Abstract
Background
We recently reported, using a receiver operating characteristic area metric, the first meta-analytic comparison of the relative accuracy of cervical and anal cytology in detecting moderate or severe histopathologic lesions by magnification directed punch biopsy. The aim of the present research was to meta-analytically examine cut-point specific operating characteristics (sensitivity, specificity) of cervical and anal cytology in detecting high grade squamous intraepithelial lesion (HSIL) histopathology by colposcope directed punch biopsy.
Methodology/Principal Findings
The primary eligibility requirement was availability of tabulated cytology (normal, atypical cells of unclear significance [ASCUS], low grade squamous intraepithelial lesion, HSIL or atypical squamous cells cannot rule out high grade [ASC-H]) and biopsy (<HSIL, ≥ HSIL) counts. Meta-analysis and meta-regression of diagnostic accuracy was performed with examination of study quality criteria and heterogeneity. Thirty-three cervical and 11 anal publications were eligible between 1990 and 2010. Meta-analytically cut-point analysis showed that using a cut-point of ASCUS the sensitivity in both settings is similar while anal cytology is less specific than cervical cytology (specificity [95% confidence interval] 0.33 [0.20–0.49] vs. 0.53[0.40–0.66], p = 0.04) for the detection of HSIL histopathology by colposcope directed punch biopsy.
Conclusions/Significance
Using a cytology cut-point of HSIL or ASC-H, anal cytology is less sensitive but comparably specific to cervical cytology. However, using a cut-point of ASCUS, differences in accuracy were of borderline significance.
Introduction
In response to increasing rates of invasive anal cancer among HIV-infected persons [1], [2], a growing number of HIV clinics are implementing screening programs for anal cancer and its precursors modeled on procedures used in cervical cancer screening [3]. We recently reported, using a receiver operating characteristic (ROC) area metric, the first meta-analytic comparison of the relative accuracy of cervical and anal cytology in detecting moderate or severe (high grade squamous intraepithelial lesion -HSIL) histopathologic lesions by magnification directed punch biopsy [4]. While ROC area is a useful summary measure of test discrimination, a more clinically useful metric would be based on test sensitivity and specificity at varying cytology cut-points. We therefore conducted a secondary meta-analytic comparison of the previously published summary data tables. The aim of the present research was to meta-analytically examine cut-point specific operating characteristics (sensitivity [SE], specificity [SP]) of cervical and anal cytology in detecting HSIL histopathology by colposcopic and high resolution anoscopic (HRA) directed punch biopsy.
Methods
Eligible studies were identified by MEDLINE citation of relevant publications between 1990 and 2010 published in the English literature as described in flowchart of included studies in our original publication [4]. Briefly, the index test was cytologic sampling of cervicovaginal or anal canal tissues. The reference standard was defined as colposcope magnified and directed punch biopsy of the uterine cervix or anal canal, respectively. The addition of endocervical curettage sampling was allowed for colposcopy studies. Cytology diagnostic categories include negative (“no atypical or malignant cells” [NAMC]), atypical squamous cells of uncertain significance (ASCUS), atypical squamous cells can’t rule out high grade (ASC-H), low grade squamous intraepithelial lesion (LSIL), and HSIL. “Cases” are defined as those with histopathologic evidence of HSIL or greater by colposcope directed punch biopsy. Cases included cervical or anal intraepithelial neoplasia 2 (CIN 2 or AIN 2), CIN/AIN 3, Carcinoma in situ or invasive carcinoma. A primary study eligibility requirement was availability of cross- tabulated cytology (normal, ASCUS, LSIL, HSIL or ASC-H) and biopsy (<HSIL, ≥ HSIL) counts. Source study quality was rated using QUADAS criteria as previously published [4].
We meta-analytically compared the joint sensitivity and specificity of cervical and anal cytology for biopsy confirmed high grade dysplasia or carcinoma. In this analysis, cytology as the index test was treated as a four-level ordinal measure (NAMC, ASCUS, LSIL, ASC-H or HSIL or carcinoma) and histopathology as the reference standard, as a dichotomous outcome ( <HSIL, ≥ HSIL). Meta-analysis and meta-regression of diagnostic accuracy was performed using metandi and midas, respectively, implemented in Stata 11.2. Heterogeneity was examined using the I2statistic [5]. To illustrate the cut-point dependent tradeoffs between sensitivity and specificity, we plotted the ROC curves for anal and cervical cytology and calculated the corresponding ROC areas using the trapezoidal rule implemented using the integ function in Stata [6].
Results
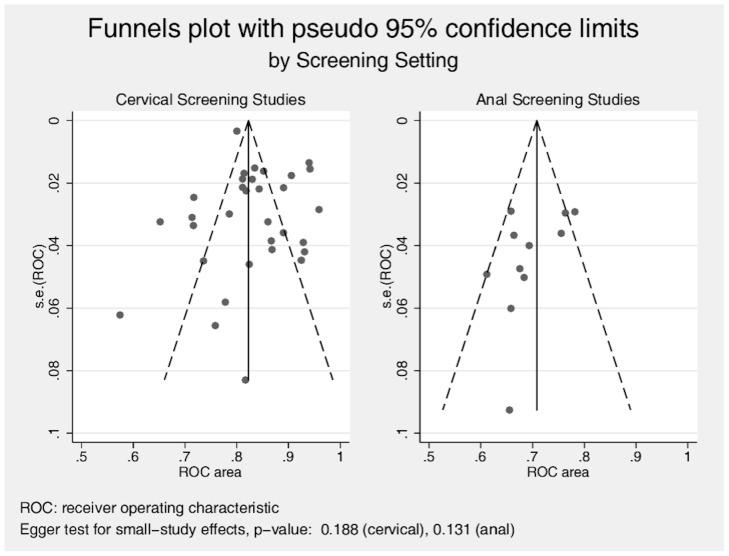
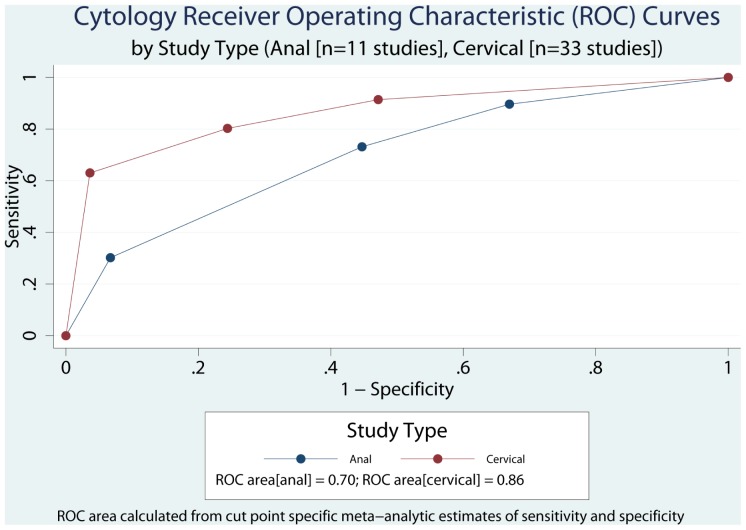
Thirty-three cervical and 11 anal publications were eligible according to the MEDLINE search algorithm and evaluation of review papers, Figures 1 and 2. Table S1 (supplementary information) presents the data extraction results and summary metric (cytology-biopsy ROC area) organized by study type (cervical and anal [7]–[50]. Table 1 presents the principal meta-analytic comparisons of the ability of cervical and anal cytology to differentiate between high grade and non-high grade histology by colposcope directed biopsy of uterine cervix and anal canal respectively, using different cytology cut-points. Using a cytology cut-point of either HSIL or ASC-H, cervical cytology, compared to anal cytology, had better sensitivity but comparable specificity to correctly identify HSIL histological lesions. However, using a cut-point of ASCUS, differences in accuracy were of borderline significance (cervical vs. anal: p = 0.04; I2 = 68). Study heterogeneity was large in both screening settings. Funnel plots (Figure 3), demonstrate that relatively more of the cervical screening studies fall outside the pseudo 95% confidence intervals than is observed for the anal screening studies, however, the relative symmetry of both funnel plots is supported by the non-significant Egger test for both. Figure 4 presents ROC curves based on alternative cytology cut-points and compares the ability of anal and cervical cytology to discriminate between histopathologic categories (<HSIL vs. ≥ HSIL).
Figure 1. Flow of Included Studies: Cervical Screening.
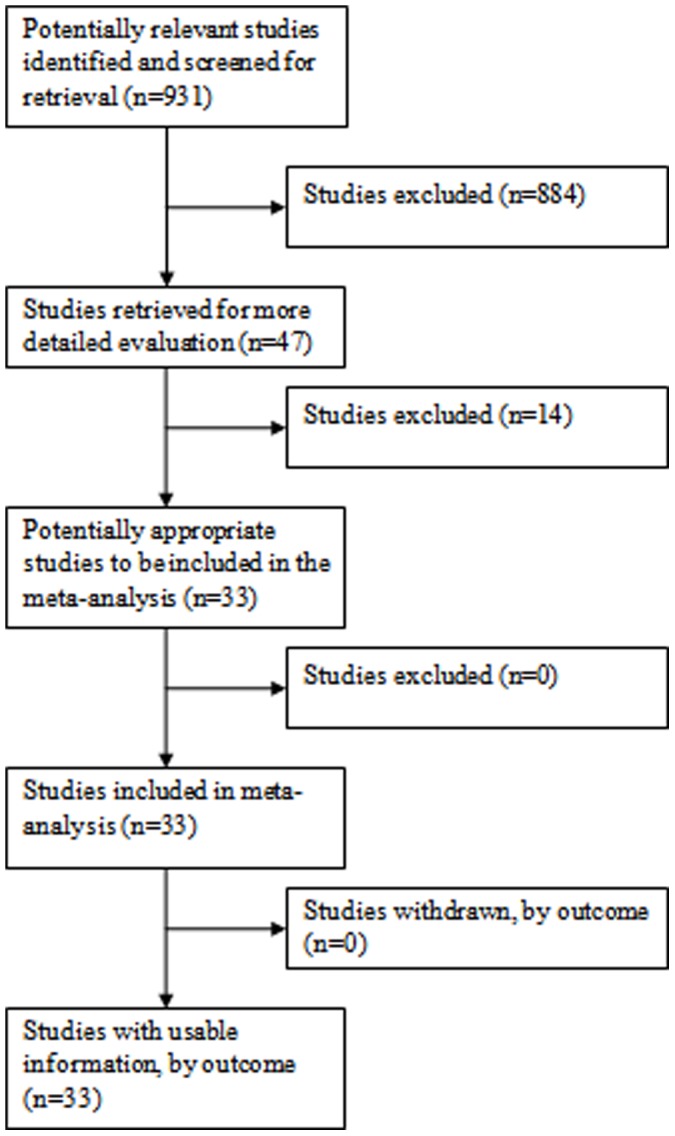
Figure 2. Flow of Included Studies: Anal Screening.
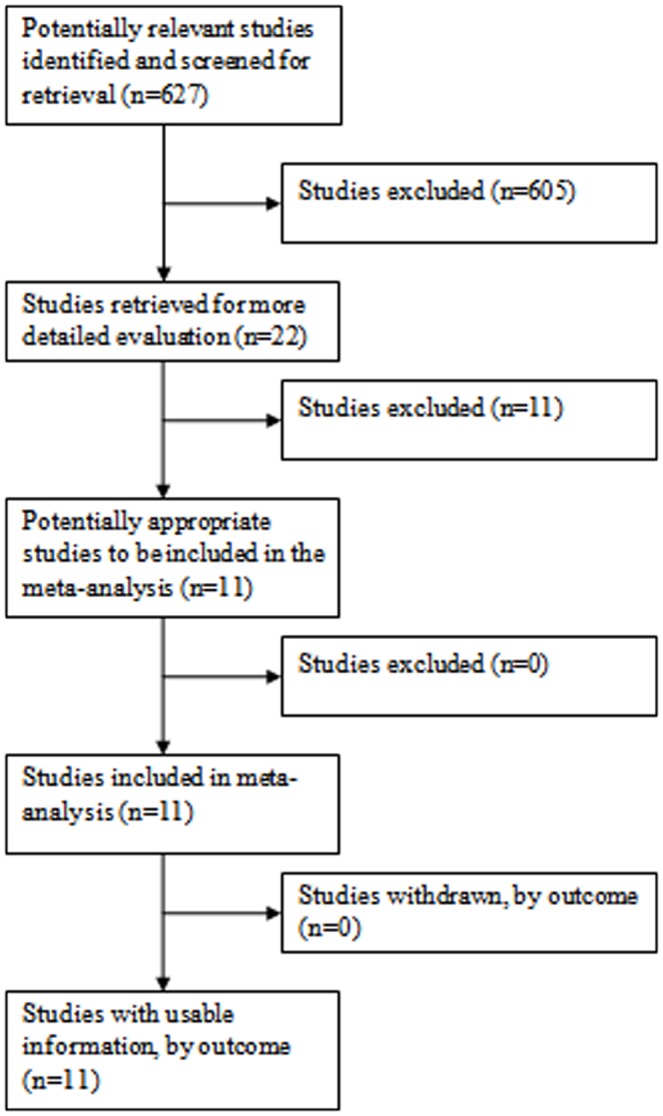
Table 1. Meta-analytically cut-point comparison of the joint sensitivity and specificity of cervical and anal cytology for biopsy confirmed high grade dysplasia.
| Sensitivity (SE) | Specificity (SP) | |||||||
| Cytology Cut-Point | Anal | Cervical | Anal | Cervical | ||||
| SE | (95% CI) | SE | (95% CI) | SP | (95% CI) | SP | (95% CI) | |
| (HSIL or ASC-H) vs. (LSIL, ASCUS, Normal)1 | 0.30 | (0.19–0.44) | 0.63 | (0.56–0.69) | 0.93 | (0.90–0.95) | 0.96 | (0.95–0.98) |
| (HSIL or ASC-H, LSIL) vs. (ASCUS, Normal)2 | 0.73 | (0.62–0.82) | 0.80 | (0.75–0.85) | 0.55 | (0.45–0.65) | 0.76 | (0.66–0.83) |
| (HSIL or ASC-H, LSIL, ASCUS) vs. (Normal)3 | 0.90 | (0.76–0.96) | 0.91 | (0.88–0.94) | 0.33 | (0.20–0.49) | 0.53 | (0.40–0.66) |
1. Joint model comparison (cervical vs. anal): p<0.001; I2 = 92.
2. Joint model comparison (cervical vs. anal): p<0.001; I2 = 82.
3. Joint model comparison (cervical vs. anal: p = 0.04; I2 = 68.
CI = Confidence Interval, HSIL = High grade squamous intraepithelial lesion, ASC-H = Atypical squamous cells can’t rule out high grade, LSIL = Low grade squamous intraepithelial lesion, ASCUS = Atypical squamous cells of uncertain significance.
Figure 3. Funnel plots with pseudo 95% confidence limits, by Screening Setting.
Figure 4. Performance of Diagnostic Receiver Operating Characteristic (ROC) areas by Screening Setting (Cervical, Anal) at different cut-points for identification of High grade squamous intraepithelial lesion histological lesions.
Discussion
Anal cancer screening has not been recommended as standard of care in HIV clinics in the United States with the exception of the State of New York [51]. Our cytology cut-point specific meta-analytic comparison of the relative accuracy of cervical and anal cytology allows the following conclusions to be made: (1) anal cytology is overall less discriminating than cervical cytology using ROC area as the metric of test discrimination; (2) if a cytology cut-point of NAMC vs. ≥ ASCUS is used, the sensitivity in both settings is nearly identical while anal cytology is meaningfully less specific than cervical cytology; (3)at cytology cut-point of (HSIL or ASC-H) vs. ≤ LSIL, specificity of anal and cervical cytology is nearly identical while sensitivity of anal cytology is meaningfully less than that of cervical cytology. In the setting of screening for anal cancer and its potentially modifiable precursor lesions, we believe that a selecting a cytology cut-point with maximal sensitivity is preferable to selecting a cut-point that maximizes specificity. The primary reason we argue this position is that, as we have previously shown [52], neither cervical nor anal histopathology obtained by colposcope magnified punch biopsy is a true “gold standard”. Punch biopsy in both settings is subject to several sources of error including sampling error, operator error, and interpretation error [53], [54]. Thus many false positive cytology results may in fact be erroneously classified because of the fallibility of the reference standard punch biopsy.
In summary, using a cytology cut-point of HSIL or ASC-H, anal cytology is less sensitive but comparably specific compared to cervical cytology. However, using a cut-point of ASCUS, differences in accuracy were of borderline significance. These results contribute to the evidence base regarding screening accuracy and might inform discussion regarding potential guidelines for screening for anal cancer and its precursors in high risk populations.
Supporting Information
Extracted Study Data and Outcome Metrics, by Study Type Cytology-Biopsy Joint Cell Frequencies.
(DOC)
Acknowledgments
We wish to thank Susan McQuillen for clinical and administrative assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported in part by the Clinical Investigation Core of the University of California San Diego Center for AIDS Research [AI036214], the CFAR Network of Integrated Clinical Systems (CNICS) [R24 AI067039-01A1], and the Pacific AIDS Education and Training Center (PAETC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.D’Souza G, Wiley DJ, Li X, Chmiel JS, Margolick JB, et al. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. Journal of acquired immune deficiency syndromes. 2008;48:491–9. doi: 10.1097/QAI.0b013e31817aebfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piketty C, Selinger-Leneman H, Grabar S, Duvivier C, Bonmarchand M, et al. Marked increase in the incidence of invasive anal cancer among HIV-infected patients despite treatment with combination antiretroviral therapy. AIDS. 2008;22:1203–11. doi: 10.1097/QAD.0b013e3283023f78. [DOI] [PubMed] [Google Scholar]
- 3.Palefsky J. Anal Cancer Info. San Francisco. University of California San Francisco. 2012:List of HRA providers. 2012. Available: http://id.medicine.ucsf.edu/analcancerinfo/all_providers.html#C. Accessed: 2012 February 21.
- 4.Mathews WC, Agmas W, Cachay E. Comparative accuracy of anal and cervical cytology in screening for moderate to severe dysplasia by magnification guided punch biopsy: a meta-analysis. PLoS One. 2011;6:e24946. doi: 10.1371/journal.pone.0024946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1998;44:837–45. [PubMed] [Google Scholar]
- 7.Adamopoulou M, Kalkani E, Charvalos E, Avgoustidis D, Haidopoulos D, et al. Comparison of cytology, colposcopy, HPV typing and biomarker analysis in cervical neoplasia. Anticancer Res. 2009;29:3401–3409. [PubMed] [Google Scholar]
- 8.Alves RR, Rabelo-Santos SH, Ribeiro AA, Carneiro MA, Ximenes Y, et al. Usefulness of repeat cytology at the time of first colposcopy. Diagn Cytopathol. 2009;37:68–73. doi: 10.1002/dc.20976. [DOI] [PubMed] [Google Scholar]
- 9.Andersson S, Sowjanya P, Wangsa D, Hjerpe A, Johansson B, et al. Detection of genomic amplification of the human telomerase gene TERC, a potential marker for triage of women with HPV-positive, abnormal Pap smears. Am J Pathol. 2009;175:1831–1847. doi: 10.2353/ajpath.2009.090122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angstetra D, Tait T, Tan J, Symonds I. Should liquid-based cytology be performed prior to colposcopy? A comparison of the accuracy, unsatisfactory rates and cost in a tertiary referral setting. Aust N Z J Obstet Gynaecol. 2009;49:681–684. doi: 10.1111/j.1479-828X.2009.01095.x. [DOI] [PubMed] [Google Scholar]
- 11.Antonishyn NA, Horsman GB, Kelln RA, Severini A. Human papillomavirus typing and viral gene expression analysis for the triage of women with abnormal results from papanicolaou test smears to colposcopy. Arch Pathol Lab Med. 2009;133:1577–1586. doi: 10.5858/133.10.1577. [DOI] [PubMed] [Google Scholar]
- 12.Baer A, Kiviat NB, Kulasingam S, Mao C, Kuypers J, et al. Liquid-based Papanicolaou smears without a transformation zone component: should clinicians worry? Obstet Gynecol. 2002;99:1053–1059. doi: 10.1016/s0029-7844(02)01998-1. [DOI] [PubMed] [Google Scholar]
- 13.Belinson JL, Pan QJ, Biscotti C, Wu LY, Pretorius RG, et al. Primary screening with liquid-based cytology in an unscreened population in rural China, with an emphasis on reprocessing unsatisfactory samples. Acta Cytol. 2002;46:470–474. doi: 10.1159/000326863. [DOI] [PubMed] [Google Scholar]
- 14.Benevolo M, Vocaturo A, Mottolese M, Mariani L, Vocaturo G, et al. Clinical role of p16INK4a expression in liquid-based cervical cytology: correlation with HPV testing and histologic diagnosis. Am J Clin Pathol. 2008;129:606–612. doi: 10.1309/BEPQXTCQD61RGFMJ. [DOI] [PubMed] [Google Scholar]
- 15.Bigras G, de Marval F. The probability for a Pap test to be abnormal is directly proportional to HPV viral load: results from a Swiss study comparing HPV testing and liquid-based cytology to detect cervical cancer precursors in 13,842 women. Br J Cancer. 2005;93:575–581. doi: 10.1038/sj.bjc.6602728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carns B, Fadare O. Papanicolaou test in the detection of high-grade cervical lesions: a re-evaluation based on cytohistologic non-correlation rates in 356 concurrently obtained samples. Int J Clin Exp Pathol. 2008;1:285–290. [PMC free article] [PubMed] [Google Scholar]
- 17.Chung JH, Park EJ, Choi YD, Kim HS, Lee YJ, et al. Efficacy assessment of CellSlide in liquid-based gynecologic cytology. Gynecol Oncol. 2005;99:597–602. doi: 10.1016/j.ygyno.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 18.Cohn JA, Gagnon S, Spence MR, Harrison DD, Kluzak TR, et al. The role of human papillomavirus deoxyribonucleic acid assay and repeated cervical cytologic examination in the detection of cervical intraepithelial neoplasia among human immunodeficiency virus-infected women. Cervical Disease Study Group of the American Foundation for AIDS Research Community Based Clinical Trials Network. Am J Obstet Gynecol. 2001;184:322–330. doi: 10.1067/mob.2001.109938. [DOI] [PubMed] [Google Scholar]
- 19.DiBonito L, Falconieri G, Tomasic G, Colautti I, Bonifacio D, et al. Cervical cytopathology. An evaluation of its accuracy based on cytohistologic comparison. Cancer. 1993;72:3002–3006. doi: 10.1002/1097-0142(19931115)72:10<3002::aid-cncr2820721023>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Guerra B, De Simone P, Gabrielli S, Falco P, Montanari G, et al. Combined cytology and colposcopy to screen for cervical cancer in pregnancy. J Reprod Med. 1998;43:647–653. [PubMed] [Google Scholar]
- 21.Guo M, Hu L, Martin L, Liu S, Baliga M, et al. Accuracy of liquid-based Pap tests: comparison of concurrent liquid-based tests and cervical biopsies on 782 women with previously abnormal Pap smears. Acta Cytol. 2005;49:132–138. doi: 10.1159/000326120. [DOI] [PubMed] [Google Scholar]
- 22.Guo M, Patel SJ, Chovanec M, Jan YJ, Tarco E, et al. A human papillomavirus testing system in women with abnormal Pap results: a comparison study with follow-up biopsies. Acta Cytol. 2007;51:749–754. doi: 10.1159/000325838. [DOI] [PubMed] [Google Scholar]
- 23.Harkness CB, Theofrastous JP, Ibrahim SN, Galvin SL, Lawrence HC. Papanicolaou and thin-layer cervical cytology with colposcopic biopsy control. A comparison. J Reprod Med. 2003;48:681–686. [PubMed] [Google Scholar]
- 24.Howard M, Sellors J, Kaczorowski J. Optimizing the hybrid capture II human papillomavirus test to detect cervical intraepithelial neoplasia. Obstet Gynecol. 2002;100:972–980. doi: 10.1016/s0029-7844(02)02315-3. [DOI] [PubMed] [Google Scholar]
- 25.Jones BA, Novis DA. Cervical biopsy-cytology correlation. A College of American Pathologists Q-Probes study of 22 439 correlations in 348 laboratories. Arch Pathol Lab Med. 1996;120:523–531. [PubMed] [Google Scholar]
- 26.Kumar K, Iyer VK, Bhatla N, Kriplani A, Verma K. Comparative evaluation of smear cytology & hybrid capture II for the diagnosis of cervical cancer. Indian J Med Res. 2007;126:39–44. [PubMed] [Google Scholar]
- 27.Lee GY, Kim SM, Rim SY, Choi HS, Park CS, et al. Human papillomavirus (HPV) genotyping by HPV DNA chip in cervical cancer and precancerous lesions. Int J Gynecol Cancer. 2005;15:81–87. doi: 10.1111/j.1048-891x.2005.14417.x. [DOI] [PubMed] [Google Scholar]
- 28.Lorenzato F, Ho L, Terry G, Singer A, Santos LC, et al. The use of human papillomavirus typing in detection of cervical neoplasia in Recife (Brazil). Int J Gynecol Cancer. 2000;10:143–150. doi: 10.1046/j.1525-1438.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 29.Mao C, Balasubramanian A, Koutsky LA. Should liquid-based cytology be repeated at the time of colposcopy? J Low Genit Tract Dis. 2005;9:82–88. doi: 10.1097/00128360-200504000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Mattosinho de Castro Ferraz Mda G, Nicolau SM, Stavale JN, Focchi J, Castelo A, et al. Cervical biopsy-based comparison of a new liquid-based thin-layer preparation with conventional Pap smears. Diagn Cytopathol. 2004;30:220–226. doi: 10.1002/dc.10409. [DOI] [PubMed] [Google Scholar]
- 31.Pan Q, Belinson JL, Li L, Pretorius RG, Qiao YL, et al. A thin-layer, liquid-based pap test for mass screening in an area of China with a high incidence of cervical carcinoma. A cross-sectional, comparative study. Acta Cytol. 2003;47:45–50. doi: 10.1159/000326474. [DOI] [PubMed] [Google Scholar]
- 32.Pimple SA, Amin G, Goswami S, Shastri SS. Evaluation of colposcopy vs cytology as secondary test to triage women found positive on visual inspection test. Indian J Cancer. 2010;47:308–313. doi: 10.4103/0019-509X.64726. [DOI] [PubMed] [Google Scholar]
- 33.Ratnam S, Franco EL, Ferenczy A. Human papillomavirus testing for primary screening of cervical cancer precursors. Cancer Epidemiol Biomarkers Prev. 2000;9:945–951. [PubMed] [Google Scholar]
- 34.Sangwa-Lugoma G, Mahmud S, Nasr SH, Liaras J, Kayembe PK, et al. Visual inspection as a cervical cancer screening method in a primary health care setting in Africa. Int J Cancer. 2006;119:1389–1395. doi: 10.1002/ijc.21972. [DOI] [PubMed] [Google Scholar]
- 35.Simsir A, Ioffe OB, Bourquin P, Brooks SE, Henry M. Repeat cervical cytology at the time of colposcopy. Is there an added benefit? Acta Cytol. 2001;45:23–27. doi: 10.1159/000327183. [DOI] [PubMed] [Google Scholar]
- 36.Sun XR, Wang J, Garner D, Palcic B. Detection of cervical cancer and high grade neoplastic lesions by a combination of liquid-based sampling preparation and DNA measurements using automated image cytometry. Cell Oncol. 2005;27:33–41. doi: 10.1155/2005/981612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor S, Kuhn L, Dupree W, Denny L, De Souza M, et al. Direct comparison of liquid-based and conventional cytology in a South African screening trial. Int J Cancer. 2006;118:957–962. doi: 10.1002/ijc.21434. [DOI] [PubMed] [Google Scholar]
- 38.Witt A, Hudelist G, Gregor H, Kucera E, Walchetseder C, et al. The detection of HPV DNA improves the recognition of cervical intraepithelial lesions. Arch Gynecol Obstet. 2003;268:29–34. doi: 10.1007/s00404-002-0320-9. [DOI] [PubMed] [Google Scholar]
- 39.Yu BK, Kuo BI, Yen MS, Twu NF, Lai CR, et al. Improved early detection of cervical intraepithelial lesions by combination of conventional Pap smear and speculoscopy. Eur J Gynaecol Oncol. 2003;24:495–499. [PubMed] [Google Scholar]
- 40.Berry JM, Palefsky JM, Jay N, Cheng SC, Darragh TM, et al. Performance characteristics of anal cytology and human papillomavirus testing in patients with high-resolution anoscopy-guided biopsy of high-grade anal intraepithelial neoplasia. Diseases of the colon and rectum. 2009;52:239–247. doi: 10.1007/DCR.0b013e31819793d9. [DOI] [PubMed] [Google Scholar]
- 41.Cranston RD, Darragh TM, Holly EA, Jay N, Berry JM, et al. Self-collected versus clinician-collected anal cytology specimens to diagnose anal intraepithelial neoplasia in HIV-positive men. Journal of acquired immune deficiency syndromes. 2004;36:915–920. doi: 10.1097/00126334-200408010-00004. [DOI] [PubMed] [Google Scholar]
- 42.Mathews WC, Sitapati A, Caperna JC, Barber RE, Tugend A, et al. Measurement characteristics of anal cytology, histopathology, and high-resolution anoscopic visual impression in an anal dysplasia screening program. Journal of acquired immune deficiency syndromes. 2004;37:1610–1615. doi: 10.1097/00126334-200412150-00014. [DOI] [PubMed] [Google Scholar]
- 43.Mathews WC, Cachay ER, Caperna J, Sitapati A, Cosman B, et al. Estimating the accuracy of anal cytology in the presence of an imperfect reference standard. PloS one. 2010;5:e12284. doi: 10.1371/journal.pone.0012284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nahas CS, da Silva Filho EV, Segurado AA, Genevcius RF, Gerhard R, et al. Screening anal dysplasia in HIV-infected patients: is there an agreement between anal pap smear and high-resolution anoscopy-guided biopsy? Diseases of the colon and rectum. 2009;52:1854–1860. doi: 10.1007/DCR.0b013e3181b98f36. [DOI] [PubMed] [Google Scholar]
- 45.Nathan M, Singh N, Garrett N, Hickey N, Prevost T, et al. Performance of anal cytology in a clinical setting when measured against histology and high-resolution anoscopy findings. AIDS. 2010;24:373–379. doi: 10.1097/QAD.0b013e328333ab8e. [DOI] [PubMed] [Google Scholar]
- 46.Palefsky JM, Holly EA, Hogeboom CJ, Berry JM, Jay N, et al. Anal cytology as a screening tool for anal squamous intraepithelial lesions. Journal of acquired immune deficiency syndromes and human retrovirology. 1997;14:415–422. doi: 10.1097/00042560-199704150-00004. [DOI] [PubMed] [Google Scholar]
- 47.Panther LA, Wagner K, Proper J, Fugelso DK, Chatis PA, et al. High resolution anoscopy findings for men who have sex with men: inaccuracy of anal cytology as a predictor of histologic high-grade anal intraepithelial neoplasia and the impact of HIV serostatus. Clinical infectious diseases. 2004;38:1490–1492. doi: 10.1086/383574. [DOI] [PubMed] [Google Scholar]
- 48.Salit IE, Lytwyn A, Raboud J, Sano M, Chong S, et al. The role of cytology (Pap tests) and human papillomavirus testing in anal cancer screening. AIDS. 2010;24:1307–1313. doi: 10.1097/QAD.0b013e328339e592. [DOI] [PubMed] [Google Scholar]
- 49.Tramujas da Costa ESI, Coelho Ribeiro M, Santos Gimenez F, Dutra FerreiraJR, Galvao RS, et al. Performance of p16INK4a immunocytochemistry as a marker of anal squamous intraepithelial lesions. Cancer cytopathology. 2011;119:167–76. doi: 10.1002/cncy.20143. [DOI] [PubMed] [Google Scholar]
- 50.Williams VM, Metcalf C, French MA, McCloskey JC. Audit of paired anal cytology and histopathology outcomes in patients referred to a public sexual health clinic. Sexual health. 2010;7:346–351. doi: 10.1071/SH09118. [DOI] [PubMed] [Google Scholar]
- 51.AIDS Institute New York Department of Health. New York State Guidelines recommendations on anal dysplasia and cancer screening. 2007. Available: http://www.hivguidelines.org/clinical-guidelines/adults/neoplastic-complications-of-hiv-infection/#V. Accessed: 2012 February 22.
- 52.Mathews WC, Cachay ER, Caperna J, Sitapati A, Cosman B, Abramson I. Estimating the accuracy of anal cytology in the presence of an imperfect reference standard. PLoS One. 2010;5:e12284. doi: 10.1371/journal.pone.0012284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lytwyn A, Salit IE, Raboud J. Interobserver agreement in the interpretation of anal intraepithelial neoplasia. Cancer. 2005;103:1447–56. doi: 10.1002/cncr.20927. [DOI] [PubMed] [Google Scholar]
- 54.Mathews WC, Sitapati A, Caperna JC, Barber RE, Tugend A, et al. Measurement characteristics of anal cytology, histopathology, and high-resolution anoscopic visual impression in an anal dysplasia screening program. Journal of acquired immune deficiency syndromes. 2004;37:1610–5. doi: 10.1097/00126334-200412150-00014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Extracted Study Data and Outcome Metrics, by Study Type Cytology-Biopsy Joint Cell Frequencies.
(DOC)