Abstract
Background:
TAS-102 consists of α, α, α-trifluorothymidine (TFT) and an inhibitor of thymidine phosphorylase (TPI). We conducted a dose-escalation phase I study in Japanese patients with advanced solid tumours.
Methods:
TAS-102 was administered twice daily on days 1–5 and days 8–12 in a 28-day cycle to patients with solid tumours refractory to standard chemotherapy, to determine its maximum tolerated dose (MTD), dose-limiting toxicities (DLTs), and pharmacokinetics (PKs). MTD was evaluated in cycle 1.
Results:
Safety and PKs were evaluated in 21 patients treated with TAS-102 at 30, 40, 50, 60, or 70 mg m−2 per day. DLTs, such as grade 4 leucopenia, grade 4 neutropenia, and grade 4 thrombocytopenia, were observed in two patients at doses of 30 and 70 mg m−2. α, α, α-trifluorothymidine and TPI exposures increased dose dependently, and the percentage of decrease in neutrophil count and TFT exposure were significantly correlated. The disease control rate was 50.0% with a median progression-free survival of 2.4 months in 18 colorectal cancer patients. The dose of TAS-102 was not increased above 70 mg m−2 per day because of the increased tendency for grade 3 and 4 neutropenia, and 70 mg m−2 per day was the recommended dose for phase II studies.
Conclusions:
TAS-102 at 70 mg m−2 per day was tolerated in Japanese patients with advanced solid tumours. Phase II studies are ongoing in patients with colorectal cancer.
Keywords: phase I study, pharmacokinetics, TAS-102, TFT, TPI
α, α, α-trifluorothymidine (TFT), an analogue of thymidine, exhibits two mechanisms of anti-tumour action as follows: inhibition of thymidylate synthase, which is similar to the mechanism of action of 5-fluorouracil (5-FU), and creation of single-strand DNA breaks by incorporating the triphosphate form of TFT into DNA (Fujiwara and Heidelberger, 1970a; Fujiwara et al, 1970b). The anti-tumour effects of TFT on colon cancer cell lines and xenograft models refractory to 5-FU (Emura et al, 2004b; Temmink et al, 2007) are thought to be because of incorporation of TFT into DNA. The reason that TFT is able to affect DNA is that TFT is resistant to DNA glycosylase as compared with 5-FU (Suzuki et al, 2011). It is also reported that TFT incorporation into DNA causes instability of DNA (Markley et al, 2011). Thus, TFT has been proposed to be effective in patients refractory to 5-FU treatment.
In the initial phase I/II studies of TFT performed in the 1960s, different schedules of intravenous TFT administration were evaluated in chemotherapy-naive patients with metastatic breast cancer and metastatic colorectal cancer. Although early clinical trials showed some anti-tumour activity of TFT, further development of this agent was not undertaken because of inadequate information about the pharmacokinetic (PK) and toxicity profiles (Heidelberger et al, 1970; Ansfield and Ramirez, 1971; Emura et al, 2004a). On the other hand, concomitant administration of TFT and thymidine phosphorylase inhibitor (TPI; 5-chloro-6- (2-iminopyrrolidin-1-yl) methyl-2, 4 (1H 3H) -pyrimidinedione hydrochloride) showed an improvement in the PK profile of TFT; thus, the plasma concentrations and anti-tumour activity of TFT increased because of inhibition of TFT degeneration (Fukushima et al, 2000). TAS-102 is an oral anti-cancer drug consisting of TFT and TPI combined at a molar ratio of 1 : 0.5. Initial clinical studies of TAS-102 were performed in 111 patients using various dosing schedules.
On the basis of the results of five phase I studies and in consideration of the safety and efficacy, a dose of 50 mg m−2 per day was defined as the recommended dose (RD); TAS-102 was given twice a day within 1 h after a meal for 5 days a week for 2 weeks, followed by a 2-week rest (Green et al, 2006; Hong et al, 2006; Overman et al, 2008a; 2008b). Granulocytopenia was consistently identified as a dose-limiting toxicity (DLT).
The primary objective of this phase I study was to establish the maximum tolerated dose (MTD) and DLTs in Japanese patients to determine the optimal phase II dose, and the secondary objective was to examine the PKs and preliminary efficacy of TAS-102.
Patients and methods
Patient population
The eligibility criteria in this study were as follows: (1) Japanese patients with advanced or metastatic solid tumours confirmed by histological or cytological examination for which standard treatments have failed or no standard treatment exists; (2) age, from 20 to 74 years; (3) Eastern Cooperative Oncology Group performance status score of no more than 2; (4) a life expectancy of more than 12 weeks; (5) no chemotherapy within 3 weeks before participating in the study; (6) measurable or evaluable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0; (7) adequate liver function (bilirubin levels⩽1.5 mg dl−1 and transaminase levels ⩽2.5 times the upper limit of normal or ⩽5 times in the case of liver metastases); (8) adequate renal function (creatinine levels ⩽1.5 mg dl−1); and (9) adequate bone marrow function (absolute neutrophil counts ⩾2000 cells per mm3, platelet counts ⩾100 000 cells per mm3, and haemoglobin levels ⩾9.0 g dl−1). Patients with central nervous system metastasis, those with a history of extensive radiation therapy within the past 6 weeks, and pregnant women were excluded from this study.
Pre-treatment evaluation and study procedures
Baseline evaluations, including computed tomography, chest radiography, and electrocardiography, were performed within 4 weeks before treatment, and medical histories, physical examinations, and laboratory tests were performed within 1 week before treatment.
Patients were considered to be evaluable if they were given TAS-102 at least once. Toxicity was evaluated according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0. The study protocol was approved by independent ethics committees and the government authorities. All patients provided written informed consent. This trial was conducted in accordance with the Declaration of Helsinki (October 1996; JapicCTI-No.: JapicCTI-111545).
Study design, DLT, and MTD
This study was conducted using a conventional 3+3 dose-escalation study design. The starting dose of 30 mg m−2 per day was selected on the basis of the results of a previous study performed in the United States (Green et al, 2006). If a DLT occurred at a particular dose level, additional three patients were enroled at the same dose level. If two or more DLTs occurred at a dose level, the next lower dose level was determined to be the MTD. Intra-patient dose escalation was not allowed.
A DLT was defined as the occurrence of any of the following toxicities during the first course of TAS-102 administration. Non-haematological toxicity (excluding nausea/vomiting) of grade 3 or more, nausea/vomiting of grade 3 or more uncontrolled by aggressive anti-emetic support, grade 4 neutropenia lasting 5 days or more, febrile (⩾38.5 °C) neutropenia of grade 3 or more, grade 4 thrombocytopenia, or unresolved toxicities causing more than a 2-week delay of the next scheduled dose.
Before the next cycle of therapy could be initiated, patients were required to have a non-haematological toxicity of grade ⩽1, excluding alopecia, a platelet count >100 000 cells per μl, and a granulocyte count >1500 cells per μl. For patients who developed grade 3 or 4 toxicities, the TAS-102 dose was reduced by 10 mg per day for the next cycle to a minimum dose of 30 mg m-2 per day.
Study treatment
The actual dose of TAS-102 for each patient was set to the nearest dose by a 10-mg increment. The 28-day cycle of treatment involved administration of TAS-102 twice a day within 1 h after a meal for 5 days a week for 2 weeks, followed by a 2-week rest. This treatment cycle was repeated every 4 weeks until disease progression or an unacceptable toxicity was observed. During cycle 1, the prophylactic administration of granulocyte-colony stimulating factor (G-CSF) or antibiotics was prohibited.
Pharmacokinetic analysis
Pharmacokinetic studies were performed during cycle 1. Blood samples were collected at 0, 0.25, 0.5, 1, 2, 4, 6, 8, and 10 h after TAS-102 administration on days 1 and 12. A urine sample was collected on day 1 before administration, and from 0 to 10 h after administration. The concentrations of TFT, TPI, and an inactive form of TAS-102 (FTY) in the plasma, and TFT and TPI concentrations in the urine were measured at the Toray Research Center, Inc. (Tokyo, Japan) using a validated liquid chromatography-coupled tandem mass spectrometry method.
Pharmacokinetic parameters of TFT and TPI in the plasma were determined by non-compartmental methods (WinNonlin v. 5.2; Pharsight, Mountain View, CA, USA). The maximum plasma concentrations (Cmax) and time to maximum plasma concentrations (Tmax) were determined from the highest concentration, and the time of at which it was observed. The area under the concentration-time curve (AUC0–10) was calculated using the linear trapezoidal method from 0 to 10 h, and AUCinf was calculated using the linear trapezoidal rule from time zero to the time of the last quantifiable concentration, which was followed by extrapolation to infinity. The elimination half-life (t1/2) was estimated from ln(2)/Ke, where the terminal phase elimination rate constant (Ke) was estimated using log-linear regression during the terminal phase. The oral clearance (CL/F) was the body weight-normalised dose (mg kg−1) divided by the AUCinf determined on day 1.
The volume of distribution (Vd/F) was CL/F divided by Ke. The cumulative excretion of TFT and TPI in the urine (Ae) was the total amount of urinary excretion of each compound over the 10-h period after administration divided by the administered dose amount (% of dose).
Statistics
The number of patients in each cohort was based on a standard 3+3 design for dose-escalation studies. We planned to enrol 21 patients to assess the safety and tolerability of TAS-102, depending on the observed toxicities.
The DLT analysis set consisted of patients who had completed the first cycle in the DLT evaluation period. All patients who received TAS-102 at least once were included in the safety analysis. Pharmacokinetic analyses were performed on patients who received the scheduled dosage of TAS-102 and recorded dosing and sampling times correctly (PK analysis set). The efficacy analysis set consisted of patients with measurable disease at baseline. Descriptive statistics were provided for all endpoints by cohort. Continuous measurements were summarised with the central tendency (mean or median) and variability (s.d. or s.e.m.). Categorical data were summarised using frequency counts and percentages of patients. Correlations between the percentage decrease in neutrophil count and the log-transformed Cmax or AUCinf values of TFT on day 12 in course 1 were investigated by linear regression analyses (SAS v8.02 software, SAS Institute, Inc., Cary, NC, USA). The per cent change of neutrophil count was (pre-treatment count – nadir)/(pre-treatment count) × 100, where the nadir of the neutrophil count was the minimum value in course 1.
Results
Patient characteristics
Between March 2006 and July 2007, a total of 21 patients were enroled (Table 1). The median age of the patients was 59 years (range, 38–68 years). The performance status of all patients was 0–2. The median number of prior chemotherapies was 3. Of the 21 patients, 18 had colorectal cancer. All patients had progressive disease during prior 5-FU treatment. All patients with colorectal cancer were refractory to the three conventional cytotoxic agents, 5-FU, irinotecan, and oxaliplatin, and the three patients were refractory to anti-epidermal growth factor receptor or vascular endothelial growth factor monoclonal antibody.
Table 1. Patient characteristics.
| Characteristics | n (%) |
|---|---|
| Sex | |
| Male | 14 (66.7) |
| Female | 7 (33.3) |
| Age | |
| Median (range) | 59 (38–68) |
| ECOG PS | |
| 0 | 16 (76.2) |
| 1 | 4 (19.0) |
| 2 | 1 (4.8) |
| Primary lesion | |
| Colon/rectum | 12 (57.1)/6 (28.6) |
| Gastric | 1 (4.8) |
| Gastric+prostate | 1 (4.8) |
| Oesophagus | 1 (4.8) |
| Histological type | |
| Well differentiated | 6 (28.6) |
| Moderately differentiated | 12 (57.1) |
| Poorly differentiated | 2 (9.5) |
| Squamous cell | 1 (4.8) |
| Number of prior therapy | |
| Median (range) | 3 (2–6) |
| ⩽2 | 6 (28.6) |
| ⩾3 | 15 (71.4) |
| Prior therapy | |
| Include fluropyrimidine, irinotecan, oxaliplatin | 18 (85.7) |
| Other | 3 (14.3) |
Abbreviation: ECOG PS=Eastern Cooperative Oncology Group performance status.
TAS-102 administration
We administered 66 treatment courses. All patients received at least one dose of TAS-102. The median TAS-102 treatment duration was 68 days (30 mg m−2 per day cohort, 29 days; 40 mg m−2 per day cohort, 40 days; 50 mg m−2 per day cohort, 47 days; 60 mg m−2 per day cohort, 119 days; and 70 mg m−2 per day cohort, 79 days). Treatment was discontinued in all patients because of progressive disease.
Safety and efficacy
All patients were evaluable for safety. Adverse events were observed in all patients. Although four patients died within 90 days of the first administration, the causes of their deaths were not related to the treatment. There were four treatment-related serious adverse events, namely leucopenia, neutropenia, thrombocytopenia, and pneumonia, in a patient at the 30 mg m−2 dose level in the first course. The major treatment-related adverse events are listed in Table 2. The main toxicities were leucopenia (81% of patients), neutropenia (71.4%), decreased red blood cell counts (66.7%), decreased haematocrit (66.7%), nausea (66.7%), decreased appetite (61.9%), lymphocytopenia (61.9%), and decreased haemoglobin levels (57.1%).
Table 2. The most common treatment-related adverse events.
| 30 ( n =6) | 40 ( n =3) | 50 ( n =3) | 60 ( n =3) | 70 ( n =6) | Total ( n =21) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg m−2 per day) | All | ⩾G3 | ⩾G4 | All | ⩾G3 | ⩾G4 | All | ⩾G3 | ⩾G4 | All | ⩾G3 | ⩾G4 | All | ⩾G3 | ⩾G4 | All (%) | ⩾G3 (%) | ⩾G4 (%) |
| Haematological toxicities | ||||||||||||||||||
| Leucopenia | 4 | 1 | 2 | 2 | 1 | 3 | 1 | 6 | 4 | 17 (81.0) | 7 (33.3) | |||||||
| Neutropenia | 2 | 1 | 2 | 2 | 1 | 3 | 3 | 6 | 4 | 15 (71.4) | 8 (38.1) | 1 (4.8) | ||||||
| Red blood cell count decreased | 2 | 2 | 3 | 2 | 5 | 1 | 14 (66.7) | 2 (9.5) | ||||||||||
| Haematocrit decreased | 3 | 2 | 3 | 1 | 2 | 4 | 1 | 14 (66.7) | 1 (4.8) | |||||||||
| Lymphocytopenia | 3 | 1 | 1 | 3 | 2 | 4 | 3 | 13 (61.9) | 4 (19.0) | |||||||||
| Anaemia | 4 | 1 | 2 | 2 | 2 | 4 | 4 | 12 (57.1) | 7 (33.3) | |||||||||
| Thrombocytopenia | 2 | 1 | 1 | 1 | 1 | 5 | 9 (42.9) | 1 (4.8) | 1 (4.8) | |||||||||
| Blood albumin level decreased | 1 | 1 | 1 | 1 | 4 (19.0) | |||||||||||||
| Blood bilirubin level increased | 1 | 1 | 2 | 4 (19.0) | ||||||||||||||
| Aspartate aminotransferase level increased | 1 | 1 | 1 | 1 | 4 (19.0) | |||||||||||||
| Blood alkaline phosphatase level increased | 1 | 1 | 1 | 1 | 4 (19.0) | |||||||||||||
| Protein total decreased | 1 | 1 | 1 | 3 (14.3) | 1 (4.8) | |||||||||||||
| Monocyte count increased | 1 | 1 | 2 | 3 (14.3) | ||||||||||||||
| Alanine aminotransferase increased | 1 | 1 | 1 | 3 (14.3) | ||||||||||||||
| Nonhaematological toxicities | ||||||||||||||||||
| Nausea | 4 | 2 | 3 | 5 | 14 (66.7) | |||||||||||||
| Appetite loss | 5 | 1 | 3 | 5 | 13 (61.9) | 1 (4.8) | ||||||||||||
| Fatigue | 3 | 1 | 2 | 1 | 2 | 8 (38.1) | 1 (4.8) | |||||||||||
| Vomiting | 2 | 1 | 2 | 1 | 6 (28.6) | |||||||||||||
| Diarrhoea | 1 | 2 | 1 | 1 | 1 | 5 (23.8) | 1 (4.8) | |||||||||||
| Albuminuria | 1 | 1 | 2 | 1 | 5 (23.8) | |||||||||||||
| Stomatitis | 1 | 1 | 1 | 3 (14.3) | ||||||||||||||
| Weight loss | 1 | 2 | 3 (14.3) | |||||||||||||||
| Bloating | 2 | 1 | 3 (14.3) | |||||||||||||||
In the initial cohort at a dose level of 30 mg m−2 per day, one of the three patients developed DLTs, involving grade 4 leucopenia, neutropenia, and thrombocytopenia. An additional three patients were enroled at the same dose level, and no DLTs were observed. At a dose of 40, 50, and 60 mg m−2, no DLTs occurred in any of the three patients enroled. At a dose of 70 mg m−2, one patient developed a DLT involving grade 4 neutropenia. However, no DLTs were observed in the additional three patients enroled at the same dose level. The second course was delayed in three of the six patients, and the third course was delayed in four of the four patients in the 70 mg m−2 treatment cohort because of the development of grade 3 or 4 neutropenia.
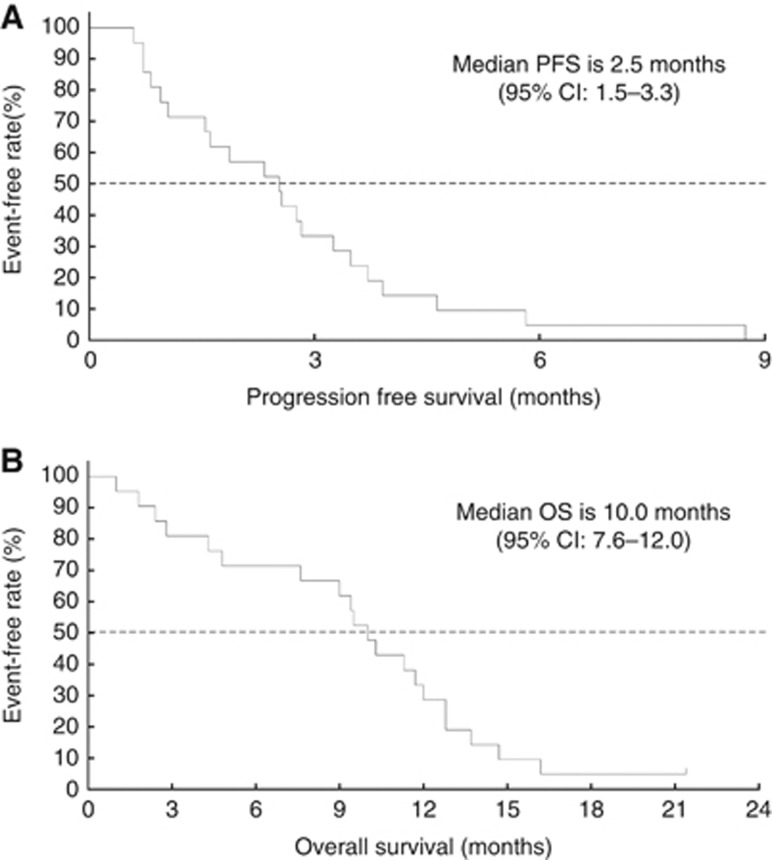
All patients were evaluable for tumour response according to RECIST version 1.0. Although no complete or partial responses were observed, 11 patients achieved stable disease, resulting in a disease control rate (DCR) of 52.3%. The treatment could be continued for 12 weeks in 8 patients. The median progression-free survival (PFS) and overall survival (OS) were 2.6 months and 10.2 months, respectively (Figure 1).
Figure 1.
Kaplan–Meier plots of the median progression-free survival (PFS) (A) and median overall survival (OS) (B) in all patients.
In colorectal cancer patients (n=18), the DCR was 50.0%, including 6 patients who were able to continue treatment over 12 weeks. The median PFS and OS in this cohort were 2.4 and 9.8 months, respectively.
Pharmacokinetics
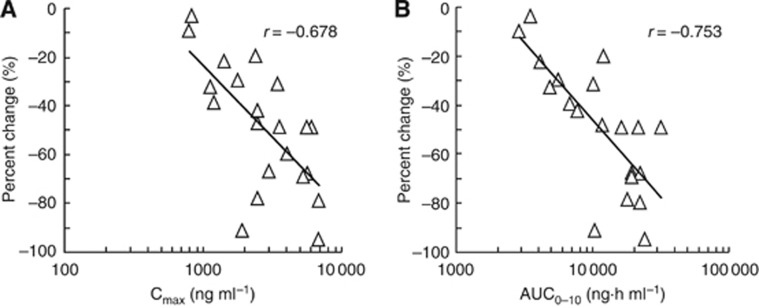
Pharmacokinetics were evaluated using plasma and urine samples (Table 3). Systemic concentrations of TFT and TPI increased linearly in correlation with increasing oral doses. Plasma TFT concentrations tended to increase with repeated administrations, and on day 12, AUC0–10 of TFT was about 2.6 times higher than that measured on day 1. In contrast, there were no obvious changes in the PK parameters of TPI and FTY after repeated administrations. These tendencies were similar to the results after repeated, once daily administration. A significant inverse correlation was observed between the percent change in neutrophil count and TFT Cmax (r=−0.678, P<0.001) or AUC0–10 (r=−0.753, P<0.001; Figure 2). The cumulative percentage of the TFT and TPI doses excreted in the urine from 0 to 10 h after drug administration was 1% to 8% and 19% to 23%, respectively.
Table 3. Pharmacokinetic parameters of TFT, TPI, and an inactive form (FTY) after administration of TAS-102 on day 1 and day 12.
| Dose (mg m−2 per day) | 30 ( n =6) | 40 ( n =3) | 50 ( n =3) | 60 ( n =3) | 70 ( n =6) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day | 1 | 12 | 1 | 12 | 1 | 12 | 1 | 12 | 1 | 12 |
| TFT | ||||||||||
| Cmax (ng ml−1) | 1009±491 | 1205±421 | 1840±737 | 2747±610 | 2450±1021 | 2757±1173 | 3677±1459 | 5437±1685 | 3338±767 | 4752±1697 |
| Tmax (h) | 1.7±1.3 | 1.6±0.7 | 1.2±0.8 | 1.7±0.6 | 1.5±0.9 | 1.3±0.6 | 1.2±0.8 | 1.3±0.6 | 1.3±0.5 | 1.9±1.6 |
| AUC0–10 (ng h ml−1) | 2037±773 | 5478±2849 | 4347±535 | 9994±2109 | 4281±1380 | 8656a±NA | 8229±1441 | 23672±7844 | 8678±1786b | 20950±2237 |
| AUCinf (ng h ml−1) | 2055±793 | — | 4373±568 | — | 4297±1387 | — | 8435±1645 | — | 8672±1710 | — |
| t1/2 (h) | 1.39±0.38 | 2.44±1.57 | 1.17±0.15 | 1.52±0.34 | 1.49±0.59 | 1.96±0.10 | 1.88±0.73 | 2.33±1.26 | 1.41±0.38 | 1.97±0.51 |
| CL/F (l h−1 kg−1) | 0.168±0.034 | — | 0.124±0.035 | — | 0.178±0.055 | — | 0.103±0.014 | — | 0.118±0.018 | — |
| Vd/F (l kg−1) | 0.324±0.095 | — | 0.204±0.030 | — | 0.384±0.175 | — | 0.273±0.089 | — | 0.234±0.054 | — |
| Ae (%) | 3.59±3.82 | — | 4.85±5.61 | — | 7.64±4.49 | — | 0.96a±NA | — | 3.69±3.42b | — |
| TPI | ||||||||||
| Cmax (ng ml−1) | 25.8±14.7 | 44.1±51.8 | 43.1±6.5 | 41.8±14.7 | 54.2±28.5 | 50.2±13.1 | 136.1±77.5 | 99.6±43.8 | 76.6±32.1 | 70.0 ±43.4 |
| Tmax (h) | 2.6±1.6 | 2.8±1.5 | 1.7±0.6 | 2.7±1.2 | 1.7±0.6 | 2.7±1.2 | 2.7±1.2 | 2.7±1.2 | 2.3±0.8 | 2.3±0.8 |
| AUC0–10 (ng h ml−1) | 117±84 | 234±283 | 166±29 | 161±41 | 214±79 | 300a±NA | 521±338 | 447±278 | 281±99b | 317±182 |
| AUCinf (ng h ml−1) | 129±96 | — | 170±29 | — | 222±79 | — | 542±360 | — | 302±96 | — |
| t1/2 (h) | 2.27±0.74 | 2.89±0.83 | 1.53±0.17 | 1.82±0.18 | 1.78±0.27 | 4.01±3.57 | 1.66±0.37 | 2.21±0.62 | 1.67±0.22 | 2.37±0.93 |
| CL/F (l h−1 kg−1) | 1.52±0.67 | — | 1.52±0.50 | — | 1.66±0.56 | — | 0.91 ±0.40 | — | 1.83±1.06 | — |
| Vd/F (l kg−1) | 4.90 ±2.37 | — | 3.30 ±0.93 | — | 4.31±1.85 | — | 2.06±0.62 | — | 4.42±2.68 | — |
| Ae (%) | 19.4 ±12.2 | — | 22.9 ±5.1 | — | 20.0 ±9.6 | — | 20.0a±NA | — | 19.0 ±7.5b | — |
| FTY | ||||||||||
| Cmax (ng ml−1) | 248±83 | 198±49 | 453±91 | 398±4 | 645±23 | 470±174 | 753±293 | 512±41 | 878±228 | 560±92 |
| Tmax (h) | 2.1±1.6 | 2.3±0.8 | 1.7±0.6 | 2.0 ±0.0 | 1.5±0.9 | 1.7±0.6 | 1.5±0.9 | 1.2±0.8 | 2.0 ±0.0 | 2.3±1.4 |
| AUC0–10 (ng h ml−1) | 993±392 | 1301±524 | 1740±172 | 2259±411 | 1901±316 | 2401a±NA | 2653±537 | 3095±538 | 3165±341b | 3622±1094 |
| AUCinf (ng h ml−1) | 1016±407 | — | 1776±216 | — | 1915±327 | — | 2710±559 | — | 3492±693 | — |
| t1/2 (h) | 1.34±0.30 | 4.57±2.74 | 1.32±0.40 | 4.55±2.90 | 1.18±0.18 | 4.79±2.50 | 1.62±0.32 | 9.60±5.31 | 1.57±0.38 | 7.27±2.95 |
Abbreviations: TFT=trifluorothymidine; TPI=thymidine phosphorylase inhibitor; AUC=area under the concentration-time curve; NA=not applicable.
Cumulative urinary excretion rate was calculated within 10 h.
n=2.
n=5.
Figure 2.
Relationship between pharmacokinetic parameters of trifluorothymidine (TFT; Cmax and AUCinf) and the percent change of the neutrophil count of patients treated with 30–70 mg m−2 per day of TAS-102 on day 12 in 1 course. (A) Relationship between TFT Cmax and the percent change of the neutrophil count. (B) Relationship between TFT AUCinf and the percent change of the neutrophil count. Each symbol denotes an individual value.
Discussion
TAS-102, a thymidine analogue, is an orally administered anti-tumour drug. On the basis of this phase I trial, the RD for the subsequent phase II trial of TAS-102 in Japanese patients was determined to be 70 mg m−2 per day twice a day for 5 days a week for 2 weeks, followed by 2-week rest. The treatment was well tolerated at the phase II doses.
The common grade 3 and 4 adverse events were haematological toxicities. Two patients experienced DLTs during cycle 1; one patient developed grade 4 neutropenia, leucopenia, and thrombocytopenia after treatment at a dose of 30 mg m−2 per day, and the other patient experienced grade 4 neutropenia and leucopenia after treatment at a dose of 70 mg m−2 per day. Thus, the safety profiles of TAS-102 in the Japanese patients were similar to those of the American patients reported in previous studies (Green et al, 2006; Hong et al, 2006; Overman et al, 2008a; 2008b). Of the 21 patients, 3 were treated with G-CSF 3 weeks after the first administration of each cycle. Two of these three patients received G-CSF at cycle 1 to treat neutropenia evaluated as DLTs and neutropenia resolved. Seven patients received antibiotics prophylactically to treat the adverse events. Blood tests performed 14 days or later after the first administration were useful for finding the nadir of toxicities, because the toxicities tended to be observed about 21 days after the first administration in each course. TAS-102 treatment could be continued in almost all patients with adequate management of toxicity without developing serious adverse events. In this study, we did not examine the effects of a dose of 80 mg m−2 per day, because grade 3 neutropenia was observed in three of six patients at a dose of 70 mg m−2 per day, and the higher dose (80 mg m−2 per day) was thought not to be tolerated on the basis of the similarity in the toxicities observed in this study and those in the US study (Green et al, 2006).
Dose-dependent increases in TFT and TPI exposures were observed for doses up to 70 mg m−2 per day. Although the number of patients included in the PK evaluation was limited and the administration schedules differed, it seems that there are no large differences in the PK parameter of TFT, the active component of TAS-102, between the Japanese and American patients, because CL/F, Tmax, and t1/2 values of American patients at a dose of 50 mg m−2 per administration are approximately comparable to those of Japanese patients at a dose range from 15 to 35 mg m−2 per administration. However, the RD in this study was higher than that in the US study, which had the same schedule design as this study (Green et al, 2006). The data obtained from several phase I studies in the United States provided information about managing various adverse events and about when to perform blood tests, and when to skip drug administrations. Therefore, in this trial, patients were hospitalised during cycle 1 for evaluation of DLTs and blood tests, and observation of symptoms was performed more closely and frequently compared with that in the US study. Thus, our study suggested a different RD as compared with the US study. Therefore, we are going to perform the dose-matching study in the United States to determine the optimal dose for global study.
Only 1% to 8% and approximately 20% of the administered doses were excreted into the urine as TFT and TPI, respectively. TPI and FTY exposure on day 12 did not show obvious alterations with repeated administration of TAS-102, but TFT exposure on day 12 was 2.6 times that on day 1. Moreover, the TFT exposure on day 12 was significantly correlated with the percent change of neutrophil count from baseline. These results suggest that the triphosphate form of TFT is incorporated into DNA in the neutrophils in a concentration-dependent manner, which results in sequential increases in single-strand DNA breaks. Therefore, 70 mg m−2 per day, the maximum dose in this study, is expected to be effective in a phase II study.
Although partial responses and complete responses were not confirmed, stable disease was observed in 11 patients. The DCR was 33.3% each at doses of 30, 40, and 50 mg m−2 per day; 100.0% at a dose of 60 mg m−2 per day; and 66.7% at a dose of 70 mg m−2 per day. These findings indicate the tendency that the DCR correlated with the dose. The median PFS in all patients was 2.5 months. The median PFS was 1.7 months at doses ⩽50 mg m−2 per day and 3.3 months at doses ⩾60 mg m−2 per day. These result suggest that TAS-102 has a potent anti-tumour effect at a dose of >60 mg m−2 per day. Although this agent did not reach the MTD, 70 mg m−2 per day was determined as the RD for the subsequent studies.
In this study, 18 of the 21 patients had colorectal cancer. The DCR, median PFS, and OS in the colorectal cancer patients was 50.0%, 2.4 months, and 9.8 months, respectively; however, the median OS of panitumumab and cetuximab in colorectal cancer patients refractory to 5-FU, irinotecan, and oxaliplatin was 8.6 and 6.4 months, respectively (Saltz et al, 2004; Hecht et al, 2007). These results suggest that TAS-102 showed anti-tumour effects in colorectal cancer patients refractory to standard treatment and thus may be a promising agent, and it is meaningful to conduct the phase II trials of TAS-102 on colorectal cancer patients.
Recently, chemotherapy based on 5-FU, such as FOLFOX and FOLFIRI, have been established as a first- and second-line therapy for colorectal cancer, but tertiary and subsequent therapies remain to established. Although cetuximab and panitumumab were effective in colorectal cancer patients refractory to standard therapy, these agents were not effective in patients with K-ras mutations. These results suggest that the efficacy of TAS-102 in patients refractory to 5-FU may be because of the differences in anti-tumour mechanisms of both agents, and the efficacy was presumed not to be affected by the K-ras status. The recent randomised phase II trial for colorectal cancer in Japan based on our phase I data has shown promising preliminary results (Kuboki et al, 2011). The PK and safety profiles reported in this phase I trial are important to determine the optimal dose and schedule used in a clinical setting. Studies on combination of TAS-102 with other molecular-target drugs should be performed in the future.
In conclusion, our Phase I study showed that TAS-102 was well tolerated up to doses of 70 mg m−2 per day in Japanese patients with advanced solid tumours. The safety and efficacy of TAS-102 for colorectal cancer patients should be investigated in phase II and III trials.
Acknowledgments
Financial support for this research was provided by Taiho Pharmaceutical Co., Ltd. We thank Masanobu Ito and Kazuo Koba for their kind advice.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Presented at the 20th EORTC-NCI-AACR Symposium (EJC, Supplements 2008; 6 (12): 134–135).
The authors declare no conflict of interest.
References
- Ansfield FJ, Ramirez G (1971) Phase I and II studies of 2'-deoxy-5-(trifluoromethyl)-uridine (NSC-75520). Cancer Chemother Rep 55: 205–208 [PubMed] [Google Scholar]
- Emura T, Nakagawa F, Fujioka A, Ohshimo H, Yokogawa T, Okabe H, Kitazato K (2004a) An optimal dosing schedule for a novel combination antimetabolite, TAS-102, based on its intracellular metabolism and its incorporation into DNA. Int J Mol Med 13: 249–255 [PubMed] [Google Scholar]
- Emura T, Suzuki N, Yamaguchi M, Ohshimo H, Fukushima M (2004b) A novel combination antimetabolite, TAS-102, exhibits antitumor activity in FU-resistant human cancer cells through a mechanism involving FTD incorporation in DNA. Int J Oncol 25: 571–578 [PubMed] [Google Scholar]
- Fujiwara Y, Heidelberger C (1970a) Fluorinated pyrimidines. XXXVII. The incorporation of 5-trifluoromethyl-2'-deoxyuridine into the deoxyribonucleic acid vaccinia virus. Mol Pharmacol 6: 281–291 [PubMed] [Google Scholar]
- Fujiwara Y, Oki T, Heidelberger C (1970b) Fluorinated pyrimidines. XXXVII. Effect of 5-trifluoromethyl-2'-deoxyuridine on the synthesis of deoxyribonucleic acid of mammalian cells in culture. Mol Pharmacol 6: 273–280 [PubMed] [Google Scholar]
- Fukushima M, Suzuki N, Emura T, Yano S, Kazuno H, Tada Y, Yamada Y, Asao T (2000) Structure and activity of specific inhibitors of thymidine phosphorylase to potentiate the function of antitumor 2'-deoxyribonucleosides. Biochem Pharmacol 59: 1227–1236 [DOI] [PubMed] [Google Scholar]
- Green MC, Pusztai L, Theriault LR, Adinin RB, Hofweber M, Fukushima M, Mita A, Bindra N, Hortobagyi GN (2006) Phase I study to determine the safety of oral administration of TAS-102 on a twice daily (BID) schedule for five days a week (wk) followed by two days rest for two wks, every (Q) four wks in patients (pts) with metastatic breast cancer (MBC). J Clin Oncol. ASCO Annual Meeting Proceedings Part I 24: 10576 [Google Scholar]
- Hecht JR, Patnai A, Berlin J, Venook A, Malik I, Tchekmedyian S, Navale L, Amado RG, Meropol NJ (2007) Panitumumab monotherapy in patients with previously treated metastatic colorectal cancer. Cancer 110: 980–988 [DOI] [PubMed] [Google Scholar]
- Heidelberger C, Dexter DL, Wolberg WH (1970) Clinical pharmacology of 5-trifluoromethyl-2'-deoxyuridine (F3Tdr). Proc Am Assoc Cancer Res 11: 35. [PubMed] [Google Scholar]
- Hong DS, Abbruzzese JL, Bogaard K, Lassere Y, Fukushima M, Mita A, Kuwata K, Hoff PM (2006) Phase I study to determine the safety and pharmacokinetics of oral administration of TAS-102 in patients with solid tumors. Cancer 107: 1383–1390 [DOI] [PubMed] [Google Scholar]
- Kuboki Y, Yoshino T, Yamazaki K, Nishina T, Komatsu Y, Baba H, Tsuji A, Yamaguchi K, Muro K, Ohtsu A (2011) A multicenter, randomized, double-blind, phase II study of TAS-102 plus best supportive care (BSC) versus placebo plus BSC in patients with chemotherapy-refractory metastatic colorectal cancer. EJC 47: 392 [Google Scholar]
- Markley JC, Chirakul P, Sologub D, Sigurdsson ST (2011) Incorporation of 20-Deoxy-5-(trifluoromethyl)uridine and 5-Cyano-20-deoxyuridine into DNA. Bioorg Med Chem Lett 11: 2453–2455 [DOI] [PubMed] [Google Scholar]
- Overman MJ, Kopetz S, Varadhachary G, Fukushima M, Kuwata K, Mita A, Wolff RA, Hoff P, Xiong H, Abbruzzese JL (2008a) Phase I clinical study of three times a day oral administration of TAS-102 in patients with solid tumors. Cancer Invest 26: 794–799 [DOI] [PubMed] [Google Scholar]
- Overman MJ, Varadhachary G, Kopetz S, Thomas MB, Fukushima M, Kuwata K, Mita A, Wolff RA, Hoff PM, Xiong H, Abbruzzese JL (2008b) Phase 1 study of TAS-102 administered once daily on a five-day-per-week schedule in patients with solid tumors. Invest New Drugs 26: 445–454 [DOI] [PubMed] [Google Scholar]
- Saltz LB, Meropol NJ, Loehrer PJ, Needle MN, Kopit J, Mayer RJ (2004) Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22: 1201–1208 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Emura T, Fukushima M (2011) Mode of action of trifluorothymidine (TFT) against DNA replication and repair enzymes. Int J Oncol 39: 263–270 [DOI] [PubMed] [Google Scholar]
- Temmink OH, Emura T, de Bruin M, Fukushima M, Peters GJ (2007) Therapeutic potential of the dual-targeted TAS-102 formulation in the treatment of gastrointestinal malignancies. Cancer Sci 98: 779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]