Abstract
Objective
To relate dietary fat types to cognitive change in healthy community-based elders.
Methods
Among 6,183 older participants in the Women’s Health Study, we related intake of major fatty acids (FAs) (saturated [SFA], mono-unsaturated [MUFA], total poly-unsaturated [PUFA], trans-unsaturated) to late-life cognitive trajectory. Serial cognitive testing, conducted over 4 years, began 5 years post-dietary assessment. Primary outcomes were global cognition (averaging tests of general cognition, verbal memory and semantic fluency) and verbal memory (averaging tests of recall). We used analyses of response profiles and logistic regression to estimate multivariable-adjusted differences in cognitive trajectory and risk of worst cognitive change (worst 10%) by fat intake.
Results
Higher SFA intake was associated with worse global cognitive (p-linear-trend=0.008) and verbal memory (p-linear-trend=0.01) trajectories. There was a higher risk of worst cognitive change, comparing highest vs. lowest SFA quintiles: the multivariable-adjusted odds ratio (OR) (95% confidence interval, CI) was 1.64 (1.04,2.58) for global cognition and 1.65 (1.04,2.61) for verbal memory. By contrast, higher MUFA intake was related to better global cognitive (p-linear-trend<0.001) and verbal memory (p-linear-trend=0.009) trajectories, and lower OR (95% CI) of worst cognitive change in global cognition (0.52 [0.31,0.88]) and verbal memory (0.56 [0.34,0.94]). Total fat, PUFA, and trans fat intakes were not associated with cognitive trajectory.
Interpretation
Higher SFA intake was associated with worse global cognitive and verbal memory trajectories, while higher MUFA intake was related to better trajectories. Thus, different consumption levels of the major specific fat types, rather than total fat intake itself, appeared to influence cognitive aging.
INTRODUCTION
The continuum of cognitive decline is important in dementia research; early decline is likely more amenable than clinical-level impairments to preventive or disease-modifying interventions.1–3 Emerging evidence links dietary fat to late-life cognition; mechanisms may involve lipid profiles,4, 5 inflammation6–8, cardiovascular health9–14 or neuroprotection15, 16. While these potential links are compelling, it is challenging to implement long-term randomized trials of varying intakes of major fatty acids (FA). Thus, consensus has emerged that more well-conducted, large-scale prospective studies with serial cognitive assessments are needed to address long-term relations of fats to cognitive aging.17
We examined relations of major fat types to cognitive change over 4 years among ~6,000 older, community-dwelling participants of the Women’s Health Study (WHS). We hypothesized: worse cognitive trajectories among women with higher vs. lower consumption of saturated FA (SFA) and trans fats (“bad fats”) and better trajectories among women with higher vs. lower intake of monounsaturated FA (MUFA) and polyunsaturated FA (PUFA) (“good fats”).
METHODS
Participants
Women’s Health Study
The WHS was a randomized, double-blind, placebo-controlled 2×2 trial of aspirin and vitamin E supplements for primary prevention of heart disease and cancer18. From 1992–1995, 39,876 US female health professionals, aged ≥45 years, were randomized to one of four factorial groups. All were initially free of cancer (except nonmelanoma skin cancer), myocardial infarction, stroke, transient cerebral ischemia, liver disease, renal disease, peptic ulcer, and gout; women using corticosteroids, anticoagulants, or vitamin A and E supplements were excluded. Participants completed annual questionnaires updating information on health and lifestyle factors and clinical outcomes. The trial was completed on March 31, 2004; total follow-up was >99%.19
The Cognitive Sub-study
In 1998, cognitive testing began among WHS participants aged ≥65 years. Of 7,175 age-eligible participants, 6,377 (89%) completed the initial assessment. Follow-up assessments occurred in 2000 and 2002: 5,692 (89%) of those who completed the initial assessment also participated in a second wave of assessment; 5,226 women (82%) participated in wave 3. The mean duration was 2 years between each wave. This study was approved by the Institutional Review Board of Brigham and Women’s Hospital (Boston, MA).
The Cognitive Function Assessment
Cognitive exams were conducted via telephone by hypotheses-blind interviewers and consisted of: (1) Telephone Interview for Cognitive Status (TICS); (2) immediate and (3) delayed recall trials of the East Boston Memory Test; (4) delayed recall trial of the TICS 10-word list; and (5) category fluency. The TICS20 (range: 0–41 points) is a test of general cognitive function, similar to the Mini-Mental State Examination21, and has high reliability and validity. The East Boston Memory Test (EBMT)22 is a verbal (episodic) memory task of paragraph recall (range: 0–12 points) and involves immediate and 15-minute delayed recalls. The 15-minute delayed recall of the TICS 10-word list (range: 0–10 points) also assesses verbal memory. Lastly, category fluency (naming as many different animals as possible in one minute) captures language and executive functions such as abstract conceptualization and use of strategy.23
Reliability and Validity of Telephone Cognitive Assessments
To examine test-retest reliability, we administered the TICS twice after a one-month interval among 35 similar older women; the Pearson correlation was 0.7 (p<0.001). Regarding inter-rater reliability, intraclass correlations were >0.95 on each test. In a validity study, 61 well-educated older women completed both telephone-based and comprehensive (21-test) in-person assessments; the Pearson correlation was 0.81 between the global scores based on telephone vs. in-person tests. Also, expected relations of age and APOE ε424 to telephone-based cognition have been observed. In a further validation, cognitive impairment determined by telephone assessment was strongly associated with clinically-diagnosed dementia three years later25.
Ascertainment of Diet
A 131-item, semi-quantitative food-frequency questionnaire (FFQ) was administered at WHS baseline. For each item, portion size was specified, and participants were asked how often, on average, during the past year they consumed that amount. We computed nutrient scores by multiplying the frequency of consumption of each food unit by the nutrient content of that portion size according to US Department of Agriculture food composition tables, supplemented with information from manufacturers. Details on development, use, reproducibility and validity of the FFQ have been published previously.26, 27
Ascertainment of covariates
Information on covariates was obtained from annual questionnaires. Validation work demonstrated high accuracy of self-reported conditions (e.g., diabetes)28.
Population for Analysis
We excluded 194 women from the cognitive sub-study (n=6,377) with incomplete FFQ data. Thus, there were 6,183 participants for the analysis who completed initial testing; of these, 5,532 (89%) completed wave 2; 5,084 (82%) completed wave 3 (Supplemental Fig. 1).
Statistical Analysis
We categorized SFA, MUFA, PUFA (primarily comprised of linolenic acid), trans fat and total fat into quintiles. We conducted analyses using the multivariate nutrient density method,29 in which fats are expressed as percentages of total energy and analyzed in the same model, along with protein as a percentage of energy and total energy intake (i.e., isocaloric); coefficients can be interpreted as the effect of substituting a specific amount of energy from fat for the same amount of energy from carbohydrates. This is the preferred analytic method for dietary components comprising relatively large proportions of calories27, 29. Primary outcomes were: global score, calculated by averaging z-scores from the TICS, delayed 10-word recall, immediate and delayed EBMT and category fluency tests; verbal memory, calculated by averaging z-scores of the EBMT and 10-word immediate and delayed recall trials. Outcomes were normally-distributed.
First, we examined mean scores across the three assessments, by fat quintiles, while adjusting for trial design variables (aspirin/vitamin E randomization status) as well as socio-demographic factors (age at initial testing, education, high household income, race) found to be the greatest potential confounders. Scores were repeated continuous outcomes, and we modeled the effect of fat intake using time-by-fat quintile interaction terms. Because the pattern of scores was non-linear (likely due to learning effects typically concentrated in the early test administrations, such as between the first and second interviews30), we used general linear models of response profiles to estimate the means, and modeled timepoints as binary indicator variables (i.e., time 1, 2 or 3)30, 31. This approach imposes minimal structure on outcome trends, permits valid estimation of effects in non-linear data and can handle unbalanced patterns of longitudinal observations due to missing responses. We fitted models by maximum likelihood, incorporating longitudinal correlations within participants, using unstructured covariance matrices. For statistical testing, we used Wald tests31, and examined linear trends for fat quintiles continuously (participants in a given quintile were assigned the median value). Secondarily, because initial cognitive score was related to performance during follow-up, we repeated the above approach including interaction terms of initial (time 1) score-x-follow-up period (i.e., time 2 or 3). Analyses were conducted utilizing PROC MIXED in SAS, version 9.2 (SAS Institute, Inc., Cary, NC, USA).
We considered other variables (based on the literature and distributions in our sample) for the multivariable-adjusted models. Thus, for primary analysis, fully-adjusted models included: age at first cognitive interview (years), highest attained education (bachelor’s degree or above vs. associate’s degree), aspirin and vitamin E randomization assignment, race (white/non-white), household income (≥$50,000 per year/less), body mass index (BMI) (<25, 25.0–29.9, or ≥30 kg/m2), current smoking (yes/no), postmenopausal hormone use (ever/never), hypertension (self-reported history, use of antihypertensive medications, or systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg; yes/no), elevated cholesterol (self-reported history, use of lipid-lowering medications, or blood cholesterol >240 mg/dL; yes/no), depression (self-reported history; yes/no), diabetes (self-reported history; yes/no), daily alcohol consumption (≥1 drinks/day), and moderate or above frequency of exercise (≥1 times/week). Of note, self-reported household income (reported in categories as high as $100K+/yr or as low as <$10K/yr) was not available in 400 women; as our objective was to account for higher vs. lower income, these participants were placed in the reference (under $50K) group. Similarly, women missing self-reported white race or depression information (53 and 74, respectively) were placed in the non-white race (reference) and non-depressed (reference) categories, rather than excluded.
In a secondary analysis, we calculated odds ratios (ORs) of worst change in cognitive performance over 4 years (i.e., between the first and third testing waves). This was defined as being in the bottom 10% of the distributions of the global or verbal memory change scores. Such a population-based 10% cutpoint is common in cognitive research32 and has high sensitivity and specificity for impairment.33 Logistic regression models adjusted for covariates described above as well as the time between assessments (years). However, given the influence of initial score on the amount of absolute change that can be observed, we constructed an additional model including a term for residual initial score (after adjustment for intake of fats, protein, energy and the other covariates in linear regression), to account for initial performance while reducing bias. These analyses were restricted to the subset within our study population who completed both first and third testing waves (n=5,072 for global score; n=5,069 for verbal memory). We also addressed effects of substituting energy (e.g., 5%) from “good” fat (MUFA, PUFA) for that same energy amount from “bad” fat (SFA, trans fat) on the ORs (with confidence limits) of worst change, using the estimates, standard errors and covariances for the different fats obtained directly from the multivariate nutrient density models27.
We repeated the primary analysis models after excluding 455 women who developed cardiovascular disease (from the beginning of the WHS parent study to the end of the cognitive sub-study), as CVD is a major risk factor for late-life cognitive dysfunction34 and strongly related to fat intake.14, 35, 36 CVD included all medical record-confirmed non-fatal myocardial infarction, non-fatal stroke, cardiovascular-related deaths, or vascular disease as evidenced by coronary artery bypass graft or percutaneous transluminal coronary angioplasty or stenting.19
Finally, we addressed possible interactions by two key factors: 1) age, as prior work37 in a similar cohort revealed significant interactions between age and fat intake on cognitive decline; 2) history of elevated cholesterol, due to its central relation to fat intake.
RESULTS
Baseline characteristics
Women with higher intakes of all fats had higher BMI, higher prevalence of current smoking, and lower prevalence of moderate-or-above exercise. Women with higher SFA and MUFA intakes had lower prevalence of hypercholesterolemia and higher prevalence of daily alcohol consumption. Those with higher PUFA intake had higher prevalence of daily alcohol and lower prevalence of high income. Finally, women with higher trans fat intake had lower prevalence of high income and education (Table 1).
Table 1.
Characteristics of the Sample at Dietary Assessment, by Quintiles of Major Fat Types (n=6,183)*
| Saturated fat Quintiles | Mono-unsaturated fat Quintiles | Poly-unsaturated fat Quintiles | Trans fat Quintiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 1 | 3 | 5 | 1 | 3 | 5 | 1 | 3 | 5 | |
| Mean age at study entry (years) | 66.5 | 66.3 | 65.9 | 66.4 | 66.1 | 66.1 | 66.5 | 66.1 | 66.3 | 66.6 | 66.1 | 66.0 |
| Mean age at initial cognitive testing (years) | 72.1 | 71.9 | 71.6 | 72.0 | 71.7 | 71.8 | 72.1 | 71.8 | 72.0 | 72.3 | 71.7 | 71.6 |
| Mean body mass index (kg/m2) | 24.8 | 25.9 | 26.3 | 25.1 | 25.8 | 26.1 | 25.4 | 25.7 | 25.8 | 25.1 | 25.8 | 26.2 |
| Household income ≥$50,000/year | 26 | 23 | 23 | 26 | 25 | 25 | 29 | 23 | 23 | 29 | 26 | 21 |
| Caucasian race | 94 | 97 | 97 | 95 | 97 | 97 | 96 | 97 | 96 | 95 | 97 | 97 |
| Randomized to aspirin | 52 | 50 | 50 | 51 | 49 | 50 | 52 | 50 | 52 | 50 | 52 | 50 |
| Randomized to vitamin E | 53 | 50 | 48 | 52 | 50 | 49 | 49 | 50 | 50 | 52 | 51 | 51 |
| Bachelor’s degree or higher education | 39 | 31 | 30 | 38 | 34 | 31 | 37 | 34 | 31 | 40 | 35 | 28 |
| History of hypertension | 39 | 41 | 41 | 39 | 39 | 42 | 39 | 41 | 42 | 40 | 39 | 44 |
| History of elevated cholesterol | 54 | 42 | 35 | 50 | 42 | 39 | 43 | 43 | 41 | 48 | 42 | 42 |
| History of diabetes | 4 | 3 | 4 | 5 | 4 | 3 | 4 | 3 | 4 | 4 | 4 | 3 |
| Current smoking | 5 | 9 | 19 | 5 | 9 | 18 | 8 | 9 | 13 | 6 | 11 | 14 |
| Current hormone use | 43 | 42 | 36 | 43 | 42 | 38 | 41 | 41 | 40 | 44 | 41 | 37 |
| History of depression | 6 | 7 | 7 | 6 | 6 | 6 | 7 | 5 | 6 | 5 | 6 | 6 |
| Exercise level | ||||||||||||
| ≥4 times per week | 19 | 10 | 7 | 18 | 11 | 8 | 14 | 12 | 11 | 21 | 11 | 7 |
| 1–3 times per week | 36 | 29 | 22 | 34 | 29 | 20 | 32 | 28 | 25 | 35 | 28 | 23 |
| Rare or never | 45 | 61 | 71 | 48 | 61 | 71 | 55 | 60 | 63 | 44 | 61 | 71 |
| Alcohol use | ||||||||||||
| 1 or more drinks/day | 10 | 12 | 16 | 10 | 11 | 18 | 11 | 12 | 16 | 12 | 13 | 12 |
| 1–6 drinks/week | 23 | 31 | 29 | 23 | 31 | 30 | 26 | 31 | 28 | 27 | 31 | 28 |
| 1–3 drinks/month or less, or never | 67 | 57 | 55 | 67 | 58 | 52 | 64 | 57 | 56 | 61 | 57 | 60 |
| Mean total calories (kcal/day) | 1769 | 1753 | 1744 | 1768 | 1754 | 1740 | 1761 | 1754 | 1750 | 1765 | 1752 | 1747 |
| Total fat (median % energy) | 22.4 | 29.5 | 36.4 | 22.1 | 29.5 | 37.1 | 24.3 | 29.7 | 34.4 | 23.8 | 30.0 | 33.9 |
| Protein (median % energy) | 17.9 | 18.5 | 18.5 | 18.5 | 18.4 | 18.2 | 18.5 | 18.5 | 17.7 | 19.0 | 18.6 | 17.0 |
| Carbohydrate (median % energy) | 59.3 | 51.9 | 44.7 | 59.3 | 52.0 | 44.3 | 56.8 | 51.8 | 47.5 | 56.6 | 51.1 | 48.7 |
| SFA† (median % energy) | 7.0 | 9.8 | 13.1 | 7.3 | 9.9 | 12.3 | 8.8 | 9.9 | 10.5 | 7.7 | 10.2 | 11.2 |
| MUFA† (median % energy) | 8.0 | 11.0 | 13.6 | 7.8 | 11.0 | 14.4 | 8.9 | 11.1 | 12.8 | 8.4 | 11.1 | 12.9 |
| PUFA† (median % energy) | 5.1 | 5.8 | 6.0 | 4.6 | 5.7 | 6.8 | 4.1 | 5.7 | 7.7 | 5.1 | 5.7 | 6.2 |
| Trans fat† (median % energy) | 0.65 | 1.06 | 1.37 | 0.64 | 1.07 | 1.46 | 0.84 | 1.10 | 1.20 | 0.55 | 1.04 | 1.84 |
| Median PUFA/SFA† ratio | 0.75 | 0.59 | 0.45 | 0.66 | 0.58 | 0.55 | 0.45 | 0.57 | 0.77 | 0.69 | 0.56 | 0.56 |
Figures are percentages of respondents, unless stated otherwise. Due to rounding, percentages may not add to 100.
SFA = saturated fatty acid; MUFA = mono-unsaturated fatty acid; PUFA = poly-unsaturated fatty acid; Trans = trans-unsaturated fatty acid.
Prospective analyses of cognitive change
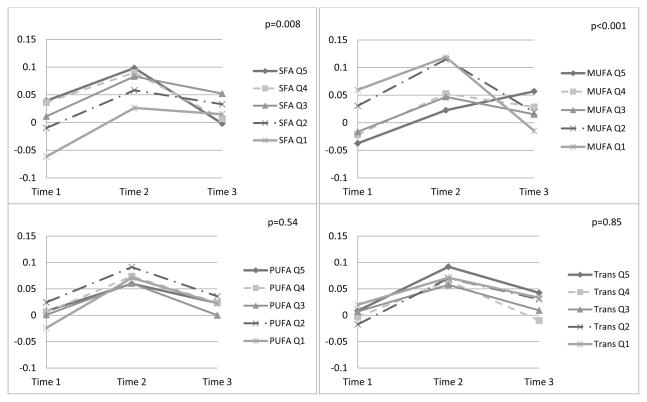
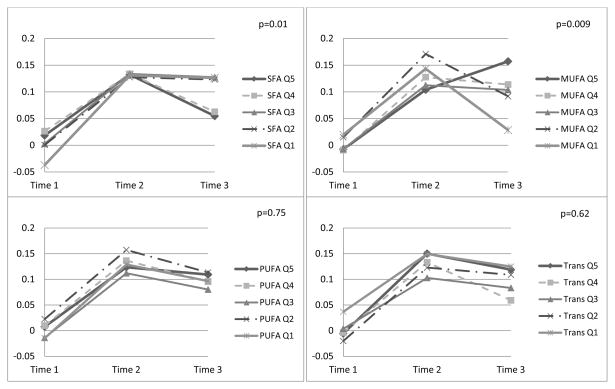
Lower SFA and higher MUFA intakes were significantly related to more favorable global and verbal memory scores over time, after adjusting for socio-demographic variables (data not shown). Multivariable-adjusted results were similar (Figures 1 and 2). Wald p-values for linear trends of time-by-fat quintiles interactions illustrated significantly worse trajectories with higher SFA intakes for global score (p=0.008) and verbal memory (p=0.01). Similarly, trajectories were more favorable among those with higher MUFA intake, for global score (p<0.001) and verbal memory (p=0.009). There were no associations of PUFA, trans fat or total fat with cognitive change. Results were similar when models included adjustment for initial score-by-follow-up time interactions (Supplemental Figure 2, results shown for SFA and MUFA only).
Figure 1. Multivariable-adjusted Least-squares Means Global Cognitive* Scores over 4 years, by Quintiles of Fat Types (n=6,172)†.
* Global score combines results of the TICS (Telephone Interview for Cognitive Status), category fluency, immediate and delayed recall trials of East Boston Memory Test (EBMT), and delayed recall trial of the TICS 10-word list; verbal memory score combines results of the immediate and delayed recall trials of EBMT and the TICS 10-word list; mean (SD) span between the 1st and 3rd assessments was 4.0 (0.3) years. Adjusted least-squares means were obtained from the repeated measures analysis models involving N=6,172 participants.
† SFA = saturated fatty acid; MUFA = mono-unsaturated fatty acid; PUFA = poly-unsaturated fatty acid; Trans = trans fatty acid; Q1 = lowest quintile of intake; Q5 = highest quintile of intake; models adjusted for mean-centered age at initial cognitive assessment (continuous, in years), educational attainment (bachelor’s degree or above versus associate’s degree), race (white/non-white), annual household income (≥$50,000/less), randomized treatment assignment (aspirin, vitamin E), other fat intake, protein intake, total energy intake, body mass index (<25, 25.0–29.9, or ≥30 kg/m2), current cigarette smoking (yes/no), postmenopausal hormone use (ever/never), history of hypertension (yes/no), history of elevated cholesterol (yes/no), history of depression (yes/no), history of diabetes (yes/no), daily alcohol consumption (yes/no), exercise (≥1 times per week/less), and all covariate-by-time interactions. P-values are from the Wald tests of interactions between fat type consumption level (medians-per-quintile) and time. N.B.: N=11 women were missing data at initial cognitive testing on components tests required to compute the global score.
Figure 2. Multivariable-adjusted Least-squares Means Verbal Memory* Scores over 4 years, by Quintiles of Fat Types (n=6,176)†.
* Global score combines results of the TICS (Telephone Interview for Cognitive Status), category fluency, immediate and delayed recall trials of East Boston Memory Test (EBMT), and delayed recall trial of the TICS 10-word list; verbal memory score combines results of the immediate and delayed recall trials of EBMT and the TICS 10-word list; mean (SD) span between the 1st and 3rd assessments was 4.0 (0.3) years. Adjusted least-squares means were obtained from the repeated measures analysis models involving N=6,176 participants.
† SFA = saturated fatty acid; MUFA = mono-unsaturated fatty acid; PUFA = poly-unsaturated fatty acid; Trans = trans fatty acid; Q1 = lowest quintile of intake; Q5 = highest quintile of intake; models adjusted for mean-centered age at initial cognitive assessment (continuous, in years), educational attainment (bachelor’s degree or above versus associate’s degree), race (white/non-white), annual household income (≥$50,000/less), randomized treatment assignment (aspirin, vitamin E), other fat intake, protein intake, total energy intake, body mass index (<25, 25.0–29.9, or ≥30 kg/m2), current cigarette smoking (yes/no), postmenopausal hormone use (ever/never), history of hypertension (yes/no), history of elevated cholesterol (yes/no), history of depression (yes/no), history of diabetes (yes/no), daily alcohol consumption (yes/no), exercise (≥1 times per week/less), and all covariate-by-time interactions. P-values are from the Wald tests of interactions between fat type consumption level (medians-per-quintile) and time. N.B.: N=7 women were missing data at initial cognitive testing on components tests required to compute the verbal memory composite.
For further illustration of differences in cognitive outcomes by fat consumption, the mean differences in change over 4 years can be obtained directly from the models (Table 2). For example, multivariable-adjusted mean differences (95% confidence intervals [CI]) in 4-year change were −0.12 (−0.20, −0.03) standard units for global score and −0.13 (−0.23, −0.03) standard units for verbal memory, comparing the highest vs. lowest SFA quintiles. Comparing women in the highest vs. lowest MUFA quintile, the adjusted mean differences (95% CI) in change were 0.17 (0.07,0.26) standard units for global score and 0.16 (0.04,0.27) standard units for verbal memory. To help interpret these differences, we contrasted them with the estimate for the relation of age to cognitive change. Estimates for 4-year cognitive change comparing women in the highest vs. lowest SFA quintiles were similar to those for women 5-to-6 years apart at the start of testing (i.e., 5–6 added years of aging). By contrast, mean differences in change comparing the extreme MUFA quintiles were equivalent to 6-to-7 fewer years of aging.
Table 2.
Multivariable-adjusted* Mean Differences (95% Confidence Intervals) in 4-year Cognitive Change†, by Fat Quintiles.
| GLOBAL SCORE† (in standard units) | VERBAL MEMORY† (in standard units) | |
|---|---|---|
| SFA‡ | ||
| Quintile 1 | 0.00 | 0.00 |
| Quintile 2 | −0.03 (−0.10, 0.03) | −0.04 (−0.12, 0.04) |
| Quintile 3 | −0.04 (−0.11, 0.04) | −0.04 (−0.12, 0.05) |
| Quintile 4 | −0.11 (−0.19, −0.03) | −0.13 (−0.22, −0.03) |
| Quintile 5 | −0.12 (−0.20, −0.03) | −0.13 (−0.23, −0.03) |
| MUFA‡ | ||
| Quintile 1 | 0.00 | 0.00 |
| Quintile 2 | 0.06 (−0.00, 0.13) | 0.07 (−0.01, 0.15) |
| Quintile 3 | 0.11 (0.03, 0.18) | 0.10 (0.01, 0.19) |
| Quintile 4 | 0.12 (0.04, 0.21) | 0.11 (0.02, 0.21) |
| Quintile 5 | 0.17 (0.07, 0.26) | 0.16 (0.04, 0.27) |
| PUFA‡ | ||
| Quintile 1 | 0.00 | 0.00 |
| Quintile 2 | −0.03 (−0.09, 0.02) | −0.02 (−0.09, 0.05) |
| Quintile 3 | −0.05 (−0.11, 0.01) | −0.02 (−0.09, 0.05) |
| Quintile 4 | −0.03 (−0.09, 0.03) | −0.03 (−0.10, 0.05) |
| Quintile 5 | −0.03 (−0.10, 0.04) | −0.01 (−0.09, 0.07) |
| Trans‡ | ||
| Quintile 1 | 0.00 | 0.00 |
| Quintile 2 | 0.03 (−0.03, 0.10) | 0.04 (−0.03, 0.11) |
| Quintile 3 | −0.01 (−0.08, 0.05) | −0.01 (−0.09, 0.07) |
| Quintile 4 | −0.02 (−0.09, 0.05) | −0.03 (−0.11, 0.06) |
| Quintile 5 | 0.02 (−0.05, 0.09) | 0.04 (−0.05, 0.12) |
Models adjusted for age at 1st cognitive assessment (years), educational attainment (bachelor’s degree or above vs. associate’s degree), race (white/non-white), annual household income (≥$50,000/less), randomized treatment assignment (aspirin, vitamin E), other fat intake, protein intake, total energy intake, body mass index (<25, 25.0–29.9, or ≥30 kg/m2), current cigarette smoking (yes/no), postmenopausal hormone use (ever/never), history of hypertension (yes/no), history of elevated cholesterol (yes/no), history of depression (yes/no), history of diabetes (yes/no), daily alcohol consumption (yes/no), exercise (≥1 times per week/less), and all covariate-by-time interactions. For all fat types, the reference category=quintile 1 (lowest quintile of intake).
Global score combines results of TICS (Telephone Interview for Cognitive Status), category fluency, immediate and delayed recall trials of East Boston Memory Test (EBMT), and delayed recall trial of the TICS 10-word list. Verbal memory score combines results of the immediate and delayed recall trials of the EBMT and the TICS 10-word list. Mean (SD) interval between first and third waves of cognitive assessment = 4.0 (0.3) years. Adjusted mean differences were obtained from the repeated measures analysis models involving N=6,172 participants for the global score and N=6,176 participants for verbal memory.
SFA = saturated fatty acid; MUFA = mono-unsaturated fatty acid; PUFA = poly-unsaturated fatty acid; Trans = trans-unsaturated fatty acid.
Findings from analyses addressing worst cognitive change were consistent with results from primary analyses (Table 3). Women with the highest vs. lowest SFA intake had 60–70% greater odds of worst change on global score and verbal memory. By contrast, women with the highest vs. lowest MUFA intake had 40–50% lower odds of worst change. Results were the same in models that adjusted for residual initial cognitive scores (data not shown). Finally, when estimating impacts of “good” fat vs. “bad” fat substitutions, we found that replacing 5% of energy from SFA with the same amount of energy from MUFA was associated with significantly lower odds of worst change: ORs were 0.47 (95% CI=0.25,0.89) for global score and verbal memory. There were no significant associations of substituting trans fat with MUFA or PUFA for either outcome. (Substitution data shown in Supplemental Table 1).
Table 3.
Multivariable-adjusted Odds Ratios (95% CI) of Worst Cognitive Change over 4 years, by Quintiles of Major Fats*
| Fat Type and Cognitive Outcome | Quintile of Fat Intake | Linear Trend | ||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | P-value | |
| Saturated fat | ||||||
| Global score† | 1.0 | 1.23 (0.88, 1.71) | 1.28 (0.88, 1.87) | 1.54 (1.02, 2.33) | 1.64 (1.04, 2.58) | 0.02 |
| Verbal memory† | 1.0 | 1.35 (0.97, 1.89) | 1.39 (0.95, 2.02) | 1.63 (1.08, 2.47) | 1.65 (1.04, 2.61) | 0.02 |
| Mono-unsaturated fat | ||||||
| Global score† | 1.0 | 0.96 (0.68, 1.34) | 0.73 (0.49, 1.08) | 0.66 (0.42, 1.03) | 0.52 (0.31, 0.88) | 0.006 |
| Verbal memory† | 1.0 | 0.89 (0.64, 1.24) | 0.65 (0.44, 0.97) | 0.67 (0.43, 1.04) | 0.56 (0.34, 0.94) | 0.02 |
| Poly-unsaturated fat | ||||||
| Global score† | 1.0 | 1.28 (0.95, 1.73) | 1.23 (0.90, 1.69) | 1.33 (0.96, 1.85) | 1.37 (0.97, 1.94) | 0.09 |
| Verbal memory† | 1.0 | 1.11 (0.83, 1.49) | 0.93 (0.68, 1.27) | 1.11 (0.80, 1.53) | 1.03 (0.73, 1.46) | 0.82 |
| Trans fat | ||||||
| Global score† | 1.0 | 0.90 (0.66, 1.23) | 0.94 (0.68, 1.32) | 1.08 (0.76, 1.52) | 0.76 (0.52, 1.11) | 0.49 |
| Verbal memory† | 1.0 | 0.85 (0.62, 1.17) | 1.05 (0.75, 1.45) | 1.00 (0.71, 1.41) | 0.72 (0.49, 1.06) | 0.32 |
Models adjusted for mean-centered age at initial cognitive assessment (continuous, in years), educational attainment (bachelor’s degree or above versus associate’s degree), aspirin and vitamin E randomization assignment, race (white/non-white), household income level (≥$50,000 per year/less), body mass index (kg/m2), cigarette smoking (current/past/never), postmenopausal hormone use (ever/never), history of hypertension (yes/no), history of elevated cholesterol (yes/no), history of depression (yes/no), history of diabetes (yes/no), alcohol consumption (none/rare, 1–6 per week, >=7 per week), exercise frequency (never/rare, 1–3 times per week, >=4 times per week), other fat intake, protein intake, total energy intake, and time span between 1st and 3rd cognitive assessment (years). For all fat types, the reference category=lowest (1st) quintile of intake.
Global score combines results of the TICS (Telephone Interview for Cognitive Status), category fluency, immediate and delayed recall trials of East Boston Memory Test (EBMT), and delayed recall trial of the TICS 10-word list; verbal memory score combines results of the immediate and delayed recall trials of EBMT and the TICS 10-word list; mean (SD) span between the 1st and 3rd assessments was 4.0 (0.3) years. Of 5,084 women who participated in waves 1 and 3, there were 12 missing data on components tests required to compute the global score (N=5,072 for analysis) and 15 missing data on components tests required to compute the verbal memory composite (N=5,069 for analysis).
In analyses excluding women with CVD, results were similar to those from the primary analyses: i.e., better trajectories in global score (n=5,717) and verbal memory (n=5,721) with lower SFA and higher MUFA intakes (p-linear-trends≤0.01; data not shown).
There were no significant three-way-interactions for age (above/below median age-at-initial testing [71 years]) or hypercholesterolemia with fat intake and time (data not shown). Of note, there appeared to be differences in initial global scores by SFA and MUFA intakes – driven by performance among persons below median age (e.g., p-interaction<0.01 for age-x-SFA intake on initial global score only; data not shown in Figure 1); no such differences or interactions were observed for verbal memory.
DISCUSSION
In this study of community-dwelling older women, higher saturated fat intake was associated with a poorer 4-year trajectory of global cognition and verbal memory. By contrast, higher MUFA intake was related to better global cognitive and verbal memory trajectory. The magnitude of relations found for extreme fat quintiles and cognitive change were equivalent to ~6 years of aging. Regarding worst global cognitive or verbal memory 4-year change, there was a 60–70% higher risk comparing the highest vs. lowest SFA quintiles, but a 40–50% lower risk comparing the highest vs. lowest MUFA quintiles. There were no associations of PUFA, trans fat or total fat with cognitive change.
The results regarding SFA are similar to those from prior large-scale studies38–40 that examined “bad” fats with comparable methodology (e.g., adjusting for presence of other fats). For example, Morris et al.38 observed that increasing SFA (p-trend=0.04) and trans fat intakes (p-trend=0.07) were linearly associated with faster global cognitive decline over 5.6 years among 2,560 age-65+ participants. Similarly, among 1,486 older women of the Nurses’ Health Study with type 2 diabetes, higher SFA and trans intakes were associated with worse cognitive decline.40 Finally, Eskelinen et al.39 reported a 2-fold elevated risk of MCI (mild cognitive impairment) among 1,341 participants with high vs. low mid-life SFA intake; although the analysis was cross-sectional, the 21-year interval between diet assessment (mean age=50 years) and cognitive examination may have approximated prospective development of MCI. Regarding our null findings for trans fats, a potential explanation is their narrower distribution among these generally healthy women, compared to that in other cohorts38, 40. Although trans fat intake can be quite high among Americans,41 the median percentage of energy from trans fat in the highest quintile for our cohort was 1.8%.
There are limited data available from larger-scale prospective studies regarding “good” fats. Solfrizzi et al.42, 43 identified significant relations of higher MUFA and PUFA intakes to better cognitive aging. Devore et al.40 observed inverse associations of higher MUFA consumption (p-trend=0.06) and higher intake of PUFA relative to SFA (p-trend=0.03) with global cognitive change among older diabetic women. Navqi et al. found significantly less 3-year memory decline among 482 Women’s Health Initiative participants in the highest vs. lowest MUFA intake quartiles (SFA and trans fat were not significantly related to cognitive change; associations for PUFAs were not reported)44. Morris et al.38 did not find significant associations of MUFA or PUFA with global cognitive decline, but estimates suggested inverse relations. Vercambre and colleagues37 reported inverse relations of MUFA and PUFA to 5-year global cognitive decline among 2,551 women with CVD or risk factors – but only among the oldest (73–91 years). Variability in study designs may partly explain inconsistency in findings. For example, investigators used different methods for defining fat types (e.g., total PUFA, PUFA from spreads45, linolenic acid (n-6 PUFA) only46), may address different sub-groups (e.g., those with diabetes40), or may not account for other fat types – as is recommended by experts in the field.38 Finally, unsaturated fats may be more susceptible to random misclassification when using only a one-time or a lower-precision diet instrument.
Strengths of this study include its prospective design, large sample, well-validated FFQ, availability of numerous health and lifestyle covariates, high follow-up and focus on late-life cognitive change. Limitations should also be considered. First, repeated diet assessments were not available – increasing random measurement error, which could attenuate associations. Second, reverse causation is possible; however, there was a 5-year lag between the FFQ and initial cognitive assessment, and it seems unlikely that many women had substantial cognitive impairment at study entry, as all WHS participants had successfully completed a pre-randomization run-in phase that scrutinized compliance to assigned treatment. Third, generalizability of findings among these mostly Caucasian women is an issue. Although it seems unlikely that basic biological relations would differ greatly, further research on dietary fat and cognition among racial/ethnic minorities and men is needed. Lastly, residual confounding is possible, and the data should be interpreted with appropriate caution.
In conclusion, these data suggest that elevated SFA intake is related to worse late-life cognitive trajectory, and increased MUFA intake is related to better cognitive aging. Thus, decreasing SFA and increasing MUFA merit further consideration in promoting healthy cognitive aging, and dietary patterns that incorporate higher intake of “good” fats (e.g., Mediterranean)47 should be further addressed in cognitive aging research. Findings from this large-scale prospective study help to address the identified need for an expanded, stronger evidence base on dietary factors and cognitive decline.17, 48
Supplementary Material
Supplemental Figure 1. Determination of population for analysis.
* Eligible as of January 1, 1998. Most ineligibility (98.5%) was due to the age restriction; only 1.5% of deaths or losses to follow-up had occurred by the start of the cognitive sub-study.
† Participation rates among those with dietary data are identical to rates for the entire cognitive sub-study as reported in Kang JH et al. BMJ. 2007;334(7601):987. N=594 completed waves 1 and 2 but not wave 3; n=146 completed waves 1 and 3 but not wave 2.
Supplemental Figure 2. Least-squares Means Test Scores over 4 years, by Quintiles of Saturated and Mono-unsaturated Fat Intakes: Multivariable-adjusted with Initial Cognitive Score-x-Follow-up Time Interaction*.
* SFA = saturated fatty acid; MUFA = mono-unsaturated fatty acid; PUFA = poly-unsaturated fatty acid; Trans = trans fatty acid; Q1 = lowest quintile of intake; Q5 = highest quintile of intake; models adjusted for mean-centered age at initial cognitive assessment (continuous, in years), educational attainment (bachelor’s degree or above versus associate’s degree), race (white/non-white), annual household income (≥$50,000/less), randomized treatment assignment (aspirin, vitamin E), other fat intake, protein intake, total energy intake, all covariate-by-time interactions, and interaction terms for initial cognitive score-x-follow-up time indicators. P-values are from the Wald tests of interactions between fat type consumption level (medians-per-quintile) and time.
† Global score combines results of the TICS (Telephone Interview for Cognitive Status), category fluency, immediate and delayed recall trials of East Boston Memory Test (EBMT), and delayed recall trial of the TICS 10-word list; verbal memory score combines results of the immediate and delayed recall trials of EBMT and the TICS 10-word list. The mean (SD) span between the 1st and 3rd assessments was 4.0 (0.3) years. Adjusted least-squares means were obtained from the repeated measures analysis models involving N=6,172 participants for the global score and N=6,176 participants for verbal memory.
Multivariable-adjusted* Odds Ratios (95% Confidence Intervals) of Worst Cognitive Change over 4 years, by Percent of Total Energy Substitutions†.
* Models adjusted for mean-centered age at initial cognitive assessment (continuous, in years), educational attainment (bachelor’s degree or above versus associate’s degree), aspirin and vitamin E randomization assignment, race (white/non-white), household income level (≥$50,000 per year/less), body mass index (kg/m2), cigarette smoking (current/past/never), postmenopausal hormone use (ever/never), history of hypertension (yes/no), history of elevated cholesterol (yes/no), history of depression (yes/no), history of diabetes (yes/no), alcohol consumption (none/rare, 1–6 per week, >=7 per week), exercise frequency (never/rare, 1–3 times per week, >=4 times per week), other fat intake, protein intake, total energy intake, and time span between 1st and 3rd cognitive assessment (years). For all fat types, the reference category=lowest (1st) quintile of intake.
† Odds ratios reflect estimated effect of substituting in a portion of total energy (i.e., daily caloric intake) from one fat type for another type.
‡ Global score combines results of the TICS (Telephone Interview for Cognitive Status), category fluency, immediate and delayed recall trials of East Boston Memory Test (EBMT), and delayed recall trial of the TICS 10-word list; verbal memory score combines results of the immediate and delayed recall trials of EBMT and the TICS 10-word list; mean (SD) span between the 1st and 3rd assessments was 4.0 (0.3) years. Of 5,084 women who participated in waves 1 and 3, there were 12 missing data on components tests required to compute the global score (N=5,072 for analysis) and 15 missing data on components tests required to compute the verbal memory composite (N=5,069 for analysis).
Acknowledgments
This work was supported by research grants HL043851 (JEB), CA047988 (JEB), HL080467 (JEB), and AG015933 (FG) from the National Institutes of Health (NIH). Dr. Okereke is supported by Career Development Award K08 AG029813 from the NIH. The funding agencies did not play any role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. Dr. Okereke had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
None of the authors report any potential financial or personal conflicts of interest pertaining to this manuscript.
References
- 1.DeKosky ST, Marek K. Looking backward to move forward: early detection of neurodegenerative disorders. Science. 2003;302:830–834. doi: 10.1126/science.1090349. [DOI] [PubMed] [Google Scholar]
- 2.Lleo A, Greenberg SM, Growdon JH. Current pharmacotherapy for Alzheimer’s disease. Annu Rev Med. 2006;57:513–533. doi: 10.1146/annurev.med.57.121304.131442. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 4.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288:2569–2578. doi: 10.1001/jama.288.20.2569. [DOI] [PubMed] [Google Scholar]
- 5.Harris WS. n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr. 1997;65:1645S–1654S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 6.Blok WL, Katan MB, van der Meer JW. Modulation of inflammation and cytokine production by dietary (n-3) fatty acids. J Nutr. 1996;126:1515–1533. doi: 10.1093/jn/126.6.1515. [DOI] [PubMed] [Google Scholar]
- 7.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 8.Pischon T, Hankinson SE, Hotamisligil GS, et al. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–160. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- 9.de Lorgeril M, Renaud S, Mamelle N, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343:1454–1459. doi: 10.1016/s0140-6736(94)92580-1. [Erratum, Lancet 1995; 1345:1738] [DOI] [PubMed] [Google Scholar]
- 10.de Lorgeril M, Salen P, Martin JL, et al. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 11.Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993;88:523–533. doi: 10.1161/01.cir.88.2.523. [DOI] [PubMed] [Google Scholar]
- 12.Singh RB, Dubnov G, Niaz MA, et al. Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): a randomised single-blind trial. Lancet. 2002;360:1455–1461. doi: 10.1016/S0140-6736(02)11472-3. [DOI] [PubMed] [Google Scholar]
- 13.von Schacky C, Angerer P, Kothny W, et al. The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;130:554–562. doi: 10.7326/0003-4819-130-7-199904060-00003. [DOI] [PubMed] [Google Scholar]
- 14.Mozaffarian D, Katan MB, Ascherio A, et al. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 15.Oksman M, Iivonen H, Hogyes E, et al. Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiol Dis. 2006;23:563–572. doi: 10.1016/j.nbd.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Calon F, Lim GP, Yang F, et al. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institutes of Health State-of-the-Science Conference Statement. NIH State-of-the-Science Conference: Preventing Alzheimer’s Disease and Cognitive Decline. 2010 doi: 10.7326/0003-4819-153-3-201008030-00260. http://consensus.nih.gov/2010/alzstatement.htm. [DOI] [PubMed]
- 18.Buring JE, Hennekens CH. The Women’s Health Study: summary of the study design. Journal of Myocardial Ischemia. 1992;4:27–29. [Google Scholar]
- 19.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 20.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsych, Neuropsychol, Behav Neurol. 1988;1:111–117. [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Albert M, Smith LA, Scherr PA, et al. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s Disease. Intern J Neuroscience. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 23.Royall DR, Lauterbach EC, Cummings JL, et al. Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2002;14:377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- 24.Kang JH, Logroscino G, De Vivo I, et al. Apolipoprotein E, cardiovascular disease and cognitive function in aging women. Neurobiol Aging. 2005;26:475–484. doi: 10.1016/j.neurobiolaging.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Kang JH, Cook N, Manson J, et al. A randomized trial of vitamin E supplementation and cognitive function in women. Arch Intern Med. 2006;166:2462–2468. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]
- 26.London SJ, Sacks FM, Caesar J, et al. Fatty acid composition of subcutaneous adipose tissue and diet in postmenopausal US women. Am J Clin Nutr. 1991;54:340–345. doi: 10.1093/ajcn/54.2.340. [DOI] [PubMed] [Google Scholar]
- 27.Willett W. Nutritional epidemiology. 2. New York: Oxford University Press; 1998. [Google Scholar]
- 28.Liu S, Lee IM, Song Y, et al. Vitamin E and risk of type 2 diabetes in the women’s health study randomized controlled trial. Diabetes. 2006;55:2856–2862. doi: 10.2337/db06-0456. [DOI] [PubMed] [Google Scholar]
- 29.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 30.Katsumata Y, Todoriki H, Higashiuesato Y, et al. Metabolic Syndrome and Cognitive Decline Among the Oldest Old in Okinawa: In Search of a Mechanism. The KOCOA Project. J Gerontol A Biol Sci Med Sci. 2012;67:126–134. doi: 10.1093/gerona/glr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzmaurice GM, Laird NM, Ware JH. Modelling the mean: analyzing response profiles. In: Fitzmaurice GM, Laird NM, Ware JH, editors. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons; 2004. pp. 103–139. [Google Scholar]
- 32.Yaffe K, Krueger K, Sarkar S, et al. Cognitive function in postmenopausal women treated with raloxifene. N Engl J Med. 2001;344:1207–1213. doi: 10.1056/NEJM200104193441604. [DOI] [PubMed] [Google Scholar]
- 33.Ganguli M, Belle S, Ratcliff G, et al. Sensitivity and specificity for dementia of population-based criteria for cognitive impairment: the MoVIES project. J Gerontol. 1993;48:M152–161. doi: 10.1093/geronj/48.4.m152. [DOI] [PubMed] [Google Scholar]
- 34.Nash DT, Fillit H. Cardiovascular disease risk factors and cognitive impairment. Am J Cardiol. 2006;97:1262–1265. doi: 10.1016/j.amjcard.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 35.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7:e1000252. doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491–1499. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 37.Vercambre M-N, Grodstein F, Kang JH. Dietary fat intake in relation to cognitive change in high-risk women with cardiovascular disease or vascular factors. Eur J Clin Nutr. 2010 doi: 10.1038/ejcn.2010.113. Epub date: 21 July 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris MC, Evans DA, Bienias JL, et al. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology. 2004;62:1573–1579. doi: 10.1212/01.wnl.0000123250.82849.b6. [DOI] [PubMed] [Google Scholar]
- 39.Eskelinen MH, Ngandu T, Helkala EL, et al. Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int J Geriatr Psychiatry. 2008;23:741–747. doi: 10.1002/gps.1969. [DOI] [PubMed] [Google Scholar]
- 40.Devore EE, Stampfer MJ, Breteler MM, et al. Dietary fat intake and cognitive decline in women with type 2 diabetes. Diabetes Care. 2009;32:635–640. doi: 10.2337/dc08-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remig V, Franklin B, Margolis S, et al. Trans fats in America: a review of their use, consumption, health implications, and regulation. J Am Diet Assoc. 2010;110:585–592. doi: 10.1016/j.jada.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 42.Solfrizzi V, Colacicco AM, D’Introno A, et al. Dietary intake of unsaturated fatty acids and age-related cognitive decline: a 8.5-year follow-up of the Italian Longitudinal Study on Aging. Neurobiol Aging. 2006;27:1694–1704. doi: 10.1016/j.neurobiolaging.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 43.Solfrizzi V, Panza F, Torres F, et al. High monounsaturated fatty acids intake protects against age-related cognitive decline. Neurology. 1999;52:1563–1569. doi: 10.1212/wnl.52.8.1563. [DOI] [PubMed] [Google Scholar]
- 44.Naqvi AZ, Harty B, Mukamal KJ, et al. Monounsaturated, trans, and saturated Fatty acids and cognitive decline in women. J Am Geriatr Soc. 2011;59:837–843. doi: 10.1111/j.1532-5415.2011.03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laitinen MH, Ngandu T, Rovio S, et al. Fat intake at midlife and risk of dementia and Alzheimer’s disease: a population-based study. Dement Geriatr Cogn Disord. 2006;22:99–107. doi: 10.1159/000093478. [DOI] [PubMed] [Google Scholar]
- 46.Kalmijn S, Feskens EJ, Launer LJ, Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am J Epidemiol. 1997;145:33–41. doi: 10.1093/oxfordjournals.aje.a009029. [DOI] [PubMed] [Google Scholar]
- 47.Scarmeas N, Stern Y, Mayeux R, et al. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams JW, Plassman BL, Burke J, et al. AHRQ Publication No. 10-E005. Rockville, MD: Agency for Healthcare Research and Quality; Apr, 2010. Preventing Alzheimer’s Disease and Cognitive Decline. Evidence Report/Technology Assessment No. 193. (Prepared by the Duke Evidence-based Practice Center under Contract No. HHSA 290-2007-10066-I.) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Determination of population for analysis.
* Eligible as of January 1, 1998. Most ineligibility (98.5%) was due to the age restriction; only 1.5% of deaths or losses to follow-up had occurred by the start of the cognitive sub-study.
† Participation rates among those with dietary data are identical to rates for the entire cognitive sub-study as reported in Kang JH et al. BMJ. 2007;334(7601):987. N=594 completed waves 1 and 2 but not wave 3; n=146 completed waves 1 and 3 but not wave 2.
Supplemental Figure 2. Least-squares Means Test Scores over 4 years, by Quintiles of Saturated and Mono-unsaturated Fat Intakes: Multivariable-adjusted with Initial Cognitive Score-x-Follow-up Time Interaction*.
* SFA = saturated fatty acid; MUFA = mono-unsaturated fatty acid; PUFA = poly-unsaturated fatty acid; Trans = trans fatty acid; Q1 = lowest quintile of intake; Q5 = highest quintile of intake; models adjusted for mean-centered age at initial cognitive assessment (continuous, in years), educational attainment (bachelor’s degree or above versus associate’s degree), race (white/non-white), annual household income (≥$50,000/less), randomized treatment assignment (aspirin, vitamin E), other fat intake, protein intake, total energy intake, all covariate-by-time interactions, and interaction terms for initial cognitive score-x-follow-up time indicators. P-values are from the Wald tests of interactions between fat type consumption level (medians-per-quintile) and time.
† Global score combines results of the TICS (Telephone Interview for Cognitive Status), category fluency, immediate and delayed recall trials of East Boston Memory Test (EBMT), and delayed recall trial of the TICS 10-word list; verbal memory score combines results of the immediate and delayed recall trials of EBMT and the TICS 10-word list. The mean (SD) span between the 1st and 3rd assessments was 4.0 (0.3) years. Adjusted least-squares means were obtained from the repeated measures analysis models involving N=6,172 participants for the global score and N=6,176 participants for verbal memory.
Multivariable-adjusted* Odds Ratios (95% Confidence Intervals) of Worst Cognitive Change over 4 years, by Percent of Total Energy Substitutions†.
* Models adjusted for mean-centered age at initial cognitive assessment (continuous, in years), educational attainment (bachelor’s degree or above versus associate’s degree), aspirin and vitamin E randomization assignment, race (white/non-white), household income level (≥$50,000 per year/less), body mass index (kg/m2), cigarette smoking (current/past/never), postmenopausal hormone use (ever/never), history of hypertension (yes/no), history of elevated cholesterol (yes/no), history of depression (yes/no), history of diabetes (yes/no), alcohol consumption (none/rare, 1–6 per week, >=7 per week), exercise frequency (never/rare, 1–3 times per week, >=4 times per week), other fat intake, protein intake, total energy intake, and time span between 1st and 3rd cognitive assessment (years). For all fat types, the reference category=lowest (1st) quintile of intake.
† Odds ratios reflect estimated effect of substituting in a portion of total energy (i.e., daily caloric intake) from one fat type for another type.
‡ Global score combines results of the TICS (Telephone Interview for Cognitive Status), category fluency, immediate and delayed recall trials of East Boston Memory Test (EBMT), and delayed recall trial of the TICS 10-word list; verbal memory score combines results of the immediate and delayed recall trials of EBMT and the TICS 10-word list; mean (SD) span between the 1st and 3rd assessments was 4.0 (0.3) years. Of 5,084 women who participated in waves 1 and 3, there were 12 missing data on components tests required to compute the global score (N=5,072 for analysis) and 15 missing data on components tests required to compute the verbal memory composite (N=5,069 for analysis).