1. Introduction
Acute renal failure (ARF) and chronic renal disease (CRD) are distinctly different disease processes and current therapy for both disorders is suboptimal. ARF arises from toxic or ischemic (usually simultaneous) tubule damage from antibiotics, chemo-therapeutic agents, or shock during infectious or major operative procedures. The development of ARF in a hospitalized patient results in a 5 to 8 fold higher risk of death (Humes 1995; Chertow et al. 1998; Bates et al. 2001) with overall mortality rates exceeding 50%. However, if the patient survives the episode of ARF, the regenerative repair processes within the kidney can result in a return of kidney function in 90 to 95% of patients with this acute disorder. The cause of death in patients with acute renal failure is usually the development of SIRS, with resulting cardiovascular collapse, ischemic damage to vital organs, and MOF (Humes 2000; Humes et al. 2002). The propensity of patients with ARF to develop SIRS and sepsis suggests that renal metabolic function, specifically renal tubule cell function secondary to ATN, plays a critical immunomodulatory role in individuals under stress states. This function is not replaced with standard dialysis therapy.
Unlike ARF, CRD is an irreversible process of progressive kidney damage commonly from diabetes and hypertension which leads to end-stage renal disease (ESRD). Patients with ESRD on dialysis continue to have major medical, social and economic problems with life expectancies of only 4–5 years (Iglehart 1993, 1998; Xue et al. 2001; Cukor et al. 2007). While the ideal standard for kidney replacement therapy for ESRD remains organ transplant, with less than 10,000 kidney transplants completed each year and the current number of patients awaiting transplantation approaching 60,000 (Collins et al. 2005), the need far exceeds availability. For all disease processes, available renal replacement therapies consisting of hemofiltration, hemodialysis or chronic ambulatory peritoneal dialysis are non-physiologic and fail to address the homeostatic, regulatory, metabolic, and endocrine functions of the kidney. Patients with ESRD are at high risk for cardiovascular and infectious diseases despite conventional renal replacement therapy. A recent clinical trial failed to show survival benefit from increased doses of dialysis above current standard of care (Humes 1995) suggesting that there are important metabolic derangements not adequately treated with conventional dialytic treatment. Survival of renal transplant recipients far exceeds that of age-, sex-, and risk-matched controls awaiting transplant, clearly demonstrative of the metabolic function provided by the kidney beyond filtration. ESRD currently effects over 430,000 U.S patients and has an annual cost of more than 25 billion dollars and patient numbers are predicted to increase to 2.24 million by 2030 (Schrier et al. 2004). The potential success of renal cell therapy lies in the growing appreciation that most disease processes are not due to the lack of a single protein but develop due to alterations in complex interactions of a variety of cell products. The renal tubule cell’s roles in glutathione reclamation, glutathione peroxidase synthesis, and activation of vitamin D3, with its important immunoregulatory functions, are well-recognized pathways to maintain important tissue integrity and host defense under stress conditions (Humes 2000; Humes et al. 2002). A less recognized role of the kidney, chiefly the renal tubule, is its potential immunoregulatory function. The kidney is derived embryologically from dorsal mesoderm, a collection of cells also important in the development of bone marrow stem cells (Turpen et al. 1982; Saxen 1987). The maturation of cells responsible for erythropoietin synthesis and activation of 1,25-(OH)2 vitamin D3 in the kidney is reflective of this embryonic origin. Phylogenetically, in bony fish and amphibians without lymph systems, the kidney is the major antibody-producing organ (Smith et al. 1967; Zapata 1979; Turpen et al. 1982). Not surprisingly, mammalian renal proximal tubule cells are immunologically active. They are antigen-presenting cells (Bishop et al. 1988; Wuthrich et al. 1990) that possess co-stimulatory molecules (Wahl et al. 2002) and that synthesize and process a variety of inflammatory cytokines (Prodjosudjadi et al. 1995; Leonard et al. 1999; van Kooten et al. 1999). The development of renal epithelial cell (REC) therapy to replace the endocrine and immunologic functions of the kidney, in addition to hemofiltration, promises to add significant value to the current suboptimal treatments of renal failure. Candidate biological markers that are known to be dysregulated in ESRD, correlate with poor outcome and are linked to known mechanisms of disease, include markers of inflammation and oxidative stress.
A methodology to isolate and grow REC from adult human kidneys has already been developed (Smith et al. 2006) and the described methods used to provide the renal cell component of tissue engineered devices evaluated in large animal and clinical studies (Tumlin et al. 2008). However, using standard cell processing techniques, REC isolated from human transplant discards do not have the expansion capability required to provide cell therapy for the large patient population suffering from ARF and ESRD. The ability to differentiate a totipotent embryonic stem cell down an intricately orchestrated kidney specific path is still in its infancy and does not provide a timely solution to cell sourcing for renal replacement therapy. Clinical and experimental observations suggest that REC progenitor cells have immense proliferative potential as evidenced by complete tubule regeneration to reform a fully functional and differentiated epithelium after severe nephrotoxic or ischemic injury (Duffield et al. 2005). This regeneration potential has been attributed to hematopoetic or mesenchymal stem cells rather than resident renal progenitor cells with controversial results (Poulsom et al. 2001; Gupta et al. 2002; Kale et al. 2003). The most pertinent observation from these studies was that injection of mesenchymal stem cells post ischemic injury enhances the regenerative potential and recovery of tubules, occurring without actual incorporation of these cells into the regenerated tubules, possibly due to a paracrine effect (Bi et al. 2007; Imai et al. 2007).
Current opinion is that precursors responsible for the repopulation of tubules reside within the kidney, possibly maintained within a specialized niche (Al-Awqati et al. 2006; Sagrinati et al. 2006), though the defining characteristics and mechanism are still under investigation (Duffield et al. 2005; Al-Awqati et al. 2006; Challen et al. 2006). Regardless of source, these resident kidney progenitor cells have stem cell-like characteristics, with a high capacity for self-renewal and the ability to differentiate under defined conditions into mature somatic cells as evidenced by tubule regeneration (Hall et al. 1989; Potten et al. 1990). An approach to more closely mimic the in-vivo environment promises to greatly enhance REC propagation potential and is the basis of these studies.
2. Materials and Methods
2.1 Tissue Procurement
Human cadaver kidneys, rejected for organ transplantation due to anatomic or fibrotic defects, were procured from the National Disease Research Interchange (NDRI). Additional donor acceptance criteria included: adults <80 years of age, blood tests negative for adventitious viruses, non-septic, normal creatinine and blood urea nitrogen levels, no other indices of kidney disease, and time between cross clamp and the beginning of tissue processing ≤36 hours. Tissue from 6 donors was simultaneously processed using EP and the standard protocol. The method modifications are highlighted in Table 1.
Table 1.
Comparison of Standard vs. Enhanced Propagation Protocols
| Step | Standard Method | Enhanced Propagation |
|---|---|---|
| Enzymatic Digestion | Collagenase <600uM particle size |
Liberase/Blendzyme Single cell →212uM particle size |
| Centrifugation | 50g (exclusive) | 300g (inclusive) |
| Plating Density | 500uL pellet / 100mm plate | 4-32uL pellet/100mm plate |
| Media Change | 24hr post plating Non-adherent cells removed by aspiration |
7 days post plating Non-adherent cells retained |
| Matrix | Collagen IV/adsorbed FCS | Endogenous Matrix |
| Sub-Culture | ~1×107 cells seeded and 2×107 recovered per T75 flask Pass ratio 1:2 |
1.25×106 plated, and 2×107 cells recovered per T75 Flask Pass ratio ~ 1:16 |
| Differentiation Factor Retinoic Acid | Added prior to Primary passage. Cells remain differentiated through passages |
Signals the end of precursor amplification. Added on device or test well after confluence (contact inhibition). |
2.2 Cell isolation
Kidneys were dissected clean of all external tissue, capsule removed, cortex cut from medulla and cortex weight was documented. Finely minced cortex was digested at 1g tissue/mL of pre warmed enzyme (collagenase for Standard or Liberase for EP)/DNase (0.239 Wunchst units and 250 Kunitz units/mL) at 37°C for 20 minute cycles. Using the standard protocol, the digestion was quenched with cold Dulbecco's Modified Eagle Medium (DMEM) and all tissue aggregates >850um returned to the digestion flask and cycles repeated. The quenched slurry was subsequently sieved without further enzymatic digestion and remaining tissue aggregates greater than 600um was discarded. For EP, the enzymatic digestion proceeded uninterrupted with only the <212um sieved filtrate being quenched as it passed into the collection beaker containing cold DMEM. To evaluate the relationship of aggregate and cell yield, EP slurry was further fractionated by differential sieving into 150–212, 90–150, 38–90 and <38um fractions. For the standard method, tissue was washed by centrifugation at 50xg to preferentially retain intact tubules, while EP tissue was centrifuged at 300xg, which is inclusive of single cells and aggregates that may contain damaged and therefore more buoyant tubules.
2.3 Primary Plating
REC were cultured in UltraMDCK Media (UM, Lonza) supplemented with ½× the manufacturer’s recommended dilution for insulin, transferrin, ethanolamine and selenium supplement (ITES, Lonza), 60ng/L epidermal growth factor (hrEGF, R&D systems), 10−9M triiodothyronine and 1x penicillin-streptomycin. Standard plating density was 1mL of 50xg pellet/24mLof media, 20ml per T75 flask and non-adherent fraction was aspirated as a result of the first media change after 24 hours in culture. For EP plating, cells were further diluted 1:16 by suspending 1mL of the 300xg pellet/384mL of media, 20mL per T75 flask. For three donors, initial plating density was evaluated over a wide range by continued 1:2 dilution until achieving 1:516 dilution. Partial media changes (50%) were performed on an as needed basis with need assessed by monitoring glucose (Stanbio), lactate (Van Den Hamer et al. 1958; Krieg et al. 1967) and pH of culture media. Non-adherent cells were retained from primary EP culture through first passage. Sub-Culture: For both methods, sub-culture was accomplished as follows: cells were rinsed free of divalent cations, enzymatically released from plates with 0.05% trypsin-versene (Lonza) by incubation for 20minutes at room temperature and reaction inhibited by 0.1%(w/v) soybean trypsin inhibitor(Gibco). Upon reaching tight confluence, retinoic acid (RA) was added to standard cultures 24 hours before first passage and cultures passed at 1:2, thus maintaining confluency between 50 and 100%. Enhanced propagation cultures did not receive RA at confluence and were subcultured at 105cells/mL, 12mL/100mm plate and passaged when 50–75% confluent, maintaining confluency between 6.25 and 75%. For both standard and EP cultures, RA was added to media just prior to use at a concentration of 10−7M.
2.4 Assessment of Identity and Function
Tests were made on increasingly passaged EP cultures, while those generated using standard protocols were tested at fifth passage, the point at which standard populations were integrated into devices for clinical studies. For all tests EP cells were seeded at confluent density, 106cells/mL/5cm2. RA was added after 2 days and cells cultured for an additional 5 days prior to testing. For cytokine evaluation, cell media was supplemented with 0.1% bovine serum albumin both with and without 10ug/mL lipopolysaccharide (LPS), supernatants removed at 24hr, clarified by centrifugation and concentration of IL-8 determined by ELISA (R&D Systems). A quantitative γGT enzyme assay was developed using γ-glutamy-p-nitoanalide, a commercially available,γGT specific colorometrc substrate (Stanbio) (Szasz 1974). Activity was assayed in whole cell lysates prepared using a non-ionic detergent buffer and normalized with protein concentration (BioRad). One unit of activity is defined as the conversion of 1uMol of reagent /minute/mg protein at 37°C. For immunohistochemistry (IHC), cells were fixed in 4% paraformaldehyde, permeated with 0.1%Triton X-100 and subsequently labeled with antibodies to REC specific markers including acetylated tubulin (AT1, Zymed), zonna occludens-1 (ZO1, Zymed), γGT (Lab Vision) and aminopeptidase-N (APN, Becton Dickinson), and nuclei labeled with 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI).
2.5 Cryopreservation
Trypsinized cells were mixed at 107 cells/mL in cryoprotectant media (10%DMSO in UM), placed in a controlled rate freezing apparatus (Nalgene, Mr. Frosty) at −80°C overnight and then transferred to the vapor phase of liquid nitrogen. Cells remained frozen for at least 2 weeks prior to use in cryopreservation studies. Cells were allowed to recover by plating and passage using the EP propagation protocol before seeding at confluent density for characterization.
3. Results and Discussion
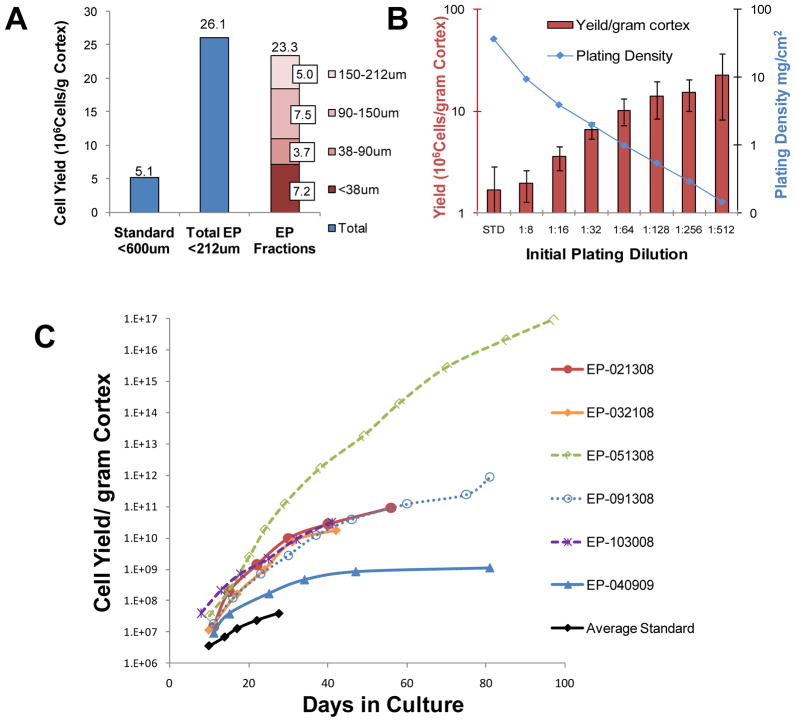
Independent culture of sieved fractions confirmed that all aggregate pools contribute significantly to the EP precursor pool. A representative plot of the yield from the primary plating of each sieved portion is shown in figure 1A. The proliferation potential of the <38um portion, in which any three dimensional stem cell niche would be destroyed, was essentially equivalent to the 38–90um portion and persisted through continued passage. Plating density was investigated in four of 6 isolates and representative results shown in figure 1B. Yield per gram cortex increased inversely to plating density at primary passage. However, cultures plated at dilutions greater than 1:16 of the standard protocol were subject to an island effect where cells become contact inhibited at the center of islands. For continued expansion, these dilute cultures required passage before the tissue culture media became depleted. Cells plated at 1.25×106cells/20mL /75cm2 flask (a concentration calculated from 1:16 of a confluent 75 cm2 flask =2×107cells) required weekly passage, essentially eliminating the need for media changes between passage days and dramatically decreasing the reagents, supplies, effort and therefore cost of maintenance over more dilute cultures. For the remainder of this report all assays for identification and efficacy focus on EP cells plated at 1:16 and subcultured at 1.25×106cells/20mL/75cm2 flask.
Figure 1.
Cell Yield Calculations. Application of the Enhanced Propagation (EP) protocol for the isolation and propagation of renal epithelial progenitor cells to human cadaver kidneys greatly increased yield. (A): Yield increase is observed at first passage. Progenitor cells are present in all sieved cell fractions (shown by fraction (red) far right) including the <38um portion in which any three dimensional stem cell niche would be destroyed. Representative results from 09/13/2008 isolate are shown. (B): Cell yield per gram cortex was proportional to dilution factor at primary plating. The results are shown average±SE for n=3 isolates. (C) The growth curves of six individual EP isolates and the Average Standard are plotted as total cell number through time. Though growth potential varies greatly between isolates, the plots clearly demonstrate that a far greater cell mass was obtainable using EP techniques. Note the logarithmic scale displayed on the vertical axis (cell yield/gram cortex). For Figures A and B, primary yield is defined as the number of cells resulting from the initial plating evaluated counted post trypsinization prior to subculture.
In Figure 1C, growth curves of REC progenitors from each donor are plotted as cell yield/gram cortex through culture time along with the average yield from the standard method for comparison. All EP cultures maintained exponential growth for at least 6 weeks with the exception of the 04/09/09 isolate, the only isolate that was terminated due to senescence. The others were terminated because they did not form epithelial monolayers when plated at higher density on tissue culture surfaces. Instead, they had a greater affinity for each other and aggregated into three-dimensional structures in suspension. Yield exceeded 1016 cells/gram cortex from the only kidney obtained due to an anatomical defect, while the average yield from diseased kidneys ranged from 1.1×109 to 8.8×1011 cells/gram cortex, representing an increase of 10.3±1.4 doublings over standard methods. Because the cell numbers obtained using the EP methods were sufficient by the time the effect was observed, further propagation and analysis of these cells were not pursued. Plots clearly demonstrate the far greater cell mass obtainable with EP techniques. Note the logarithmic scale displayed on the vertical axis (cell yield/gram cortex). The cell yields for both methods and relevant donor information are provided in Table 2.
Table 2.
Relevant Donor History and Yield
| Isolation Date | Yield Cell Number/g Cortex | Additional Doublings Fresh/CRYO | Cause of Death | AGE / Sex | Relevant Donor Historya | Glomerular Sclerosis | ||
|---|---|---|---|---|---|---|---|---|
| Standard | EP | EP CRYO | ||||||
| 02/13/2008 | 6.3 × 107 | 9.2 × 1010 | 3.0 × 108 | 10.4 / 5.6 | Anoxia | 57F | D, HT, T, EtOH, A | 6% |
| 03/21/2008 | 1.3x 107 | 3.7 × 1010 | 2.7 × 1010 | 11.5 / 11.0 | Trauma | 50M | HT | 4% |
| 05/13/2008 | 2.6 × 107 | 9.2 × 1016 | 6.3 × 1012 | 31.7 / 17.9 | CVAb | 65M | 0% | |
| 09/13/2008 | 4.3 × 107 | 8.8 × 1011 | 2.2 × 1010 | 14.3 / 9.0 | Anoxia | 51F | HT, T, EtOH | Biopsy Not Performed |
| 10/30/2008 | 5.3 × 107 | 3.2 × 1010 | 6.6 × 1010 | 9.2 / 10.3 | CVA | 52F | D, HT, T, EtOH, A | 14% |
| 04/09/2009 | 2.0 × 107 | 1.1 × 109 | Not Determined | 5.8 / ND | CVA | 78M | D, HT, T, RS | 20% |
Cell mass generated from enhanced propagation (EP) methods is diminished by cryopreservation (CRYO), but still far exceeds that of the standard protocol. Yield is highly dependent on prior disease state of donor and ranges from >1016 cells/gram cortex derived from a relatively healthy donors to <1010 from a kidney known to be diseased. At a projected cell therapy dose of 108 cells, the 05/13/2008 donor would yield over 108 devices from a single gram of cortex.
Diabetes (D), Hypertension (HT), Tobacco Use (T), Alcohol Use (EtOH), Asthma (A), Renal Stent (RS).
Cardiovascular Accident (CVA)
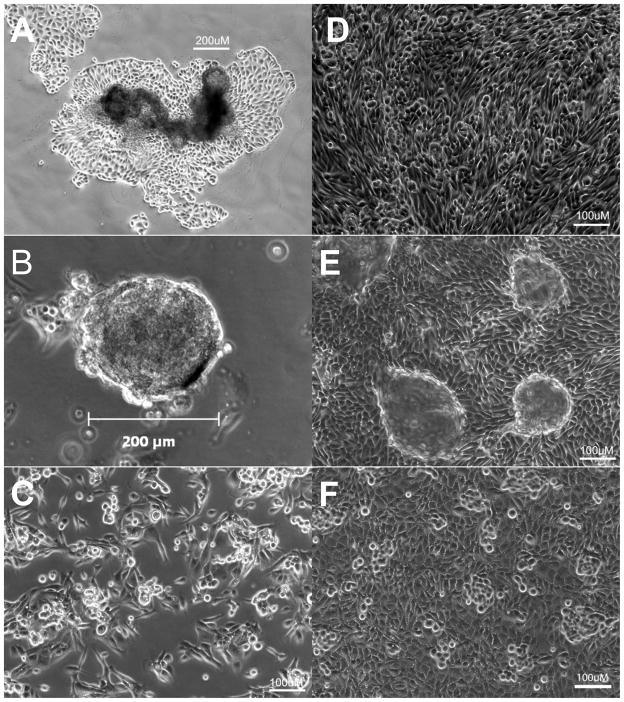
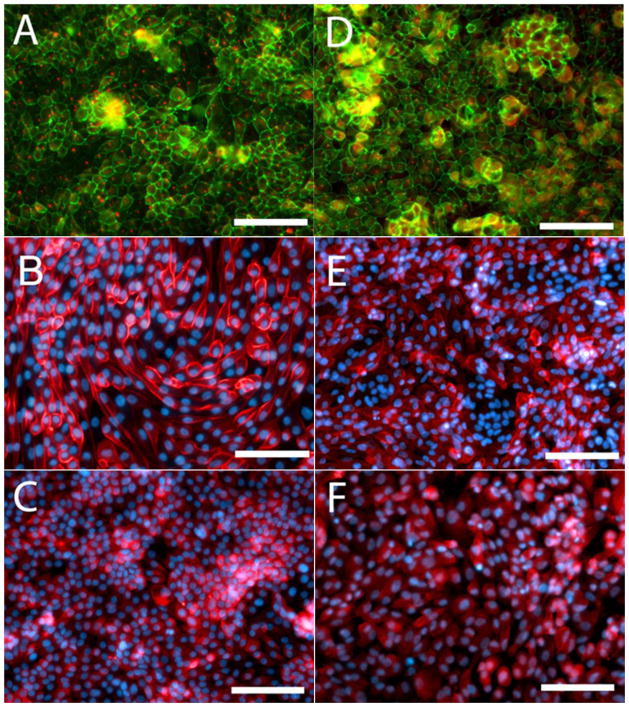
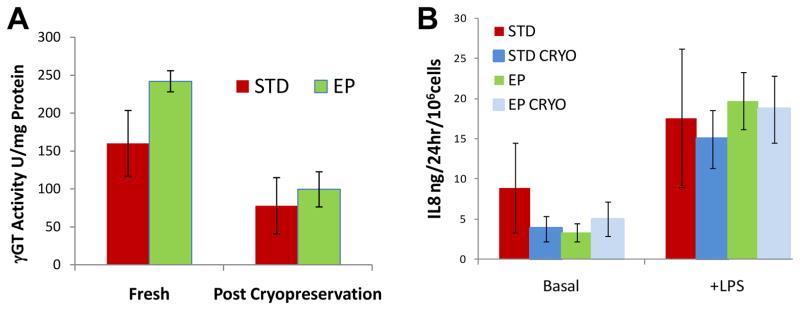
During primary propagation at low density, REC progenitor cells are highly motile, forming adherent islands, floating rafts and non-adherent spheroids (Figure 2A-C). Using standard methods part of the progenitor pool is discarded as a result of the initial media change and the non-adherent structures did not have time to form. As cell density increases, the cells coalesce into islands with cuboidal centers and spindle shaped cells on the outer edges (supplemental online video 1). When plated at higher density with RA for functional testing, the cells quickly develop the familiar cobblestone appearance associated with REC with occasional doming (Figure 2D, E and supplemental online video 2). As shown in Figure 3A, advanced EP cultures retained the ability to form polarized epithelium as evidenced by formation of apical central cilia revealed by punctuate AT1 labeling and the distinctive, spider web pattern of tight junctions was demonstrated with ZO1. The brush border enzymes APN and γGT persisted in advanced EP cultures (Figure 3B, C). The results from the γGT enzyme assay complemented IHC results with 160±43 and 242±37U/mg protein for the standard and EP cells respectively. No trend was observed as the cultures aged, however for both populations, there was a significant decrease in γGT enzyme activity detected in cells post cryopreservation, dropping to 78±14 and 99±23U/mg protein for the standard and EP cells respectively (Figure 4A).
Figure 2. Morphology of REC propagated using the EP protocol.
In primary culture REC progenitors form non-adherent floating rafts (A) and hollow spheroids (B) as well as adherent spindle shaped cells. Post cryopreservation, progenitor cells kept at low density remain in exponential growth phase retaining spindle shaped morphology (C). When plated at density with RA, previously cryopreserved cells obtain the cobblestone morphology of differentiated REC (D) with occasional doming (E). Standard culture at passage 5 shown is shown for comparison (F).
Figure 3. Immunohistochemical Comparison of Renal Epithelial Cells (REC) propagated using the Enhanced Propagation (EP) Protocol compared to Standard Protocol.
Left Panel (A, B, C) EP, Right Panel (D, E, F) Standard. EP generated REC form polarized epithelial monolayers as demonstrated by acetylated tubulin positive apical central cilia (Red) while zona occludens (Green) is concentrated in tight junctions having a distinct spider web pattern (A,D). Expression of renal brush border enzymes aminopeptidase N (B and E, Red) and γGT (C and F, Red) persist. DAPI counterstaining is shown in B, E, C and F (Blue). Photos are representative of advanced EP cultures at yields greater than 100× that possible with the standard protocol compared to passage 5 standard cultures (passage historically used for therapy). Scale bar=100μm.
Figure 4.
Retention of Therapeutic Potential through Cryopreservation. (A): γ-glutamyl transpeptidase (γGT) enzyme activity was detected in renal epithelial cells generated from both standard (STD) and enhanced propagation (EP) cultures but was greatly diminished post cryopreservation(CRYO). (B): Addition of lipopolysaccharide (LPS) stimulated IL8 secretion over basal secretion level in all cultures tested and was not affected by cyropreservation. The average results ± SE for n=4 donors are shown.
EP generated REC retained the ability to respond to endotoxin challenge through advanced culture. LPS stimulated IL8 secretion for all populations tested, however the amount of IL8 released was highly variable across donors for the standard populations, averaging 6.3±3.4 and 17.6±8.6 ng/106cells/24hr for basal and stimulated respectively. EP rates were more consistent between donors with the average being 3.4±1.1 and 19.7±3.5 ng/106cells/24hr for basal and stimulated and remained within the secretion ranges detected for the standard cultures. The ability to respond to endotoxin challenge persisted through cryopreservation with basal and stimulated rates being 6.3±3.4 and 19.1±9.6 ng/106cells/24hr for standard and 5.0±2.1 and 18.7±4.2 ng/106cells/24hr for basal and stimulated EP populations (Figure 4B).
Cryopreservation had a detrimental effect on proliferation potential. In addition to a low initial recovery rate of 80% (8×106 viable cells from each vial containing 107 cells upon cryopreservation) the overall growth potential was greatly diminished. For the two isolates with the lowest potential, growth rates were unaffected. However, for the isolates having the most proliferation potential prior to cryopreservation (02/13/08, 5/13/08 and 09/13/08), the growth potential was reduced between 2 and 4 orders of magnitude (Table 2).
Application of EP protocols to human kidneys has significantly increased cell yields compared to the standard method. In aggressively digested kidney cortex, the <38um fraction which consists of mostly single cells still retained a high potential for proliferation, indicating that the retention of a three dimensional stem cell niche is not a requirement for transient amplification of renal progenitors. Yield was inversely proportional to plating density, while partial media changes increased yields, indications that more than one factor may be influencing REC progenitor propagation.
Unfortunately, the available kidney pool for REC therapy consists of donors having preexisting serious health issues, including diabetes, hypertension and sclerosis of the kidney. Although donor health had a large impact on progenitor cell amplification, the post cryopreservation cell yields from sub optimal donors exceeded 1010 cells/gram cortex while retaining the appropriate morphology and ability to express surrogate efficacy markers. With a cell load per device expected to be 108 cells, and cortex weight of about 50g per kidney, this correlates to more than 5,000 devices from a single donor kidney, a vast improvement to the 1–10 devices demonstrated using standard methods. The only kidney donor with no known disease (05/13/08) yielded over 1016 cells per gram of cortex.
With amplification of renal progenitor cells to this magnitude, the EP protocol could be successfully applied to tissue obtained from a kidney biopsy for autologous device manufacture for chronic applications. Kidney biopsies are routinely taken for diagnostic purposes and range from a few milligrams for a needle biopsy to around to 50mg for a wedge biopsy. If the method was applied successfully to the non-diseased 05/13/08 isolate, 6.3 x1012 cells or approximately 63,000 autologous devices could theoretically be manufactured from a 50mg wedge biopsy. For proof of principle, the EP method was applied to an 11mg simulated needle biopsy taken from the whole donor kidney prior to dissection on 04/09/09. Even though the simulated biopsy was from the kidney of the oldest donor, with the most pre-existing health issues and had the lowest overall yield, application of EP method still yielded enough cells (>108) for one autologous renal cell therapy device (data not shown).
To compare cells from standard methods and those generated using the EP protocol surrogate markers were evaluated in addition to establishing equivalent morphology. LPS stimulated IL8 secretion and γGT enzyme activity were chosen because they correlate with the ability to respond to inflammation and oxidative stress, mechanisms that are known to be dysregulated in ESRD and correlate with poor outcomes. Additionally basal IL8 secretion was used as a release criterion for previous clinical trials involving REC (Tumlin et al. 2008). Because the immunoregulatory role of the kidney is very complex and response is highly individualized to the animal or patient (Humes et al. 2003), the usefulness of a surrogate marker panel in relation to actual therapeutic benefit remains unclear. Nevertheless, correlation of in vitro measurements to efficacy will be required for transition of REC therapy to the clinical setting and therefore the pursuit of surrogate markers remains a topic of emphasis. Because γGT enzyme activity and ability to respond to endotoxin challenge in expanded EP cultures are retained through cryopreservation, these functions provide very promising efficacy markers. EP derived cells in a bioartificial renal cell system are currently being evaluated using a porcine model of sepsis, where cells are in contact with the ultrafiltate in a hemodialysis circuit. In this model, renal cell therapy with standard cells increased survival time from 6.7 to 11.3 hours (Buffington et al. 2009). Additionally, cells are being evaluated in an ovine model of ESRD, where cell therapy is delivered via peritoneal dialysis. (Song et al. 2009) Results from these lines of experimentation will be used to further validate the use of IL8 secretion and γGT activity as efficacy markers.
The enhanced propagation method was developed to provide an adequate and reliable supply of functional REC for integration into a cell therapy device. While cryopreservation affected the potential cell yield, only the very basic of cryopreservation techniques were employed. Cell function and yield are expected to improve with optimized cryopreservation protocols. Cells derived from EP populations reconstituted post cryopreservation were successfully integrated into a renal cell therapy device and retained the correct morphology through long term in vitro maintenance in a perfusion circuit and ex-vivo extracorporeal hemodialysis and peritoneal dialysis circuits. Application of the EP protocol to human cadaver kidneys had greatly enhanced cell proliferation, clearly demonstrating a robust cell source for renal cell therapy in two prospective delivery systems.
Emphasis was not placed on understanding the mechanisms responsible for the observed increase in yield. More investigation into the factors influencing REC amplification is merited and may provide further advancement in renal cell progenitor technology as well insight into progenitor amplification in other organ systems.
4. Summary
As envisioned, application of the EP protocol to human transplant discard kidneys increased cell yield dramatically over the standard method for all 6 donors tested. The generated cell number from each kidney was highly variable and based on available information, correlated with overall donor health. Yield exceeded 1016 cells/gram cortex from the only kidney obtained due to an anatomical defect, while the average yield from diseased kidneys ranged from 1.1×109 to 8.8×1011 cells/gram cortex, representing an increase of 10.3±1.4 doublings over standard methods. Surrogate markers of efficacy, LPS stimulated IL8 secretion and γGT enzyme activity, were chosen because they represent the ability to respond to endotoxin challenge and oxidative stress. IL8 secretion and γGT activity persisted through advanced EP cultures. Stimulated IL8 secretion was unaffected by cryopreservation, butγGT activity and overall cell yield were significantly reduced. However, the negative effects of cryopreservation may be resolved with the application of more advanced cryopreservation methods. Cells derived from EP populations reconstituted post cryopreservation were successfully integrated into a renal cell therapy device and retained the correct morphology through long term in vitro maintenance in a perfusion circuit and ex-vivo extracorporeal hemodialysis and peritoneal dialysis circuits. Application of the EP protocol to REC expansion has solved the problem of cell sourcing as the rate limiting factor to the manufacture of cell based therapies targeting renal diseases and may provide a method for autologous device fabrication from core kidney biopsies.
Supplementary Material
Acknowledgments
This work was made possible with NIH-NIDDK SBIR grants, 1R43DK082050-01, 1R43DK074289-01, 1R43DK074289-02, 1R43DK080529-03, and 1R43DK080529-04; and DoD W81XWH-05-2-01. We acknowledge use of human tissues provided by the National Disease Research Interchange (NDRI), with support from NIH grant 5 U42 RR006042-20.
Footnotes
Disclaimers:
Deborah A Buffington and H D Humes are shareholders of Innovative BioTherapies, Inc.
Author Contribution
Angela J. Westover: Conception and design, financial support, collection and assembly of data, data analysis and interpretation and manuscript writing.
Deborah A. Buffington: Administrative support, data analysis and interpretation and final approval of manuscript.
H. D. Humes: Financial support, data analysis and interpretation and final approval of manuscript.
References
- Excerpts from the united states renal data system 1998 annual data report. Am J Kidney Dis. 1998;32(2 Suppl 1):S1–162. [PubMed] [Google Scholar]
- Al-Awqati Q, Oliver JA. The kidney papilla is a stem cells niche. Stem Cell Rev. 2006;2(3):181–184. doi: 10.1007/s12015-006-0046-3. [DOI] [PubMed] [Google Scholar]
- Bates DW, Su L, Yu DT, Chertow GM, Seger DL, Gomes DR, Dasbach EJ, Platt R. Mortality and costs of acute renal failure associated with amphotericin b therapy. Clin Infect Dis. 2001;32(5):686–693. doi: 10.1086/319211. [DOI] [PubMed] [Google Scholar]
- Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18(9):2486–2496. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- Bishop GA, Waugh JA, Hall BM. Expression of hla antigens on renal tubular cells in culture. Ii. Effect of increased hla antigen expression on tubular cell stimulation of lymphocyte activation and on their vulnerability to cell-mediated lysis. Transplantation. 1988;46(2):303–310. doi: 10.1097/00007890-198808000-00022. [DOI] [PubMed] [Google Scholar]
- Buffington DA, Hageman G, Lou L, Wang M, Ding F, Song J, Jung J, Smith PL, Westover A, Charles L, Humes HD. Design of a compact, cryoperservable, bioartificial renal cell system. J Am Soc Nephrol. 2009;20:27A. [Google Scholar]
- Challen GA, Bertoncello I, Deane JA, Ricardo SD, Little MH. Kidney side population reveals multilineage potential and renal functional capacity but also cellular heterogeneity. J Am Soc Nephrol. 2006;17(7):1896–1912. doi: 10.1681/ASN.2005111228. [DOI] [PubMed] [Google Scholar]
- Chertow GM, Levy EM, Hammermeister KE, Grover F, DJ Independent association between acute renal and mortality following cardiac surgery. Am J Med. 1998;104:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- Collins AJ, Kasiske B, Herzog C, Chavers B, Foley R, Gilbertson D, Grimm R, Liu J, Louis T, Manning W, Matas A, McBean M, Murray A, St Peter W, Xue J, Fan Q, Guo H, Li S, Roberts T, Snyder J, Solid C, Wang C, Weinhandl E, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Johnson R, Sheets D, Forrest B, Berrini D, Constantini E, Everson S, Frederick P, Eggers P, Agodoa L. Excerpts from the united states renal data system 2004 annual data report: Atlas of end-stage renal disease in the united states. Am J Kidney Dis. 2005;45(1 Suppl 1):A5-7, S1–280. doi: 10.1053/j.ajkd.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Cukor D, Cohen SD, Peterson RA, Kimmel PL. Psychosocial aspects of chronic disease: Esrd as a paradigmatic illness. J Am Soc Nephrol. 2007;18(12):3042–3055. doi: 10.1681/ASN.2007030345. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Bonventre JV. Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int. 2005;68(5):1956–1961. doi: 10.1111/j.1523-1755.2005.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME. A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int. 2002;62(4):1285–1290. doi: 10.1111/j.1523-1755.2002.kid569.x. [DOI] [PubMed] [Google Scholar]
- Hall PA, Watt FM. Stem cells: The generation and maintenance of cellular diversity. Development. 1989;106(4):619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- Humes HD. Acute renal failure: Prevailing challenges and prospects for the future. Kidney Int Suppl. 1995;50:S26–32. [PubMed] [Google Scholar]
- Humes HD. Bioartificial kidney for full renal replacement therapy. Semin Nephrol. 2000;20(1):71–82. [PubMed] [Google Scholar]
- Humes HD, Fissell WH, Weitzel WF. The bioartificial kidney in the treatment of acute renal failure. Kidney Int Suppl. 2002;(80):121–125. doi: 10.1046/j.1523-1755.61.s80.22.x. [DOI] [PubMed] [Google Scholar]
- Humes HD, Weitzel WF, Bartlett RH, Swaniker FC, Paganini EP. Renal cell therapy is associated with dynamic and individualized responses in patients with acute renal failure. Blood Purif. 2003;21(1):64–71. doi: 10.1159/000067864. [DOI] [PubMed] [Google Scholar]
- Iglehart JK. The american health care system. Teaching hospitals. N Engl J Med. 1993;329(14):1052–1056. doi: 10.1056/NEJM199309303291428. [DOI] [PubMed] [Google Scholar]
- Imai E, Iwatani H. The continuing story of renal repair with stem cells. J Am Soc Nephrol. 2007;18(9):2423–2424. doi: 10.1681/ASN.2007070769. [DOI] [PubMed] [Google Scholar]
- Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112(1):42–49. doi: 10.1172/JCI17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg AF, Rosenblum LJ, Henry JB. Lactate dehydrogenase isoenzymes a comparison of pyruvate-to-lactate and lactate-to-pyruvate assays. Clin Chem. 1967;13(3):196–203. [PubMed] [Google Scholar]
- Leonard M, Ryan MP, Watson AJ, Schramek H, Healy E. Role of map kinase pathways in mediating il-6 production in human primary mesangial and proximal tubular cells. Kidney Int. 1999;56(4):1366–1377. doi: 10.1046/j.1523-1755.1999.00664.x. [DOI] [PubMed] [Google Scholar]
- Potten CS, Loeffler M. Stem cells: Attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195(2):229–235. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- Prodjosudjadi W, Gerritsma JS, Klar-Mohamad N, Gerritsen AF, Bruijn JA, Daha MR, van Es LA. Production and cytokine-mediated regulation of monocyte chemoattractant protein-1 by human proximal tubular epithelial cells. Kidney Int. 1995;48(5):1477–1486. doi: 10.1038/ki.1995.437. [DOI] [PubMed] [Google Scholar]
- Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the bowman's capsule of adult human kidneys. J Am Soc Nephrol. 2006;17(9):2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- Saxen L. Organogenesis of the kidney. New York: Cambridge University Press; 1987. [Google Scholar]
- Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351(2):159–169. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- Smith AM, Potter M, Merchant EB. Antibody-forming cells in the pronephros of the teleost lepomis macrochirus. J Immunol. 1967;99(5):876–882. [PubMed] [Google Scholar]
- Smith PL, Buffington DA, Humes HD. Kidney epithelial cells. Methods Enzymol. 2006;419:194–207. doi: 10.1016/S0076-6879(06)19009-6. [DOI] [PubMed] [Google Scholar]
- Song J, Jung J, Ding F, Westover A, Hageman G, Lou L, Rojas A, Charles L, Smith PL, Buffington DA, Humes HD. Uremic animal model of bioartificial renal cell system (brecs) using continuous flow peritoneal dialysis-based extracorporeal circuit. J Am Soc Nephrol. 2009;20:106A. [Google Scholar]
- Szasz G. Methods of enzymatic analysis. New York: Academic Press, Inc; 1974. [Google Scholar]
- Tumlin J, Wali R, Williams W, Murray P, Tolwani AJ, Vinnikova AK, Szerlip HM, Ye J, Paganini EP, Dworkin L, Finkel KW, Kraus MA, Humes HD. Efficacy and safety of renal tubule cell therapy for acute renal failure. J Am Soc Nephrol. 2008;19(5):1034–1040. doi: 10.1681/ASN.2007080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpen JB, Knudson CM. Ontogeny of hematopoietic cells in rana pipiens: Precursor cell migration during embryogenesis. Dev Biol. 1982;89(1):138–151. doi: 10.1016/0012-1606(82)90302-5. [DOI] [PubMed] [Google Scholar]
- Van Den Hamer CJ, Elias RW. A method for the determination of d(-)lactic acid. Biochim Biophys Acta. 1958;29(3):556–562. doi: 10.1016/0006-3002(58)90012-x. [DOI] [PubMed] [Google Scholar]
- van Kooten C, van der Linde X, Woltman AM, van Es LA, Daha MR. Synergistic effect of interleukin-1 and cd40l on the activation of human renal tubular epithelial cells. Kidney Int. 1999;56(1):41–51. doi: 10.1046/j.1523-1755.1999.00514.x. [DOI] [PubMed] [Google Scholar]
- Wahl P, Schoop R, Bilic G, Neuweiler J, Le Hir M, Yoshinaga SK, Wuthrich RP. Renal tubular epithelial expression of the costimulatory molecule b7rp-1 (inducible costimulator ligand) J Am Soc Nephrol. 2002;13(6):1517–1526. doi: 10.1097/01.asn.0000017901.77985f. [DOI] [PubMed] [Google Scholar]
- Wuthrich RP, Glimcher LH, Yui MA, Jevnikar AM, Dumas SE, Kelley VE. Mhc class ii, antigen presentation and tumor necrosis factor in renal tubular epithelial cells. Kidney Int. 1990;37(2):783–792. doi: 10.1038/ki.1990.46. [DOI] [PubMed] [Google Scholar]
- Xue JI, Ma JZ, Louis TA, Collins AJ. Forecast of the number of patients with end-stage renal disease in the united states to the year 2010. J Am Soc Nephrol. 2001;12:2753–2758. doi: 10.1681/ASN.V12122753. [DOI] [PubMed] [Google Scholar]
- Zapata A. Ultrastructural study of the teleost fish kidney. Dev Comp Immunol. 1979;3(1):55–65. doi: 10.1016/s0145-305x(79)80006-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.