Abstract
Background:
The Nottinghamshire Lymphoma Registry contains the details of all the patients diagnosed with lymphoma (since 1 January 1973) within a defined geographical area with a population of 1.1 million. It was therefore possible to study the outcome of treatment for Hodgkin’s disease for three 10-year cohorts (1973–1982, 1983–1992 and 1993–2002).
The aims of the study were to compare survival time among the three patient cohorts, to identify prognostic factors and to estimate relative survival.
Methods:
A total of 745 patients diagnosed between 1973 and 2002 were analysed for survival. Survivorship was estimated by the Kaplan-Meier method and parametric survival models. An accelerated failure-time regression was used for multivariate analysis.
Results:
Overall, patients were observed for 9.8 (0.3–34.82) years (median(range)), on average. One, five and fifteen-year disease-specific survival was found to be 87% (85–90%), 77% (74–80%) and 70% (67–74%), respectively. For those for diagnosed between 1973 and 1982, the 15-year survival was found to be 57% for 1983–1992, it was 74% and for 1993–2002, it was 83% (P<0.001). The difference remained significant after adjusting for prognostic factors. The actuarial risk of developing a second malignancy at 20 years was for the 1973–1982 cohort, 12.4%, and for the 1983–1992 cohort, 18.8%.
Conclusion:
Treatment advances and effective management of toxicities of treatment over time, have resulted in a significantly longer survival for patients with Hodgkin’s disease diagnosed within a defined population.
Keywords: Hodgkin’s disease, survival analysis, accelerated-failure time, chemotherapy, radiotherapy, second malignancies
The majority of patients with Hodgkin’s disease (HD) have been cured of their disease in the UK since the introduction of combination chemotherapy for advanced disease in the early 1970s (Roy et al, 2000). Before this, in many centres in the UK, single-agent chemotherapy was used as a palliative treatment. The only potentially curative treatment was radiotherapy. In Nottinghamshire, with the introduction of the Medical School in the University of Nottingham, combined chemotherapy was routinely used for Hodgkin’s disease from 1972 onwards. For the start of this study, 1 January 1973 was chosen, because 1973 was the first full year using combination chemotherapy as standard therapy for patients with advanced HD. Nottinghamshire has a relatively stable population so that it has been possible to follow patients long term (over 30 years).
Survival data from large referral centres and from trial organisations tends to overestimate the survival, particularly in an older age group (Roy et al, 2000). The aim of this observational cohort study was to investigate the long-term survival of patients with Hodgkin’s disease.
In particular, we were interested in whether statistical differences between three separate cohorts (1973–1982, 1983–1992, 1993–2002) could be identified, and whether such differences would remain significant after adjusting for known prognostic factors.
Materials and methods
The Lymphoma Registry in Nottinghamshire
Most of the population of Nottinghamshire and adjacent parts of Derbyshire, Lincolnshire and Leicestershire are served by three hospitals: University Hospital (UHN) and City Hospital (NCH) Nottingham (now both part of Nottingham University Hospitals NHS Trust) and Kings Mill Hospital (KMH) (part of Sherwood Forest NHS Trust), Sutton-in-Ashfield. The served population is 1.1 million and has been stable during 1973–2002.
The registry was set up by Dr EM Bessell and AJ Moloney (now Head of Radiotherapy Physics, Clinical Director, Department of Clinical Oncology, Stoke-on-Trent, Staffordshire) in 1986, and data was entered prospectively from this date. Data from 1973–1986 was added retrospectively from radiotherapy notes held in the department and hospital notes from the three hospitals involved. The majority of patients were alive when the retrospective data were added. From 1986–1999, the data was entered from lymphoma clinics in each of the three hospitals; laptop computers were used in each clinic and the main computer updated every 6 months.
A printout from each histopathology department was obtained annually to ensure completeness of the registry. A dedicated data administrator has been employed since the registry was established. Since 1999, the data has been entered from the lymphoma multidisciplinary team meetings at the City Hospital (NCH). All patients diagnosed at the three hospitals are discussed at this meeting. The cause of death was determined by examination of the hospital notes (rather than the death certificate). The data administrator discussed all uncertain cases with the lead clinician (EM Bessell). The date, but not the type of any second malignancy, was recorded. Non-melanomatous skin cancers were not recorded.
Histopathology
The histopathology in Nottingham has been reported by histopathologists with a special interest in lymphoma throughout the study period (C Elston, IO Ellis, KA MacLennan and others), and has been reviewed at multidisciplinary team meetings since the 1980s. All cases are now seen by three haematopathologists (S O’Connor, D Clark and V Sovani). Patients have been entered into trials of the British National Lymphoma Investigation (BNLI) and other national trial organisations in the UK, where histological review was routine. Four major Nottingham reviews have been carried out on patients with Hodgkin’s disease. The slides were reviewed on 212 patients with Stage IA or IIA disease. In 188 patients, Hodgkin’s disease was confirmed (89%) and 24 were found not to be Hodgkin’s disease (22, non-Hodgkin’s lymphoma (NHL); 1, reactive; 1, carcinoma). The details of two patients were therefore removed from the registry and 22 patients were reclassified as NHL (Bessell et al, 1991; Bessell et al, 1998). All patients, aged 70 years and over, diagnosed with Hodgkin’s disease were reviewed. The histological sections of 39 patients were therefore reviewed. There were then 28 patients with Hodgkin’s disease, 10 with NHL (mostly peripheral T cell) and one was a carcinoma (Forsyth et al, 1997).
The third review was of 11 patients with advanced Hodgkin’s disease who were treated with chemotherapy and relapsed locally. These patients then received involved field radiotherapy. All 11 patients on review had Hodgkin’s disease (MacMillan and Bessell, 1994). The fourth review was done for this paper. The histological sections of ten patients with lymphocyte-depleted Hodgkin’s disease were reviewed (by S O’Connor). All 10 were reviewed as Hodgkin’s disease (six nodular sclerosing grade I, three nodular sclerosing grade II, 1 classical Hodgkin’s disease, not otherwise specified).
Management of patients with Hodgkin’s disease in the 3 separate 10-year periods
1973–1982
Advanced Hodgkin’s disease was treated with either MOPP (DeVita et al, 1970), MVPP (Nicholson et al, 1970) or LOPP (Hancock et al, 1992). Stages IA, IIA, IIIA were treated initially with radiotherapy alone in most cases. Staging laparotomy was carried out in 51 (29%) of the 176 patients without B symptoms. The chemotherapy was supervised mostly by general physicians with an interest in haematology; some chemotherapy and all the radiotherapy was given in the Department of Radiotherapy and Oncology in Nottingham. The radiotherapy given was mostly a mantle field, inverted Y or total nodal irradiation except for patients entered into the randomised BNLI trial of involved field vs extended field radiotherapy (Hoskin et al, 2005).
1983–1992
Advanced Hodgkin’s disease was treated with LOPP, LOPP/EVAP (Hancock et al, 1992), ChIVPP/PABLOE (Hancock et al, 2001) or ABVD (Canellos et al, 1992). Radiotherapy alone was given to patients with IA or non-bulky IIA (<10 cm) disease. Bulky IIA, IIB and IIIA/B with most of the disease above the diaphragm was treated with chemotherapy and radiotherapy. High-dose chemotherapy (BEAM and autologous bone marrow transplantation) was started in 1986. Seven patients had a staging laparotomy between 1983 and 1985. The radiotherapy given was mostly involved field. When a mantle field was used, the inferior border was 5 cm below the lower limit of disease (usually T7/T8 or T8/T9 to reduce cardiac irradiation.
1993–2002
Advanced Hodgkin’s disease was treated mostly with CHIVPP/PABLOE or ABVD (Johnson et al, 2005). Radiotherapy was replaced by ABVD (3–4 cycles) and involved field radiotherapy, alone in this period, except for patients with IA or IIA lymphocyte predominant disease (Fermé et al, 2007).
The radiotherapy technique did not change significantly from the previous decade. The dose of radiotherapy throughout the 30-year period has been 35–40 Gy in 20 fractions over 4 weeks.
Statistical analysis
Long-term survival studies may be analysed using either the traditional approach (cohort survival) (Cox, 1972), or a relatively recent alternative method (period survival, not presented here) that corrects for recent survival experiences by using the expected survival of a reference population, based on common characteristics (age, gender, area etc) (Brenner et al, 2004). As the primary endpoint of the analysis, survival time was considered as disease-specific (DSS), cancer-specific (CSS) and overall (OS) survival since the date of diagnosis. Alive patients were censored at the date of the last follow-up.
Univariate approaches, including Kaplan–Meier survival curves and life-tables, were implemented for estimating unadjusted survival probabilities. Survival tests (Cox-Mantel and Peto-Peto) were used for assessing differences among the patient cohorts having explored test hypotheses (e.g., proportionality).
Finally, a multivariate regression model was selected, among various candidates and an adjusted analysis was conducted. It should be noticed that a robust model evaluation was developed to assess model fit of the four most favourable survival models: Semiparametric (Cox) (Cox, 1972), flexible-parametric (Royston and Parmar, 2002), parametric Accelerated-time failure (gamma) (Kleinbaum and Klein, 2005) and parametric piece-wise (exponential) (Blossfeld and Rohwer, 1995). Model fit can be seen in Figure 1.
Figure 1.
Comparison of fit using cohort-adjusted survival curves.
Despite the popularity of the Cox regression in cancer research, a thorough evaluation suggested that it was not the best model. Both the Akaike information criterion (AIC) and the model- fit graph indicated the AFT model as the most recommended (Collett, 2000). As the major assumption of this model (proportional hazards) was rejected for two covariates (age and stage), a parametric alternative model was explored. Because of non-proportional-hazard erroneous assumption, AFT models avoid bias; however, they require a suitable distribution approximation of the survival time (parametric). In this study, a generalised gamma distribution was used in the multivariate analysis. The independent variables were patient (age, sex), disease (stage, histology, B-symptom) and treatment (therapy type) characteristics and a cohort-indicator variable (1973/82, 1983/92, 1993/02). Time ratios were estimated for facilitating interpretation. Such ratios are exponential coefficients (as the hazard ratios) known as acceleration factors. However, they declare an increase/decrease in delay of experiencing an event (death), rather than an increase/decrease in risk of such event, depending on whether the ratio is greater/lower than 1. Thus, a time ratio greater than one indicates that the exposure to that factor is beneficial as it delays death (Kleinbaum and Klein, 2005).
A significant level of 5% was adopted for all analyses. The analysis was performed in Stata v.10. (Cleves, 2008).
Results
Although 768 patients were found to be diagnosed and treated between January 1973 and December 2002, after a careful data-cleaning, only 745 patients were taken into the analysis stage (the main exclusion was deaths occurring within one week of diagnosis).
Table 1 presents patient characteristics stratified by the three patient-cohorts. Early cohorts, mid-cohort and late-cohort included 253 (33%), 272 (35%) and 243 (32%) patients correspondingly. The median (range) time of observation was 9.8 years (max 34.82).
Table 1. Patient characteristics.
| 1973–82 | 1983–92 | 1993–2002 | Total | |
|---|---|---|---|---|
| Freq. (%) | Freq. (%) | Freq. (%) | Freq. (%) | |
| Age group | ||||
| <26 | 66 (26.1) | 72 (26.6) | 61 (25.1) | 199 (25.9) |
| 26 to 45 | 81 (32) | 105 (38.7) | 94 (38.7) | 280 (36.5) |
| 46 to 65 | 70 (27.7) | 60 (22.1) | 65 (26.7) | 195 (25.4) |
| 66+ | 36 (14.2) | 34 (12.5) | 23 (9.5) | 93 (12.1) |
| Pearson χ2(6)=6.3939, P=0.381 | ||||
| Gender | ||||
| Female | 80 (31.6) | 105 (38.6) | 100 (41.2) | 285 (37.1) |
| Male | 173 (68.4) | 167 (61.4) | 143 (58.8) | 483 (62.9) |
| Pearson χ2(2)=5.2278, P=0.073 | ||||
| Treat | ||||
| Chemo | 126 (49.8) | 133 (48.9) | 129 (53.8) | 388 (50.7) |
| Combi | 29 (11.5) | 65 (23.9) | 56 (23.3) | 150 (19.6) |
| Radio | 98 (38.7) | 74 (27.2) | 55 (22.9) | 227 (29.7) |
| Pearson χ2(4)=24.7184, P<0.001 | ||||
| Stage | ||||
| I | 62 (24.5) | 53 (19.5) | 63 (25.9) | 178 (23.2) |
| II | 44 (17.4) | 97 (35.7) | 110 (45.3) | 251 (32.7) |
| III | 78 (30.8) | 65 (23.9) | 36 (14.8) | 179 (23.3) |
| IV | 30 (11.9) | 40 (14.7) | 29 (11.9) | 99 (12.9) |
| Unsp. | 39 (15.4) | 17 (6.3) | 5 (2.1) | 61 (7.9) |
| Pearson χ2(8)=77.3425, P<0.001 | ||||
| B symptoms | ||||
| No | 188 (74.3) | 193 (71.2) | 146 (61.3) | 527 (69.2) |
| Yes | 65 (25.7) | 78 (28.8) | 92 (38.7) | 235 (30.8) |
| Pearson χ2(2)=10.4978, P=0.005 | ||||
| Who | ||||
| Lymphocyte Pred. | 24 (9.5) | 22 (8.1) | 24 (9.9) | 70 (9.1) |
| Mixed Cell. | 61 (24.1) | 54 (19.9) | 39 (16) | 154 (20.1) |
| Nodular Scl. | 127 (50.2) | 184 (67.6) | 177 (72.8) | 488 (63.5) |
| Unsp. | 41 (16.2) | 12 (4.4) | 3 (1.2) | 56 (7.3) |
| Pearson χ2(6)=58.5400, P<0.001 | ||||
| Status | ||||
| Alive | 71 (28.1) | 132 (48.5) | 168 (69.1) | 371 (48.3) |
| Died of disease | 105 (41.5) | 70 (25.7) | 35 (14.4) | 210 (27.3) |
| Died of other cancer | 30 (11.9) | 12 (4.4) | 8 (3.3) | 50 (6.5) |
| Died of other causes | 30 (11.9) | 40 (14.7) | 9 (3.7) | 79 (10.3) |
| 4. Lost to follow-up | 17 (6.7) | 18 (6.6) | 23 (9.5) | 58 (7.6) |
| Pearson χ2(8)=111.5255, P=0.000 | ||||
| Sample size | 253 | 272 | 243 | 768 |
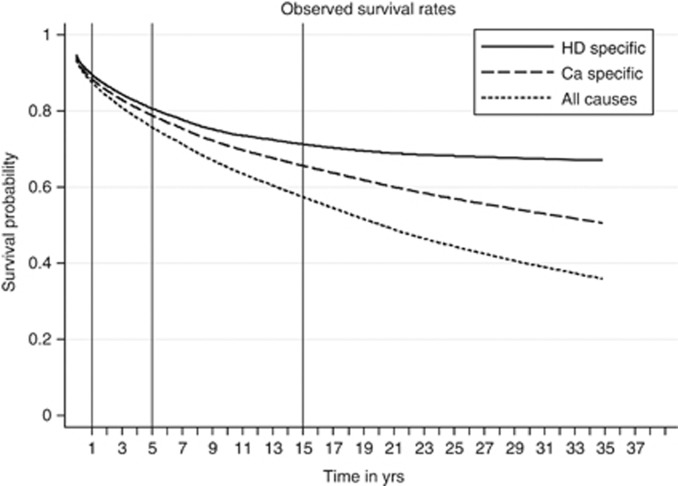
Table 2 presents one, five and fifteen-year survival rates (and 95% confidence intervals) for three types of survivorship: DSS, CSS and OS. In addition to this, corresponding Kaplan–Meier survival curves are illustrated at Figures 1 and 2.
Table 2. Patient survival.
| Survival | Year 1 | Year 5 | Year 15 |
|---|---|---|---|
| DSS | |||
| Cohort 1973–82 | 0.7808 | 0.647 | 0.5742 |
| Cohort 1983–92 | 0.8849 | 0.7926 | 0.7363 |
| Cohort 1993–2002 | 0.9536 | 0.8822 | 0.8258 |
| Log-rank (χ2) =37.82, P<0.001, Peto-Peto (χ2)=40.6, P<0.001, Wald test (χ2)=34.60, P<0.001 | |||
| CaSS | |||
| Cohort 1973–82 | 0.7549 | 0.6217 | 0.5226 |
| Cohort 1983–92 | 0.8816 | 0.7822 | 0.7096 |
| Cohort 1993–02 | 0.9495 | 0.8696 | 0.7524 |
| Log-rank (χ2) =40.38, P<0.001, Peto-Peto (χ2)=45.29, P<0.001, Wald test (χ2)=35.67, P<0.001 | |||
| Any cause | |||
| Cohort 1973–82 | 0.751 | 0.5968 | 0.4503 |
| Cohort 1983–92 | 0.8709 | 0.7417 | 0.6112 |
| Cohort 1993–02 | 0.9334 | 0.845 | 0.7115 |
| Log-rank (χ2) =32.22, P<0.001, Peto-Peto (χ2)=40.6, P<0.001, Wald test (χ2)=31.2, P<0.001 | |||
Figure 2.
Survival probabilities for all patients.
Next, cohort differences were investigated. Survival differences across the three cohorts found to be highly significant as both Wilcoxon and Cox-Manthel (log-rank) test indicated. It should be noted that the hypothesis of proportional hazards was marginally rejected, in the case of late-cohort. As a result, univariate analysis encouraged a multivariable analysis based on disease and patient factors.
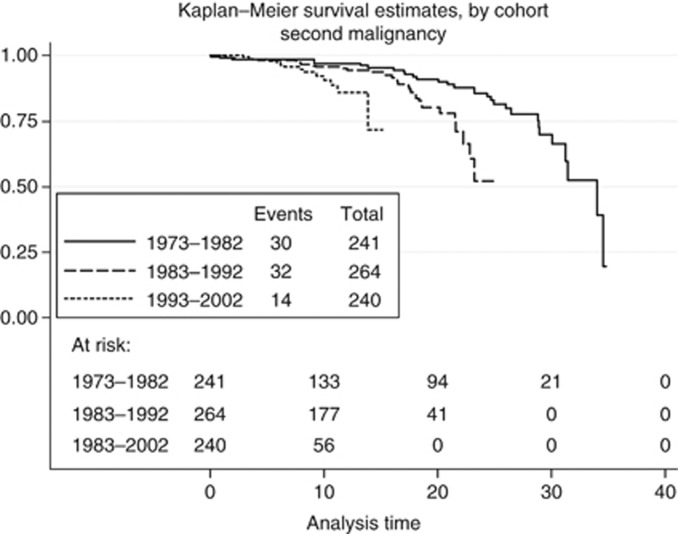
In addition to survivorship, time to second malignancy was also considered (Figure 3). Among the 745 patients, second malignancy was observed in only 76 (10.2%). Cohort estimates are presented in Tables 3 and 4. Univariate differences were found to be significant (x2=7.389, P=0.025). Also, time differences to second malignancy found to be significant (Wilcoxon x2=6.581 P=0.0372). We should note that the observation time for second malignancy was extended until June 2010. If a comparison is made with the data in Table 1, it can be seen that all the 30 malignancies diagnosed in the cohort of patients (1973–1982) resulted in death, whereas, in the cohort (1983–1992), only 12 of 32 malignancies resulted in death, and, in the cohort (1993–2002), only 8 of 14 malignancies resulted in death.
Figure 3.
Time (between diagnosis) and second malignancy stratified by cohort.
Table 3. Frequency of second malignancy.
| 1973–82 | 1983–92 | 1993–2002 | ||||
|---|---|---|---|---|---|---|
| Freq ( N ) | (%) | Freq ( N ) | (%) | Freq ( N ) | (%) | |
| Second malignancy | ||||||
| No | 211 | 87.6 | 232 | 87.9 | 226 | 94.2 |
| Yes | 30 | 12.4 | 32 | 12.1 | 14 | 5.8 |
Pearson χ2(2)=7.3889, P=0.025
Table 4. Time to second malignancy.
| Survival | Year 1 | Year 5 | Year 15 |
|---|---|---|---|
| Cohort 1973–82 | 0.7999 | 0.6627 | 0.5882 |
| Cohort 1983–92 | 0.9049 | 0.8105 | 0.7530 |
| Cohort 1993–2002 | 0.9536 | 0.8822 | 0.8258 |
| Log-rank (χ2) =37.82, P<0.001, Peto-Peto (χ2)=40.6, P<0.001, Wald test (χ2)=34.60, P<0.001 | |||
Finally, regression models were used to provide adjusted estimates for the three cohorts after controlling for age (at diagnosis), gender, tumour staging, treatment type, histological type and B symptoms. Results from the accelerated time-failure regression model are listed in Table 5 in the form of time ratios (exponentiated coefficients). Results indicated that after adjusting for related factors, cohort (survival) differences remained highly significant. In particular, the shift from the early cohort (1973–82, base-category) to mid cohort (1983–92) is associated with an almost two-fold delay of death event otherwise, prolonged survival (i.e., experiencing of death decelerates over time by a factor of 1.92), and from early cohort to the late (1993–02), with an almost 5-times prolonged survival (P<0.001). Covariates associated with accelerated (faster) time impact on death were increasing age, higher than stage I tumour, presence of B symptom and other-than lymphocyte predominant histology. In contrast, covariates of decelerated (delayed) time found to be female gender (not significant), combined chemotherapy and radiotherapy (not significant), and radiotherapy. The proportion of female patients increased with each successive cohort. Male-to-female ratio 2.16, 1973–1982; 1.59, 1983–1992; 1.43, 1993–2002.
Table 5. Accelerated-failure time model.
| Factor | Time ratio univariate | 95% CI | Time ratio multivariate | 95% CI | P -value |
|---|---|---|---|---|---|
| Cohort | |||||
| Cohort(73–82) | 1.00 | 1.00 | |||
| Cohort(83–92) | 4.85*** | 1.93, 12.22 | 1.92* | 1.02, 3.62 | 0.045 |
| Cohort(93–02) | 22.58*** | 8.84, 57.71 | 4.60*** | 2.28, 9.30 | <0.001 |
| Age group | |||||
| Age gp (<26) | 1.00 | 1.00 | |||
| Age gp(26–45) | 0.64 | 0.26, 1.57 | 0.81 | 0.39, 1.66 | 0.556 |
| Age gp(46–65) | 0.07*** | 0.03, 0.18 | 0.14*** | 0.06, 0.30 | <0.001 |
| Age gp(66+) | 0.004*** | 0.001, 0.011 | 0.02*** | 0.01, 0.06 | <0.001 |
| Gender | |||||
| Male | 1.00 | 1.00 | 0.85, 2.51 | ||
| Female | 1.26 | 0.51, 3.13 | 1.46 | 0.85, 2.51 | 0.170 |
| Treatment | |||||
| Chemotherapy | 1.00 | 1.00 | |||
| Combi (chemo+radio) | 23.22*** | 9.36, 57.65 | 2.06 | 0.99, 4.28 | 0.053 |
| Radiotherapy | 27.14*** | 11.83, 62.28 | 2.63* | 1.24, 5.57 | 0.011 |
| B symptom | |||||
| No | 1.00 | 1.00 | |||
| Yes | 0.39* | 0.17, 0.91 | 0.42** | 0.23, 0.76 | 0.005 |
| Who | |||||
| Lymphocyte | 1.00 | 1.00 | |||
| Mixed Cell | 0.22* | 0.06, 0.88 | 0.31* | 0.11, 0.85 | 0.022 |
| Nodular Scl | 0.49 | 0.14, 1.75 | 0.37* | 0.15, 0.92 | 0.032 |
| Unspecified | 0.001*** | 0.0003, 0.008 | 0.03*** | 0.01, 0.14 | <0.001 |
| Stages | |||||
| Stage I | 1.00 | 1.00 | |||
| Stage II | 0.33* | 0.13, 0.83 | 0.37* | 0.16, 0.84 | 0.018 |
| Stage III | 0.06*** | 0.02, 0.15 | 0.29** | 0.12, 0.68 | 0.005 |
| Stage IV | 0.02*** | 0.006, 0.089 | 0.22** | 0.08, 0.63 | 0.005 |
| Stage V | 0.001*** | 0.0004, 0.004 | 0.06*** | 0.01, 0.26 | <0.001 |
*P<0.05, **P<0.01, ***P<0.001. If TR<1, event occurs faster as time accelerates. If TR>1, event delays as time decelerates.
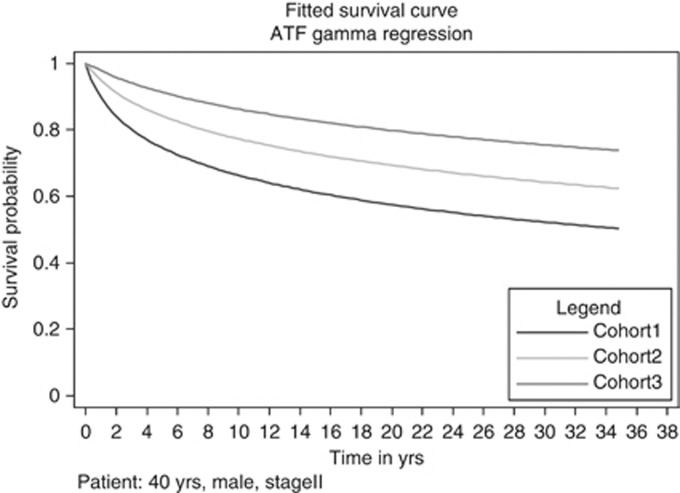
Last, as a way to illustrate the survival disparities among the three cohorts, we attempted a between-cohort comparison of the fitted/predicted survival time of a typical HD patient in each of the three available cohorts. Figure 4 illustrates the predicted survival for a male, diagnosed with a stage II disease, at the age of 40 years, treated with chemotherapy and radiotherapy, having nodular sclerosing histology. As the graph suggests, transparent differences in predicted survival are observed over time.
Figure 4.
Comparison of survival probabilities for a typical patient.
Discussion
The cure rate for HD in Nottinghamshire has improved significantly over a 30-year period with patients treated in the most recent cohort, (1993–2002) having a cure rate of 75% (15 year cause specific survival). This compares with a cure rate of 52% in the era 1973–1982. Our results suggest a clear improvement in survival time was observed. After adjusting for other factors, regarding time to death, a 4-fold deceleration was found for the latest cohort compared with the earliest (95% CI 2.3–9.3).
This improvement in survival is likely to be due to the introduction of Doxorubicin in the early 1980s, better management of toxicity such as neutropenic sepsis, and possibly increased dose intensity of chemotherapy (this was not demonstrated for ABVD chemotherapy in the United Kingdom Lymphoma Group trial LY09, Owadally et al, 2010). There is insufficient data on the registry to clarify this situation further. There is no evidence supporting a change in the natural history of HD. It is not clear why the proportion of female patients with HD has been increasing in more recent cohorts except that, in the general population in Nottinghamshire (over the age of 70 years), the female-to-male ratio has been increasing (Forsyth et al, 1997).
The risk of second malignancy has been estimated for each of the three cohorts. At 20 years, the risk of second malignancy is 12.4% for the 1973–1982 cohort, but because all second malignancies resulted in death, it is probable that this is an underestimate. The risk of second malignancy in the second cohort (1983–1992) at 20 years is 18.8%. The actuarial risk of second malignancy seems to be increasing (Figure 4), but this may be due to the more accurate registration of second malignancies on the lymphoma registry that do not result in death. The estimates for the actuarial risk of second malignancy are similar to other series of patients with Hodgkin’s disease treated with radiotherapy, chemotherapy or chemoradiotherapy (Swerdlow et al, 1992; Mauch et al, 1996; Bonadonna et al, 2005; Allemani et al, 2006; Franklin et al, 2006; Hodgson et al, 2007; Sénécal et al, 2008). These series give a long-term risk of 20–30% for second malignancy.
There are relatively few publications on the long-term survival of patients with HD. Some of these include patients treated in the 1960s, when combination chemotherapy was not used routinely and radiotherapy was used with larger fields and less precise imaging. In addition, even fewer publications are based on a defined population from a registry. Using national cancer registration data with limited access to the hospital notes may be more inaccurate for lymphomas than for solid malignancies (Trent Regional Cancer Registry data). There is agreement in the literature that, for the era 1973–2002, the 15-year disease-specific survival or relative survival is about 70% (Mauch et al, 1995; Van Spronsen et al, 1997; Aleman et al, 2003; Provencio et al, 2008). The survival of patients with HD entered onto the British National Lymphoma Investigation data base (which would have included some Nottinghamshire patients) during the period 1970–1987 was similar to that obtained from the population-based UK National Cancer Registry only for patients less than 45 years of age. Older patients were found to have a much better survival (relative survival 39%) than those from the National Cancer Registry (relative survival 27%). This demonstrates that data bases set up to analyse clinical trials often contain patients with a more favourable prognosis (Roy et al, 2000). There is evidence that the prognosis for patients over 45 years of age with HD is improving in recent cohorts but is still less favourable than for younger patients (Brenner et al, 2008).
The prognostic factors for HD are well known. The Hasenclever prognostic index is widely used in managing patients with advanced HD (Hasenclever and Diehl, 1998). This includes 7 factors, a serum albumin level of <40 g l−1, a haemoglobin level of <10.5 g dl−1, male sex, age ⩾45 years, stage IV disease, leucocytosis (⩾15.0 × 109 l−1) and lymphopaenia (<0.6 × 109 l−1).
For early stage HD (IA and IIA), the following factors associated with a good prognosis have been found: lymphocyte predominant and nodular sclerosing histology, young age <40 years, non-bulky disease (<8 cm), three or fewer nodal sites and an erythrocyte sedimentation rate of <30 mm h−1 (Haybittle et al, 1985).
In our series, the covariates associated with a poorer prognosis (Table 5) in the accelerated-failure time model were earlier methods of treatment, increasing age of the patient (particularly over the age of 45 years), chemotherapy alone (without radiotherapy), B symptoms, histology other than lymphocyte predominant HD, and increasing stage of disease. Male gender was not associated with a worse prognosis. There has been much debate about the role of consolidation radiotherapy in the treatment of advance HD, but in the UKLG LY09 trial of ABVD vs ChlVPP/PABLOE, consolidation radiotherapy was associated with better outcomes across all prognostic groups in multivariate analysis (Johnson et al, 2010). In our series, combined chemotherapy and radiotherapy compared with chemotherapy alone caused a two-fold delay in death (95% Cls 0.99–4.28).
The improvement in survival seen in the three consecutive cohorts in our series is independent of all of these prognostic factors.
Acknowledgments
We are indebted to the generous financial support of Mr & Mrs Kelly Bloom and family of Nottinghamshire for the lymphoma registry.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
The authors declare no conflict of interest.
References
- Aleman BMP, van den Belt-Dusebout AW, Klokman WJ, Van’t Veer MB, Bartelink H, van Leeuwen FE (2003) Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol 21: 3431–3439 [DOI] [PubMed] [Google Scholar]
- Allemani C, Sant M, DeAngelis R, Marcos-Gragera R, Coebergh JW (2006) Hodgkin’s survival in Europe and the US: prognostic significance of morphologic groups. Cancer 107: 352–360 [DOI] [PubMed] [Google Scholar]
- Bessell EM, MacLennan KA, Toghill PJ, Ellis IO, Fletcher J, Dowling FD (1991) Suprahyoid Hodgkin’s disease stage IA. Radiother Oncol 22: 190–194 [DOI] [PubMed]
- Bessell EM, Moloney AJ, Ellis IO, Fletcher J, Dowling F (1998) Prognosis factors affecting disease-free survival in patients with Hodgkin’s disease stages IA and IIA treated initially with radiotherapy alone in a single centre during 1973-1992. Radiother Oncol 49: 15–19 [DOI] [PubMed] [Google Scholar]
- Blossfeld H-P, Rohwer GT (1995) Techniques of Event history Modelling: New Approaches to Casual Analysis. Erlbaum: Mahwah, N.M [Google Scholar]
- Bonadonna G, Viviani S, Bonfante V, Gianni AM, Valagussa P (2005) Survival in Hodgkin’s disease patients – report of 25 years of experience at the Milan Cancer Institute. Eur J Cancer 41: 998–1006 [DOI] [PubMed] [Google Scholar]
- Brenner H, Arndt V, Gefelleer O, Hakulinen T (2004) An alternative approach to age adjustment of cancer survival rates. Eur J Cancer 40: 2317–2322 [DOI] [PubMed] [Google Scholar]
- Brenner H, Gefeller O, Hakulinen T (2004) Period analysis for ‘up-to-date’ cancer survival data: theory, empirical evaluation, computational realisation and applications. Eur J Cancer 40: 326–335 [DOI] [PubMed] [Google Scholar]
- Brenner H, Gondos A, Pulse D (2008) Ongoing improvement in long-term survival of patients with Hodgkin’s disease at all ages and recent catch up of older patients. Blood 111: 2977–2983 [DOI] [PubMed] [Google Scholar]
- Canellos GP, Anderson JR, Propert KJ, Nissen N, Cooper MR, Henderson ES, Green MR, Gottlieb A, Peterson BA (1992) Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD or MOPP alternating with ABVD. N. Eng. J Med 327: 1478–1484 [DOI] [PubMed] [Google Scholar]
- Cleves MA (2008) An Introduction to Survival Analysis using Stata 2nd edn Stata Press: College Station, TX, p 372 [Google Scholar]
- Collett DR (2000) Applied Survival Analysis – A Practical Approach. Control Clin Trials 21: 56–58 [Google Scholar]
- Cox DR (1972) Regression Models and Life-Tables. J Roy Statist Soc Ser B Stat Methodol 34: 187 [Google Scholar]
- DeVita VT, Serpick AA, Carbone PP (1970) Combination chemotherapy in the treatment of Hodgkin’s disease. (1970) Ann Intern. Med 73: 881–895 [DOI] [PubMed] [Google Scholar]
- Fermé C, Eghbali H, Meerwaldt JH, Rieux C, Bosq J, Berger F, Girinsky T, Brice P, van’teer MB, Walewski JA, Lederlin P, Tirelli U, Carde P, van den Neste E, Gryan E, Monconduit M, Diviné M, JMM Raemaekers, Salles G, Noordijk EM, Creemers G-J, Gabarre J, Hagenbeek A, Reman O, Blanc M, Thomas J, Vié B, Kluin-Nelemans JC, Viseu F, Baars JW, Poortmans P, Lugtenburg PJ, Carrie C, Jaubert J, Henry-Amar M (2007) Chemotherapy plus involved-field radiation in early stage Hodgkin’s disease. N Eng J Med 357: 1916–1927 [DOI] [PubMed] [Google Scholar]
- Forsyth PD, Bessell EM, Moloney AJ, Leach IH, Davies JM, Fletcher J (1997) Hodgkin’s disease in patients older than 70 years of age; a registry based analysis. Europ J Cancer 33: 1638–1642 [DOI] [PubMed] [Google Scholar]
- Franklin J, Pluetschow A, Paus M, Specht L, Anselmo AP, Aviles A, Biti G, Bogatyreva T, Bonadonna G, Brillant C, Cavalieri E, Diehl V, Eghbali H, Fermé C, Henry-Amar M, Hoppe R, Howard S, Meyer R, Niedzwiecki D, Pavlovsky S, Radford J, Raemaekers J, Ryder D, Schiller P, Shakhtarina S, Valagussa P, Wilimas J, Yahalom J (2006) Second malignancy risk associated with treatment of Hodgkin’s lymphoma: meta-analysis of the randomised trials. Ann- Oncol 17: 1749–1760 [DOI] [PubMed] [Google Scholar]
- Hancock BW, Gregory WM, Cullen MH, Hudson GV, Burton A, Selby P, MacLennan KA, Jack A, Bessell EM, Smith P, Linch DC (2001) ChIVPP alternating with PABLOE is superior to PABLOE alone in the initial treatment of advanced Hodgkin’s disease: results of a British National Lymphoma Investigation/Central Lymphoma Group randomised controlled trial. Br J Cancer 184: 1293–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock BW, Vaughan Hudson G, Vaughan Hudson B, Bennett MH, MacLennan KA, Haybittle JL, Anderson L, Linch DC (1992) LOPP alternating with EVAP is superior to LOPP alone in the initial treatment of advanced Hodgkin’s disease: results of a British National Lymphoma Investigations trial. J Clin Oncol 10: 1252–1258 [DOI] [PubMed] [Google Scholar]
- Hasenclever D, Diehl V (1998) A prognostic score for advanced Hodgkin’s disease. N Eng J Med 339: 1506–1514 [DOI] [PubMed] [Google Scholar]
- Haybittle JL, Hayhoe FG, Easterling MJ, Jelliffe AM, Bennett MH, Vaughan Hudson G, Vaughan Hudson B, MacLennan KA (1985) Review of British National Lymphoma Investigation studies of Hodgkin’s disease and development of prognostic index. Lancet 1: 967–972 [DOI] [PubMed] [Google Scholar]
- Hodgson DE, Gilbert ES, Dores GM, Schonfeld SJ, Lynch CF, Storm H, Hall P, Langmark F, Pukkala E, Andersson M, Kaijser M, Joensuu H, Fossa SD, Travis LB (2007) Long-term solid cancer risk among 5-year survivors of Hodgkin’s lymphoma. J Clin-Oncol 25: 1489–1497 [DOI] [PubMed] [Google Scholar]
- Hoskin PJ, Smith P, Maughan TS, Gilson D, Vernon C, Syndikus I, Linch DC (2005) Long-term results of a randomised trial of involved field radiotherapy versus extended field radiotherapy in stage I and II Hodgkin’s lymphoma. Clin Oncol (R Coll Radiol) 17: 47–53 [DOI] [PubMed] [Google Scholar]
- Johnson PW, Radford JA, Cullen MH, Sydes MR, Walewski J, Jack AS, MacLennan KA, Stenning SP, Clawson S, Smith P, Ryder D, Hancock BW (2005) United Kingdom Lymphoma Group LY09 Trial Comparison of ABVD and alternating or hybrid multidrug regimens for the treatment of advanced Hodgkin’s lymphoma results of the United Kingdom Lymphoma Group LY09 trial. J Clin Oncol 23: 9208–9218 [DOI] [PubMed] [Google Scholar]
- Johnson PWM, Sydes MR, Hancock BW, Cullen M, Radford JA, Stenning SP (2010) Consolidation radiotherapy in patients with advanced Hodgkin’s lymphoma: survival data from the UKLG LY09 randomized controlled trial (ISRCTN97144519). J Clin Oncol 28: 3352–3359 [DOI] [PubMed] [Google Scholar]
- Kleinbaum DG, Klein M (2005) Survival Analysis: a Self-Learning Text 2nd edn Springer: New York, NY [Google Scholar]
- MacMillan CH, Bessell EM (1994) The effectiveness of radiotherapy for localised relapse in patients with Hodgkin’s disease (IIB-IVB) who obtained a complete response with chemotherapy alone as initial treatment. Clin Oncol (R Coll Radiol) 6: 147–150 [DOI] [PubMed] [Google Scholar]
- Mauch PM, Kalish LA, Marcus KC, Coleman CN, Shulman LN, Krill E, Come S, Silver B, Canellos GP, Tarbell NJ (1996) Second malignancies after treatment for laparotomy stages IA-IIIB Hodgkin’s disease: long-term analysis of risk factors and outcome. Blood 87: 3625–3632 [PubMed] [Google Scholar]
- Mauch PM, Kalish LA, Marcus KC, Shulman LN, Krill E, Tarbell NJ, Silver B, Weinstein H, Come S, Canellos GP, Coleman CN (1995) Long-term survival in Hodgkin’s disease relative impact of mortality, second tumours, infection and cardiovascular disease. Cancer J Sci Am 1: 33–42 [PubMed] [Google Scholar]
- Nicholson WM, Beard ME, Crowther D, Stansfeld AG, Vartan CP, Malpas JS, Fairley GH, Scott RB (1970) Combination chemotherapy in generalised Hodgkin's disease. Br Med J 3(5713): 7–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owadally WS, Sydes MR, Radford JA, Hancock BW, Cullen MH, Stenning SP, Johnson PWM (2010) Initial dose intensity has limited impact on the outcome of ABVD Chemotherapy for advanced Hodgkin lymphoma: data from UKLG LY09 21. Ann Oncol 21(3): 568–573 [DOI] [PubMed] [Google Scholar]
- Provencio M, Millan I, Espana P, Sanchez AC, Sanchez JJ, Cantos B, Vargas JA, Bellas C, Garcia V, Sabin P, Bonilla F (2008) Analysis of competing risks of causes of death and their variation over different time periods in Hodgkin’s disease. Clin Cancer Res 14: 5300–5301 [DOI] [PubMed] [Google Scholar]
- Roy P, Vaughan Hudson G, Vaughan Hudson B, Esteve J, Swerdlow AJ (2000) Long-term survival in Hodgkin’s disease patients. A comparison of relative survival in patients in trials and those recorded in population-based cancer registers. Eur J Cancer 36: 384–389 [DOI] [PubMed] [Google Scholar]
- Royston P, Parmar MKB (2002) Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med 21: 2175–2197 [DOI] [PubMed] [Google Scholar]
- Sénécal D, Jais P, Desablens B, Berthou C, Casassus P, Moles MP, Delwail V, Gastinne T, Colonna P, Andrieu JM (2008) Twenty-year disease and treatment – associated mortality rates of patients with Hodgkin’s lymphoma of clinical stages IIIB and IV prospectively treated with 3-month anthracycline-based chemotherapy followed by extended high-dose radiation. Cancer 112: 846–855 [DOI] [PubMed] [Google Scholar]
- Van Spronsen DJ, Dijkema IM, Vrints LW, Hofstra G, Crommelin MA, Erdkamp FL, Coebergh JW, Breed WP (1997) Improved survival of Hodgkin’s patients in south-east Netherlands since 1972. Eur J Cancer 33: 436–441 [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Douglas AJ, Vaughan Hudson G, Vaughan Hudson B, Bennett MH, MacLennan KA (1992) Risk of second primary cancers after Hodgkin’s disease by type of treatment: analysis of 2846 patients in the British National Lymphoma Investigation. BMJ 304: 1137–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]