Abstract
OBJECTIVE
To assess renal injury in a pig model after treatment with a clinical dose of shock waves using a narrow focal zone (≈ 3 mm) lithotriptor (Modulith SLX, Karl Storz Lithotripsy).
MATERIALS AND METHODS
The left kidney of anaesthetized female pigs were treated with 2000 or 4000 shock waves (SWs) at 120 SWs/min, or 2000 SWs at 60 SWs/min using the Storz SLX.
Measures of renal function (glomerular filtration rate and renal plasma flow) were collected before and 1 h after shock wave lithotripsy (SWL) and the kidneys were harvested for histological analysis and morphometric quantitation of haemorrhage in the renal parenchyma with lesion size expressed as a percentage of functional renal volume (FRV).
A fibre-optic probe hydrophone was used to determine acoustic output and map the focal width of the lithotriptor.
Data for the SLX were compared with data from a previously published study in which pigs of the same age (7–8 weeks) were treated (2000 SWs at 120 or 60 SWs/min) using an unmodified Dornier HM3 lithotriptor.
RESULTS
Treatment with the SLX produced a highly focused lesion running from cortex to medulla and often spanning the full thickness of the kidney. Unlike the diffuse interstitial haemorrhage observed with the HM3, the SLX lesion bore a blood-filled core of near-complete tissue disruption devoid of histologically recognizable kidney structure.
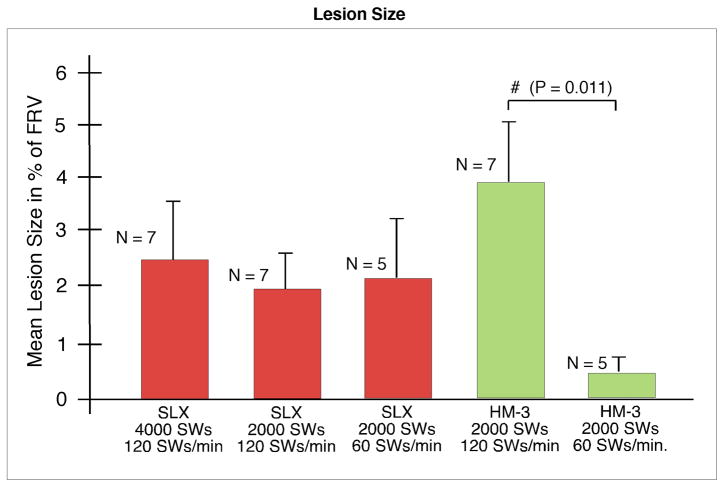
Despite the intensity of tissue destruction at the core of the lesion, measures of lesion size based on macroscopic determination of haemorrhage in the parenchyma were not significantly different from kidneys treated using the HM3 (2000 SWs, 120 SWs/min: SLX, 1.86 ± 0.52% FRV; HM3, 3.93 ± 1.29% FRV).
Doubling the SW dose of the SLX from 2000 to 4000 SWs did not significantly increase lesion size. In addition, slowing the firing rate of the SLX to 60 SWs/min did not reduce the size of the lesion (2.16 ± 0.96% FRV) compared with treatment at 120 SWs/min, as was the case with the HM3 (0.42 ± 0.23% FRV vs 3.93 ± 1.29% FRV).
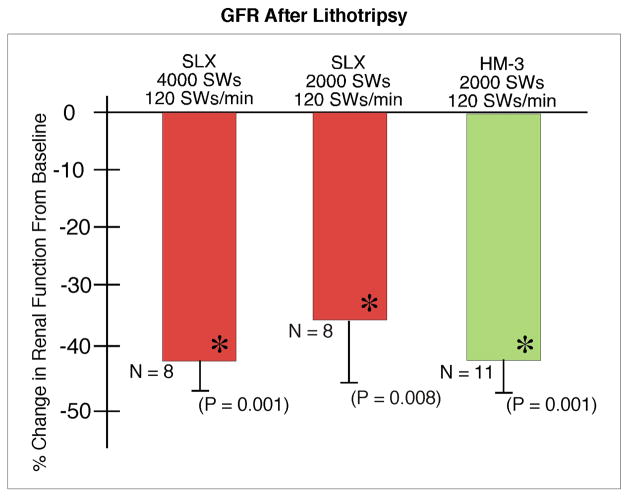
Renal function fell significantly below baseline in all treated groups but was similar for both lithotriptors.
Focal width of the SLX (≈ 2.6 mm) was about one-third that of the HM3 (≈ 8 mm) while peak pressures were higher (SLX at power level 9: P+ ≈ 90 MPa, P− ≈ −12 MPa; HM3 at 24 kV: P+ ≈ 46 MPa, P−≈−8 MPa).
CONCLUSIONS
The lesion produced by the SLX (narrow focal width, high acoustic pressure) was a more focused, more intense form of tissue damage than occurs with the HM3.
Slowing the SW rate to 60 SWs/min, a strategy shown to be effective in reducing injury with the HM3, was not protective with the SLX.
These findings suggest that the focal width and acoustic output of a lithotriptor affect the renal response to SWL.
Keywords: shock wave lithotripsy, renal injury, narrow focal zone
INTRODUCTION
Renal injury is an unfortunate but expected consequence of shock wave lithotripsy (SWL). All patients experience at least mild haematuria, some develop subcapsular or perinephric haematomas, and in rare cases excessive bleeding can develop, requiring intervention [1–6]. SWL injury has not been well studied in patients but there is a wealth of information describing the renal response to SWs in experimental animals. The most thorough characterization has been conducted in the pig model where the severity of tissue damage and size of the haemorrhagic lesion are dependent on many factors, including treatment settings for power and shock wave (SW) rate, the sequence of SW delivery, the number of SWs and the size of the kidney [7–10]. This work in assessing treatment variables has helped to estimate the potential for injury in the clinical setting and has revealed treatment strategies that significantly reduce tissue damage [8,9,11–13]. Thus, there is a growing understanding of how treatment settings contribute to injury in SWL. However, little has been done in a systematic way to compare the injuries produced by different lithotriptors.
Lithotriptors are not all the same. The SWs of all lithotriptors have similar features, but the acoustic output and dimensions of the focal zone produced by different machines can be very different [14]. Focal width is a critical feature of a lithotriptor and in working terms describes how tightly SW energy is focused in the patient. Focal width is important because it affects the mechanisms at play in stone breakage. Shear stress contributing to stone breakage is enhanced when the focal width is wider than the stone [15,16]. Also, since respiratory motion moves the stone in and out of the focal zone, a lithotriptor with larger focal width has an improved chance of hitting the target [17]. Indeed, patient studies have suggested that focal width can affect outcomes with lower stone-free rates for narrow focal width lithotriptors [18–22]. Focal width has also been implicated in SWL injury, with the suggestion of an increased occurrence of adverse effects with narrow focal width machines [23,24].
The focal widths of current lithotriptors cover a broad range, from ≈ 2.1 mm (Wolf Piezolith P3000) to ≈ 20 mm (LithoGold LG-380). Most machines are reported have a focal width of about 6–10 mm and it is not uncommon to find considerable variance for the values reported for a given machine. For example, reported values of focal width for the unmodified Dornier HM3 lithotriptor (Dornier Medical Systems, Kennesaw, GA, USA) run from ≈ 8 to ≈ 12 mm, the difference being due to how the measurements were conducted [14,25]. Accurate measures require rigorous mapping of the pressure field with a fibre-optic probe hydrophone and this is not an assessment often performed beyond the characterization required for the licensing and approval of a new lithotriptor [26].
The Storz Modulith SLX (Karl Storz Lithotripsy, Atlanta, GA, USA) is an electromagnetic lithotriptor that has gained considerable popularity within the urology community. This machine emerged during the wave of technical development spurred by interest in making SWL an anaesthesia-free procedure. Since discomfort during SWL is due largely to cutaneous sensation, the strategy used by many manufacturers was to widen the aperture of the shock source to spread the area of contact between the acoustic pulse and the body. This reduced pain at the skin but also narrowed the focal zone [14]. The SLX has a focal width of only ≈ 3 mm and produces higher acoustic pressures (P+≈ 90 MPa) than broader focal width machines (i.e. LG-380: FW ≈ 20 mm, P+≈ 20 MPa; XiXin CS2012: FW ≈ 18 mm, P+ ≈ 17 MPa; HM3: FW ≈ 8 mm, P+≈ 40 MPa) [26,27].
As kidney injury has not been adequately assessed for a narrow focal zone lithotriptor, we used the pig model to characterize the renal response to SWs for the SLX. SWs were administered under conditions that simulated clinical SWL at settings for SW number, power level and SW rate that have been reported for treating patients using this lithotriptor [28]. Data for morphology, lesion size and renal function were compared with similar, previously published data for pigs treated using the Dornier HM3 [10]. The study included assessment of the renal response to slow SW rate, a treatment strategy shown to protect against renal trauma in the pig model [9,10].
MATERIALS AND METHODS
The surgical and treatment protocols used in the present study were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine. Surgical procedures for the placement of vascular and ureteric catheters have been described previously [7], as have procedures for morphological evaluation [29], quantitation of lesion volume [30] and measures of renal function in SWL [27].
Twenty-one female farm pigs, 7–8 weeks of age (Hardin Farms, Danville, IN, USA) were treated using a Storz Modulith SLX-T lithotriptor at power level 9 (PL-9) and received 2000 SWs at 120 SWs/min (n = 8), 4000 SWs at 120 SWs/min (n = 8) or 2000 SWs at 60 SWs/min (n = 5).
Coupling the animal to the treatment head followed a consistent protocol. The water cushion above the shock source was set at inflation value zero, mineral oil (4 mL) was placed in the centre of the water cushion and the table was lowered to bring the treatment head in contact with the undersurface of the patient water bath. If bubbles formed at this interface, the procedure was repeated until the acoustic window was clear. The skin over the kidney was shaved smooth to minimize accumulation of bubbles at this surface. The animal was placed supine, warm tap water was added to the bath and bubbles were smoothed away by gentle hand massage.
Contrast medium (Renografin 60% or Isovue 300, Bracco Diagnostics, Princeton, NJ, USA) was injected through a ureteric catheter to highlight the renal collecting system, and a lower pole calyx was targeted using biplanar X-ray fluoroscopy. SWs were administered without stopping. Targeting was checked every 500 SWs on-the-fly and, if needed, adjustments in position were made with slight movements of the treatment table.
Kidney function was determined immediately before and at 1 h after SW treatment. Urine and plasma samples were analysed for inulin and para-aminohippurate (PAH) concentrations. Inulin clearances, PAH clearance and PAH extraction (EPAH) were determined and used to calculate GFR and renal plasma flow (RPF).
Kidneys were perfusion-fixed in situ with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH = 7.4), quickly removed and submerged in fresh fixative for subsequent determination of lesion size or routine histological processing for haematoxylin and eosin-stained paraffin sections. The area of haemorrhagic lesion in systematically selected sections was used to determine lesion volume expressed as percentage of total functional volume (FRV) of renal parenchyma [30].
Body-weight, blood pressure and renal function measures were summarized as means and standard errors of the mean (SEMs). Baseline measures were compared using ANOVA. Changes in blood pressure and renal function measures from baseline were examined using paired t-tests within each group. Renal function measures collected at 1 h were normalized to baseline values and compared between groups using ANOVA. Mean lesion size ± SEM was calculated in each group of pigs. Comparison of lesion sizes between groups was performed with a Kruskal–Wallis test, a non-parametric ANOVA for non-normally distributed data. P < 0.05 was considered to indicate statistical significance.
For comparison, we have included data for lesion size, GFR, EPAH and RPF from a previously published study in which pigs of the same age (7–8 weeks) were treated (2000 SWs, 24 kV, 120 SWs/min; or 2000 SWs, 24 kV, 60 SWs/min) using an unmodified Dornier HM3 lithotriptor [10,25].
Acoustic characterization of the SLX was conducted in well-degassed water (dissolved gas 10–30% saturation) within a Lexan test chamber (15 in × 13 in × 14 in) fitted with a Mylar window. The chamber was supported by a frame spanning the opening in the patient table (water bath removed), such that the treatment head could be coupled with LithoClear gel (Sonotech, Inc.) directly to the Mylar acoustic window. Waveforms over a complete range of power settings were collected using a fibre-optic probe hydrophone FOPH-500 (RP Acoustics, Leutenbach, Germany) and stored using a Tektronix (TDS 5034) digital oscilloscope [31]. For mapping the acoustic field at the focal plane of the lithotriptor, the tip of the FOPH was moved in 0.25–1.0 mm steps over a total excursion of 36 mm. For this the lithotriptor was fired at 0.5 Hz, PL-9. Similar measurements were conducted in the open water bath of the Dornier HM3. The IEC standard (−6 dB zone) was used to define the dimensions of the focal zone [32].
RESULTS
Body-weights and baseline blood pressures were not significantly different between groups (Table 1). Mean blood pressure changes were also similar between the groups over the course of the experiment, except for a slightly greater fall in the animals treated with 2000 SWs (120 SWs/min) using the SLX.
Table 1.
Mean (SEM) body-weight and blood pressure in the five treatment groups. The asterisk (*) indicates that post-SWL blood pressure is significantly different from pre-SWL baseline measurements from the group. Data for the Dornier HM3 were previously published by Connors et al. [10].
| SLX 4000 SWs |
SLX 2000 SWs |
HM3 2000 SWs |
|||
|---|---|---|---|---|---|
|
|
|||||
| Variable | 120 SWs/min | 120 SWs/min | 60 SWs/min | 120 SWs/min | 60 SWs/min |
| Body- weight, kg | 14.8 (0.2) | 14.3 (0.6) | 13.1 (0.3) | 14.8 (0.5) | 13.3 (0.5) |
| Blood pressure, mmHg | |||||
| Baseline | 68.7 (2.0) | 64.5 (1.6) | 69.7 (11.0) | 68.2 (1.9) | |
| +1 h | 64.9 (3.4) | 61.3 (1.6)* | 66.6 (3.0) | 64.0 (1.8) | |
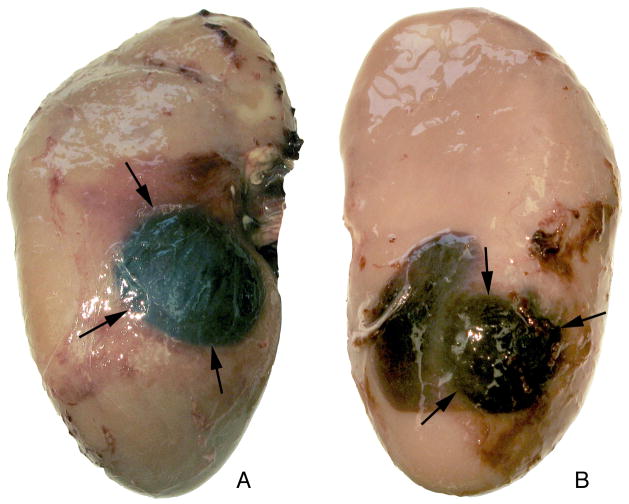
Most kidneys (15/21) treated with the SLX showed frank subcapsular haematomas and almost half (nine of 21) had haematomas on both the anterior and posterior surfaces (Fig. 1). Occurrence of dual haematomas did not increase with increased dose and was not related to SW rate, as five of eight kidneys treated with 2000 SWs (120 SWs/min) and only one of eight kidneys receiving 4000 SWs (120 SWs/min) developed haematomas at both surfaces, while three of five kidneys treated at the slow SW rate (2000 SWs, 60 SWs/min) had haematomas on both the anterior and posterior surfaces. For the HM3, all kidneys treated at 120 SWs/min (n = 7) and only one of the kidneys treated at 60 SWs/min (n = 5) formed haematomas, and all were just on one surface.
FIG. 1.
Subcapsular haematomas in kidney treated with the Storz SLX (2000 SW, 120 SWs/min). Photographs show haematomas (arrows) on the anterior (A) and posterior (B) surfaces of kidney, corresponding to entry and exit points on the SW axis. The images show the haematoma pattern found in nine of 21 kidneys treated with the SLX.
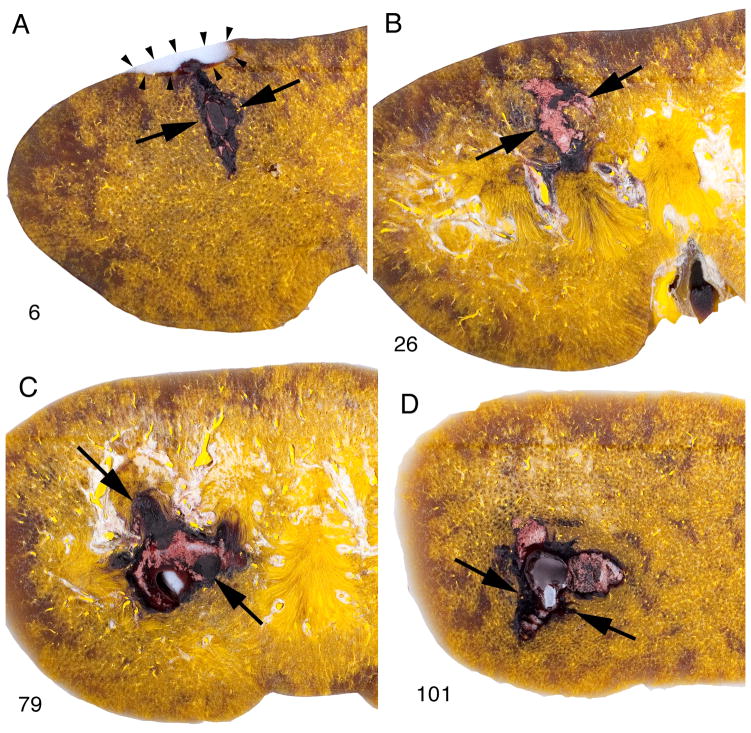
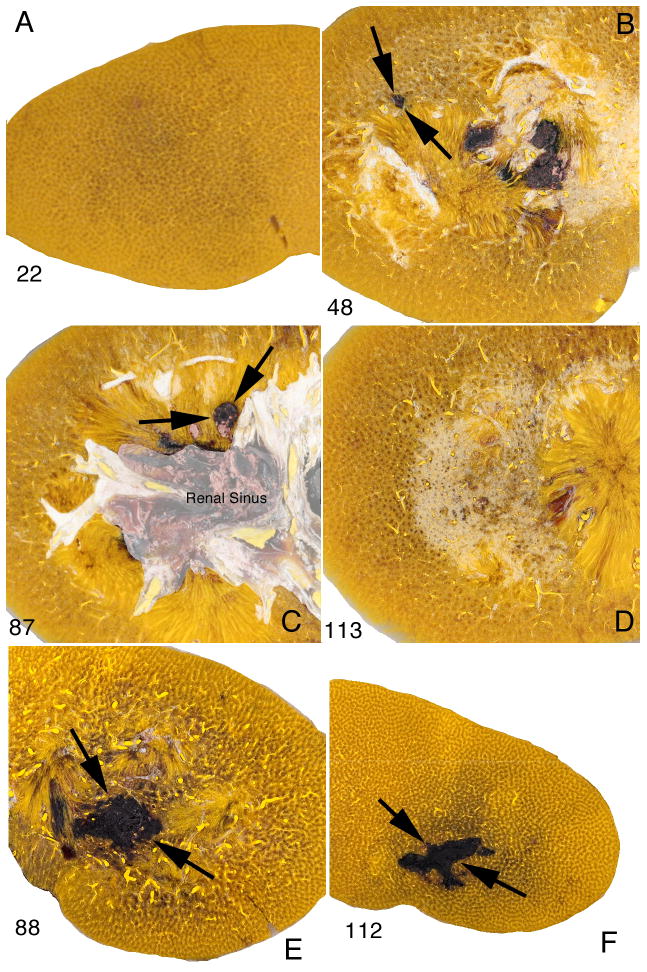
The parenchymal lesion with the SLX showed similar morphological characteristics regardless of the dose or SW rate. The lesion typically extended from the cortex to the medulla, and in kidneys with dual haematomas a discrete path of haemorrhage could be followed in serial sections across the full thickness of the kidney (Fig. 2). In this kidney treated with 2000 SWs (120 SWs/min, PL-9), the lesion started in continuity with a subcapsular haematoma at the posterior surface and coursed through the parenchyma to the cortex of the opposite side. Macroscopically the site of injury was highly delineated with a sharp boundary between the lesion and adjacent parenchyma with little diffuse haemorrhage in the surrounding tissue. Even at low magnification the lesion appeared to be focused and intense, with a core that was severely damaged. There was similar lesion morphology in kidneys treated at 60 SWs/min using the SLX (Fig. 3e,f). By contrast, the lesion observed in kidneys treated using the HM3 at 60 SWs/min was not nearly as severe and amounted to scattered spots of haemorrhage in the cortex and medulla (Fig. 3b,c) with few areas of complete disruption of tissue as seen with the SLX.
FIG. 2.
Path of lesion in pig kidney treated with the Storz SLX (2000 SWs, 120 SWs/min). These macroscopic images are of four sections (nos 6, 26, 79, 101) taken from 157 serial sections spanning the full thickness (anterior to posterior) of the kidney. The lesion can be tracked beginning at the posterior side (no. 6) where the parenchymal lesion (flanked by arrows) is continuous with an indentation caused by a subcapsular haematoma (blood washed out during processing). The lesion in sections 79 and 101 includes a region (arrowhead) in which haemorrhage was displaced during processing leaving a plug of embedding wax. This could only occur with complete ablation of tissue. In some cases such a channel could be tracked to the surface of the kidney. The lesion size for this kidney was determined to be 3.15% FRV.
FIG. 3.
Comparison of lesion in pig kidneys treated at 60 SWs/min with Dornier HM3 and Storz SLX lithotriptors. Frames A–D show macroscopic images of four tissue sections (nos 22, 48, 87, 113) among 160 serial sections from a kidney treated at 60 SWs/min using the HM3 (2,000 SWs, 24 kV). The parenchymal lesion is limited to discrete regions in sections 48 and 87 (arrows). The lesion size in this kidney treated at slow SW rate measured 0.5% FRV compared with a mean value of 3.93% FRV for kidneys treated at 120 SWs/min as previously reported (Connors et al. [10]). Frames E and F show two sections (nos 88, 112) from a kidney likewise treated at 60 SWs/min but using the SLX (2000 SWs, PL-9). The lesion (flanked by arrows) is seen as a region of concentrated haemorrhage surrounded by seemingly unaffected parenchyma. The lesion volume in this kidney was 4.1% FRV.
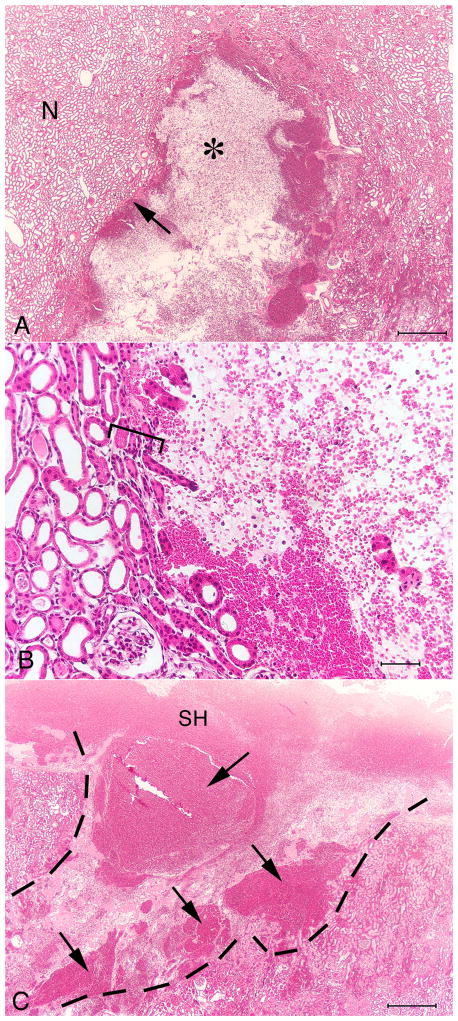
On histological examination, the SLX lesion showed an abrupt transition between injured tissue and the adjacent, seemingly unaffected, parenchyma (Fig. 4). The core of the lesion was often devoid of recognizable histological structure–that is, renal tubules, renal corpuscles and blood vessels were completely destroyed.
FIG. 4.
Histology of renal cortex from a pig kidney treated using the Storz SLX (2000 SWs, 120 SWs/min). (A) The haemorrhagic lesion (*) surrounded by intact kidney parenchyma. The portion of the lesion shown in this frame measures ≈ 2.5 mm × 3.0 mm. (B) The margin of the lesion, showing an abrupt transition between intact renal tubules and the core of the lesion largely devoid of organized structures. The transition from intact parenchyma to zone of complete tissue disruption occurs over a distance of just a few tubule diameters (square bracket). (C) Continuity can be seen between the parenchymal lesion (dashed line) and a subcapsular haematoma (SH). Several areas of intense haemorrhage are evident in the lesion (arrows). Bars, 0.5 mm (A), 0.05 mm (B), 0.5 mm (C).
Lesion sizes of the three SLX-treated groups ranged from 1.86% to 2.55% FRV (Fig. 5). Although the tissue damage produced by the SLX was more destructive to histological structures within the core of the lesion than was the case with the HM3, lesion size determined by measuring interstitial haemorrhage within the renal parenchyma was not different for the two lithotriptors (Fig. 5). That is, comparison of lesion size for the two 2000 SWs, 120 SWs/min groups showed no significant difference (SLX, 1.86 ± 0.52% FRV; HM3, 3.93 ± 1.29% FRV). Doubling the SW dose of the SLX from 2000 to 4000 SWs did not significantly increase lesion size. In addition, slowing the firing rate of the SLX to 60 SWs/min did not reduce the size of the lesion (2.16 ± 0.96% FRV) compared with treatment at 120 SWs/min, as was the case with the HM3 (0.42 ± 0.23% FRV vs 3.93 ± 1.29% FRV) [10].
FIG. 5.
Lesion size in pig kidneys treated using the Storz SLX and Dornier HM3 lithotriptors. Data are presented as percentage of functional renal volume (% FRV) determined by morphometric analysis of parenchymal haemorrhage (excluding the renal calyceal system). The lesion sizes for 2000 SWs at 120 SWs/min were not significantly different between the SLX and the HM3. Doubling the dose to 4000 SWs did not increase the lesion size with the SLX. Slowing the SW rate did not reduce the lesion size, as happened with the HM3. The hash (#) indicates a significant difference between groups. N indicates number of individual kidneys sectioned and quantified in each group. Data for the Dornier HM3 were published previously by Connors et al. [10].
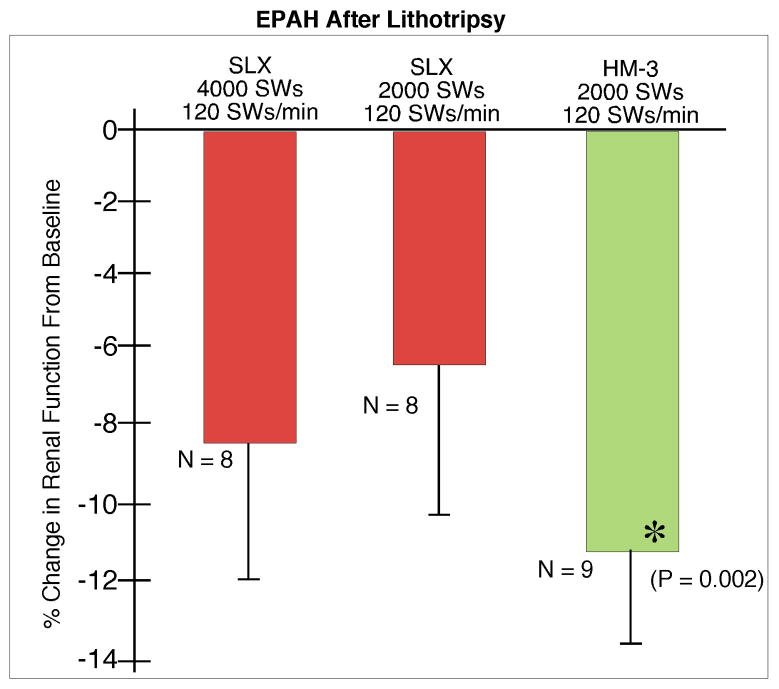
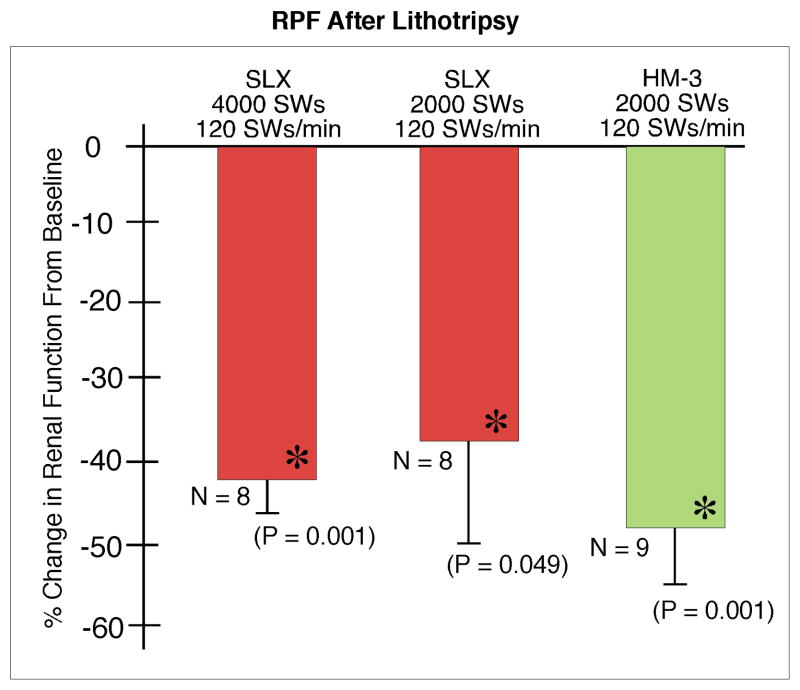
Baseline renal function was similar for all groups. GFR (Fig. 6) fell significantly at 1 h in kidneys treated at 120 SWs/min and the fall was similar among the groups. EPAH (Fig. 7) fell by 11.4 ± 2.35% in treated kidneys of the HM3 2000 SW group at 1 h post-treatment, but this change was not significantly different from the fall seen in kidneys treated with the SLX. Renal plasma flow (Fig. 8) also fell significantly at 1 h in the shocked kidneys in all three groups treated at 120 SWs/min and the fall was similar in all the groups.
FIG. 6.
Glomerular filtration rate at 1 h after treatment with the Storz SLX or Dornier HM3 lithotriptor. There was no significant difference in measurements of GFR among groups. N indicates the number of animals per group. The asterisk (*) indicates that post-SWL renal function is significantly different from pre-SWL baseline measurements for the group. Data for the Dornier HM3 were previously published by Connors et al. [10].
FIG. 7.
PAH extraction (EPAH) at 1 h after treatment with Storz SLX or Dornier HM3 lithotriptor. There was no significant difference in measurements of EPAH among groups. N indicates number of animals per group. The asterisk (*) indicates that post-SWL renal function is significantly different from pre-SWL baseline measurements for the group. Data for Dornier HM3 were previously published by Connors et al. [10].
FIG. 8.
Renal plasma flow at 1 h after treatment with the Storz SLX or Dornier HM3 lithotriptor. There was no significant difference in measurements of RPF among groups. N indicates number of animals per group. The asterisk (*) indicates that post-SWL renal function is significantly different from pre-SWL baseline measurements. Data for Dornier HM3 were previously published by Connors et al. [10].
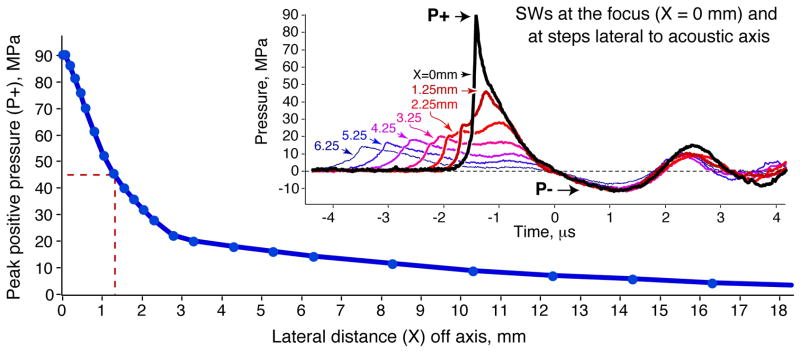
Mapping of the lateral distribution of peak positive pressure for the SLX showed the − 6 dB (half-amplitude) width of the focal zone at PL-9 (P+ ≈ 90 MPa) to be ≈ 2.6 mm (Fig. 9). Peak negative pressure was P−≈−12 MPa. Similar measures for the HM3 fired at 18 kV (P+ ≈ 32–34 MPa), also using the FOPH, gave a focal width of ≈ 8 mm [27]. Thus, for similar measures at similar but not equivalent output, the focal width of the SLX was about one-third that of the HM3. Peak pressures for the HM3 fired at 24 kV were P+ ≈ 46 MPa and P−≈−8 MPa.
Fig. 9.
Peak positive pressure (P+) at PL-9 as a function of lateral distance off the acoustic axis. The − 6 dB width (i.e. pressure half-maximum amplitude; dashed lines) of the acoustic field was ≈ 2.6 mm (1.3 mm radius from the axis). The inset shows temporal profiles of SWs at various distances from the acoustic axis. Waveforms are aligned so that the transition from positive to negative pressure is at the zero point on the timescale. The duration of the positive-pressure phase is longer for waveforms collected off axis. Comparing waveforms at 0 and 1.25 mm, the P+ declines quickly off-axis: the P+ at 1.25 mm off-axis (≈ 45 MPa) was about half the amplitude recorded at the focus of the lithotriptor (90 MPa).
DISCUSSION
Shock wave lithotripsy renal injury in the pig model has been studied in much greater detail for the Dornier HM3 than for any other lithotriptor. The findings have been consistent and repeatable and provide a useful standard in assessing injury caused by other lithotriptors [27,33,34]. With the caveat that morphological features of the HM3 lesion are dependent on numerous factors, including the SW dose (SW number and kV), the size of the kidney, protocol for SW delivery and rate of SW administration, the pattern of tissue damage tends to exhibit a recognizable, predictable set of characteristics. The lesion can extend across the full thickness of the kidney, subcapsular haematomas are common, and, although injury to cortical structures almost always occurs, the renal papilla is the area most readily injured. The HM3 lesion is focal in the sense that the injury follows the path of the focal zone, but at the histological level, it is diffuse. Kidneys treated with a typical dose of SWs (2000 SWs, 24 kV, 120 SWs/min) using the HM3 show areas of parenchymal interstitial haemorrhage in which there might be numerous small foci of ruptured vessels and broken renal tubules, but such areas of damage contain intact, recognizable structures. This pattern of diffuse injury is in stark contrast to what occurred in pigs treated using the Storz SLX. Injury with the SLX was particularly intense in that foci of damage often showed complete disruption of tissue with areas entirely devoid of histologically recognizable structures. These kidneys exhibited a 3- to 4-mm-diameter tunnel-like lesion with a crisp boundary between intact tissue at the periphery and homogenized cellular debris in the interior. In some cases the lesion traversed the full thickness of the renal parenchyma and was continuous with subcapsular haematomas on both the anterior and posterior surfaces of the kidney.
Quantitation of lesion volume (Fig. 5) showed that injury with the SLX was not significantly different from that produced by the HM3. However, the method of assessment we employed does not account for the intensity of injury and cannot discriminate between mild interstitial haemorrhage and frank bleeding into a wound site. The method measures the area of haemorrhage visible in systematically selected tissue sections of known thickness from which the volume of the haemorrhagic tissue is calculated [30]. As such, a given area of mild injury in which the interstitium surrounding intact renal structures (i.e. tubules, renal corpuscles) contains extravasated blood cells, an injury that is characteristic of the diffuse lesion with the HM3, will yield the same lesion value as a similar area in which blood has pooled into a void created by homogenization of the parenchyma, as occurred with the SLX.
Why the lesion with the SLX was more focused and more intense than the diffuse injury seen with the HM3 is probably due to multiple factors. In measurements with the FOPH, the focal width of the SLX (≈ 3 mm) was considerably narrower than that of the HM3 (≈ 8 mm). Thus, with the SLX the zone of highest acoustic pressure will be directed to a very narrow region. Indeed, the SLX produces the narrowest focal width of any electromagnetic lithotriptor and, among current lithotriptors, only the Wolf Piezolith 3000, a piezoelectric device with selectable focal width capability, can be set to generate a narrower focal zone (2.1 mm) [14]. Also, SWs were fired at a power setting (PL-9) with the SLX that generated peak pressures (P+ ≈ 90 MPa, P− ≈ − 12 MPa) considerably greater than were generated by the HM3 fired at 24 kV (P+≈ 46 MPa, P−≈−8 MPa). In addition, like other electromagnetic lithotriptors, the SLX delivers exceptionally consistent pulses from shot to shot. By contrast, the performance of caged electrodes of the type used with the HM3 was far less consistent, with irregularities in the path of arc discharge (i.e. spark jitter) producing dramatic fluctuations in acoustic pressures due to mm-scale wandering of the focal zone [14]. That is, the location of the zone of high pressure within the kidney is much more consistent with the SLX than with the HM3. Thus, the lesion created by the SLX might be more focused and more intense than injury caused by the HM3 because the focal zone is narrower, acoustic pressures are higher and successive SWs are more consistently on target.
The shot-to-shot consistency of the SLX could also explain why increasing the dose from 2000 to 4000 SWs did not significantly increase the volume of the lesion (Fig. 5). That is, if the first 2000 SWs were sufficient to cause detectable haemorrhage and with each successive SW the focal zone hit the same region of tissue, the measured lesion at 4000 SWs could yield the same volume. The intensity of the lesion – the completeness of tissue disruption within the focal volume – might be expected to increase as the number of SWs increases, but the measured volume of damage would remain the same.
Although the focused lesion created by the SLX was more pronounced than the injury seen with the HM3, this is not to say that such a lesion is more consequential. Very little is known about the long-term effects of lithotripsy injury regardless of the type of lithotriptor used. Animal studies have shown that SW-induced renal injury leads to parenchymal fibrosis, that scarring is dose-dependent and that a typical clinical dose of 2000 SWs in the pig model can cause atrophy of the renal papilla [1,5,6]. The focal ablation of renal structure as seen with the SLX would surely lead to fibrotic change. Whether this form of injury will result in greater permanent scarring or different long-term alterations in renal structure or function than the more diffuse, but clearly disruptive, injury seen with the HM3 is not known.
In the present study we used the SLX at its highest power setting (PL-9). Published reports confirm that the SLX and its predecessor, the Modulith SL20, have been used at this setting to treat renal stones [28,35,36]. However, current recommendations for the more recent Storz Modulith SLX-F2 lithotriptor are to use the highest setting only for ureteric stones [37]. It is possible that the unique morphological damage from the SLX in the present study was related to the use of the high power setting, and that injury at lower settings could be different. This will have to be ascertained in a future study.
Animal studies with the HM3 have shown that a variety of treatment strategies can be used to reduce the renal injury associated with SWL [12]. As injury is dose-dependent, a reduction in the number of SWs, or treatment at a reduced power setting, will lessen tissue trauma [2,38]. So too will initiating treatment at low power before ramping it up, or instituting a brief pause in treatment after a small priming dose, followed by the main dose [8,12]. Slowing the rate of SW administration is also effective in reducing injury, and pigs treated at 30–60 SWs/min showed significantly lower injury than pigs treated at 120 SWs/min [9,10].
Slow SW rate is a particularly attractive option in clinical SWL because it is uncomplicated, easy to control and has the added advantage of improved stone breakage outcomes [39]. Previous experimental animal studies of the effect of SW rate on renal injury have all been performed using the HM3. In the current study, however, we observed that slowing the SW rate with the SLX from 120 to 60 SWs/min did not reduce the volume of the haemorrhagic lesion (Fig. 5). Indeed, the histological characteristics of the lesion at 60 SWs/min were not different from the pattern of damage that occurred at 120 SWs/min. That is, like the SLX lesion at faster SW rates, the lesion at 60 SWs/min consisted of a highly focused region of tissue erosion largely devoid of recognizable renal structures (Fig. 3).
The mechanism by which slow SW rate is protective with the HM3 has yet to be determined, so it is difficult to suggest why the tissue response was different with the SLX. Until this can be investigated in much greater detail, once can only assume that just as the intensity of the lesion appears to be dependent on the acoustic characteristics of the lithotriptor, so is the renal response to insult. This is not to say that narrow focal width or small focal volume alone prevented a protective response to slow SW rate. It could be, instead, that the high acoustic pressures that were used in these experiments are the key factor – or perhaps narrow focal width and raised pressure work together to mitigate a protective response. What seems most important is the idea that the renal response to SWs can be affected by the acoustic features of the lithotriptor. One would hope that treatment variables will eventually be identified to allow improved safety regardless of the acoustic output and focal volume of the lithotriptor in question.
Measurements of acute changes in renal haemodynamics in SLX-treated animals showed significant reductions from baseline values regardless of the dose of SWs applied. That is, increasing the dose did not affect the response. This is similar to what has been observed in pigs treated with the HM3 at 2000 and 8000 SWs (24 kV, 2 Hz) [7]. Thus, even though the acoustic output and dimensions of the focal zone are substantially different for these two lithotriptors, and despite the fact that severity of the lesion differed between the two machines, they induced a similar haemodynamic response.
In conclusion, SW treatment in the pig model using a Storz SLX lithotriptor operated at similar settings to clinical treatment in SWL created a more focused, more intense lesion than occurred using the Dornier HM3. Tissue damage with the SLX was more severe within the core of the lesion than was seen with the HM3, but the volume of the haemorrhagic lesion was not significantly different for these two lithotriptors. While slowing the SW rate reduced injury with the HM3, this strategy was not protective with the SLX. These findings suggest that the acoustic output and dimensions of the focal zone of a lithotriptor affect the severity of renal injury. Further study will be needed to determine the role played by focal width of a lithotriptor in the long-term consequences of SWL injury.
‘What is known on the subject?’
Of all the SW lithotripters manufactured to date, more research studies have been conducted on and more is known about the injury (both description of injury and how to manipulate injury size) produced by the Dornier HM-3 than any other machine. From this information have come suggestions for treatment protocols to reduce shock wave (SW)-induced injury for use in stone clinics. By contrast, much less is known about the injury produced by narrow-focus and high-pressure lithotripters like the Storz Modulith SLX. In fact, a careful study looking at the morphology of the injury produced by the SLX itself is lacking, as is any study exploring ways to reduce renal injury by manipulating SW delivery variables of this lithotripter.
‘What does the study add?’
The present study quantitates the lesion size and describes the morphology of the injury produced by the SLX. In addition, we report that reducing the SW delivery rate, a manoeuvre known to lower injury in the HM-3, does not reduce lesion size in the SLX.
Acknowledgments
This project was supported by grants from the National Institutes of Health (P01-DK43881 and R01-DK67133). Special thanks are due to Dr Trevor M. Soergel, Metropolitan Urology, Louisville, KY, USA, for making the Storz SLX lithotriptor available for these studies, and to Dr Robin Cleveland for advice on methods for acoustic characterization of the SLX lithotriptor.
Abbreviations
- FRV
functional renal volume
- PAH
para-aminohippurat
- PL-9
power level 9
- RPF
renal plasma flow
- SEM
standard errors of the mean
- SWL
shock wave lithotripsy
- SWs
shock waves
References
- 1.Evan AP, Willis LR, Lingeman JE, McAteer JA. Renal trauma and the risk of long-term complications in shock wave lithotripsy. Nephron. 1998;78:1–8. doi: 10.1159/000044874. [DOI] [PubMed] [Google Scholar]
- 2.Connors BA, Evan AP, Willis LR, Blomgren PM, Lingeman JE, Fineberg NS. The effect of discharge voltage on renal injury and impairment caused by lithotripsy in the pig. J Am Soc Nephrol. 2000;11:310–8. doi: 10.1681/ASN.V112310. [DOI] [PubMed] [Google Scholar]
- 3.Lingeman J, Delius M, Evan A, Gupta M, Sarica K, Strohmaier W, McAteer J, Williams J. Bioeffects and physical mechanisms of SW effects in SWL. In: Segura J, Conort P, Khoury S, Pak C, Preminger GM, Tolley D, editors. Stone Disease: First International Consultation on Stone Disease. Heath Publications; Paris: 2003. pp. 251–286. [Google Scholar]
- 4.Lingeman JE, Matlaga BR, Evan AP. Surgical management of upper urinary tract calculi. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 9. II. Philadelphia: Saunders; 2007. pp. 1431–507. Chapt 44. [Google Scholar]
- 5.Evan AP, Willis LR. Extracorporeal shock wave lithotripsy: Complications. In: Smith AD, Badlani GH, Bagley DH, Clayman RV, Docimo SG, Jordan GH, Kavoussi LR, Lee BR, Lingeman JE, Preminger GM, Segura JW, editors. Smith’s Textbook on Endourology. Hamilton, Ontario, Canada: BC Decker, Inc; 2007. pp. 353–365. [Google Scholar]
- 6.McAteer JA, Evan AP. The acute and long-term adverse effects of shock wave lithotripsy. Seminars in Nephrology. 2008;28:200–213. doi: 10.1016/j.semnephrol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willis LR, Evan AP, Connors BA, Blomgren PM, Fineberg NS, Lingeman JE. Relationship between kidney size, renal injury, and renal impairment induced by shock wave lithotripsy. J Am Soc Nephrol. 1999;10:1753–62. doi: 10.1681/ASN.V1081753. [DOI] [PubMed] [Google Scholar]
- 8.Willis LR, Evan AP, Connors BA, Handa RK, Blomgren PM, Lingeman JE. Prevention of lithotripsy-induced renal injury by pretreating kidneys with low-energy shock waves. J Am Soc Nephrol. 2006;17:663–73. doi: 10.1681/ASN.2005060634. [DOI] [PubMed] [Google Scholar]
- 9.Evan AP, McAteer JA, Connors BA, Blomgren PM, Lingeman JE. Renal injury during shock wave lithotripsy is significantly reduced by slowing the rate of shock wave delivery. BJU Int. 2007;100:624–8. doi: 10.1111/j.1464-410X.2007.07007.x. [DOI] [PubMed] [Google Scholar]
- 10.Connors BA, Evan AP, Blomgren PM, Handa RK, Willis LR, Gao S, McAteer JA, Lingeman JE. Extracorporeal shock wave lithotripsy at 60 shock waves/min reduces renal injury in a porcine model. BJU Int. 2009;104:1004–8. doi: 10.1111/j.1464-410X.2009.08520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAteer JA, Evan AP, Connors BA, Pishchalnikov YA, Williams JC, Lingeman JE. Treatment protocols to reduce injury and improve stone breakage in SWL. In: Evan AP, Lingeman JE, McAteer JA, Williams JC, editors. Renal Stone Disease 2: Proceedings of the 2nd International Urolithiasis Research Symposium; 2008. pp. 243–248. AIP Proceedings 1049. [Google Scholar]
- 12.McAteer JA, Evan AP, Williams JC, Jr, Lingeman JE. Treatment protocols to reduce renal injury during shock wave lithotripsy. Current Opin Urology. 2009;19:192–195. doi: 10.1097/mou.0b013e32831e16e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lingeman JE, McAteer JA, Gnessin E, Evan AP. Shock Wave Lithotripsy: Advances in Technology and Technique. Nature Rev Urol. 2009;6:660–70. doi: 10.1038/nrurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleveland RO, McAteer JA. The physics of shock wave lithotripsy. In: Smith AD, Badlani GH, Bagley DH, Clayman RV, Docimo SG, Jordan GH, Kavoussi LR, Lee BR, Lingeman JE, Preminger GM, Segura JW, editors. Smith’s Textbook on Endourology. Hamilton, Ontario, Canada: BC Decker, Inc; 2007. pp. 317–332. [Google Scholar]
- 15.Cleveland RO, Sapozhnikov OA. Modeling elastic wave propagation in kidney stones with application to shock wave lithotripsy. J Acoust Soc Am. 2005;118:2667–2676. doi: 10.1121/1.2032187. [DOI] [PubMed] [Google Scholar]
- 16.Sapozhnikov OA, Maxwell AD, MacConaghy B, Bailey MR. A mechanistic analysis of stone fracture in lithotripsy. J Acoust Soc Am. 2007;121:1190–1202. doi: 10.1121/1.2404894. [DOI] [PubMed] [Google Scholar]
- 17.Cleveland RO, Anglade R, Babayan RK. Effect of stone motion on in vitro comminution efficiency of the Storz Modulith SLX. J Endourol. 2004;18:629–33. doi: 10.1089/end.2004.18.629. [DOI] [PubMed] [Google Scholar]
- 18.Tan EC, Tung KH, Foo KT. Comparative studies of extracorporeal shock wave lithotripsy by Dornier HM3, EDAP LT 01 and Sonolith 2000 devices. J Urol. 1991;146:294–297. doi: 10.1016/s0022-5347(17)37774-1. [DOI] [PubMed] [Google Scholar]
- 19.Bierkens AF, Hendrikx AJ, de Kort VJ, de Reyke T, Bruynen CA, Bouve ER, Beek TV, Vos P, Berkel HV. Efficacy of second generation lithotriptors: a multicenter comparative study of 2,206 extracorporeal shock wave lithotripsy treatments with the Siemens Lithostar, Dornier HM4, Wolf Piezolith 2300, Direx Tripter X-1 and Breakstone lithotriptors. J Urol. 1992;148:1052–1056. doi: 10.1016/s0022-5347(17)36814-3. [DOI] [PubMed] [Google Scholar]
- 20.Gerber R, Studer UE, Danuser H. Is newer always better? A comparative study of 3 lithotriptor generations. J Urol. 2005;173:2013–6. doi: 10.1097/01.ju.0000158042.41319.c4. [DOI] [PubMed] [Google Scholar]
- 21.Ng CF, Thompson TJ, McLornan L, Tolley DA. Single-center experience using three shockwave lithotripters with different generator designs in management of urinary calculi. J Endourol. 2006;20:1–8. doi: 10.1089/end.2006.20.1. [DOI] [PubMed] [Google Scholar]
- 22.Hoag CC, Taylor WN, Rowley VA. The efficacy of the Dornier Doli S lithotripter for renal stones. Can J Urol. 2006;13:3358–3363. [PubMed] [Google Scholar]
- 23.Ueda S, Matsuoka K, Yamashita T, Kunimi H, Noda S, Eto K. Perirenal hematomas caused by SWL with EDAP LT-01 lithotripter. J Endourol. 1993;7:11–15. doi: 10.1089/end.1993.7.11. [DOI] [PubMed] [Google Scholar]
- 24.Dhar NB, Thornton J, Karafa MT, Streem SB. A multivariate analysis of risk factors associated with subcapsular hematoma formation following electromagnetic shock wave lithotripsy. J Urol. 2004;172:2271–4. doi: 10.1097/01.ju.0000143459.03836.2d. [DOI] [PubMed] [Google Scholar]
- 25.Cleveland RO, Bailey MR, Fineberg N, Hartenbaum B, Lokhandwalla M, McAteer JA, Sturtevant B. Design and characterization of a research electrohydraulic lithotripter patterned after the Dornier HM3. Rev Scientific Instr. 2000;71:2514–2525. [Google Scholar]
- 26.Schultheiss R, Doerffel M. Standards for lithotripter performance. In: Evan AP, Lingeman JE, McAteer JA, Williams JC, editors. Renal Stone Disease 2: Proceedings of the 2nd International Urolithiasis Research Symposium. 2008. pp. 226–237. AIP Proceedings 1049. [Google Scholar]
- 27.Evan AP, McAteer JA, Connors BA, Pishchalnikov YA, Handa RK, Blomgren P, Willis LR, Williams JC, Jr, Lingeman JE, Gao S. Independent assessment of a wide-focus, low-pressure electromagnetic lithotriptor: absence of renal bioeffects in the pig. BJU Int. 2007;101:382–8. doi: 10.1111/j.1464-410X.2007.07231.x. [DOI] [PubMed] [Google Scholar]
- 28.Matin SF, Yost A, Streem SB. Extracorporeal shock-wave lithotripsy: a comparative study of electrohydraulic and electromagnetic units. J Urol. 2001;166:2053–6. doi: 10.1016/s0022-5347(05)65504-8. [DOI] [PubMed] [Google Scholar]
- 29.Shao Y, Connors BA, Evan AP, Willis LR, Lifshitz DA, Lingeman JE. Morphological changes induced in the pig kidney by extracorporeal shock wave lithotripsy: nephron injury. Anat Rec. 2003;275A:979–89. doi: 10.1002/ar.a.10115. [DOI] [PubMed] [Google Scholar]
- 30.Blomgren PM, Connors BA, Lingeman JE, Willis LR, Evan AP. Quantitation of shock wave lithotripsy-induced lesion in small and large pig kidneys. Anat Rec. 1997;249:341–8. doi: 10.1002/(SICI)1097-0185(199711)249:3<341::AID-AR4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 31.Pishchalnikov YA, McAteer JA, Williams JC., Jr Effect of firing rate on the performance of shock wave lithotriptors. BJU Int. 2008;102:1681–1686. doi: 10.1111/j.1464-410X.2008.07896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.IEC 61846 Ultrasonics - Pressure pulse lithotripters - Characteristics of fields. International Electrotechnical Commission; Geneva, Switzerland: 1998. [Google Scholar]
- 33.Handa RK, McAteer JA, Willis LR, Pishchalnikov YA, Connors BA, Ying J, Lingeman JE, Evan AP. Dual-head lithotripsy in synchronous mode: Acute effect on renal function and morphology in the pig. Brit J Urol Int. 2007;99:1134–1142. doi: 10.1111/j.1464-410X.2006.06736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Handa RK, McAteer JA, Connors BA, Pishchalnikov YA, Gao S, Evan AP. Assessment of renal injury with a clinical dual-head lithotripter delivering 240 shock waves per minute. J Urol. 2009;181:884–889. doi: 10.1016/j.juro.2008.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohrmann KU, Rassweiler JJ, Manning M, Mohr G, Henkel TO, Junemann KP, Alken P. The clinical introduction of a third generation lithotriptor: Modulith SL 20. J Urol. 1995;153:1379–83. [PubMed] [Google Scholar]
- 36.Coz F, Orvieto M, Bustos M, Lyng R, Stein C, Hinrichs A, San Francisco I. Extracorporeal shockwave lithotripsy of 2000 urinary calculi with the Modulith SL-20: success and failure according to size and location of stones. J Endourol. 2000;14:239–246. doi: 10.1089/end.2000.14.239. [DOI] [PubMed] [Google Scholar]
- 37.Elkoushy MA, Hassan JA, Morehouse DD, Anidjar M, Andonian S. Factors determining stone-free rate in shock wave lithotripsy using standard focus of Storz Modulith SLX-F2 lithotripter. Urology. 2011;77 doi: 10.1016/j.urology.2011.03.005. (In Press) [DOI] [PubMed] [Google Scholar]
- 38.Connors BA, Evan AP, Blomgren PM, et al. Reducing shock number dramatically decreases lesion size in a juvenile kidney model. J Endourol. 2006;20:607–11. doi: 10.1089/end.2006.20.607. [DOI] [PubMed] [Google Scholar]
- 39.Semins MJ, Trock BJ, Matlaga BR. The effect of shock wave rate on the outcome of shock wave lithotripsy: a meta-analysis. J Urol. 2008;179:194–197. doi: 10.1016/j.juro.2007.08.173. [DOI] [PubMed] [Google Scholar]