Abstract
By sequentially applying sonic hedgehog (C25II) and CHIR99021 (GSK3β inhibitor) to induce the midbrain floor plate progenitors and fibroblast growth factor 8 (FGF8) to promote dopaminergic differentiation in a chemically defined medium, we have established a robust system for generation of midbrain dopamine (DA) neurons from human and rhesus monkey embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). We found that CHIR99021 specifies diencephalon to hindbrain fates in a concentration-dependent manner and only a narrow concentration range of CHIR99021 at a particular window is necessary to induce the midbrain floor plate progenitors, expressing Corin, En1, FoxA2 and Lmx1a. FGF8 enhances the dopaminergic fate of the progenitors, thus generating DA neurons with midbrain characteristics, including expression of TH, Lmx1a/b, FoxA2, FoxP1, Nurr1 and En1 as well as typical electrophysiological properties. More than half of these DA neurons expressed A9 DA neuron markers Girk2 and ALDH1a1. The new strategy will allow generation of enriched populations of functional midbrain DA neurons from both human and monkey PSCs for disease modeling, drug testing, and potential cell therapy.
Keywords: Parkinson’s disease, drug discovery, neural patterning, transplantation
INTRODUCTION
During neural development, midbrain dopamine (DA) neurons originate from the floor plate [1], a group of cells located at the ventral midline of the neural tube [2]. The floor plate in the mesencephalon, as opposed to other parts of the brain and spinal cord, is unique because of its neurogenic potential [1, 3]. This neurogenic potential is endowed by the transcriptional code expressed by the progenitors, including Lmx1a, FoxA2, En1, and Otx2, which in turn is controlled by two regulatory feedback loops (Wnt1-Lmx1a and SHH-FoxA2) [4]. In particular, wnt1 induces expression of Otx2, which represses Gbx2 to position and maintain mid-hindbrain organizer and represses Nkx2.2, which delimits the midbrain DA progenitor domain from the more laterally located progenitors of serotonin neurons [5]. It induces the expression of Lmx1a which either induces the proneural gene Ngn2 though Msx1 [3, 4] or inhibits the neuroepithelia from acquiring other alternative cell fates by repressing Nkx6.1 [3, 6].
This developmental principle forms the guideline for differentiating midbrain DA neurons from (human and non-human) primate pluripotent stem cells, including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). Numerous reports show differentiation of TH (tyrosine hydroxylase)-expressing DA neurons from hESCs and iPSCs, mainly by treatment of neural precursors with sonic hedgehog (SHH), a ventralizing morphogen, and fibroblast growth factor 8 (FGF8), a morphogen important for the formation of the isthmus [7–13]. Nevertheless, most reports did not assess the expression of midbrain markers, including En-1 and Ptx3, in the DA neurons, or the rate of En1/TH co-labeled neurons was very low. This suggests that the combination of FGF8 and SHH can induce the dopaminergic identity but it is not sufficient to restrict the neurons to the midbrain fate. Further efforts were made to induce the midbrain fate by addition of retinoic acid [12] or blockade of the FGF signaling in the early stage of differentiation [14], which could enhance the expression midbrain-related genes. But the effect was very limited.
As discussed above, activation of wnt signaling may enable midbrain patterning of neural precursors. Indeed, forced expression of Pitx3 and Nurr1 [15] or Lmx1a [16, 17], downstream targets of Wnt1, promotes generation of DA neurons that carry some midbrain characteristics. However, effective non-genetic approaches for activating wnt pathway were not available until the identification of an effective small molecule inhibitor of GSK3β, CHIR99021 [18, 19]. By treating floor plate progenitors [20] with CHIR99021 (3 uM), Studer and colleagues showed robust generation of TH-expressing neurons that also are positive for FoxA2 and Lmx1a [21]. However, the authors did not show that these DA neurons express the key midbrain marker En1. In the meantime, we found that high concentrations of CHIR (>1 uM) restricted the human precursors to the hindbrain and the TH neurons do not carry midbrain characteristics. Only a narrow range of CHIR concentration at a particular developmental stage will restrict the precursors to the midbrain floor plate progenitors which, in the presence of FGF8, acquire DA neurons with most of the known midbrain DA neuron characteristics. This protocol is readily reproducible in different hESC lines, iPSCs, and rhesus monkey iPSCs.
MATERIALS AND METHODS
DA neuron differentiation from primate ESCs and iPSCs
Human ESCs (WA09, passages 23–45) and iPSCs (IMR90-4, passages 45–55) [22], as well as rhesus iPSCs, were maintained on mouse embryonic fibroblast feeder in a stem cell growth medium [23]. The human iPSCs were established using lentiviral approaches [22]. The rhesus monkey iPSCs were generated using the retroviral vectors developed by Yamanaka [24]. For DA neuron differentiation, PSCs at about 10% confluence (1 day after passaging) on MEF feeder layer were cultured in the neural induction medium consisting of DMEM/F12, N2 supplement, non-essential amino acids and heparin (Invitrogen, Carlsbad, CA, USA) in the presence of SB431542 (10uM) and LDN193189 (200nM, both from Stemgent), similar to that reported by Studer and colleagues [25]. To pattern the differentiating cells to the midbrain floor plate progenitors, SHH (C25II, 500ng/ml, R&D) and CHIR99021 (Stemgent) were added to the cultures from day 1 for 8 days. On day 8, individual colonies of epithelial cells formed. These epithelial colonies were gently blown off with a pipette and expanded as floating clusters in suspension in the same medium for an additional 4 days (D9–12). On day 12, some of the floor plate progenitor clusters were plated onto laminin-coated coverslips overnight for immunocytochemical analysis. Then, CHIR99021 was removed, SHH was reduced to 20n/ml, and FGF8b (100ng/ml) was added to the culture to expand the progenitors in suspension for 16 days (D12–28). The cultures were fed every other day and the progenitor clusters triturated into smaller cell aggregates with a polished glass Pasteur pipette every week. To differentiate the progenitors to DA neurons, the progenitor spheres were dissociated by incubation in ACCUTASE (Innovative Cell Technologies) at 37°C for 5 minutes and plating onto glass coverslips that were coated with polyornithine and laminin in the neurobasal medium supplemented with N2, B27, brain derived neurotrophic factor (BDNF, 10 ng/ml), glial cell line derived neurotrophic factor (GDNF, 10 ng/ml), ascorbic acid (200 uM), cAMP (1uM) and transforming growth factor (TGFβ3, 1 ng/ml).
Immunocytochemistry and cell counting
Immunofluorescenct staining on coverslip cultures was described previously [10, 11]. The primary antibodies were listed in Supplementary Table 1. The immunostaining was developed with appropriate fluorescencein-tagged secondary antibodies (Invitrogen, all at1:2000). Images were collected with a Nikon C1 (Tokyo, Japan) or a Leica TSC SP5 (Germany) confocal laser-scanning microscope. Cell counting was performed in optical fields randomly selected by using ImageJ (W. Rasband, NIMH, Bethesda, MD, USA) and replicated in different cell lines. Statistical analyses were performed using one-way ANOVA (Tukey or Dunnett for multiple comparisons) in SPSS13.0.
Electrophysiology
Whole-cell patch-clamp recordings were performed in PSC-derived neurons at 10 weeks. Briefly, neurons were held at −70mV to record the Na+/K+ channel activities, and at 0 mV to record the spontaneous release with the voltage-clamp mode. For recording action potentials, cells were held at in a current-clamp mode, and the steps of currents from − 60 pA to + 60 pA were injected into the cell. The bath solution consisted of 127 mM NaCl, 1.2 mM KH2PO4, 1.9 mM KCl, 26 mM NaHCO3, 2.2 mM CaCl2, 1.4 mM MgSO4, 10 mM glucose, 290 mOsm and 95% O2/5% CO2. Recording pipettes were filled with an intracellular solution containing 20 mM KCl, 121 mM K+-gluconate, 10 mM Na+-HEPES, 10 mM BAPTA, 4 mM Mg2+-ATP, pH 7.2 and 290 mOm. Bicuculline (20 μM; Sigma) or 6-cyano-7-nitroquinoxiline-2, 3-dione (CNQX, 20 μM; RBI) was applied to the bath solution to demonstrate inhibitory (GABAergic) and excitatory (Glutaminergic) response, respectively. 1% biocytin (Sigma) was injected into cells during recording to identify neuronal types. An Olympus BX51WI microscope was used to visualize neurons. A MultiClamp 700B amplifier (Axon instruments, Molecular Devices, Sunnyvale, CA, USA) was used to investigate the voltage clamp and current clamp recordings. Signals were filtered at 4 kHz and sampled at 100 kHz using a Digidata 1322A analog-digital converter (Axon instruments). Data were analyzed with pClamp 9.0 (Axon instruments). Capacitance and series resistance was compensated by 50–80% using an amplifier circuitry. Data were presented as mean ± SEM.
RESULTS
SHH alone induces hPSCs to ventral/floor plate progenitors but without midbrain characteristics
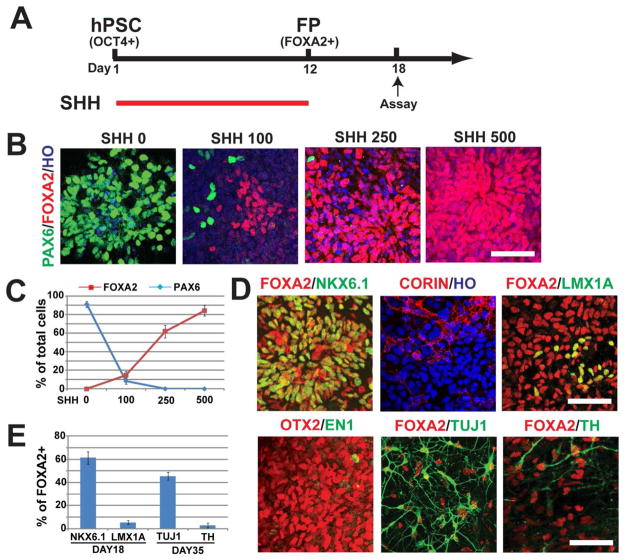
Floor plate cells, identified by FoxA2 expression, have been generated from hESC by exposure to high concentrations of SHH [20]. Using a similar protocol (treatment with SHH from D1–12 and examined at D18) (Fig. 1A), we indeed found that the differentiating cells exhibited an epithelial morphology (Supplementary Fig. 1A) and that the majority cells (84.2±5.6%) were FoxA2-positive when treated with 500 ng/ml SHH as compared to lower concentrations (Fig. 1B, C). Among them, most (95.4±3.2%) co-expressed a rostral marker Otx2 (Supplementary Fig. 1B). Interestingly, only 34.3±5.7% of the total cells expressed Corin (Fig. 1D), a transmembrane domain-containing cell-surface protease expressed in the ventral midline of the neural tube and regarded as a floor plate marker [1]. These results suggest that a large proportion of the FoxA2 cells are not floor plate cells. Indeed, 63.7±7.4% of the FoxA2 cells co-expressed Nkx6.1 (Fig. 1D, E), another ventral marker expressed in the basal plate locating dorsal to the floor plate [26, 27]. Furthermore, few FoxA2 progenitors co-expressed either Lmx1a or En1 (Fig. 1D, E, Supplementary Fig. 1B), which are expressed in the midbrain floor plate region. Thus, high concentrations of SHH alone can pattern NE cells to ventral progenitors, including some (34%) floor plate cells, likely of diencephalic identity, but hardly any of them are midbrain floor plate cells. Further differentiation of these progenitors for two weeks resulted in generation of FoxA2-expressing neurons but few were positive for TH (<2%, Fig. 1D, E).
Fig. 1. Induction of ventral/floor plate progenitors by SHH.
(A) Experimental paradigm showing differentiation of floor plate (FP) cells from hPSCs by SHH from D1–12. (B–C) Immunostaining and quantification of FoxA2 (red) and Pax6 (green) cells at day 18 after treatment with varying concentrations of SHH. (D–E) Immunostaining and quantification of cells expressing floor plate progenitor markers at day 18 and neuronal markers (Tuj1 and TH) at day 35 after treatment with 500 ng/ml SHH. Data presented in C & E are mean ± s.e.m., n=3 (independent experiments). Bar = 50 um.
A specific CHIR99021 concentration at a particular window is necessary to pattern the midbrain fate
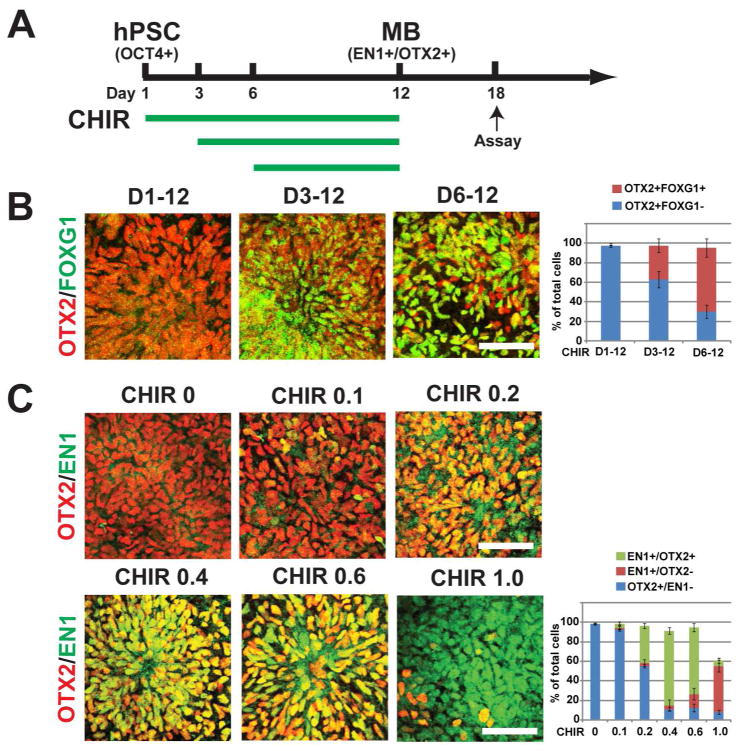
Given the anterior nature of the ventral progenitors, we hypothesize that activation of Wnt signaling, which patterns the mid-hind brain fate [28, 29], induces the midbrain identity of the progenitors. By using CHIR99021 (0.4 uM), a GSK3β inhibitor known to activate Wnt signaling, from D1, 3, and 6 until 12 and analyzing co-expression of Otx2 and En1 (as a criterion for midbrain progenitors) at D18 (Fig. 2A), we found that the anterior transcription factor FoxG1 was largely retained in cells that were treated with CHIR from D3 or D6 whereas FoxG1 was repressed in cells that were cultured in the presence of CHIR from day 1 (Fig. 2B). These FoxG1 negative cells also expressed En1 (see below). Thus, early treatment with CHIR is crucial for efficient midbrain patterning of the progenitors.
Fig. 2. Induction of midbrain progenitors by CHIR99021.
(A) Experimental paradigm showing differentiation of cells treated with CHIR99021 from D1–12. (B) Immunostaining and quantification of Otx2 (red) and FoxG1 (green) cells at day 18 after treatment with CHIR from day 1, 3, or 6. (C) Immunostaining and quantification of Otx2 (red) and En1 (green) after treatment with different concentrations of CHIR from day 1. Data presented in B & C: mean ± s.e.m., n=3 (independent experiments). Bar =50um
We then assessed the concentration-dependent effects of CHIR on anterior-posterior (A–P) patterning of the progenitors by treating the cells from day 1 to 12 and analyzing at day 18. With increasing CHIR concentrations (0.1–0.4 uM) the proportion of Otx2/En1+ cells increased to 76.5±3.5% whereas the Otx2+/En1- population, indicative of forebrain identity, decreased. With further increase in CHIR concentrations (from 0.6 uM), the percentage of Otx2/En1+ cells began to decrease. At 1 uM CHIR, 65.3±6.1% of the total cells expressed En1 but only 6% of the cells were positive for both Otx2 and En1 (Fig. 2C). The En1 positive but Otx2 negative population is suggestive of hindbrain characteristics. These results indicate that CHIR patterns the precursors from anterior to posterior in a dose-dependent manner and that a narrow range of CHIR concentrations (0.2–0.6 uM) is necessary to pattern the differentiating PSCs to the midbrain fate.
In the midst of the manuscript preparation, Studer and colleagues reported that treatment with 3 uM of CHIR from day 3 to 12 induces robust DA neuronal differentiation under the very similar condition [21]. To discern the discrepancy, we further increased the CHIR concentrations in our culture system. Under the cultures treated with 2 or 3 uM of CHIR, few cells expressed En1 (less than 3% in 2uM CHIR treated group) and nearly all the cells expressed HoxA2 and HoxB1, especially in the cultures treated with 3 uM CHIR (Supplementary Fig. 1C), transcription factors largely expressed in the hindbrain [30]. Similar to the time-dependent study (Fig. 2B), treatment of the cultures with 2 or 3 uM of CHIR from day 3 or day 6 did not significantly alter the expression of FoxG1, indicating an anterior identity of the progenitors. Collectively, our results indicate that not only a specific CHIR concentration but also a particular window of CHIR application is necessary to pattern the differentiating PSCs to the midbrain fate.
Coordinated action of SHH and CHIR induces robust midbrain floor plate progenitors
The prior sets of experiments demonstrate that specific concentrations of SHH and CHIR induce the ventral and midbrain fate, respectively. We therefore hypothesize that coordinated application of both may induce the midbrain floor plate progenitors. By treating the cultures for 12 days with 500 ng/ml SHH and 0.4uM CHIR (Fig. 3A), we found that the vast majority of the cells were FoxA2 positive (92.6±3.8%). In contrast to the cultures treated with SHH alone, very few Nkx6.1 positive cells were detected (4.3±1.7%) and the FoxA2 cells rarely (<2%) co-labeled with Nkx6.1 (Fig. 3B). The floor plate marker, corin, was highly expressed in most cells (86%) and Lmx1a was now induced in the FoxA2 cells (Fig. 3C). Importantly, the majority of FoxA2 and corin cells co-expressed En1, Otx2, Lmx1a, and nestin at D18 (Fig. 3D, Supplementary Fig. 2A and 2B). These results indicate that coordinated action of SHH and CHIR induces a progenitor pool with real midbrain floor plate characteristics. The midbrain floor plate progenitors also can be induced in a human iPSC line (Supplementary Fig. 3A).
Fig. 3. Coordinated action of SHH and CHIR induces robust midbrain floor plate progenitors.
(A) Experimental paradigm showing differentiation of midbrain floor plate cells by combined treatment with SHH and CHIR99021 from D1–12. (B) Immunostaining and quantification of FoxA2 (red) and Nkx6.1 (green) cells at day 18 after treatment with varying concentrations of SHH and 0.4 uM of CHIR. (C) Immunostaining and quantification of Corin (red) as well as FoxA2/Lmx1a cells at day 18 after treatment with SHH with or without CHIR. (D) Immunostaining and quantification of FoxA2 (blue) that carry other midbrain floor plate related markers. Data presented in B, C, D: mean ± s.e.m., n=3 (independent experiments). Bar =50um
FGF8 promotes dopaminergic differentiation of the midbrain floor plate progenitors
To initiate neurogenesis of the midbrain floor plate progenitors, we first withdrew SHH and CHIR from D13 and examined the expression of pro-neural genes Ngn2 and Mash1 which are expressed by human midbrain DA progenitors [31]. Examination at day 22 revealed that 36.4% of the cells expressed Ngn2 and 32.3% of these cells co-expressed Mash1 (Supplementary Fig. 2C). Further differentiation for 2 weeks (Fig. 4A) resulted in generation of βIII-tubulin+ neurons (41.7%). Nevertheless, only 8.3% of the neurons were positive for TH positive. Similarly, the expression of a dopaminergic transcription factor Nurr1 was low (Fig. 4B). These results suggest that while the midbrain floor progenitors can become neurons upon removal of the morphogens, they do not automatically become dopaminergic neurons.
Fig. 4. FGF8 promotes dopaminergic differentiation of the midbrain floor plate progenitors.
(A) Experimental paradigm showing differentiation of midbrain floor plate progenitors and DA neurons. (B) Immunostaining for and quantification of FoxA2 cells that express Nurr1 at day 28 or TH/Tuj at day 35 after treatment with or without FGF8. Bar =50um
FGF8 has been traditionally regarded as a factor for patterning the midbrain (isthmus) identity [32–34] but it has also been suggested to instruct the proliferative progenitors to acquire a dopaminergic phenotype [35]. We hypothesize that FGF8 promotes the dopaminergic fate of the specified midbrain floor plate progenitors. By addition of FGF8 (100 ng/ml) after removal of CHIR and reduction of SHH (to 20 ng/ml) from day 13–28, we observed a significant increase in the proportion of Nurr1-FoxA2 positive cells (86.7±8.7%) as compared to the cultures without FGF8 treatment at day 28 (Fig. 4B). Indeed, large proportions (58.7±5.4%) of the differentiated neurons were positive for TH and FoxA2 at day 36 as compared to the control cultures without FGF8 treatment (Fig. 4B). As an additional control, we found that in cultures treated with 1.0uM CHIR and SHH, in which the vast majority of cells co-expressed FoxA2 and En1 but not Otx2 (Supplementary Fig. 1D), only 8.9±2.7% of total cells were positive for TH and 17.4±3.7 were 5-HT positive neurons (Supplementary Fig. 1E, F) even in the presence of FGF8. Thus, FGF8 promotes the dopaminergic differentiation of the midbrain floor plate progenitors but not more caudal progenitors.
Human PSC-derived neurons exhibit midbrain DA neuron characteristics
After 5 weeks of differentiation (day 35), 43.6±6.2% of total cells became positive for TH (Fig. 5A). Nearly all these TH-positive neurons co-expressed FoxA2 (96.7±1.8%), Lmx1a (96.5±2.3%), Lmx1b (93.4±3.4%), Nurr1 (95.3±2.4%), En1 (96.2±1.1%), and FoxP1 (87.3±4.7%) (Fig. 5A, B). This result was reproduced in an iPSC line (IMR90-4) (Supplementary Fig. 3B). Furthermore, many of these TH positive cells expressed Girk2 (56.3±6.7%) and ALDH1a1 (61.4±4.8%) (Fig. 5A, B), markers that are relatively enriched in A9 midbrain DA neurons [36–39]. These TH/Girk2 co-labeled cells also expressed En1 (Fig. 5A), validating the A9 identity of these DA neurons. In addition, these TH positive neurons expressed synapsin (Fig. 5A), a membrane glycoprotein essential for synapse formation. Few 5-HT (2.4±1.2%) and GABA (5.3±2.7%) positive neurons were observed (Supplementary Fig. 1E–G). About 15% cells remain positive for nestin at this stage (Supplementary Fig. 1H), suggestive of neural progenitors. Together, these results strongly suggest that the DA neurons generated under the particular protocol are indeed of the midbrain identity and many of them carry A9 DA neuron characteristics.
Fig. 5. Human PSC-derived neurons exhibit midbrain DA neuron characteristics.
(A) Immunostaining of day 42 cultures with markers for midbrain DA neurons and Synapsin (day 52). The inset is magnified in upper-left of the synapsin figure. (B) Quantification of the data presented in A. (C) DA neurons after 10 weeks of culture display spontaneous action potential spiking that is accompanied by a sub-threshold oscillatory potential (− 35 mV). (D) Immunostaining shows the expression of TH and En1 in the biocytin-labeled, recorded neuron (arrow). Bar =50um
Electrophysiological recording at 10 weeks of cultures indicated that among the 12 neurons patched, the mean cell capacitance (Cap) was 47.41 ± 7.27 pF, resting membrane potential (RMP) −54 ± 6 mV, and the spontaneous action potential frequency (Spon. APs Freq.) 2.64 ± 0.15 Hz (Supplementary Fig. 4A). Both inward Na+ and outward K+ currents were observed in these cells by voltage clamp (Supplementary Fig. 4B). Action potentials were triggered by injection of current steps from − 60 pA to + 60 pA (Supplementary Fig. 4C). Importantly, the DA neurons displayed spontaneous action potential spikes (Fig. 5C), and this spiking was accompanied by a slow, sub-threshold oscillatory potential (− 35 mV). This electrophysiological property is consistent with characteristics of immature midbrain DA neurons in vivo [40, 41]. Postsynaptic spontaneous currents were observed, and they were partially blocked by CNQX and further fully eliminated by combination of CNQX and bicuculline (Supplementary Fig. 4D), revealing both excitatory (glutamatergic) and inhibitory (GABAergic) neurotransmission inputs, respectively. Immunocytochemical analysis of biocytin-labeled cells confirmed the midbrain DA neuronal identity with expression of both TH and En1 (Fig. 5D). Therefore, our electrophysiological data further confirm the midbrain DA neuronal identity.
Midbrain DA neurons are induced from rhesus monkey iPSCs
To further evaluate the general applicability of the strategy described above, we treated the rhesus monkey iPSCs with SHH (500 ng/ml) and CHIR (0.4 uM) from D1–16 using a differentiation protocol modified from human cell cultures (Fig. 5A). On D21, we observed that rhesus iPSCs were efficiently patterned to the midbrain floor plate progenitors that expressed FoxA2, Corin, Otx2, En1, and Lmx1a (Supplementary Fig. 5B, D). Further differentiation of these progenitors resulted in generation of TH+ DA neurons that were also positive for FoxA2, En1, Lmx1a, and Nurr1 (Supplementary Fig. 5C, E). Thus, the strategy developed in the present study can efficiently induce human and monkey iPSCs to midbrain DA neurons.
DISCUSSION
Significant efforts have been made to differentiate human ESCs and iPSCs to midbrain DA neurons with numerous reports on generation of DA neurons following treatments with SHH and FGF8 [7–13, 42–44]. While there are indeed TH-expressing DA neurons, these DA neurons are mostly non-midbrain DA neurons, at least they do not carry the basic midbrain markers, including En1. It is known for a while that Wnt activation is critical for patterning the mid-hind brain fate during embryonic development [5, 45]. Nevertheless, non-genetic means were not very effective in activating the wnt pathway until the identification of a small molecule inhibitor of GSK3β (that activates wnts) CHIR99021 [18, 19]. Indeed, our present finding, as well as a recent report [21], indicates that CHIR can effectively pattern the primate (human and non-human) primitive neuroepithelia to the mid-hind brain progenitors in a dose-dependent manner. Importantly, our present study reveals that a very narrow concentration range of CHIR at a particular developmental stage is necessary for inducing the midbrain fate. Too high or too low concentrations of CHIR will result in either hindbrain or forebrain (diencephalons) identities, respectively. Furthermore, the midbrain floor plate progenitors induced by CHIR and SHH do not automatically become DA neurons and FGF8 promotes the dopaminergic identity of the differentiated neurons. Realization of the fundamental principles of midbrain DA neuron development and nuances in morphogen applications has led to the production of true midbrain DA neurons from human and monkey PSCs, as evidenced by cellular and electrophysiological features of the DA neurons in the present study.
Induction of midbrain floor plate cells is a prerequisite for generating midbrain DA neurons. Floor plate cells express FoxA2 and high concentrations of SHH can efficiently induce human PSCs to FoxA2-expressing cells [20, 46], as also shown in our present study. We found, however, that two-thirds of the FoxA2-expressing cells are not floor plate cells as they co-express Nkx6.1, transcription factor expressed by progenitors in the basal plate [47, 48], but not corin, a protein that is more restricted to midline floor plate cells [1]. Nkx6.1 suppresses floor plate cell differentiation as indicated by the loss of corin expression when Nkx6.1 is expressed in the entire neural tube under the Nestin enhancer [6]. Hence, repression of Nkx6.1 will enhance proper floor plate differentiation, and in the midbrain Lmx1a is known to inhibit Nkx6.1 [3, 6]. Indeed, when Lmx1a is induced by CHIR, most of the FoxA2+ cells no longer express Nkx6.1 and they now express Corin (Fig. 3C). Wnt1 (activated by CHIR) and Lmx1a form an autoregulatory feedback loop [4]. In culture, a wide concentration range of CHIR99021 (0.2 ~ 3uM) could induce Lmx1a expression in absence of SHH [21] (also our present study). Hence Lmx1a-expressing floor plate progenitors (FoxA2+) are not necessarily midbrain progenitors. Our present study demonstrates that only a very narrow concentration range of CHIR induces the midbrain fate, as revealed by co-expression of Otx2 and En1. Hence, co-expression with En1 is necessary to define the identity of midbrain floor plate progenitors in vitro. It is presently not clear why treatment with 3 uM of CHIR by Studer and colleagues resulted in robust generation of DA neurons although they did not show En1 expression in these DA neurons [21]. Otx2 represses Nkx2.2 [5], thus preventing the serotonin fate of the progenitors. Lmx1a represses Nkx6.1 via Msx1 [1, 3], thus preventing the alternative differentiation of the progenitors toward red nucleus and oculomotor neurons. This is also evident in our present study that few 5-HT cells were observed under our protocol. Together, the specific concentration of CHIR induces the floor plate progenitors (FoxA2+/Corin+) to express Lmx1a, En1, and Otx2, thus restricting the progenitors to the DA neuronal progenitor fate.
One would expect that these transcriptionally restricted midbrain floor plate progenitors will become DA neurons automatically. Indeed, we observed some midbrain DA neurons but the majority of the progenitors do not express TH (Fig. 4B). This result seems to contradict the observations made in vivo [1, 3]. Joksimovic et al reported that floor plate cells expressing Otx2 and Lmx1a, induced by stabilizing β-catinin, generate DA neurons only in the hindbrain but not in the spinal cord [6]. One major difference between the hindbrain and the spinal cord is the presence of FGF8 in the hindbrain (isthmus) but not the spinal cord [49, 50], suggesting that FGF8 enables midbrain floor plate progenitors to acquire a dopaminergic fate. Indeed, we found that FGF8 significantly increased the expression of Nurr1 and production of TH-expressing DA neurons. Thus, FGF8 is important in promoting the dopaminergic fate of the midbrain floor plate progenitors in culture. As discussed above, our progenitors are largely restricted to the midbrain fate by a particular concentration of CHIR. FGF8 promotes the dopaminergic fate from midbrain floor plate progenitors but not diencephalic or hindbrain progenitors [35]. That explains why a large population of our progenitors (86%) becomes positive for Nurr1 and FoxA2 at day 28. This is also evident from our present observation that FGF8 does not enhance the dopaminergic fate of the progenitors of hindbrain/spinal cord that are patterned with high concentrations of CHIR. Due to the presence of nestin positive neural progenitors that proliferate in subsequent cultures whereas Nurr1-expressing cells divide minimally, the TH+ DA neuron proportion is lower than 86%. This phenomenon is similar to a previous report that Corin+ cells sorted from the mesencephalic floor plate at E13.5 rat embryo express Nurr1 (85–90%), but only 53.6±3.4% of the neurons are TH positive in additional 6 days of differentiation [1]. Still, if one human PSC is differentiated for 6 weeks, we estimate that approximately 300 midbrain DA neurons are generated. Collectively, our in vitro differentiation of human PSCs faithfully recapitulates the midbrain DA neuron specification process. We have recently shown that appropriately specified neuronal progenitors from hESCs project axons toward their specific targets in a long distance in the adult mouse brain [51]. The authentic midbrain DA neurons generated in the present study now presents a golden opportunity to address if they are truly capable to projecting to the striatum when placed in the midbrain. The efficient generation of midbrain DA neurons from monkey iPSCs opens an unprecedented opportunity to address personalized regenerative therapy in non-human primates in a long term.
CONCLUSION
Our present study demonstrates clearly that coordination of SHH and a particular concentration of CHIR99021 at an early stage can effectively pattern both human and monkey neuroepithelia to the midbrain floor plate progenitors. FGF8 enables the dopaminergic fate of the progenitors. The DA neurons generated in this way carry most of the known midbrain DA neuron characteristics, including co-expression of TH with FoxA2, Lmx1a and En1, as well as characteristic electrophysiological properties. This new method allows generation of true midbrain DA neurons for disease modeling, drug testing, and potential cell therapy. Technically, our results also indicate that a reliable marker for floor plate cells, especially in vitro, should be positive for both FoxA2 and corin but not Nkx6.1. To be midbrain floor plate progenitors, these cells should additionally carry Otx2 and En1 but not forebrain or hindbrain markers.
Supplementary Material
Acknowledgments
This study was supported in part by the NIH-NINDS (NS045926), the Parkinson’s Disease Foundation, Center for Stem Cells and Regenerative Medicine at the University of Wisconsin Madison, the NICHD (P30 HD03352), National natural science foundation of China (30901615), and NIH-NCRR grant P51 RR000167 (Wisconsin National Primate Research Center, University of Wisconsin-Madison).
Footnotes
Author Contribution: J.X.: conception and design, collection and/or assembly of data, data analysis and interpretation, and manuscript writing; Y.L., H.L. and H.C.: collection and/or assembly of data and data analysis and interpretation; M.E.E.: collection of non-human primate data; and S.-C.Z.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript.
References
- 1.Ono Y, et al. Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development. 2007;134(17):3213–25. doi: 10.1242/dev.02879. [DOI] [PubMed] [Google Scholar]
- 2.Placzek M, Briscoe J. The floor plate: multiple cells, multiple signals. Nat Rev Neurosci. 2005;6(3):230–40. doi: 10.1038/nrn1628. [DOI] [PubMed] [Google Scholar]
- 3.Andersson E, et al. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124(2):393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 4.Chung S, et al. Wnt1-lmx1a forms a novel autoregulatory loop and controls midbrain dopaminergic differentiation synergistically with the SHH-FoxA2 pathway. Cell Stem Cell. 2009;5(6):646–58. doi: 10.1016/j.stem.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prakash N, et al. A Wnt1-regulated genetic network controls the identity and fate of midbrain-dopaminergic progenitors in vivo. Development. 2006;133(1):89–98. doi: 10.1242/dev.02181. [DOI] [PubMed] [Google Scholar]
- 6.Nakatani T, et al. Lmx1a and Lmx1b cooperate with Foxa2 to coordinate the specification of dopaminergic neurons and control of floor plate cell differentiation in the developing mesencephalon. Dev Biol. 2010;339(1):101–13. doi: 10.1016/j.ydbio.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Perrier AL, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101(34):12543–8. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Gomez JA, et al. Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells. 2007;25(4):918–28. doi: 10.1634/stemcells.2006-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonntag KC, et al. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells. 2007;25(2):411–8. doi: 10.1634/stemcells.2006-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan Y, et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23(6):781–90. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang D, et al. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells. 2008;26(1):55–63. doi: 10.1634/stemcells.2007-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper O, et al. Differentiation of human ES and Parkinson’s disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol Cell Neurosci. 2010;45(3):258–66. doi: 10.1016/j.mcn.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy NS, et al. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12(11):1259–68. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 14.Jaeger I, et al. Temporally controlled modulation of FGF/ERK signaling directs midbrain dopaminergic neural progenitor fate in mouse and human pluripotent stem cells. Development. 2011;138(20):4363–74. doi: 10.1242/dev.066746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinat C, et al. Cooperative transcription activation by Nurr1 and Pitx3 induces embryonic stem cell maturation to the midbrain dopamine neuron phenotype. Proc Natl Acad Sci U S A. 2006;103(8):2874–9. doi: 10.1073/pnas.0511153103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai J, et al. The role of Lmx1a in the differentiation of human embryonic stem cells into midbrain dopamine neurons in culture and after transplantation into a Parkinson’s disease model. Stem Cells. 2009;27(1):220–9. doi: 10.1634/stemcells.2008-0734. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Danes A, et al. Efficient Generation of A9 Midbrain Dopaminergic Neurons by Lentiviral Delivery of LMX1A in Human Embryonic Stem Cells and Induced Pluripotent Stem Cells. Hum Gene Ther. 2011;23(1):56–69. doi: 10.1089/hum.2011.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying QL, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453(7194):519–23. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ring DB, et al. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes. 2003;52(3):588–95. doi: 10.2337/diabetes.52.3.588. [DOI] [PubMed] [Google Scholar]
- 20.Fasano CA, et al. Efficient derivation of functional floor plate tissue from human embryonic stem cells. Cell Stem Cell. 2010;6(4):336–47. doi: 10.1016/j.stem.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Kriks S, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480(7378):547–51. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 23.Hu BY, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A. 2010;107(9):4335–40. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Chambers SM, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–80. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez-Balaguer A, et al. Shh dependent and independent maintenance of basal midbrain. Mech Dev. 2009;126(5–6):301–13. doi: 10.1016/j.mod.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Puelles E. Genetic control of basal midbrain development. J Neurosci Res. 2007;85(16):3530–4. doi: 10.1002/jnr.21363. [DOI] [PubMed] [Google Scholar]
- 28.Nordstrom U, Jessell TM, Edlund T. Progressive induction of caudal neural character by graded Wnt signaling. Nat Neurosci. 2002;5(6):525–32. doi: 10.1038/nn0602-854. [DOI] [PubMed] [Google Scholar]
- 29.Muhr J, et al. Convergent inductive signals specify midbrain, hindbrain, and spinal cord identity in gastrula stage chick embryos. Neuron. 1999;23(4):689–702. doi: 10.1016/s0896-6273(01)80028-3. [DOI] [PubMed] [Google Scholar]
- 30.Irving C, Mason I. Signalling by FGF8 from the isthmus patterns anterior hindbrain and establishes the anterior limit of Hox gene expression. Development. 2000;127(1):177–86. doi: 10.1242/dev.127.1.177. [DOI] [PubMed] [Google Scholar]
- 31.Nelander J, Hebsgaard JB, Parmar M. Organization of the human embryonic ventral mesencephalon. Gene Expr Patterns. 2009;9(8):555–61. doi: 10.1016/j.gep.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Guo Q, et al. Fgf8b-containing spliceforms, but not Fgf8a, are essential for Fgf8 function during development of the midbrain and cerebellum. Dev Biol. 2009;338(2):183–92. doi: 10.1016/j.ydbio.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato T, Joyner AL, Nakamura H. How does Fgf signaling from the isthmic organizer induce midbrain and cerebellum development? Dev Growth Differ. 2004;46(6):487–94. doi: 10.1111/j.1440-169x.2004.00769.x. [DOI] [PubMed] [Google Scholar]
- 34.Wurst W, Bally-Cuif L. Neural plate patterning: upstream and downstream of the isthmic organizer. Nat Rev Neurosci. 2001;2(2):99–108. doi: 10.1038/35053516. [DOI] [PubMed] [Google Scholar]
- 35.Lahti L, et al. Cell-autonomous FGF signaling regulates anteroposterior patterning and neuronal differentiation in the mesodiencephalic dopaminergic progenitor domain. Development. 2012;139(1):894–905. doi: 10.1242/dev.071936. [DOI] [PubMed] [Google Scholar]
- 36.Mendez I, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005;128(Pt 7):1498–510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCaffery P, Drager UC. High levels of a retinoic acid-generating dehydrogenase in the meso-telencephalic dopamine system. Proc Natl Acad Sci U S A. 1994;91(16):7772–6. doi: 10.1073/pnas.91.16.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inanobe A, et al. Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J Neurosci. 1999;19(3):1006–17. doi: 10.1523/JNEUROSCI.19-03-01006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schein JC, Hunter DD, Roffler-Tarlov S. Girk2 expression in the ventral midbrain, cerebellum, and olfactory bulb and its relationship to the murine mutation weaver. Dev Biol. 1998;204(2):432–50. doi: 10.1006/dbio.1998.9076. [DOI] [PubMed] [Google Scholar]
- 40.Guzman JN, et al. Robust pacemaking in substantia nigra dopaminergic neurons. J Neurosci. 2009;29(35):11011–9. doi: 10.1523/JNEUROSCI.2519-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nedergaard S, Flatman JA, Engberg I. Nifedipine- and omega-conotoxin-sensitive Ca2+ conductances in guinea-pig substantia nigra pars compacta neurones. J Physiol. 1993;466:727–47. [PMC free article] [PubMed] [Google Scholar]
- 42.Hargus G, et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc Natl Acad Sci U S A. 2010;107(36):15921–6. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morizane A, et al. A simple method for large-scale generation of dopamine neurons from human embryonic stem cells. J Neurosci Res. 2010;88(16):3467–78. doi: 10.1002/jnr.22515. [DOI] [PubMed] [Google Scholar]
- 44.Zeng X, et al. Dopaminergic differentiation of human embryonic stem cells. Stem Cells. 2004;22(6):925–40. doi: 10.1634/stemcells.22-6-925. [DOI] [PubMed] [Google Scholar]
- 45.Joksimovic M, et al. Wnt antagonism of Shh facilitates midbrain floor plate neurogenesis. Nat Neurosci. 2009;12(2):125–31. doi: 10.1038/nn.2243. [DOI] [PubMed] [Google Scholar]
- 46.Denham M, et al. Gli1 is an inducing factor in generating floor plate progenitor cells from human embryonic stem cells. Stem Cells. 2010;28(10):1805–15. doi: 10.1002/stem.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ang SL, Rossant J. HNF-3 beta is essential for node and notochord formation in mouse development. Cell. 1994;78(4):561–74. doi: 10.1016/0092-8674(94)90522-3. [DOI] [PubMed] [Google Scholar]
- 48.Weinstein DC, et al. The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell. 1994;78(4):575–88. doi: 10.1016/0092-8674(94)90523-1. [DOI] [PubMed] [Google Scholar]
- 49.Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121(2):439–51. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- 50.Shamim H, et al. Sequential roles for Fgf4, En1 and Fgf8 in specification and regionalisation of the midbrain. Development. 1999;126(5):945–59. doi: 10.1242/dev.126.5.945. [DOI] [PubMed] [Google Scholar]
- 51.Ma L, et al. Human Embryonic Stem Cell-Derived GABA Neurons Correct Locomotion Deficits in Quinolinic Acid-Lesioned Mice. Cell Stem Cell. 10(4):455–64. doi: 10.1016/j.stem.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.