Abstract
Background:
Melanoma incidence has increased rapidly in the last decades, and predictions show a continuing increase in the years to come. The aim of this study was to assess trends in melanoma incidence, Breslow thickness (BT), and melanoma survival among young and elderly patients in the Netherlands.
Methods:
Patients diagnosed with invasive melanoma between 1994 and 2008 were selected from the Netherlands Cancer Registry. Incidence (per 100 000) over time was calculated for young (<65 years) and elderly patients (⩾65 years). Distribution of BT for young and elderly males and females was assessed. Regression analysis of the log-transformed BT was used to assess changes over time. Relative survival was calculated as the ratio of observed survival to expected survival.
Results:
Overall, 40 880 patients were included (42.3% male and 57.7% female). Melanoma incidence increased more rapidly among the elderly (5.4% estimated annual percentage change (EAPC), P<0.0001) than among younger patients (3.9% EAPC, P<0.0001). The overall BT declined significantly over time (P<0.001). Among younger patients, BT decreased for almost all locations. Among elderly males, BT decreased for melanomas in the head and neck region (P=0.001) and trunk (P<0.001), but did not decrease significantly for the other regions. Among elderly females, BT only decreased for melanomas at the trunk (P=0.01). The relative survival of elderly patients was worse compared with that of younger patients (P<0.001).
Conclusion:
Melanoma incidence increases more rapidly for elderly than for younger patients and the decline in BT is less prominent among elderly patients than among young patients. Campaigns in the Netherlands should focus more on early melanoma detection in the elderly.
Keywords: melanoma, Breslow thickness, elderly, population-based
The incidence of cutaneous melanoma has increased in the last decades and estimates predict a continuing increase in the coming years (De Vries et al, 2005). High incidence rates are found in populations of predominantly European origin, with white populations in Australia and New Zealand having the highest incidence rates and Asian and Black populations having the lowest (Parkin et al, 1997).
In Australia, despite of rising incidence rates, mortality rates seem to have reached a plateau, possibly because of the fact that most newly diagnosed melanomas are thinner melanomas, which usually do not lead to death (Giles et al, 1996).
The most important risk factors for melanoma still are increased exposure to the sun and sunburns during childhood. Significantly increased melanoma risk is associated with sun-bed and sun-lamp exposure (Bulliard, 2000; Gallagher et al, 2005).
In the Netherlands, the total number of melanoma patients is expected to increase from 2400 patients in 2000 to 4800 patients in 2015, and reached 3500 patients in 2005 (De Vries et al, 2005). In contrast with Australia, this increase in incidence rate is accompanied by a rising mortality rate.
Several influences, such as increased melanoma awareness and an increased life expectancy, might cause a rising incidence of melanoma. Also, in order to get a vague idea about the true increase in the incidence of melanoma, it is essential to know how many pigmented lesions are excised. Marks et al (1997) concluded that ‘Among people aged 21–40 years the ratio of benign naevi to melanomas among excised lesions was 27.2, compared to 1.4 in those aged 60 years and over.’ Thus, the increase in incidence in the elderly cannot be explained by overtreatment.
Besides, because incidence of melanomas among all Breslow thickness (BT) categories increased, as well as the mortality rates, the melanoma epidemic in the Netherlands seems to be real and not due overdiagnosis (Hollestein et al, 2012).
Various studies have already shown that the melanoma incidence and mortality among elderly individuals (>65 years) is growing rapidly (Armstrong and Kricker, 1994; Gaudette and Gao, 1998). As the geriatric population increases in most industrialised nations (life expectancy almost doubled during the last century), melanoma will become an important health issue for the elderly age group in this century (Kelly, 1998; Chang et al, 2003; Testori et al, 2009).
The most important predictors of mortality, included in the American Joint Committee on Cancer (AJCC) staging, are tumour characteristics such as BT, ulceration, mitotic rate, and the presence of metastases (Balch et al, 2001; Francken et al, 2004).
However, BT has been shown to be the single most important prognostic factor for survival (De Vries et al, 2007). Therefore, the main aim of this study was to assess differences in BT and survival between young and elderly patients with invasive melanoma in the Netherlands between 1994 and 2008.
Material and methods
Patients
Patients diagnosed with invasive melanoma between 1994 and 2008 were selected from the Netherlands Cancer Registry, which covers all patients in the Netherlands. The nationwide Dutch network and registry of histopathology and cytopathology (PALGA) regularly submits reports of all diagnosed malignancies to the cancer registries. The national hospital discharge databank, which receives discharge diagnoses of admitted patients from all Dutch hospitals, completes case ascertainment. After notification, well-trained registry personnel collect data on diagnosis, staging, and treatment from the medical records, including pathology and surgery reports, using the registration and coding manual of the Dutch Association of Comprehensive Cancer Centres. For the present study, patients with their first primary melanoma were selected. Stage was defined according to the AJCC staging system. The study was approved by the local medical ethics committee.
Statistical analysis
Incidence of invasive melanoma per 100 000 Dutch individuals was calculated for young (younger than 65 years) and elderly (65 years and older) patients in the Netherlands. Because BT measurement was missing for 9.5% of patients, we used multiple imputation (five imputations) to generate a complete data set. A model was built that included sex, location, stage, year of diagnosis, age, and status as predictors to assess the patients with missing BT measurements. Median BT was calculated according to sex, age, and localisation, as well as Breslow distribution over time. Because the TNM Classification of Malignant Tumours changed in 2003, stage distribution before and after 2003 was not comparable and therefore a composite stage variable was not used in the analysis. The BT was divided according to the AJCC staging system into the following size categories: ⩽1.0, 1.0–2.0, 2.0–4.0, and >4.0 mm. Patients with an unknown primary were excluded from the analysis.
The BT distributions for young and elderly males and females were assessed over time. Because BT is skewed toward smaller tumours (most melanomas are ⩽1.0 mm), BT was log-transformed. A regression analysis was modelled with log (Breslow) to assess the changes over the years of incidence according to age, sex, and location.
Vital status and date of last follow-up were established either directly from the patient’s medical record or through linkage of cancer registry data with municipal population registries (follow-up until 1 January 2009), which record information on vital status. Relative survival is the preferred way to describe the prognosis of (elderly) patients with melanoma, as it takes into account the risk of dying from causes other than melanoma. Relative survival was calculated as the ratio of the observed survival among cancer patients to the survival that would have been expected based on the corresponding general population (with respect to age, sex, and year of diagnosis). National lifespan tables were used to estimate expected survival (Ederer II method). Relative excess risks (RERs) of death for year of diagnosis were estimated using a Poisson regression model.
Results
This study includes 40 880 patients diagnosed with melanoma between 1994 and 2008. Table 1 depicts the characteristics of the melanoma patients in the Netherlands between 1994 and 2008, of which 42% were male and 58% were female (Table 1). The median age was 54 years (range 0–105 years). The trunk was the most frequently involved melanoma site (36%). From 1994 to 1996, 5907 new melanoma patients were diagnosed (15%), increasing to 11 023 (27%) in the period from 2006 to 2008. Almost one-half (49%) of newly diagnosed melanoma patients had a BT ⩽1.0 mm, whereas for 12%, the BT was >4.0 mm for thick melanomas.
Table 1. Characteristics of melanoma patients in the Netherlands, 1994–2008.
|
Original data
|
Multiple imputation | ||
|---|---|---|---|
| Characteristic | N | % | % |
| Sex | |||
| Male | 17 305 | 42.3 | 42.3 |
| Female | 23 575 | 57.7 | 57.7 |
| Age | |||
| ⩽40 years | 9378 | 23.0 | 23.0 |
| 41–54 years | 11 506 | 28.1 | 28.1 |
| 55–64 years | 8217 | 20.1 | 20.1 |
| ⩾65 years | 11 779 | 28.8 | 28.8 |
| Location | |||
| Head and neck | 5612 | 13.7 | 13.7 |
| Trunk | 14 693 | 35.9 | 35.9 |
| Upper extremities | 8163 | 20.0 | 20.0 |
| Lower extremities | 12 246 | 30.0 | 30.0 |
| Other | 166 | 0.4 | 0.4 |
| Year | |||
| 1994–1996 | 5907 | 14.5 | 14.5 |
| 1997–1999 | 6720 | 16.4 | 16.4 |
| 2000–2002 | 7779 | 19.0 | 19.0 |
| 2003–2005 | 9451 | 23.1 | 23.1 |
| 2006–2008 | 11 023 | 27.0 | 27.0 |
| Breslow category | |||
| ⩽1.0 mm | 19 325 | 47.3 | 48.7 |
| 1.0–2.0 mm | 8622 | 21.1 | 22.7 |
| 2.0–4.0 mm | 5598 | 13.7 | 16.7 |
| >4.0 mm | 3451 | 8.4 | 11.9 |
| Unknown | 3884 | 9.5 | |
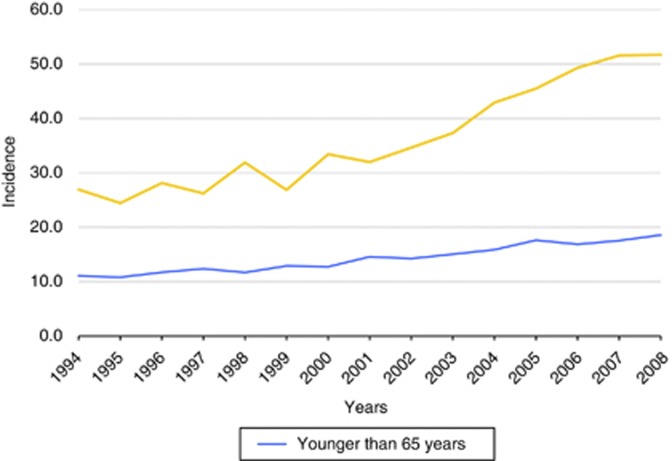
Melanoma incidence increased more rapidly among elderly patients (⩾65 years) than among younger patients (Figure 1). In 1994, melanoma incidence was 189 among elderly patients and 144 among younger patients, and in 2008, these numbers increased to 362 and 241, respectively. The estimated annual percentage change for young patients was 3.9% (P<0.0001) and for the elderly was 5.4% (P<0.0001). Especially since 2002, melanoma incidence appears to be increasing at a faster rate among the elderly than among their younger counterparts.
Figure 1.
Incidence of invasive melanoma among young and elderly patients.
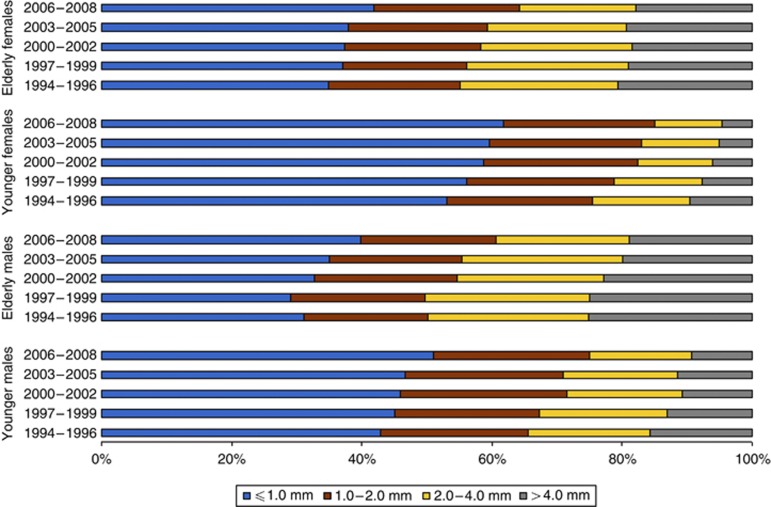
Figure 2 shows the proportion of patients that were diagnosed with thin, median, and thick melanomas over time. The percentage of young patients with a thick melanoma (Breslow >4.0 mm) has declined over time (Figure 2). In 1994, 16% of males and 10% of females had a thick melanoma; by 2008, these incidences had declined to 9% and 5%, respectively (P<0.001).
Figure 2.
Distribution of BT over time among young and elderly patients.
A decline was also observed among the elderly population, and especially among elderly females, although it was not as precipitous as among young patients. In 1994, 25% of elderly males and 20% of elderly females had a thick melanoma, compared with 19% and 18%, respectively, in 2008 (P<0.001). Although younger patients generally had thinner melanomas than elderly patients, the proportion of thin melanomas increased in both age groups. In 1994, 43% of young males and 53% of young females had a thin melanoma (Breslow ⩽1.0 mm); these numbers increased to 51% and 62%, respectively, in 2008. In 1994, 31% of elderly males and 34% of elderly females had a thin melanoma, compared with 40% and 42%, respectively, in 2008 (P<0.001).
Table 2 depicts the changes in BT over time according to age, sex, and location. The BT significantly decreased over time for head and neck, trunk, and upper extremities among young males (P<0.001), and for trunk, upper extremities, and lower extremities among young females (P<0.001) (Table 2). However, among elderly males, a significant decrease in BT over the years was only observed for melanomas in the head and neck (P=0.001) and trunk (P<0.001), and for elderly females, a decrease in BT is noted only on the trunk (P=0.01).
Table 2. Changes in Breslow thickness (log transformation) over time according to age, sex, and lesion location.
| Regression coefficient (95% CI) | P -value | ||
|---|---|---|---|
| Young | |||
| Males | |||
| Head and neck | −0.43 | −0.66 to −0.20 | <0.001 |
| Trunk | −0.26 | −0.37 to −0.14 | <0.001 |
| Upper extremities | −0.36 | −0.56 to −0.16 | <0.001 |
| Lower extremities | −0.16 | −0.41 to −0.1 | 0.2 |
| Females | |||
| Head and neck | −0.21 | −0.51 to 0.1 | 0.2 |
| Trunk | −0.31 | −0.45 to 0.17 | <0.001 |
| Upper extremities | −0.51 | −0.70 to −0.32 | <0.001 |
| Lower extremities | −0.33 | −0.46 to −0.19 | <0.001 |
| Elderly | |||
| Males | |||
| Head and neck | −0.35 | −0.56 to −0.14 | 0.001 |
| Trunk | −0.42 | −0.60 to −0.25 | <0.001 |
| Upper extremities | −0.20 | −0.47 to 0.1 | 0.2 |
| Lower extremities | −0.28 | −0.61 to 0.1 | 0.1 |
| Females | |||
| Head and neck | −0.18 | −0.44 to 0.1 | 0.2 |
| Trunk | −0.36 | −0.62 to −0.10 | 0.01 |
| Upper extremities | −0.15 | −0.37 to 0.06 | 0.2 |
| Lower extremities | −0.13 | −0.32 to 0.1 | 0.2 |
| Overall | |||
| Males and females | |||
| All locations | −0.25 | −0.30 to −0.20 | <0.001 |
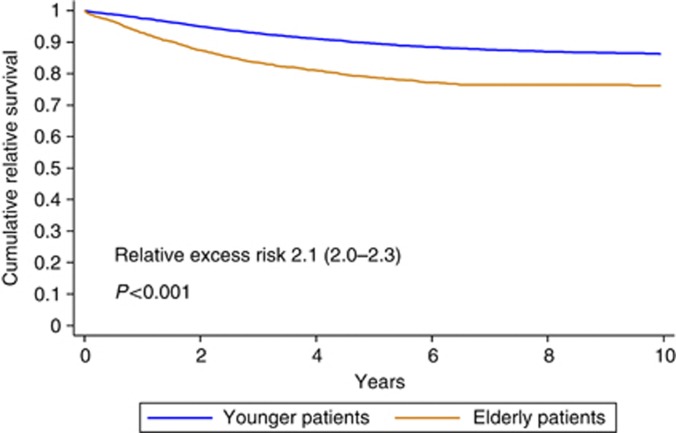
Relative survival is worse among elderly patients than among patients younger than 65 years (RER 2.1 for elderly patients; 95% CI 2.0–203; P<0.001) (Table 3). Adjusted for BT, location, sex, year, lymph node status, and distant metastases, RER remained higher among the elderly (RER 1.7; 95% CI 1.6–1.8; P<0.001). The RER for elderly (65 years and older) vs young patients was significantly worse for the elderly in all BT categories (⩽1.0, 1.0–2.0, 2.0–4.0, and >4.0 mm) and locations (head and neck, trunk, upper extremities, and lower extremities).
Table 3. Relative excess risk (RER) for elderly (65 years and older) vs young patients, stratified according to Breslow category and lesion location.
| Stratification | Adjusted RERa elderly vs young | P -value (adjusted a ) |
|---|---|---|
| Breslow thickness | ||
| ⩽1.0 mm | 1.9 (1.4–2.6) | <0.001 |
| 1.0–2.0 mm | 1.6 (1.3–2.0) | <0.001 |
| 2.0–4.0 mm | 1.6 (1.4–1.9) | <0.001 |
| >4.0 mm | 1.7 (1.5–1.9) | <0.001 |
| Location | ||
| Head and neck | 1.7 (1.5–2.0) | <0.001 |
| Trunk | 1.7 (1.5–1.8) | <0.001 |
| Upper extremities | 1.5 (1.3–1.8) | <0.001 |
| Lower extremities | 2.0 (1.8–2.4) | <0.001 |
Adjusted for sex, year, N, M, Breslow thickness, and location (when not stratified for that factor).
Figure 3 depicts the cumulative relative survival among the young and elderly melanoma patients.
Figure 3.
Cumulative relative survival among young and elderly melanoma patients.
Cumulative relative survival is significantly worse for elderly patients (RER 2.1; 95% CI 2.0–2.3; P<0.001).
Discussion
Melanoma incidence increases more rapidly among the elderly than among younger individuals. Additionally, rising incidence is accompanied by decreasing BT mainly in younger individuals. The decrease in melanoma BT was less prominent among the elderly, which is still frequently diagnosed with thick melanomas. For most melanoma locations, BT in the elderly has not declined. Especially in elderly men, the proportion of thick melanomas has declined only minimally. The high proportion of thick melanomas only partly explains the worse survival among elderly melanoma patients; even within several strata of thickness, survival remains worse among the elderly than among younger patients.
In general, for males and females, young and old, the percentage of thin melanomas increased over the period 1994–2008. This is in accordance with trends in several population-based studies in various other industrialised countries. Lasithiotakis et al (2006), who studied 1980 patients diagnosed with melanoma in southern Germany in the period 1976–2003, observed that median BT decreased steadily during that period (P<0.01). Buettner et al (2005) studied all 45 483 melanoma patients diagnosed between 1976 and 2000, and also noted a significant decrease in median BT (P<0.0001).
In our study, the highest proportion of thin melanomas was observed in young females, a finding that is shared by many other studies (De Vries et al, 2008). Women are likely more alert to skin changes, resulting in thinner melanomas at diagnosis. Various studies have also shown that female patients were more likely to discover their melanoma or melanoma recurrence by themselves, even when adjusted for localisation (Koh et al, 1992; Francken et al, 2007).
A trend towards prognostically more favourable melanomas has been described in the United States, Australia, and Europe, and can most likely be attributed to increased awareness in the population leading to earlier detection and treatment (Shafir et al, 1982; MacKie et al, 1992).
The most prominent finding in our data was that elderly patients present with significantly thicker melanomas, a phenomenon described in both Europe and Australia (Hersey et al, 1991; Melia et al, 1995; Hanrahan et al, 1997; Kelly, 1998; Chang et al, 2003; Testori et al, 2009; Criscione and Weinstock, 2010).
The question arises: why do elderly individuals fail so comprehensively to follow the trend of the younger age group towards early detection? In the first place when it comes to primary prevention in the elderly, damage as sunburns during childhood has already occurred. As far as secondary prevention is concerned, elderly patients have more difficulty recognising or detecting changes in their skin and therefore delay visits to their general practitioner (Criscione and Weinstock, 2010). Difficulty in detecting melanoma could be related to deteriorating vision, increased isolation, decreased flexibility, and awareness or possibly a lack of motivation. Another difficulty for elderly patients is often the development of multiple seborrhoeic keratoses that may appear similar to melanoma. Additionally, melanomas are found on the back in 48% of cases in elderly men, which does not facilitate detection (Kelly, 1998).
Despite physical deterioration, it has been demonstrated that older people should be very well able to detect skin changes associated with early melanoma (Hanrahan et al, 1997). This finding does suggest that public education campaigns and increased alertness in the context of elderly care might be useful to encourage the detection of skin changes. Although previous educational campaigns have been effective at promoting awareness, they have mainly been focused on younger individuals, with a continuous lack of specific attention directed towards elderly individuals (Kelly, 1998).
For instance, in the summer of 1989, a public campaign for melanoma was organised along the Dutch coast. Evaluating the results, a temporary rise in incidence and a decrease in melanoma thickness was seen, but the campaign did not improve mortality rates on short notice. For elderly, it could not be concluded what the long-term effects are of sun damage (van der Rhee et al, 1999).
Various studies report age as an independent prognostic factor, perhaps presenting a surrogate for declining host defence mechanism associated with advancing age (Testori et al, 2009). Treatment outcome is also influenced negatively, because older patients often receive a delayed diagnosis or incorrect staging, possibly due to a reluctance to treat aggressively because of patients’ co-morbidities or disabilities (Chang et al, 2003; Testori et al, 2009). Numerous studies have already demonstrated that elderly patients, especially those over 60 years of age, have a lower disease-specific survival rate (Cohen et al, 1987; Loggie et al, 1991; Austin et al, 1994).
In Australia, skin cancer has been identified as a national health priority, and awareness in the population is at a high level, thanks to health-promotion activities (Rassaby et al, 1983). In contrast, studies demonstrate that melanoma awareness in Europe is not at such a high level, and focus has been mainly on young individuals (Levi et al, 1995). This hypothesis might explain the high percentage of thick melanomas in the elderly, which (given the rising incidence) could have been caused by sun exposure in the era before melanoma became a health priority.
In conclusion, melanoma incidence has increased much more rapidly among the elderly than among younger individuals. Additionally, in contrast to younger patients, in which rising incidence is accompanied by a decline in BT, elderly patients still have the highest proportion of thick melanomas and a decline in BT among the elderly has not yet occurred. More research should be done in the pathways to the diagnosis of thick melanomas in the elderly. In this way, custom-made campaigns could be designed to prevent the ongoing increase of BT in the elderly.
Acknowledgments
We would like to thank the Netherlands Cancer Registry (NCR).
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Presented as an oral presentation at the 15th Congress of the European Society of Surgical Oncology (ESSO), 15–17 September 2010, Bordeaux, France.
The authors declare no conflict of interest.
References
- Armstrong BK, Kricker A (1994) Cutaneous melanoma. Cancer Surv 19–20: 219–240 [PubMed] [Google Scholar]
- Austin PF, Cruse CW, Lyman G, Schroer K, Glass F, Reintgen DS (1994) Age as a prognostic factor in the malignant melanoma population. Ann Surg Oncol 1: 487–494 [DOI] [PubMed] [Google Scholar]
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF (2001) Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 19: 3635–3648 [DOI] [PubMed] [Google Scholar]
- Buettner PG, Leiter U, Eigentler TK, Garbe C (2005) Development of prognostic factors and survival in cutaneous melanoma over 25 years: an analysis of the Central Malignant Melanoma Registry of the German Dermatological Society. Cancer 103: 616–624 [DOI] [PubMed] [Google Scholar]
- Bulliard JL (2000) Site-specific risk of cutaneous malignant melanoma and pattern of sun exposure in New Zealand. Int J Cancer 85: 627–632 [DOI] [PubMed] [Google Scholar]
- Chang CK, Jacobs IA, Vizgirda VM, Salti GI (2003) Melanoma in the elderly patient. Arch Surg 138: 1135–1138 [DOI] [PubMed] [Google Scholar]
- Cohen HJ, Cox E, Manton K, Woodbyry M (1987) Malignant melanoma in the elderly. J Clin Oncol 5: 100–106 [DOI] [PubMed] [Google Scholar]
- Criscione VD, Weinstock MA (2010) Melanoma thickness trends in the United States 1988–2006. J Invest Dermatol 130: 793–797 [DOI] [PubMed] [Google Scholar]
- De Vries E, Houterman S, Janssen-Heijnen ML, Nijsten T, van de Schans SA, Eggermont AM, Coebergh JW (2007) Up-to-date survival estimates and historical trends of cutaneous malignant melanoma in the south-east of The Netherlands. Ann Oncol 18: 1110–1116 [DOI] [PubMed] [Google Scholar]
- De Vries E, Nijsten TE, Visser O, Bastiaannet E, van Hattem S, Janssen-Heijnen ML, Coebergh JW (2008) Superior survival of females among 10,538 Dutch melanoma patients is independent of Breslow thickness, histologic type and tumor site. Ann Oncol 19: 583–589 [DOI] [PubMed] [Google Scholar]
- De Vries E, van de Poll-Franse LV, Louwman WJ, de Gruijl FR, Coebergh JW (2005) Predictions of skin cancer incidence in the Netherlands up to 2015. Br J Dermatol 152: 481–488 [DOI] [PubMed] [Google Scholar]
- Francken AB, Shaw HM, Accortt NA, Soong SJ, Hoekstra HJ, Thompson JF (2007) Detection of first relapse in cutaneous melanoma patients: implications for the formulation of evidence-based follow-up guidelines. Ann Surg Oncol 14: 1924–1933 [DOI] [PubMed] [Google Scholar]
- Francken AB, Shaw HM, Thompson JF, Soong SJ, Accortt NA, Azzola MF, Scolyer RA, Milton GW, McCarthy WH, Colman MH, McGovern VJ (2004) The prognostic importance of tumor mitotic rate confirmed in 1317 patients with primary cutaneous melanoma and long follow-up. Ann Surg Oncol 11: 426–433 [DOI] [PubMed] [Google Scholar]
- Gallagher RP, Spinelli JJ, Lee TK (2005) Tanning beds, sunlamps, and risk of cutaneous malignant melanoma. Cancer Epidemiol Biomarkers Prev 14: 562–566 [DOI] [PubMed] [Google Scholar]
- Gaudette LA, Gao RN (1998) Changing trends in melanoma incidence and mortality. Health Rep 10: 29–41 [PubMed] [Google Scholar]
- Giles GG, Armstrong BK, Burton RC, Staples MP, Thursfield VJ (1996) Has mortality from melanoma stopped rising in Australia? Analysis of trends between 1931 and 1994. BMJ 312: 1121–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan PF, Hersey P, Menzies SW, Watson AB, D'Este CA (1997) Examination of the ability of people to identify early changes of melanoma in computer-altered pigmented skin lesions. Arch Dermatol 133: 301–311 [PubMed] [Google Scholar]
- Hersey P, Sillar RW, Howe CG, Burton RC, Darbar SV, Foster HM, Collins SM, Bradley DE, Owens D (1991) Factors related to the presentation of patients with thick primary melanomas. Med J Aust 154: 583–587 [DOI] [PubMed] [Google Scholar]
- Hollestein LM, van den Akker SA, Nijsten T, Karim-Kos HE, Coebergh JW, de Vries E (2012) Trends of cutaneous melanoma in the Netherlands: increasing incidence rates among all Breslow thickness categories and rising mortality rates since 1989. Ann Oncol 23: 524–530 [DOI] [PubMed] [Google Scholar]
- Kelly JW (1998) Melanoma in the elderly- a neglected public health challenge. Med J Aust 169: 403–404 [PubMed] [Google Scholar]
- Koh HK, Miller DR, Geller AC, Clapp RW, Mercer MB, Lew RA (1992) Who discovers melanoma? Patterns from a population-based survey. J Am Acad Dermatol 26: 914–919 [DOI] [PubMed] [Google Scholar]
- MacKie R, Hunter JA, Aitchison TC, Hole D, Mclaren K, Rankin R, Blessing K, Evans AT, Hutcheon AW, Jones DH (1992) Cutaneous malignant melanoma, Scotland, 1979–89. The Scottish Melanoma Group. Lancet 339: 971–975 [DOI] [PubMed] [Google Scholar]
- Marks R, Jolley D, McCormack C, Dorevitch AP (1997) Who removes pigmented skin lesions? J Am Acad Dermatol 36: 721–726 [DOI] [PubMed] [Google Scholar]
- Melia J, Cooper EJ, Frost T, Graham-Brown R, Hunter J, Marsden A, Du Vivier A, White J, Whitehead S, Warin AP (1995) Cancer Research Campaign health education programme to promote the early detection of cutaneous malignant melanoma. Br J Dermatol 132: 414–421 [DOI] [PubMed] [Google Scholar]
- Lasithiotakis KG, Leiter U, Gorkievicz R, Eigentler T, Breuninger H, Metzler G, Strobel W, Garbe C (2006) The incidence and mortality of cutaneous melanoma in Southern Germany: trends by anatomic site and pathologic characteristics, 1976 to 2003. Cancer 107: 1331–1339 [DOI] [PubMed] [Google Scholar]
- Levi F, Franceschi S, Te VC, Randimbison L, La VC (1995) Trends of skin cancer in the Canton of Vaud, 1976–92. Br J Cancer 72: 1047–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggie B, Ronan SG, Bean J, Das Gupta TK (1991) Invasive cutaneous melanoma in elderly patients. Arch Dermatol 127: 1188–1193 [PubMed] [Google Scholar]
- Parkin D, Whelan S, Ferlay J (1997) Cancer Incidence in Five Continents VII. IACR Sci Publ No 143. International Agency for Research on Cancer: Lyon [Google Scholar]
- Rassaby J, Larcombe I, Hill D, Walker M (1983) Slip, Slop, Slap! Health education about skin cancer. Cancer Forum 7: 63–69 [Google Scholar]
- van der Rhee HJ, van der Spek-Keijser LMT, van Westering R, Coebergh JWW (1999) Increase in and stabilization of incidence and mortality of primary cutaneous malignant melanoma in western Netherlands, 1980–95. Br J Dermatol 140: 463–467 [DOI] [PubMed] [Google Scholar]
- Shafir R, Hiss J, Tsur H, Bubis JJ (1982) The thin malignant melanoma: changing patterns of epidemiology and treatment. Cancer 50: 817–819 [DOI] [PubMed] [Google Scholar]
- Testori A, Soteldo J, Sances D, Mazzarol G, Trifirò G, Zonta M, Rastrelli M, Schenone F, Verrecchia F (2009) Cutaneous melanoma in the elderly. Melanoma Res 19: 125–134 [DOI] [PubMed] [Google Scholar]