Abstract
Haemophilia B is a bleeding disorder caused by a functional deficiency of the clotting factor IX. A full length human factor IX complementary DNA clone containing all the natural mRNA sequences plus some flanking intron sequences was constructed with a metallothionein promoter and introduced into transgenic mice by microinjection into the pronuclei of fertilised eggs. The transgenic mice expressed high levels of messenger RNA, gamma-carboxylated and glycosylated protein, and biological clotting activity that are indistinguishable from normal human plasma factor IX. This study demonstrates the feasibility of expressing highly complex heterologous proteins in transgenic mice. It also provides the groundwork for the production of large amounts of human factor IX in larger transgenic livestock for therapeutic use, and the investigation of alternative genetic therapies for haemophilia B.
Full text
PDF




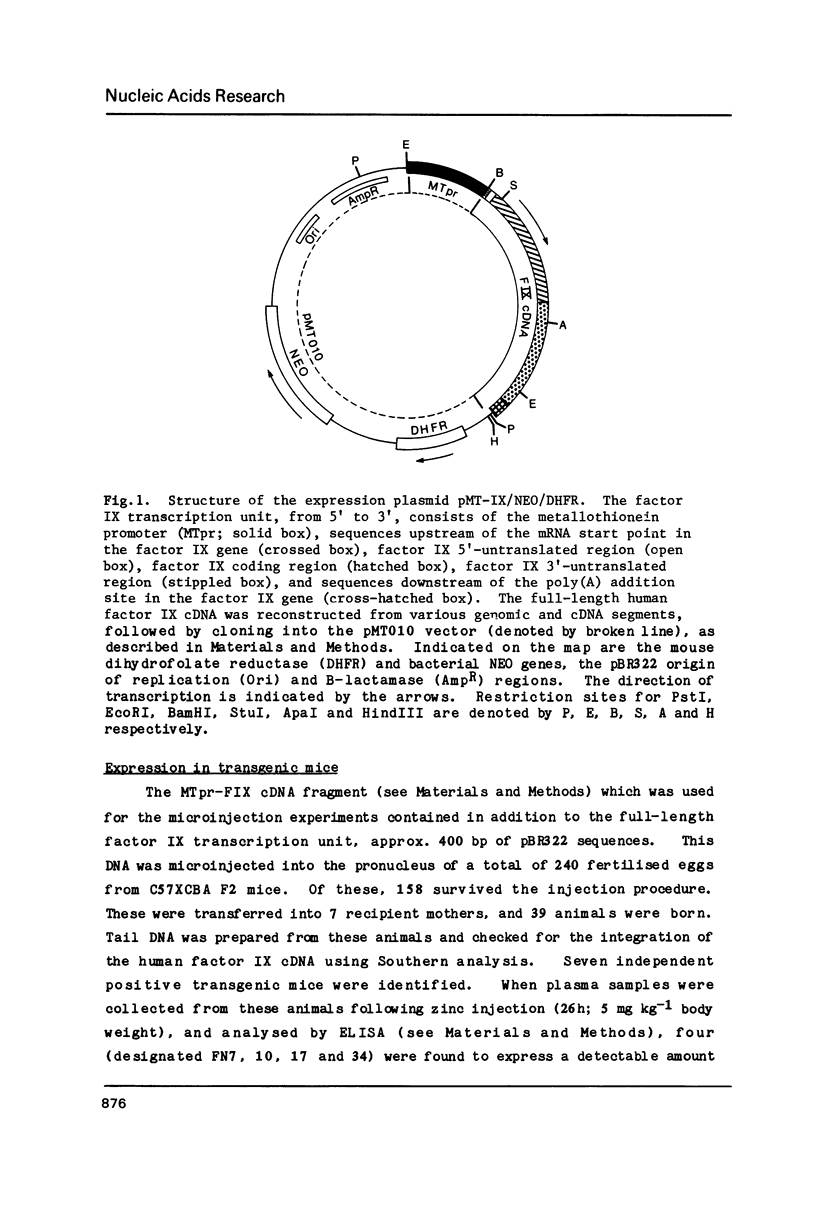
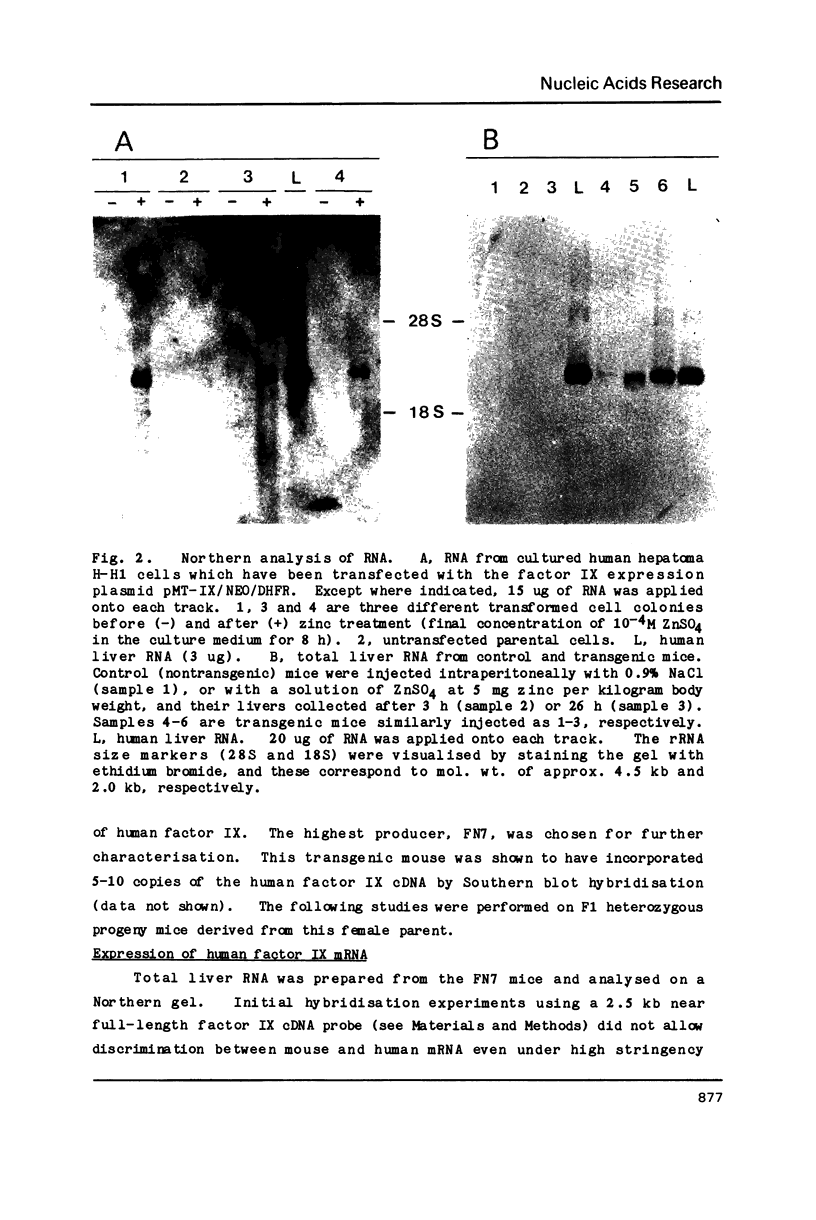


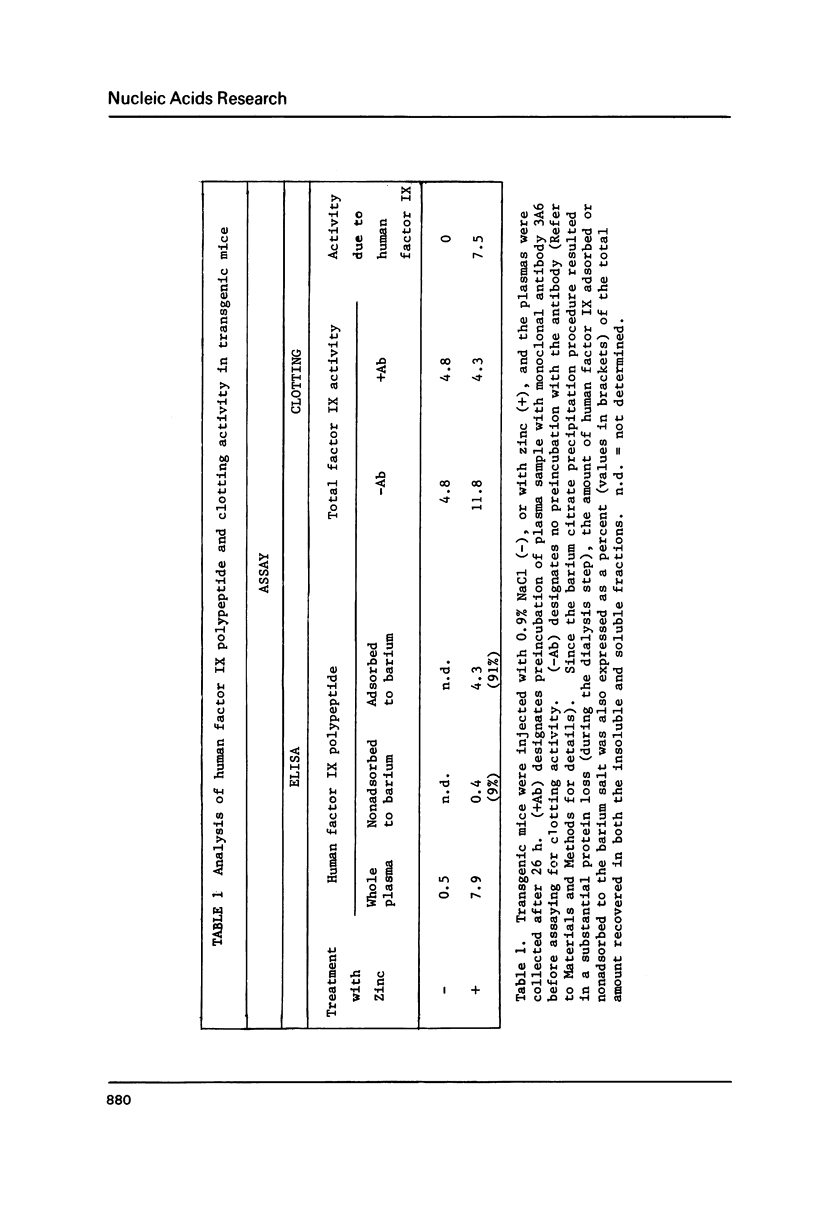




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson D. S., Austen D. E., Brownlee G. G. Expression of active human clotting factor IX from recombinant DNA clones in mammalian cells. Nature. 1985 Jun 20;315(6021):683–685. doi: 10.1038/315683a0. [DOI] [PubMed] [Google Scholar]
- Anson D. S., Choo K. H., Rees D. J., Giannelli F., Gould K., Huddleston J. A., Brownlee G. G. The gene structure of human anti-haemophilic factor IX. EMBO J. 1984 May;3(5):1053–1060. doi: 10.1002/j.1460-2075.1984.tb01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj S. P., Rapaport S. I., Brown S. F. Isolation and characterization of human factor VII. Activation of factor VII by factor Xa. J Biol Chem. 1981 Jan 10;256(1):253–259. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Busby S., Kumar A., Joseph M., Halfpap L., Insley M., Berkner K., Kurachi K., Woodbury R. Expression of active human factor IX in transfected cells. Nature. 1985 Jul 18;316(6025):271–273. doi: 10.1038/316271a0. [DOI] [PubMed] [Google Scholar]
- Chavin S. I., Weidner S. M. Blood clotting factor IX. Loss of activity after cleavage of sialic acid residues. J Biol Chem. 1984 Mar 25;259(6):3387–3390. [PubMed] [Google Scholar]
- Choo K. H., Cotton R. G., Danks D. M., Jennings I. G. Genetics of the mammalian phenylalanine hydroxylase system. Studies of human liver phenylalanine hydroxylase subunit structure and of mutations in phenylketonuria. Biochem J. 1979 Aug 1;181(2):285–294. doi: 10.1042/bj1810285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo K. H., Cotton R. G. Genetics of the mammalian phenylalanine hydroxylase system: I. Isolation of phenylalanine hydroxylase-deficient tyrosine auxotrophs from rat hepatoma cells. Somatic Cell Genet. 1977 Sep;3(5):457–470. doi: 10.1007/BF01539118. [DOI] [PubMed] [Google Scholar]
- Choo K. H., Filby R. G., Jennings I. G., Peterson G., Fowler K. Vectors for expression and amplification of cDNA in mammalian cells: expression of rat phenylalanine hydroxylase. DNA. 1986 Dec;5(6):529–537. doi: 10.1089/dna.1.1986.5.529. [DOI] [PubMed] [Google Scholar]
- Choo K. H., Gould K. G., Rees D. J., Brownlee G. G. Molecular cloning of the gene for human anti-haemophilic factor IX. Nature. 1982 Sep 9;299(5879):178–180. doi: 10.1038/299178a0. [DOI] [PubMed] [Google Scholar]
- Costantini F., Lacy E. Gene transfer into the mouse germ-line. J Cell Physiol Suppl. 1982;1:219–226. doi: 10.1002/jcp.1041130430. [DOI] [PubMed] [Google Scholar]
- Dobner P. R., Kawasaki E. S., Yu L. Y., Bancroft F. C. Thyroid or glucocorticoid hormone induces pre-growth-hormone mRNA and its probable nuclear precursor in rat pituitary cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2230–2234. doi: 10.1073/pnas.78.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernlund P., Stenflo J. Beta-hydroxyaspartic acid in vitamin K-dependent proteins. J Biol Chem. 1983 Oct 25;258(20):12509–12512. [PubMed] [Google Scholar]
- Hammer R. E., Pursel V. G., Rexroad C. E., Jr, Wall R. J., Bolt D. J., Ebert K. M., Palmiter R. D., Brinster R. L. Production of transgenic rabbits, sheep and pigs by microinjection. Nature. 1985 Jun 20;315(6021):680–683. doi: 10.1038/315680a0. [DOI] [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Jaye M., de la Salle H., Schamber F., Balland A., Kohli V., Findeli A., Tolstoshev P., Lecocq J. P. Isolation of a human anti-haemophilic factor IX cDNA clone using a unique 52-base synthetic oligonucleotide probe deduced from the amino acid sequence of bovine factor IX. Nucleic Acids Res. 1983 Apr 25;11(8):2325–2335. doi: 10.1093/nar/11.8.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama K., Ericsson L. H., Enfield D. L., Walsh K. A., Neurath H., Davie E. W., Titani K. Comparison of amino acid sequence of bovine coagulation Factor IX (Christmas Factor) with that of other vitamin K-dependent plasma proteins. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4990–4994. doi: 10.1073/pnas.76.10.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Wasley L. C., Furie B. C., Furie B., Shoemaker C. B. Expression, purification, and characterization of recombinant gamma-carboxylated factor IX synthesized in Chinese hamster ovary cells. J Biol Chem. 1986 Jul 25;261(21):9622–9628. [PubMed] [Google Scholar]
- Kurachi K., Davie E. W. Isolation and characterization of a cDNA coding for human factor IX. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6461–6464. doi: 10.1073/pnas.79.21.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Isaacs B. S., Esmon C. T., Johnson A. E. Derivatives of blood coagulation factor IX contain a high affinity Ca2+-binding site that lacks gamma-carboxyglutamic acid. J Biol Chem. 1984 May 10;259(9):5698–5704. [PubMed] [Google Scholar]
- Suttie J. W. Mechanism of action of vitamin K: synthesis of gamma-carboxyglutamic acid. CRC Crit Rev Biochem. 1980;8(2):191–223. doi: 10.3109/10409238009105469. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka A., Giddings J. C., Thomas J. E., Fujimura Y., Bloom A. L. Immunoassays of factor IX antigen using monoclonal antibodies. Br J Haematol. 1985 Feb;59(2):265–275. doi: 10.1111/j.1365-2141.1985.tb02993.x. [DOI] [PubMed] [Google Scholar]
- de la Salle H., Altenburger W., Elkaim R., Dott K., Dieterlé A., Drillien R., Cazenave J. P., Tolstoshev P., Lecocq J. P. Active gamma-carboxylated human factor IX expressed using recombinant DNA techniques. Nature. 1985 Jul 18;316(6025):268–270. doi: 10.1038/316268a0. [DOI] [PubMed] [Google Scholar]