Abstract
Aims/hypothesis
Hyperglycaemia and dyslipidaemia are common metabolic abnormalities in adults with type 1 diabetes and both increase cardiovascular disease (CVD) risk. The hypothesis of this study was that change in HbA1c over 6 years would be associated with change in fasting lipids in adults with type 1 diabetes.
Methods
The Coronary Artery Calcification in Type 1 Diabetes (CACTI) study examined 652 patients with type 1 diabetes (54% female); 559 and 543 had follow-up visits at 3 and 6 years. Baseline age (mean ± SD) was 37±9 years, diabetes duration 23±9 years, and HbA1c 8.0±1.3%. Use of dyslipidaemia medication was 17%, 32%, and 46% at the three visits. Separate longitudinal mixed models were fitted to examine the relationship between change in HbA1c and change in fasting total cholesterol (TC), HDL-cholesterol (HDL-c), LDL-cholesterol (LDL-c), log triacylglycerols (TG), and non-HDL-cholesterol (non- HDL-c). Because of an interaction between dyslipidaemia medication use and association of HbA1c with lipids, results were stratified by dyslipidaemia medication use.
Results
Among patients not using dyslipidaemia medication, a higher HbA1c was associated with significantly worse levels of the lipids TC, LDL-c, TG and non-HDL-c (per 1% change in HbA1c, TC 0.101 mmol/l, 95% CI 0.050, 0.152; LDL-c 0.103 mmol/l, 95% CI 0.058, 0.148; TG 0.052 mmol/l, 95% CI 0.024, 0.081; and non-HDL-c 0.129 mmol/l, 95% CI 0.078, 0.180) but not HDL-c (−0.20 mmol/l, 95% CI −0.047, 0.007). The associations between HbA1c and any lipid outcome among those on dyslipidaemia medication were in the same direction, but attenuated compared with persons not on medication.
Conclusions/interpretation
Change in HbA1c is significantly associated with change in fasting lipids, but dyslipidaemia medications may be required to optimise lipid and cardiovascular health.
Keywords: CVD risk, Dyslipidaemia medications, Glycaemia, Lipids, Type 1 diabetes
Introduction
Hyperglycaemia, as measured by HbA1c, is the most important determinant of vascular complications of type 1 diabetes [1-4]. In addition to hyperglycaemia, dyslipidaemia is a well-established cardiovascular disease (CVD) risk factor [5, 6]. However, few data exist in people with type 1 diabetes on the relationship of glycaemia to other CVD risk factors such as dyslipidaemia, although aggressive control of glycaemia is recommended as first line treatment of dyslipidaemia in the care of people with type 1 diabetes, with addition of lipid-lowering medications if lipid goals are not achieved [7, 8]. Although hyperglycaemia and dyslipidaemia [9] both increase CVD risk and are metabolic abnormalities frequently found in individuals with type 1 diabetes, it is unclear how changes in HbA1c are associated with alterations in lipids and whether dyslipidaemia medications modify this association.
The Diabetes Control and Complications Trial (DCCT) demonstrated that intensive (vs conventional) insulin therapy was associated with significantly reduced total cholesterol (TC), LDL-cholesterol (LDL-c) and triacylglycerols (TG) (p<0.01 for all) and decreased development of LDL-c >4.14 mmol/l in adults with type 1 diabetes, which was considered a treatment threshold at the time of the study [10], as well as a more favourable distribution of lipoprotein cholesterol [11]. Similarly, the DCCT/Epidemiology of Diabetes Control (EDIC) study demonstrated that intensive vs conventional insulin therapy was associated with a 24% reduced risk of developing incident hypertension over a median of 15.8 years of follow-up [12]. Thus, it is plausible that some of the deleterious effect of hyperglycaemia on cardiovascular health is mediated via the effects of hyperglycaemia on hypertension and dyslipidaemia [10, 12].
Therefore, to better describe the relationship of glycaemia to dyslipidaemia outside the setting of a clinical trial, we tested the hypothesis that change in HbA1c over a follow-up interval of approximately 6 years resulted in a concordant change in the lipid profile in adults with type 1 diabetes participating in the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study, independent of potential confounders, and accounting for dyslipidaemia medication usage.
Methods
Study participants
The data presented in this report were collected as part of the baseline examination of 1,416 participants in the CACTI study who were 19–56 years of age and included 652 participants with type 1 diabetes [13]. All participants were asymptomatic for coronary artery disease (CAD) and had no history of coronary artery bypass graft, coronary angioplasty, or unstable angina. Participants with diabetes generally had been diagnosed when <30 years of age, or had positive antibodies or a clinical course consistent with type 1 diabetes. Of the 652 persons enrolled at baseline, 559 (86%) participated in a second study visit ~3 years after baseline and 543 (83%) in a third study visit ~6 years after baseline, with 603 (92%) of participants having at least one follow-up study visit. Participants who did not have any follow-up data (n=49) had higher TC, LDL-c and non-HDL-cholesterol (non-HDL-c) at baseline than those with follow-up data. All participants provided informed consent and the study was approved by the Colorado Multiple Institutional Review Board.
Examination and laboratory measurements
Participants completed a baseline examination between March 2000 and April 2002. A more detailed description of the study and baseline characteristics of this cohort has been published [14]. The second and third study visits followed the same protocol and occurred between 2003 and 2005 and between 2006 and 2008, respectively.
Laboratory analyses
Blood was collected after an overnight fast and serum was stored at −70°C until assayed. Fasting plasma samples were obtained for measurement of TC, HDL-c and TG, in the University of Colorado Denver Clinical Translational Research Center core lab. Measurements of TC, HDL-c, and TG were performed enzymatically on a Hitachi 917 autoanalyser (Boehringer Mannheim Diagnostics, Indianapolis, IN, USA). LDL-c levels were calculated by the Friedewald equation for individuals with TG levels lower than 4.52 mmol/l [15] and by Lipid Research Clinics Beta Quantification [16] for those with TG≥4.52 mmol/l. Non-HDL-c was calculated as TC minus HDL-c. HbA1c levels were measured by (DCCT-calibrated) ion-exchange high-performance liquid chromatography (Bio-Rad Laboratories, Hercules, CA, USA).
Variables
Weight and height were measured using standard methods and BMI, defined as weight (kg) divided by height (m2), was calculated. Minimal waist circumference (measured at the smallest point between the tenth rib and the iliac crest over the bare skin) was measured. Intraabdominal fat and subcutaneous fat were assessed using abdominal computed tomography scan at the L4–L5 levels and volume in cubic centimeters was measured using the AccuAnalyzer software from AccuImage.
Dyslipidaemia medications
Participants brought all current medications to each study visit for documentation by study staff. Dyslipidaemia treatment was defined as current use of lipid-lowering medication, such as hydroxymethylglutaryl-CoA reductase inhibitors, bile acid sequestrants, fibric acid derivatives, ezetimibe or niacin, and was recorded at each study visit. Dyslipidaemia medication treatment decisions were made by the participants’ physicians, not by the CACTI study team.
Statistical methods
The mean and SE for each variable of interest at baseline and follow-up were estimated using the SAS Mixed procedure for linear mixed models. TG was log transformed due to non-normal distribution. Therefore, back transformed estimates were calculated as change in TG per 1% change in HbA1c from a starting level of 0.93 mmol/l (median baseline TG level for cohort.) To determine the association of change in HbA1c with change in lipids, two a priori models (basic and saturated) were constructed with separate models for change in each lipid variable (TC, HDL-c, TG, LDL-c, and non-HDL-c) as an outcome. Time-varying change variables were created for HbA1c and each lipid outcome for change from baseline to the first follow-up visit and from baseline to the second follow-up visit. The outcome of interest in all models was change in lipid levels over time. Baseline lipid levels were also included as a covariate to increase precision. In the basic model, demographic variables (sex, race/ethnicity, diabetes duration, and age as a time-varying variable) were adjusted for in addition to change in HbA1c, visit, and a change in HbA1c by visit interaction term to determine whether the association of change in HbA1c with change in lipid outcome differed by visit. To account for differences in baseline levels of HbA1c, baseline HbA1c was also included. A baseline HbA1c by time-varying change in HbA1c interaction term was tested for to determine whether the association of change in HbA1c with change in lipid was different according to baseline HbA1c, but this was not significant and was thus not included in the final models. Since the change in HbA1c by visit interaction terms were not significant, ESTIMATE statements were used to determine the average association of change in HbA1c with change in each lipid outcome over time. In the saturated model, to account for the confounding role of adiposity on lipids, we additionally adjusted for BMI, waist circumference and visceral fat as time-varying variables. All three of these measures were included to fully capture the confounding effect of adiposity on lipids, as the individual role of each adiposity measure is not the focus of this analysis. Dyslipidaemia medication usage was considered as an effect modifier for the association of HbA1c with lipid levels. A time-varying dyslipidaemia medication usage (yes/no) by change in HbA1c interaction term was included to determine whether the association of change in HbA1c with change in the lipid outcome differed according to whether or not a participant was on a dyslipidaemia medication. As significant differences existed, models stratified by dyslipidaemia medication usage status are presented. In post hoc analyses, interactions for both timevarying age and sex by change in HbA1c were explored and, since significant differences existed by sex, models stratified by sex are also presented. The Mixed procedure in SAS 9.2 (SAS Institute, Cary, NC, USA) was used for the analysis. A p value of <0.05 was considered statistically significant.
Results
Statistically significant improvements over 6 years (Table 1, p <0.0001 for all) were noted for TC (mean decrease of 0.26 mmol/l), HDL-c (mean increase of 0.13 mmol/l), LDL-c (mean decrease of 0.33 mmol/l), TG (mean decrease of 0.14 mmol/l) and non-HDL-c (mean decrease of 0.39 mmol/l). HbA1c also improved over time (mean decrease of 0.24% from baseline to first follow-up and mean decrease of 0.08% from baseline to second followup). Reported use of dyslipidaemia medications increased from 17% at baseline to 32% at the first follow-up and to 46% at the second follow-up visit. The improvements in lipid measures were noted despite increases in adiposity measures over the 6 year period.
Table 1.
Characteristics of CACTI Type 1 Diabetes Cohort
| Variable | Visit 0, n=652 | Visit 1, n=559 | Visit 2, n=543 | p value |
|---|---|---|---|---|
| Age (years) | 36.5±0.4 | 39.0±0.4 | 43.2±0.4 | NA |
| Type 1 diabetes duration (years) | 23.3±0.3 | 25.8±0.3 | 29.6±0.3 | NA |
| Time to follow-up (years) | NA | 2.5±0.4 | 6.2±0.6 | NA |
| Sex (male/female), % | 46%/54% | 46%/54% | 46%/54% | NA |
| Race/ethnicity (NHW), % | 94% | 94% | 94% | NA |
| HbA1c (%) | 7.98±0.05 | 7.74±0.05 | 7.90±0.05 | <0.0001 |
| TC (mmol/l) | 4.53±0.03 | 4.60±0.04 | 4.27±0.04 | <0.0001 |
| HDL (mmol/l) | 1.45±0.02 | 1.58±0.02 | 1.58±0.02 | <0.0001 |
| LDL (mmol/l) | 2.61±0.03 | 2.61±0.03 | 2.28±0.03 | <0.0001 |
| TGa (mmol/l) | 0.93 (0.28–7.54) | 0.75 (0.30–6.58) | 0.79 (0.28–3.92) | <0.0001 |
| Non-HDL (mg/l) | 3.08±0.03 | 3.00±0.03 | 2.69±0.04 | <0.0001 |
| Dyslipidaemia medications, yes/total, % | 113/652 (17%) | 180/559 (32%) | 250/543 (46%) | <0.0001 |
| BMI (kg/m2) | 26.2±0.2 | 26.5±0.2 | 26.9±0.2 | <0.0001 |
| Waist circumference (cm) | 85.1±0.5 | 85.2±0.5 | 88.7±0.5 | <0.0001 |
| VSF (cm3) | 10.3±0.03 | 10.5±0.03 | 10.6±0.02 | <0.0001 |
Results are presented as means±SE or count and frequency, except for ‘Time to follow-up’ (mean±SD)
p value, type 3 tests of fixed effects by visit in SAS Proc Mixed
n=603 with a baseline and either first or second visit
Geometric mean and range
NA, not applicable; NHW, non-Hispanic white; TC, total cholesterol; TG, triacylglycerols; VSF, visceral fat
The general pattern for reported dyslipidaemia medication use was that once participants started medications they continued on medications. Between the baseline and first follow-up visit, 85 additional participants were started on dyslipidaemia medications while 13 participants reported discontinuing dyslipidaemia medications. From the baseline to second follow-up visit 79 participants began and 13 discontinued dyslipidaemia medications. Of those on dyslipidaemia medications 97%, 95% and 93% were on statins at each visit.
In non-stratified models, there was a significantly different association of change in HbA1c with change in lipid outcomes (TC, LDL-c, and non-HDL-c, p<0.0001 for each HbA1c by dyslipidaemia medication use interaction term) depending on whether a participant reported use of dyslipidaemia medication. We therefore report the results of stratified mixed model analysis of the association of change in HbA1c to change in each lipid outcome (β-estimate ± SE and p value) in the basic and saturated models as shown in Table 2. Among those not on dyslipidaemia medications, statistically significant associations existed for the association between change in HbA1c and change in TC, LDL-c, TG, and non-HDL-c, but not HDL-c. In the basic model, for every 1% change in HbA1c the associated changes in lipids over 6 years were: TC +0.101 mmol/l (95% CI 0.050, 0.152, p=0.0001), HDL-c −0.20 mmol/l (95% CI −0.047, 0.007, p=0.15), LDL-c +0.103 mmol/l (95% CI 0.058, 0.148, p<0.0001), TG +0.052 mmol/l (95% CI 0.024, 0.081, p=0.0003), and non-HDL-c +0.129 mmol/l (95% CI 0.078, 0.180, p<0.0001). Similar associations existed in the saturated model (Table 2). In contrast, among those on dyslipidaemia medications, the association of change in HbA1c with change in lipid level was reduced by approximately one-half and was no longer statistically significant, though the direction of the effect was similar to persons not on medication. Figure 1 displays the association of change in HbA1c and change in lipid.
Table 2.
Longitudinal change in lipid per 1% point increase in HbA1c estimate
| Basic model β (95% CI) | p value | Saturated model β (95% CI) | p value | |
|---|---|---|---|---|
| NO lipid medications | ||||
| TC | 0.101 (0.050,0.152) | 0.0001 | 0.127 (0.072,0.182) | <0.0001 |
| HDL-c | −0.020 (−0.047,0.007) | 0.15 | −0.008 (−0.035,0.019) | 0.57 |
| LDL-c | 0.103 (0.058,0.148) | <0.0001 | 0.122 0.071,0.173) | <0.0001 |
| TGa | 0.052 (0.024,0.081) | 0.0003 | 0.043 (0.014,0.073) | 0.0004 |
| Non-HDL-c | 0.129 (0.078,0.180) | <0.0001 | 0.140 (0.085,0.195) | <0.0001 |
| YES lipid medications | ||||
| TC | 0.052 (−0.034,0.138) | 0.23 | 0.036 (−0.055,0.127) | 0.44 |
| HDL-c | −0.018 (−0.053,0.017) | 0.32 | −0.022 (−0.059,0.015) | 0.25 |
| LDL-c | 0.062 (−0.014,0.138) | 0.12 | 0.054 (−0.26,0.134) | 0.20 |
| TGa | 0.025 (−0.018,0.070) | 0.25 | 0.027 (−0.016,0.072) | 0.22 |
| Non-HDL-c | 0.072 (−0.008,0.152) | 0.09 | 0.065 (−0.021,0.151) | 0.14 |
Basic model includes sex, visit, race/ethnicity, diabetes duration, baseline HbA1c and baseline lipid (model-specific) and time-varying age, change in HbA1c, change in HbA1c × visit interaction
Saturated model includes variables included in the basic model plus time-varying BMI, waist circumference and visceral fat
Change in TG per 1% change in HbA1c from a starting level of 0.93 mmol/l
Fig. 1.
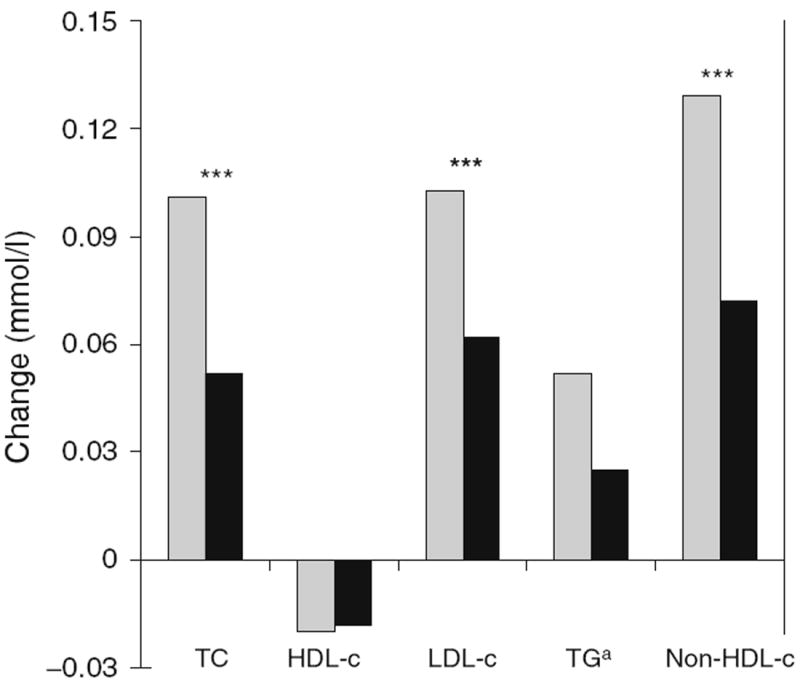
Longitudinal association of change in lipid per 1% change in HbA1c for the basic model (adjusted for sex, visit, race/ethnicity, diabetes duration, baseline HbA1c and baseline lipid [model-specific] and time-varying age, change in HbA1c, and change in HbA1c×visit interaction). Grey bars, participants receiving no lipid medication; black bars, participants receiving lipid medication. ***p<0.001 for dyslipidaemia medication status for change per 1% increase in HbA1c. aEstimated change in TG from a starting level of 0.93 mmol/l
In participants not on dyslipidaemia medications, the following variables were also significantly associated with lipid outcomes in the basic model: male sex (HDL-c, LDL-c, non-HDL-c), older age (TC, HDL-c, LDL-c and non- HDL-c) and in the saturated models larger waist (HDL-c) and higher visceral fat (HDL-c, TG and non-HDL-c). Among participants on dyslipidaemia medications the following variables were significantly associated with lipid outcomes in the basic model: race/ethnicity other than non-Hispanic white (TG), higher baseline HbA1c (all) and higher baseline lipid levels (all); and in the saturated models: male sex (HDL-c), older age (TC), higher baseline HbA1c (all) and higher baseline lipid levels (all).
In post hoc analyses, significant differences existed in the association ofHbA1c with lipids, and models stratified by sex are presented in Tables 3 and 4. In men, there was a stronger association of HbA1c with TC, LDL-c and non-HDL-c. HbA1c was not associated with HDL-c in either sex. In men, HbA1c was associated with TG in those not on dyslipidaemia medications in the basic but not the saturated model, and was of borderline significance in those on dyslipidaemia medications, whereas in women HbA1c was significantly associated with TG in those not on dyslipidaemia medications, but not in those on dyslipidaemia medications.
Table 3.
Longitudinal change in lipid per 1% point increase in HbA1c estimate in men
| Lipid | NO lipid medications
|
YES lipid medications
|
||||||
|---|---|---|---|---|---|---|---|---|
| Basic
|
Saturated
|
Basic
|
Saturated
|
|||||
| β (95% CI) | p value | β (95% CI) | p value | β (95% CI) | p value | β (95% CI) | p value | |
| TC | 0.220 (0.130, 0.310) | <0.0001 | 0.232 (0.132, 0.332) | <0.0001 | 0.141 (0.025, 0.257) | 0.02 | 0.121 (−0.002, 0.244) | 0.059 |
| HDL-c | 0.011 (−0.030, 0.052) | 0.62 | 0.017 (−0.030, 0.064) | 0.49 | −0.034 (−0.075, 0.007) | 0.11 | −0.037 (−0.080, 0.006) | 0.10 |
| LDL-c | 0.193 (0.109, 0.277) | <0.0001 | 0.214 (0.118, 0.310) | <0.0001 | 0.148 (0.047, 0.249) | 0.005 | 0.136 (0.024, 0.248) | 0.02 |
| TGa | 0.058 (0.005, 0.113) | 0.03 | 0.024 (−0.030, 0.083) | 0.40 | 0.056 (−0.005, 0.120) | 0.07 | 0.064 (0.002, 0.131) | 0.046 |
| Non-HDL-c | 0.217 (0.123, 0.311) | <0.0001 | 0.223 (0.117, 0.329) | <0.0001 | 0.172 (0.064, 0.280) | 0.002 | 0.161 (0.047, 0.275) | 0.007 |
Basic model includes sex, visit, race/ethnicity, diabetes duration, baseline HbA1c and baseline lipid (model specific) and time-varying age, change in HbA1c, change in HbA1c × visit interaction
Saturated model includes variables included in the basic model plus time-varying BMI, waist circumference and visceral fat
Change in TG per 1% change in HbA1c from a starting level of 0.93 mmol/l
Table 4.
Longitudinal change in lipid per 1% point increase in HbA1c estimate in women
| Lipid | NO lipid medications
|
YES lipid medications
|
||||||
|---|---|---|---|---|---|---|---|---|
| Basic
|
Saturated
|
Basic
|
Saturated
|
|||||
| β (95% CI) | p value | β (95% CI) | p value | β (95% CI) | p value | β (95% CI) | p value | |
| TC | 0.066 (0.001, 0.131) | 0.049 | 0.095 (0.024, 0.166) | 0.009 | −0.054 (−0.191, 0.083) | 0.44 | 0.057 (−0.084, 0.198) | 0.43 |
| HDL-c | −0.025 (−0.060, 0.10) | 0.16 | −0.010 (−0.045, 0.025) | 0.57 | −0.010 (−0.075, 0.055) | 0.77 | 0.12 (−0.057, 0.081) | 0.73 |
| LDL-c | 0.075 (0.018, 0.132) | 0.01 | 0.090 (0.031, 0.149) | 0.003 | −0.035 (−0.155, 0.085) | 0.57 | −0.037 (−0.159, 0.085) | 0.55 |
| TGa | 0.051 (0.018, 0.086) | 0.002 | 0.047 (0.013, 0.084) | 0.008 | −0.020 (−0.079, 0.043) | 0.52 | −0.006 (−0.064, 0.057) | 0.85 |
| Non-HDL-c | 0.101 (0.040, 0.162) | 0.001 | 0.112 (0.049, 0.175) | 0.0005 | −0.057 (−0.188, 0.074) | 0.40 | −0.050 (−0.181, 0.081) | 0.46 |
Basic model includes sex, visit, race/ethnicity, diabetes duration, baseline HbA1c and baseline lipid (model specific) and time-varying age, change in HbA1c, change in HbA1c×visit interaction
Saturated model includes variables included in the basic model plus time-varying BMI, waist circumference and visceral fat β-coefficients, 95% CI and p value for 1% change in HbA1c estimate
Change in TG per 1% change in HbA1c from a starting level of 0.93 mmol/l
Discussion
We found that over 6 years of follow-up an increase in glycaemia was positively associated with change in TC, LDL-c, TG and non-HDL-c in adults with type 1 diabetes who were not on dyslipidaemia medications. This strong association was not observed in patients who were on dyslipidaemia medications. Although glycaemic control is important for reduction of vascular complications, we believe these data suggest that the effect of dyslipidaemia treatment on lipids is stronger than the effect of glycaemia on lipids and, therefore, may obscure the effect of glucose control on lipids. Therefore, this suggests that both glucose control and treatment with dyslipidaemia medications (if lipids exceed treatment goals) in adults with type 1 diabetes should be emphasised to achieve lipid and cardiovascular health. Furthermore, given the magnitude of the association of change in HbA1c with change in lipids, for some patients with significantly elevated lipids it is likely that intensification of glycaemic control may be insufficient to achieve lipid treatment goals [7, 17].
In post hoc analyses, we found that the association of HbA1c with TC, LDL-c and non-HDL-c was of reduced magnitude in women as compared with men. HbA1c was not associated with HDL-c in either men or women, and the association of HbA1c with TG was significant only in women not on dyslipidaemia medications, whereas men had a more variable pattern. Sex-based differences in CVD risk in type 1 diabetes have been previously reported [18-20] and we have reported that insulin resistance was associated with a proatherogenic lipoprotein subfraction cholesterol distribution in women [21]. Further investigation of sexbased differences in CVD risk in type 1 diabetes is needed.
CACTI is an observational cohort, and use of dyslipidaemia medications increased from 17% to 46% over the mean 6 years of follow-up, demonstrating increased use of dyslipidaemia medications for lipid and cardiovascular health in type 1 diabetes. Not surprisingly, the fasting lipid profile improved over the study period despite increases in adiposity measures. In contrast, there was minimal average improvement in HbA1c over the study period. In the DCCT trial, TC, LDL-c and TG were significantly lower in the intensively treated arm than in the conventionally treated arm [10]. In addition, no participants were on statins at the end of the DCCT in 1993, while in 2004 in EDIC 33% and 34% in the intensive and conventional arms, respectively, were on statins [22]. The improved lipid profile in CACTI over time is likely due to increased use of dyslipidaemia medications, not improved glycaemic control.
Data clearly suggest that care for type 1 diabetes has improved in the past two decades, as evidenced by reduced rates of microvascular disease [23-25]; however, substantially less progress has been made in reduction of macrovascular disease rates [25-27], despite the DCCT/EDIC study showing that intensive glycaemic control over a mean of 6.5 years reduced CVD complications by 57% after a mean of 17 years of follow-up [22]. People with type 1 diabetes continue to have excess morbidity and mortality, with a dramatic increase in CVD mortality risk compared with people without diabetes [27].
To describe the clinical course of type 1 diabetes in the post-DCCT age of intensive insulin therapy, data from 161 participants of the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study (n=88 at the 2006 follow-up) were compared with those from the DCCT/EDIC Study [28]. The EDC reported a mean HbA1c similar to that of the conventional arm of the DCCT (9%) until EDC year 8 (1994–1996), when HbA1c fell by 0.5%, subsequent to publication of initial data from the DCCT demonstrating reduced microvascular complications with intensive control [28]. Subjects in the observational EDC reported a decrease in HbA1c from 9.0% to 8.3%, an increase in BMI from 24 to 28 kg/m2, and an increase in statin use from 1% to 38% from 1986–1988 to 2006, similar to the CACTI data. The authors emphasise that, based on these findings, current outcomes for people with type 1 diabetes should be much improved in comparison to cohorts who had onset of type 1 diabetes in the 1950s to 1970s.
In addition to glycaemic control, dyslipidaemia is another important CVD risk factor with extensive data to support its role as a target to improve cardiovascular health in people with type 1 diabetes [5]. Despite abundant data on the importance of controlling dyslipidaemia, adequate control of this CVD risk factor is frequently not achieved [9]. Our results add to those of the DCCT/EDIC and EDC studies by providing data on glycaemic and lipid control in a large observational cohort in the early 21st century. Moreover, we investigate the interplay of glycaemic control and dyslipidaemia medication use on the fasting lipid profile over a 6 year period in a large cohort of adults with type 1 diabetes. As the DCCT/EDIC study has shown that hyperglycaemia is a risk factor for hypertension [12], our data support a similar role for hyperglycaemia as a risk factor for a more atherogenic lipid profile; however, the association of glycaemia with lipids is attenuated by dyslipidaemia medication use in CACTI, whereas in the DCCT/EDIC study the association of intensive therapy with reduced incidence of hypertension remained significant in sensitivity analyses when accounting for use of antihypertensive medications [12].
While intensive treatment of glycaemia has been shown to reduce vascular complications in type 1 diabetes [22], there is also concern that intensive control is associated with weight gain, leading to adverse effects on blood pressure and lipids [29]. In the CACTI cohort, HbA1c was relatively stable, adiposity measures increased and lipids improved over time. How this improved lipid profile translates into changes in health outcomes remains an important future research objective. Limited clinical trial data exist on the effect of dyslipidaemia medications on lipids and outcomes in type 1 diabetes, but the Heart Protection Study included 615 participants with type 1 diabetes. Though the study was underpowered to detect an effect in subgroup analysis, the magnitude of the risk reduction was similar to that in type 2 diabetes [30], consistent with a subsequent meta-analysis [31].
As noted, CACTI is an observational cohort study and not the result of a randomised clinical trial; therefore our data have limitations to consider when applying these findings to clinical scenarios. While 8% of participants did not have at least one follow-up visit, minimal differences exist between those who did and did not follow up.We also did not measure the effect of change in diet and exercise on change in lipid levels in these analyses, although these effects would likely be captured in the adiposity measures, which were adjusted for in the analyses. Nonetheless, CACTI participants represent one of the largest cohorts of persons with type 1 diabetes followed over 6 years who have had detailed and repeated measures of glycaemia, lipids, dyslipidaemia medications and adiposity. Our analyses stratified by sex were post hoc and should be further investigated. Finally, sensitivity analyses were performed inwhich participants with serumcreatinine >2.0 mg/dl or microalbuminuria were removed from the analysis, and the associations of change in HbA1c with change in lipids were similar.
In conclusion, over a 6 year period in the CACTI cohort, change in glycaemia was associated with a concomitant change in TC, LDL-c, TG and non-HDL-c, which was not observed in the participants who were on dyslipidaemia medications. These data suggest that there is a statistically strong association between change in glycaemia and change in lipid levels; however, this association may not be as clinically relevant compared with the stronger effect of treatment with dyslipidaemia medications on lipid levels. Therefore, both glycaemic control and treatment with dyslipidaemia medications, when indicated, in adults with type 1 diabetes may be required to optimise lipid and cardiovascular health and improve patient outcomes. Further data are needed to inform clinical decisions on lipid lowering in type 1 diabetes.
Acknowledgments
Support for this study was provided by the National Institutes of Health National Heart Lung Blood Institute grant R01 HL61753, HL79611, and Diabetes Endocrinology Research Center Clinical Investigation Core P30 DK57516. The study was performed at the Adult General Clinical Research Center at University of Colorado Denver supported by grant no. NIH-M01-RR00051, at the Barbara Davis Center for Childhood Diabetes and at Colorado Heart Imaging Center in Denver (CO, USA). D. M. Maahs was supported by grant no. K23 DK075360.
Abbreviations
- CACTI
Coronary Artery Calcification in Type 1 Diabetes
- CAD
Coronary artery disease
- CVD
Cardiovascular disease
- DCCT
Diabetes Control and Complications Trial
- EDC
Epidemiology of Diabetes Complications
- EDIC
Epidemiology of Diabetes Control
- HDL-c
HDL-cholesterol
- LDL-c
LDL-cholesterol
- TC
Total cholesterol
- TG
Triacylglycerols
Footnotes
The results presented in this paper have not been published previously in whole or part, except in abstract format (American Diabetes Association Scientfic Sessions, Orlando, FL, USA, 2010).
Duality of interest D. M. Maahs has research support from Merck for a separate trial of dyslipidaemia medications in adolescents with type 1 diabetes. None of the other authors has a duality of interest associated with this manuscript.
Contributor Information
D. M. Maahs, Email: David.Maahs@ucdenver.edu, Barbara Davis Center for Childhood Diabetes, University of Colorado Denver, Mail Stop A140, PO Box 6511, Aurora, CO 80045, USA; Department of Epidemiology, Colorado School of Public Health, University of Colorado Denver, Aurora, CO, USA; The Children’s Hospital Denver, Aurora, CO, USA.
L. G. Ogden, Colorado School of Public Health, Department of Biostatistics and Informatics, University of Colorado Denver, Aurora, CO, USA
D. Dabelea, Department of Epidemiology, Colorado School of Public Health, University of Colorado Denver, Aurora, CO, USA
J. K. Snell-Bergeon, Barbara Davis Center for Childhood Diabetes, University of Colorado Denver, Mail Stop A140, PO Box 6511, Aurora, CO 80045, USA
S. R. Daniels, Department of Epidemiology, Colorado School of Public Health, University of Colorado Denver, Aurora, CO, USA The Children’s Hospital Denver, Aurora, CO, USA.
R. F. Hamman, Department of Epidemiology, Colorado School of Public Health, University of Colorado Denver, Aurora, CO, USA
M. Rewers, Barbara Davis Center for Childhood Diabetes, University of Colorado Denver, Mail Stop A140, PO Box 6511, Aurora, CO 80045, USA Department of Epidemiology, Colorado School of Public Health, University of Colorado Denver, Aurora, CO, USA.
References
- 1.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snell-Bergeon JK, Hokanson JE, Jensen L, et al. Progression of coronary artery calcification in type 1 diabetes: the importance of glycemic control. Diab Care. 2003;26:2923–2928. doi: 10.2337/diacare.26.10.2923. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125:177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Standards of medical care in diabetes—2008. Diab Care. 2008;31(Suppl 1):S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 6.Maahs DM, Wadwa RP, Bishop F, Daniels SR, Rewers M, Klingensmith GJ. Dyslipidemia in youth with diabetes: to treat or not to treat? J Pediatr. 2008;153:458–465. doi: 10.1016/j.jpeds.2008.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diab Care. 2007;30:162–172. doi: 10.2337/dc07-9917. [DOI] [PubMed] [Google Scholar]
- 8.Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diab Care. 2008;31:811–822. doi: 10.2337/dc08-9018. [DOI] [PubMed] [Google Scholar]
- 9.Wadwa RP, Kinney GL, Maahs DM, et al. Awareness and treatment of dyslipidemia in young adults with type 1 diabetes. Diab Care. 2005;28:1051–1056. doi: 10.2337/diacare.28.5.1051. [DOI] [PubMed] [Google Scholar]
- 10.The Diabetes Control and Complications Trial (DCCT) Research Group. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol. 1995;75:894–903. doi: 10.1016/s0002-9149(99)80683-3. [DOI] [PubMed] [Google Scholar]
- 11.Purnell JQ, Marcovina SM, Hokanson JE, et al. Levels of lipoprotein(a), apolipoprotein B, and lipoprotein cholesterol distribution in IDDM. Results from follow-up in the Diabetes Control and Complications Trial. Diabetes. 1995;44:1218–1226. doi: 10.2337/diab.44.10.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Boer IH, Kestenbaum B, Rue TC, et al. Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch Intern Med. 2008;168:1867–1873. doi: 10.1001/archinternmed.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maahs DM, Kinney GL, Wadwa P, et al. Hypertension prevalence, awareness, treatment, and control in an adult type 1 diabetes population and a comparable general population. Diab Care. 2005;28:301–306. doi: 10.2337/diacare.28.2.301. [DOI] [PubMed] [Google Scholar]
- 14.Dabelea D, Kinney G, Snell-Bergeon JK, et al. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003;52:2833–2839. doi: 10.2337/diabetes.52.11.2833. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–503. [PubMed] [Google Scholar]
- 16.Hainline A, Jr, Miller DT, Mather A. The Coronary Drug Project. Role and methods of the Central Laboratory. Control Clin Trials. 1983;4:377–387. doi: 10.1016/0197-2456(83)90023-5. [DOI] [PubMed] [Google Scholar]
- 17.De Backer G, Ambrosioni E, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2003;24:1601–1610. doi: 10.1016/s0195-668x(03)00347-6. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd CE, Kuller LH, Ellis D, Becker DJ, Wing RR, Orchard TJ. Coronary artery disease in IDDM. Gender differences in risk factors but not risk. Arterioscler Thromb Vasc Biol. 1996;16:720–726. doi: 10.1161/01.atv.16.6.720. [DOI] [PubMed] [Google Scholar]
- 19.Colhoun HM, Rubens MB, Underwood SR, Fuller JH. The effect of type 1 diabetes mellitus on the gender difference in coronary artery calcification. J Am Coll Cardiol. 2000;36:2160–2167. doi: 10.1016/s0735-1097(00)00986-4. [DOI] [PubMed] [Google Scholar]
- 20.Laing SP, Swerdlow AJ, Slater SD, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia. 2003;46:760–765. doi: 10.1007/s00125-003-1116-6. [DOI] [PubMed] [Google Scholar]
- 21.Maahs DM, Hokanson JE, Wang H, et al. Lipoprotein subfraction cholesterol distribution is proatherogenic in women with type 1 diabetes and insulin resistance. Diabetes. 2010;59:1771–1779. doi: 10.2337/db09-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maahs DM, Rewers M. Editorial: Mortality and renal disease in type 1 diabetes mellitus—progress made, more to be done. J Clin Endocrinol Metab. 2006;91:3757–3759. doi: 10.1210/jc.2006-1730. [DOI] [PubMed] [Google Scholar]
- 24.Hovind P, Tarnow L, Rossing K, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diab Care. 2003;26:1258–1264. doi: 10.2337/diacare.26.4.1258. [DOI] [PubMed] [Google Scholar]
- 25.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55:1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 26.Libby P, Nathan DM, Abraham K, et al. Report of the National Heart, Lung, and Blood Institute–National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation. 2005;111:3489–3493. doi: 10.1161/CIRCULATIONAHA.104.529651. [DOI] [PubMed] [Google Scholar]
- 27.Rewers M. Why do people with diabetes die too soon? More questions than answers. Diab Care. 2008;31:830–832. doi: 10.2337/dc08-0245. [DOI] [PubMed] [Google Scholar]
- 28.Nathan DM, Zinman B, Cleary PA, et al. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005) Arch Intern Med. 2009;169:1307–1316. doi: 10.1001/archinternmed.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial. JAMA. 1998;280:140–146. doi: 10.1001/jama.280.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5,963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 31.Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]


