Abstract
The requirements of micronutrients for biomass and hydrocarbon production in Botryococcus braunii UTEX 572 were studied using response surface methodology. The concentrations of four micronutrients (iron, manganese, molybdenum, and nickel) were manipulated to achieve the best performance of B. braunii in laboratory conditions. The responses of algal biomass and hydrocarbon to the concentration variations of the four micronutrients were estimated by a second order quadratic regression model. Genetic algorithm calculations showed that the optimal level of micronutrients for algal biomass were 0.266 µM iron, 0.707 µM manganese, 0.624 µM molybdenum and 3.38 µM nickel. The maximum hydrocarbon content could be achieved when the culture media contained 10.43 µM iron, 6.53 µM manganese, 0.012 µM molybdenum and 1.73 µM nickel. The validation through an independent test in a photobioreactor suggests that the modified media with optimised concentrations of trace elements can increase algal biomass by 34.5% and hydrocarbon by 27.4%. This study indicates that micronutrients play significant roles in regulating algal growth and hydrocarbon production, and the response surface methodology can be used to optimise the composition of culture medium in algal culture.
Introduction
Microalgae have recently been receiving much attention in an attempt to explore their use as a potential feedstock for biofuel production [1], [2]. Botryococcus braunii is a green colonial microalga found in freshwater lakes, reservoirs, and ponds [3], [4] and is classified into A, B and L races depending on the type of hydrocarbons synthesized [5]. Race A produces C23–C33 odd numbered n-alkadienes, mono-, tri-, tetra-, and pentaenes and race B produces C30–C37 triperpenes while race L produces C40 tetraperpenes [5]. This species is characterised by a conspicuous ability to synthesise and accumulate a variety of hydrocarbons [6], [7], [8]. These hexane-soluble hydrocarbons have the potential to be converted into biofuels by catalytic cracking [9]. However, the great variation of hydrocarbon content in B. braunii (0.1∼86% of dry weight) provides an opportunity to explore the optimal growing conditions to maximise hydrocarbon production for a given B. braunii strain [10], [11], [12]. Therefore, it is necessary to identify the most efficient growing conditions for sustainable mass and hydrocarbon production in B. braunii.
The requirements for macronutrients by B. braunii have been intensively studied in the past a few decades. Largeau et al. [13] pointed out that the phosphorus (0.46 mM) in the Chu 13 medium was not limiting through the stationary growth phase in B. braunii, while the nitrogen concentration of 0.5 mM NO3 - is only adequate to sustain the growth of B. braunii for 10 days and the initial concentration of 8 mM NO3 - is required to maintain the growth of growth B. braunii for 35 days. Ammonia can inhibit botryococcene biosynthesis in the B. braunii race B [14], but the replacement of nitrite nitrogen for nitrate nitrogen benefits the growth of race A B. braunii [15]. Air enriched with 1% CO2 can enhance algal growth by doubling algal biomass and achieving 5-fold hydrocarbon production compared to aeration without CO2 enrichment [16]. Dayanada et al. [17] reported that the N: P ratio played a significant role in both biomass and hydrocarbon production in B. braunii and the N: P ratio of 1∶4 by weight favoured hydrocarbon production while the N:P ratio of 1∶0.5 by weight increased the yield of algal biomass.
Given the depth of understanding in the growth requirement for macronutrients in B. braunii, it is surprising that the requirements for trace elements are little known. Trace elements such as iron, molybdenum and manganese can play critical roles in a variety of metabolic pathways involving utilization of light, nitrogen, phosphorus, and CO2 [18], [19]. Among trace elements, iron is essential for photosynthetic electron transport, respiratory electron transport, nitrate and nitrite reduction, and detoxification of reactive oxygen species [20], [21], [22]. Mojaat et al. [23] demonstrated that the addition of iron to the Dunaliella salina culture medium stimulated β-carotene production. The iron enrichment in the Chlorella vulgaris culture could increase algal growth and lipid accumulation [24], where the total lipid content of algae grown in the medium supplemented with 1.2×10−5 M FeCl3 reached 56.6% of the dry biomass, which was a 3–7 fold increase compared to the medium without iron enrichment. Manganese is another important component in algal photosynthesis and also presents in enzymes to remove toxic superoxide radicals to sustain algal growth [25]. Chernikova et al. [26] reported that manganese (MnCl2) enhanced the capacity to accumulate inorganic minerals and catalysed protein synthesis in Spirulina platensis. Molybdenum is coupled with iron in the enzymes for nitrate reduction, and its deficiency diminishes the nitrate uptake mechanism and interferes with lipid synthesis [27]. Nickel can facilitate nitrogen uptake to enhance the growth of Thalassiosira weissflogii when urea is the nitrogen source, suggesting the positive role of Ni in enhancing algal growth [28]. Berges et al. [29] also reported that the addition of nickel and molybdenum to the algal culture medium increased the overall primary productivity. Coincidently, in a field survey, Wake and Hillen [3] found that wherever the B. braunii bloom occurred in the Darwin River reservoir, the nickel concentration in the environment was always higher than that in adjacent water bodies where no B. braunii bloomed, suggesting this trace element may trigger the occurrence of B. braunii. However, no laboratory testing has been conducted so far to test the need of nickel to enhance the growth of B. braunii in the laboratory since the early field survey work of Wake and Hillen’s in the 1980’s.
Optimization of micronutrient requirements is an important undertaking prior to the establishment of sustainable production of B. braunii on a large scale. The conventional method to optimise the level of multiple nutrients in algal culture has been focussed on one-factor-at-a-time approach, studying the effect of one nutrient on the response of algae by keeping the other nutrients constant. However, this approach is time consuming and does not take into account interactions between nutrients, which usually results in poor optimization results [30], [31].
Techniques in experimental design are critical to identify key nutrients required for algal growth. In this study we used the response surface methodology (RSM) [32] to explore the requirement of micronutrients in the culture of B. braunii because the RSM approach can optimise the nutrient requirement with low input of time and resources [33], [34], [35]. This approach has been widely used in optimization of plant nutrients [36], [37], bacterial medium composition [38], enzymatic hydrolysis [39], [40], synthesis of polymers [41], food processing [42], [43] and operation conditions for photobioreactors [44]. The RSM approach has also been used for medium optimisation in algal culture. Azma et al. [45] optimised the culture medium for Tetraselmis suecica by RSM and increased algal production by two times. Similarly, by using RSM, Isleten-Hosoglu et al. [46] optimised the carbon and nitrogen concentrations for Chlorella saccharophila and improved biomass production by 7.7 fold.
The objectives of this study were to (1) estimate the roles of the four micronutrients iron, manganese, molybdenum, and nickel in regulating the responses of algal biomass and hydrocarbon, and (2) identify the optimum requirements of micronutrients for the cultivation of B. braunii to maximise hydrocarbon production.
Methods
Materials and Procedures
Botryococcus braunii UTEX 572 was obtained from the University of Texas Culture Collection, USA. The basic macronutrients for algal growth were adapted from the Bold 3N medium, which also contains micronutrients including 5.35 µM Fe, 6.36 µM Mn, and 0.31 µM Mo [47]. All chemicals were of analytical regent grade. To avoid the effect of other unknown trace elements, soil residuals were not added into the medium in this study. The experiment for model construction was conducted at 24±1°C with illumination provided by fluorescent lights at 150 µmol/m2/s at 12 h light and 12 h dark. The algal growth experiments lasted 3 weeks.
The dry weight of algal cells was measured by vacuum filtration onto pre-weighed Whatman® GF/C filters [48]. The filters with algal cells were freeze-dried, weighed, and expressed as algal biomass (g/L). Hydrocarbons in dry biomass were extracted on glass filters using η-hexane [48]. Solvents were removed from the extracts by a rotary evaporator and the residues were rinsed with η-hexane. Hydrocarbon fractions were purified by passing the samples through an alumina gel plug and eluting with η-hexane. Solvents were evaporated under a stream of nitrogen to dry, and the pure hydrocarbon fractions were measured gravimetrically and expressed as hydrocarbon content (%, w/w).
Experimental Design
Central composite design (CCD) is one type of RSM approach [49] which allows estimating the polynomial regression between independent variables and dependant variables [50]. In this study, a 24 CCD with 24 runs and six replications of the centre points were used to determine the optimal concentrations of iron, manganese, molybdenum, and nickel on the yield of algal biomass and hydrocarbon production (Fig. 1). The coded and corresponding actual values are given in Table 1. The corresponding central composite experimental design and their values are shown in Table 2. All the design points except the centre point (0, 0, 0, 0) were run in three replications. Due to the restriction of modeling protocol, only one mean value of the three replicates for each dependent variable was allowed to enter the model. Therefore, the degree of freedom of the triplicate for each non-centrepoint could not be used for pure error calculation. Experiments were repeated six times at the central point to provide an estimate of pure error [51], [52], [53], [54] thus providing adequate degree of freedom (df = 5) for pure error calculation (Tables 3 and 4).
Figure 1. Illustration of the central composite design (only 3 out of the 4 dimensions are shown).
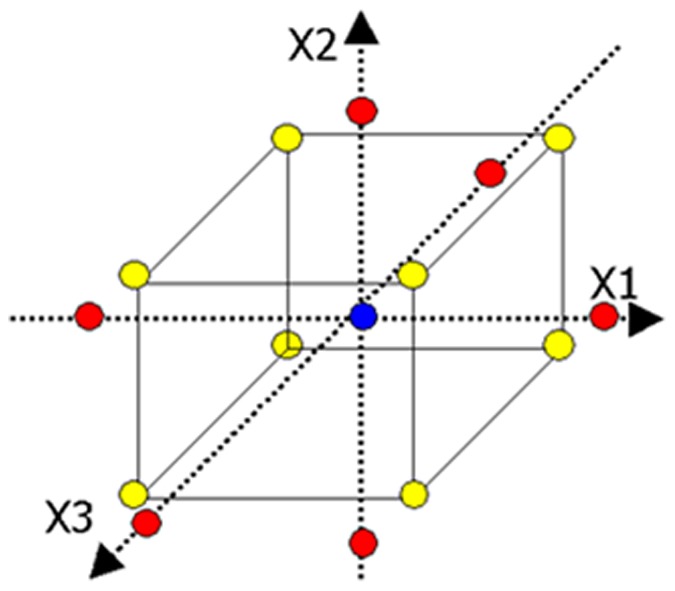
Table 1. Coded and actual values of experimental variables used in the central composite experimental design.
| Independentvariables | Symbols | Levels | ||||
| −1.72* | −1 | 0 | 1 | 1.72* | ||
| Fe (µM) | x1 | 0.03 | 2.39 | 5.35 | 8.31 | 10.44 |
| Mn (µM) | x2 | 0.02 | 2.67 | 6.36 | 10.05 | 12.70 |
| Mo (µM) | x3 | 0 | 0.13 | 0.31 | 0.50 | 0.62 |
| Ni (µM) | x4 | 0 | 0.71 | 1.69 | 2.68 | 3.39 |
Alpha values used for the axial points in this study.
Table 2. Central composite design matrix and the responses of biomass and hydrocarbon production to Fe (x1), Mn (x2), Mo (x3) and Ni (x4).
| Runs | Independent variables | Responses | ||||
| Coded levels | Biomass(g/L) | Hydrocarbon (%, w/w) | ||||
| x1 | x2 | x3 | x4 | |||
| 1 | 1 | 1 | 1 | 1 | 0.246 | 14.82 |
| 2 | −1 | −1 | 1 | 1 | 0.292 | 14.31 |
| 3 | 1 | −1 | −1 | 1 | 0.251 | 15.45 |
| 4 | −1 | 1 | −1 | 1 | 0.296 | 14.56 |
| 5 | 1 | −1 | 1 | −1 | 0.124 | 13.99 |
| 6 | −1 | 1 | 1 | −1 | 0.120 | 13.42 |
| 7 | 1 | 1 | −1 | −1 | 0.136 | 14.83 |
| 8 | −1 | −1 | −1 | −1 | 0.125 | 13.86 |
| 9 | 1 | −1 | 1 | 1 | 0.257 | 13.96 |
| 10 | −1 | 1 | 1 | 1 | 0.320 | 14.12 |
| 11 | 1 | 1 | −1 | 1 | 0.248 | 14.19 |
| 12 | −1 | −1 | −1 | 1 | 0.306 | 14.00 |
| 13 | 1 | 1 | 1 | −1 | 0.116 | 13.96 |
| 14 | −1 | −1 | 1 | −1 | 0.121 | 15.26 |
| 15 | 1 | −1 | −1 | −1 | 0.105 | 14.68 |
| 16 | −1 | 1 | −1 | −1 | 0.126 | 13.96 |
| 17 | 1.72 | 0 | 0 | 0 | 0.215 | 20.23 |
| 18 | −1.72 | 0 | 0 | 0 | 0.231 | 19.24 |
| 19 | 0 | 1.72 | 0 | 0 | 0.123 | 12.25 |
| 20 | 0 | −1.72 | 0 | 0 | 0.121 | 11.59 |
| 21 | 0 | 0 | 1.72 | 0 | 0.118 | 18.57 |
| 22 | 0 | 0 | −1.72 | 0 | 0.124 | 20.18 |
| 23 | 0 | 0 | 0 | 1.72 | 0.289 | 12.54 |
| 24 | 0 | 0 | 0 | −1.72 | 0.094 | 11.90 |
| 25* | 0 | 0 | 0 | 0 | 0.124 | 19.31 |
| 26* | 0 | 0 | 0 | 0 | 0.120 | 18.46 |
| 27* | 0 | 0 | 0 | 0 | 0.123 | 19.17 |
| 28* | 0 | 0 | 0 | 0 | 0.127 | 20.13 |
| 29* | 0 | 0 | 0 | 0 | 0.122 | 19.74 |
| 30* | 0 | 0 | 0 | 0 | 0.126 | 18.45 |
Central point values contributing to the degree of freedom for pure error calculation.
Table 3. Analysis of variance (ANOVA) for the fitted quadratic polynomial regression model for optimization of the algal biomass production.
| Source | Sum of squares | df | Mean square | F-value | Probability P -value |
| Model | 0.162049 | 14 | 0.011575 | 31.64 | <0.001 |
| Residual | 0.005488 | 15 | 0.000366 | ||
| Lack of fit | 0.005354 | 10 | 0.000535 | 20.08 | 0.002 |
| Pure error | 0.000133 | 5 | 0.000027 | ||
| Cor. total | 0.167537 | 29 | |||
| R2 = 0.967 | |||||
| Adj. R2 = 0.937 Pred. R2 = 0.824 |
Table 4. Analysis of variance (ANOVA) for the fitted quadratic polynomial regression model for optimization of the hydrocarbon production.
| Source | Sum of squares | df | Mean square | F-value | Probability P value |
| Model | 218.69 | 14 | 15.621 | 36.58 | <0.001 |
| Residual | 6.406 | 15 | 0.427 | ||
| Lack of fit | 4.127 | 10 | 0.413 | 0.91 | 0.584 |
| Pure error | 2.279 | 5 | 0.456 | ||
| Cor. total | 225.096 | 29 | |||
| R2 = 0.972 | |||||
| Adj. R2 = 0.945 Pred. R2 = 0.875 |
Data from the CCD experiment were analysed by RSM. A mathematical model with a second-order polynomial regression was developed to describe the relationships between the predicted response variables (biomass or hydrocarbon) and the independent variables (Fe, Mn, Mo and Ni). The regression equation was described as follows (Eq. 1):
| (1) |
where y is the predicted response variables (biomass or hydrocarbon production); β 0 is a constant, β i is the linear coefficient, β ii is the quadratic coefficients, β ij is the interaction coefficients of the model, respectively; xi and xj (i = 1, 4; j = 1, 4; i≠j) represent the non-coded independent variables (micronutrient concentrations).
Model Validation
The predicted models on algal biomass and hydrocarbon production of B. braunii were validated in an independent experiment using optimized micronutrient concentrations from the genetic algorithms calculations [55]. A flat plate photobioreactor (3.2 L) was used as the culture vessel under a light intensity of 300 µmol/m2/s and a mixing rate of 1.10 L/L/min. The B. braunii cells were separately inoculated into the original Bold 3N medium, the modified Bold 3N-1 medium for producing algal biomass, and the modified Bold 3N-2 for producing hydrocarbon with different micronutrient compositions (Table 5). The experimental protocols in the validation study were the same as those in the model construction. Algal biomass and hydrocarbon content were separately measured at 3-day intervals over 12 days to assess the response of algal performance to modified media. The productivities of algal biomass and hydrocarbon during the experimental period were also calculated and expressed as g/L/day. All data points in the figures were the mean of three replicates to provide a better estimate of the response of each dependent variable.
Table 5. Concentration of micronutrients in different algal culture media.
| Culture media | Micronutrients (µM) | |||
| Fe | Mn | Mo | Ni | |
| Original Bold 3N | 2.150 | 1.240 | 0.099 | 0.00 |
| Modified Bold 3N-1 | 0.276 | 0.707 | 0.624 | 3.38 |
| Modified Bold 3N-2 | 10.430 | 6.530 | 0.012 | 1.73 |
Statistical Analysis
The data analyses for model construction were performed with MINITAB 16, based on the response surface methodology. The F-test for the analysis of variance (ANOVA) was performed on experimental data to evaluate the statistical significance of the model. The significance of regression coefficients was evaluated using t-test. The contour plots described by the regression model were drawn using MATLAB 7 to illustrate the effects of the independent variables and interactive effects of each independent variable on the response variables.
Optimisation of nutrient composition in the medium was determined by the procedure of genetic algorithms (MATLAB 7), which is a computer simulation program based on the best fit theory of natural selection to generate optimal solutions to problems [55]. In simulations, the program selected the best-fit concentration of each nutrient to maximise the algal response such as biomass and hydrocarbon production. In the validation experiment, data from the original 3N medium and modified medium were analysed by quadratic regression to compare the significant differences of curves. The probability level for significant difference was set at P<0.05.
Results and Discussion
Model Fitting
The application of RSM yielded the following regression equations for biomass (Eq. 2) and hydrocarbon production (Eq. 3). A central composite design (CCD) with five coded levels for all the four factors: iron, manganese, molybdenum, and nickel were used for model simulations. The range of variables, experimental designs and results for biomass and hydrocarbon production are presented in Table 2. The second order polynomial regression equations were used to fit the dependent variables (Y biomass and Y hydrocarbon) to the independent variables x 1 (iron), x 2 (manganese), x 3 (molybdenum) and x 4 (nickel).
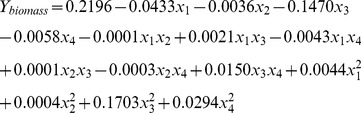 |
(2) |
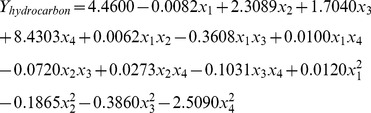 |
(3) |
The significance and adequacy of the regression model were tested using ANOVA. These two regression models could significantly predict algal biomass (P<0.001) and hydrocarbon production (P<0.001) from the four micronutrients (Tables 3 and 4). The predicted R2 (0.824 for Eq. 2 and 0.875 for Eq. 3) agreed well with the adjusted model R2 (0.937 for Eq. 2 and 0.945 for Eq. 3), suggesting a close correlation between the observed values and the predicted values. Therefore, we can use the regression models to predict algal biomass and hydrocarbon production from the amount of micronutrients in the culture medium.
Effect of Micronutrients on Algal Biomass
The regression coefficients of the model for biomass prediction are presented in Table 6. The linear effect of x1 and the quadric effect of x12 and x42 had significant effects (P<0.001) on Y bioamss followed by the interaction effect of x1x4 (P = 0.019). Other terms of the model had no significant effect on Y bioamss. Negative coefficients of x1 and interaction term x1x4 decreased Y bioamss. However, the quadratic terms of x12 and x42 had positive effects on Y bioamss.
Table 6. Results of regression analysis of the full second-order polynomial model for optimization of algal biomass production with Fe (x1), Mn (x2), Mo (x3) and Ni (x4).
| Model term | Coefficients estimated | P-value | t-Statistic |
| intercept | 0.2196 | <0.001 | 5.04 |
| x1 | −0.0433 | <0.001 | −5.93 |
| x2 | −0.0036 | 0.547 | 0.55 |
| x3 | −0.1471 | 0.249 | −1.20 |
| x4 | −0.0058 | 0.795 | −0.26 |
| x1x2 | −0.0001 | 0.999 | −0.00 |
| x1x3 | 0.0021 | 0.813 | 0.24 |
| x1x4 | −0.0043 | 0.019 | −2.62 |
| x2x3 | 0.0001 | 0.992 | 0.01 |
| x2x4 | −0.0003 | 0.798 | −0.26 |
| x3x4 | 0.0149 | 0.581 | 0.56 |
| x12 | 0.0044 | <0.001 | 8.64 |
| x22 | 0.0004 | 0.290 | 1.10 |
| x32 | 0.1703 | 0.269 | 1.15 |
| x42 | 0.0294 | <0.001 | 6.22 |
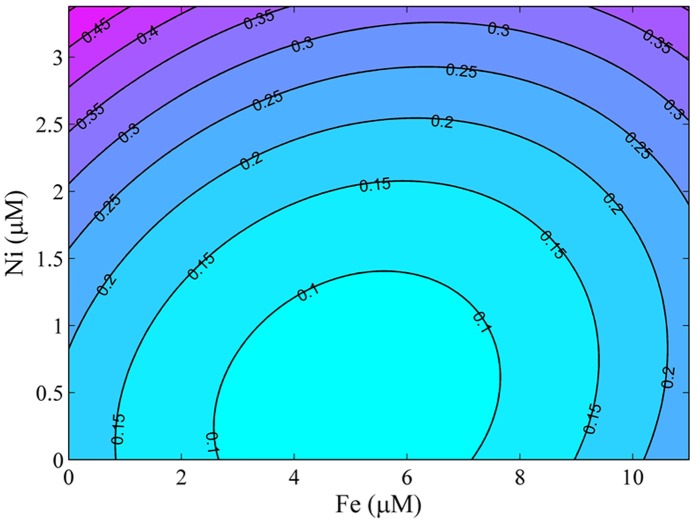
The interaction between two independent variables (Fe and Ni) and the response variable (biomass) was shown by the contour plots generated by keeping the independent variables (Mn and Mo) as constants (Fig. 2). The algal biomass was sensitive to the change of Fe and Ni concentrations. As the concentration of Ni increased, algal biomass increased progressively. The Fe in the medium at either low or high concentrations increased algal biomass when Ni concentrations were high.
Figure 2. Contour plot showing biomass prediction from Fe (x 1) Ni (x 4) with other independent variables Mn (x2) and Mo (x3) being constant.
In this study, the positive relationship between algal biomass and Ni concentrations corroborates an early report by Wake and Hillen [3] that the B. braunii bloom occurred in waters with the nickel concentration of 0.1 mg/L. In other studies, however, nickel accumulation in cells has been shown to cause a detrimental effect on algal growth as nickel is toxic to some physiological processes [56]. Wong et al. [57] reported that both Chlorella vulgaris and Chlorella miniata were capable of cell division after being treated with wastewater containing nickel for 24 h, but the growth rate was reduced in proportion to the concentrations of nickel in the wastewater. Despite this inhibition effect of nickel on other algal species, the present study does suggest that the use of nickel stimulated the growth of B. braunii.
Effect of Micronutrients on Hydrocarbon Production
The regression coefficients of the model for hydrocarbon production are presented in Table 7. The linear effect of x2 and x4, and the quadric effect of x22 and x42 had significant effects (P<0.001) on Y hydrocarbon. Other terms of the model had no significant effect on Y hydrocarbon. Positive coefficient of x2 and x4 indicated their role to enhance Y hydrocarbon. However, the quadratic terms of x12 and x42 had negative effects on Y hydrocarbon.
Table 7. Results of regression analysis of the full second-order polynomial regression model for optimization of hydrocarbon production with Fe (x1), Mn (x2), Mo (x3) and Ni (x4).
| Model term | Coefficients estimated | P-value | t-Statistic |
| intercept | 4.4600 | <0.001 | 3.00 |
| x1 | −0.0082 | 0.974 | −0.03 |
| x2 | 2.3089 | <0.001 | 11.46 |
| x3 | 1.7040 | 0.690 | 0.41 |
| x4 | 8.4303 | <0.001 | 11.17 |
| x1x2 | 0.0062 | 0.683 | 0.42 |
| x1x3 | −0.3608 | 0.245 | −1.21 |
| x1x4 | 0.0100 | 0.861 | 0.18 |
| x2x3 | −0.0720 | 0.768 | −0.30 |
| x2x4 | 0.0273 | 0.554 | 0.61 |
| x3x4 | −0.1031 | 0.911 | −0.11 |
| x12 | 0.0120 | 0.497 | 0.70 |
| x22 | −0.1865 | <0.001 | −16.22 |
| x32 | −0.3860 | 0.940 | −0.08 |
| x42 | −2.5090 | <0.001 | −15.54 |
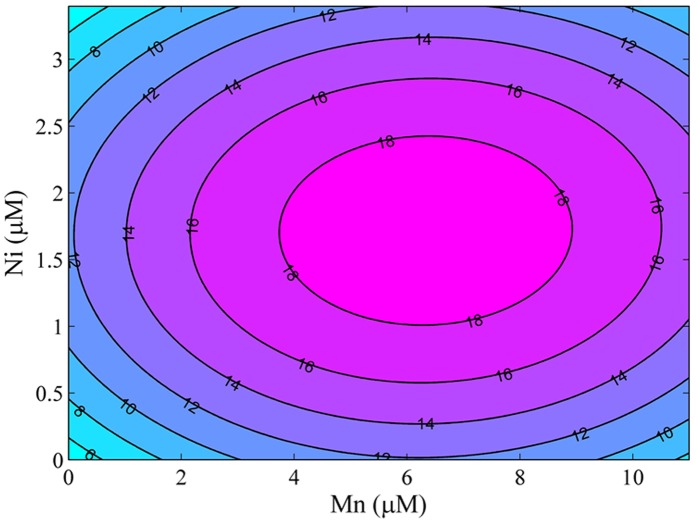
The interaction effects of two independent variables (Mn and Ni) on the response variable (hydrocarbon) are shown by the contour plots generated by keeping the independent variables (Fe and Mo) as constants (Fig. 3). Hydrocarbon production was more sensitive to the change of Mn and Ni concentrations. An increase in hydrocarbon production was observed with the increase of Mn concentrations. But this trend was reversed when the Mn concentration was above 9 µM. The effect of Ni on Y hydrocarbon followed the similar trend. With the increase of Ni concentration, Y hydrocarbon firstly increased and then decreased as a result of excessive Ni concentration. The circular profile of the contour plots indicated that the interaction between the Mn and Ni concentrations on hydrocarbon was negligible (Fig. 3).
Figure 3. Contour plot showing hydrocarbon prediction from Mn (x2) and Ni (x 4) with other independent variables Fe (x1) and Mo (x3) being constant.
The composition of the culture medium affects not only algal productivity, but also secondary metabolites [58]. This finding was consistent with result of Wang et al. [59] who found that the increase of Fe and Mn concentrations stimulated the growth of blue green algae, while a further increase in their concentrations inhibited algal growth. Cloëz et al. [60] found that lipid synthesis increased by three times after adding manganese, copper and nickel at 2 mM. On the other hand, Mohammady and Fathy [61] reported that the total lipid content in Dunaliella salina cultivated in nickel supplemented media (0.5 mg/L NiCl2) has reduced in comparison to the control. In another study, Rousch and Sommerfeld [62] found that manganese had stronger impact on the growth of a green alga (Ulothrix sp.) than nickel. However, in this study, both nickel and manganese regulated the production of hydrocarbon, though the algal biomass was only affected by nickel.
Optimisation of Micronutrients
The concentrations of these four micronutrients for producing algal biomass were optimized using the genetic algorithm calculation. The optimal medium for biomass consisted of 0.266 µM Fe, 0.707 µM Mn, 0.624 µM Mo and 3.38 µM Ni. By running the optimization simulation within the experimental range, the optimal medium for hydrocarbon production is recommended to contain 10.43 µM Fe, 6.53 µM Mn, 0.012 µM Mo and 1.73 µM Ni. It is worth noting that the optimal composition of these four micronutrients for algal biomass was different from that for hydrocarbon production. This difference highlights the importance of selecting culture medium to achieve different objectives in algal culture since the nutrient requirement differs for algae cell division and accumulation of secondary metabolites [63].
Validation of Algal Growth and Hydrocarbon Production
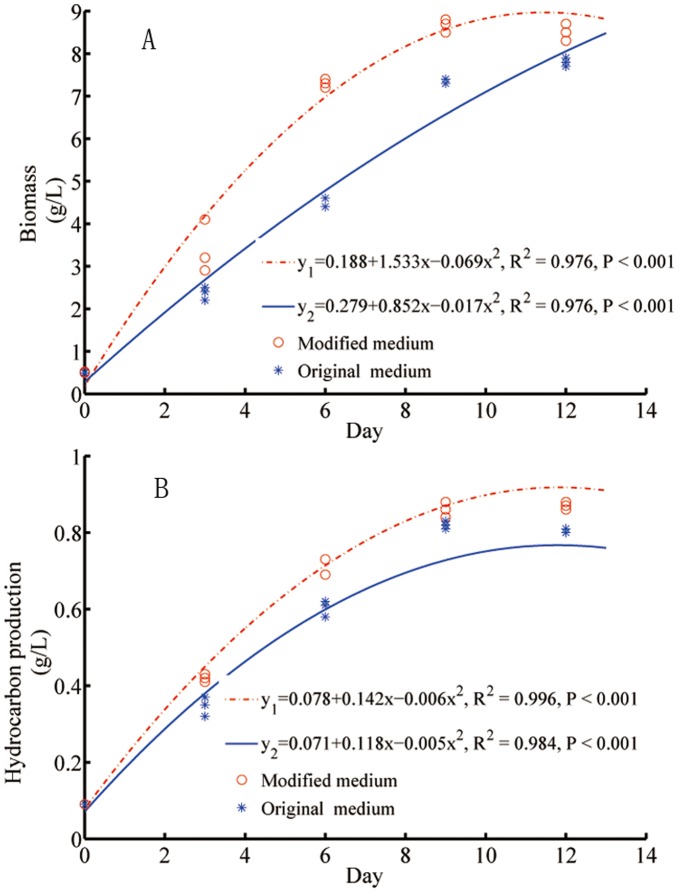
The reliability of nutrient requirement generated from the predicted models and the genetic algorithm calculations for biomass and hydrocarbon production in B. braunii were validated in an independent photobioreactor study. From day 3 to day 12, the algal biomass produced in the Bold 3N medium supplemented with 0.266 µM Fe, 0.707 µM Mn, 0.624 µM Mo, 3.38 µM Ni was significantly higher than that produced in the original Bold 3N medium (P<0.05, Fig. 4A). The maximal algal biomass productivity (1.300±0.176 g/L/day) in dry weight with modified media was significantly higher than that (0.967±0.033 g/L/day) in the original media (P<0.05, Fig. 5A).
Figure 4. Regression plots of biomass (A) and hydrocarbon (B) productions in the modified and original Bold 3N media.
Figure 5. Comparison of maximal biomass (A) and hydrocarbon (B) productivities in the modified and original Bold-3N media.
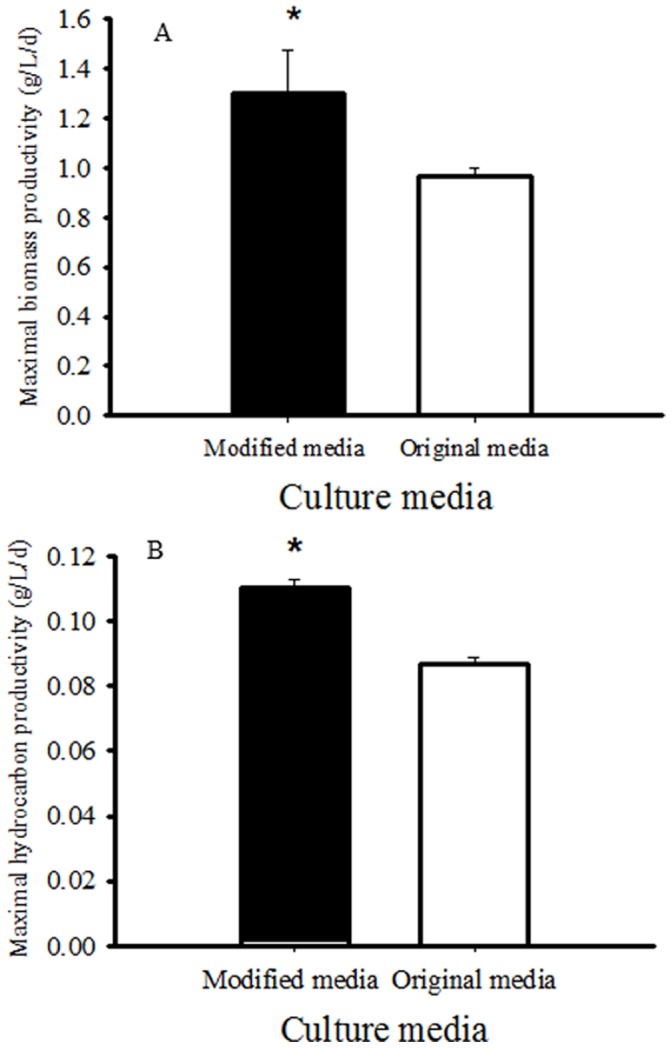
The hydrocarbon production of algae in the Bold 3N medium supplemented with 10.43 µM Fe, 6.53 µM Mn, 0.012 µM Mo and 1.73 µM Ni was significantly higher than that in the original medium from day 3 to day 12 (P<0.05, Fig. 4B). The maximal hydrocarbon productivity (0.110±0.003 g/L/day) in the modified media was significantly higher than that (0.087±0.002 g/L/day) in the original media (P<0.05, Fig. 5B).
The biomass and hydrocarbon productivity are key parameters affecting the economic feasibility of producing bioproducts from algae. The micronutrient concentrations optimised by modelling were validated in a photobioreactor, and the accuracy and reliability of the model in predicting nutrient requirements for producing algal biomass and hydrocarbon have been confirmed.
Conclusion
The application of response surface methodology (RSM) is a reliable approach to model and optimize the requirements for iron, manganese, molybdenum, and nickel in producing algal biomass and hydrocarbon in B. braunii. Nickel and iron played significant roles but manganese and molybdenum had a trivial role in algal biomass production. In contrast, nickel and manganese were more important than molybdenum and iron in regulating algal hydrocarbon production. The production of algal biomass and production of hydrocarbon require different micronutrients in the culture medium. The recommended levels of micronutrients in the Bold 3N medium are 0.266 µM iron, 0.707 µM manganese, 0.624 µM molybdenum and 3.38 µM nickel for B. braunii biomass and 10.43 µM iron, 6.53 µM manganese, 0.012 µM and 1.73 µM nickel for hydrocarbon production. The model validation showed that by using modified algal culture media, algal biomass productivity increased 1.345 fold and hydrocarbon productivity increased 1.274 fold compared with the original Bold 3N medium without addition of the trace elements.
Acknowledgments
The authors would like to thank Dr. Daniel Jardine for his advice on chemical analysis and Dr. David Kehoe for commenting on the early draft manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: These authors have no support or funding to report.
References
- 1.Chisti Y. Biodiesel from microalgae. Biotechnol Adv. 2007;25:294–306. doi: 10.1016/j.biotechadv.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Qin JG. Timmis KN, editor. Hydrocarbons from algae. 2010. editor. Microbiology of hydrocarbons, oils, lipids, and derived dompounds, vol 4: Consequences of microbial interactions with hydrocarbons, oils and lipids. Berlin: Springer, 2817–2816.
- 3.Wake LV, Hillen LW. Study of a “bloom” of the oil-rich alga Botryococcus braunii in the Darwin River reservoir. Biotechnol Bioeng. 1980;22:1637–1656. [Google Scholar]
- 4.Wake LV, Hillen LW. Nature and hydrocarbon content of blooms of the alga Botryococcus braunii occurring in Australian freshwater lakes. Aust J Mar Freshwater Res. 1991;32:353–367. [Google Scholar]
- 5.Metzger P, Largeau C. Botryococcus braunii: a rich source for hydrocarbons and related ether lipids. Appl Microbiol Biot. 2005;66:486–496. doi: 10.1007/s00253-004-1779-z. [DOI] [PubMed] [Google Scholar]
- 6.Brown AC, Knights BA, Conway E. Hydrocarbon content and its relationship to phsiological state in green alga Botrycoccus braunii. Phytochemistry. 1969;8:543–547. [Google Scholar]
- 7.Knights BA, Brown AC, Conway E, Middleditch BS. Hydrocarbons from the green form of the freshwater alga Botryococcus braunii. . Phytochemistry. 1970;9:1317–1324. [Google Scholar]
- 8.Li Y, Qin JG. Comparison of growth and lipid content in three Botryococcus braunii strains. J Appl Phycol. 2005;17:551–556. [Google Scholar]
- 9.Hillen L, Pollard G, Wake LV, White N. Hydrocracking of the oils of the oils of Botryococcus braunii to transport fuels. Biotechnol Bioeng. 1982;24:193–205. doi: 10.1002/bit.260240116. [DOI] [PubMed] [Google Scholar]
- 10.Qin JG. Bio-hydrocarbons from algae: impacts of temperature, light and salinity on algae growth. Development. 2005;5:1–26. [Google Scholar]
- 11.Qin JG, Li Y. Optimization of the growth environment of Botryococcus braunii strain CHN 357. J Freshwater Ecol. 2006;21:169–176. [Google Scholar]
- 12.Metzger P, Largeau C. Chemicals of Botryococcus braunii. In Cohen Z editor. Chemicals from microalgae. London: Taylor & Francis. 1999. pp. 205–260.
- 13.Largeau C, Casadevall E, Berkaloff C, Dhamelincourt P. Sites of accumulation and composition of hydrocarbons in Botryococcus braunii. . Phytochemistry. 1980;19:1043–1051. [Google Scholar]
- 14.Ohmori M, Wolf FR, Bassham JA. Botryococcus braunii: carbon/nitrogen metabolism as affected by ammonia addition. Arch Microbiol. 1984;140:101–106. [Google Scholar]
- 15.Yang SL, Wang J, Cong W, Cai ZL, Fan OY. Utilization of nitrite as a nitrogen source by Botryococcus braunii. . Biotechnol Lett. 2004;26:239–243. doi: 10.1023/b:bile.0000013722.45527.18. [DOI] [PubMed] [Google Scholar]
- 16.Chirac C, Casadevall E, Largeau C, Metzger P. Bacterial influence upon growth and hydrocarbon production of the green alga Botryococcus braunii. . J Phycol. 1985;21:380–387. [Google Scholar]
- 17.Dayananda C, Sarada R, Bhattacharya S, Ravishankar GA. Effect of media and culture conditions on growth and hydrocarbon production by Botryococcus braunii. . Process Biochem. 2005;40:3125–3131. [Google Scholar]
- 18.Raven JA. The iron and molybdenum use efficiencies of plant growth with different energy, carbon, and nitrogen sources. New Phytol. 1988;109:279–287. [Google Scholar]
- 19.Raven JA. Predictions of Mn and Fe use efficiencies of phototrophic growth as a function of light availability for growth and C assimilation pathway. New Phytol. 1990. 116
- 20.Maldonado MT, Price NM. Influence of N substrate on Fe requirements of marine centric diatoms. Mar Ecol Prog Ser. 1996;141:161–172. [Google Scholar]
- 21.Sunda WG, Huntsman SA. Interrelated influence of iron, light, and cell size on growth of marine phytoplankton. Nature. 1997;390:389–92. [Google Scholar]
- 22.Sunda WG, Huntsman SA. Relationships among photoperiod, carbon fixation, growth, chlorophyll a, and cellular iron and zinc in a coastal diatom. Limnol Oceanogr. 2004;49:1742–1753. [Google Scholar]
- 23.Mojaat M, Pruvost J, Foucault A, Legrand J. Effect of organic carbon sources and Fe2+ ions on growth and β-carotene accumulation by Dunaliella salina. . Biochem Eng J. 2008;39:177–184. [Google Scholar]
- 24.Liu ZY, Wang GG, Zhou BC. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. . Bioresource Technol. 2008;99:4717–4722. doi: 10.1016/j.biortech.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 25.Peers GS, Price NM. A role for manganese in superoxide dismutases and the growth of iron-deficient diatoms. Limnol Oceanogr. 2004;49:1174–1783. [Google Scholar]
- 26.Chernikova AA, Tsoglin LN, Markelova AG, Zorin SN, Mazo VK, et al. Capacity of Spirulina platensis to accumulate manganese and its distribution in dell. Russ J Plant Physl. 2006;53:800–806. [Google Scholar]
- 27.Carvalho AP, Pontes I, Gaspar H, Malcata FX. Metabolic relationships between macro- and micronutrients, and the eicosapentaenoic acid and docosahexaenoic acid contents of Pavlova lutheri. . Enzyme Microb Tech. 2006;38:358–366. [Google Scholar]
- 28.Price NM, Morel FMM. Co-limitation of phytoplankton growth by nickel and nitrogen. Limnol Oceanogr. 1991;36:1071–1077. [Google Scholar]
- 29.Berges JA, Franklin DJ, Harrison PJ. Evolution of an artificial seawater medium: improvements in enriched seawater, artificial water over the last two decades. J Phycol. 2001;37:1138–1145. [Google Scholar]
- 30.Herney-Ramirez J, Lampinen M, Vicente MA, Costa CA, Madeira LM. Experimental design to optimize the oxidation of orange II dye solution using a clay-based Fenton-like catalyst. Ind Eng Chem Res. 2008;47:284–294. [Google Scholar]
- 31.Oliveira R, Almeida MF, Santos L, Madeira LM. Experimental design of 2, 4-dichlorophenol oxidation by Fenton’s reaction. Ind Eng Chem Res. 2006;45:1266–1276. [Google Scholar]
- 32.Myers RH, Montgomery DC. Response surface methodology: process and product optimization using designed experiments, second (ed). New York: John Wiley & Sons. 704 p. 2002.
- 33.Ren J, Lin WT, Shen YJ, Wang JF, Lou XC, et al. Optimization of fermentation media for nitrite oxidizing bacteria using sequential statistical design. Bioresour Technol. 2008;99:7923–7927. doi: 10.1016/j.biortech.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 34.Kammoun R, Naili B, Bejar S. Application of a statistical design to the optimization of parameters and culture media for a-amylase production by Aspergillus oryzae CBS 819.72 grown on gruel (wheat grinding by-product). Bioresour Technol. 2008;99:602–5609. doi: 10.1016/j.biortech.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 35.Pan CM, Fan YF, Xing Y, Hou HW, Zhang ML. Statistical optimization of process parameters on biohydrogen production from glucose by Clostridium sp. Fanp2. Bioresour Technol. 2008;99:3146–3154. doi: 10.1016/j.biortech.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 36.De Rijck G, Schrevens E. Multifactorial optimisation of the nutrient solution for hydroponically grown chicory plants. Scientia Hortic. 1998;76:149–159. [Google Scholar]
- 37.Niedz RP, Hyndman SE, Evens TJ. Using a gestalt to measure the quality of in vitro responses. Sci Hortic-Amsterdam. 2007;112:349–359. [Google Scholar]
- 38.Rao KJ, Kim CH, Rhee SK. Statistical optimization of medium for the production of recombinant hirudin from Saccharomyces cerevisiae using response surface methodology. Process Biochem. 2000;35:639–647. [Google Scholar]
- 39.Kunamneni A, Singh S. Response surface optimization of enzymatic hydrolysis of maize starch for higher glucose production. Biochem Eng J. 2005;27:179–190. [Google Scholar]
- 40.Nilsang S, Lertsiri S, Suphantharika M, Assavanic A. Optimization of enzymatic hydrolysis of fish soluble concentrate by commercial proteases. J Food Eng. 2005;70:571–578. [Google Scholar]
- 41.Shieh CJ, Lai YF. Application of response surface methodology to the study of methyl glucoside polyester synthesis parameters in a solvent-free system. J Agr Food Chem. 2000;48:1124–1128. doi: 10.1021/jf990460f. [DOI] [PubMed] [Google Scholar]
- 42.Castro IA, Tirapegui J, Silva RSSF. Protein mixtures and their nutritional properties optimized by response surface methodology. Nutr Res. 2000;20:1341–1353. [Google Scholar]
- 43.Ozer EA, Herken EN, Guzel S, Ainsworth S, Ibanoglu S. Effect of extrusion process on the antioxidant activity and total phenolics in a nutritious snack food. Int J Food Sci Tech. 2006;41:289–293. [Google Scholar]
- 44.Jacob-lopes E, Lacerda LMCF, Franco TT. Biomass production and carbon dioxide fixation by Aphanothece microscopoica nageli in a bubble column photobioreactor. Biochem Eng J. 2008;40:27–34. [Google Scholar]
- 45.Azma M, Mohamed MS, Mohamad R, Rahim RA, Ariff AB. Improvement of medium composition for heterotrophic cultivation of green microalgae, Tetraselmis suecica, using response surface methodology. Biochem Eng J. 2011;53:187–195. [Google Scholar]
- 46.Isleten-Hosoglu M, Gultepe L, Elibol M. Optimization of carbon and nitrogen sources for biomass and lipid production by Chlorella saccharophila under heterotrophic conditions and development of Nile red fluorescence based method for quantification of its neutral lipid content. Biochem Eng J. 2012;61:11–19. [Google Scholar]
- 47.Provasoli L, McLaughlin JJA, Droop MR. The development of artificial media for marine algae. Arch Mikrobiol. 1957;25:392–428. doi: 10.1007/BF00446694. [DOI] [PubMed] [Google Scholar]
- 48.Okada S, Devarenne TP, Murakami M, Abe H, Chappell J. Characterization of botryococcene synthase enzyme activity, a squalene synthase-like activity from the green microalga Botryococcus braunii, race B. Arch Biochem Biophys. 2004;422:110–118. doi: 10.1016/j.abb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Wang JP, Chen YZ, Ge XW, Yu HQ. Optimization of coagulation–flocculation process for a paper-recycling wastewater treatment using response surface methodology, Colloids Surf. Afri Physicochem Eng. 2007;302:204–210. [Google Scholar]
- 50.Zheng ZM, Hu QI, Hao J, Xu F, Guo NN, et al. Statistical optimization of culture conditions for 1,3,-propanediol by Klebsiella pneumonia AC15 via central composite design. Bioresource Technol. 2008;99:1052–1056. doi: 10.1016/j.biortech.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 51.Ghadge SV, Raheman H. Process optimization for biodiesel production from mahua (Madhuca indica) oil using response surface methodology. Bioresour Technol. 2006;97:379–384. doi: 10.1016/j.biortech.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 52.Cui FJ, Li Y, Xu ZH, Xu HY, Sun K, et al. Optimization of the medium composition for production of mycelial biomass and exo-polymer by Grifola frondosa GF9801 using response surface methodology. Bioresour Technol. 2006;97:1209–1216. doi: 10.1016/j.biortech.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Gu XB, Zheng ZM, Yu HQ, Wang J, Liang FL, et al. Optimization of medium constituents for a novel lipopeptide production by Bacillus subtilis MO-01 by a response surface method. Process Biochem. 2005;40:3196–3201. [Google Scholar]
- 54.Kaushik R, Saran S, Isar J, Saxena RK. Statistical optimization of medium components and growth conditions by response surface methodology to enhance lipase production by Aspergillus carneus. J Mol Catal B: Enzyme. 2006;40:121–126. [Google Scholar]
- 55.Goldberg ED. Genetic algorithms in search optimization and machine learning. Boston: Addison-Wesley Longman Publishing. 412 p. 1989.
- 56.Jin X, Nalewajko C, Kushner DJ. Comparative study of nickel toxicity to growth and photosynthesis in nickel-resistant and -sensitive strains of Scenedesmus acutus f. alternans (Chlorophyceae). Microbial Ecol. 1996;31:103–114. doi: 10.1007/BF00175079. [DOI] [PubMed] [Google Scholar]
- 57.Wong JPK, Wong YS, Tam NFY. Nickel biosorption by two chlorella species, C. vulgaris (a commercial species) and C. miniata (a local isolate). Biores Technol. 2000;73:133–137. [Google Scholar]
- 58.Shay LK, Hunt HR, Wegner GH. Highproductivity fermentation process for cultivation industrial microorganisms. J Ind Microbiol. 1987;2:79–85. [Google Scholar]
- 59.Wang Z, Chen S, Cao X. Micro-nutrients effects on algae colony: growth rate and biomass response to various micro-nutrients and competitive inhibitions among multi-microelements. Symposim of 4th Internat Con Bioinformat Biomed Eng 1–8. 2010.
- 60.Cloëz I, Dumont O, Piciotti M, Bourre JM. Alterations of lipid synthesis in the normal and dysmyelinating trembler mouse sciatic nerve by heavy metals (Hg, Pb, Mn, Cu, Ni). Toxicology. 1987;46:65–71. doi: 10.1016/0300-483x(87)90138-7. [DOI] [PubMed] [Google Scholar]
- 61.Mohammady NGE, Fathy AA. Humic acid mitigates viability reduction, lipids and fatty acids of Dunaliella salina and Nannochloropsis salina grown under nickel stress. Internat J Bot. 2007;3:64–70. [Google Scholar]
- 62.Rousch JM, Sommerfeld MR. Effect of manganese and nickel on growth of selected algae in pH buffered medium. Water Res. 1999;33:2448–2454. [Google Scholar]
- 63.Lee YK, Ding SY. Cell cycle and accumulation of astaxanthin in Haematococcus lacustris (Chlorophyta). J Phycol. 1994;30:445–449. [Google Scholar]