Abstract
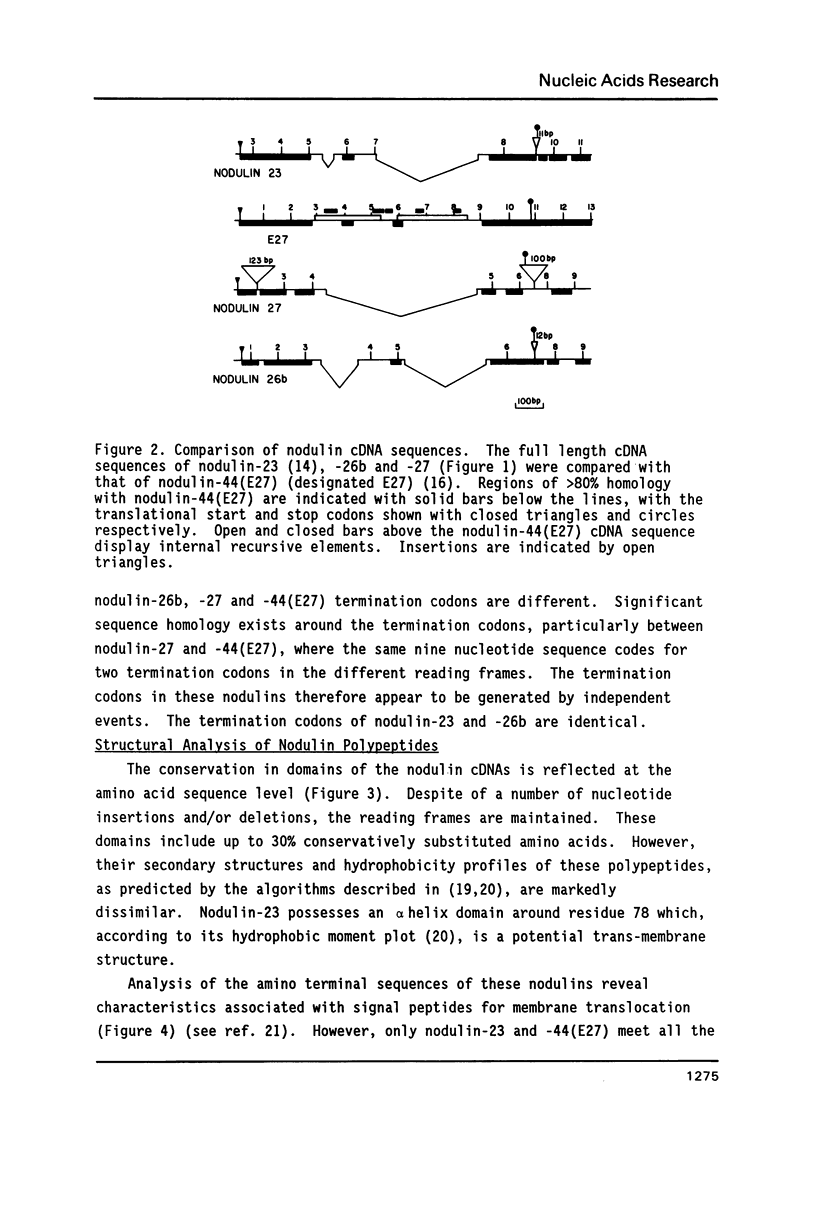
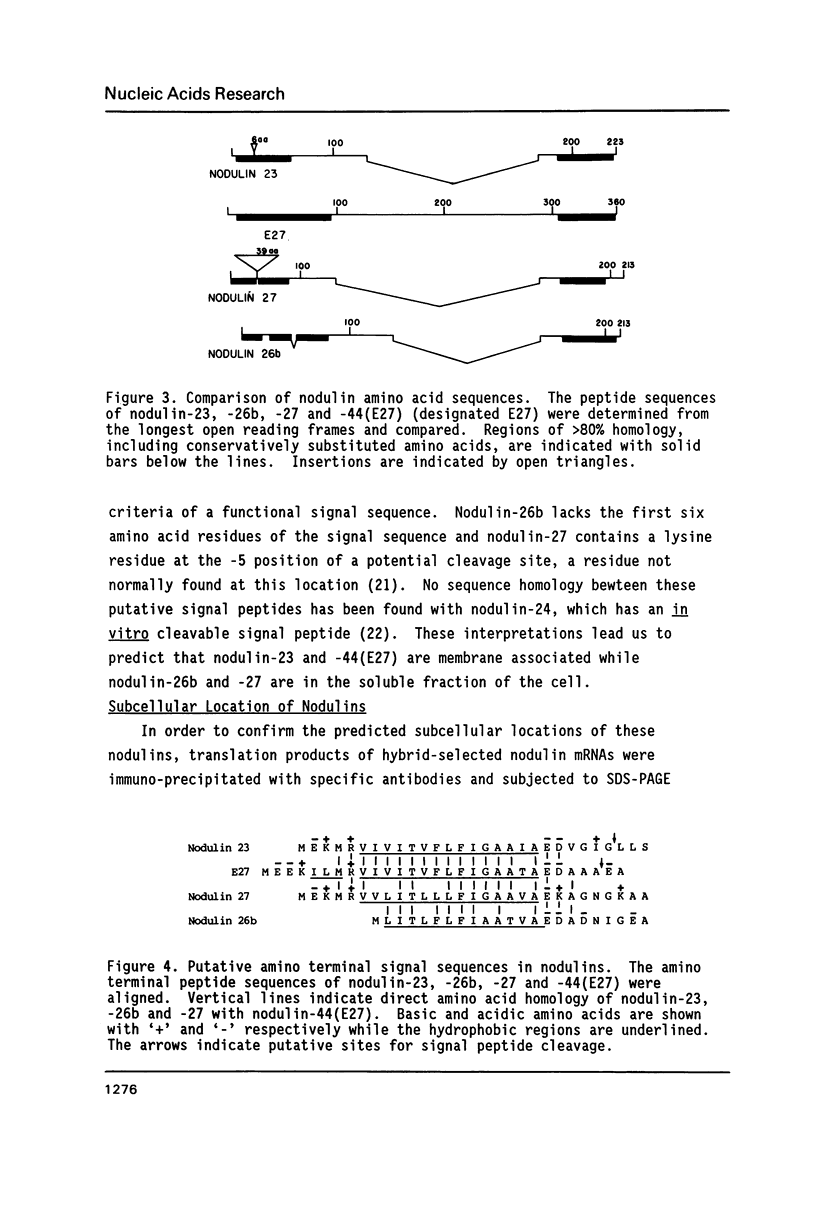
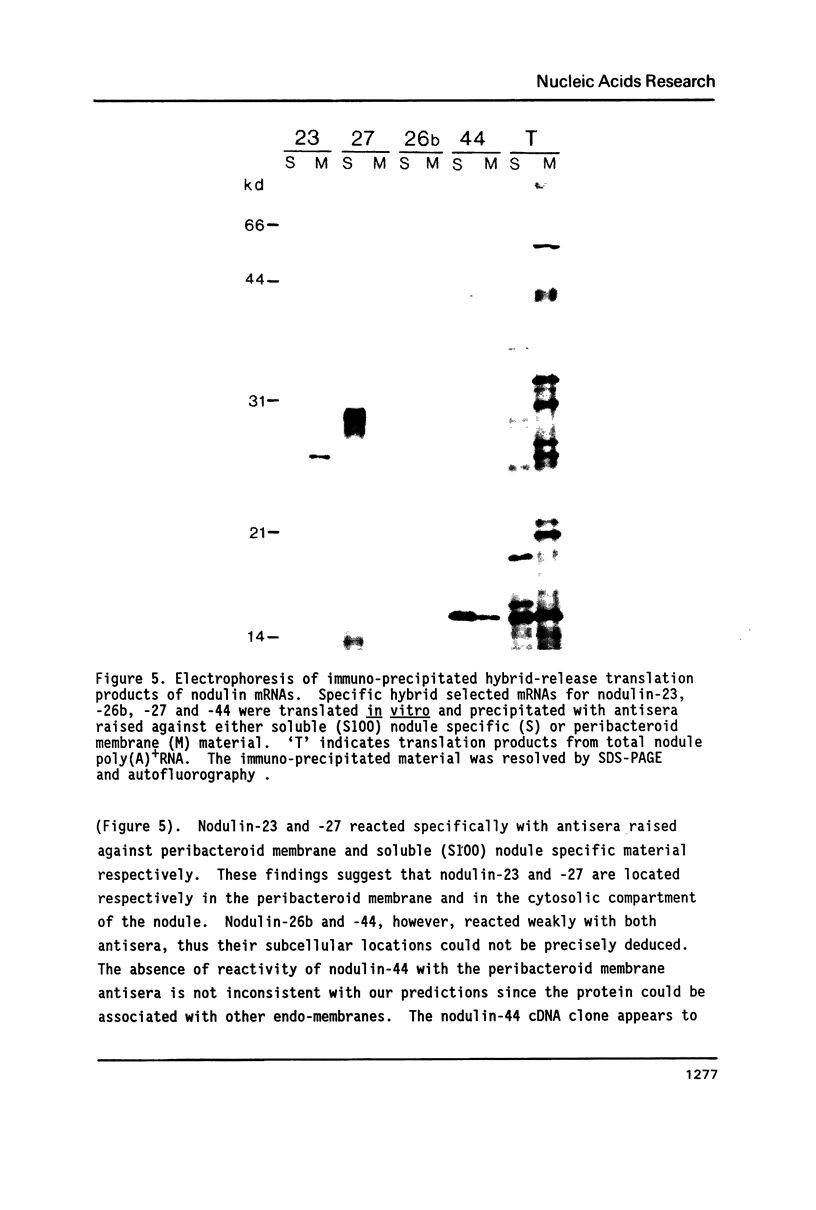
Four soybean cDNA nodule-specific clones encoding nodulin-23, -26b, -27 and -44 were observed to cross-hybridize under low stringency conditions. Nucleotide sequence analysis revealed that the cDNAs contain three distinct domains: two domains with 70 to 95% homology separated by a third domain unique to each cDNA. Despite a number of nucleotide insertions and deletions, the protein sequences are conserved in the two domains which correlate with the homologous nucleotide domains. The amino terminal domain of each nodulin contains putative signal sequences for membrane translocation, although only two (nodulin-23 and -44) meet all the criteria for a functional signal. Immuno-precipitation of hybrid-release translation products of the four cDNAs revealed that nodulin-23 is associated with the peribacteroid membrane while nodulin-27 is in the cytoplasmic fraction of the nodule. These four nodulins are members of a diverse family with conserved structural features and the genes encoding them appear to have recently evolved from a common ancestor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A., Nicola N. A., Hurrell J. G., Leach S. J. Characterization and improved separation of soybean leghemoglobins. Biochemistry. 1975 Oct 7;14(20):4444–4450. doi: 10.1021/bi00691a016. [DOI] [PubMed] [Google Scholar]
- Argos P., Narayana S. V., Nielsen N. C. Structural similarity between legumin and vicilin storage proteins from legumes. EMBO J. 1985 May;4(5):1111–1117. doi: 10.1002/j.1460-2075.1985.tb03747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger S., Verma D. P. Induction and expression of nodule-specific host genes in effective and ineffective root nodules of soybean. Biochemistry. 1981 Mar 3;20(5):1300–1306. doi: 10.1021/bi00508a040. [DOI] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Fortin M. G., Zelechowska M., Verma D. P. Specific targeting of membrane nodulins to the bacteroid-enclosing compartment in soybean nodules. EMBO J. 1985 Dec 1;4(12):3041–3046. doi: 10.1002/j.1460-2075.1985.tb04043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller F., Künstner P. W., Nguyen T., Verma D. P. Soybean nodulin genes: Analysis of cDNA clones reveals several major tissue-specific sequences in nitrogen-fixing root nodules. Proc Natl Acad Sci U S A. 1983 May;80(9):2594–2598. doi: 10.1073/pnas.80.9.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govers F., Gloudemans T., Moerman M., van Kammen A., Bisseling T. Expression of plant genes during the development of pea root nodules. EMBO J. 1985 Apr;4(4):861–867. doi: 10.1002/j.1460-2075.1985.tb03711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katinakis P., Verma D. P. Nodulin-24 gene of soybean codes for a peptide of the peribacteroid membrane and was generated by tandem duplication of a sequence resembling an insertion element. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4157–4161. doi: 10.1073/pnas.82.12.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982 Apr;62(2):672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- Lang-Unnasch N., Ausubel F. M. Nodule-specific polypeptides from effective alfalfa root nodules and from ineffective nodules lacking nitrogenase. Plant Physiol. 1985 Apr;77(4):833–839. doi: 10.1104/pp.77.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Verma D. P. Structure and chromosomal arrangement of leghemoglobin genes in kidney bean suggest divergence in soybean leghemoglobin gene loci following tetraploidization. EMBO J. 1984 Dec 1;3(12):2745–2752. doi: 10.1002/j.1460-2075.1984.tb02205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mauro V. P., Nguyen T., Katinakis P., Verma D. P. Primary structure of the soybean nodulin-23 gene and potential regulatory elements in the 5'-flanking regions of nodulin and leghemoglobin genes. Nucleic Acids Res. 1985 Jan 11;13(1):239–249. doi: 10.1093/nar/13.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T., Zelechowska M., Foster V., Bergmann H., Verma D. P. Primary structure of the soybean nodulin-35 gene encoding uricase II localized in the peroxisomes of uninfected cells of nodules. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5040–5044. doi: 10.1073/pnas.82.15.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]