Abstract
Background:
Recently developed detection system for circulating tumour cells (CTCs) using a telomerase-specific replicative adenovirus generated nonspecific green fluorescent protein (GFP) signals because of the co-presence of white blood cells (WBCs) nonspecifically infected by viruses. Here, we established a unique detection system for CTCs that completely excludes nonspecific signals.
Methods:
Blood obtained from the patients was subjected to haemolytic processes to eliminate red blood cells. The cell pellets were then infected with OBP-401, fixed, incubated with fluorescence-labelled anti-CD45 antibody to mark white blood WBCs, and examined on slides under a microscope.
Results:
Preparatory experiments with cancer cells artificially added to healthy donor samples confirmed that CD45 labelling could distinguish GFP-positive cancer cells from WBCs. In 53 patients with gynaecological cancers, CTCs were detected in 21 patients (39.6%) when CD45-positive cells were excluded as WBCs among GFP-positive cells. No CTCs were detected in samples from healthy volunteers. There was no significant correlation between CTC counts and known clinicopathological factors. The CTCs rapidly vanished after surgery or chemotherapy in most patients whose treatments were effective. In contrast, the persistence of CTCs even after treatments was tightly associated with poor response to the treatments (P<0.005).
Conclusion:
The presence of CTCs in our system may potentially be a novel therapeutic marker in gynaecological cancers.
Keywords: circulating tumour cells, telomerase, adenovirus, GFP, gynaecological cancers
Blood-borne metastasis responsible for most cancer-related deaths is initiated by intravasation of cancer cells from the primary lesion into the systemic circulation. Efforts have been made to identify the circulating tumour cells (CTCs) in peripheral blood (Racila et al, 1998; Yu et al, 2011). The clinical relevance of detecting CTCs as a prognostic and/or surrogate marker of treatment response has been established in several cancer types such as breast cancer, colorectal cancer, and prostate cancer (Cristofanilli et al, 2004, 2005; Cohen et al, 2008; de Bono et al, 2008). Core techniques have been applied to immunologically recognise CTCs with epithelial cell-specific markers, combined with reverse transcription PCR, cell size filtration, or other cytometric procedures (Attard and de Bono, 2010; Sun et al, 2011). CellSearch (Veridex, Raritan, NJ, USA) is the only method approved by the US Food and Drug Administration as an automated detection system for CTCs (Allard et al, 2004; Riethdorf et al, 2007); it consists of immunomagnetic enrichment with ferrofluids conjugated with anti-epithelial cell adhesion molecule (EpCAM) antibody and detection with a triple-stain procedure (positive staining for cytokeratin (CK8/18/19) and DAPI, and negative staining for CD45, a leucocyte common antigen).
Telomerase-specific replication-selective adenovirus is an oncolytic adenovirus vector that expresses a green fluorescent protein (GFP) gene and was developed by us (named Telomescan or OBP-401). It contains the human telomerase reverse transcriptase (hTERT) gene promoter upstream of the E1 gene in the adenovirus type 5 genome (Figure 1A). As hTERT expression is highly specific to cancer cells (Takakura et al, 1998; Kyo et al, 1999a, 1999b; Takakura et al, 2005) and the hTERT promoter has stringent cancer specificity (Takakura et al, 1999, 2005), this virus can express E1 genes preferentially in cancer cells and thereby replicate there with much higher efficiency than in healthy cells (Kawashima et al, 2004; Takakura et al, 2010). Cancer cells infected with OBP-401 have been confirmed to express GFP in not only in vivo mouse xenografts but also cytological samples in clinical practice (Kishimoto et al, 2006; Maida et al, 2009). Recently, OBP-401 was successfully applied to detect CTCs in the clinical blood samples of patients with gastric, breast, and other cancers (colon cancer, hepatocellular carcinoma, and non-small cell lung cancer) (Kojima et al, 2009; Kim et al, 2011). These hTERT-based adenoviral-mediated systems have a great advantage for detecting living CTCs, minimising the false-positive detection of healthy circulating epithelia. However, some technical problems remain, such as false-positive signals caused by white blood cells (WBCs) that cannot be excluded, because numerous WBCs contained in the clinical blood samples generate nonspecific infection of virus and telomerase is known to be activated in specific groups of blood cells (Matsumura-Arioka et al, 2005; Ge et al, 2006). This study focused on this point and developed a novel strategy to overcome these problems.
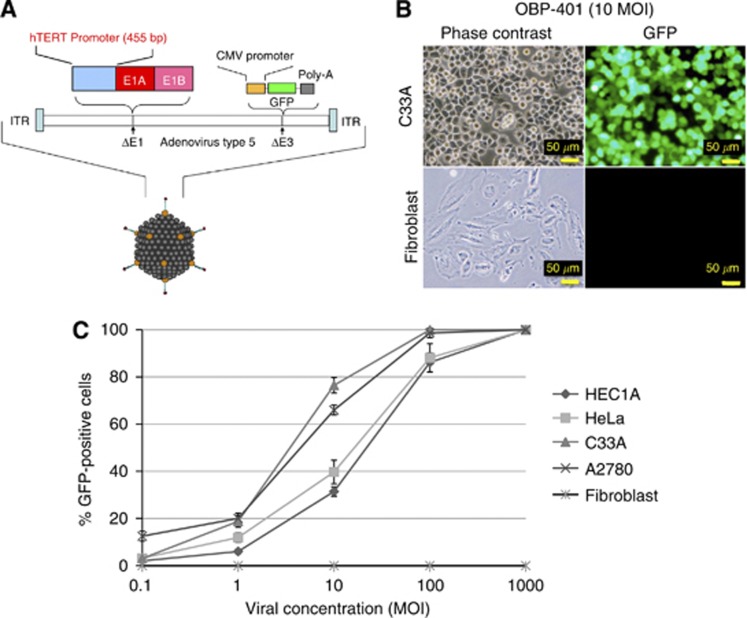
Figure 1.
Selective GFP expression in human cancer cell lines by OBP-401 infection in vitro. (A) The schematic structure of OBP-401 is shown. (B) The human cervical cancer cell line C33A and normal human fibroblasts were infected with OBP-401 at a MOI of 10 and GFP expression was assessed using a fluorescence microscope 24 h after infection. (C) The human endometrial cancer cell line HEC1A, cervical cancer cell lines HeLa and C33A, ovarian cancer cell line A2780, and normal human fibroblasts were infected with OBP-401 at the indicated MOI. The ratios of GFP-expressing cells were counted 24 h after infection. Bars indicate s.e.
Materials and methods
Cell culture
The human cervical cancer cell line C33A and ovarian cancer cell line A2780 were cultured at 37 °C under 5% CO2 in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated foetal calf serum (Sigma-Aldrich, St. Louis, MO, USA), 100 μg ml−1 streptomycin, and 100 IU ml−1 penicillin.
Viruses
OBP-401 (Telomescan) is a telomerase-specific replication-competent adenovirus, in which the hTERT gene promoter has been inserted upstream of the E1A and E1B genes linked with an internal ribosome entry site in the adenovirus type 5 genome, and the GFP gene is inserted under the cytomegalovirus promoter into the E3 region (Figure 1A; Fujiwara et al, 2006; Kishimoto et al, 2006; Watanabe et al, 2006). The construction and features of these viruses have been described in detail in our previous studies (Umeoka et al, 2004; Fujiwara et al, 2006). The viruses were purified by CsCl2 linear gradient ultracentrifugation. The viral titres were determined by a plaque-forming assay using 293 cells and the virus sample was stored at −80 °C.
Blood sample preparation
Blood samples (5 ml) were drawn into heparinised tubes and incubated with lysis buffer containing ammonium chloride (NH4Cl) for 15 min twice to remove erythrocytes. After centrifugation, the cell pellets were mixed with 105 PFUs of OBP-401 and incubated at 37 °C for 24 h with gentle rotation. Following centrifugation, the cells were fixed with 2% paraformaldehyde and 0.1% glutaraldehyde. Following washing with phosphate-buffered saline, the cells were incubated with 1/200 diluted mouse anti-human CD45 antibody (BioLegend, San Diego, CA, USA) for 20 min on ice. CD45 expression was detected by incubation with CF 555-labelled goat anti-mouse secondary antibody (Biotium, Hayward, CA, USA) for 20 min in the dark. Following washing with phosphate-buffered saline and centrifugation, cells were resuspended in 50 μl of mounting media (DAKO, Glostrup, Denmark) and placed onto slides under coverslips.
These studies were approved by the Medical Ethics Committee of Kanazawa University Graduate School of Medical Science. All patients and healthy volunteers gave written informed consent.
In vitro fluorescence imaging
Fluorescence levels were assessed with excitation/emission at 485 nm/505 nm for GFP and 550 nm/570 nm for CF 555 using a BX53 fluorescent microscope with 100-w mercury lamp unit (Olympus, Tokyo, Japan), photographed ( × 400), and counted by viewing the monitor and evaluating cell shapes or CD45 staining. High-resolution image acquisition was accomplished using an EPSON personal computer (SEIKO EPSON, Suwa, Japan). Images were processed for contrast and brightness with the use of Adobe Photoshop CS2 software (Adobe, San Jose, CA, USA), and were observed by persons who did not know the clinical information of the patients.
Patients
Patients with primary or recurrent gynaecological cancers before any treatment as well as healthy volunteers were eligible to participate in this study. Serial blood samplings were done before and after the treatments, including surgical reduction, chemotherapy, or their combination. Blood samples from healthy volunteers were also examined as negative controls. All experimental procedures including blood sampling, processing, and analysis were performed under the approval of the Medical Ethics Committee of Kanazawa University Graduate School of Medical Science. All patients and healthy volunteers gave written informed consent. The clinical characteristics of the patients are summarised in Supplementary Table 1.
Statistical analysis
To assess the statistical significance of differences in the numbers of CTCs, the χ2 test or Mann–Whitney U test were performed using the Microsoft Excel 2010 software (Microsoft, Redmond, WA, USA). A P-value of <0.05 was considered to indicate statistical significance.
Results
Optimising the condition of OBP-401 infection
OBP-401 was designed to replicate only in cells in which the hTERT promoter is activated. To confirm whether GFP expression by OBP-401 is specific in cancer cells, we first evaluated the GFP expression in various telomerase-positive gynaecological cancer cell lines and normal human fibroblasts infected by OBP-401 (Figure 1). The cells expressing GFP were counted under the fluorescent microscope 24 h after infection. No GFP-positive cells were observed in healthy fibroblasts at any viral concentration (Figure 1B). The GFP expression ratios varied among cell lines at an intermediate viral concentration (multiplicities of infection (MOI): 10). At higher viral concentrations over 100 MOI, most of the cancer cells expressed GFP, irrespective of the cell type (Figure 1C). Importantly, a cytotoxic effect was not observed within 24 h after infection, appearing at least 36 h after infection (data not shown).
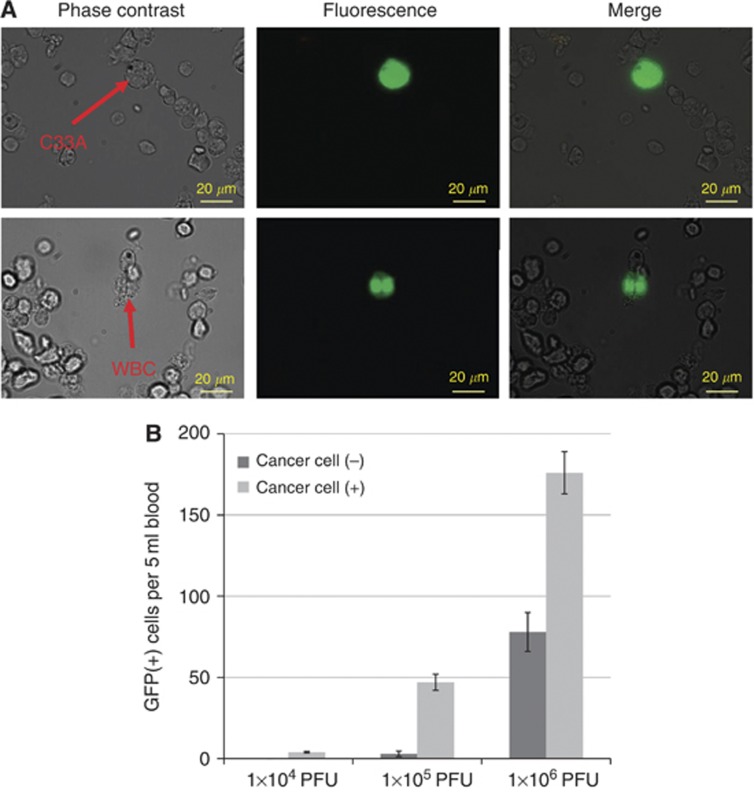
We next performed the preparatory experiments to evaluate the efficiency of OBP-401 in marking the cancer cells in peripheral blood: a total of 100 C33A human cervical cancer cells were placed in 5-ml blood samples from healthy volunteers. Red blood cells were lysed in NH4Cl buffer; subsequently, the cell pellets were recovered and infected with OBP-401 at the indicated concentrations. Green fluorescent protein-expressing cells were counted under the fluorescent microscope. In general, GFP-expressing C33A cells were observed large and strong GFP fluorescence, whereas GFP-expressing WBCs were apparently small with weaker fluorescence (Figure 2A). However, we sometimes had difficulty distinguishing these GFP signals, because we detected some large GFP-positive cells in negative control bloods. The numbers of GFP-expressing cells counted in a 5-ml blood sample containing 100 C33A cells are shown in Figure 2B. In negative control samples without C33A cells, we also counted GFP-expressing cells that gave false-positive signals. Approximately 180 GFP-positive signals were counted from the infection of 1 × 106 PFU of OBP-401, in which about 80 GFP-positive cells were considered to be false-positives by WBCs, based on the count in the negative control samples. Conversely, no false-positive signals were detected from the infection of 1 × 104 PFU of OBP-401, but the sensitivity for detecting GFP-expressing C33A cells was too low (less than 5%) in this condition. We found that 1 × 105 PFU of OBP-401 could confer optimal results, detecting ∼50% of C33A cells while minimising false-positive signals at <3% (Figure 2B). These findings indicated that increasing viral load improved the sensitivity for detecting cancer cells, while it decreased specificity, thereby suggesting its importance to effectively eliminate false-positive signals.
Figure 2.
Preparatory experiments using human blood samples containing cancer cells. (A) Phase contrast and fluorescent images of peripheral blood cells containing C33A cervical cancer cells. A total of 100 C33A cells were mixed with 5-ml whole blood samples from healthy volunteers and treated with haemolysis buffer, followed by the infection with 1 × 105 PFU of OBP-401. Green fluorescent protein-expressing cells were analysed 24 h after infection. Green fluorescent protein was mainly expressed in C33A cells (upper lane), but some WBCs expressed GFP (lower lane). (B) The number of the GFP-expressing cells in 5 ml blood with or without 100 C33A cells is shown. The samples were infected with the indicated amount of OBP-401. Bars indicate s.e.
Application of CD45 staining to distinguish GFP signals between CTCs and WBCs
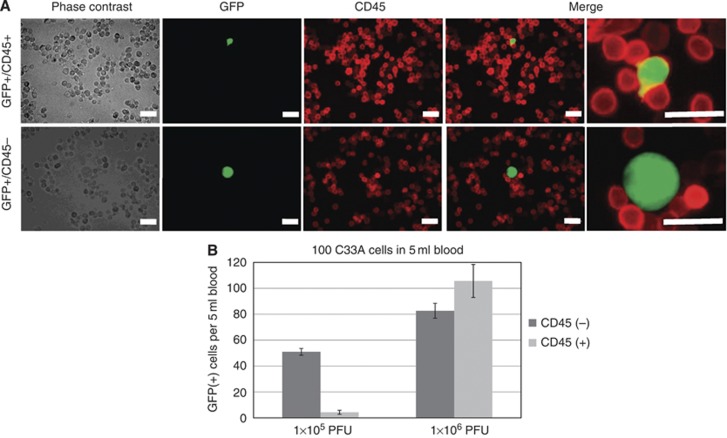
To distinguish GFP expression in CTCs from that in WBCs, we attempted co-immunostaining of CD45 after OBP-401 infection (Figure 3A). As CD45 expression is broadly observed at the surface of the WBCs, GFP-expressing WBCs should be recognised with CD45 signals (red fluorescent) at the membrane and should be distinguishable from CTCs lacking these signals in merged pictures. Preparatory experiments using artificial samples in which 100 C33A cells were added to 5-ml blood samples from healthy volunteers showed that about 50 CD45-negative/GFP-positive C33A cells were counted at a viral dose of 1 × 105 PFU and about 80 of these C33A cells were counted at a viral dose of 1 × 106 PFU (Figure 3B), whereas 4 CD45-positive/GFP-positive WBCs were detected at a viral dose of 1 × 105 PFU and about 100 of these WBCs were detected at a viral dose of 1 × 106 PFU. On the basis of these experiments, we considered the viral concentration of 1 × 105 PFU to be optimal for detecting CTCs in clinical samples. No CD45-negative/GFP-positive cell was observed in negative control samples, meaning that the specificity is extremely high (almost 100%) in this system.
Figure 3.
Application of CD45 staining to distinguish CTCs from GFP-expressing WBCs. (A) Phase contrast and fluorescent images of peripheral blood cells mixed with C33A cervical cancer cells. OBP-401-infected samples were fixed and stained with anti-CD45 antibody and CF555-labelled secondary antibody. The overlap of green (GFP) and red (CD45) fluorescence is displayed as yellow. Bar, 20 μm. (B) The number of the GFP-expressing cells with/without CD45 fluorescence in 5 ml blood mixed with 100 C33A cells is shown. The samples were infected with the indicated amount of OBP-401. Bars indicate s.e.
Detection of CTCs in patients with gynaecological cancers
We analysed fresh blood samples collected from 53 patients with both primary and recurrent gynaecological cancers as well as from 22 healthy volunteers (14 male and 8 female, mean age 31.4; s.d. 6.6). Using fluorescence microscopy, we counted GFP-expressing cells and those with CD45 immunofluorescence were judged to be WBCs. We first confirmed that no CTCs were detected in samples from healthy volunteers, but the number of GFP-positive, CD45-positive cells (WBCs) ranged from 1 to 64 in 5-ml blood samples (mean 11.5; s.d. 13.9), indicating a relatively high frequency of nonspecific signals.
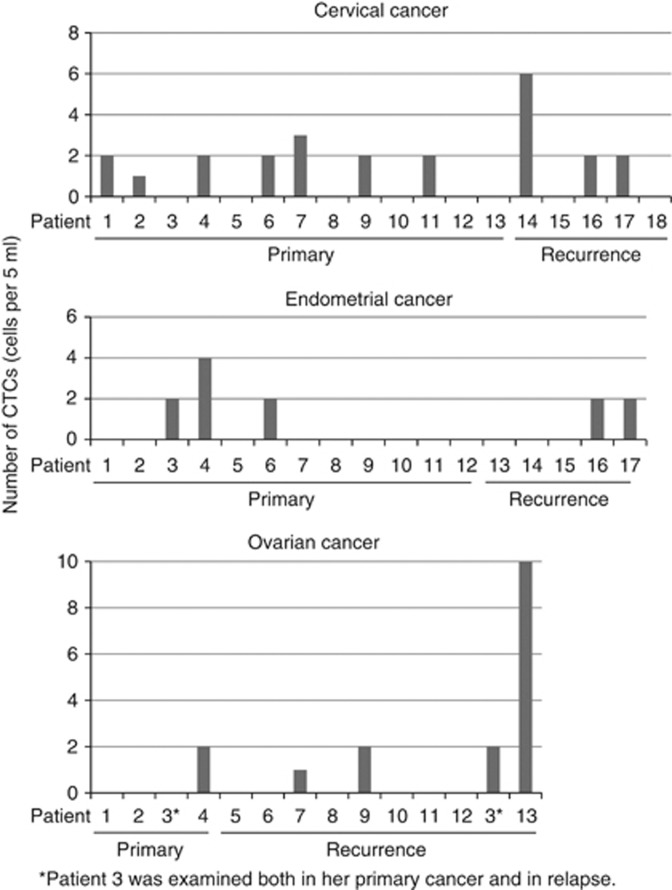
The 53 patients consisted of those with 18 cervical cancers, 17 endometrial cancers, 14 ovarian cancers, 3 vulvar cancers, and 1 vaginal cancer (Supplementary Table 1). All samples were collected before treatment. Serial evaluations were done during their treatment courses. One or more CTCs were identified from 10 of 18 (55.6%) patients with cervical cancer, 5 of 17 (29.4%) with endometrial cancer, 5 of 14 (35.7%) of ovarian cancer, and 1 of 4 (25.0%) with other cancers (Supplementary Table 2). Overall, 21 of 53 (39.6%) of patients with gynaecological cancers were CTC-positive. The number of CTCs observed in 5-ml blood samples ranged from 0 to 10 (Figure 4). There was no significant difference in the CTC-positive ratios among cancer types. No correlation was observed between CTCs and the clinicopathological characteristics of tumours (Supplementary Table 2).
Figure 4.
Numbers of CTCs detected in patients by type of cancer. Comparison of the numbers of CTCs observed in each patient. Patient no. 3 with ovarian cancer was negative for CTCs at her initial examination. After 6 months, she relapsed, at which time CTCs were detected (indicated with asterisk).
Some doubt may arise regarding the reproducibility of this system owing to the small number of CTCs detected in a sample. To verify the reproducibility, the additional control experiments were performed, in which 50 C33A cells were spiked into 50 ml of control bloods from healthy donors, divided into 10 tubes and subjected to the counting. The average of observed C33A cells in each 5ml blood was 2.2 (s.d.=0.9). At least one cancer cell was observed in every sample (data not shown).
Change in CTC counts correlates with clinical course in patients
Serial examination was performed during their treatment courses. The final evaluations of CTCs were done 4 weeks after the start of the treatments. Circulating tumour cells rapidly vanished after surgery or chemotherapy in most patients whose treatments were judged as effective including complete response and partial response by imaging diagnosis. Conversely, CTCs were sustained in patients in whom the treatments were not effective ( progressive disease). Representative cases are shown in supplementary figure 1. There was significant correlation between the outcome of the treatment and the presence of CTCs (Table 1). There was no patient whose CTC was negative at the initial evaluation but detected later during the treatment course.
Table 1. Change in CTC count in patients with positive CTCs at initial evaluation.
| Effect of treatment | CTCs no longer present | CTCs remained |
|---|---|---|
| Complete response/partial response | 15 | 1# |
| Progressive disease | 0 | 3# |
Abbreviation: CTC=circulating tumour cell.
P<0.005.
Discussion
Most of the detection systems for CTCs currently available rely on immunological recognition of epithelial-specific cell surface markers, such as EpCAM and cytokeratin. With regard to female cancers (cervical, endometrial, ovarian, and breast cancers), the detection rates of CTCs by such methods were reported as ranging from 14.4 to 90% (Judson et al, 2003; He et al, 2008; Behbakht et al, 2011; Poveda et al, 2011). Several critical problems in detecting CTCs using cell surface markers have been reported. First, there is no guarantee that the detected cells are real cancer cells; they may be merely healthy epithelia circulating in blood. Second, they may not be living cells because dead cells can still present surface antigens. Third, although the cascades of cancer metastasis formation are not fully understood, the epithelial-to-mesenchymal transition (EMT) process is believed to have a great role in these cascades (Klymkowsky and Savagner, 2009; Bonnomet et al, 2010; Iwatsuki et al, 2010) and the expression of epithelial markers is known to be downregulated during the process of EMT, probably leading to the false-negative findings (Mikolajczyk et al, 2011). The OBP-401-based method theoretically has a technical advantage for overcoming these issues, because OBP-401 infects and replicates only in the living cancer cells and the infectivity does not change during the process of EMT.
Our preparatory experiments showed that the positive ratio of GFP-positive cancer cells increased in a dose-dependent manner with OBP-401, but the ratio of GFP-positive WBCs also increased. There are some reports about the induction of hTERT expression in growth-stimulated healthy lymphocytes (Matsumura-Arioka et al, 2005; Ge et al, 2006). Although the frequency of GFP-positive WBCs was quite low compared with that of cancer cells, it is not ignorable because the number of WBCs contained in the blood sample is much larger than that of CTCs. These problems have been left unresolved in an OBP-401-mediated detection system. The unique technique applied in this study is the addition of a process of counterstaining with anti-CD45 antibody to mark WBCs. We could confirm that anti-CD45 staining did not interfere with the recognition of GFP-expressing cancer cells (data not shown). Merging GFP and CD45 fluorescences (red with CF555) allowed the discernment of cancer cells from WBCs (Figure 3). Therefore, we propose this step to be crucial in an OBP-401-mediated CTC detection system.
In this novel protocol, the CTC detection rate was ∼ 40% in clinical samples from patients with gynaecological cancers. The reported rates varied widely among studies, ranging from 14.4 to 90%, and the clinical significance of the presence of CTCs are still under controversy (Judson et al, 2003; He et al, 2008; Behbakht et al, 2011; Poveda et al, 2011). Although our results were near the centre of this range, the number of CTCs detected in a sample was significantly lower than that in other reports. This might be due to the higher stringency of the system to minimise nonspecific WBC signals. The reproducibility of our system was confirmed by the additional experiments after examinations with clinical samples, in which 50 C33A cells were spiked into 50 ml of bloods from healthy donors, divided into 10 tubes. At least one cancer cell was observed in each tube (mean 2.2±0.9 s.d.), supporting the reproducibility of our method.
There was no significant difference in the CTC counts among cancer types, clinical stage, or other clinicopathological findings. Notably, no statistically significant difference was observed between patients with hematogenous metastasis and those with non-hematogenous metastasis, suggesting that CTCs may appear in the early stage of cancers, irrespective of the cancer type. The persistence of CTCs after treatment, regardless of the type of treatment, was associated with poor response to the treatment, suggesting that CTCs may be a sensitive marker of treatment effect. However, it remains unclear whether the presence of CTCs can be used as a prognostic marker and we are currently analysing outcome data. Two of the important merits of our system are the cost effectiveness and technical feasibility. No special machine is required other than a fluorescent microscope, and it takes 15–20 min to test one sample. Furthermore, the CTCs can be captured under the microscope and then be used for further analysis such as whole-genome amplification and gene expression analysis. This might be attractive because we can obtain genome information from the cancers via CTCs without invasive procedures such as biopsy and surgery.
In conclusion, we developed a novel CTC detection system using a telomerase-specific replicative adenovirus combined with CD45 staining. Our system clearly resolved the technical defect of a viral-mediated CTC detection method, effectively eliminating false-positive signals by WBCs and maintaining sufficient sensitivity. The feasibility and superior cost-effectiveness of this method encourage further confirmation of the results with larger numbers of patients and longer follow-up periods to evaluate the prognostic impact.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, the Hokkoku Cancer Foundation, and the Megumi Medical Foundation of Kanazawa University.
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
The authors declare no conflict of interest.
Supplementary Material
References
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10(20): 6897–6904 [DOI] [PubMed] [Google Scholar]
- Attard G, de Bono JS (2010) Utilizing circulating tumor cells: challenges and pitfalls. Curr Opin Genet Dev 21(1): 50–58 [DOI] [PubMed] [Google Scholar]
- Behbakht K, Sill MW, Darcy KM, Rubin SC, Mannel RS, Waggoner S, Schilder RJ, Cai KQ, Godwin AK, Alpaugh RK (2011) Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a Gynecologic Oncology Group study. Gynecol Oncol 123(1): 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnomet A, Brysse A, Tachsidis A, Waltham M, Thompson EW, Polette M, Gilles C (2010) Epithelial-to-mesenchymal transitions and circulating tumor cells. J Mammary Gland Biol Neoplasia 15(2): 261–273 [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ (2008) Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 26(19): 3213–3221 [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351(8): 781–791 [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC, Fritsche HA, Hortobagyi GN, Terstappen LW (2005) Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 23(7): 1420–1430 [DOI] [PubMed] [Google Scholar]
- de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D (2008) Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 14(19): 6302–6309 [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Kagawa S, Kishimoto H, Endo Y, Hioki M, Ikeda Y, Sakai R, Urata Y, Tanaka N (2006) Enhanced antitumor efficacy of telomerase-selective oncolytic adenoviral agent OBP-401 with docetaxel: preclinical evaluation of chemovirotherapy. Int J Cancer 119(2): 432–440 [DOI] [PubMed] [Google Scholar]
- Ge Z, Liu C, Bjorkholm M, Gruber A, Xu D (2006) Mitogen-activated protein kinase cascade-mediated histone H3 phosphorylation is critical for telomerase reverse transcriptase expression/telomerase activation induced by proliferation. Mol Cell Biol 26(1): 230–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Kularatne SA, Kalli KR, Prendergast FG, Amato RJ, Klee GG, Hartmann LC, Low PS (2008) Quantitation of circulating tumor cells in blood samples from ovarian and prostate cancer patients using tumor-specific fluorescent ligands. Int J Cancer 123(8): 1968–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, Baba H, Mori M (2010) Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci 101(2): 293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson PL, Geller MA, Bliss RL, Boente MP, Downs LS, Argenta PA, Carson LF (2003) Preoperative detection of peripherally circulating cancer cells and its prognostic significance in ovarian cancer. Gynecol Oncol 91(2): 389–394 [DOI] [PubMed] [Google Scholar]
- Kawashima T, Kagawa S, Kobayashi N, Shirakiya Y, Umeoka T, Teraishi F, Taki M, Kyo S, Tanaka N, Fujiwara T (2004) Telomerase-specific replication-selective virotherapy for human cancer. Clin Cancer Res 10(1 Pt 1): 285–292 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Masago A, Tamaki Y, Akazawa K, Tsukamoto F, Sato J, Ozawa T, Tsujino Y, Noguchi S (2011) A novel approach using telomerase-specific replication-selective adenovirus for detection of circulating tumor cells in breast cancer patients. Breast Cancer Res Treat 128(3): 765–773 [DOI] [PubMed] [Google Scholar]
- Kishimoto H, Kojima T, Watanabe Y, Kagawa S, Fujiwara T, Uno F, Teraishi F, Kyo S, Mizuguchi H, Hashimoto Y, Urata Y, Tanaka N (2006) In vivo imaging of lymph node metastasis with telomerase-specific replication-selective adenovirus. Nat Med 12(10): 1213–1219 [DOI] [PubMed] [Google Scholar]
- Klymkowsky MW, Savagner P (2009) Epithelial-mesenchymal transition: a cancer researcher's conceptual friend and foe. Am J Pathol 174(5): 1588–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Hashimoto Y, Watanabe Y, Kagawa S, Uno F, Kuroda S, Tazawa H, Kyo S, Mizuguchi H, Urata Y, Tanaka N, Fujiwara T (2009) A simple biological imaging system for detecting viable human circulating tumor cells. J Clin Invest 119(10): 3172–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyo S, Kanaya T, Takakura M, Tanaka M, Inoue M (1999a) Human telomerase reverse transcriptase as a critical determinant of telomerase activity in normal and malignant endometrial tissues. Int J Cancer 80(1): 60–63 [DOI] [PubMed] [Google Scholar]
- Kyo S, Kanaya T, Takakura M, Tanaka M, Yamashita A, Inoue H, Inoue M (1999b) Expression of human telomerase subunits in ovarian malignant borderline and benign tumors. Int J Cancer 80(6): 804–809 [DOI] [PubMed] [Google Scholar]
- Maida Y, Kyo S, Sakaguchi J, Mizumoto Y, Hashimoto M, Mori N, Ikoma T, Nakamura M, Takakura M, Urata Y, Fujiwara T, Inoue M (2009) Diagnostic potential and limitation of imaging cancer cells in cytological samples using telomerase-specific replicative adenovirus. Int J Oncol 34(6): 1549–1556 [DOI] [PubMed] [Google Scholar]
- Matsumura-Arioka Y, Ohtani K, Hara T, Iwanaga R, Nakamura M (2005) Identification of two distinct elements mediating activation of telomerase (hTERT) gene expression in association with cell growth in human T cells. Int Immunol 17(2): 207–215 [DOI] [PubMed] [Google Scholar]
- Mikolajczyk SD, Millar LS, Tsinberg P, Coutts SM, Zomorrodi M, Pham T, Bischoff FZ, Pircher TJ (2011) Detection of EpCAM-negative and cytokeratin-negative circulating tumor cells in peripheral blood. J Oncol 2011: 252361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poveda A, Kaye SB, McCormack R, Wang S, Parekh T, Ricci D, Lebedinsky CA, Tercero JC, Zintl P, Monk BJ (2011) Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent advanced ovarian cancer. Gynecol Oncol 122(3): 567–572 [DOI] [PubMed] [Google Scholar]
- Racila E, Euhus D, Weiss AJ, Rao C, McConnell J, Terstappen LW, Uhr JW (1998) Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci USA 95(8): 4589–4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Janicke F, Jackson S, Gornet T, Cristofanilli M, Pantel K (2007) Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res 13(3): 920–928 [DOI] [PubMed] [Google Scholar]
- Sun YF, Yang XR, Zhou J, Qiu SJ, Fan J, Xu Y (2011) Circulating tumor cells: advances in detection methods, biological issues, and clinical relevance. J Cancer Res Clin Oncol 137(8): 1151–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura M, Kyo S, Inoue M, Wright W, Shay J (2005) Function of AP-1 in transcription of the telomerase reverse transcriptase gene (TERT) in human and mouse cells. Mol Cell Biol 25(18): 8037–8043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura M, Kyo S, Kanaya T, Hirano H, Takeda J, Yutsudo M, Inoue M (1999) Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res 59(3): 551–557 [PubMed] [Google Scholar]
- Takakura M, Kyo S, Kanaya T, Tanaka M, Inoue M (1998) Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res 58(7): 1558–1561 [PubMed] [Google Scholar]
- Takakura M, Nakamura M, Kyo S, Hashimoto M, Mori N, Ikoma T, Mizumoto Y, Fujiwara T, Urata Y, Inoue M (2010) Intraperitoneal administration of telomerase-specific oncolytic adenovirus sensitizes ovarian cancer cells to cisplatin and affects survival in a xenograft model with peritoneal dissemination. Cancer Gene Ther 17(1): 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeoka T, Kawashima T, Kagawa S, Teraishi F, Taki M, Nishizaki M, Kyo S, Nagai K, Urata Y, Tanaka N, Fujiwara T (2004) Visualization of intrathoracically disseminated solid tumors in mice with optical imaging by telomerase-specific amplification of a transferred green fluorescent protein gene. Cancer Res 64(17): 6259–6265 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Hioki M, Fujiwara T, Nishizaki M, Kagawa S, Taki M, Kishimoto H, Endo Y, Urata Y, Tanaka N (2006) Histone deacetylase inhibitor FR901228 enhances the antitumor effect of telomerase-specific replication-selective adenoviral agent OBP-301 in human lung cancer cells. Exp Cell Res 312(3): 256–265 [DOI] [PubMed] [Google Scholar]
- Yu M, Stott S, Toner M, Maheswaran S, Haber DA (2011) Circulating tumour cells: approaches to isolation and characterization. J Cell Biol 192(3): 373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.