Abstract
Background
For stage IV melanoma, systemic medical therapy (SMT) is used most frequently; surgery is considered an adjunct in selected patients. We retrospectively compared survival after surgery±SMT versus SMT alone for melanoma patients developing distant metastases while enrolled in the first Multicenter Selective Lymphadenectomy Trial.
Methods
Patients were randomized to wide excision and sentinel node biopsy, or wide excision and nodal observation. We evaluated recurrence site, therapy (selected by treating clinician), and survival after stage IV diagnosis.
Results
Of 291 patients with complete data for stage IV recurrence, 161 (55%) underwent surgery±SMT. Median survival was 15.8 vs. 6.9 months and 4-year survival was 20.8% vs. 7.0% for patients receiving surgery±SMT vs. SMT alone (p<0.0001; HR 0.406). Surgery±SMT conferred a survival advantage for patients with M1a (median >60 months vs. 12.4 months; 4-year 69.3% vs. 0; p=0.0106), M1b (median 17.9 vs. 9.1 months; 4-year 24.1% vs. 14.3%; p=0.1143), and M1c (median 15.0 vs. 6.3 months; 4-year 10.5% vs. 4.6%; p=0.0001) disease. Patients with multiple metastases treated surgically had a survival advantage, and number of operations did not reduce survival in the 67 patients (42%) who had multiple surgeries for distant melanoma.
Conclusions
Our findings suggest that over half of stage IV patients are candidates for resection and exhibit improved survival over patients receiving SMT alone, regardless of site(s) and number(s) of metastases. We have begun a multicenter randomized phase III trial comparing surgery versus SMT as initial treatment for resectable distant melanoma.
Keywords: Stage IV melanoma, metastasectomy, MSLT-I, survival
INTRODUCTION
Melanoma has become one of the most expensive cancers to diagnose, treat and follow.[1] Most of this expense represents the management of distant metastatic disease. Many medical oncologists believe that once melanoma has spread through the bloodstream to a distant site, surgery is not useful because patients already have clinically relevant circulating tumor cells and subclinical occult metastases.[2,3] Thus systemic medical therapy (SMT) remains the mainstay of management for stage IV disease.[4, 5] However, in 2008 a meta-analysis by Korn et al [6] showed no significant outcome difference for different types of medical therapy. Although promising data from recent trials of targeted therapy challenge the findings of Korn’s group [7–9], currently there is no standard approach to treatment of distant melanoma or consensus on the role of surgery.
The results of several previous studies suggest that surgical resection of distant melanoma should be undertaken whenever feasible. Data from small, single-institution prospective series indicate that 5-year survival is possible in 15–28% of patients with distant metastases that can be completely resected. [10–17] The Southwestern Oncology Group’s small multicenter phase II study of surgery for stage IV melanoma found a median survival of 21 months and an estimated 4-year survival of 31%; 62 (83.8%) of 74 candidates for the study had disease that could be completely resected, and 48% had visceral metastases.[18] These multicenter trial data confirm single-center reports of the effectiveness of surgery alone in patients with stage IV melanoma.[11–22]
Serial surgical operations for metachronous metastases can control disease, but sequential combined approaches using surgery and medical therapy have not been well explored. In the present study, we hypothesized that when melanoma recurs at a distant site, complete surgical resection is the best treatment option when feasible and may offer an opportunity for cure as compared to SMT alone. We examined this hypothesis by comparing the treatment and overall survival of patients who developed distant metastases while enrolled in the first Multicenter Selective Lymphadenectomy Trial (MSLT-I; NIH Clinical Trials Identifier: NCT00275496), an international phase III trial for patients with clinically localized cutaneous melanoma.[23]
PATIENTS AND METHODS
MSLT-I Design
As previously reported, MSLT-I randomly assigned patients with primary cutaneous melanoma to two treatment arms: wide excision and sentinel node biopsy, with immediate complete lymphadenectomy for histopathologic evidence of sentinel node metastasis; or wide excision and postoperative observation of the regional nodal basin, with delayed complete lymphadenectomy on clinical evidence of regional node metastasis.[23] Patients with tumor-positive sentinel nodes or clinical evidence of nodal involvement during follow-up could receive adjuvant systemic therapy.
Patients were monitored postoperatively by clinical examination, blood tests, and chest x-rays every 3 months for 2 years, every 4 months the third year, every 6 months during years 4 and 5, and then yearly until year 10. The number of organ sites of recurrence and the number of individual clinically detectable metastases were recorded if a patient developed stage IV recurrence
MSLT-I reached its accrual goal of 2001 patients in March 2002 and is now completing follow-up.
Design of the Retrospective Study Based on MSLT-I Data
The MSLT-I database was queried for patients who developed stage IV recurrence. We excluded patients who developed locoregional recurrence without concurrent distant disease, patients who did not receive treatment for stage IV melanoma, and patients whose stage IV treatment data were missing. Treatment was categorized as surgery alone, surgery followed by SMT, SMT followed by surgery, or SMT alone. SMT was categorized as chemotherapy, biochemotherapy, biotherapy, immunotherapy, radiation therapy, or treatment with an unspecified systemic agent. Although not systemic therapy, radiation was included in this group as a non-surgical treatment for patients with stage IV melanoma recurrence.
The primary endpoint was overall survival after diagnosis of stage IV recurrence. Distant disease-free interval (DDFI) was the interval between initial diagnosis of melanoma and first stage IV recurrence. The number of distant metastases and the number of distant recurrence organ sites at the first stage IV recurrence were evaluated. Progression-free survival (PFS) was the interval between surgical treatment for initial stage IV recurrence and diagnosis of subsequent recurrence.
Survival estimates were evaluated using the Kaplan Meier method. Univariate and multivariate analyses were performed. Chi-square tests were used to evaluate the relationship between DDFI quartiles and surgery. P values and hazard ratios for survival were calculated using Cox proportional hazard models. All reported p values are two sided, with a value of less than 0.05 considered to indicate statistical significance. Analyses were performed with the use of SAS software, version 9.1.
RESULTS
Multicenter Trial Cohort
Follow-up data were available for 1970 patients from MSLT-I: 397 of 1970 (20.1%) patients developed stage IV recurrence; 291 of the 397 (73.3%) had detailed records of treatment for stage IV recurrence.
Demographics for the 291 patients who were treated for distant melanoma metastases revealed that 63% were male and 68% were under the age of 60 years. Nearly all patients were Caucasian. Most patients had high-risk primary melanomas: Clark level IV (63%) and Breslow thickness 1.2−3.5 mm (65%) (Table 1). Of the 291 patients, 32 (10%) had M1a disease, 49 (17%) had M1b disease and 210 (73%) had M1c disease. Median survival and 4-year overall survival rate of all patients treated for stage IV disease were 11.6 months and 14.4%, respectively. The 106 patients without treatment information had a median survival of 3.5 months and 4-year overall survival rate of 13.6%.
Table 1.
Patients treated for stage IV melanoma recurrence while enrolled in MSLT-I.
| Factor | ||
|---|---|---|
| Sex | N | % |
| Female | 107 | 37 |
| Male | 184 | 63 |
| Age | ||
| <60 years | 197 | 68 |
| 60+ years | 94 | 32 |
| Primary Site | ||
| Extremity | 80 | 28 |
| Non-Extremity | 172 | 59 |
| Other | 39 | 13 |
| Unknown | 0 | 0 |
| Clark Level | ||
| II | 0 | 0 |
| III | 91 | 31 |
| IV | 182 | 63 |
| V | 18 | 6 |
| Unknown | 0 | 0 |
| Breslow thickness | ||
| <1.2 mm | 15 | 5 |
| 1.2–3.5 mm | 189 | 65 |
| >3.5 mm | 87 | 30 |
| Race | ||
| Caucasian | 290 | 99.6 |
| Other | 1 | 0.4 |
| Treatment for stage IV recurrence | ||
| Surgery only | 43 | 15 |
| Surgery, then systemic medical therapy | 85 | 29 |
| Systemic medical therapy, then surgery | 33 | 11 |
| Systemic medical therapy only | 130 | 45 |
Stage IV recurrence involved only one organ in 242 (83%) patients, two organs in 35 (12%) patients, and three or more separate organs in 14 (5%) patients. Of these, 226 (78%) patients had only one solitary distant metastasis, 43 (15%) had two solitary metastases, and 22 (7%) had three or more solitary metastases.
Treatment Profile
Of patients treated for stage IV recurrence, 43 (15%) received surgery alone, 85 (29%) received surgery followed by SMT, 33 (11%) received SMT followed by surgery, and 130 (45%) received SMT alone (Table 1). Of the 248 patients whose treatment included SMT, 138 (44%) received chemo/biochemotherapy, 80 (32%) received radiation, 18 (7%) received immunotherapy, and 8 (3%) received biotherapy; in 4 (2%) patients the details of SMT were not available. Among patients who received SMT before surgery, median interval between the two types of treatment was 241 days. The most frequent type of SMT administered before surgery was chemo/biochemotherapy (n=24, 73%).
Surgery was used for treatment of recurrence in 161 (55%) patients; of these 161 patients, 67 (42%) had more than one operation. As expected, use of surgery was inversely related to the number of distant organ sites of recurrence; surgical resection was all or part of the treatment plan for 60%, 40%, and 21% patients with 1, 2, and ≥3 organ sites of recurrence, respectively (p=0.0031). Corresponding non-surgical therapy rates were 40%, 60%, and 79%, respectively, for patients with the same number of tumor-involved organ sites (Table 2). Surgical complication rates for stage IV recurrences were not available. In the 161 patients treated surgically there were 4 deaths (2.5%) within 30 days after surgery.
Table 2.
Median survival (months) from diagnosis of stage IV recurrent melanoma in MSLT-I patients, based on treatment, number of organs involved, and number of individual metastases.
| Organ sites with metastases (N) |
Surgery | Non-surgical | P value | ||
|---|---|---|---|---|---|
| Median Survival (N) |
Percentage | Median Survival (N) |
Percentage | ||
| 1 (242) | 17.6 (144) | 60% | 7.5 (98) | 40% | <0.0001 |
| 2 (35) | 13.4 (14) | 40% | 6.6 (21) | 60% | 0.056 |
| ≥3 (14) | 4.5 (3) | 21% | 4.8 (11) | 79% | 0.36 |
|
Individual metastases |
|||||
| 1 (226) | 16.3 (134) | 59% | 7.5 (92) | 41% | <0.0001 |
| 2(43) | 17.1 (20) | 47% | 6.9 (23) | 53% | 0.0364 |
| ≥3 (22) | 13.9 (7) | 32% | 5.2 (15) | 68% | 0.085 |
Survival and Recurrence Outcomes
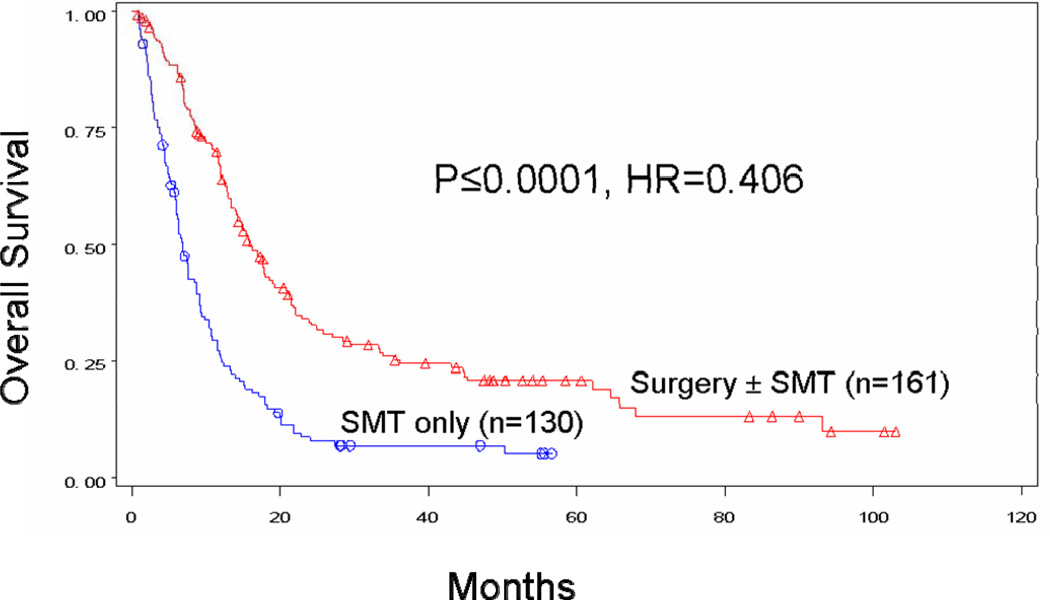
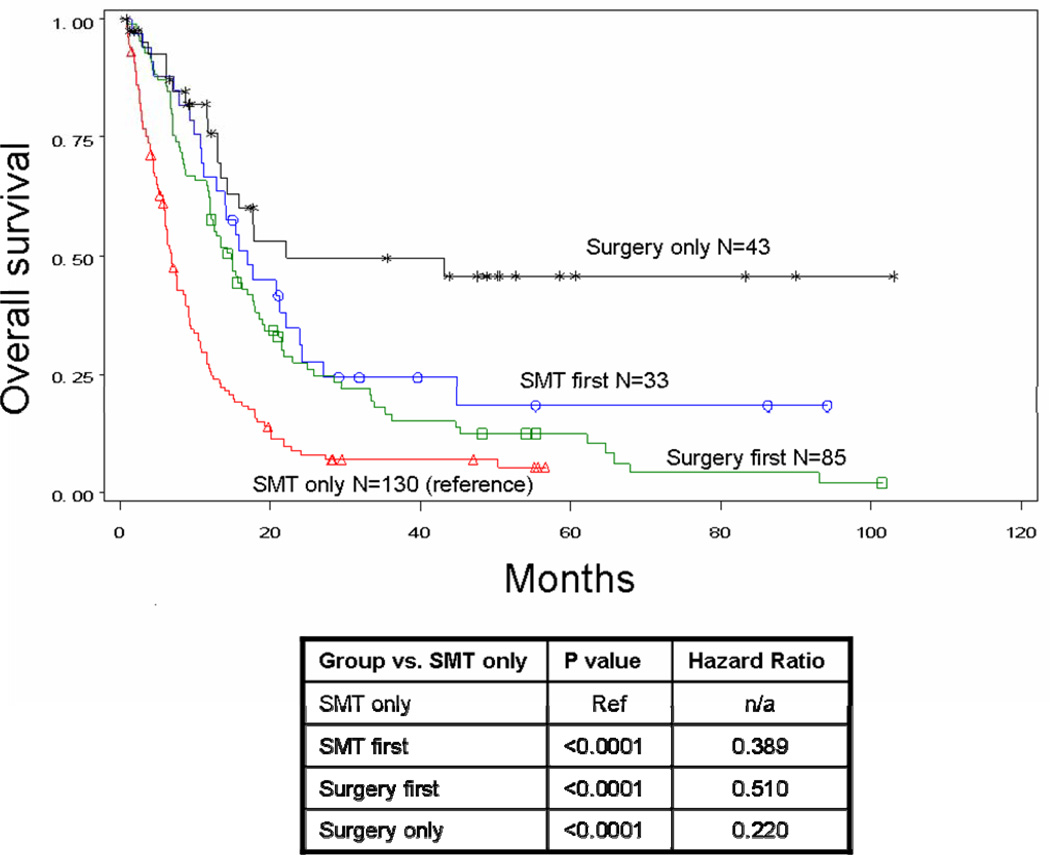
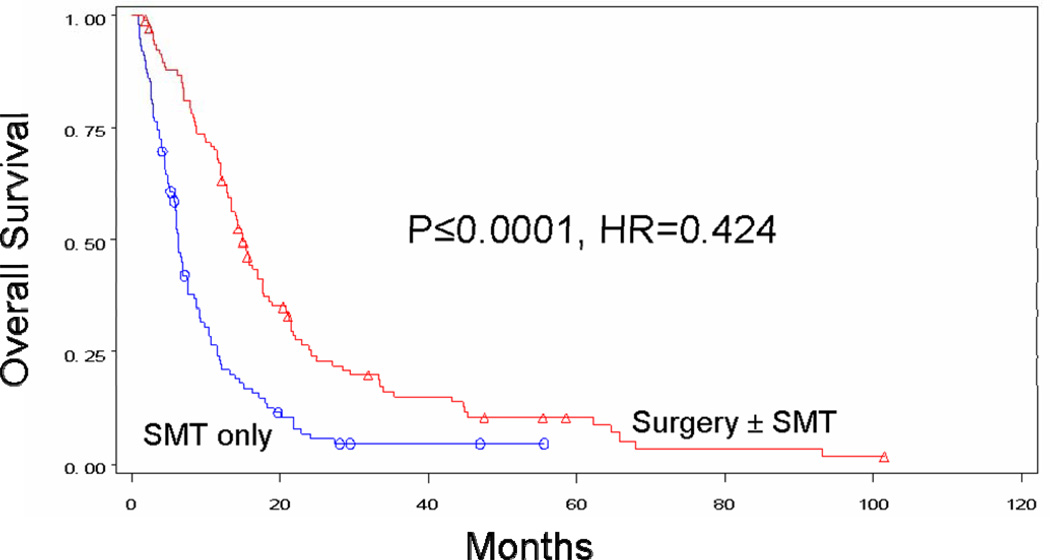
Median survival after stage IV diagnosis was 15.8 months for patients who received surgery at any point during treatment for their distant metastases and only 6.9 months for patients who received SMT alone (p<0.0001) (Figure 1A). When the surgery group was further evaluated, median survival was 22.1 months for patients who received surgery alone (p<0.0001), 17.1 months for patients who received SMT followed by surgery (p<0.0001), and 14.7 months for those who underwent surgery followed by SMT (p<0.0001). Four-year survival was 20.8% for all patients with stage IV recurrence treated surgically and 45.7%, 18.2%, and 12.3% for the respective surgical subgroups. Four-year survival for patients treated without surgery was only 7%. Order of therapy did not influence survival for patients treated with systemic therapy and surgery (p=0.2401) (Figure 1B).
Figure 1.
Figure 1A. Overall survival for patients whose recurrent stage IV melanoma was treated with surgery ± SMT (n=161, median survival 15.8 months, 4-year survival 20.8%) versus systemic medical therapy alone (n=130, median survival 6.9 months, 4-year survival 7.0%).
Figure 1B. Overall survival for patients by treatment received: surgery only (n=43, median survival 22.1 months, 4-year survival 45.7%), systemic medical therapy followed by surgery (n=33, median survival 17.1 months, 4-year survival 18.2%), surgery followed by systemic medical therapy (n=85, median survival 14.7 months, 4-year survival 12.3%), or systemic medical therapy alone (n=130, median survival 6.9 months, 4-year survival 7.0%). P value <0.0001.
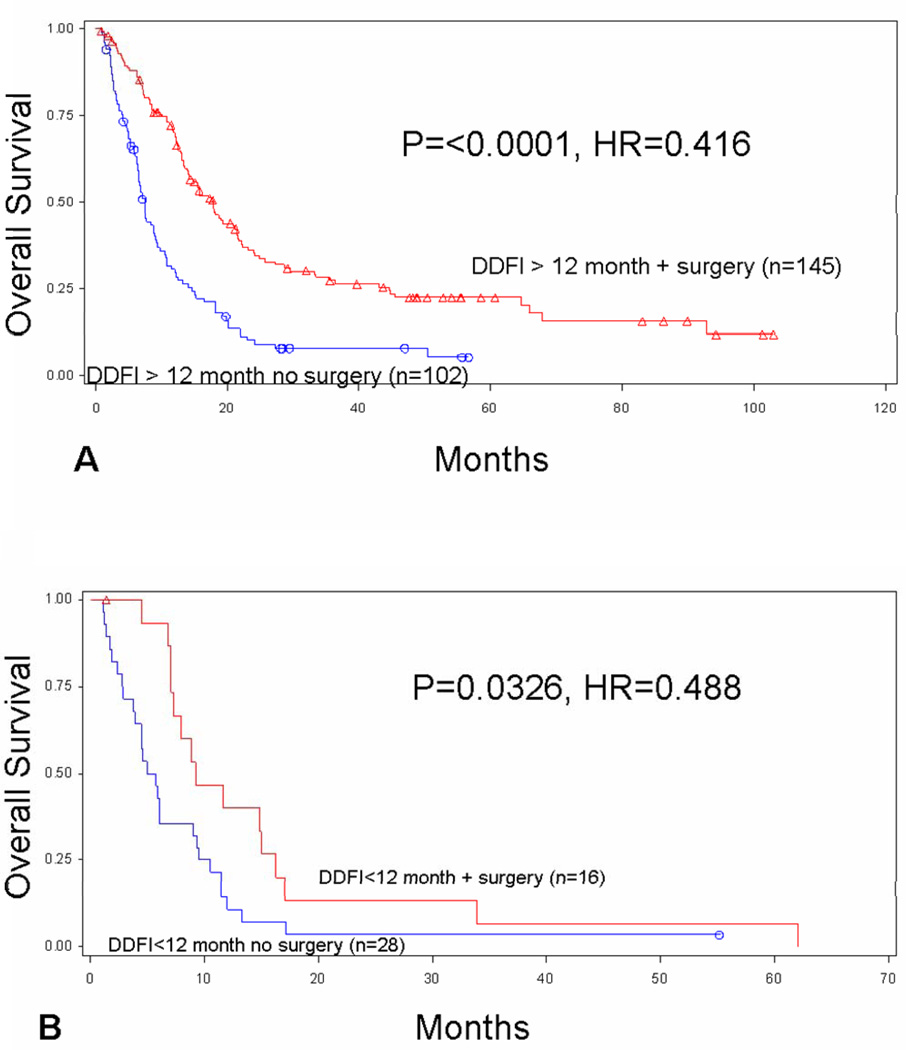
The median interval between initial diagnosis of melanoma and development of stage IV recurrence (DDFI) was 29.7 months. Survival after stage IV recurrence was associated with a longer DDFI (≥12 months) for all patients (p=0.0001). Patients with a longer DDFI were more likely to undergo surgical treatment (p=0.0296). There was a difference in survival for patients who received surgery after a short (<12 month) versus long (≥12 month) DDFI (p=0.0093), but surgery was associated with improved survival for patients with both short and long DDFI. Patients who developed distant recurrence after at least 12 months had improved median survival (17.8 vs. 7.4 months) if they were treated with surgery vs. SMT alone (p<0.0001) (Figure 2A). Patients who recurred in less than 12 months also had improved median survival (9.3 vs. 5.4 months) when treated with surgery vs. SMT alone (p=0.0326) (Figure 2B).
Figure 2.
Overall survival based on distant disease-free interval (DDFI). Overall survival is compared for patients with (A) long (≥12 months) and (B) short (<12 months) DDFI by use of surgery vs. systemic medical therapy alone for treatment of stage IV melanoma.
Median PFS and 4-year rate of PFS after surgical treatment for stage IV recurrence were 7.6 months and 18.0%, respectively.
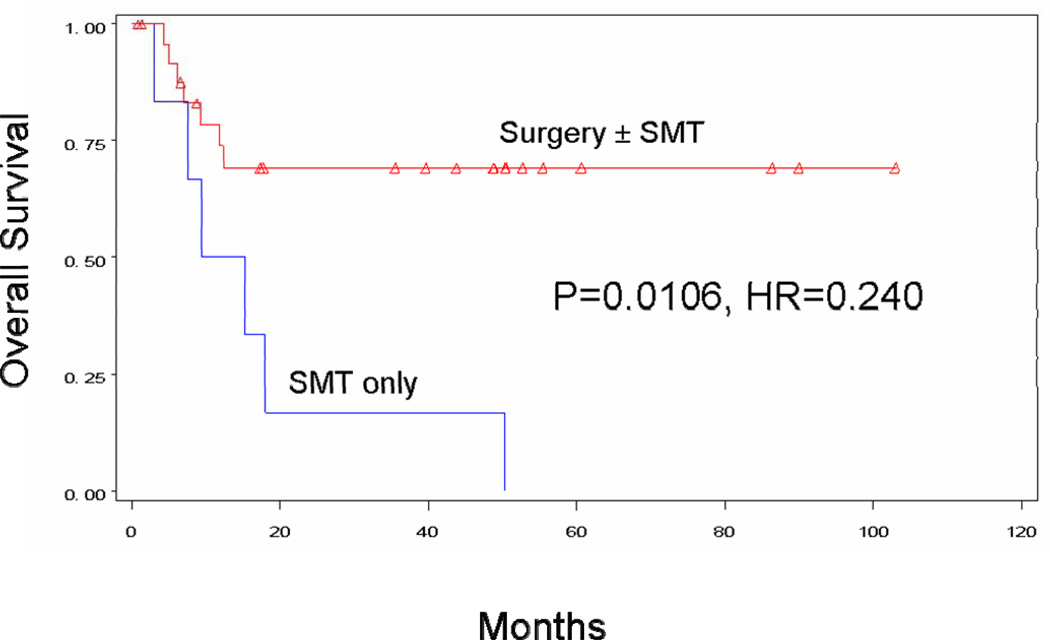
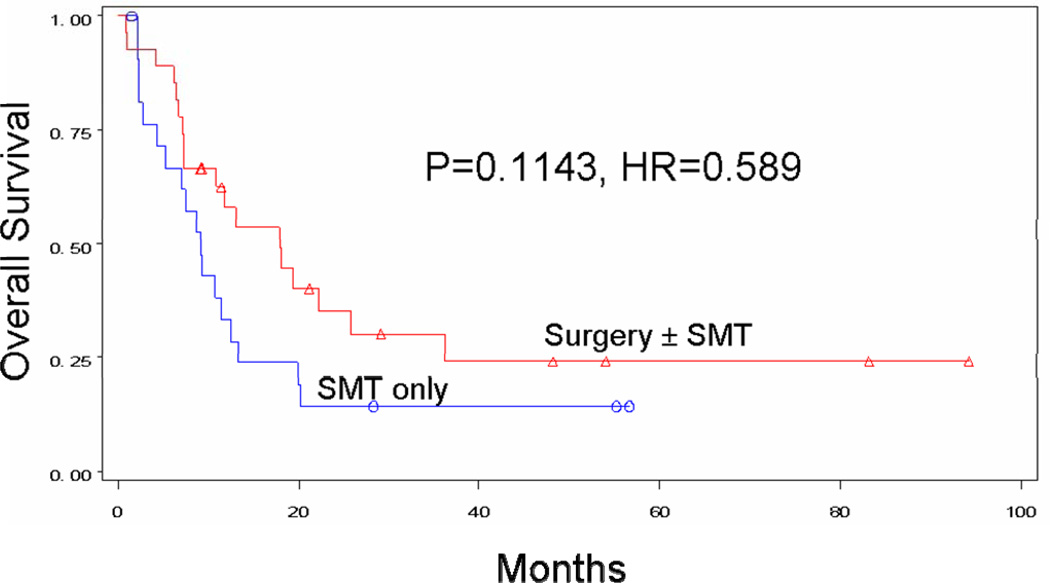
Surgical treatment was associated with improved survival for any M category. Patients with M1a metastases had a median survival of >60 months with surgery±SMT, versus 12.4 months with SMT alone (p=0.0106). Four-year survival was 69.3% for surgical patients and 0.0% for non-surgical patients (Figure 3a). For the M1b group, median survival was 17.9 months with surgery±SMT versus 9.1 months with SMT alone (p=0.1143). Four-year survival was 24.1% for surgical patients and 14.3% for nonsurgical patients (Figure 3b). For the M1c group, median survival was 15.0 months with surgery±SMT, versus 6.3 months with SMT alone (p<0.0001). Four-year survival was 10.5% for surgical patients and 4.6% for non-surgical patients (Figure 3c).
Figure 3.
Figure 3A. Overall survival for patients with M1a recurrence treated with surgery ± SMT (n=26, median survival NA, 4-year survival 69.3%) vs. SMT alone (n=6, median survival 12.4 months, 4-year survival 0%).
Figure 3B. Overall survival for patients with M1b recurrence treated with surgery ± SMT (n=27, median survival 17.9 months, 4-year survival 24.1%) vs. SMT alone (n=22, median survival 9.1 months, 4-year survival 14.3%).
Figure 3C. Overall survival for patients with M1c recurrence treated with surgery ± SMT (n=108, median survival 15.0 months, 4-year survival 10.5%) vs. SMT alone (n=102, median survival 6.3 months, 4-year survival 4.6%).
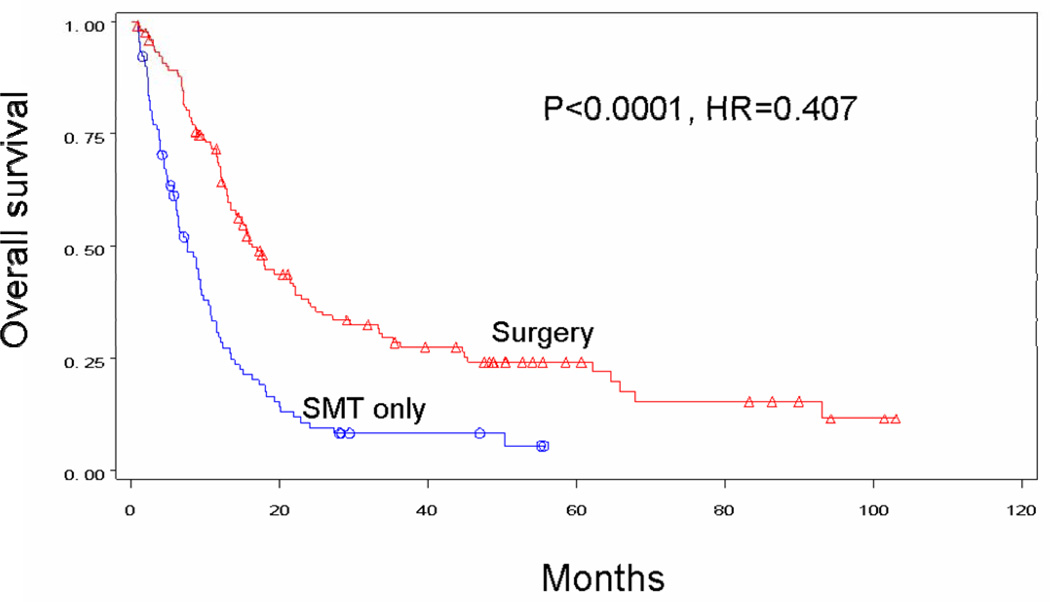
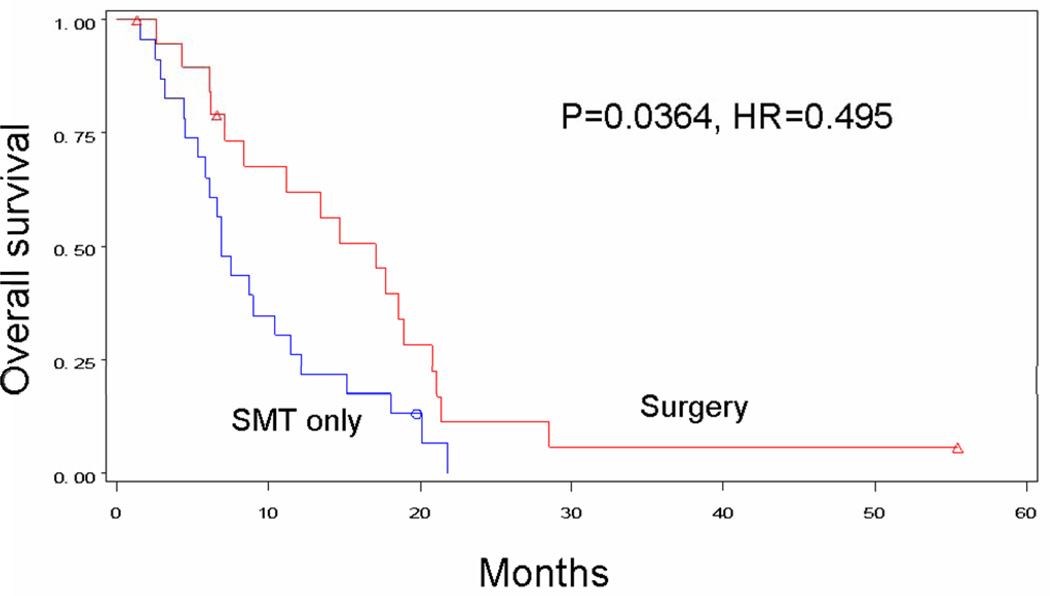
In an effort to minimize selection bias, patients with similar disease status were compared by treatment received. This included comparisons based on number of individual metastases as well as number of organ systems affected. Surgical treatment tended to be associated with improved survival regardless of the number of separate distant metastases. Median survival for patients receiving surgery±SMT versus SMT alone was 16.3 versus 7.5 months in patients with a single metastasis (p<0.0001) (Figure 4A), 17.1 versus 6.9 months in patients with two metastases (p=0.0364) (Figure 4B), and 13.9 months versus 5.2 months in patients with three metastases (p=0.0850) (Figure 4C). Interestingly, patients who had multiple operations to treat their metastases had a 21.1-month median survival and a 25.0% 4-year survival rate, as compared with 13 months and 18%, respectively, for patients receiving only one operation (p=0.0218). Survival decreased significantly for surgical patients as the number of sites of recurrence increased to two (p=0.0041) or to three or more (p=0.0001). Median survival for patients receiving surgery was 17.6 months with one organ site of distant recurrence, 13.4 months with two organ sites of recurrence, and 4.5 months with 3 or more organ sites of recurrence. By comparison, median survival for patients receiving SMT alone was only 7.5 months, 6.6 months, and 4.8 months, respectively (Table 2).
Figure 4.
Figure 4A: Overall survival for patients with stage IV recurrence of melanoma who had only one metastatic lesion treated by surgery (n=134) or SMT only (n=92).
Figure 4B: Overall survival for patients with stage IV recurrence of melanoma who had two metastatic lesions treated by surgery (n=20) or SMT only (n=23).
Figure 4C: Overall survival for patients with stage IV recurrence of melanoma who had three or greater metastatic lesions treated by surgery (n=7) or SMT only (n=15).
Factors significant in univariate analysis were used as a basis for comparing treatment groups with multivariate analysis, which revealed that treatment modality and M stage showed significance for survival. Patients treated with surgery±SMT had better survival than patients treated only with SMT (p<0.0001, HR=0.463). Patients with M1a disease showed improved survival when treated surgically (p<0.0001). Age (p=0.2465), sex (p=0.0638), and previously diagnosed stage III disease (p=0.0532) (with or without palpable lymph nodes) did not show significance for survival on multivariate analysis. A distant disease-free interval of ≥12 months was associated with improved survival. A lower number of organ sites of metastases was associated with improved survival, however the number of individual metastases was not a significant prognostic factor on multivariate analysis (Table 3).
Table 3.
Multivariate analysis for overall survival after diagnosis of distant recurrence in patients enrolled in MSLT-I
| Variable | P-value | HR |
|---|---|---|
| Male vs. Female | 0.0638 | 1.298 |
| Clinical Status of Lymph Nodes: Palpable vs. Non-palpable | 0.7960 | 0.957 |
| Age at Stage IV Diagnosis: >60 vs. ≤60 yrs | 0.2465 | 1.173 |
| Distant DFI: <12 vs. ≥12 mos | 0.0021 | 1.717 |
| Prior Stage III Disease | 0.0532 | 1.327 |
| Surgery (at any point) vs. Medical Only | <.0001 | 0.463 |
| 1–2 Organs vs. 3+ | 0.0354 | 0.338 |
| 1–2 Metastases vs. 3+ | 0.9504 | 1.027 |
| M-Stage: | ||
| M1c vs. M1a | <.0001 | 0.311 |
| M1c vs. M1b | 0.0728 | 0.714 |
DISCUSSION
The strength of this study is its size. To our knowledge, this represents the largest evaluation of multicenter prospective data from early-stage melanoma patients who were longitudinally followed up and treated for melanoma metastatic to skin, soft tissue, lung, or visceral organs. Our findings indicate a survival advantage for a surgical approach, even in patients with high-risk visceral metastases or multiple metastases that may require multiple operations for complete resection. Clearly not all patients with distant melanoma are candidates for resection, due to extensive tumor burden or poor performance status, but our data suggest that at least 55% of stage IV patients may be eligible to undergo surgery as part of their treatment plan. We therefore believe that the surgeon should play an integral role in evaluation and treatment planning for all patients with stage IV recurrence of melanoma.
One potential therapeutic advantage of resection is that it may delay disease progression by interrupting the metastatic cascade associated with hematogenous seeding of cells to other sites.[24] In addition, it immediately reduces tumor burden and thereby decreases tumor-induced immune suppression.[25] This is particularly important for tumor masses larger than 2 cm, which may prove difficult to completely eradicate with systemic therapy.[26] Finally, metastasectomy may enhance the patient’s endogenous immune defenses or response to adjuvant immunotherapy, and thus maintain a complete clinical remission.[27] Although immunological data after diagnosis of distant recurrence were not available for patients in our study, JWCI investigators have previously reported a direct correlation between specific endogenous antitumor antibody immune responses and postoperative survival of patients with stage IV melanoma.[27]
Surgery for distant metastases has been improved by development of more advanced imaging techniques that can detect lesions as small as 5–10 mm.[28] These techniques can differentiate patients with multiple vs. limited metastases, allowing surgeons to better judge the extent of disease and plan the operative procedure necessary for complete resection. In addition, modern advances in anesthesia, surgical techniques and supportive care have reduced operative mortality from multiple metastasectomy to below 1–2%, with a corresponding reduction in morbidity.[29] Finally, shorter postsurgical hospitalizations have decreased the total costs of cancer surgery, often below the costs of newer chemotherapeutic and biologic cancer therapies such as a 4-cycle course of ipilimumab, which costs approximately $120,000.
Our study was limited by a possible selection bias with respect to choice of therapy for patients at the time of stage IV diagnosis. Patients undergoing surgery for distant melanoma tend to have less extensive disease, whereas patients receiving SMT alone might be more likely to have multiple tumors. We attempted to control for this bias by comparing surgical and non-surgical cohorts based on M-stage, number of metastases, number of sites with distant metastases, and DDFI. Our study was also limited by its retrospective nature. Although the data was drawn from a carefully controlled phase III trial, the management of stage IV recurrence was not dictated or standardized in MSLT-I. We had access to recurrence dates, treatment dates, and treatment types, but not to treatment intent or treatment response. Thus it was not possible to determine which patients underwent debulking versus curative resection, or which patients had stable versus progressive disease after SMT. Although we knew the interval between SMT and surgery, we could not determine whether SMT was administered to downstage disease prior to surgery, as a primary intervention instead of surgery for resectable disease, or as a palliative measure for symptom control. We also could not determine dosing duration, side-effects, or complications of SMT.
Despite the limitations of our study, the multicenter data offer impressive evidence that inclusion of surgery in the treatment plan for stage IV recurrence confers a survival advantage and the only chance for long-term survival and cure. Single-center retrospective studies also demonstrate prolonged survival following surgery, as do findings of the prospective SWOG study.[10–22,26,29,30]. Our median OS and 4-year OS rate (15.9 months and 20.8%, respectively) compare favorably with SWOG data (median OS, 21 months; 4-year OS, 31%)[18] and are far superior to data from Korn’s meta-analysis of over 2000 patients undergoing systemic therapy for stage IV disease (median OS, 6.2 months; 1-year OS, 25.5%).[6] Further evidence to support the use of surgery for stage IV melanoma is found in the international MMAIT-IV trial (NIH Clinical Trials Identifier: NCT00052156). In this prospective randomized trial, 496 patients who had undergone complete resection of stage IV melanoma were treated adjuvantly with two types of immunotherapy. Estimated 5-year survival for the study population was 42.3%. This trial was terminated early by the Data Monitoring Safety Board based on a low probability of showing superiority of either arm.[31]
The optimal approach to distant recurrent melanoma will likely be a combination of surgery and immune-based systemic therapies. As novel targeted agents continue to demonstrate encouraging results in phase I-III trials,[7–9,32,33] more patients will become candidates for neoadjuvant or postoperative treatment regimens. Indeed, many of the new systemic agents can demonstrate reduced disease progression but do not yet create high complete response rates, leaving significant residual tumor.[7–9] The timing of surgery versus systemic treatments thus will become particularly critical, which is why we are examining the efficacy of initial surgical versus systemic treatment for resectable stage IV melanoma in a prospective phase III international trial (NIH Clinical Trials Identifier: NCT01013623). As shown in Figure 5 (supplement), patients with ≤6 metastases in ≤3 organ sites are randomized to surgery with or without BCG adjuvant immunotherapy, or to SMT. The primary endpoint is overall survival; secondary endpoints are time to progression of target lesions or development of new lesions. This trial will attempt to confirm the high survival rate following metastasectomy and establish the indications for surgical resection in treatment of stage IV melanoma.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful for the support and assistance of all members of the Multicenter Selective Lymphadenectomy Trial Group:
Independent data and safety monitoring committee — J. Kirkwood (chair), J. Daly, M. Kutner, M. Mihm, G. Smith, M. Urist, N. Beegun (patient advocate); Trial sites and principal investigators — Sydney Melanoma Unit, Sydney, Australia: J.F. Thompson; Istituto Nazionale dei Tumori de Napoli, Naples, Italy: N. Mozzillo; Netherlands Cancer Institute, Amsterdam: O.E. Nieweg; New York University, New York: D.F. Roses; University Medical Center Groningen and University of Groningen, Groningen, the Netherlands: H.J. Hoekstra; Millard Fillmore Hospital, Buffalo, NY: C.P. Karakousis; H. Lee Moffit Cancer Center, Tampa, FL: D.S. Reintgen; University of California and Mt. Zion Medical Center, San Francisco: S.P.Leong; Royal Adelaide Hospital, Adelaide, Australia: B.J. Coventry; Roswell Park Cancer Institute, Buffalo, NY: W. Kraybill; Princess Alexandra Hospital, Brisbane, Australia: M. Smithers; Henry Ford Hospital, Detroit: S.D. Nathanson; University of Texas Southwestern Medical Center at Dallas, Dallas: J.F. Huth; University of Hawaii at Manoa, Honolulu: J.H. Wong; University of Pennsylvania, Philadelphia: D.L. Fraker; Tom Baker Cancer Centre, Calgary, AB, Canada: W. Temple; City Hospital of Nürnberg, Nürnberg, Germany: E. Paul; John Wayne Cancer Institute at Saint John's Health Center, Santa Monica, CA: D.L. Morton.
Supported by grant P01 CA29605 from the National Cancer Institute, and by funding from the Melanoma Research Alliance (Washington, DC), Dr. Miriam & Sheldon G. Adelson Medical Research Foundation (Boston, MA), Lincy Foundation (Beverly Hills, CA), Amyx Foundation, Inc. (Boise, ID), Alan and Brenda Borstein (Los Angeles, CA), Mr. and Mrs. Louis Johnson (Stanfield, AZ), Heather and Jim Murren (Las Vegas, NV), and the Wayne and Gladys Valley Foundation (Oakland, CA). Dr. Howard is the Harold McAlister Charitable Foundation Fellow. Content is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the National Institutes of Health.
Footnotes
Members of the Multicenter Selective Lymphadenectomy Trial (MSLT) Group are listed in the Acknowledgments.
Presented in part at the Society of Surgical Oncology Annual Meeting, San Antonio, TX, March 2–5, 2011.
REFERENCES
- 1.Alexandrescu DT. Melanoma costs: a dynamic model comparing estimated overall costs of various clinical stages. Dermatol Online J. 2009;15 [PubMed] [Google Scholar]
- 2.Koyanagi K, O'Day SJ, Boasberg P, et al. Serial monitoring of circulating tumor cells predicts outcome of induction biochemotherapy plus maintenance biotherapy for metastatic melanoma. Clin Cancer Res. 2010;16:2402–2408. doi: 10.1158/1078-0432.CCR-10-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koyanagi K, Mori T, O'Day SJ, et al. Association of circulating tumor cells with serum tumor-related methylated DNA in peripheral blood of melanoma patients. Cancer Res. 2006;66:6111–6117. doi: 10.1158/0008-5472.CAN-05-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coit DG, Andtbacka R, Bichakjian CK, et al. Melanoma. J Natl Compr Canc Netw. 2009;7:250–275. doi: 10.6004/jnccn.2009.0020. [DOI] [PubMed] [Google Scholar]
- 5.Trinh VA. Current management of metastatic melanoma. Am J Health Syst Pharm. 2008;65:S3–S8. doi: 10.2146/ajhp080460. [DOI] [PubMed] [Google Scholar]
- 6.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 7.Hodi FS, O'Day SJ, McDermott DF, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 10.Ollila DW. Complete metastasectomy in patients with stage IV metastatic melanoma. Lancet Oncol. 2006;7:919–924. doi: 10.1016/S1470-2045(06)70938-X. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher WS, Pommier RF, Lum S, Wilmarth TJ. Surgical treatment of metastatic melanoma. Am J Surg. 1998;175:413–417. doi: 10.1016/S0002-9610(98)00041-5. [DOI] [PubMed] [Google Scholar]
- 12.Karakousis CP, Velez A, Driscoll DL, Takita H. Metastasectomy in malignant melanoma. Surgery. 1994;115:295–302. [PubMed] [Google Scholar]
- 13.Meyer T, Merkel S, Goehl J, Hohenberger W. Surgical therapy for distant metastases of malignant melanoma. Cancer. 2000;89:1983–1991. doi: 10.1002/1097-0142(20001101)89:9<1983::aid-cncr15>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- 14.Ollila DW, Essner R, Wanek LA, Morton DL. Surgical resection for melanoma metastatic to the gastrointestinal tract. Arch Surg. 1996;131:975–979. 979–980. doi: 10.1001/archsurg.1996.01430210073013. [DOI] [PubMed] [Google Scholar]
- 15.Ollila DW, Hsueh EC, Stern SL, Morton DL. Metastasectomy for recurrent stage IV melanoma. J Surg Oncol. 1999;71:209–213. doi: 10.1002/(sici)1096-9098(199908)71:4<209::aid-jso1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Wong JH, Skinner KA, Kim KA, et al. The role of surgery in the treatment of nonregionally recurrent melanoma. Surgery. 1993;113:389–394. [PubMed] [Google Scholar]
- 17.Wood TF, DiFronzo LA, Rose DM, et al. Does complete resection of melanoma metastatic to solid intra-abdominal organs improve survival? Ann Surg Oncol. 2001;8:658–662. doi: 10.1007/s10434-001-0658-4. [DOI] [PubMed] [Google Scholar]
- 18.Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: Results of Southwest Oncology Group Clinical Trial S9430. Cancer. 2011;117:4740–4746. doi: 10.1002/cncr.26111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal S, Yao TJ, Coit DG. Surgery for melanoma metastatic to the gastrointestinal tract. Ann Surg Oncol. 1999;6:336–344. doi: 10.1007/s10434-999-0336-5. [DOI] [PubMed] [Google Scholar]
- 20.Harpole DH, Jr, Johnson CM, Wolfe WG, et al. Analysis of 945 cases of pulmonary metastatic melanoma. J Thorac Cardiovasc Surg. 1992;103:743–748. discussion 748–750. [PubMed] [Google Scholar]
- 21.Leo F, Cagini L, Rocmans P, et al. Lung metastases from melanoma: when is surgical treatment warranted? Br J Cancer. 2000;83:569–572. doi: 10.1054/bjoc.2000.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricaniadis N, Konstadoulakis MM, Walsh D, Karakousis CP. Gastrointestinal metastases from malignant melanoma. Surg Oncol. 1995;4:105–110. doi: 10.1016/s0960-7404(10)80014-3. [DOI] [PubMed] [Google Scholar]
- 23.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 24.Roth JA, Silverstein MJ, Morton DL. Metastatic potential of metastases. Surgery. 1976;79:669–673. [PubMed] [Google Scholar]
- 25.Morton DL, Holmes EC, Golub SH. Immunologic aspects of lung cancer. Chest. 1977;71:640–643. doi: 10.1378/chest.71.5.640. [DOI] [PubMed] [Google Scholar]
- 26.Morton DL, Ollila DW, Hsueh EC, et al. Cytoreductive surgery and adjuvant immunotherapy: a new management paradigm for metastatic melanoma. CA Cancer J Clin. 1999;49:101–116. 165. doi: 10.3322/canjclin.49.2.101. [DOI] [PubMed] [Google Scholar]
- 27.Hsueh EC, Gupta RK, Yee R, et al. Does endogenous immune response determine the outcome of surgical therapy for metastatic melanoma? Ann Surg Oncol. 2000;7:232–238. doi: 10.1007/BF02523659. [DOI] [PubMed] [Google Scholar]
- 28.Gulec SA, Faries MB, Lee CC, et al. The role of fluorine-18 deoxyglucose positron emission tomography in the management of patients with metastatic melanoma: impact on surgical decision making. Clin Nucl Med. 2003;28:961–965. doi: 10.1097/01.rlu.0000099805.36471.aa. [DOI] [PubMed] [Google Scholar]
- 29.Thompson JF, Morton DL, Balch CM, Ross MI, editors. Surgical excision of distant melanoma metastases. St. Louis: Quality Medical Publishing; 2009. [Google Scholar]
- 30.Rose DM, Essner R, Hughes TM, et al. Surgical resection for metastatic melanoma to the liver: the John Wayne Cancer Institute and Sydney Melanoma Unit experience. Arch Surg. 2001;136:950–955. doi: 10.1001/archsurg.136.8.950. [DOI] [PubMed] [Google Scholar]
- 31.Morton DL, Mozzillo N, Thompson JF, Kelley MC, Faries M, Wagner J, Schneebaum S, Schuchter L, Gammon G, Elashoff R MMAIT Clinical Trials Group. An International, randomized, phase III trial of bacillus Calmette-Guerin (BCG) plus allogenieic melanoma vaccine (MCV) or placebo after complete resection of melanoma metastatic to regional or distant sites. Journal of Clinical Oncology 2007 ASCO Annual Meeting Proceedings Part I Vol 25, No 18S (June 20 Supplement) 2007:8508. [Google Scholar]
- 32.Tarhini AA, Iqbal F. CTLA-4 blockade: therapeutic potential in cancer treatments. Onco Targets Ther. 2010;3:15–25. doi: 10.2147/ott.s4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.