Abstract
The identification of stem-cell-like cancer cells through conventional methods that depend on stem-cell markers is often unreliable. We developed a mechanical method of selecting tumourigenic cells by culturing single cancer cells in fibrin matrices of ~100 Pa in stiffness. When cultured within these gels, primary human cancer cells or single cancer cells from mouse or human cancer cell lines grew within a few days into individual round colonies that resembled embryonic stem-cell colonies. Subcutaneous or intravenous injection of 10 or 100 fibrin-cultured cells in syngeneic or severe-combined-immunodeficiency mice led to the formation of solid tumours at the site of injection or at the distant lung organ much more efficiently than control cancer cells selected using conventional surface marker methods or cultured on conventional rigid dishes or on soft gels. Remarkably, as few as 10 such cells were able to survive and form tumours in the lungs of wild-type non-syngeneic mice.
Keywords: cancer, matrix stiffness, metastasis, mechanical force, tumour-repopulating cells
The notion of “stem cell-like cancer cells” or tumourigenic cells has been based on the observation that only a very small fraction of cells from a tumour can seed and generate a tumour in a SCID (Severe Combined Immunodeficiency) mouse1–3. These tumourigenic cells are often speculated to be the key players in the relapses after chemotherapy or surgery. However, the idea of stem cell-like cancer cells is rather controversial4, 5. A report shows that more than 25% of melanoma cells from human subjects, not just a small fraction, with no expression of a stem-cell marker CD133, can seed and generate a tumour in a NOD-SCID interleukin-2 receptor gamma chain null (IL2rγ−/−) mouse6, casting doubts on whether stem-cell-like cancer cells truly exist or whether they are biologically relevant to cancer. In contrast, a tiny heterogeneous subpopulation of cells from human colon cancer patients exhibits non-stochastic self-renewing capabilities and tumourigenicity, although these behaviors are also not correlated with stem-cell markers7. Other reports show, however, that non-stem-like cancer cells appear to spontaneously and stochastically turn into stem-like cancer cells de novo8, 9, suggesting that there is a bi-directional conversion between stem and nonstem states, further complicating the concept of stem cell-like cancer cells. As a result, existing methods based on the correlative stem cell-like cancer cell markers are unreliable. Therefore, new approaches are highly desirable for the study of tumourigenic cells.
We have recently demonstrated that mechanical forces can regulate mouse embryonic stem (ES) cell differentiation and self-renewal independent of soluble factors and that mouse ES cells are ~10-fold softer than their differentiated counterpart cells10. Soft substrates maintain self-renewal of ES cells but stiff substrates promote differentiation of ES cells by upregulation of endogenous mechanical forces11. It is known that tumours are in general stiffer than normal tissues because of a hardened stroma12, which is stiffened by extracellular matrix crosslinking13 and by actomyosin-driven collagen deposition14, suggesting that tumour cell differentiation might be regulated by the rigidity of substrates. Hence we hypothesize that if there were self-renewing tumourigenic cells among cancer cells, we might be able to use a mechanical strategy of soft substrates to select tumourigenic cells out from a pool of cancer cells.
Soft 3D fibrin gels generate multicellular tumour spheroids
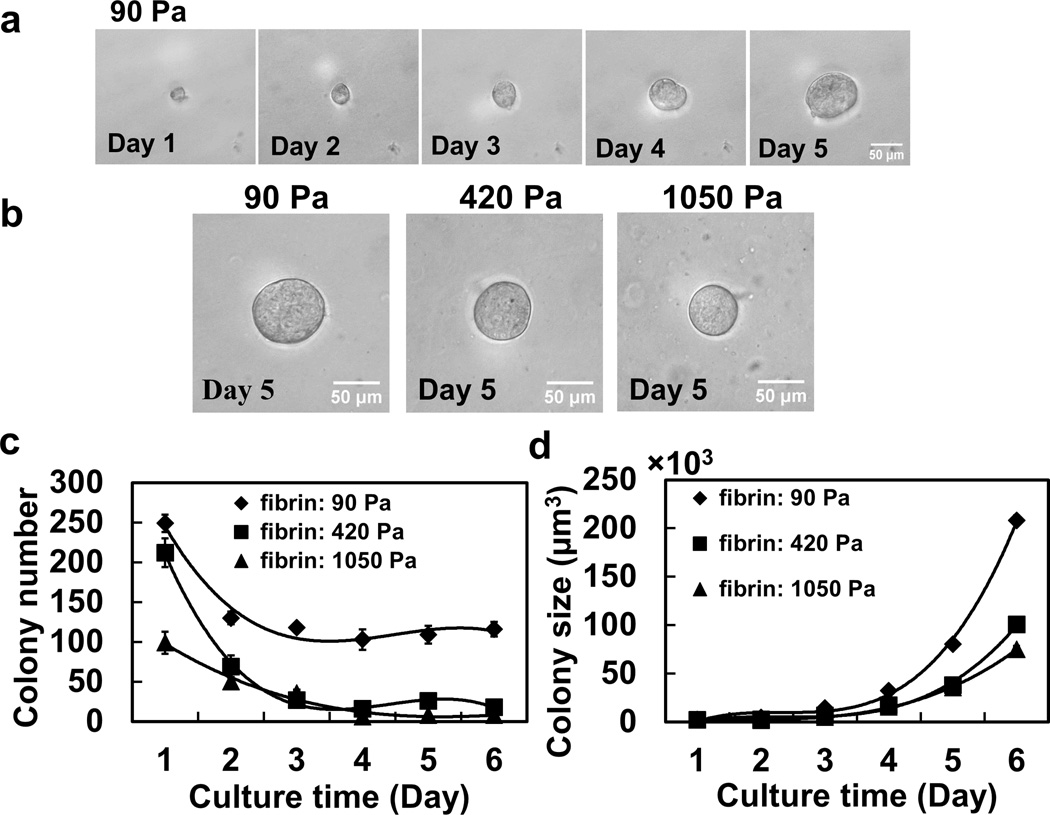
We prepared salmon fibrin gels (purified fibrinogen activated by thrombin) of different concentrations which were mixed with dilute murine B16-F1 melanoma cells. Salmon fibrins have been known for their nontoxicity and low immunogenicity and thus are excellent flexible scaffolds for transplanted cells in different animal species15, 16. It has been reported that fibrin gels of 1, 4, 8 mg/ml correspond to 90, 420, 1050 Pa in elastic stiffness17. We determined that fibrin gels of 90 Pa (1 mg/ml) were the optimal gels for cancer cell proliferation and spheroid formation (Fig. 1a, b). About 1250 of B16-F1 cancer cells in 125 µl MEM medium, which were trypsinized from conventional 2D rigid dishes, were mixed with 125 µl fibrinogen solution (2 mg/ml fibrinogen, activated by 0.5 Units of thrombin). The cells were trapped individually in the 3D fibrin gel and maintained in MEM cell culture medium containing 10% FBS. Inside the soft fibrin gel (90 Pa), about 100–150 spheroid colonies (~8–12% of all seeded cancer cells) formed and maintained from Day 2 up to Day 6 (Fig. 1c); some cells at the bottom of the gel near the rigid dish exhibited spread morphology. In contrast, inside stiff gels (420 or 1050 Pa), the spheroid colony number decreased dramatically to only a few as culture time increased, consistent with the data that percentage of apoptotic colonies increased over time (Supplementary Information, Fig. S1). Softer gels led to larger tumour spheroid size than stiffer gels (Fig. 1b, d). For each colony (that grew from a single cell) within fibrin gels of different stiffness, on Day 5, there were more proliferating cells in the softest gel (90-Pa) than in the stiffer ones (420-Pa or 1050-Pa) (Supplementary Information, Fig. S2), suggesting that larger colonies within the softer gels are primarily a result of more proliferating cells per colony. Both trypan blue dye exclusion assay and annexin V apoptosis detection by flow cytometry showed the complete viability of these cells (Supplementary Information, Fig. S3). The spheroid colony number and size dramatically increased after culturing the cells from the 5-day 3D fibrin gels for a second generation or a third generation (Supplementary Information, Fig. S4, S5), suggesting that the 3D fibrin gel selected cells had a better capacity to form spheroid colonies and grew more rapidly. Tumour spheroid colonies also formed within 3D collagen-1 gels of similar stiffness (94 or ~160 Pa), but the size of the colony was considerably smaller and the proliferation rate was much lower than within soft 3D fibrin gels (Supplementary Information, Fig. S6, S7). Similar to murine B16-F1 melanoma cells, murine H22 hepatocarcinoma cells and murine EL4 lymphoma cells, two suspension growth-dependent cell lines, and murine P815 lymphoblast-like mastocytoma cells, an adherent growth cell line, as well as human A2780 ovarian cancer cells and human HepG2 liver carcinoma cells, two adherent growth cell lines, all formed spheroid colonies within the soft 90-Pa 3D fibrin gel (Supplementary Information, Fig. S8). Importantly, primary tumour cells, isolated from one breast cancer patient and one leukemia patient also formed similar spheroid colonies in soft 3D fibrin gels (Supplementary Information Fig. S8), although the growth rate was somewhat lower than that of cancer cell lines, suggesting that this strategy can be generalized to many types of cancer cell lines and of primary cancer cells, including those that usually only grow in suspension. In contrast, when we cultured B16-F1 cells on top of 2D fibrin substrates of similar stiffness (90, 420, or 1050 Pa), colony growth rates were much lower than those within the 3D fibrin gels (Supplementary Information, Fig. S9). When B16 cells were plated on top of collagen-coated soft polyacrylamide gels, only very small round colonies formed (Supplementary Information, Fig. S10), suggesting that collagen gels (either 2D or 3D) are not as effective as fibrin gels in selecting and growing B16 cells into spheroid colonies. In addition, control B16-F1 cancer cells seem to spread and proliferate better on stiffer 2D polyacrylamide gels (Supplementary Information, Fig. S10), consistent with published reports that stiffer substrates promote spreading and proliferation of differentiated cells and mesenchymal stem cells20, contrary to what we observed inside the 3D fibrin gels where the selected B16-F1 cells proliferated better in softer gels. Importantly, the 2D soft substrates (of any matrix proteins) cannot be used for suspension growth-dependent cancer cells, making them non-ideal for selecting and growing soft-substrate conditioned cancer cells. Together, these findings suggest that 3D soft fibrin gels are unique in promoting multicellular tumour spheroids formation and growth.
Fig. 1. Multicellular tumour spheroid formation in soft 3D fibrin gels.
(a) A single B16-F1 cell grew into a multicellular tumour spheroid within 90-Pa 3D fibrin gel during culture course from Day 1 to Day 5. (b) Multicellular B16-F1 tumour spheroid formation after 5 days in culture within soft 3-D fibrin gels of different stiffness. (c) Multicellular tumour spheroid (round colony) number as a function of culture time: Day 1 to Day 6. The 90-Pa fibrin gel appears to be optimal for sustaining spheroid colony number. Mean ± s.e.m., n=6 (for 90-Pa gels) or 3 (for 420-Pa or 1050-Pa gels) independent experiments. There are no significant differences between 90-Pa and 420- Pa gels at Day 1 (p>0.2); there are significant differences at Day 2 through Day 6 (all p<0.022). There are significant differences between 90-Pa and 1050-Pa at Day 1 through Day 6 (all p<0.018). Between 420-Pa and 1050-Pa gels, there are differences only at Day 1 (p=0.037) and at Day 5 (p=0.00082). (d) Colony size of multicellular tumour spheroid as a function of culture time and fibrin stiffness. Apparently 90-Pa fibrin best promotes tumour growth. Mean ± s.e.m.; n=6 (for 90-Pa gels) or 3 (for 420-Pa or 1050-Pa gels) independent experiments. The stiffness of 3D fibrin gels with concentrations of 1 mg/ml, 4 mg/ml and 8 mg/ml is 90, 420 and 1050 Pa, respectively. There are no significant differences at Day 1 between 90 Pa and 420 Pa (p=0.27) or between 90-Pa and 1050-Pa gels (p=0.33); from Day 2 through Day 6, there are significant differences between 90-Pa and 420-Pa (p<0.002 for all paired comparisons) and between 90 Pa and 1050 Pa gels (all p<0.0012). The data in (c) and (d) are fitted by the 3rd order polynomial functions (solid lines), the parameters of which are given in the Methods section.
Tumourigenic ability of B16-F1 melanoma spheroids
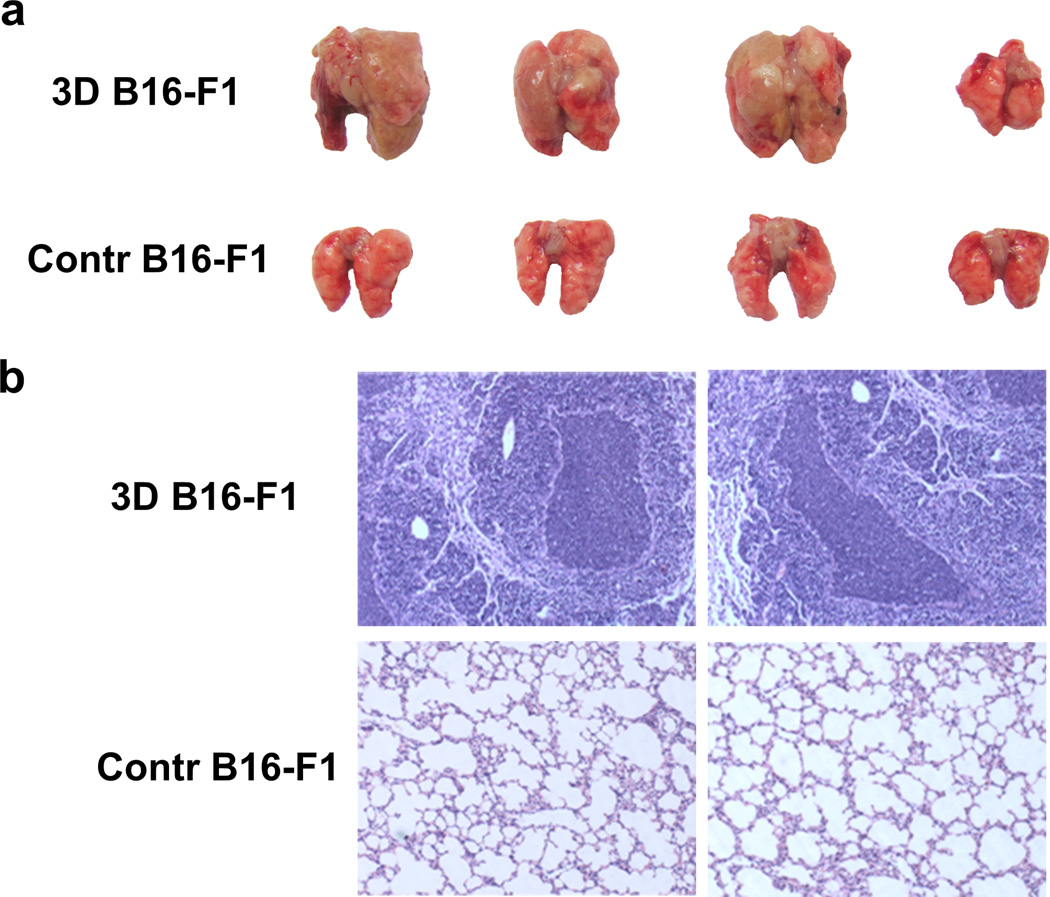
We wondered whether the above B16-F1 melanoma spheroids formed in the 3D soft fibrin gel acquired more efficient tumourigenicity than those cultured on conventional 2D rigid dishes. For this purpose, single colonies were picked out from 3D 90-Pa fibrin gels via pipetting and each time 10 or 100 of such cells were subcutaneously inoculated to normal C57BL/6 mice. We found that 100 or even 10 of the above cells could form subcutaneous melanoma with relatively high frequency (6/12 or 3/12, respectively). These cells grew very rapidly in vivo, with palpable tumours by 15 days for 100 injected cells and 21 days for 10 injected cells. Injecting the 2nd generation 3D soft fibrin gel cultured B16 cells into C57BL/6 mice also resulted in tumour formation at 10 or 100 cells per mouse. In contrast, subcutaneously injecting 100 of B16-F1 cells (dissociated directly from the conventional 2D rigid dishes) per mouse, formed no tumour (0/12); injecting 10 such cells per mouse could not form any tumour either (0/12) (Table 1). At least 10000 B16 cells from rigid plastic dishes were required to form a tumour efficiently (Table 2); when we used limiting dilution assays6 to quantify the frequency of tumours for each condition of 3D soft, 2D soft, or plastic, we found that the highest frequency of tumour formation occurred in the 3D soft fibrin gel condition (Table 2). A cardinal feature of malignant melanoma is its metastatic propensity to lung. Using a well-characterized model of experimental lung metastasis by intravenously injecting cancer cells18, we delivered 100 or 10 of 3D soft gel cultured B16-F1 melanoma cells per mouse into C57BL/6 mice via tail vein. The formation of metastatic tumours was examined on Day 60. Tumour formation efficiency was relatively high: ~40% of mice formed tumours (5 out of 12 mice) for 100 cells, and ~16% of mice (2 out of 12 mice) formed tumours for 10 cells. In contrast, 10 or 100 control cells per mouse could not form any lung metastatic tumour (0/12) (Table 1). Most remarkably, B16-F1 tumour cells (from C57BL/6 mice) selected from the 3D soft fibrin gels were even capable of forming tumours in nonsyngenic BALB/c mice (Fig. 2a and 2b), although they grew slower than in SCID mice. To exclude the possibility that fibrin itself might play a role in tumourigenicity of the cancer cells, control B16-F1 cells were mixed with the soft 90-Pa fibrin gel and treated the same way as above the B16-F1 cells cultured in the 90-Pa 3D fibrin gel. 100 cells were then subcutaneously or intravenously injected to C57BL/6 mice (n=12). Under such conditions, no tumours were formed in mice. Therefore, these data suggest that B16-F1 melanoma cells selected from the 3D soft fibrin gels acquire strikingly efficient tumourigenic capacity. The above differential tumour formation in the lung could not be a result of poor access of the cells to the lung, because similar numbers of cells from either soft fibrin gels or the rigid plastic reached the lung within 4 hr after injection (Supplementary Information, Fig. S11). However, B16 cells from 3D soft fibrin gels appeared to survive better in the lung 12 hr and 24 hr post-injection than those from the rigid plastic (Supplementary Information, Fig. S11). To determine if this method can be generalized, we tested other cancer cell lines, including H22 hepatocarcinoma cells (BALB/c background) and human A2780 ovarian cancer cells. Both H22 and A2780 cancer cells from the 90-Pa 3D fibrin gel showed dramatic increases in tumourigenicity compared to their control counterparts from the conventional 2D rigid dishes (Supplementary Information, Table S1). In contrast, subcutaneously injecting 100 of the B16-F1 cells selected and grown from 94-Pa 3D collagen-1 gels did not result in any tumour formation in C57BL/6 mice (n=10), even when observed for more than 90 days. Thus, the soft fibrin gels, rather than the soft collagen-1 gels, of low rigidity of ~100 Pa might be used as a general strategy to select and grow tumourigenic cells out from a pool of cancer cells by in vitro 3D culture.
Table 1. Tumourigenicity of 3D fibrin gel cultured B16-F1 cells in C57BL/6 mice.
| Mouse Model | C57BL/6 mice | ||
|---|---|---|---|
| Cell line | s.c.* | i.v.* | |
| B16-F1 (100 cells) | 3D | 6/12 | 5/12 |
| Control | 0/12 | 0/12 | |
| B16-F1 (10 cells) | 3D | 3/12 | 2/12 |
| Control | 0/12 | 0/12 | |
s.c.: subcutaneous injection; i.v.: intravenous injection.
Table 2. 3D soft fibrin gels promote more efficient tumourigenicity.
B16-F1 cells were cultured on 2D soft fibrin gels (2D soft fibrin) (90-Pa) or in 3D soft fibrin gels (3D soft fibrin) (90-Pa) or on the rigid plastic (Plastic), all for 5 days. They were then subcutaneously injected into C57BL/6 mice with different cell numbers. CD133+CD44+ the B16-F1 cells were cultured for 5 days on rigid plastic and labeled with FITC-conjugated anti-CD133 and PE-conjugated anti-CD44 antibodies, and then sorted by FACS. The tumour formations were counted as a function of injected cell number.
| Cell number | 2D soft fibrin |
3D soft fibrin |
Plastic | CD133+ CD44+ |
|---|---|---|---|---|
| 100 | 0/6 | 2/6 | 0/6 | 0/6 |
| 1000 | 1/6 | 5/6 | 0/6 | 2/6 |
| 10000 | 5/6 | 6/6 | 3/6 | 4/6 |
| 100000 | 6/6 | 6/6 | 6/6 | 6/6 |
Fig. 2. Tumour metastasis of 3D cultured B16-F1 cells in lung tissue of BALB/c mice.
(a) B16-F1 tumour spheroids formed in soft 3D fibrin gel after 5-day culture were injected into tail vein of BALB/c mice with 10 cells. Mice were sacrificed after 2 months of injection and lung tissue image was recorded. B16-F1 cells cultured in 2D rigid dish were used as control. The results shown were a representative from three independent experiments. (b) Lung tissues of the above B16-F1 tumour spheroid treated BALB/c mice were analyzed by H&E staining. B16-F1 cells cultured on 2D rigid dish were used as control.
Upregulation of stem cell markers in B16-F1 spheroid cells
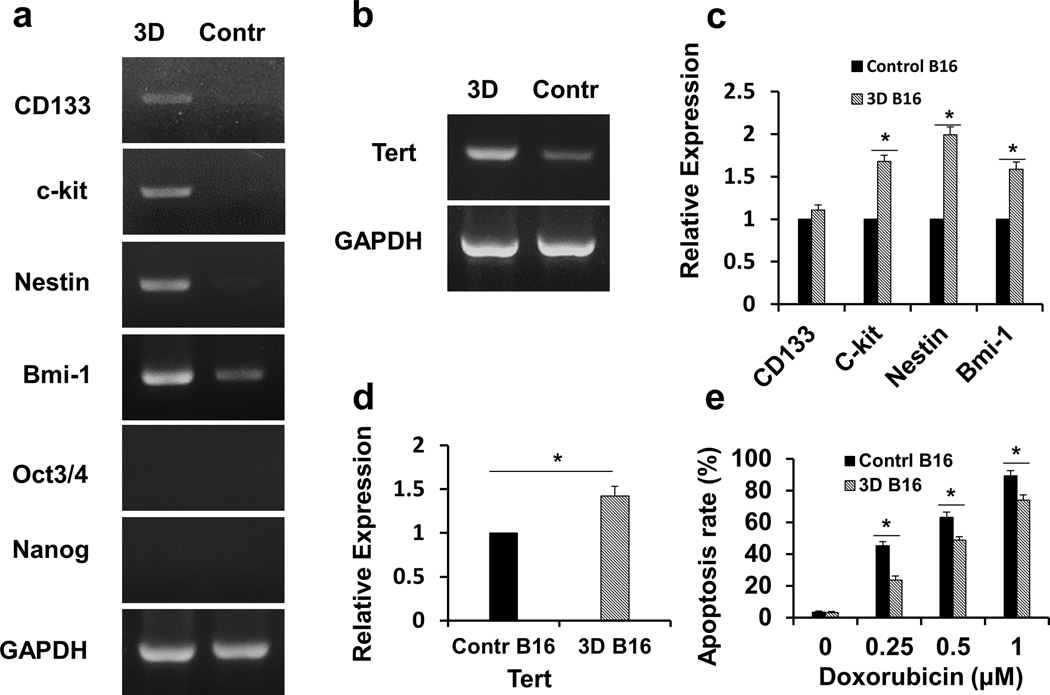
The above in vivo tumour formation data suggest that cells within the spheroids formed in the soft 3D fibrin gel may share some features of a stem cell. To further test this idea, B16-F1 melanoma cells were trapped in the 90-Pa fibrin gel and cultured for 5 days. The formed spheroids were picked out and the cells were used for RNA isolation. A panel of stem cell markers Oct3/4, Nanog, CD133, nestin, Bmi-1 and c-kit were determined by RT-PCR. The expression of Oct3/4 or Nanog was not detected in either 3D fibrin gel or 2D rigid dish cultured B16-F1 cells (Fig. 3a), but CD133, nestin, Bmi-1 and c-kit were upregulated, when compared with the controls (Fig. 3a), In line with the RT-PCR result, upregulation of nestin, Bmi-1 and c-kit was further confirmed with real time RT-PCR, although the increase in CD133 was not significant (Fig. 3c). Telomerase enzyme activity is known to be expressed in ES cells and stem-cell-like cancer cells19. When we analyzed the expression of murine telomerase reverse transcriptase subunit (mTERT), the catalytic component of telomerase, we found that mTERT was upregulated in the cells from the soft 3D fibrin gel (Fig. 3b and 3d). In addition to Oct3/4 and Nanog, we examined expression of three other self-renewal markers c-myc, Rex-1, and Sox2 in B16-F1 cells. Rex-1 was not detected and c-myc was equally expressed in the cells from the 3D soft fibrin gels and from the rigid plastic. Interestingly, Sox2 was only expressed by the cells from 3D soft fibrin gels (Supplementary Information, Fig. S12), suggesting that this unique microenvironment might be promoting self-renewal of these tumourigenic cells via Sox2. Moreover, silencing Sox2, c-kit, Nestin, or Bmi-1 in cells on 2D soft fibrin gels (90 Pa) via siRNA transfection promoted spreading of the round colony (Supplementary Information, Fig. S13). Since published reports have shown that colony spreading is necessary for inhibition of self-renewal of ES cells and for onset of differentiation of ES cells10, 11, the results suggest that these self-renewal markers, especially Sox2, are required for the phenotypes of the cells in soft fibrin gels.
Fig. 3. Upregulation of stem cell-associated genes in B16-F1 spheroid cells cultured in 3D fibrin gel.
(a) Stem cell marker expression in B16-F1 spheroid cells. Total mRNA of B16-F1 spheroid cells at Day 5 was extracted and used for the detection of Nanog, Oct3/4, CD133, nestin, Bmi-1 and c-kit mRNA expression by RT-PCR. B16-F1 cells cultured in 2D rigid dish were used as control. Three independent experiments showed similar results. (b) Murine telomerase reverse transcriptase subunit (mTERT) expression of B16-F1 spheroid cells. mTERT mRNA expression was measured by RT-PCR; representative images of 3 independent experiments. (c) and (d) Stem cells markers and mTERT expression in B16-F1 cells are quantified by real-time PCR. Same mRNA sample of B16-F1 tumour spheroid cells was used as above. B16-F1 cells cultured in 2D rigid dish were used as control. Mean ± s.e.m., *p <0.05, compared with control cells. (e) Apoptotic analysis of doxorubicin-treated 3D B16-F1 cells. Different concentrations of doxorubicin were added to the B16-F1 cell culture medium during the last 18 hours of 5-day culture in the 90-Pa 3D fibrin gels or conventional 2D rigid dish. Cells were collected and stained with FITC conjugated Annexin-V for apoptotic detection by flow cytometry. Mean ± s.e.m..; n=3 independent experiments; *p <0.05, compared with control cells.
It is known that “cancer stem cells” are more resistant to chemotherapeutic drug-induced apoptosis. To determine if these 3D-fibrin gel selected cells are more drug-resistant, different concentrations of doxorubicin or cisplatin were added during the last 18 hr of 5-day culture in the 90-Pa 3D fibrin gels. In line with the expression of stem cell-associated surface markers, B16-F1 cells from 3D fibrin gels were more resistant to apoptosis, compared to those from 2D rigid dish (Fig. 3e; Supplementary Information, Fig. S14).
To further test the possibility of self-renewing capacity of these tumour-repopulating cells, we conducted serial transplantation in mice. 100000 B16-F1 melanoma cells, isolated from the primary tumour that was formed by injecting 100 B16 cells from 3D soft fibrin gels, also generated tumour in C57BL/6 mice. Such serial transplantation could be successive to at least 3 generations. Together, these data suggest that the cells from spheroids formed in the 3D soft fibrin gel acquire self-renewing capacities.
Substrate rigidity regulates tractions but not stiffness of tumourigenic cells
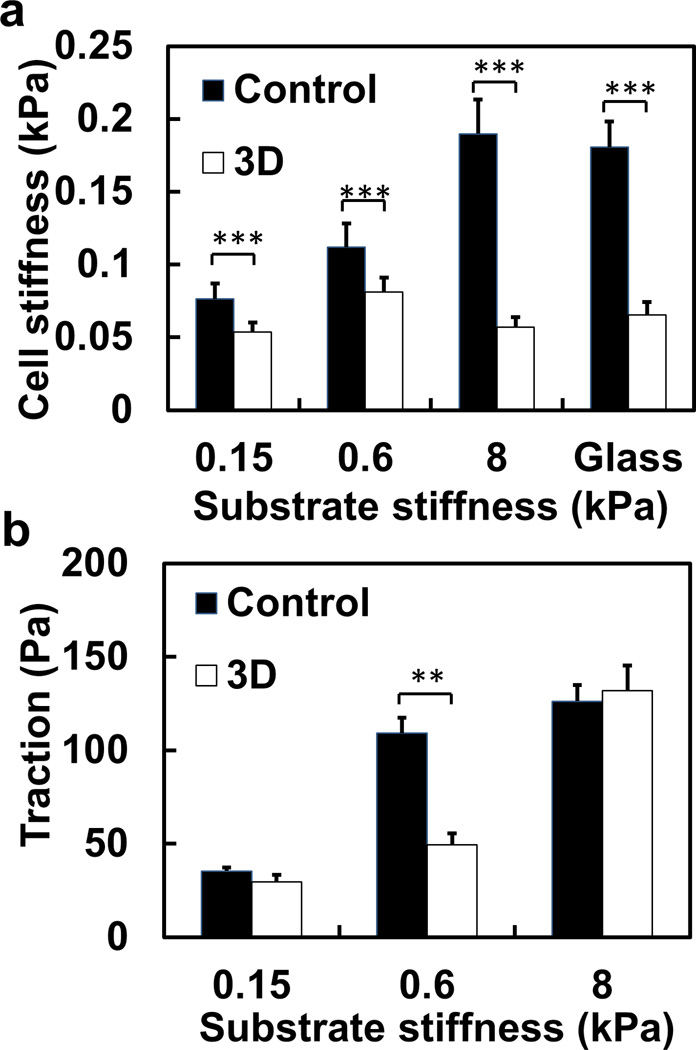
The importance of substrate rigidity in stem cell differentiation and self-renewal is becoming increasingly evident10, 20, 21. To determine the biophysical mechanisms of B16-F1 cells possessing stem cell-like features and tumourigenicity after being cultured within the soft 3D fibrin gel, we re-plated these 3D-fibrin gel cultured tumourigenic cells (after 5-day culture) on a 2D flexible substrate and quantified their mechanical stiffness and tractions. Their intrinsic cell stiffness (defined as stiffness on rigid surface) was ~0.05 kPa on the rigid glass, about 25% of the control cancer cell stiffness (~0.2 kPa) (Fig. 4a). Importantly, these tumourigenic cells did not stiffen much with substrate stiffness (Fig. 4a), but their tractions increased dramatically (by ~3-fold) when substrate stiffness increased from 0.6 to 8 kPa, similar to a recent finding in mouse ES cells22. In contrast, both stiffness and tractions of control cancer cells increased proportionally with substrate stiffness (Fig. 4a, b). Interestingly, although they are ~4-fold softer, the B16-F1 cells selected from the soft 3D fibrin gel showed similar F-actin distribution as the control cells (Supplementary Information, Fig. S15). Together with the data of low cell stiffness, round morphology on 2D rigid substrates, and formation of spheroid colonies within soft 3D gels, all these suggest that these tumourigenic cells possess some key phenotypic and biophysical features of ES cells, without expressing ES cell markers Oct3/4 and Nanog (see Fig. 3a).
Fig. 4. 3D fibrin-gel cultured B16-F1 cells do not stiffen but elevate tractions with substrate rigidity.
(a) Cell stiffness (shear modulus) as a function of substrate stiffness. 3D fibrin gel cultured B16-F1 cells do not stiffen much with substrate rigidity, whereas control cancer cells do. Mean ± s.e.m., 5 independent experiments; at least 150 cells per experiment. (b) Both 3D fibrin gel cultured B16-F1 cells and control cells elevate tractions on stiffer substrates. The cells were plated for 6 hr before experiments in both (a) and (b). Mean ± s.e.m.; 3 independent experiments, n> 60 cells for each value (**, p<0.01; ***, p<0.001.)
To further explore the cellular and molecular mechanisms of soft fibrin-gel selected cells, we measured expression of αvβ3 integrin, which binds fibrin23. There were no differences in αvβ3 expression between fibrin-gel cultured cells and control cells (Supplementary Information, Fig. S16). However, B16-F1 cells depended on αvβ3 integrin to attach and grow in the 3D soft fibrin gels, as a blocking antibody to αvβ3, but not a blocking antibody to β1 (α1/α2β1 engaging collagen-124), completely blocked spheroid colony formation and growth in a dose-dependent manner (Supplementary Information, Fig. S17). This result suggests that although these cells may synthesize and secrete other matrix proteins during the 5-day culturing period, they primarily depend on fibrin-αvβ3 integrin interactions for survival and growth. In addition, inhibition of ROCK with Y-27632 decreased spheroid colony number and colony growth for both 3D and 2D soft fibrin gels in a dose-dependent manner (Supplementary Information, Fig. S18), suggesting that RhoA-dependent cell contractility might be associated with the selection and growth of these tumourigenic cells in the 3D soft fibrin gels.
The establishment of robust and reliable methods to identify and isolate tumour-repopulating cells remains a major challenge for cancer research. While a number of cell surface markers have been proved useful for the isolation of subsets enriched for “stem cell-like cancer cells”, the use of those surface markers is controversial and their relevance to tumour-repopulating cancer cells is not clear. In the present study, we introduced an alternative mechanical method to select and grow tumour-repopulating cells from a pool of cancer cells, without using surface marker labeling or other intrusive methods that might alter cell functions or phenotypes themselves. By culturing cancer cells within very soft 3D fibrin gels that are as soft as the intrinsic stiffness of those cancer cells, we have demonstrated that the selected cells in the form of spheroid exhibit highly efficient tumourigenicity and are able to survive and form tumours in syngeneic or SCID mice, either injected subcutaneously or intravenously. Most strikingly, only as few as 10 such cells are needed to form tumours in the lungs of normal wild type non-syngeneic mice, suggesting that these tumourigenic cells from multicellular tumour spheroids are able to survive the harsh environment in the lung and grow multiple large tumour colonies in vivo. The soft fibrin gel-based method described in this study possesses some advantage over conventional stem cell surface marker-based methods, since the sorted CD133+CD44+ B16 cells could not form a tumour with 100 cells; even when 1000 cells were transplanted only one third of mice grew tumour (Table 2). In addition to selection and enrichment, our data suggest the possibility of “priming”. If we assume that ~10% of the cancer cells in the general population are tumour-repopulating cells (based on our in vitro data in colony formation in Fig. 1), then one would predict that 100 cells from the plastic should be sufficient to form tumours since 10 cells from the 3D soft fibrin gels are sufficient to generate tumours. However, we find that 1000 cells from the rigid plastic cannot form any tumour at all (0 out of 6 mice) and that it takes 10,000 cells to be 50% efficient (3 out of 6 mice) in generating tumours, suggesting that there is some “priming” by the 3D soft fibrin gels. Consistent with this interpretation, B16-F1 cells from the 3D soft fibrin gels, but not from the rigid plastic, express Sox2, which might be essential for self-renewal and growth of these tumour-repopulating cells in vivo, since knocking down Sox2 leads to differentiation of these soft fibrin cultured, self-renewing B16-F1 cells. Our findings that the B16-F1 melanoma cells from 3D soft fibrin gels can form efficient tumours in wild type nonsyngeneic mice might also be partially explained by a finding that in vitro melanoma spheroid cells do elicit lower allogeneic response from immune cells25. In contrast, as many as >20,000 cells are needed to generate tumours in immunodeficient IL-2rγ −/− mice from the tumour-initiating cells7. Even in vitro transformation of a subpopulation of human fibroblasts leading to a multipotent cell type with stem cell-like cancer cell properties, it still takes at least 100 cells to generate tumours in NSG (NOD-SCID gamma) mice26.
What is the mechanism of the long-term survival and growth in the lung for mechanically selected tumourigenic cells in this study? In vitro transwell study does not reveal a difference in transmigration through 8-µm membrane pores between 3D fibrin gel cultured cells and control cells (Supplementary Information Fig. S19), suggesting that the high colonization efficiency and long-term survival of these soft tumourigenic cells cannot be recapitulated by the models of the transwell assays, consistent with the in vivo data that both control cells and 3D soft fibrin gel cultured cells gain access to the lung from the blood within a few hours post-injection (Supplementary Information, Fig. S11). However, the softness (or the high cell deformability, which is the inverse of cell stiffness) of these tumourigenic cells does make them easier (less energy needed) to change cell shape to penetrate the endothelial monolayer in the lung and to move through the dense extracellular matrices of the colonized tissues. This interpretation is supported by our finding that these tumourigenic cells do not stiffen in response to a much stiffer substrate (Fig. 4a) (as they might encounter in vivo), similar to the behavior found in mouse ES cells22. Importantly, these cells do dramatically elevate their tractional forces with substrate stiffness (Fig. 4b), which is necessary for the tumourigenic cells to invade or to plow through the dense extracellular matrices. Although both the cells from the 3D soft fibrin gels and control cancer cells can reach the lung tissue, whether there are differences in the exact location of these cells in the lung microenvironment and in the local mechanical properties of the tissue is not clear at this time. It also remains to be determined the relationship between the softness of these soft-fibrin cultured cells and self-renewing gene expression and long-term growth in vivo. In addition, the anti-apoptotic feature of these 3D-fibrin gel selected cells (Fig. 3e) may also contribute to the long-term survival of these tumour-repopulating cells in the lung of the mice. Recently it is shown that JAK1 and ROCK mediated high contractility of the round, amoeboid-like cancer cells plays a critical role at the leading edge of collective migration of invading cancer cells27. Thus it will be interesting to determine the signaling pathways during migration and invasion of these soft but high force-generating tumourigenic cells. Such high tumourigenicity efficiency cannot be ascribed to the specific effect of fibrin gel in shielding the cancer cells from animal host immune response, since a mixture of fibrin gel with control cancer cells did not elevate the efficiency of tumourigenicity. Our findings show that soft 3D fibrin gels are much better than soft 3D collagen-1 gels in selecting tumourigenic cells and promoting tumourigenic cell proliferation. This suggests that although stiffness of 3D substrates is a critical factor in determining whether a tumour spheroid colony forms or not, other factors such as integrin subsets, polymer size and orientation are additional important factors in regulating tumourigenic cell proliferation rates, invasion, and metastasizing potential28, 29. In addition, it is known that salmon fibrin, besides having a unique non-linear elasticity behavior17, possesses some unique advantages: salmon fibrin has been successfully used in several animal models of neuronal wound healing, and are probably low in immunogenicity and low in virus infection propensity16, suggesting that salmon fibrin gels might be used in in vivo physiologic conditions of different animal species to study cancer progression and metastasis.
Numerous reports show that fibrin is present in connective tissue stroma in human malignant tumours30, that fibrin and fibrinogen increase the survival and metastatic potential of circulating tumour cells31, 32, that fibrin-fibronectin complexes promote lung metastasis33, and that fibrinogen depletion downregulates pulmonary tumour formation in wild type mice34. In our study, fibrin was confirmed to be expressed in the stroma of B16-F1 melanoma (Supplementary Information, Fig. S20). In addition, we found that the activity of AKT1/2 kinase, an important signaling molecule involved in melanoma cell proliferation and migration, was elevated in B16-F1 cells from 3D soft fibrin gels, compared to that from the rigid plastic or to that from the 3D soft collagen gels (Supplementary Information, Fig. S21). Unlike the cells from the rigid plastic or from the 3D collagen gels, the cells from the 3D soft fibrin gels do not change their AKT activity after treatment of EGF. Such AKT1/2 pathways seemed to be critical for B16 spheroid development in 3D soft fibrin gels, since a selective AKT1/2 inhibitor suppressed the spheroid growth (Supplementary Information, Fig. S22). Therefore, the above published reports, together with our current study, suggest that fibrin may play a significant role in cancer that has been underappreciated.
It is of considerable interests that the optimal stiffness of 3D fibrin gel for tumourigenic cell proliferation and spheroid formation is ~90 Pa (=30 Pa shear modulus), which is less than 1/6 of the control B16-F1 cancer cell stiffness, and ~one half of the stiffness of tumourigenic cells cultured in the 3D fibrin gels (Fig. 4). These findings suggest that these tumourigenic cells may prefer a soft microenvironment for their expansion, resembling the characteristics of mouse embryonic stem cells that soft substrates promote their self-renewal and pluripotency10, 11. Our results are consistent with a recent report that soft 3D collagen gel substrates are more conducive than stiff ones in facilitating tumour spheroid formation35, supporting the postulate of physical microenvironment barrier (e.g., extracellular matrix) in inhibition of cancer progression36. The discrepancies between our findings in these tumourigenic cells and the report that stiffening of extracellular matrix facilitates mammary epithelial cell transformation and tumour progression12 might lie in the different substrate-rigidity dependent growth behaviors between the tumour-repopulating soft tumourigenic cells and the stiffer differentiated cancer cells.
Our findings that ~10% of mouse B16-F1 melanoma cells have extremely high tumourigenic ability are consistent with a previous report6 on ~25% of human melanoma cells with tumourigenic ability. However, the high tumourigenicity efficiency of those single cells is only achieved in IL-2rγ −/− NOD/SCID mice6. In contrast, our present study demonstrates that healthy syngeneic mice and even non-syngeneic mice grow tumour after implantation of a few 3D soft fibrin gel selected cells. Our present work suggests that it is possible to decrease the stochastic nature of state transitions between stem-like and non-stem like cancer cells8 when the mechanical properties of their microenvironment are better defined and controlled. The findings that tumourigenic cells are soft and generate low tractions are in accord with recent reports on the role of mechanical forces (exogenous or endogenous) in stem cell fate10, 20 and in tumour progression12–14. A recent report shows that 2D soft substrates do not select a rigidity-independent subgroup from rigidity-dependent cells37. Our 3D soft substrates, on the other hand, do select a tumourigenic subgroup from a pool of cancer cells. Recently it is shown that TAZ is necessary for self-renewal and tumour-initiation in breast cancer cells38 and YAP/TAZ appears to be important in matrix-rigidity dependent differentiation of mesenchymal stem cells39. It will be interesting to see if TAZ plays a role in tumourigenicity of the tumour-repopulating cells from the 3D soft fibrin gels. It still remains to be elucidated how 3D soft fibrin selected tumour-repopulating cells can survive and generate efficient tumour colonization40 locally and at distant organs.
In summary, these data show that a 3D fibrin gel, by virtue of its softness relative to the cancer cell stiffness, can control differentiation behaviors and proliferation rates of tumour cell subsets. This mechanical method is useful in culturing, selecting, and growing tumourigenic cells out of cancer cell lines that are capable of form efficient tumours in syngeneic, SCID, or normal wild-type non-syngeneic mice with as few as 10 cells, independent of stem cell markers Thus, the present approach not only provides a useful platform for understanding the underlying mechanisms of tumourigenicity and metastasis41,42 but also opens a new avenue in the field of tumour-repopulating cancer cell study.
Methods
Ethics Statement
The animal studies were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Animal Care and Use Committee of Tongji medical College. All efforts were made to minimize suffering.
The Clinical tumour specimens were acquired from patients with breast cancer or with T-cell acute lymphoblastic leukemia, which was approved by the Ethical Committee of the Medical Faculty of Tongji Medical College. Informed consent was obtained in accordance with the Declaration of Helsinki from all subjects. The specimens were de-identified before being given to the experimentalists.
Animals and cell lines
Four-week old C57BL/6 mice, SCID mice, Nude mice, and BALB/c mice, all female, were purchased from Wuhan University Center for Animal Experiment. All animals received humane care in compliance with the Principles of Laboratory Animal Care Formulated by the National Society of Medical Research and the guide for the US National Institutes of Health. The protocol was approved by the Animal Care and Use Committee of Wuhan University. Human ovarian cancer cell line A2780, human liver carcinoma cell line HepG2, murine lymphoma cell line EL4, and murine hepatocarcinoma cell line H22, murine lymphoblast-like mastocytoma cell line P815 were purchased from China Center for Type Culture Collection (CCTCC, Wuhan, China); murine melanoma cell line B16-F1 were purchased from either CCTCC or ATCC.
Conventional 2D rigid dish and 3D fibrin gel cell culture of tumour cells
For conventional 2D cell culture, B16-F1 cells were maintained in rigid dish with MEM cell culture medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) at 37°C with 5% CO2. For 3D fibrin gel cell culture, salmon fibrinogen and thrombin were purchased from Searun Holdings Company, Freeport, ME. Detailed methods are described in the Supplementary Information.
RT-PCR and real-time PCR analysis
Total RNA of B16 cell spheroids from 3D fibrin gel culture were extracted using Trizol reagent according to the supplier’s instruction (Invitrogen). The relative quantity of mRNA was determined by reverse transcription-PCR (RT-PCR; 30 cycles, One-step RT-PCR kit, QIAGEN, Germany). Oct3/4, Nanog, CD133, nestin, Bmi-1, c-kit, TERT, c-myc, Rex-1, Sox2, integrins αv, β3, α5, and β1 mRNA expressions were examined. The mRNA of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control. The primer sequences are shown in Table S2.
For real-time RT-PCR assays, the cDNA sequences of all detected genes were retrieved from the NCBI database. For sample analysis, the threshold was set based on the exponential phase of products, and the CT value for samples was determined. The resulting data were analyzed with the comparative CT method for relative gene expression quantification against GAPDH.
Analysis of tumourigenicity
Single B16-F1 cell spheroids were picked out from 3D fibrin gel via pipetting. The spheroids were harvested and pipetted to single cells. The cell number was counted under microscopy and then suspended in 0.9% NaCl solution with appropriate cell density. Then we diluted it to 50 or 500 cells/ml and 200 µl solution was used for injection. 10 or 100 of cells were subcutaneously inoculated or intravenously injected from tail vein to normal C57BL/6 mice, respectively. Immunocompetent BALB/c mice and immunodeficient SCID mice and Nude mice were also used for tumourigenicity assay of B16-F1 cell spheroids. Tumour initiating abilities of 3D fibrin gel cultured human ovarian cancer A2780 cell spheroids and murine hepatocarcinoma H22 cell spheroids were also tested. H22 cell line was intramuscularly inoculated into BALB/c and SCID mice with 100 cells per mouse, respectively. A2780 cells were injected to peritoneal cavity of Nude mice with 100 cells per mouse. Tumour growth of injected mice was carefully monitored every day and tail vein injected mice were sacrificed and observed for lung tumour formation after 2–3 months.
Quantification of cell stiffness
Cell stiffness was measured by applying an oscillatory magnetic field (the applied stress σ = 15.5 Pa at 0.3 Hz) and measuring the resultant oscillatory bead displacement using published protocols10. Cell stiffness is the inverse of cell deformation or cell deformability.
Traction measurement
We followed the protocols of cell traction quantification published previously10.
A two-tailed Student’s t-test was used for all statistics.
Additional methods can be found in Supplementary Information.
Supplementary Material
Acknowledgments
We would like to thank Dr. Jessamine Winer for help with fibrin gel protocols. This work was supported by funds from Huazhong University of Science and Technology, from National Basic Research Program of China (2012CB932500) (to B.H.), from China Natural National Science Foundation (30911120482) (to B.H.), from the Fundamental Research Funds for the Central Universities (HUST-2011TS027) (to B.H.), and from USA NIH grant GM072744 (to N.W.).
Footnotes
Author Contributions:
NW and BH conceived the project; BH, NW, JL, and YT designed the experiments. BH, JL, YT, HZ, YZ, PX, JC, YCP, and KT carried out the experiments and analyzed the data. NW, BH, JL, and YT wrote the manuscript.
The authors declare that they do not have any competing financial interests.
References
- 1.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt P, et al. Eradication of melanomas by targeted elimination of a minor subset of tumour cells. Proc. Natl. Acad. Sci. USA. 2011;108:2474–2479. doi: 10.1073/pnas.1009069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Civenni G, et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumour heterogeneity and long-term growth. Cancer Res. 2011;71:3098–3109. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- 4.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat. Med. 2009;15:1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 5.Shipitsin M, Polyak K. The cancer stem cell hypothesis: in search of definitions, markers, and relevance. Lab Invest. 2008;88:459–463. doi: 10.1038/labinvest.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quintana E, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieter SM, et al. Distinct types of tumour-initiating cells form human colon cancer tumours and metastases. Cell Stem Cell. 2011;9:357–365. doi: 10.1016/j.stem.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Gupta PB, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Chaffer CL, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc. Natl. Acad. Sci. USA. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhury F, et al. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 2010;9:82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhury F, et al. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS. One. 2010;5:e15655. doi: 10.1371/journal.pone.0015655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Levental KR, et al. Matrix crosslinking forces tumour progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuel MS, et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumour growth. Cancer Cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uibo R, et al. Soft materials to treat central nervous system injuries: Evaluation of the suitability of non-mammalian fibrin gels. Biochim. Biophys. Acta. 2009;1793:924–930. doi: 10.1016/j.bbamcr.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ju YE, Janmey PA, McCormick ME, Sawyer ES, Flanagan LA. Enhanced neurite growth from mammalian neurons in three-dimensional salmon fibrin gels. Biomaterials. 2007;28:2097–2108. doi: 10.1016/j.biomaterials.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winer JP, Oake S, Janmey PA. Non-linear elasticity of extracellular matrices enables contractile cells to communicate local position and orientation. PLoS. One. 2009;4:e6382. doi: 10.1371/journal.pone.0006382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maeda M, Suga T, Takasuka N, Hoshi A, Sasaki T. Effect of bis(bilato)-1,2-cyclohexanediammineplatinum(II) complexes on lung metastasis of B16-F10 melanoma cells in mice. Cancer Lett. 1990;55:143–147. doi: 10.1016/0304-3835(90)90024-r. [DOI] [PubMed] [Google Scholar]
- 19.Hiyama E, Hiyama K. Telomere and telomerase in stem cells. Br. J. Cancer. 2007;96:1020–1024. doi: 10.1038/sj.bjc.6603671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert PM, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poh YC, Chowdhury F, Tanaka TS, Wang N. Embryonic stem cells do not stiffen on rigid substrates. Biophys. J. 2010;99:L01–L03. doi: 10.1016/j.bpj.2010.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weisel JW. Fibrinogen and fibrin. Adv. Pro. Chem. 2005;70:247–299. doi: 10.1016/S0065-3233(05)70008-5. [DOI] [PubMed] [Google Scholar]
- 24.Hong H, Stegemann JP. 2D and 3D collagen and fibrin biopolymers promote specific ECM and integrin gene expression by vascular smooth muscle cells. J. Biomater. Sci. Polym. Ed. 2008;19:1279–1293. doi: 10.1163/156856208786052380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramgolam K, et al. Melanoma spheroids grown under neural crest cell conditions are highly plastic migratory/invasive tumour cells endowed with immunomodulator function. PLoS One. 2011;6:e18784. doi: 10.1371/journal.pone.0018784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scaffidi P, Misteli T. In vitro generation of human cells with cancer stem cell properties. Nat. Cell Biol. 2011;13:1051–1061. doi: 10.1038/ncb2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanz-Moreno V, et al. ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumour cells and stroma. Cancer Cell. 2011;20:229–245. doi: 10.1016/j.ccr.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Mierke CT, Frey B, Fellner M, Herrmann M, Fabry B. Integrin α5β1 facilitates cancer cell invasion through enhanced contractile forces. J. Cell Sci. 2011;124:369–383. doi: 10.1242/jcs.071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pathak A, Kumar S. Biophysical regulation of tumour cell invasion: moving beyond matrix stiffness. Integ. Biol. 2011;3:267–278. doi: 10.1039/c0ib00095g. [DOI] [PubMed] [Google Scholar]
- 30.Costantini V, Zacharski LR. The role of fibrin in tumour metastasis. Cancer Metastasis Rev. 1992;11:283–290. doi: 10.1007/BF01307183. [DOI] [PubMed] [Google Scholar]
- 31.Palumbo JS, Degen JL. Fibrinogen and tumour cell metastasis. Haemostasis. 2001;31(Suppl 1):11–15. [PubMed] [Google Scholar]
- 32.Palumbo JS, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumour cells. Blood. 2005;105:178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 33.Malik G, et al. Plasma fibronectin promotes lung metastasis by contributions to fibrin clots and tumour cell invasion. Cancer Res. 2010;70:4327–4334. doi: 10.1158/0008-5472.CAN-09-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Sluis GL, et al. Endogenous activated protein C is essential for immune-mediated cancer cell elimination from the circulation. Cancer Lett. 2011;306:106–110. doi: 10.1016/j.canlet.2011.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang Y, et al. A cell-instructive hydrogel to regulate malignancy of 3D tumour spheroids with matrix rigidity. Biomaterials. 2011;32:9308–9315. doi: 10.1016/j.biomaterials.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 36.Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tilghman RW, et al. Matrix rigidity regulates cancer cell growth and cellular phenotype. PLoS. One. 2010;5:e12905. doi: 10.1371/journal.pone.0012905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cordenonsi M, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 39.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 40.Valastyan S, Weinberg RA. Tumour metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer. 2011;11:512–522. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 43.Mijailovich SM, Kojic M, Zivkovic M, Fabry B, Fredberg JJ. A finite element model of cell deformation during magnetic bead twisting. J. Appl. Physiol. 2002;93:1429–1436. doi: 10.1152/japplphysiol.00255.2002. [DOI] [PubMed] [Google Scholar]
- 44.Senger DR, et al. The alpha(1)beta(1) and alpha(2)beta(1) integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumour angiogenesis. Am J Pathol. 2002;160:195–204. doi: 10.1016/s0002-9440(10)64363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.