Abstract
Background
Lymph node assessment (LNA), including sentinel lymph node biopsy (SLNB), is controversial in patients undergoing lumpectomy for ductal carcinoma in situ (DCIS). Our goal was to identify factors influencing LNA in these patients.
Methods
We used the Surveillance Epidemiology and End Results database to identify all female patients treated with lumpectomy for DCIS from 2000–2008. We excluded patients without histologic confirmation, including those diagnosed at autopsy, and those for whom LNA status was unknown. Multivariate logistic regression models predicted use of LNA. Likelihood of undergoing LNA was reported as odds ratios (OR) with 95% confidence intervals (CI.
Results
A total of 62,935 patients met inclusion criteria. Approximately 15% (N = 9726) had regional LNA at the time of lumpectomy, with 12% (N =7294) undergoing SLNB. Factors associated with an increased likelihood of undergoing LNA included treatment in the Southeast (OR 1.25, CI−1.04–1.22), treatment after the year 2000, grade II (OR 2.71, CI 2.48–2.96), III (OR 2.38, CI 2.18–2.59), or IV (OR 2.61, CI 2.37–2.88) tumors, DCIS size 2–5 cm (OR 1.49, CI−1.37–1.62) or > 5 cm (OR 2.16, CI−1.78–2.61), and ER negative (OR 1.29, CI−1.16–1.43) or PR negative (OR 1.22, CI 1.11–1.33) tumors. Factors associated with a decreased likelihood of undergoing regional LNA were age > 60 (OR 0.83, CI −0.79–0.87), and Asian (OR 0.88, CI 0.81–0.96) race. Factors predictive of LNA in general were also predictive of SLNB.
Conclusion
Although LNA is controversial for patients undergoing lumpectomy for DCIS, it is utilized in 15% of cases. Further research establishing for the benefit of LNA in DCIS patients treated with lumpectomy is needed.
Keywords: sentinel lymph node biopsy, ductal carcinoma in situ, SEER, lymph node assessment, lumpectomy
Introduction
Lymph node assessment (LNA), including sentinel lymph node biopsy (SLNB), is controversial among patients undergoing lumpectomy for the treatment of ductal carcinoma in situ (DCIS). While opponents argue that DCIS has no potential to metastasize to regional lymph nodes, proponents of LNA counter that sampling error and missed underlying invasion may necessitate additional surgery for lymph node staging; at definitive surgery, DCIS may be upstaged to invasive carcinoma in 10% to 38% of cases(1–5). Even among patients with pure DCIS without evidence of invasion, sentinel node metastases may be found in 1% to 7%, although their oncologic significance is unknown (6, 7).
Recognizing the importance of invasion risk assessment on the use of LNA, the American Society of Clinical Oncology (ASCO) established guidelines for the use of SLNB in cases of DCIS in 2005 (8). The guidelines specifically do not recommend the routine use of SLNB for DCIS patients undergoing lumpectomy, but instead suggest SLNB for DCIS lesions > 5 cm or those associated with suspected or proven microinvasion.
We sought to determine how frequently LNA, particularly SLNB, is used among women undergoing lumpectomy for DCIS and to identify specific patient, tumor, and geographic factors that influence the use of LNA in these patients using a national, population-based database.
Methods
We used the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) database to identify all women undergoing lumpectomy for the treatment of DCIS from 2000 to 2008. We identified patients as having undergone LNA if one or more lymph nodes were removed and examined. Patients were only identified as having had a SLNB if it was specifically stated or coded under one of two field categories. We excluded patients without histologic confirmation, including those diagnosed at autopsy, and those for whom the status of nodal assessment was unknown. All size measurements reported represent that of the primary tumor in situ component.
We used multivariate logistic regression to evaluate the relationship between tumor- and patient-related factors on the likelihood of undergoing LNA. Covariates evaluated included age (≤ 60, older than 60), race (white, black, Asian, Hispanic, native American, other), year of diagnosis (2000–2008), grade (I, II, III, IV, unknown), size of DCIS (< 2 cm, 2–5 cm,> 5 cm, unknown), estrogen receptor (ER) and progesterone receptor (PR) status (positive, negative, borderline, unknown), and regional location (West, Midwest, Northeast, Southeast). Likelihood of undergoing LNA was reported as odds ratios (OR) with 95% confidence intervals (CI).
Since information in the SEER registry contains de-identified patient data, this study was exempt from institutional review board approval.
Results
Table 1 depicts the patient- and tumor-related characteristics of the cohort. Briefly, a total of 62,935 patients met inclusion criteria. The mean age was 60 years, with most of these being white women (75%) residing in areas reporting to Western or Northeastern SEER registries (77%). Approximately 15% (N = 9726) of patients had a regional LNA at the time of lumpectomy, with 75% (N = 7294) of these being a SLNB. Tumor grade was unknown for 19% of patients. Of those patients for whom tumor grade was known, 69% had grade II or III tumors. A majority of patients (59%) did not have complete data on the size of their DCIS focus. Among patients for whom the size of the DCIS was known, 84% had areas of DCIS less than 2 cm. By contrast, 2% had DCIS greater than 5 cm. Only 4% of all patients who underwent LNA or SLNB had DCIS measuring ≥ 5 cm. In general, DCIS is regarded as being hormone receptor positive. As such, it was not tested and therefore unknown in the majority (58–61%) of patients.
Table 1.
Patient and tumor characteristics
| Patient Characteristic | Total, N=62935 (%) |
SLNBX, N=7294 (%) |
LNA, N=9726 (%) |
|---|---|---|---|
| Age | |||
| ≤60 years | 33353(53) | 4192(57) | 5547(57) |
| >60 years | 29582(47) | 3102(43) | 4179(43) |
| Race | |||
| White | 47101(75) | 5520(76) | 7276(75) |
| Asian or Pacific Islander | 5467(9) | 614(8) | 784(8) |
| Black | 5396(9) | 551(8) | 809(8) |
| Hispanic | 4241(7) | 552(8) | 774(8) |
| Native American/Alaskan | 228(<1) | 17(<1) | 28(<1) |
| Unknown | 502(1) | 40(<1) | 55(<1) |
| Grade | |||
| I | 7858(12) | 493(7) | 736(8) |
| II | 21542(24) | 1982(27) | 2685(28) |
| III | 14055(22) | 2402(33) | 3080(32) |
| IV | 7823(12) | 1444(20) | 1760(18) |
| Unknown | 11657(19) | 973(13) | 1465(15) |
| DCIS Size | |||
| <2cm | 21977(35) | 3054(42) | 3830(39) |
| 2–5cm | 3573(6) | 771(11) | 969(10) |
| >5cm | 517(<1) | 142(2) | 184(2) |
| Unknown | 36868(59) | 3327(46) | 4743(49) |
| SEER Registry | |||
| West | 34376(55) | 4347(60) | 5484(56) |
| Midwest | 6538(10) | 633(9) | 900(9) |
| Northeast | 14015(22) | 1323(18) | 1968(20) |
| Southeast | 8006(13) | 991(14) | 1374(14) |
| ER | |||
| Positive | 22308(35) | 3189(44) | 4018(41) |
| Borderline | 70(<1) | 10(<1) | 14(<1) |
| Negative | 3965(6) | 986(14) | 1196(12) |
| Unknown | 36592(58) | 3109(43) | 4498(46) |
| PR | |||
| Positive | 18302(29) | 2475(34) | 3168(33) |
| Borderline | 170(<1) | 22(<1) | 30(<1) |
| Negative | 6316(10) | 1405(19) | 1704(18) |
| Unknown | 38147(61) | 3392(47) | 4824(50) |
Abbreviations: SLNB - Sentinel lymph node biopsy; LNA -Lymph node assessment, ER-Estrogen receptor, PR- progesterone receptor, DCIS- ductal carcinoma in situ
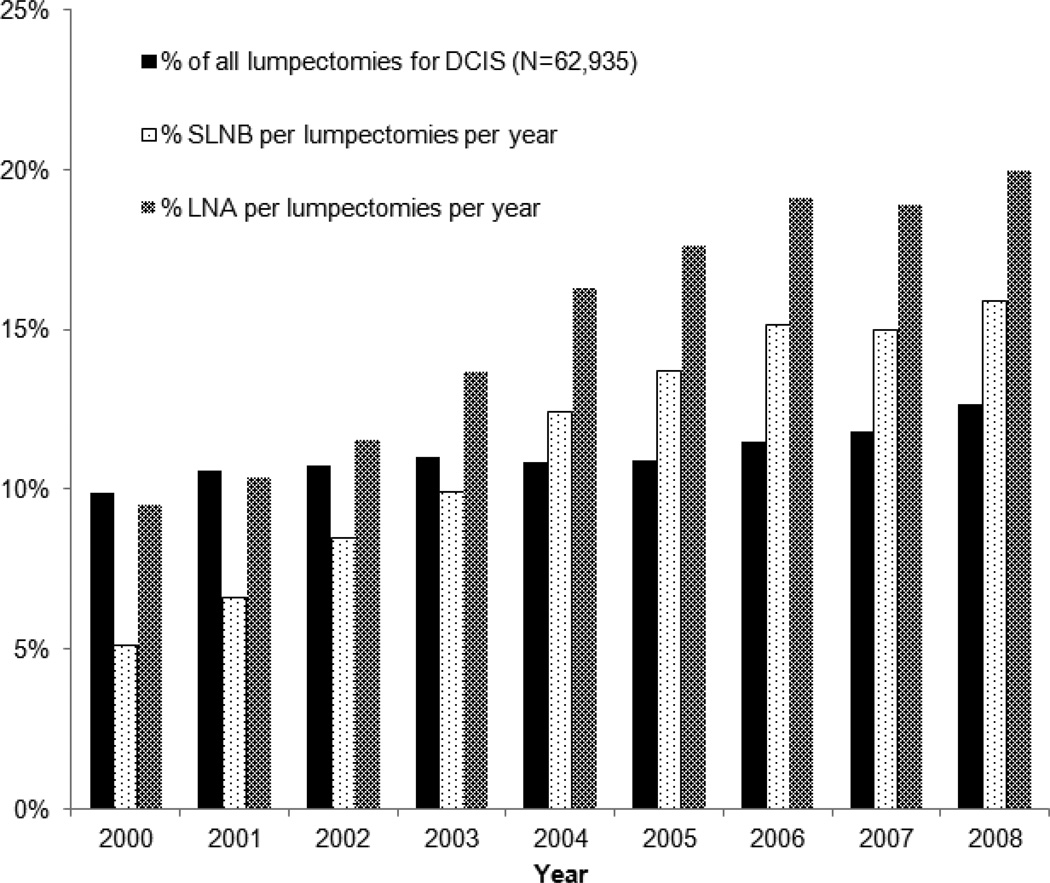
From 2000 to 2008, the rate of LNA and SLNB increased without an associated increase in the use of lumpectomy for DCIS (see Figure 1). LNA increased from 10% to 20% of all cases of DCIS treated with lumpectomy from the year 2000 to 2008. Over the same time period, use of SLNB increased from 5% to 16% of all lumpectomy cases.
Figure 1.
Trend in LNA and SLNB over time period of study. Percentage of LNA and SLNB represent proportion of each performed per total lumpectomies per given year. Abbreviations: LNA- Lymph node assessment, SLNB - Sentinel lymph node biopsy.
Tables 2 depicts the results of multivariate logistic regression models assessing the likelihood of undergoing LNA or SLNB. An increased likelihood of undergoing a LNA or SLNB was associated with the following factors: year of diagnosis, advancing or unknown tumor grade, increasing size of DCIS, southeast geography, and negative ER and PR status. Age greater than 60 and Asian race/ethnicity were the only factors associated with a decreased likelihood of undergoing regional LNA and SLNB.
Table 2.
Patient and tumor factors associated with odds of lymph node assessment and sentinel node biopsy
| SLNB | LNA | |||
|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | |
| Age | ||||
| ≤60 years | Reference | Reference | ||
| >60 years | 0.82 | 0.78–0.86 | 0.83 | 0.79–0.87 |
| Year Diagnosis | ||||
| 2000 | Reference | Reference | ||
| 2001 | 1.33 | 1.14–1.54 | 1.11 | 0.99–1.24 |
| 2002 | 1.72 | 1.49–1.98 | 1.24 | 1.10–1.38 |
| 2003 | 2.18 | 1.90–2.50 | 1.61 | 1.44–1.79 |
| 2004 | 2.12 | 1.83–2.47 | 1.58 | 1.40–1.79 |
| 2005 | 2.43 | 1.93–2.61 | 1.67 | 1.47–1.89 |
| 2006 | 2.54 | 2.19–2.96 | 1.85 | 1.64–2.10 |
| 2007 | 2.53 | 2.17–2.95 | 1.84 | 1.63–2.09 |
| 2008 | 2.73 | 2.35–3.18 | 2 | 1.76–2.26 |
| Race | ||||
| White | Reference | Reference | ||
| Asian or PI | 0.86 | 0.78–0.95 | 0.88 | 0.81–0.96 |
| Black | 0.85 | 0.77–0.94 | 0.95 | 0.87–1.03 |
| Hispanic | 1.03 | 0.94–1.14 | 1.18 | 1.08–1.28 |
| Native American/Alaskan | 0.63 | 0.37–1.01 | 0.84 | 0.55–1.25 |
| Unknown | 0.6 | 0.40–0.86 | 0.61 | 0.43–0.84 |
| Grade | ||||
| I | Reference | Reference | ||
| II | 2.83 | 2.55–3.15 | 2.71 | 2.48–2.96 |
| III | 2.62 | 2.36–2.91 | 2.38 | 2.18–2.59 |
| IV | 2.96 | 2.65–3.31 | 2.61 | 2.37–2.88 |
| Unknown | 1.51 | 1.35–1.69 | 1.49 | 1.36–1.64 |
| DCIS Size | ||||
| <2cm | Reference | Reference | ||
| 2–5cm | 1.4 | 1.28–1.54 | 1.49 | 1.37–1.62 |
| >5cm | 1.89 | 1.53–2.31 | 2.16 | 1.78–2.61 |
| Unknown | 0.93 | 1.35–1.69 | 0.94 | 0.88–1.00 |
| SEER Registry | ||||
| West | Reference | Reference | ||
| Midwest | 0.83 | 0.76–0.91 | 0.95 | 0.87–1.03 |
| Northeast | 0.83 | 0.77–0.89 | 0.98 | 0.92–1.04 |
| Southeast | 1.13 | 1.04–1.22 | 1.25 | 1.16–1.34 |
| ER | ||||
| Positive | Reference | Reference | ||
| Borderline | 1 | 0.46–2.04 | 1.16 | 0.58–2.20 |
| Negative | 1.25 | 1.11–1.39 | 1.29 | 1.16–1.43 |
| Unknown | 0.69 | 0.59–0.80 | 0.8 | 0.7–0.92 |
| PR | ||||
| Positive | Reference | Reference | ||
| Borderline | 0.81 | 0.49–1.28 | 0.84 | 0.54–1.28 |
| Negative | 1.26 | 1.14–1.40 | 1.22 | 1.11–1.33 |
| Unknown | 1.19 | 1.03–1.37 | 1.09 | 0.95–1.24 |
Abbreviations: OR- Odds ratio, CI- 95% Confidence Interval
Discussion
Despite a paucity of evidence indicating a benefit, approximately 15% of women in our study underwent LNA at the time of lumpectomy for DCIS. We identified several factors that increase the likelihood of receiving a LNA. Many of these, such as increasing DCIS size, advancing tumor grade, negative hormone receptor status, and younger age reflect risk factors of underlying invasive carcinoma (2, 4, 9, 10). In particular, size of the DCIS component has been identified by the ASCO as a factor that may influence the use of a LNA, particularly SLNB. These guidelines allow for the use of LNA for DCIS foci greater than 5 cm in size. In our current series, however, only 1% of patients satisfied this criterion.
Potential risk factors for occult invasive carcinoma which we did not or could not identify from SEER data include the presence of comedo necrosis (11), microinvasion (2, 12) and palpable disease. Although these factors remain relatively rare, they are not without clinical significance. Schroen et al (13) surveyed and analyzed data from 459 American College of Surgeons members regarding their use of SLNB for DCIS. Overall, 79% of surgeons reported offering SLNB to patients with DCIS. When asked about factors influencing their decision to perform a SLNB for DCIS 84% reported the presence of microinvasion, 55% cited palpable disease, and 38% comedo necrosis.
Interestingly, our study noted age-related, racial, and regional disparities in use of LNA and SLNB. Several studies have documented age-related disparities in all treatment modalities for breast cancer(14, 15), including LNA and SLNB (16), independent of medical co-morbidities. While these previous studies demonstrated age-based disparities in treatment, these were most commonly noted among the most elderly subgroups. By contrast, we observed disparities in patients as young as 60 years old. Lack of access to Medicare insurance in the 60–65 year old subgroup may partially account for these disparities (17). However, Mandelblatt et al. demonstrated that patient-perceived ageism by their physician may also account for these differences (14). Other large epidemiological studies have demonstrated that racial/ethnic minorities, especially blacks, are less likely to undergo SLNB for early stage breast cancer (16–18). In our current study, both blacks and Asians were less likely to undergo SLNB compared to whites. While the scope of our study is limited in its ability to account for such differences, this disparity may be independent of socioeconomic status, and tumor characteristics (16). Regional disparities in treatment may also represent time-related shifting in geographic distributions of underserved, minority women that are less likely to receive standard of care. For example, in 2005, Southern women were less likely to receive SLNB as standard of care for breast cancer than Southern women in 1998 (18). In our study, they were more likely to undergo controversial LNA and SLNB for DCIS .
We also noted an increase in the rate of LNA within the time period of our study, even after publication of the 2005 ASCO guidelines. Despite this, the overall rate of LNA for DCIS appears to have decreased over the last two decades. In a SEER analysis encompassing the years 1988 to 2002, Porembka et al (19) reported LNA rates of 22% in patients undergoing lumpectomy for DCIS. During most of the time period of their study, however, SEER did not specifically code for the use of SLNB. The majority of LNAs in Porembka’s study, therefore, were axillary lymph node dissections (ALNDs). In 2002, when SEER was coding for both ALND and SLNB, 67% of LNAs were still ALNDs, but there was a growing trend in use of SLNB. By contrast, 75% of LNAs performed within the time period of our study were done using SLNB. The advent of SLNB has made LNA easier to do and has decreased the rate of complications relative to axillary lymph node dissection (20). This ease and perceived lack of morbidity are likely responsible for its increased use. Approximately 60,000 patients are diagnosed with DCIS each year (21). If we assume that 80% are eligible for breast conservation, then as many as 48,000 patients could receive lumpectomy for DCIS. If SLNB was performed on all of these patients, we would expect 2,304 (20%) to be positive due to previously occult invasive disease and another 1,536 (4%) to be positive in the setting of pure DCIS. Such aggressive use of SLNB would result in 3,360 (7%) patients with lymphedema using ACOSOG Z0010 estimates, as well as additional thousands with other morbidities, such as paresthesias and seromas (22). We would hope and expect that use of LNA and SLNB for patients undergoing lumpectomy for DCIS will decrease over time, with increased recognition of the improving diagnostic accuracy of core needle and vacuum assisted biopsies and the published morbidity rates of SLNB. It is possible, however, that indications for SLNB may change if it’s demonstrated that a SLNB is a more sensitive screening test for occult invasive cancer that is missed on routine pathologic examination, but identified on a more thorough evaluation prompted by a positive sentinel lymph node on immunohistochemistry (23).
Our study’s results should be interpreted with an understanding of the limitations inherent to studies utilizing population-based data such as SEER. SEER only represents 17 cancer registries nationwide and represents 26% of the total U.S. population. In addition, while, the population reporting to SEER is comparable to the general population of the United States with respect to measures of poverty and education; it over represents minority racial/ethnic groups, foreign-born, and urban populations (Yu (24) et al). Our patient population was also limited because we excluded cases of DCIS prior to 2000. We chose these years because they incorporated years before and after ASCO guidelines for the use of SLNB for DCIS and represented a more modern era of LNA. SEER data provide no information regarding individual patients’ past medical or family history that may predispose them to have higher risks of invasive breast cancer. Such patients may have been seen as having a significantly high risk of invasive disease to warrant LNA. Furthermore, we are unable to analyze all patients that received lumpectomy for DCIS. Some of these patients were undoubtedly found to have invasive breast cancer on final pathology, and SEER would code these patients according to their most invasive tumor component without regard for associated DCIS. The current analysis represents only patients that carried a diagnosis of DCIS after definitive surgery, and may represent, by definition, a lower risk group with respect to clinical predictors of invasive disease. Data completeness could have influenced our data. We had a large proportion of patients with unknown DCIS size of DCIS extent. It is possible that these patients were determined to have high-risk disease on the basis of their physical examination or imaging results. We did not exclude these patients from analysis since these predictors may not necessarily be available to all surgeons at the time of surgery for DCIS.
Due to improved breast cancer awareness and mammographic screening, the incidence of DCIS in women has steadily increased since 1970s with 32.5 out of 100,000 women being diagnosed every year (25). As more woman present for treatment for DCIS, further research directed at identifying the subgroup of women who should receive SLNB at time of initial operation as well as research directed at determining the oncologic significance of a positive SLNB will be necessary to minimize costs and morbidity of potential reoperation. We hoped to have helped in this matter by identifying disparities between current consensus guidelines and actual practice as well identifying common risk factors considered to be important in the decision to perform LNA or SLNB.
Acknowledgments
Supported by Grant Number UL1 RR024146 from the National Center for Research Resources (NCRR) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NNCRR or NIH. Information on NCRR is available athttp://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the Seventh Annual Academic Surgical Congress, Las Vegas, NV, February 14–16, 2012.
References
- 1.Mittendorf EA, Arciero CA, Gutchell V, et al. Core biopsy diagnosis of ductal carcinoma in situ: an indication for sentinel lymph node biopsy. Curr Surg. 2005;62:253–257. doi: 10.1016/j.cursur.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Wilkie C, White L, Dupont E, et al. An update of sentinel lymph node mapping in patients with ductal carcinoma in situ. Am J Surg. 2005;190:563–566. doi: 10.1016/j.amjsurg.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Moran CJ, Kell MR, Kerin MJ. The role of sentinel lymph node biopsy in ductal carcinoma in situ. Eur J Surg Oncol. 2005;31:1105–1111. doi: 10.1016/j.ejso.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Yen TW, Hunt KK, Ross MI, et al. Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: a guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg. 2005;200:516–526. doi: 10.1016/j.jamcollsurg.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Dillon MF, McDermott EW, Quinn CM, et al. Predictors of invasive disease in breast cancer when core biopsy demonstrates DCIS only. J Surg Oncol. 2006;93:559–563. doi: 10.1002/jso.20445. [DOI] [PubMed] [Google Scholar]
- 6.Intra M, Rotmensz N, Veronesi P, et al. Sentinel node biopsy is not a standard procedure in ductal carcinoma in situ of the breast: the experience of the European institute of oncology on 854 patients in 10 years. Ann Surg. 2008;247:315–319. doi: 10.1097/SLA.0b013e31815b446b. [DOI] [PubMed] [Google Scholar]
- 7.Sakr R, Barranger E, Antoine M, et al. Ductal carcinoma in situ: value of sentinel lymph node biopsy. J Surg Oncol. 2006;94:426–430. doi: 10.1002/jso.20578. [DOI] [PubMed] [Google Scholar]
- 8.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Miyake T, Shimazu K, Ohashi H, et al. Indication for sentinel lymph node biopsy for breast cancer when core biopsy shows ductal carcinoma in situ. Am J Surg. 2011;202:59–65. doi: 10.1016/j.amjsurg.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Hoorntje LE, Schipper ME, Peeters PH, et al. The finding of invasive cancer after a preoperative diagnosis of ductal carcinoma-in-situ: causes of ductal carcinoma-in-situ underestimates with stereotactic 14-gauge needle biopsy. Ann Surg Oncol. 2003;10:748–753. doi: 10.1245/aso.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Renshaw AA. Predicting invasion in the excision specimen from breast core needle biopsy specimens with only ductal carcinoma in situ. Arch Pathol Lab Med. 2002;126:39–41. doi: 10.5858/2002-126-0039-PIITES. [DOI] [PubMed] [Google Scholar]
- 12.van la Parra RF, Ernst MF, Barneveld PC, et al. The value of sentinel lymph node biopsy in ductal carcinoma in situ (DCIS) and DCIS with microinvasion of the breast. Eur J Surg Oncol. 2008;34:631–635. doi: 10.1016/j.ejso.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Schroen AT, Brenin DR. Breast cancer treatment beliefs and influences among surgeons in areas of scientific uncertainty. Am J Surg. 2010;199:491–499. doi: 10.1016/j.amjsurg.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Mandelblatt JS, Kerner JF, Hadley J, et al. Variations in breast carcinoma treatment in older medicare beneficiaries: is it black or white. Cancer. 2002;95:1401–1414. doi: 10.1002/cncr.10825. [DOI] [PubMed] [Google Scholar]
- 15.Haggstrom DA, Quale C, Smith-Bindman R. Differences in the quality of breast cancer care among vulnerable populations. Cancer. 2005;104:2347–2358. doi: 10.1002/cncr.21443. [DOI] [PubMed] [Google Scholar]
- 16.Reeder-Hayes KE, Bainbridge J, Meyer AM, et al. Race and age disparities in receipt of sentinel lymph node biopsy for early-stage breast cancer. Breast Cancer Res Treat. 2011;128:863–871. doi: 10.1007/s10549-011-1398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halpern MT, Chen AY, Marlow NS, et al. Disparities in receipt of lymph node biopsy among early-stage female breast cancer patients. Ann Surg Oncol. 2009;16:562–570. doi: 10.1245/s10434-008-0205-7. [DOI] [PubMed] [Google Scholar]
- 18.Chen AY, Halpern MT, Schrag NM, et al. Disparities and trends in sentinel lymph node biopsy among early-stage breast cancer patients (1998–2005) J Natl Cancer Inst. 2008;100:462–474. doi: 10.1093/jnci/djn057. [DOI] [PubMed] [Google Scholar]
- 19.Porembka MR, Abraham RL, Sefko JA, et al. Factors associated with lymph node assessment in ductal carcinoma in situ: analysis of 1988–2002 seer data. Ann Surg Oncol. 2008;15:2709–2719. doi: 10.1245/s10434-008-9947-5. [DOI] [PubMed] [Google Scholar]
- 20.Crane-Okada R, Wascher RA, Elashoff D, et al. Long-term morbidity of sentinel node biopsy versus complete axillary dissection for unilateral breast cancer. Ann Surg Oncol. 2008;15:1996–2005. doi: 10.1245/s10434-008-9909-y. [DOI] [PubMed] [Google Scholar]
- 21.Goyal S, Vicini F, Beitsch PD, et al. Ductal carcinoma in situ treated with breast-conserving surgery and accelerated partial breast irradiation: comparison of the Mammosite registry trial with intergroup study E5194. Cancer. 2011;117:1149–1155. doi: 10.1002/cncr.25615. [DOI] [PubMed] [Google Scholar]
- 22.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13:491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Cody HSVZ, Kimberly J. Point: Sentinel Lymph Node Biopsy Is Indicated for Patients With DCIS. The Journal of the National Comprehensive Cancer Network. 2003:9. doi: 10.6004/jnccn.2003.0018. [DOI] [PubMed] [Google Scholar]
- 24.Yu JB, Gross CP, Wilson LD, et al. NCI SEER public-use data: applications and limitations in oncology research. Oncology (Williston Park) 2009;23:288–295. [PubMed] [Google Scholar]
- 25.Virnig BA, Tuttle TM, Shamliyan T, et al. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102:170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]