Abstract
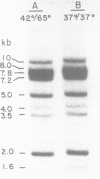
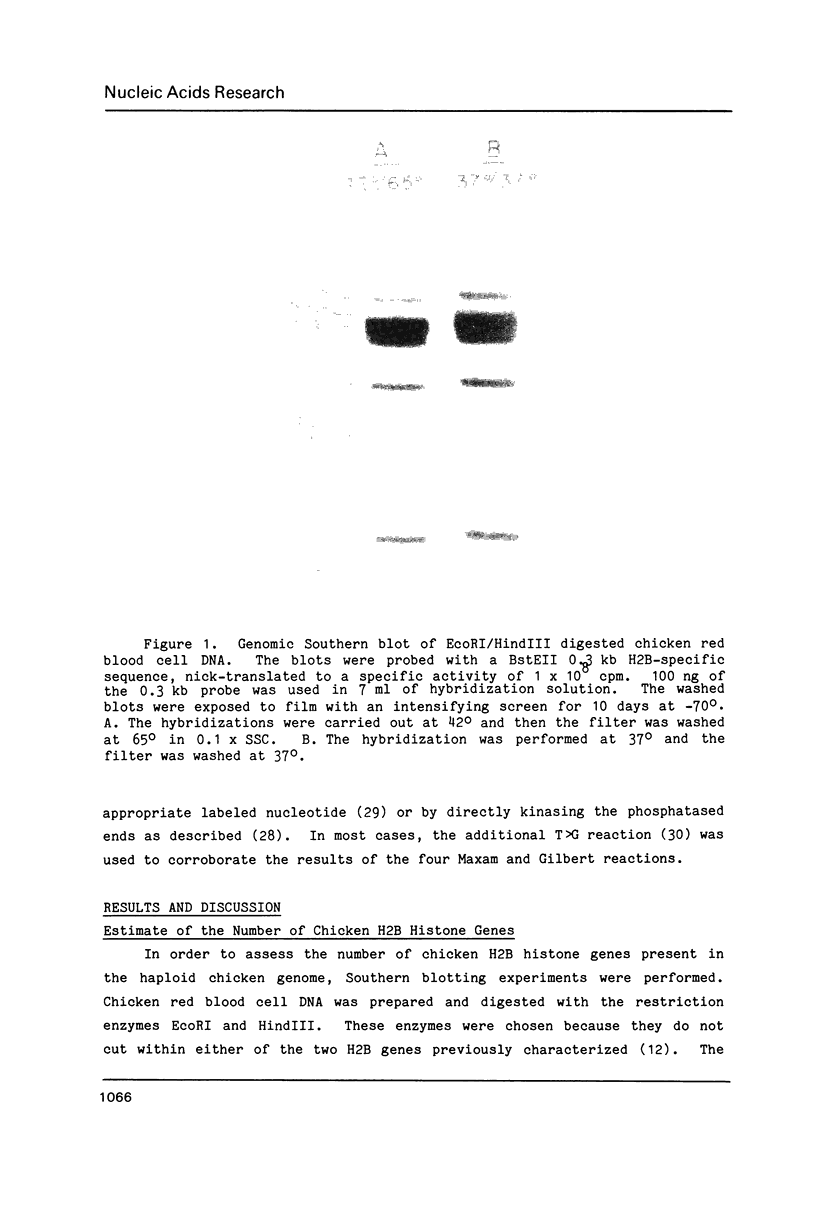
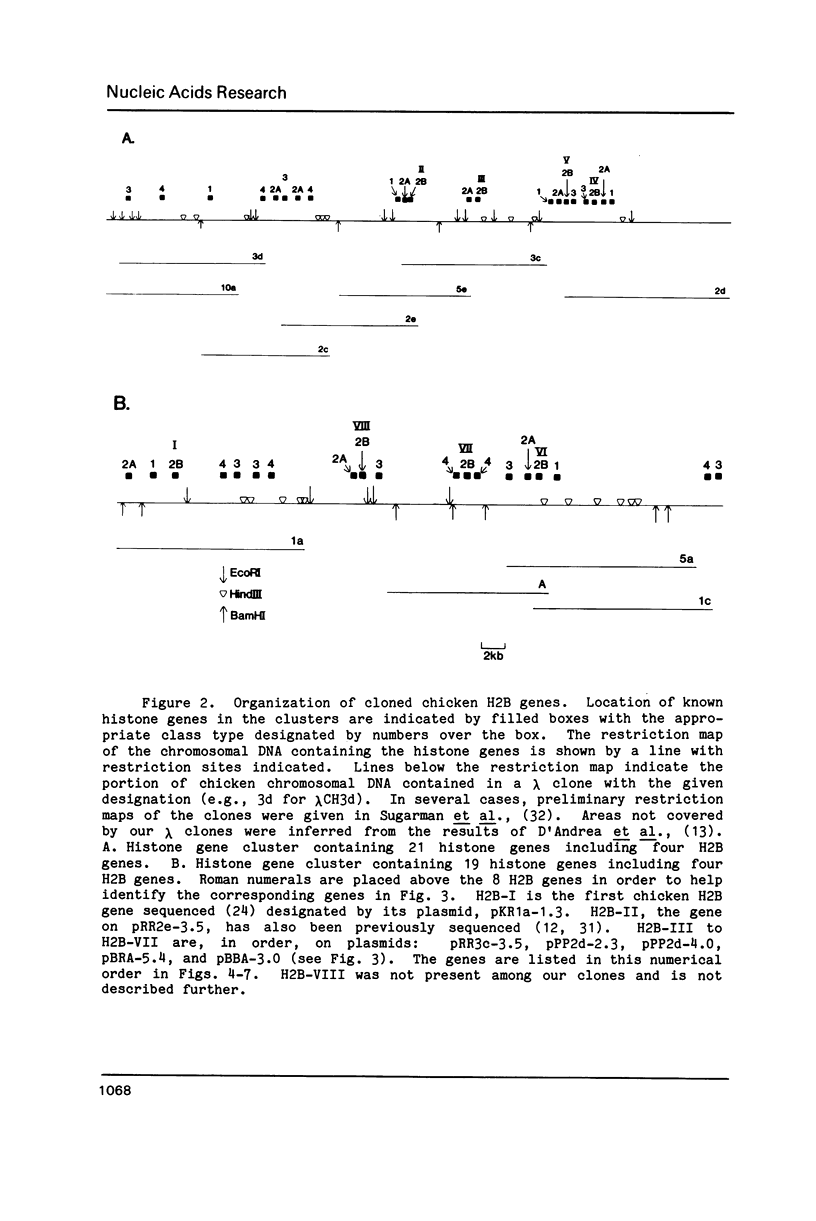
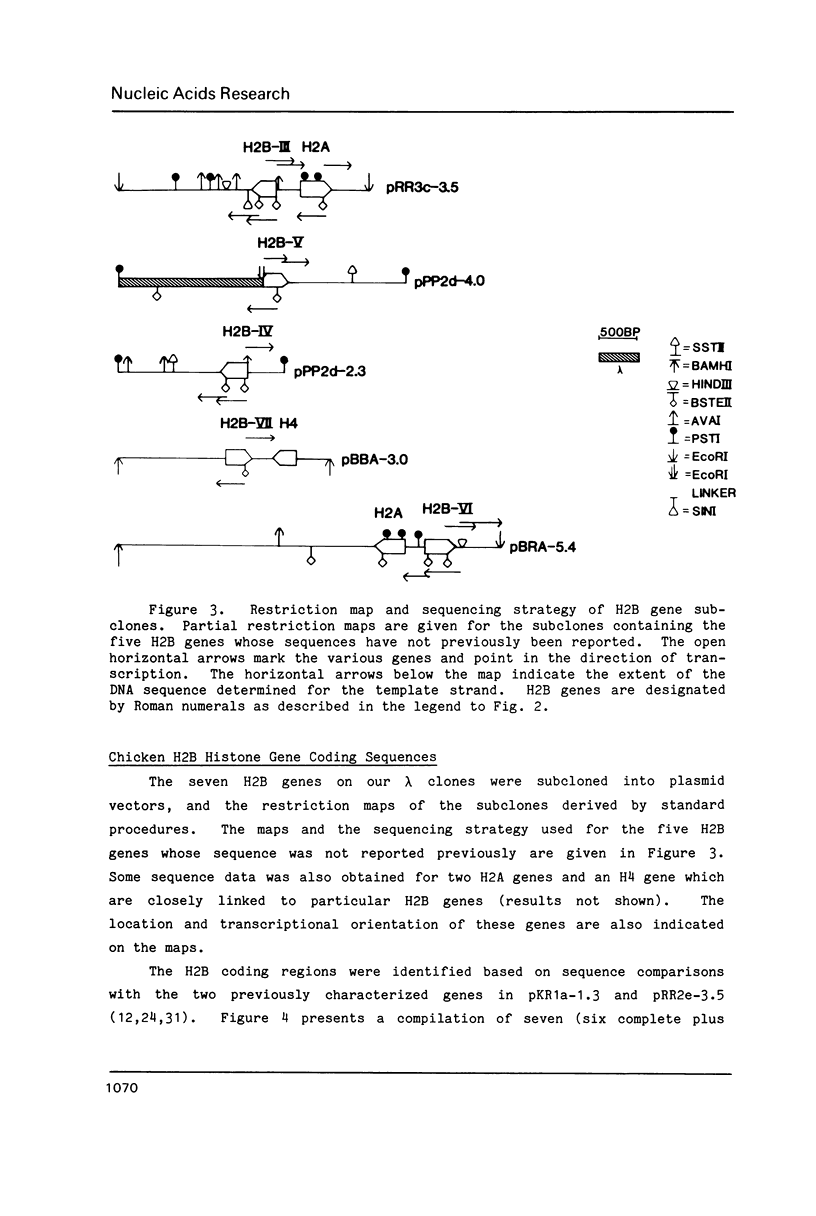
The results of Southern blotting experiments confirm that the chicken H2B histone gene family contains eight highly homologous members. One or two more sequences which are considerably divergent from the others appear to exist in the chicken genome. Seven of the eight H2B genes have been cloned and sequenced. All seven genes fall in two histone gene clusters, but no common arrangement exists for the clusters themselves. Three different H2B protein variants are encoded by these seven genes. The nucleotide sequence homology among the genes within their coding sequences appears to exceed that required for the corresponding protein sequences, suggesting that histone H2B mRNA sequence and structure are both selected during evolution. An analysis of the 5' flanking sequence data reveals that these genes possess CCAAT and TATA boxes, elements commonly associated with genes transcribed by RNA polymerase II. In addition, these genes all share an H2B-specific element of the form: ATTTGCATA. The 3' sequences of these genes contain the hyphenated symmetrical dyad homology and downstream purine-rich sequence shared by histone genes in general.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brush D., Dodgson J. B., Choi O. R., Stevens P. W., Engel J. D. Replacement variant histone genes contain intervening sequences. Mol Cell Biol. 1985 Jun;5(6):1307–1317. doi: 10.1128/mcb.5.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs G., Nocente-McGrath C., Lieber T., Holt C., Knowles J. A. Sea urchin (lytechinus pictus) late-stage histone H3 and H4 genes: characterization and mapping of a clustered but nontandemly linked multigene family. Cell. 1982 Dec;31(2 Pt 1):383–393. doi: 10.1016/0092-8674(82)90132-5. [DOI] [PubMed] [Google Scholar]
- D'Andrea R. J., Coles L. S., Lesnikowski C., Tabe L., Wells J. R. Chromosomal organization of chicken histone genes: preferred associations and inverted duplications. Mol Cell Biol. 1985 Nov;5(11):3108–3115. doi: 10.1128/mcb.5.11.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle A. J., Graves R. A., Marzluff W. F., Johnson L. F. Regulation of histone mRNA production and stability in serum-stimulated mouse 3T6 fibroblasts. Mol Cell Biol. 1983 Nov;3(11):1920–1929. doi: 10.1128/mcb.3.11.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Dolan M., Sugarman B. J., Dodgson J. B., Engel J. D. Chromosomal arrangement of the chicken beta-type globin genes. Cell. 1981 Jun;24(3):669–677. doi: 10.1016/0092-8674(81)90093-3. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Engel J. D., Dodgson J. B. Histone genes are clustered but not tandemly repeated in the chicken genome. Proc Natl Acad Sci U S A. 1981 May;78(5):2856–2860. doi: 10.1073/pnas.78.5.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. D., Sugarman B. J., Dodgson J. B. A chicken histone H3 gene contains intervening sequences. Nature. 1982 Jun 3;297(5865):434–436. doi: 10.1038/297434a0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Grandy D. K., Engel J. D., Dodgson J. B. Complete nucleotide sequence of a chicken H2b histone gene. J Biol Chem. 1982 Aug 10;257(15):8577–8580. [PubMed] [Google Scholar]
- Groppi V. E., Jr, Coffino P. G1 and S phase mammalian cells synthesize histones at equivalent rates. Cell. 1980 Aug;21(1):195–204. doi: 10.1016/0092-8674(80)90127-0. [DOI] [PubMed] [Google Scholar]
- Harvey R. P., Robins A. J., Wells J. R. Independently evolving chicken histone H2B genes: identification of a ubiquitous H2B-specific 5' element. Nucleic Acids Res. 1982 Dec 11;10(23):7851–7863. doi: 10.1093/nar/10.23.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. P., Whiting J. A., Coles L. S., Krieg P. A., Wells J. R. H2A.F: an extremely variant histone H2A sequence expressed in the chicken embryo. Proc Natl Acad Sci U S A. 1983 May;80(10):2819–2823. doi: 10.1073/pnas.80.10.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N., Zernik M., Roeder R. G. The structure of the human histone genes: clustered but not tandemly repeated. Cell. 1981 Jun;24(3):661–668. doi: 10.1016/0092-8674(81)90092-1. [DOI] [PubMed] [Google Scholar]
- Hentschel C. C., Birnstiel M. L. The organization and expression of histone gene families. Cell. 1981 Aug;25(2):301–313. doi: 10.1016/0092-8674(81)90048-9. [DOI] [PubMed] [Google Scholar]
- Hereford L., Fahrner K., Woolford J., Jr, Rosbash M., Kaback D. B. Isolation of yeast histone genes H2A and H2B. Cell. 1979 Dec;18(4):1261–1271. doi: 10.1016/0092-8674(79)90237-x. [DOI] [PubMed] [Google Scholar]
- Iwai K., Ishikawa K., Hayashi H. Amino-acid sequence of slightly lysine-rich histone. Nature. 1970 Jun 13;226(5250):1056–1058. doi: 10.1038/2261056b0. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Robins A. J., D'Andrea R., Wells J. R. The chicken H5 gene is unlinked to core and H1 histone genes. Nucleic Acids Res. 1983 Feb 11;11(3):619–627. doi: 10.1093/nar/11.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Perry M., Thomsen G. H., Roeder R. G. Genomic organization and nucleotide sequence of two distinct histone gene clusters from Xenopus laevis. Identification of novel conserved upstream sequence elements. J Mol Biol. 1985 Oct 5;185(3):479–499. doi: 10.1016/0022-2836(85)90065-8. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Affolter M., Renaud J. Genomic organization of the genes coding for the six main histones of the chicken: complete sequence of the H5 gene. J Mol Biol. 1983 Nov 15;170(4):843–859. doi: 10.1016/s0022-2836(83)80191-0. [DOI] [PubMed] [Google Scholar]
- Salser W. Globin mRNA sequences: analysis of base pairing and evolutionary implications. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):985–1002. doi: 10.1101/sqb.1978.042.01.099. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Török I. A photoinduced cleavage of DNA useful for determining T residues. Nucleic Acids Res. 1982 Dec 20;10(24):7959–7964. doi: 10.1093/nar/10.24.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittman D. B., Chiu I. M., Pan C. J., Cohn R. H., Kedes L. H., Marzluff W. F. Isolation of two clusters of mouse histone genes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4078–4082. doi: 10.1073/pnas.78.7.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. R., Calvo J. M. Nucleotide sequence of the E coli gene coding for dihydrofolate reductase. Nucleic Acids Res. 1980 May 24;8(10):2255–2274. doi: 10.1093/nar/8.10.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sugarman B. J., Dodgson J. B., Engel J. D. Genomic organization, DNA sequence, and expression of chicken embryonic histone genes. J Biol Chem. 1983 Jul 25;258(14):9005–9016. [PubMed] [Google Scholar]
- Urban M. K., Franklin S. G., Zweidler A. Isolation and characterization of the histone variants in chicken erythrocytes. Biochemistry. 1979 Sep 4;18(18):3952–3960. doi: 10.1021/bi00585a017. [DOI] [PubMed] [Google Scholar]
- Urban M. K., Zweidler A. Changes in nucleosomal core histone variants during chicken development and maturation. Dev Biol. 1983 Feb;95(2):421–428. doi: 10.1016/0012-1606(83)90043-x. [DOI] [PubMed] [Google Scholar]
- Wang S. W., Robins A. J., d'Andrea R., Wells J. R. Inverted duplication of histone genes in chicken and disposition of regulatory sequences. Nucleic Acids Res. 1985 Feb 25;13(4):1369–1387. doi: 10.1093/nar/13.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudt L. P., Pastink A., Kempers-Veenstra A. E., Jansen A. E., Mager W. H., Planta R. J. The genes coding for histone H3 and H4 in Neurospora crassa are unique and contain intervening sequences. Nucleic Acids Res. 1983 Aug 25;11(16):5347–5360. doi: 10.1093/nar/11.16.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. S., Bonner W. M. Separation of basal histone synthesis from S-phase histone synthesis in dividing cells. Cell. 1981 Dec;27(2 Pt 1):321–330. doi: 10.1016/0092-8674(81)90415-3. [DOI] [PubMed] [Google Scholar]
- Zernik M., Heintz N., Boime I., Roeder R. G. Xenopus laevis histone genes: variant H1 genes are present in different clusters. Cell. 1980 Dec;22(3):807–815. doi: 10.1016/0092-8674(80)90557-7. [DOI] [PubMed] [Google Scholar]
- van Helden P. D., Strickland W. N., Strickland M., von Holt C. The complete amino-acid sequence of histone H2B from erythrocytes of the adult domestic fowl Gallus domesticus. Biochim Biophys Acta. 1982 Apr 21;703(1):17–20. doi: 10.1016/0167-4838(82)90004-8. [DOI] [PubMed] [Google Scholar]