed] [Google Scholar]
- 40.Amado D, Mingozzi F, Hui D, et al. Safety and efficacy of subretinal readministration of a viral vector in large animals to treat congenital blindness. Sci. Transl. Med. 2010;2(21):21ra16. doi: 10.1126/scitranslmed.3000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barker SE, Broderick CA, Robbie SJ, et al. Subretinal delivery of adeno-associated virus serotype 2 results in minimal immune responses that allow repeat vector administration in immunocompetent mice. J. Gene. Med. 2009;11(6):486–497. doi: 10.1002/jgm.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai X, Conley SM, Naash MI. Gene therapy in the retinal degeneration slow model of retinitis pigmentosa. Adv. Exp. Med. Biol. 2010;664:611–619. doi: 10.1007/978-1-4419-1399-9_70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.