Abstract
Riboflavin is an important water soluble vitamin (B2) required for metabolic reactions, normal cellular growth, differentiation and function. Mammalian brain cells cannot synthesize riboflavin and must import from systemic circulation. However, the uptake mechanism, cellular translocation and intracellular trafficking of riboflavin in brain capillary endothelial cells are poorly understood. The primary objective of this study is to investigate the existence of riboflavin-specific transport system and delineate the uptake and intracellular regulation of riboflavin in immortalized rat brain capillary endothelial cells (RBE4). The uptake of [3H]-Riboflavin is sodium, temperature and energy dependent but pH independent. [3H]-Riboflavin uptake is saturable with Km and Vmax values of 19 ± 3 µM and 0.235 ± 0.012 picomoles/min/mg protein, respectively. The uptake process is inhibited by unlabelled structural analogs (lumiflavin, lumichrome) but not by structurally unrelated vitamins. Ca++/calmodulin and protein kinase A (PKA) pathways are found to play an important role in the intracellular regulation of [3H]-Riboflavin. Apical and baso-lateral uptake of [3H]-Riboflavin clearly indicate that riboflavin specific transport system is predominantly localized on the apical side of RBE4 cells. A 628 bp band corresponding to riboflavin transporter is revealed in RT-PCR analysis. These findings, for the first time report the existence of a specialized and high affinity transport system for riboflavin in RBE4 cells. Blood-brain barrier (BBB) is a major obstacle limiting drug transport inside the brain as it regulates drug permeation from systemic circulation. This transporter can be utilized for targeted delivery in enhancing brain permeation of highly potent drugs on systemic administration.
Keywords: Riboflavin, rat brain capillary endothelial cells, blood-brain barrier, functional and molecular aspects
Introduction
Drug delivery to the brain has been a challenging task due to the presence of blood-brain barrier (BBB), which prevents the entry of hydrophilic drug molecules from systemic circulation (Wolburg et al., 1994). It expresses tight junctions which form an impassable barrier between endothelial cells for paracellular drug transport (Dallasta et al., 1999). ABC transporters such as P-gp (P-glycoprotein), MRP (multidrug resistant proteins) and BCRP (breast cancer resistant protein) can also modulate drug uptake across BBB, thereby causing lower brain accumulation of therapeutic agents (Shen and Zhang, 2010).
In the last few decades, transporter targeted drug delivery approach has gained considerable attention for delivering drugs that are poorly permeable across physiological barriers. Several nutrient transport systems have been identified on BBB. Nutrient transporters such as amino acids (Smith, 2000); (Wu and Pardridge, 1999), nucleoside (Chishty et al., 2003), sodium dependent multivitamin (Park and Sinko, 2005), glucose (Agus et al., 1997) and monocarboxylic acid (Terasaki et al., 1991) transporters have been reported to be expressed on BBB. Various nutrients and xenobiotics required for cellular growth and maintenance are usually translocated through these transporters (Lee, 2000). Thus, BBB not only poses a formidable barrier for the passage of therapeutic agents but also selectively facilitates supply of nutrients to the brain. Utilization of these transporters for drug delivery might be a useful approach for enhancing drug availability from systemic circulation into the brain (Gynther et al., 2008; Takada et al., 1992). Riboflavin (vitamin B2) is a water soluble vitamin which is required in a variety of metabolic reactions. This vitamin plays an important role in maintaining normal function as well as differentiation and growth of mammalian cells (Hariharan et al., 2006). Riboflavin is a precursor of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) co-enzymes. These flavin co-enzymes are highly essential for the metabolism of amino acids, carbohydrates, lipids and in the conversion of other vitamins (pyridoxine) to their co-enzyme forms (Merrill et al., 1981). These flavin co-enzymes are also an integral part of electron transport chain responsible for energy production (Joosten and van Berkel, 2007).
Mammalian cells do not synthesize riboflavin and depend on exogenous sources unlike fungi, bacteria and plants (Bereswill et al., 1999). Recently, deficiency of riboflavin has been reported to cause a rare neurological disorder known as Brown-Vialetto-Van Laere syndrome (BVVLS) (Bosch et al., 2011). It is an inherited or acquired genetic disorder caused due to mutation in C20orf54 gene resulting in neurological abnormalities (Anand et al., 2012; Horvath, 2012). Oral administration of riboflavin has significantly improved clinical symptoms and abnormalities in patients with BVVLS (Anand et al., 2012; Bosch et al., 2011). Expression of riboflavin specific transporter on brain capillary endothelial cells might have an essential role in enhancing brain absorption of riboflavin from systemic circulation. However, uptake and intracellular trafficking of riboflavin has not been investigated across rat brain capillary endothelial cells. The uptake of riboflavin has already been reported in various tissues such as intestine (Said et al., 1994), cornea (Hariharan et al., 2006), retina (Kansara et al., 2005), colon (Said et al., 2000), liver (Said et al., 1998), kidney (Kumar et al., 1998) and placenta (Huang and Swaan, 2001).
Recently, riboflavin specific carrier system has been targeted to improve the permeability of therapeutic agents across biological membranes. Huang et al. have conjugated riboflavin to rhodamine and examined the uptake of [3H]-Riboflavin in presence of unlabelled riboflavin and rhodamine-riboflavin conjugates on the microvillous membrane of BeWo cells (Huang et al., 2003). Rhodamine-riboflavin conjugate produced significant inhibition of [3H]-Riboflavin uptake while rhodamine did not have any effect on the uptake process. It is probably due to the carrier mediated uptake of rhodamine-riboflavin conjugate via riboflavin transport system. Riboflavin modified bovine serum albumin exhibited significantly higher transport across distal pulmonary epithelium relative to unmodified bovine serum albumin in anesthetized rats (Wangensteen et al., 1996).
For riboflavin uptake studies, immortalized rat brain endothelial cells (RBE4) have been selected as it is a well established in vitro model for brain capillary endothelial cells. Expression of L-system amino acid (Reichel et al., 2002), organic cation/carnitine (OCTN2) (Friedrich et al., 2003), reduced folate (Araujo et al., 2010), serotonin (Brust et al., 2000), nucleoside transport systems (Chishty et al., 2003) and insulin receptor (Yu et al., 2006) has already been reported on RBE4 cells. However there are no reports regarding the expression of riboflavin transport system on RBE4 cells. In this study, we therefore investigated the functionality and molecular expression of riboflavin transport system on RBE4 cells. Results obtained from this study may indicate involvement of a specialized and high affinity carrier transport system for the translocation of riboflavin. Furthermore, this carrier system was highly specific for riboflavin and its structural analogs. Therefore this system may be differentially targeted for improving drug delivery across BBB.
Results
Time and temperature dependency
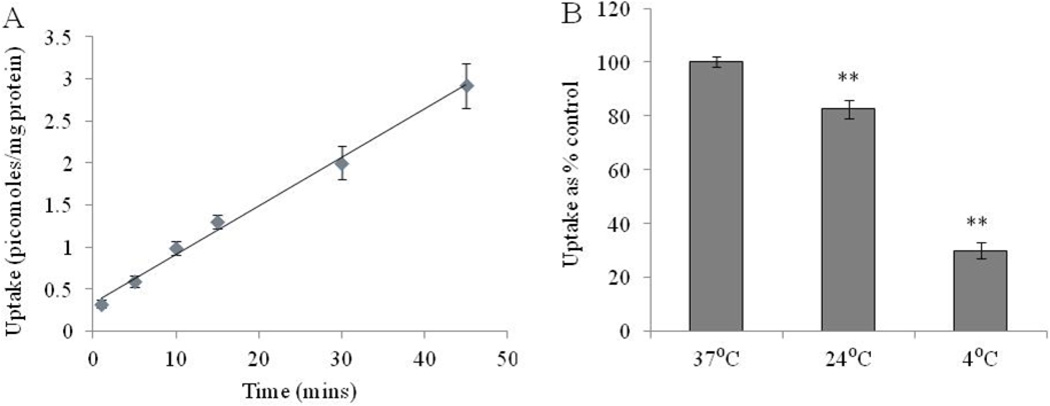
The uptake of [3H]-Riboflavin in RBE4 cells is linear up to 45 min (r2 = 0.994) as seen in Fig. 1A. Incubation time of 15 min is selected for further uptake studies. For temperature dependence, the uptake of [3H]-Riboflavin is performed at different temperatures. Fig. 1B clearly shows that the uptake of [3H]-Riboflavin is maximal at 37° C. [3H]-Riboflavin uptake diminished significantly on reducing the temperature to 24° C and 4° C.
Fig. 1A.
Uptake of [3H]-Riboflavin as a function of time in RBE4 cells in DPBS (pH 7.4) at 37° C. Fig. 1B: Temperature dependent uptake study of [3H]-Riboflavin in RBE4 cells in DPBS (pH 7.4). The uptake is expressed as percentage of control (37° C). Each data point is expressed as mean ± standard deviation (n=5–6). Asterisk (**) represents significant difference from the control (p < 0.01).
pH and ion dependency
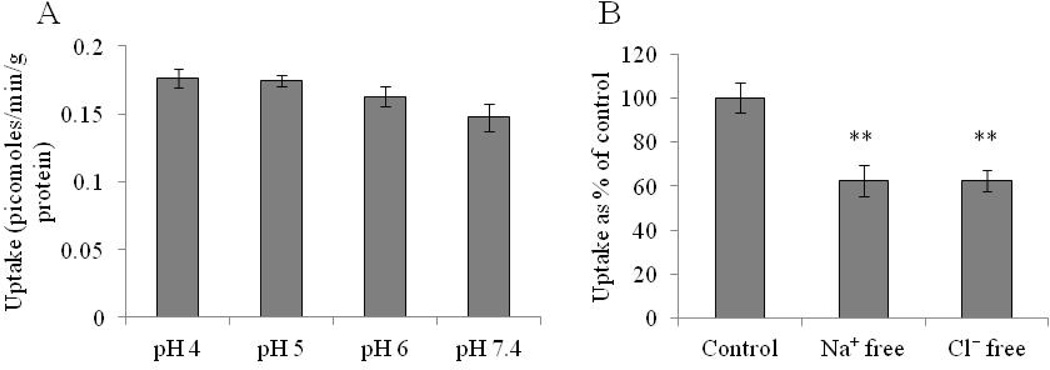
In order to investigate the involvement of an inward driven proton gradient for [3H]-Riboflavin uptake, the study is carried out at different pH in the range 4–7.4. As seen in Fig. 2A, [3H]-Riboflavin uptake is not altered by the pH of the solution indicating that the process is pH independent. Based on these results, subsequent uptake studies are carried out at pH 7.4. Effect of sodium ions on [3H]-Riboflavin uptake is examined by replacing sodium ions with equimolar quantities of choline chloride and KH2PO4 in DPBS buffer.
Fig. 2.
Fig. 2A: pH dependent uptake study of [3H]-Riboflavin in RBE4 cells in DPBS (4–7.4) at 37° C. Fig. 2B: Ion dependent uptake study of 3H-Riboflavin in RBE4 cells in sodium and chloride free DPBS (pH 7.4) at 37° C. The uptake is expressed as percentage of control (DPBS). Each data point is expressed as mean ± standard deviation (n=4). Asterisk (**) represents significant difference from the control (p < 0.01).
For studying the effect of chloride ions, chloride ions are replaced with equimolar quantities of Na2HPO4, KH2PO4 and calcium acetate in DPBS buffer. Fig. 2B clearly indicates that the uptake is highly dependent on sodium and chloride ions. A marked reduction (40%) in the uptake rate of [3H]-Riboflavin is observed in the absence of either sodium or chloride ions in the bathing media, respectively.
Concentration dependency
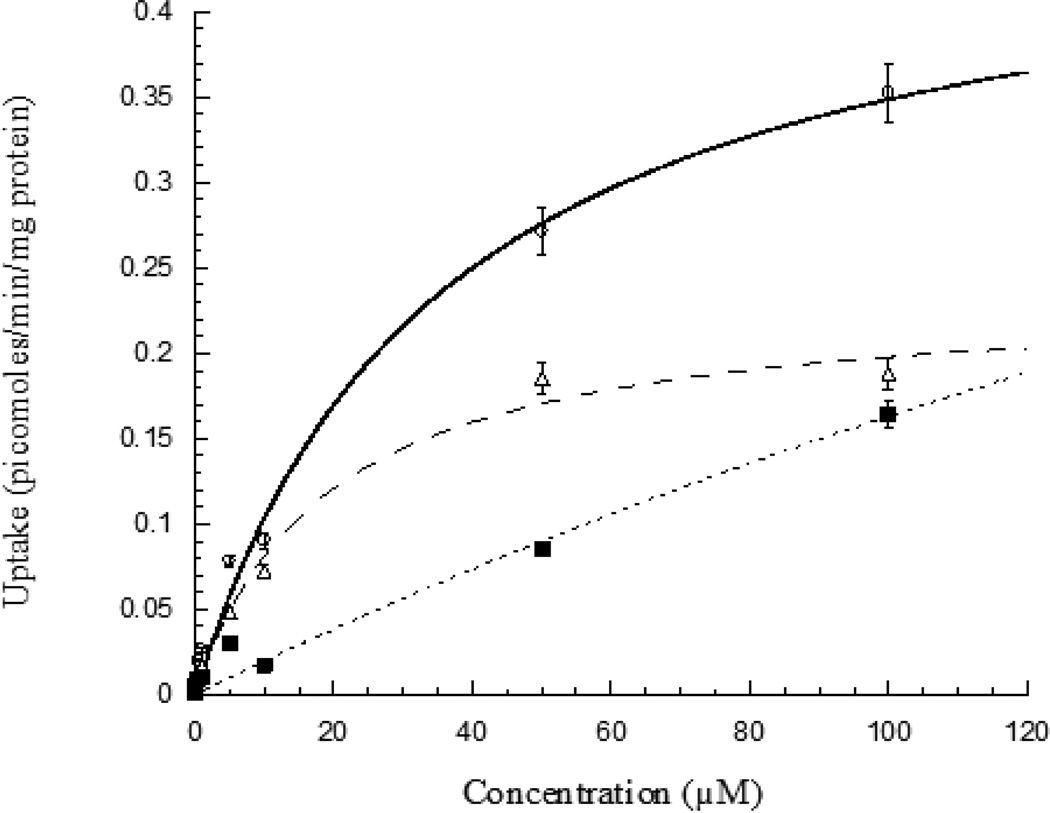
The involvement of a carrier mediated transport system for [3H]-Riboflavin uptake in RBE4 cells is also determined. Uptake study involving saturation kinetics is performed in the presence of unlabelled riboflavin over the concentration range 10 nM-100 µM. [3H]-Riboflavin uptake appears to be concentration dependent and involves a saturable carrier mediated process (Fig. 3). The kinetic parameters, Km and Vmax values are calculated to be 19 ± 3 µM and 0.235 ± 0.012 picomoles/min/mg protein, respectively.
Fig. 3.
Concentration dependent uptake of [3H]-Riboflavin in RBE4 cells in presence of unlabelled riboflavin (10 nM −100 µM), DPBS (pH 7.4) at 37° C. Each data point is expressed as mean ± standard deviation (n=4). (○) represents total uptake, (Δ) Michaelis-Menten component and (■) linear non saturable component.
Effects of metabolic and membrane inhibitors
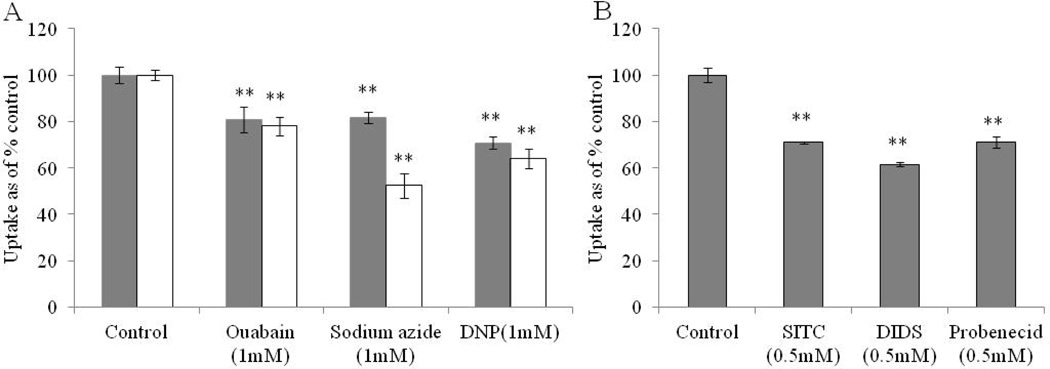
In order to delineate energy dependence, [3H]-Riboflavin uptake is carried out in presence of metabolic inhibitors such as ouabain, DNP and sodium azide. In the first method, cells are pretreated with metabolic inhibitors and uptake is carried out in bathing media containing only [3H]-Riboflavin. In the second method, cells are pretreated with metabolic inhibitors for 1 h and [3H]-Riboflavin uptake is carried out in bathing media containing 1 mM concentration of metabolic inhibitors. Fig. 4A clearly indicates that the uptake process is energy dependent. A significant difference in the reduction of [3H]-Riboflavin uptake is observed in the presence of metabolic inhibitors in the incubation buffer.
Fig. 4.
Fig. 4A: Energy dependent uptake study of [3H]-Riboflavin in RBE4 cells in DPBS (pH 7.4) and 37° C. Solid bar: cells pre-incubated with metabolic inhibitors: Ouabain, Sodium azide and 2,4-dinitrophenol (DNP) for 1 h. Unfilled bar: cells pre-incubated with metabolic inhibitors for 30 min followed by uptake of [3H]-Riboflavin in DPBS containing 1 mM metabolic inhibitors. Fig. 4B: Effect of membrane inhibitors: 4-acetamindo-4’-isothiocyanostilbene-2,2’-disulfonic acid (SITC) (0.5 mM), 4,4’-di-isothiocyanostilbene-2,2’- disulphonic acid (DIDS) (0.5 mM) and probenecid (0.5 mM) on the uptake of [3H]-Riboflavin in RBE4 cells in DPBS (pH 7.4) at 37° C. The uptake is expressed as percentage of control (DPBS). Each data point is expressed as mean ± standard deviation (n=4). Asterisk (**) represents significant difference from the control (p < 0.01).
We also investigated the effect of membrane inhibitors (SITC, DIDS, probenecid) and endocytotic inhibitor (colchicine) on [3H]-Riboflavin uptake. SITC, DIDS and probenecid significantly reduce [3H]-Riboflavin uptake by 70%, 61% and 71%, respectively. This result clearly indicates that anion exchanger may be involved in the uptake process (Fig. 4B). [3H]-Riboflavin uptake remains unaltered when cells are pre-incubated with colchicine (100 µM) suggesting that receptor mediated endocytosis may not involved in the uptake process (control: 100 ± 6%, colchicine 100 µM: 94 ± 8% - data not shown).
Substrate specificity
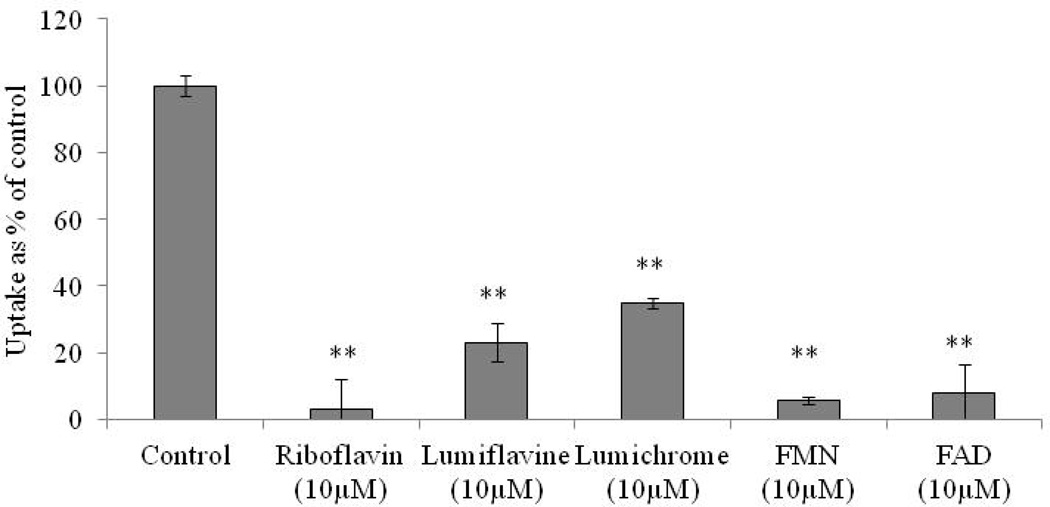
The substrate specificity of the riboflavin transport system is also studied by carrying out [3H]-Riboflavin uptake in presence of various structural analogs and unlabelled vitamins. The uptake is significantly diminished in the presence of unlabelled riboflavin (10 µM). Structural analogs lumiflavin and lumichrome also exhibit significant affinity towards this transport system. The uptake is significantly reduced to 23% and 35% in presence of lumiflavin and lumichrome. FMN and FAD also produce significant inhibition in the uptake process by 95% and 92%, respectively (Fig. 5). Also, D-ribose at the concentration of 10 µM does not produce any significant inhibition in uptake process (control 100 ± 10% and D-ribose 101 ± 3% - data not shown). The effect of various unlabelled vitamins such as ascorbic acid, thiamine, niacin, pyridoxine, pantothenic acid, biotin and folic acid on [3H]-Riboflavin uptake is also investigated. None of these unlabelled vitamins appear to alter the uptake process (Table 1).
Fig. 5.
Uptake of [3H]-Riboflavin in RBE4 cells in presence of lumiflavin (10 µM), lumichrome (10 µM), FMN (10 µM) and FAD (10 µM) in DPBS (pH 7.4) at 37° C. The uptake is expressed as percentage of control (DPBS). Each data point is expressed as mean ± standard deviation (n=4). Asterisk (**) represents significant difference from the control (p < 0.01).
Table 1.
Effect of various vitamins on [3H]-Riboflavin uptake in RBE4 cells. Uptake of [3H]-Riboflavin on RBE4 cells in presence of various unlabelled vitamins (10 µM) in DPBS (pH 7.4) at 37° C. The uptake is expressed as percentage of control (DPBS). Each data point is expressed as mean ± standard deviation (n=4). P<0.01 is considered as statistically significant from control. NS represents not statistically significant from control.
| Vitamins (10 µM) | Uptake as % of control |
Statistics |
|---|---|---|
| Control Riboflavin Ascorbic acid Thiamine Niacin Pyridoxine Pantothenic acid Biotin Folic acid |
100 ± 10 3 ± 7 100 ± 8 103 ± 3 116 ± 5 113 ± 11 100 ± 7 100 ± 13 87 ± 9 |
- P<0.01 NS NS NS NS NS NS NS |
Effect of intracellular regulatory pathways
The role of intracellular regulatory pathways such as Ca++/calmodulin pathway, PKA, PTK and PKC pathway in [3H]-Riboflavin uptake is also examined. For Ca++/calmodulin pathway, cells are pre-incubated for 1 h with modulators such as calmidazolium (Cal), KN-62 and TFP. Cal, KN-62 and TFP significantly lower [3H]-Riboflavin uptake in a concentration dependent manner. Involvement of PKA pathway in the intracellular regulation of riboflavin is examined by pre-incubating cells with forskolin and IBMX. PKA pathway regulators, FOR and IBMX causes significant inhibition of [3H]-Riboflavin uptake. This suggests that PKA pathway may be involved in the intracellular regulation of riboflavin in RBE4 cells (Table 2).
Table 2.
Uptake of [3H]-Riboflavin on RBE4 cells in presence of various concentrations of Ca++/calmodulin pathway and PKA modulators in DPBS (pH 7.4) at 37° C. The uptake is expressed as percentage of control (DPBS). Each data point is expressed as mean ± standard deviation (n=4). P<0.01 is considered as statistically significant from control. NS represents not statistically significant from control.
| Pathway | Modulators | Uptake as % of control |
Statistics |
|---|---|---|---|
| Ca++/ Calmodulin | Control Cal (10 µM) Cal (25 µM) Cal (50 µM) Cal (100 µM) Cal (250 µM) KN-62 (10 µM) KN-62 (25 µM) KN-62 (50 µM) TFP (100 µM) TFP (250 µM) |
100 ± 4 105 ± 6 91 ± 1 65 ± 3 55 ± 3 38 ± 5 80 ± 2 67 ± 2 69 ± 5 70 ± 3 65 ± 6 |
- NS NS P<0.01 P<0.01 P<0.01 P<0.01 P<0.01 P<0.01 P<0.01 P<0.01 |
| PKA | Control FOR (50 µM) FOR (100 µM) IBMX (2.5 mM) IBMX (5 mM) |
100 ± 10 85 ± 3 67 ± 2 61 ± 3 50 ± 3 |
- P<0.01 P<0.01 P<0.01 P<0.01 |
The involvement of PTK pathway is also examined by pre-incubating cells with genistein, genistein and tyrphostin A 25. Cells are pre-incubated with PMA for delineating the role of PKC pathway in modulating [3H]-Riboflavin uptake. Table 3 clearly summarizes that PTK regulators, genistin genistein and tyrphostin A 25 and PKC pathway activator, PMA does not produce any significant change in [3H]-Riboflavin uptake. These results clearly suggest that PTK and PKC pathways have no significant role in the intracellular regulation of riboflavin uptake.
Table 3.
Uptake of [3H]-Riboflavin on RBE4 cells in presence of various concentrations of PTK and PKC pathway modulators in DPBS (pH 7.4) at 37° C. The uptake is expressed as percentage of control (DPBS). Each data point is expressed as mean ± standard deviation (n=4). P<0.01 is considered as statistically significant from control. NS represents not statistically significant from control.
| Pathway | Modulators | Uptake as % of control |
Statistics |
|---|---|---|---|
| PTK | Control Genistin (50 µM) Genistin (100 µM) Genistein (50 µM) Genistein (100 µM) Tyrphostin A25 (50 µM) Tyrphostin A25 (100 µM) |
100 ± 3 88 ± 3 91 ± 9 96 ± 3 96 ± 5 102 ± 2 101 ± 4 |
- NS NS NS NS NS NS |
| PKC | Control PMA (5 µM) PMA (10 µM) PMA (50 µM) |
100 ± 4 106 ± 1 101 ± 3 112 ± 3 |
- NS NS NS |
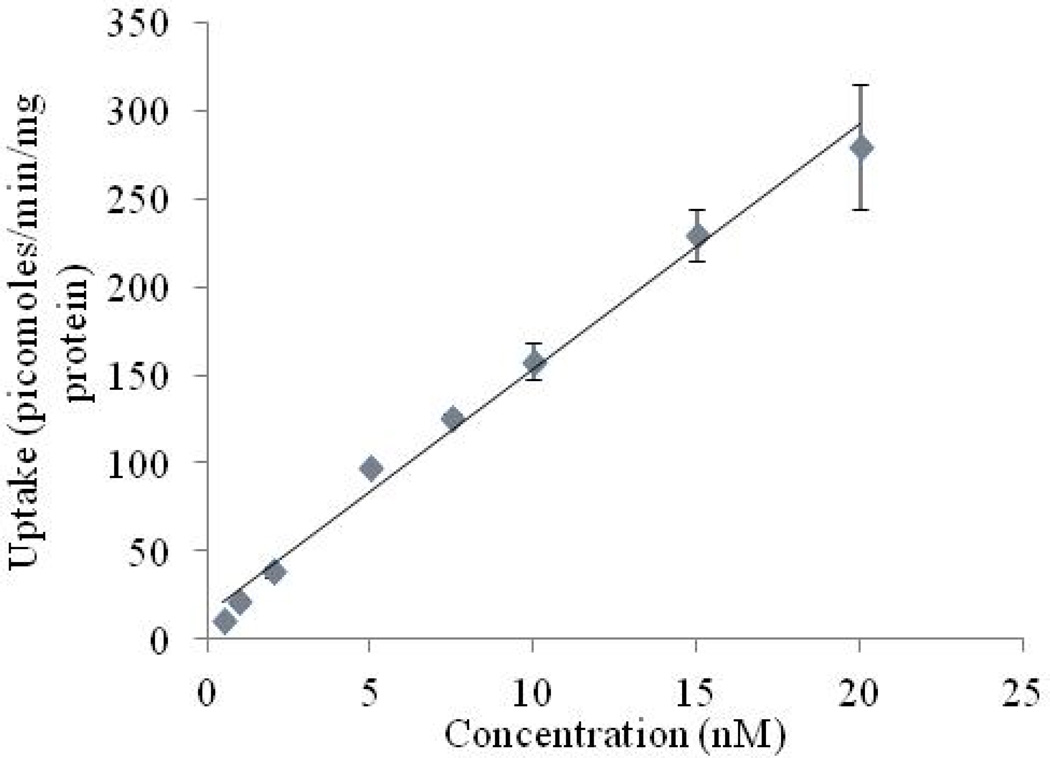
Expression of second riboflavin-specific high affinity carrier system
In order to investigate the presence of a second high affinity transporter specific for riboflavin, we carried out [3H]-Riboflavin uptake study at relatively lower concentration. A linear increase in uptake of [3H]-Riboflavin is observed within the concentration range, 0.5–200 nM (Fig. 6).
Fig. 6.
Uptake of [3H]-Riboflavin (0.5–200 nM) in RBE4 cells in DPBS (pH 7.4) at 37° C. Each data point is expressed as mean ± standard deviation (n=4).
Polarized distribution of riboflavin-specific transporter
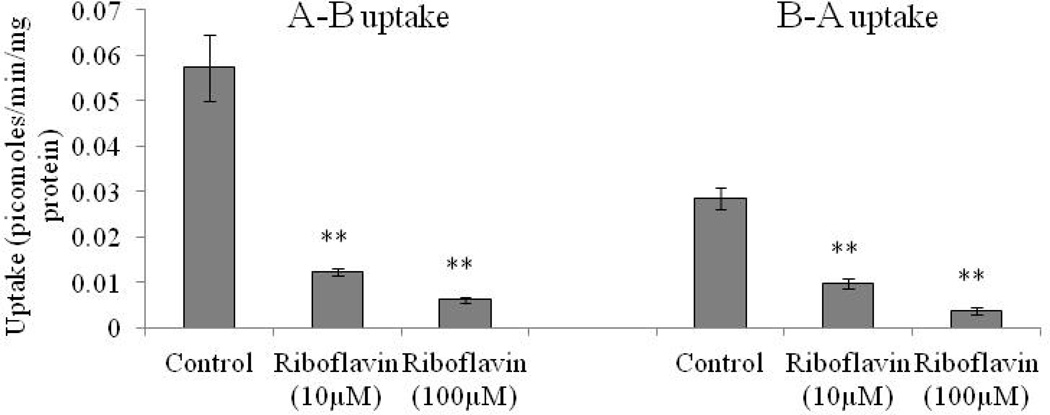
For determining polarized distribution of riboflavin transport system on RBE4 cells, we carried out apical to baso-lateral and baso-lateral to apical uptake of [3H]-Riboflavin. A-B uptake of [3H]-Rf is significantly higher (two-fold) than B-A uptake as seen in Fig. 7. Unlabelled riboflavin (10 and 100 µM) significantly diminish [3H]-Riboflavin uptake from the apical side relative to baso-lateral side (Fig. 7).
Fig. 7.
A–B and B–A uptake of [3H]-Riboflavin in RBE4 cells in absence and presence of 10 and 100 µM unlabelled riboflavin in DPBS (pH 7.4) at 37° C. Each data point is expressed as mean ± standard deviation (n=4). Asterisk (**) represents significant difference from the control (p < 0.01).
RT-PCR analysis
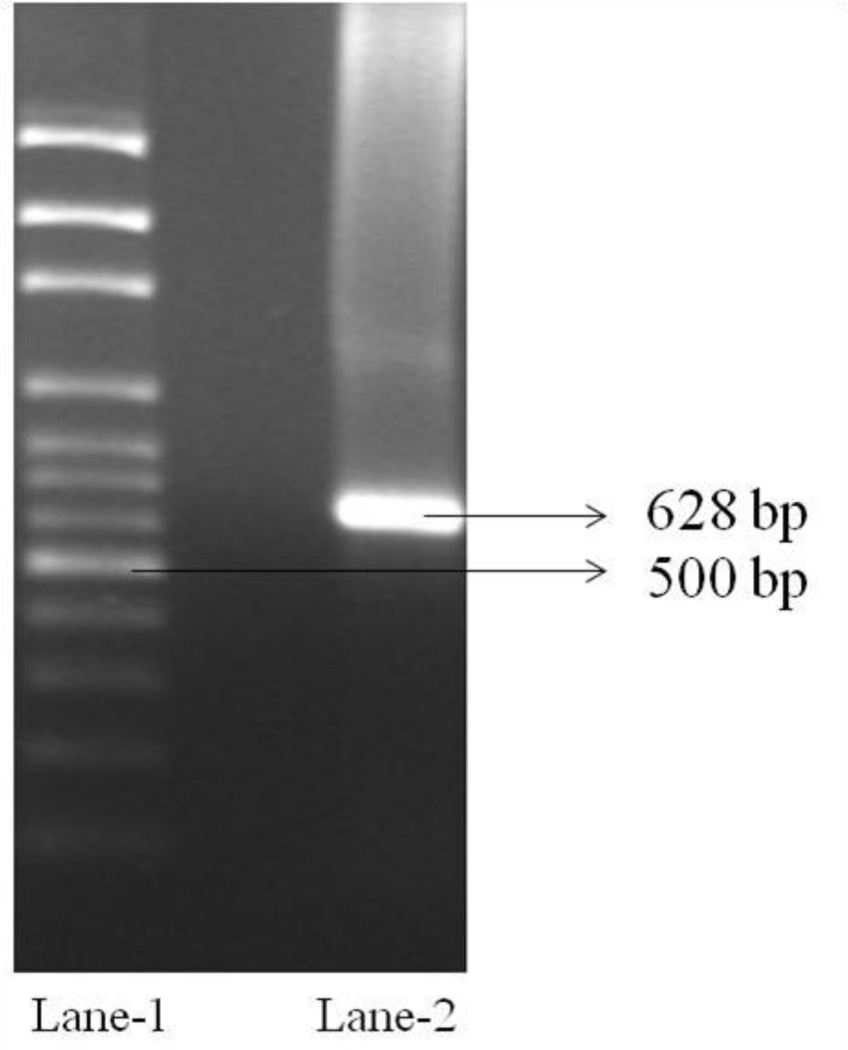
RT-PCR analysis is carried out to confirm the existence of riboflavin transport system in RBE4 cells. The PCR product is analyzed by 3% agarose gel electrophoresis using ethidium bromide. The cDNA produced from total RNA is PCR amplified with primers specific for rat riboflavin transport system. The PCR product obtained at 628 bp confirms the expression of riboflavin specific transport system on RBE4 cells (Fig. 8).
Fig. 8.
RT-PCR analysis showing the presence of riboflavin transport system in RBE4 cells. Lane 1: Molecular ladder (100 bp). Lane 2: PCR product (628 bp) obtained from total RNA isolated from RBE4 cells with primers specific for rat riboflavin specific transport system
Discussion
Riboflavin is a water soluble vitamin required in normal metabolic reactions, growth and function in mammalian cells. It is a precursor of flavin co-enzymes which are involved in cellular energy production. Riboflavin transport system have been identified and characterized on various tissues including intestine, colon, retina, cornea, liver and kidney. However, the existence of this transport system on brain endothelial cells has not been reported till to date. Riboflavin specific high affinity transport system, if expressed on brain endothelial cells can be further targeted to enhance brain accumulation of poorly permeable drugs from systemic circulation. In this article, we report the presence of a carrier mediated transport system for riboflavin on the luminal side of rat brain capillary endothelial cells.
Time dependent study clearly depicts a linear increase in the uptake of riboflavin up to 45 min (Fig. 1A). The rate is significantly more rapid at physiological temperature of 37°C suggesting that the uptake process is temperature dependent (Fig. 1B). The uptake process remains unaltered within the pH range 4 to 7.4 (Fig. 2A). This result suggests that the uptake process is pH independent which is consistent with observations in corneal cells (Hariharan et al., 2006). Absence of sodium and chloride ions in DPBS buffer significantly lowers the uptake of riboflavin (Fig. 2B). These findings clearly demonstrate that sodium and chloride ions may play an important role in the translocation of riboflavin.
The concentration dependent uptake study of riboflavin in RBE4 cells clearly demonstrate that the process is saturable in the micromolar range with Km and Vmax of 19 ± 3 µM and 0.235 ± 0.012 picomoles/min/mg protein, respectively (Fig. 3). This result confirms the involvement of a carrier mediated transport system for the translocation of riboflavin in the micromolar range. The uptake is significantly inhibited in cells exposed to metabolic inhibitors such as ouabain, (a known Na+/K+ ATPase inhibitor), sodium azide (oxidative phosphorylation inhibitor) and 2,4-dinitrophenol (intracellular ATP reducer) (Fig. 4A). Thus, riboflavin uptake is dependent on a motive energy force and seemed to be directly coupled to ATP energy sources.
Previous reports have proposed that riboflavin might be transported in anionic form through cell membranes (Lowy and Spring, 1990; Spector, 1982). We therefore carried out the uptake of riboflavin in presence of membrane inhibitors such as DIDS, SITC and probenecid. These inhibitors significantly inhibited the uptake of riboflavin, suggesting that anionic exchangers may also play an important role in the translocation of riboflavin (Fig. 4B). Endocytotic inhibitor, colchicine does not alter the riboflavin uptake indicating that the process may not undergo receptor mediated endocytosis. Summarizing these observations, one can suggest the involvement of a temperature, sodium and energy dependent, high affinity-carrier mediated riboflavin transport system which may be saturable in the micromolar range.
In order to delineate the structural requirement of the transport system, the uptake is carried out in presence of structurally related compounds such as lumiflavin, lumichrome, FMN and FAD. These structural analogs cause significant inhibition of riboflavin uptake as seen in Figs. 5. Such inhibition with lumiflavin and lumichrome indicates that the removal or substitution of ribityl side chain with a methyl group at position 10 of isoalloxazin ring may not lower the affinity of these compounds for the riboflavin transport system. Similarly, addition of phosphate groups and adenine on the ribityl side chain did not alter the interaction of FMN and FAD with the transporter. Riboflavin uptake remains unchanged in presence of D-ribose. These results clearly suggest that isoalloxazine ring appears to play an important role in transporter recognition while the side chain ribityl group may not be essential. Therefore, the side chain ribityl group can serve as a potential site for drug conjugation without compromising transporter recognition by riboflavin transport system. Structurally unrelated vitamins do not significantly alter riboflavin uptake, suggesting that the transport system is highly specific for riboflavin and its structurally related compounds (Table 1).
Several articles have suggested the importance of intracellular regulatory pathways such as Ca++/calmodulin, PKA, PTK and PKC in governing the activity of various nutrient transport system (Feschenko et al., 2000; Kansara et al., 2008; Luo et al., 2008; Said et al., 2005). For this reason, we investigated the possible role of these signaling pathways in the regulation of riboflavin uptake. The involvement of Ca++/Calmodulin pathways is evident as calmidazolium, KN-62 and trifluoperazine significantly inhibited the uptake of riboflavin. With increasing concentration of IBMX and forskolin, a significant inhibition in riboflavin uptake is observed, indicating that the process is also regulated by PKA pathway (Table 2).
Lack of generating significant inhibition in riboflavin uptake by PTK pathway modulators (genistin and tyrphostin A25) and PKC pathway modulators (PMA) rule out their possible roles in the intracellular regulation of riboflavin (Table 3). Most of these signaling pathways are always engaged in various cellular functions and therefore specific mechanism through which Ca++/Calmodulin and PKA pathways control the intracellular regulation of riboflavin is poorly understood. Extensive cross-signaling is observed at cellular levels between cAMP and calmodulin-mediated signaling pathways. Hence the inhibition of riboflavin uptake via this pathway can be a manifestation of intertwined regulation of these processes (Kansara et al., 2008).
A second riboflavin specific carrier system, rRFT2 active at nanomnolar concentration range has been reported in small intestine cells (Yamamoto et al., 2009). In order to examine the presence of this isoform, we carried out uptake of riboflavin in nanomolar range. Uptake of riboflavin in RBE4 cells is found to be linear within concentration range 5–200 nM (Fig. 6). This result indicates that the second riboflavin-specific carrier system may not be functional in RBE4 cells.
To delineate the polarized distribution of riboflavin specific transport system, apical and baso-lateral uptake of riboflavin is carried out in RBE4 cells. Fig. 7 clearly illustrates that the apical uptake of riboflavin is higher than the baso-lateral uptake. Furthermore, 10 and 100 µM concentration of cold riboflavin produce more inhibition on the apical uptake relative to baso-lateral uptake. These observations clearly suggest a greater polarized distribution of riboflavin transport system on the apical side of RBE4 cells. More polarized distribution of riboflavin transport system on the apical (lumenal) side of brain endothelial capillary cells can be beneficial in enhancing drug accumulation from systemic circulation. Finally, RT-PCR analysis confirms the molecular evidence of riboflavin specific carrier system in RBE4 cells. The PCR product obtained at 628 bp is specific for riboflavin transport system (Fig. 8).
In summary, this investigation clearly demonstrates the functional evidence of riboflavin specific high affinity carrier transport system in RBE4 cells. This transport system requires coupling to an electrochemical Na+ gradient and ATP sources for transporting riboflavin. This system is pH independent and highly specific for riboflavin and its structural analogs. Ca++/calmodulin and PKA pathway appears to play an important role in modulating riboflavin uptake.
Higher polarized distribution of this transporter is found on the luminal surface of brain capillary endothelial cells. Hence, this transporter may serve as a potential target for enhancing brain permeability of wide range of compounds such as anti HIV protease inhibitors and anti cancer drugs. Taken together, this study clearly suggests that RBE4 cells express a carrier mediated transport system for riboflavin. This cell line may also serve as a valuable in vitro tool for screening riboflavin-conjugated therapeutic prodrugs.
Experimental procedure
Materials
[3H]-Riboflavin, (20 nM) was obtained from Moravek Biochemicals, Inc. (Bra, CA). The growth medium Dulbecco’s modified minimum essential medium (DMEM) and non-essential amino acids (NEAA) were purchased from Invitrogen (CA). Fetal bovine serum (FBS) was procured from Atlanta biological. Unlabelled riboflavin, ascorbic acid, thiamine, niacin, pyridoxine, pantothenic acid, biotin, folic acid, lumichrome, lumiflavin, flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD), choline chloride, ouabain, sodium azide, 2,4-dinitrophenol (DNP), 4-acetamindo-4’-isothiocyanostilbene-2,2’-disulfonic acid (SITC), 4,4’-di-isothiocyanostilbene-2,2’-disulphonic acid (DIDS), probenecid, calcium-calmodulin pathway modulators (calmidazolium, KN-62, and trifluoperazine [TFP]), protein kinase (PKC and PKA) pathway modulators (phorbol-12-myristate-13-acetate [PMA], forkolin, 3-isobutyl-1-methylxanthine [IBMX]), protein kinase inhibitors (PTK) modulators (genistin tyrphostin A25 and genistein), triton X-100, HEPES, D-glucose, penicillin, streptomycin, sodium bicarbonate and other chemicals were procured from Sigma Chemical Co. (St. Louis, MO).
Culture flasks (75 cm2), 12-well plates (3.8 cm2 growth area/well) and polyester Transwells (pore size of 0.4 ím and 12 mm diameter) were obtained from Costar (Bedford, MA, USA). All other chemicals purchased were of special reagent grade and used without further purification.
Cell culture
RBE4 cells were grown in 75 cm2 tissue culture flask in DMEM, supplemented with 10% FBS (heat inactivated), penicillin (100 units/mL), streptomycin (100 µg/mL), 1% nonessential amino acids, 20 mM HEPES and 29 mM NaHCO3 and maintained at 7.4. Cells were maintained in incubator at 37 °C in an atmosphere of 5% CO2 and 90% relative humidity. The culture medium was changed every alternate day until cells reached 80% confluency.
Uptake (cellular accumulation) studies
For uptake experiments, RBE4 cells of passages 12–30 were plated at a density of 3 × 106 cells in 12 well culture plates. Uptake of [3H]-Riboflavin was conducted on a fully confluent monolayer of RBE4 cells. Following medium aspiration, cell monolayers were washed with 2 mL of Dulbecco’s phosphate buffered saline (DPBS) (130 mM NaCl, 0.03 mM KCl, 7.5 mM Na2HPO4, 1.5 mM KH2PO4, 1 mM CaCl2, 0.5 mM MgSO4, and 5 mM glucose, pH 7.4) for three times at 37 °C (each wash of 10 min). Uptake studies were initiated by incubating cells with a solution (0.3µCi/mL) of [3H]-Riboflavin in DPBS (pH 7.4) at 37°C. Following incubation, the solution was quickly aspirated and cells were washed with ice-cold stop solution (210 mM KCl, 2 mM HEPES, pH of 7.4) to terminate the uptake process.
One mL of lysis buffer (0.1% Triton-X solution in 0.3% NaOH) was added to each well and plates were kept overnight at room temperature. Further, 500 µL aliquot was transferred to scintillation vials containing 3 mL of scintillation cocktail. The radioactivity associated with cells was analyzed with a scintillation counter (Beckman Instruments Inc., Model LS-6500; Fullerton, CA). Furthermore, the rate of uptake was normalized with protein count which was quantified with BioRad protein estimation kit (BioRad protein; Hercules, CA).
Time and temperature dependence
To determine the optimum time, [3H]-Riboflavin uptake is performed over various time periods (1, 5, 10, 15, 30 and 45 min). [3H]-Riboflavin uptake was then carried out according to the method described earlier. The uptake process is terminated at respective time points.
The effect of temperature on the uptake of [3H]-Riboflavin is delineated by carrying out the uptake study at different temperatures i.e. 4°, 24° and 37° C. The temperature of the buffer is adjusted to 4°, 24° and 37° C before initiation of the study.
pH and ion dependence
The effect of pH is studied by adjusting pH of the buffer to 4, 5, 6 and 7.4. In order to study the effect of sodium ions on [3H]-Riboflavin uptake, NaCl and Na2HPO4 in DPBS is replaced by equimolar quantities of choline chloride and KH2PO4, respectively. The role of chloride ion is studied by adding equimolar quantities of Na2HPO4, KH2PO4 and calcium acetate to substitute for NaCl, KCl and CaCl2 in DPBS at pH 7.4.
Concentration dependence
For concentration dependent study, various concentrations of unlabelled riboflavin (10 nM to 100 µM) in DPBS, pH 7.4 was spiked with [3H]-Riboflavin (0.3 µCi/mL). The data obtained is used to determine Km and Vmax by fitting to a modified Michaelis-Menton equation as mentioned in “Data Analysis” section.
Effect of energy and membrane transport inhibitors
To delineate the role of metabolic inhibitors on the uptake of [3H]-Riboflavin, cells are preincubated with ouabain (ATPase inhibitor), sodium azide (oxidative phosphorylation inhibitor) and 2,4 dinitrophenol (intracellular ATP reducer). After preincubation, solutions are removed and uptake study is carried out in two different ways. In the first study, uptake of [3H]-Riboflavin is carried out alone and in the second study [3H]-Riboflavin uptake is carried in the presence of 1mM metabolic inhibitors. In order to study the effect of anion transport inhibitors, cells are pre-incubated with SITC, DIDS and probenecid for 1 h. Pre-incubation with 100 µM colchicine is needed to delineate if endocytosis is involved in the uptake process.
Substrate specificity
The structural requirements for interaction with riboflavin transport system are delineated by performing [3H]-Riboflavin uptake in presence of various unlabelled vitamins and structural analogs. Structural analogs such as lumiflavin, lumichrome, FMN and FAD of concentration 10 µM are prepared in DPBS and spiked with [3H]-Riboflavin (0.3 µCi/mL). A similar [3H]-Riboflavin uptake study is conducted in the presence of various unlabelled vitamins such as ascorbic acid, thiamine, niacin, pyridoxine, pantothenic acid, biotin and folic acid.
Intracellular regulation
Involvement of intracellular regulatory pathways such as Ca++/calmodulin, PTK, PKC and PKA pathway in [3H]-Riboflavin uptake was also investigated. The concentration of pathway regulators were selected from previously published work (Kansara et al., 2005). Cells are pre-incubated for 1 h with calmidazolium (Cal), KN-62 and TFP for studying the involvement of Ca++/calmodulin pathway in the intracellular regulation of [3H]-Riboflavin uptake. Cells are pre-incubated with forskolin and IBMX for 1 h in order to study the involvement of PKA pathway. For PTK pathway determination, cells are pre-incubated with intracellular regulators such as genistein, genistin and tyrphostin A25 for 1 h. The role of PKC pathway is delineated by pre-incubating cells with PMA. [3H]-Riboflavin uptake study is then carried out according to the method described earlier.
Polarized distribution of Riboflavin transport system
For determining polarized distribution of riboflavin transport system in RBE4 cells, we carried out apical to baso-lateral (A-B) and baso-lateral to apical uptake (B-A) of [3H]-Riboflavin in the absence and presence of unlabelled riboflavin. For A-B and B-A [3H]-Riboflavin uptake, cells are seeded and maintained at 37 °C in an atmosphere of 5% CO2 and 90% relative humidity on transwells inserts.
In A–B [3H]-Riboflavin uptake, 0.5 mL of radioactive solution (0.3 µCi/mL) is added in the apical chamber and for B-A [3H]-Riboflavin uptake, 1.5 mL of radiolabelled solution (0.3 µCi/mL) is added to the basal chamber of 12-well Transwell plates. [3H]-Riboflavin uptake study is then carried out according to the method described previously.
RT-PCR analysis
RT-PCR studies are performed to identify and confirm the presence of riboflavin specific transport system. Total mRNA is isolated from cultured cells utilizing a standard protocol (Sugawara et al., 2000). Cells are lysed in 1 mL of Trizol® reagent. The lysate is transferred into an eppendorf, mixed with chloroform and centrifuged. The aqueous phase is separated and isopropanol is added to facilitate RNA precipitation. RNA pellet is washed twice with 1 mL of 75% ethanol and dissolved in DNase/RNase-free water. The concentration of RNA is obtained with the help of Nanodrop (Thermo Fisher Scientific, Wilmington, DE, USA).
RT-PCR analysis is performed based on previously published methods from our laboratory (Paturi et al., 2010). One µg of RNA is reverse transcribed using M-MLV reverse transcriptase and oligodT as a primer. The conditions for reverse transcription are initial denaturation at 70°C for 5 min, reverse transcription at 42°C for 1 h and final extension at 72°C for 5 min. Three µL of cDNA is then used for amplification using the 10 µM forward and reverse primers with the help of Taq polymerase. Sequence, template and length of forward and reverse primer are shown in Table 4. The conditions for PCR amplification are denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 1 min, for 35 cycles, and a final extension at 72°C for 10 min. The product obtained was sequenced from both directions by SeqWright with an automated Perkin-Elmer Applied Biosystems 3730×1 Prism TM DNA sequencer to confirm molecular identity of riboflavin specific transport system in RBE4 cells.
Table 4.
Sequence, template and length of forward and reverse primer.
| Primer | Sequence (5’-3’) | Template | Length | Tm (°C) |
|---|---|---|---|---|
| Forward Reverse |
AGGGCTGTATGGCCTCTGTCT GAAGGAGGCCTGGCGTAGTG |
1470–1490 2097–2078 |
21 20 |
56.65 56.78 |
Data analysis
In order to determine Km and Vmax associated with the uptake of [3H]-Riboflavin, concentration dependency data is fitted in modified Michaelis-Menten equation as shown in Eq. 1.
| Eq. 1 |
v represents the total uptake, Vmax denotes the maximum uptake rate for the carrier-mediated process, Km is Michaelis-Menten constant which is the concentration at half saturation, Kd is non-saturable diffusion rate constant and C is substrate concentration. In the above equation, (Vmax *C)/(Km + C) represents carrier mediated saturable process whereas, Kd*C provides the non-saturable component. Data is analyzed with a non-linear least-square regression analysis program (KaleidaGraph, version 3.09, Synergy Software, Reading, PA). The kinetic parameters are calculated and substituted in the equation 1 to determine saturable and non-saturable component of the total uptake.
Statistical analysis
All uptake experiments are conducted atleast in quadruplicate and results are expressed as mean ± S.D. The kinetic parameters Km and Vmax are expressed as mean ± S.D. Student’s t-test is applied to determine the statistical significance between two groups. A difference between mean values is considered to differ significantly if the P value is ≤0.01.
Highlights.
-
➢
Specialized and high affinity transport system for riboflavin in RBE4 cells
-
➢
Functional and molecular characteristics of riboflavin transport system
-
➢
Targeted drug delivery for enhancing brain permeation of therapeutic drugs
-
➢
RBE4 cells may serve as an in vitro model for screening riboflavin conjugated drugs
Acknowledgement
This work was supported by NIH grant RO1 AI071199. We would like to thank Dr P. O. Couraud, INSERM, France for supplying RBE4 cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agus DB, Gambhir SS, Pardridge WM, Spielholz C, Baselga J, Vera JC, Golde DW. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest. 1997;100:2842–2848. doi: 10.1172/JCI119832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand G, Hasan N, Jayapal S, Huma Z, Ali T, Hull J, Blair E, McShane T, Jayawant S. Early use of high-dose riboflavin in a case of Brown-Vialetto-Van Laere syndrome. Developmental medicine and child neurology. 2012;54:187–189. doi: 10.1111/j.1469-8749.2011.04142.x. [DOI] [PubMed] [Google Scholar]
- Bereswill S, Hinkelmann S, Kist M, Sander A. Molecular analysis of riboflavin synthesis genes in Bartonella henselae and use of the ribC gene for differentiation of Bartonella species by PCR. J Clin Microbiol. 1999;37:3159–3166. doi: 10.1128/jcm.37.10.3159-3166.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch AM, Abeling NG, Ijlst L, Knoester H, van der Pol WL, Stroomer AE, Wanders RJ, Visser G, Wijburg FA, Duran M, Waterham HR. Brown-Vialetto-Van Laere and Fazio Londe syndrome is associated with a riboflavin transporter defect mimicking mild MADD: a new inborn error of metabolism with potential treatment. Journal of inherited metabolic disease. 2011;34:159–164. doi: 10.1007/s10545-010-9242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust P, Friedrich A, Krizbai IA, Bergmann R, Roux F, Ganapathy V, Johannsen B. Functional expression of the serotonin transporter in immortalized rat brain microvessel endothelial cells. J Neurochem. 2000;74:1241–1248. doi: 10.1046/j.1471-4159.2000.741241.x. [DOI] [PubMed] [Google Scholar]
- Chishty M, Begley DJ, Abbott NJ, Reichel A. Functional characterisation of nucleoside transport in rat brain endothelial cells. Neuroreport. 2003;14:1087–1090. doi: 10.1097/01.wnr.0000072843.93264.ff. [DOI] [PubMed] [Google Scholar]
- Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Nelson JA, Achim CL. Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1915–1927. doi: 10.1016/S0002-9440(10)65511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feschenko MS, Stevenson E, Sweadner KJ. Interaction of protein kinase C and cAMP-dependent pathways in the phosphorylation of the Na,K-ATPase. J Biol Chem. 2000;275:34693–34700. doi: 10.1074/jbc.M005869200. [DOI] [PubMed] [Google Scholar]
- Friedrich A, Prasad PD, Freyer D, Ganapathy V, Brust P. Molecular cloning and functional characterization of the OCTN2 transporter at the RBE4 cells, an in vitro model of the blood-brain barrier. Brain Res. 2003;968:69–79. doi: 10.1016/s0006-8993(02)04271-3. [DOI] [PubMed] [Google Scholar]
- Gynther M, Laine K, Ropponen J, Leppanen J, Mannila A, Nevalainen T, Savolainen J, Jarvinen T, Rautio J. Large neutral amino acid transporter enables brain drug delivery via prodrugs. J Med Chem. 2008;51:932–936. doi: 10.1021/jm701175d. [DOI] [PubMed] [Google Scholar]
- Hariharan S, Janoria KG, Gunda S, Zhu X, Pal D, Mitra AK. Identification and functional expression of a carrier-mediated riboflavin transport system on rabbit corneal epithelium. Curr Eye Res. 2006;31:811–824. doi: 10.1080/02713680600899655. [DOI] [PubMed] [Google Scholar]
- Horvath R. Update on clinical aspects and treatment of selected vitamin-responsive disorders II (riboflavin and CoQ(10)) Journal of inherited metabolic disease. 2012 doi: 10.1007/s10545-011-9434-1. [DOI] [PubMed] [Google Scholar]
- Huang SN, Swaan PW. Riboflavin uptake in human trophoblast-derived BeWo cell monolayers: cellular translocation and regulatory mechanisms. J Pharmacol Exp Ther. 2001;298:264–271. [PubMed] [Google Scholar]
- Huang SN, Phelps MA, Swaan PW. Involvement of endocytic organelles in the subcellular trafficking and localization of riboflavin. J Pharmacol Exp Ther. 2003;306:681–687. doi: 10.1124/jpet.103.051581. [DOI] [PubMed] [Google Scholar]
- Joosten V, van Berkel WJ. Flavoenzymes. Curr Opin Chem Biol. 2007;11:195–202. doi: 10.1016/j.cbpa.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Kansara V, Pal D, Jain R, Mitra AK. Identification and functional characterization of riboflavin transporter in human-derived retinoblastoma cell line (Y-79): mechanisms of cellular uptake and translocation. J Ocul Pharmacol Ther. 2005;21:275–287. doi: 10.1089/jop.2005.21.275. [DOI] [PubMed] [Google Scholar]
- Kansara V, Paturi D, Luo S, Gaudana R, Mitra AK. Folic acid transport via high affinity carrier-mediated system in human retinoblastoma cells. Int J Pharm. 2008;355:210–219. doi: 10.1016/j.ijpharm.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar CK, Yanagawa N, Ortiz A, Said HM. Mechanism and regulation of riboflavin uptake by human renal proximal tubule epithelial cell line HK-2. Am J Physiol. 1998;274:F104–F110. doi: 10.1152/ajprenal.1998.274.1.F104. [DOI] [PubMed] [Google Scholar]
- Lee VH. Membrane transporters. Eur J Pharm Sci. 2000;11(Suppl 2):S41–S50. doi: 10.1016/s0928-0987(00)00163-9. [DOI] [PubMed] [Google Scholar]
- Lowy RJ, Spring KR. Identification of riboflavin transport by MDCK cells using quantitative fluorescence video microscopy. J Membr Biol. 1990;117:91–99. doi: 10.1007/BF01871568. [DOI] [PubMed] [Google Scholar]
- Luo S, Wang Z, Kansara V, Pal D, Mitra AK. Activity of a sodium-dependent vitamin C transporter (SVCT) in MDCK-MDR1 cells and mechanism of ascorbate uptake. Int J Pharm. 2008;358:168–176. doi: 10.1016/j.ijpharm.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill AH, Jr, Lambeth JD, Edmondson DE, McCormick DB. Formation and mode of action of flavoproteins. Annu Rev Nutr. 1981;1:281–317. doi: 10.1146/annurev.nu.01.070181.001433. [DOI] [PubMed] [Google Scholar]
- Park S, Sinko PJ. The blood-brain barrier sodium-dependent multivitamin transporter: a molecular functional in vitro-in situ correlation. Drug Metab Dispos. 2005;33:1547–1554. doi: 10.1124/dmd.105.005231. [DOI] [PubMed] [Google Scholar]
- Paturi DK, Kwatra D, Ananthula HK, Pal D, Mitra AK. Identification and functional characterization of breast cancer resistance protein in human bronchial epithelial cells (Calu-3) International journal of pharmaceutics. 2010;384:32–38. doi: 10.1016/j.ijpharm.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel A, Abbott NJ, Begley DJ. Evaluation of the RBE4 cell line to explore carrier-mediated drug delivery to the CNS via the L-system amino acid transporter at the blood-brain barrier. J Drug Target. 2002;10:277–283. doi: 10.1080/10611860290031930. [DOI] [PubMed] [Google Scholar]
- Said HM, Ma TY, Grant K. Regulation of riboflavin intestinal uptake by protein kinase A: studies with Caco-2 cells. Am J Physiol. 1994;267:G955–G959. doi: 10.1152/ajpgi.1994.267.6.G955. [DOI] [PubMed] [Google Scholar]
- Said HM, Ortiz A, Ma TY, McCloud E. Riboflavin uptake by the human-derived liver cells Hep G2: mechanism and regulation. J Cell Physiol. 1998;176:588–594. doi: 10.1002/(SICI)1097-4652(199809)176:3<588::AID-JCP15>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Said HM, Ortiz A, Moyer MP, Yanagawa N. Riboflavin uptake by human-derived colonic epithelial NCM460 cells. Am J Physiol Cell Physiol. 2000;278:C270–C276. doi: 10.1152/ajpcell.2000.278.2.C270. [DOI] [PubMed] [Google Scholar]
- Said HM, Wang S, Ma TY. Mechanism of riboflavin uptake by cultured human retinal pigment epithelial ARPE-19 cells: possible regulation by an intracellular Ca2+-calmodulin-mediated pathway. J Physiol. 2005;566:369–377. doi: 10.1113/jphysiol.2005.085811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Zhang W. ABC transporters and drug efflux at the blood-brain barrier. Reviews in the neurosciences. 2010;21:29–53. doi: 10.1515/revneuro.2010.21.1.29. [DOI] [PubMed] [Google Scholar]
- Smith QR. Transport of glutamate and other amino acids at the blood-brain barrier. J Nutr. 2000;130:1016S–1022S. doi: 10.1093/jn/130.4.1016S. [DOI] [PubMed] [Google Scholar]
- Spector R. Riboflavin transport by rabbit kidney slices: characterization and relation to cyclic organic acid transport. J Pharmacol Exp Ther. 1982;221:394–398. [PubMed] [Google Scholar]
- Sugawara M, Nakanishi T, Fei YJ, Huang W, Ganapathy ME, Leibach FH, Ganapathy V. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J Biol Chem. 2000;275:16473–16477. doi: 10.1074/jbc.C000205200. [DOI] [PubMed] [Google Scholar]
- Takada Y, Vistica DT, Greig NH, Purdon D, Rapoport SI, Smith QR. Rapid high-affinity transport of a chemotherapeutic amino acid across the blood-brain barrier. Cancer Res. 1992;52:2191–2196. [PubMed] [Google Scholar]
- Terasaki T, Takakuwa S, Moritani S, Tsuji A. Transport of monocarboxylic acids at the blood-brain barrier: studies with monolayers of primary cultured bovine brain capillary endothelial cells. J Pharmacol Exp Ther. 1991;258:932–937. [PubMed] [Google Scholar]
- Wangensteen OD, Bartlett MM, James JK, Yang ZF, Low PS. Riboflavin-enhanced transport of serum albumin across the distal pulmonary epithelium. Pharm Res. 1996;13:1861–1864. doi: 10.1023/a:1016093310707. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Neuhaus J, Kniesel U, Krauss B, Schmid EM, Ocalan M, Farrell C, Risau W. Modulation of tight junction structure in blood-brain barrier endothelial cells. Effects of tissue culture, second messengers and cocultured astrocytes. J Cell Sci. 1994;107(Pt 5):1347–1357. doi: 10.1242/jcs.107.5.1347. [DOI] [PubMed] [Google Scholar]
- Wu D, Pardridge WM. Blood-brain barrier transport of reduced folic acid. Pharm Res. 1999;16:415–419. doi: 10.1023/a:1018829920158. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Inoue K, Ohta KY, Fukatsu R, Maeda JY, Yoshida Y, Yuasa H. Identification and functional characterization of rat riboflavin transporter 2. J Biochem. 2009;145:437–443. doi: 10.1093/jb/mvn181. [DOI] [PubMed] [Google Scholar]
- Yu Y, Kastin AJ, Pan W. Reciprocal interactions of insulin and insulin-like growth factor I in receptor-mediated transport across the blood-brain barrier. Endocrinology. 2006;147:2611–2615. doi: 10.1210/en.2006-0020. [DOI] [PubMed] [Google Scholar]