Abstract
Tumor-associated angiogenesis is a complex process that involves the interplay among several molecular players such as cell-surface heparan sulfate proteoglycans, vascular endothelial growth factors and their cognate receptors. PI-88, a highly sulfonated oligosaccharide, has been shown to have potent anti-angiogenic activity and is currently in clinical trials. However, one of the major drawbacks of large oligosaccharides such as PI-88 is that their synthesis often requires numerous complex synthetic steps. In this study, several novel polysulfonated small molecule carbohydrate mimetics, which can easily be synthesized in fewer steps, are identified as promising inhibitors of angiogenesis in an in vitro tube formation assay.
Keywords: Angiogenesis, Matrigel, Small molecules, Inhibitors, Polysulfonated molecules
The inhibition of tumor-associated angiogenesis has been one of the primary methods of controlling cancers for several decades.[1–3] Heparan sulfate proteoglycans (HSPGs), composed of sulfonated glycosaminoglycan (GAG) chains attached to a protein core, are key players in stimulating tumor-associated angiogenesis.[2, 4] HSPGs facilitate cell signalling by acting as co-receptors for a variety of pro- and anti-angiogenic factors such as FGF, VEGF, and endostatin.[5–8] Infact, cell surface HS is essential for endothelial tube formation in vitro.[9]
Several studies have utilized HS-/Heparin-based drugs to control angiogenesis.[10] Recently, ReGeneraTing Agents (RGTAs) were found to enhance angiogenesis by increasing the affinity of VEGF-165 for its cognate receptor.[11] In contrast, low molecular weight heparin was found to have anti-angiogenic properties in rat corneas.[12] PI-88, a highly sulfonated oligosaccharide (phosphomannopentose sulfate) which entered clinical trials, has potent anti-angiogenic properties.[13] Additionally, JG3 (oligomannurate sulfate), a recently discovered marine-derived oligosaccharide, inhibits heparanase-associated angiogenesis.[14] In vivo testing of PG545, an HS mimetic, also showed promise in a recent preclinical study in a murine tumor model.[15] These and other heparin-based inhibitors demonstrate the power of sulfonated saccharides in anti-cancer treatments. However, nearly all HS mimetics discovered thus far as angiogenesis inhibitors are high molecular weight oligo- or poly- saccharide derivatives.
High molecular weight HS/heparin derivatives are notoriously difficult to prepare in a homogenous form. Additionally, distinct HS sequences possessing different sulfation patterns and/or chain lengths may have agonistic or antagonistic effects.[16–17] Finding medically relevant HS/heparin derivatives requires exhaustive library screening– a task made difficult by the problems of synthesis. Thus, we reasoned that small molecules that 1) mimic HS; 2) are much smaller than oligosaccharides; 3) are homogenous; 4) are easily prepared; and 5) function as angiogenesis inhibitors, would be more clinically effective than current HS/Heparin-based oligosaccharide drugs. Previously we found novel small molecule fluoro-xylosides that potently reduced tumor-associated angiogenesis by inhibiting HS biosynthesis in vitro.[18] However, these molecules required cellular entry to be effective. Small molecule angiogenesis inhibitors that can assert their action outside cells are far more desirable than carbohydrate-based oligo- and poly- saccharides because of their potentially favorable pharmacokinetic properties.
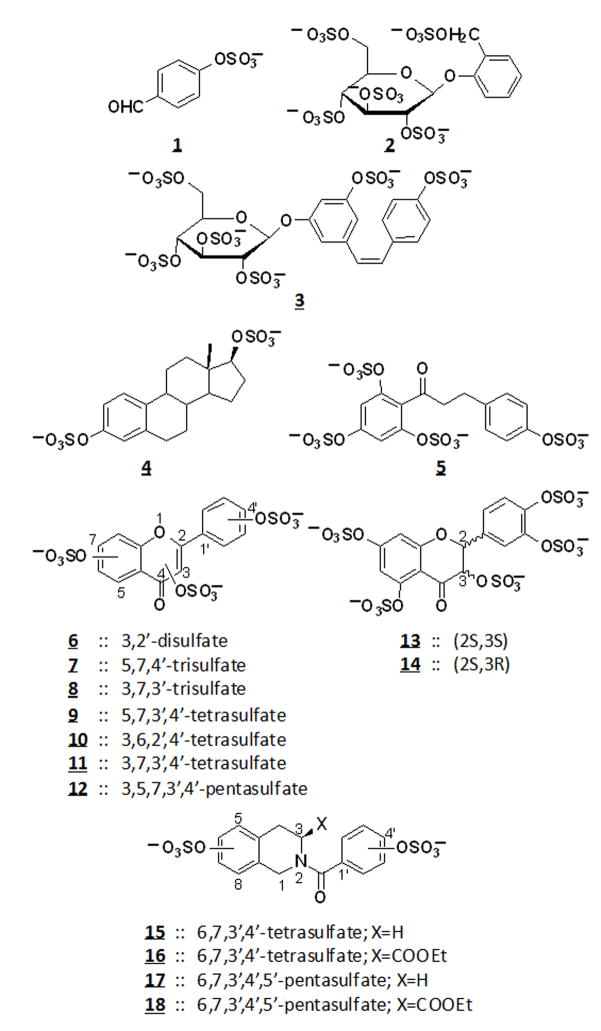
To test this hypothesis, we designed a library of 18 sulfonated non-carbohydrate small molecules that can be expected to mimic heparin/HS due to their highly charged nature. Using a microwave sulfonation protocol that we previously developed and rigorously characterized, we synthesized molecules belonging to the flavone, flavan, chalcone, stillbene, styrene, and isoquinoline scaffolds – representing significant diversity at the three-dimensional level (Figure 1).[19–21] Furthermore, the synthesized molecules contain 1–5 sulfate groups and are under 500 Da in size; therefore, they have similar charge density, sulfate functionality, and size as HS/heparin di-/tri- saccharides and should mimic HS/heparin functions.
Figure 1.
Chemical structures of the library of sulfonated small molecules.
These molecules were screened in an in vitro assay of tumor-associated angiogenesis which utilizes reduced growth factor basement membrane extract (RGF-BME) – derived from the Englebreth-Holm-Swarm (EHS) mouse sarcoma. When bovine lung microvascular endothelial cells (BLMVEC) are cultured on RGF-BME, they spontaneously form tube-like structures. The development of these ‘tubes’ in vitro mimics an important step in the formation of blood vessels in vivo. An extensive tube-like network with significant branching and tube length indicates normal angiogenesis. On the other hand, disjointed groups of cells forming short tubes that are not interconnected exemplify the inhibition of angiogenesis. Several previous studies have utilized this matrigel tube formation assay.[18, 22]
We initially screened the library at a number of different concentrations and found that several molecules completely abolished tube formation. Control wells contained either no compound (positive control) or Sulforaphane (negative control). Sulforaphane, found in broccoli and other cruciferous vegetables, is a potent anti-cancer agent provided by the assay manufacturer.[23] Screening of the 18 molecules (Figure 1) led to the identification of 4, 5, 6, 7 and 9 as potent inhibitors of angiogenesis at 100 μM (Figure 2). While untreated wells and inactive mimetics showed significant branching and interconnectivity, endothelial cells treated with these sulfonated molecules were dispersed and formed small cell clumps without much network formation.
Figure 2.
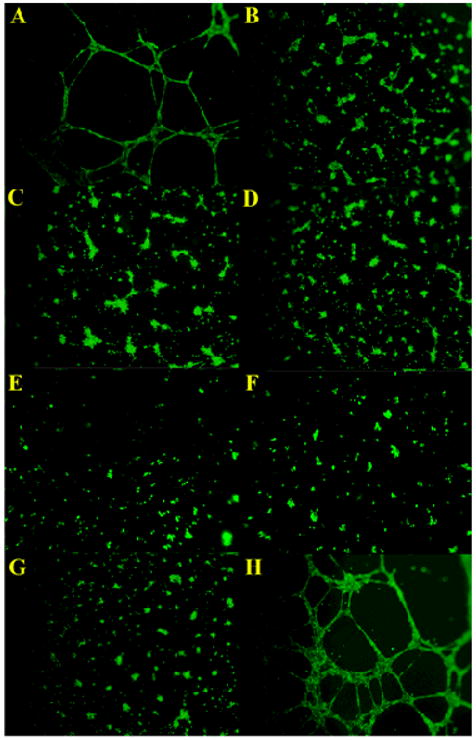
An in vitro tube formation assay was utilized to identify angiogenesis inhibitors. Wells were treated at a concentration of 100 μM. Representative panels in this figure are: (A) Positive untreated control, (B) Sulforaphane negative control, (C) 9, (D) 4, (E) 5, (F) 6, (G) 7, and (H) 12.
Based on our findings, it is possible to glimpse into structure – activity relationships that play a role in vascular tube formation; although, this process is considerably complex and involves a large number of probable mechanisms. The active molecules, 4, 5, 6, 7 and 9, carry two, three, or four sulfate groups per scaffold (Figure 1). However, the inhibitory activity was not proportional to number of sulfate groups as several tetra- and penta- sulfonated molecules (e.g., 10–18) were found to be inactive. A comparison of structures of the molecules that exhibit inhibitory activity shows that the minimal ‘pharmacophore’ appears to be two sulfate groups at an optimal distance of 5–10 Å as found in scaffolds 4, 5, and 7. This suggests that structural selectivity is involved in the process. Additionally, due to the highly charged nature of these molecules, they probably inhibit tube formation via a chelation or competition mechanism – outcompeting cell-surface heparan sulfates for pro-angiogenic factors such as FGF. We have previously shown that disruption of cell surface HS by heparitinases and by GAG biosynthesis inhibitors halts tube-formation.[9, 18] The current study presents an alternative approach which utilizes sulfonated small molecules to inhibit tube formation by competing with the functions of cell surface HS.
This work presents the first small, synthetic, non-saccharide, highly sulfonated heparin/HS mimetics that possess anti-angiogenic function. The compounds in the current study are likely to be clinically superior to current carbohydrate-based high molecular weight drugs which have shown significant anti-cancer potential in clinical trails.[13] Molecules 4, 5, 6, 7, and 9 have lower molecular weights, can potentially modulate a variety of signalling pathways, are easy to synthesize, and probably exert their activity outside cells without the need for cell penetration. Due to these properties, it is expected that the current molecules will have more favorable pharmacokinetic properties and clinical application. Additional scaffolds will be developed to identify more potent angiogenesis inhibitors and to ascertain the mechanism of action of these molecules. In vivo testing of these compounds will lead to the identification of potential drug candidates for further studies.
Experimental Information
Tube formation assay
A premixed solution of 1 × 105 BLMVEC, heparin/HS mimetic inhibitors, and MCDB-131 media were added to matrigel in a 96 well plate in duplicate. After incubation for 16 hrs at 37 °C, cells were imaged with an Olympus IX81.
Synthesis
All tested compounds were synthesized in one step from their phenolic and/or alcoholic precursors using microwave-assisted synthesis, as described previously.[19] Briefly, the precursor and trimethylamine – sulfur trioxide complex at a molar ratio of 1:6 per –OH group were mixed in acetonitrile and exposed to microwaves (50W) at 90°C for 30 min. The purity of the sulfonated compounds was assayed using reverse polarity capillary electrophoresis, as previously described[20] and found to be >95%. (See supplementary material for details).
Supplementary Material
Acknowledgments
This work was supported by the NIH grant PO1-HL107152 to B.K/U.R.D and by NIH grants HL099420 and HL090586, AHA grant EIA 0640053N, and grant 6-46064 from the A. D. Williams Foundation to U.R.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Folkman J. N Eng J Med. 1971;285:1182. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Iozzo RV, Sanderson RD. J Cell Mol Med. 2011;15:1013. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka K, Konno Y, Kuraishi Y, Kimura I, Suzuki T, Kiniwa M. Bioorg Med Chem Lett. 2002;12:623. doi: 10.1016/s0960-894x(01)00810-1. [DOI] [PubMed] [Google Scholar]
- 4.Raman K, Kuberan B. Curr Chem Biol. 2010;4:20. doi: 10.2174/187231310790226206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakato H, Kimata K. Biochim Biophys Acta. 2002;1573:312. doi: 10.1016/s0304-4165(02)00398-7. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J, Shing Y. Adv Exp Med Biol. 1992;313:355. doi: 10.1007/978-1-4899-2444-5_34. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki T, Larsson H, Kreuger J, Salmivirta M, Claesson-Welsh L, Lindahl U, Hohenester E, Timpl R. EMBO J. 1999;18:6240. doi: 10.1093/emboj/18.22.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasisekharan R, Ernst S, Venkataraman G. Angiogenesis. 1997;1:45. doi: 10.1023/A:1018318914258. [DOI] [PubMed] [Google Scholar]
- 9.Raman K, Kuberan B. Biochem Biophys Res Commun. 2010;398:191. doi: 10.1016/j.bbrc.2010.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenzie EA. Br J Pharmacol. 2007;151:1. doi: 10.1038/sj.bjp.0707182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouet V, Meddahi-Pelle A, Miao HQ, Vlodavsky I, Caruelle JP, Barritault D. J Biomed Mater Res A. 2006;78:792. doi: 10.1002/jbm.a.30723. [DOI] [PubMed] [Google Scholar]
- 12.Lepri A, Benelli U, Bernardini N, Bianchi F, Lupetti M, Danesi R, Del Tacca M, Nardi M. J Ocul Pharmacol. 1994;10:273. doi: 10.1089/jop.1994.10.273. [DOI] [PubMed] [Google Scholar]
- 13.Chow LQ, Gustafson DL, O’Bryant CL, Gore L, Basche M, Holden SN, Morrow MC, Grolnic S, Creese BR, Roberts KL, Davis K, Addison R, Eckhardt SG. Cancer Chemother Pharmacol. 2008;63:65. doi: 10.1007/s00280-008-0712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao H, Liu H, Chen Y, Xin X, Li J, Hou Y, Zhang Z, Zhang X, Xie C, Geng M, Ding J. Cancer Res. 2006;66:8779. doi: 10.1158/0008-5472.CAN-06-1382. [DOI] [PubMed] [Google Scholar]
- 15.Dredge K, Hammond E, Handley P, Gonda TJ, Smith MT, Vincent C, Brandt R, Ferro V, Bytheway I. Br J Cancer. 2011;104:635. doi: 10.1038/bjc.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu D, Shriver Z, Venkataraman G, El Shabrawi Y, Sasisekharan R. Proc Natl Acad Sci U S A. 2002;99:568. doi: 10.1073/pnas.012578299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azizkhan RG, Azizkhan JC, Zetter BR, Folkman J. J Exp Med. 1980;152:931. doi: 10.1084/jem.152.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raman K, Ninomiya M, Nguyen TK, Tsuzuki Y, Koketsu M, Kuberan B. Biochem Biophys Res Commun. 2010;404:86. doi: 10.1016/j.bbrc.2010.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raghuraman A, Riaz M, Hindle M, Desai UR. Tetrahedron Lett. 2007;48:6754. doi: 10.1016/j.tetlet.2007.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunnarsson GT, Riaz M, Adams J, Desai UR. Bioorg Med Chem. 2005;13:1783. doi: 10.1016/j.bmc.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 21.Liang A, Thakkar JN, Desai UR. J Pharm Sci. 2010;99:1207. doi: 10.1002/jps.21908. [DOI] [PubMed] [Google Scholar]
- 22.Garonna E, Botham KM, Birdsey GM, Randi AM, Gonzalez-Perez RR, Wheeler-Jones CP. PLoS One. 2011;6:e18823. doi: 10.1371/journal.pone.0018823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asakage M, Tsuno NH, Kitayama J, Tsuchiya T, Yoneyama S, Yamada J, Okaji Y, Kaisaki S, Osada T, Takahashi K, Nagawa H. Angiogenesis. 2006;9:83. doi: 10.1007/s10456-006-9034-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.