Abstract
Objectives:
To measure intensive care unit (ICU) admission, intubation, decompressive craniotomy, and outcomes at discharge in a large population-based study of children with ischemic and hemorrhagic stroke.
Methods:
In a retrospective study of all children enrolled in a Northern Californian integrated health care plan (1993–2003), we identified cases of symptomatic childhood stroke (age >28 days through 19 years) from inpatient and outpatient electronic diagnoses and radiology reports, and confirmed them through chart review. Data regarding stroke evaluation, management, and outcomes at discharge were abstracted. Intensive care unit (ICU) admission, intubation, and decompressive neurosurgery rates were measured, and multivariate logistic regression was used to identify predictors of critical care usage and outcomes at discharge.
Results:
Of 256 cases (132 hemorrhagic and 124 ischemic), 61% were admitted to the ICU, 32% were intubated, and 11% were treated with a decompressive neurosurgery. Rates were particularly high among children with hemorrhagic stroke (73% admitted to the ICU, 42% intubated, and 19% received a decompressive neurosurgery). Altered mental status at presentation was the most robust predictor for all 3 measures of critical care utilization. Neurologic deficits at discharge were documented in 57%, and were less common after hemorrhagic than ischemic stroke: 48% vs 66% (odds ratio 0.5, 95% confidence interval 0.3–0.8). Case fatality was 4% overall, 7% among children admitted to the ICU, and was similar between ischemic and hemorrhagic stroke.
Conclusions:
ICU admission is frequent after childhood stroke and appears to be justified by high rates of intubation and surgical decompression.
Pediatric stroke occurs in 2–13/100,000 children annually,1,2 and while hospital case series have reported high rates of neurologic deficits and case fatality,3,4 data from population-based cohorts are limited. After a large stroke, neurologic deterioration may progress over several days from swelling, intracranial hypertension, and secondary brain injury. Although adult neurocritical care after a stroke has received increasing attention, little has been reported regarding critical care management practices of childhood stroke. However, children may have several reasons to require critical care management of elevated intracranial pressures: 1) children have a high proportion of hemorrhagic strokes,1,5 2) ischemic strokes in children are predominantly large-vessel,6,7 and 3) children lack the cerebral atrophy of aging that provides space to accommodate mass effect. We hypothesized that the frequency of ICU admission and interventions such as intubation and surgical decompression would be high after pediatric stroke, even in a population-based cohort. The ability to predict how often and which childhood strokes are at highest risk of requiring critical care interventions could be lifesaving.
The objective of this study was to determine rates and clinical predictors of critical care utilization—ICU admission, intubation, and decompressive craniotomy—in a population-based cohort of children with ischemic and hemorrhagic stroke. Because ICU admission could reflect either a true need for critical care or physician discomfort with an uncommon diagnosis, we also assessed correlations with case fatality and discharge neurologic deficits as measures of disease severity.
METHODS
Study design and setting.
This study was a cross-sectional analysis of critical care usage at the time of the index strokes within the Kaiser Pediatric Stroke Study (KPSS), a retrospective cohort study of childhood stroke. The KPSS study population included all 2.3 million children from birth through 20 years of age enrolled in the Kaiser Permanente Medical Care Program (KPMCP) from January 1993 through December 2003. KPMCP is an integrated health care delivery system that provides care to roughly one-third of the population in Northern California. Because KPMCP maintains extensive electronic records and provides comprehensive care, it is an ideal setting for a population-based study of critical care utilization after a relatively uncommon event such as pediatric stroke. Medical records from out-of-plan facilities for Kaiser patients are also generally available in the chart as a part of the Kaiser medical record. Within this population, KPSS identified cases of children diagnosed with symptomatic stroke by methods described below and in prior reports.1,7 For the purposes of this analysis, neonatal strokes (<28 days of life) were excluded because many strokes that occur at birth are identified months later and therefore have different care requirements.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the institutional review boards of the KPMCP Division of Research (Oakland, CA) and the University of California, San Francisco. Since there was no direct contact with patients and this retrospective study involved minimal risk, written informed patient consent was waived by both institutional review boards.
Case ascertainment.
Cases of symptomatic stroke were ascertained through electronic searches of KPMCP patient databases for hospital discharge diagnosis (coded by a medical records analyst) and outpatient diagnosis (coded by physicians), and keyword searches of all electronic head imaging reports (MRI and CT). Potential cases were chart reviewed, with independent confirmation of the stroke diagnosis by 2 neurologists and arbitration of disagreements by a third. The inclusion criteria for stroke were 1) documented clinical presentation consistent with stroke, such as a sudden onset focal neurologic deficit, headache, or seizure, and 2) CT or MRI scan showing a focal ischemic infarct or hemorrhage in a location and of a maturity consistent with the neurologic signs and symptoms. Ischemic strokes included both arterial ischemic stroke and venous sinus thrombosis, while hemorrhagic strokes included intracerebral, intraventricular, and subarachnoid hemorrhage (but not subdural or epidural hemorrhage). Because trauma is an important cause of intracerebral and subarachnoid hemorrhage in children, we included cases of traumatic hemorrhagic stroke, as long as the hemorrhage was symptomatic. Cases were excluded if the stroke occurred outside of the study period.
Data abstraction.
Using a standardized protocol, data were abstracted by a single pediatric nurse medical records analyst from all available medical records from all KPMCP facilities and out-of-plan facilities. All relevant records were reviewed by a single pediatric vascular neurologist (H.J.F.) to classify the stroke type (hemorrhagic or ischemic) and confirm abstracted data. Clinical data included age at the time of the stroke, gender, and race. ICU admission was admission to the ICU during the acute stroke hospitalization. Intubation was defined as mechanical ventilation in the ICU, and did not include intubation for a procedure under sedation. Decompressive neurosurgery was defined as either craniotomy for removal of a hematoma or infarcted brain or craniectomy (temporary removal of part of the skull), with the intent of relieving raised intracranial pressure. Craniotomy performed purely for surgical excision of a vascular lesion was not included in this definition. Outcome at discharge was defined as status at the end of stroke admission, and was recorded as case fatality (all cause deaths by the end of stroke admission), persistent deficit (any residual deficit documented in the medical record), or normal. Stroke presentation was presence of symptoms recorded by a nurse or physician of altered mental status, speech abnormalities, hemiparesis, gait abnormalities, headache, or seizure. Altered mental status was defined as an altered level of alertness, obtundation, or coma. Premorbid condition was a composite variable that indicated a prior diagnosis of cardiac disease, hematologic disease, vascular disease, and rheumatologic or autoimmune disease at the time of the stroke.
Data analysis.
Dichotomous variables were compared using χ2 or Fisher exact test. Univariate and multivariable logistic regression models were used to identify predictors of critical care. Our primary outcome measures were ICU admission, intubation, and decompressive neurosurgery. Because their secondary complications and neurocritical care requirements may differ, we reported frequencies of critical care usage stratified by ischemic vs hemorrhagic stroke. Stroke type was also analyzed as a covariate in our logistic regression models. Additional predictors were age, gender, race, symptoms at stroke presentation, premorbid condition, inpatient status at the time of the stroke, and length of time until stroke diagnosis. To indicate number of cases with missing data, denominators are reported for each outcome and vary slightly. Time to diagnosis was included as a predictor to control for its potential effect on the outcome of ICU admission: children with a delayed diagnosis of stroke may be less likely to be admitted to an ICU simply because sufficient time has passed during which they were stable. Time to stroke diagnosis was treated as a binary variable, with diagnosis either on or not on the date of the stroke. For multivariable analyses, we included all predictors significant to a p = 0.05 level on univariate screening, as well as age, race, and gender. We performed tests for interaction on significant predictors in the multivariable model to determine independence. A sensitivity analysis of ICU admission was performed excluding children who were already in the ICU prior to stroke. Additionally, a sensitivity analysis was performed excluding traumatic hemorrhagic stroke, because these children might be admitted to the ICU for extracranial trauma. Logistic regression was used to analyze ICU admission, intubation, and decompressive neurosurgery as predictors of neurologic deficit or death at discharge. These analyses were adjusted for hemorrhagic stroke, but additional multivariable analysis was not performed because of colinearity of predictors. α Was set at 0.05 except for tests for interaction (set at 0.10).
RESULTS
Descriptive summary.
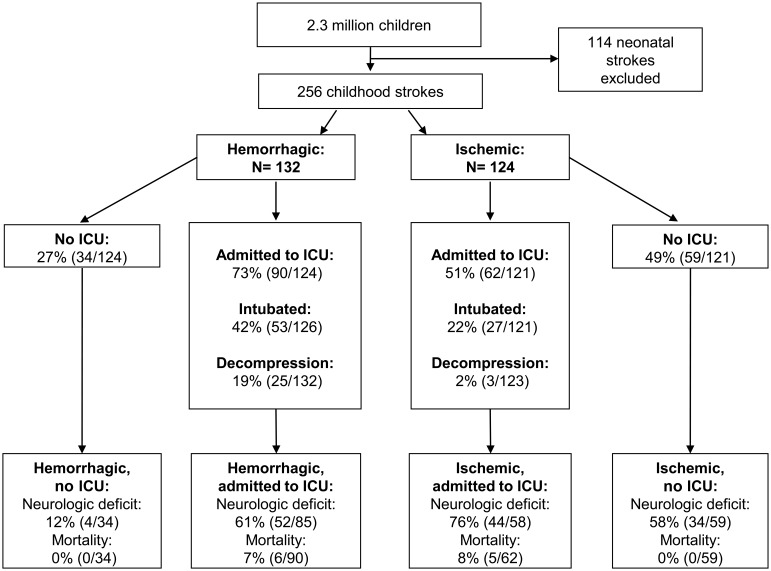
Within a population of 2.3 million children, 256 childhood stroke cases (132 hemorrhagic and 124 ischemic) were identified and included in our analysis. The median age was 13 years with an interquartile range of 7 to 17 years. Demographic and clinical characteristics are described in table 1. Overall, 62% of the children were admitted to the ICU, 32% were intubated, and 11% were treated with a decompressive neurosurgery. There were no temporal changes in pediatric stroke ICU utilization across the decade (data not shown). Twenty-seven children (10%) were admitted to the hospital and 10 children (4%) were in the ICU when the stroke occurred. Compared to children with ischemic stroke, those with hemorrhagic stroke were more likely to be admitted to the ICU (p = 0.001), intubated (p = 0.001), and treated with decompressive surgery (p < 0.001) (figure). Demographics of children with missing outcomes did not differ from the overall cohort (data not shown).
Table 1.
Demographics and clinical characteristics of childhood stroke in a population of children enrolled in Kaiser Permanente Medical Care Program, 1993–2003
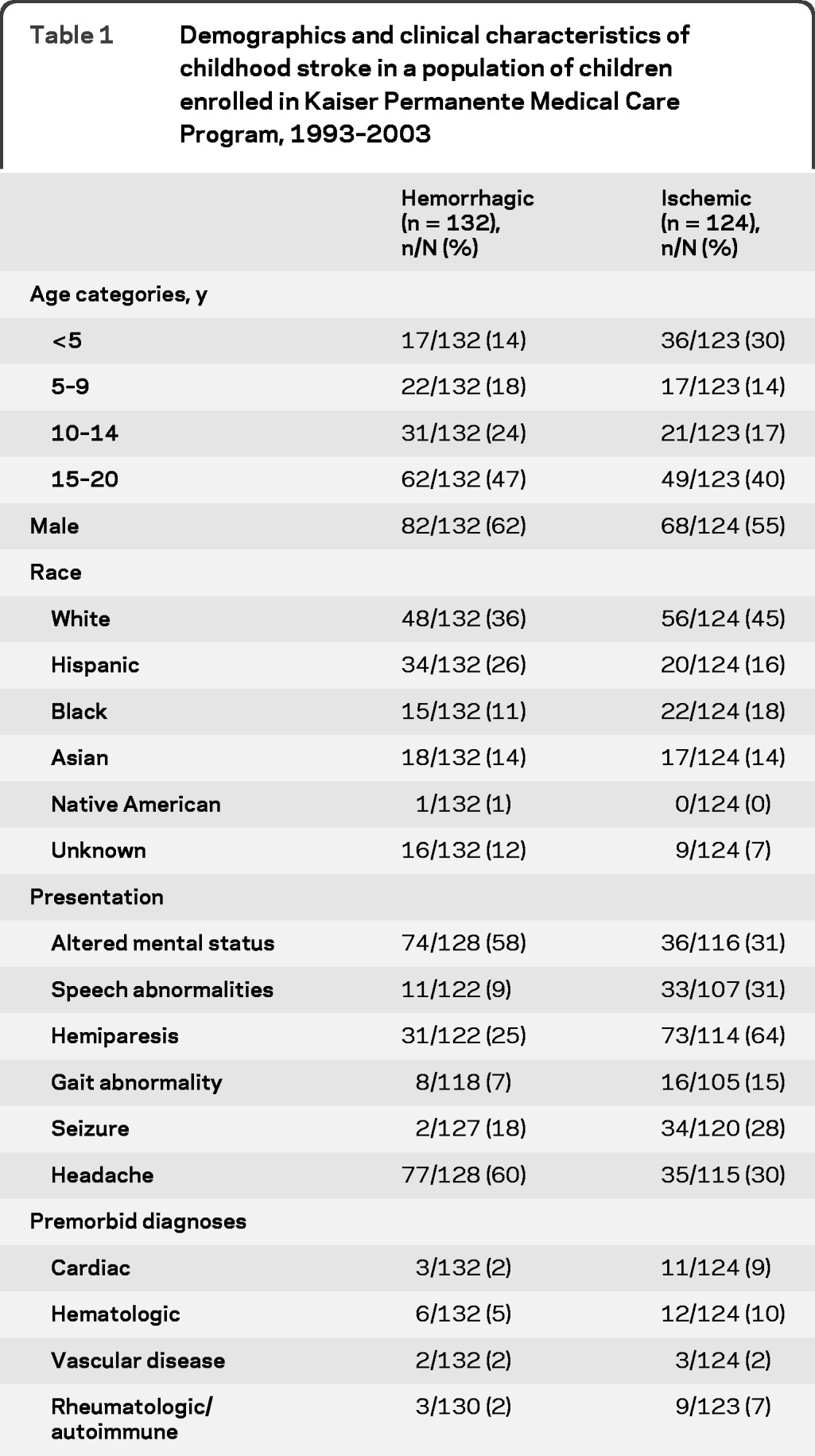
Figure. Intensive care unit (ICU) admission, intubation, surgical decompression, and short-term outcomes for children with strokes in Kaiser Permanente Medical Care Program, 1993–2003.
Predictors of ICU admission.
Data regarding ICU admission were available for 245 and missing for 11 children. Younger age, hemorrhagic stroke, altered mental status on presentation, lack of a premorbid condition, and early diagnosis of stroke were predictors of ICU admission on univariate analysis. The risk factors independently associated with ICU admission after multivariable analysis were younger age and presentation with altered mental status (table 2). Analyzed as a continuous variable, the odds of ICU admission after stroke decreased by 7% for each 1-year increase in age. If children already in the ICU prior to stroke were excluded from the analysis, having a premorbid diagnosis was protective from ICU admission (odds ratio [OR] 0.3, 95% confidence interval [CI] 0.1–0.8) but the other covariates were largely unchanged. Because traumatic hemorrhagic strokes might be admitted to the ICU for extracranial trauma, the multivariable analysis was also repeated excluding traumatic hemorrhagic stroke (n = 37); however, hemorrhagic stroke still predicted ICU admission (adjusted OR 2.9; 95% CI 1.3–6.5).
Table 2.
Univariate and multivariate risks for ICU admission after stroke in children enrolled in Kaiser Permanente Medical Care Program, 1993–2003
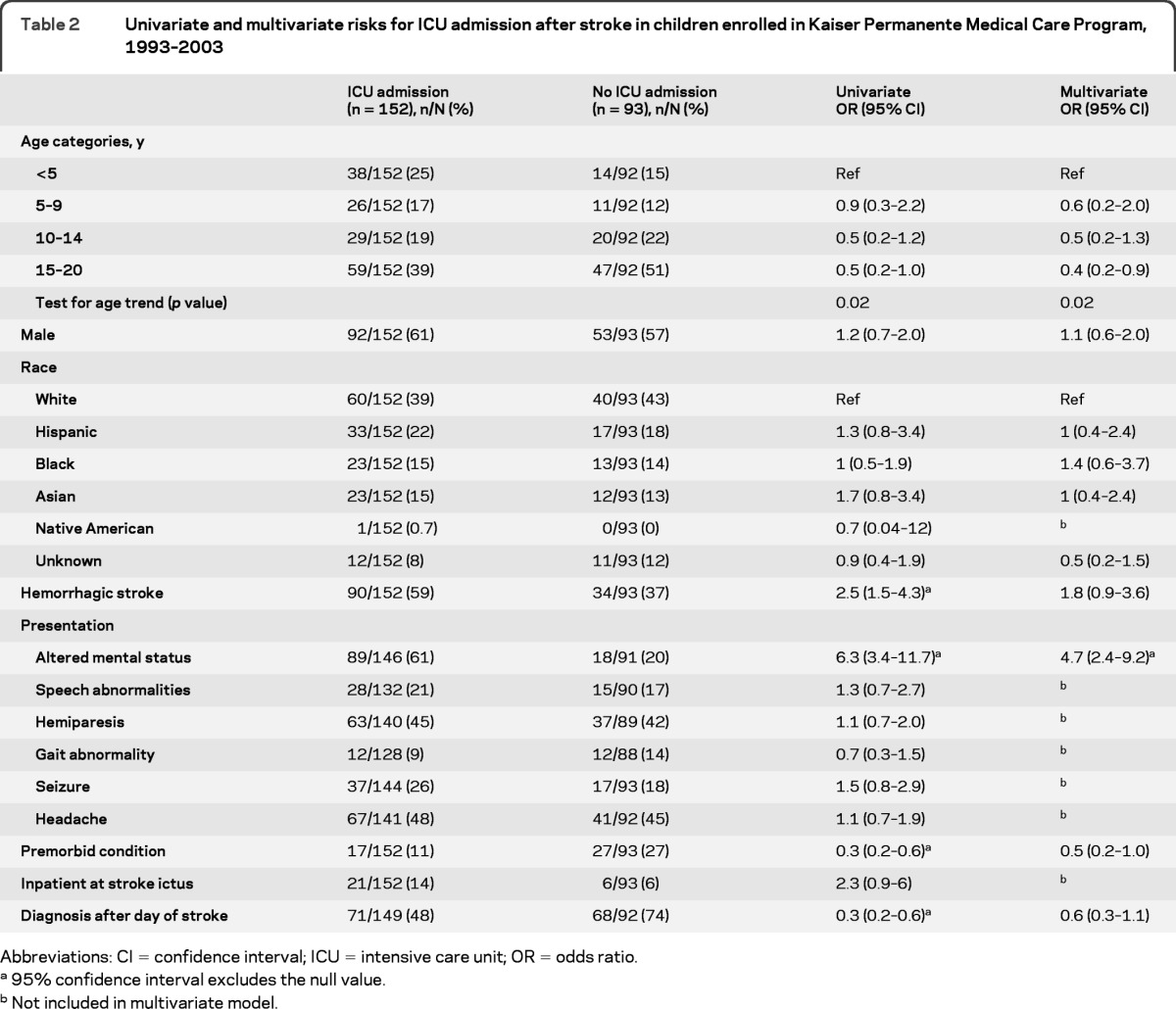
Abbreviations: CI = confidence interval; ICU = intensive care unit; OR = odds ratio.
95% confidence interval excludes the null value.
Not included in multivariate model.
Predictors of intubation.
Data regarding intubation were available for 247 and missing for 9 children. Hemorrhagic stroke, presentation with altered mental status, presentation with speech abnormalities, inpatient status at stroke ictus, and earlier stroke diagnosis all predicted intubation on univariate analysis (table 3). On multivariate analysis, presentation with altered mental status and inpatient status at the time of the stroke remained associated with intubation.
Table 3.
Univariate and multivariate risks of intubation after stroke in children enrolled in Kaiser Permanente Medical Care Program, from 1993–2003
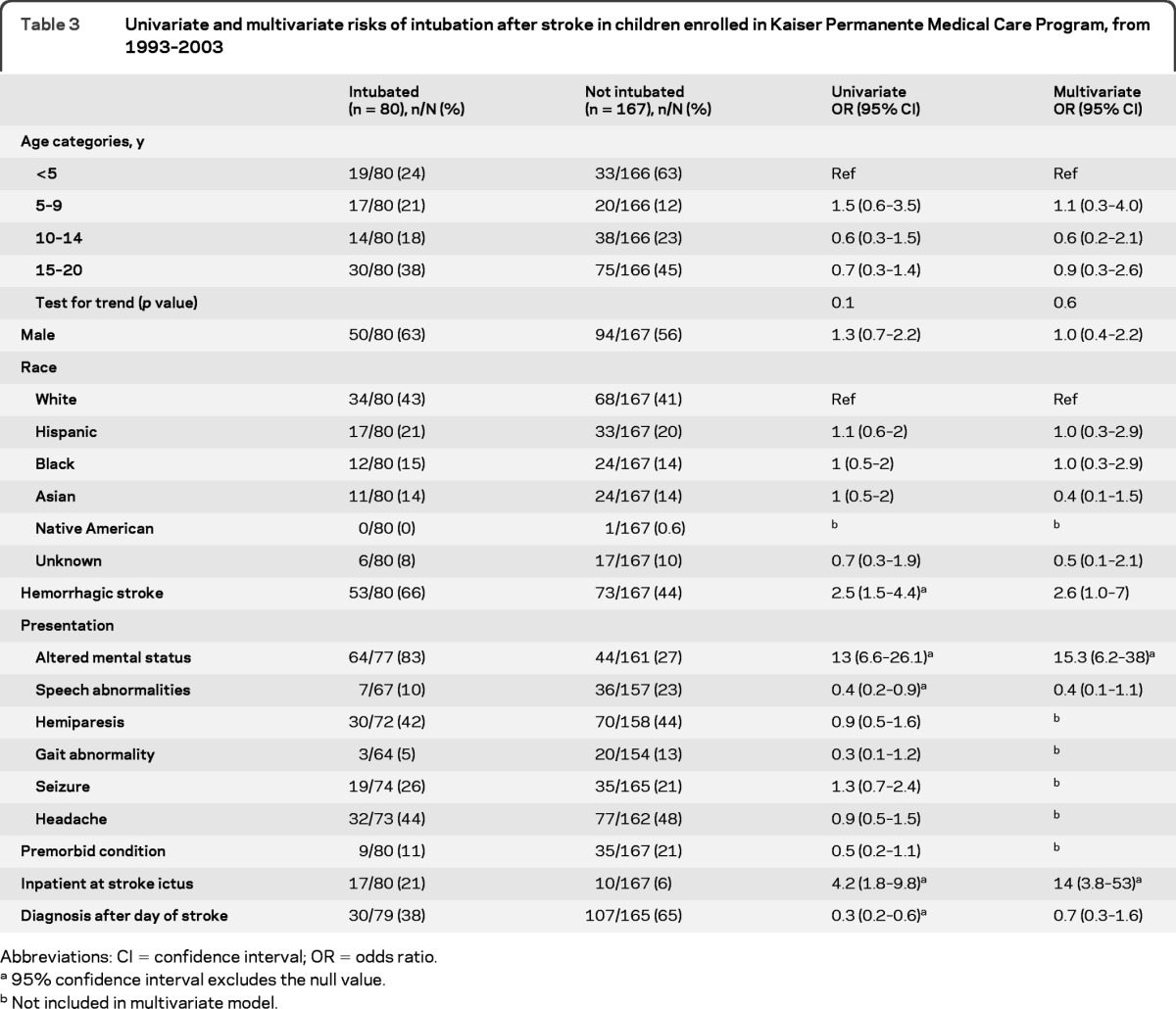
Abbreviations: CI = confidence interval; OR = odds ratio.
95% confidence interval excludes the null value.
Not included in multivariate model.
Predictors of decompressive neurosurgery.
Data regarding decompressive neurosurgery were available for 255 and missing in only 1 child. Hemorrhagic stroke, altered mental status, headache, and earlier diagnosis all predicted decompressive neurosurgery on univariate analysis (table 4). After adjustment in our multivariable analysis, children with hemorrhagic stroke were 5-fold more likely to undergo neurosurgical decompression compared to children with ischemic stroke. Altered mental status and headache also predicted decompressive neurosurgery on multivariable analysis. The median time to surgery was 1 day poststroke, with a range of 0 to 10 days (interquartile range 0–3 days). Among hemorrhagic stroke cases, decompressive neurosurgeries were performed more frequently in children who had intraparenchymal hemorrhage (24%, 24/99), compared to 3% (1/33) of children who had only subarachnoid or intraventricular blood (OR 10, 95% CI 1.3–79). Additionally, children with a nontraumatic hemorrhage received decompressive neurosurgery more frequently (24%, 23/95) than children with traumatic hemorrhage (5%, 2/37; OR 5.6, 95% CI 1.2–25).
Table 4.
Univariate and multivariate risks of neurosurgical decompression after stroke in children enrolled in Kaiser Permanente Medical Care Program, 1993–2003
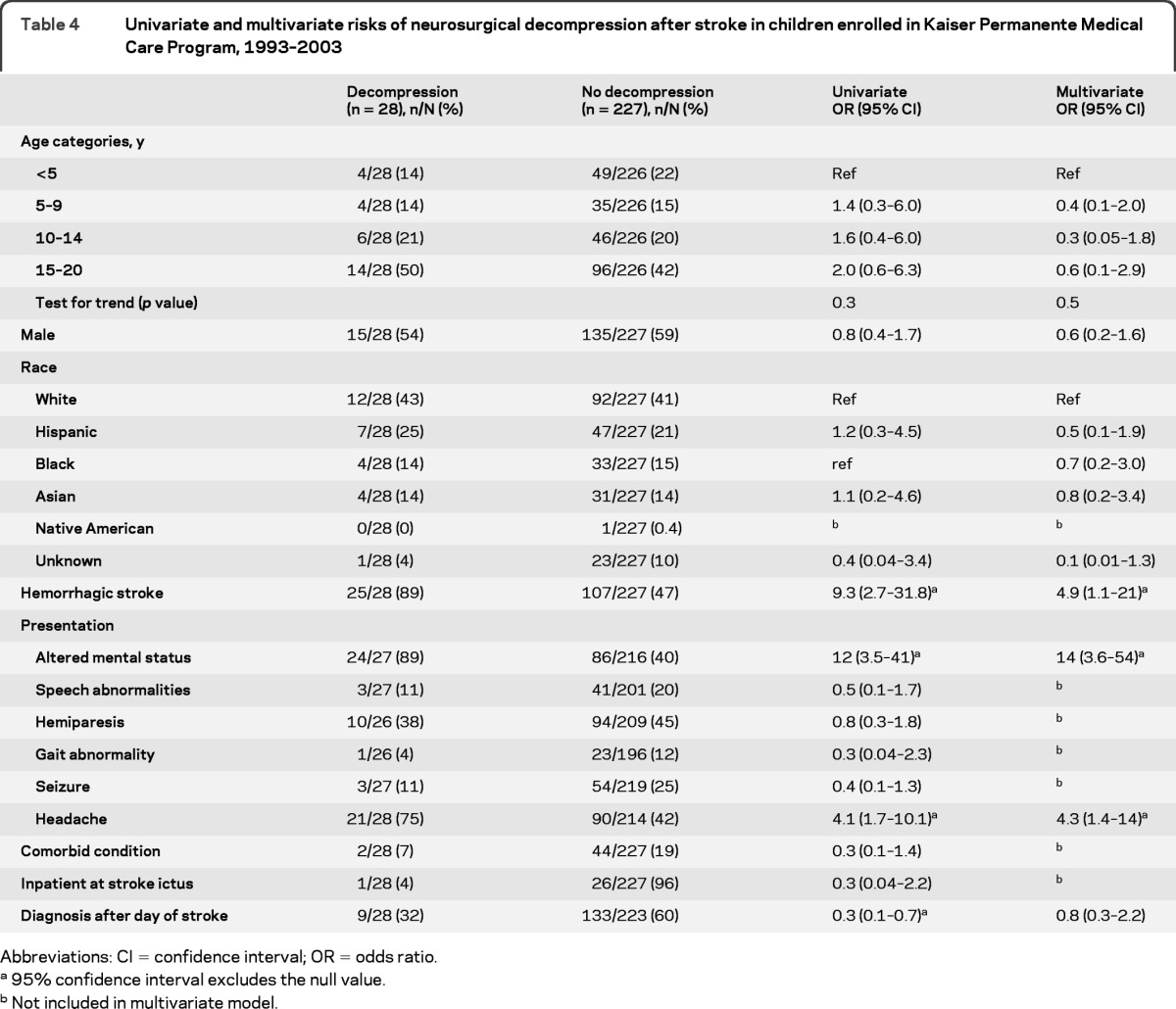
Abbreviations: CI = confidence interval; OR = odds ratio.
95% confidence interval excludes the null value.
Not included in multivariate model.
Markers of illness severity.
The median length of ICU admission for a child with a stroke was 4 days (range 0–70). Children with hemorrhagic stroke had longer ICU admissions, with a median of 5 days compared to 3 for ischemic stroke (p = 0.02, Wilcoxon rank-sum). Median total length of stay was 6 days (range 0–182), with no significant difference between stroke types.
Neurologic deficits at discharge were documented in 57% of children with stroke, and were less common after hemorrhagic than ischemic stroke: 48% vs 66% (OR 0.5, 95% CI 0.3–0.8). Children with ICU admission were 4-fold more likely to have neurologic deficits at discharge even after adjustment for hemorrhagic stroke: 67% vs 41% of those not admitted to the ICU (adjusted OR 4.4, 95% CI 2.4–8.2). Intubation (OR 5.9, 95% CI 3–12) and decompressive surgery (OR 2.6, 95% CI 1.02–6.4) also predicted deficits at discharge (adjusted for hemorrhagic stroke).
Twenty-two children were not admitted to the hospital after their stroke diagnosis. Of these, 12 presented with headache or seizure; in 20, stroke was not diagnosed on the day of the event. Median time to diagnosis for those not admitted to the hospital was 12 days.
Overall case fatality was 4% (95% CI 1.8%–6.7%), and was not different between children with ischemic vs hemorrhagic stroke (p = 0.8). Children admitted to the ICU had a 7% (n = 11) case fatality. Six deaths were attributable to the stroke as the primary cause. The other 5 deaths were attributed to trauma (n = 2), cancer (n = 2), and septic shock (n = 1). Case fatalities were higher in children treated with intubation (10/80 vs 1/167 not intubated, OR 23.7, 95% CI 3–188) and decompressive surgery (4/28 vs 7/227 without neurosurgery, OR 5.2, 95% CI 1.4–19). Both inpatient status (OR 3, 95% CI 1.1–8.5) and ICU admission (OR 6.6, 95% CI 1.3–34.5) prior to the stroke were also associated with case fatality.
DISCUSSION
In the United States, an estimated 63%8 of adult stroke patients are admitted to an ICU. In the pediatric stroke literature, reports describing therapeutic interventions have focused on antithrombotic treatment for acute ischemic stroke,3,9,10 but published rates regarding other critical care has been limited. American Heart Association clinical guidelines for management of stroke in infants and children11 report little evidence regarding critical care management. A single recent study examined pediatric ischemic and hemorrhagic stroke admissions through the Kids Inpatient Database, and estimated that 1/3 of children received any type of “intensive care” (defined as mechanical ventilation, advanced monitoring, or blood product administration).12 In our population-based Northern Californian cohort, children with stroke had high rates of critical care utilization: ICU admission (61%), intubation (32%), and decompressive neurosurgery (11%), particularly among children with hemorrhagic stroke. Altered mental status at presentation was the most robust predictor, independently associated with of all 3 of our markers of critical care utilization.
The overall acute case fatality after stroke in our study was 4%, and 7% among those admitted to the ICU. For comparison, published case fatality rates for overall medical admissions to pediatric ICUs in the United Kingdom,13 Sweden,14 and the Netherlands15 range from 2% to 5%. Prior hospital series of childhood stroke have reported case fatality rates of 2%–9% for ischemic stroke3,12,16 and 5%–34% for hemorrhagic stroke,12,17,18 but have not stratified outcomes of strokes admitted for critical care. Our study validates that even in a population-based cohort without referral bias, childhood stroke is a highly morbid disease.
Frequent ICU admission after childhood stroke appears to be justified by high rates of intubation and surgical decompression. While the high critical care utilization we observed could reflect either severity of illness or medical providers' discomfort with an uncommon diagnosis, our findings suggest that children with stroke have true critical care needs. In adult stroke studies, intubated patients have been shown to have poorer prognosis19 and high mortality.20 Similarly, all 3 critical care usage measures in our study were associated with neurologic deficits at discharge and death after childhood stroke. In other words, children with acute stroke are often critically ill.
In contrast to hemorrhagic stroke, relatively few of the children with ischemic stroke received neurosurgical decompression. However, practices regarding decompressive neurosurgery may have changed since our data collection (through 2003). Historically, decompressive surgery was a controversial treatment for refractory intracranial hypertension in ischemic stroke, and the infrequency in our study may reflect that controversy. Since 2007, pooled analysis of 3 trials of surgical decompression for malignant middle cerebral artery syndrome (the DECIMAL, DESTINY, and HAMLET trials) have shown a survival benefit compared to conservative medical treatment,21 and younger age predicted better functional outcome in a 2004 case series.22 Our study may underestimate the current frequency of decompressive neurosurgeries for treatment of refractory intracranial hypertension after childhood ischemic stroke.
In adults, the neurocritical care literature suggests that patients with both hemorrhagic23 and ischemic strokes24 have better outcomes when they are cared for by specialized neurocritical care teams. Neurocritical care fellowship training has emerged to manage adult neurologic emergencies. Neonatal neurocritical care has also recently emerged because of the specialized needs of critically ill newborns with brain injury.25,26 Now, to address the needs of older children with acute brain injury, some child neurologists and pediatric intensivists have called for the development of pediatric neurocritical care as a subspecialty.26–28 It is unknown whether specialized pediatric neurocritical care would improve childhood stroke outcomes, but the high rates of critical care utilization and poor outcomes in our cohort suggest that research focused on the critical care management of childhood stroke would be vital to the care of the majority of these patients and support a need for further studies in this area.
Our study was limited in several ways. First, we could only measure critical care utilization, and make inferences about critical care needs. The decision to admit a patient to the ICU, intubate, or take a patient to surgery is somewhat subjective, and different providers will have different thresholds for these interventions. Secondly, our stroke cohort included cases from 1993 through December 2003, and may not reflect recent changes in pediatric stroke critical care. However, compared to studies from hospital case series or registry data, our population-based study cohort should tend to include less severe cases, such as the 22 children who were not admitted to the hospital. If these cases had been excluded from the study, our measures of intensive care would appear to be even greater. Even so, children with stroke in our study still appear to be frequently critically ill with a high critical care usage. Thus far, critical care for childhood stroke has received less attention in comparison to its adult counterpart. This study is a novel assessment of childhood stroke care and is a step toward recognizing the importance of optimizing critical care after pediatric stroke. A prospective assessment of critical care needs in future pediatric stroke studies could help identify targets for improving outcomes. Further studies are needed to establish best practices for stroke care in the pediatric ICU setting, and determine whether specialized pediatric neurocritical care services might improve outcomes in children with stroke.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Barbara Rowe for data abstraction and management.
GLOSSARY
- CI
confidence interval
- ICU
intensive care unit
- KPMCP
Kaiser Permanente Medical Care Program
- KPSS
Kaiser Pediatric Stroke Study
- OR
odds ratio
Footnotes
Editorial, page 400
AUTHOR CONTRIBUTIONS
Christine Fox: study concept, analysis and interpretation of data, drafting and revising the manuscript for content. S. Claiborne Johnston: interpretation of data and revising the manuscript for content. Stephen Sidney: revision of the manuscript for content. Heather Fullerton: study concept and design, interpretation of data, and revising the manuscript for content.
DISCLOSURE
C. Fox is supported by award number K12NS001692 from the National Institute of Neurological Disorders and Stroke, received research support from the Dubois Foundation, and received travel funding from the Children's Hemiplegia & Stroke Association and the Child Neurology Society. S.C. Johnston is the Executive Vice Editor of the Annals of Neurology and serves on the editorial advisory boards for Journal Watch Neurology and Journal of Hospital Medicine. S. Sidney is employed by the Kaiser Permanente Medical Group and was involved in the development of a cardiovascular surveillance system in the Cardiovascular Disease Research Network, funded by the National Heart, Lung and Blood Institute. He is funded by research grants N01 HC-48050 and NHLBI RC2HL101666, funding from the Food and Drug Administration, funding from the AHA/UCSF for stroke research, HJLBI/Wake Forest University, NIDDK, NHLBI/University of Minnesota, Thrasher Foundation/UCSF, NJLBI/Northwestern University grant R01HL086792, AHA-FTF grant #09BGIA2260784/UCSF, and NIH #201-WH-4-2121. H. Fullerton has research funding for childhood stroke studies from NIH/NINDS grants NINDS K02 NS053883, R01 NS062820HJ, R01 NS062820-02S1, and R01 HL096789-01RJ, the Thrasher Research Foundation grant RAS-A116619, and the AHA Midwest Affiliate Predoctoral Fellowship grant 11-PAF03495. She serves on the DSMB for Berlin Heart. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: ethnic and gender disparities. Neurology 2003; 61: 189– 194 [DOI] [PubMed] [Google Scholar]
- 2.Giroud M, Lemesle M, Gouyon JB, Nivelon JL, Milan C, Dumas R. Cerebrovascular disease in children under 16 years of age in the city of Dijon, France: a study of incidence and clinical features from 1985 to 1993. J Clin Epidemiol 1995; 48: 1343– 1348 [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg NA, Bernard TJ, Fullerton HJ, Gordon A, deVeber G. Antithrombotic treatments, outcomes, and prognostic factors in acute childhood-onset arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol 2009; 8: 1120– 1127 [DOI] [PubMed] [Google Scholar]
- 4.deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol 2000; 15: 316– 324 [DOI] [PubMed] [Google Scholar]
- 5.Fang J, Alderman MH, Keenan NL, Croft JB. Declining us stroke hospitalization since 1997: national hospital discharge survey, 1988–2004. Neuroepidemiology 2007; 29: 243– 249 [DOI] [PubMed] [Google Scholar]
- 6.Amlie-Lefond C, Bernard TJ, Sebire G, et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the international pediatric stroke study. Circulation 2009; 119: 1417– 1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics 2007; 119: 495– 501 [DOI] [PubMed] [Google Scholar]
- 8.Rundek T, Nielsen K, Phillips S, Johnston KC, Hux M, Watson D. Health care resource use after acute stroke in the Glycine Antagonist in Neuroprotection (GAIN) Americas trial. Stroke 2004; 35: 1368– 1374 [DOI] [PubMed] [Google Scholar]
- 9.Kuhle S, Mitchell L, Andrew M, et al. Urgent clinical challenges in children with ischemic stroke: analysis of 1065 patients from the 1–800-noclots pediatric stroke telephone consultation service. Stroke 2006; 37: 116– 122 [DOI] [PubMed] [Google Scholar]
- 10.Strater R, Kurnik K, Heller C, Schobess R, Luigs P, Nowak-Gottl U. Aspirin versus low-dose low-molecular-weight heparin: antithrombotic therapy in pediatric ischemic stroke patients: a prospective follow-up study. Stroke 2001; 32: 2554– 2558 [DOI] [PubMed] [Google Scholar]
- 11.Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children: a scientific statement from a special writing group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke 2008; 39: 2644– 2691 [DOI] [PubMed] [Google Scholar]
- 12.Statler KD, Dong L, Nielsen DM, Bratton SL. Pediatric stroke: clinical characteristics, acute care utilization patterns, and mortality. Childs Nerv Syst 2011; 27: 565– 573 [DOI] [PubMed] [Google Scholar]
- 13.Sands R, Manning JC, Vyas H, Rashid A. Characteristics of deaths in paediatric intensive care: a 10-year study. Nurs Crit Care 2009; 14: 235– 240 [DOI] [PubMed] [Google Scholar]
- 14.Gullberg N, Kalzen H, Luhr O, et al. Immediate and 5-year cumulative outcome after paediatric intensive care in Sweden. Acta Anaesthesiol Scand 2008; 52: 1086– 1095 [DOI] [PubMed] [Google Scholar]
- 15.Naghib S, van der Starre C, Gischler SJ, Joosten KF, Tibboel D. Mortality in very long-stay pediatric intensive care unit patients and incidence of withdrawal of treatment. Intens Care Med 2010; 36: 131– 136 [DOI] [PubMed] [Google Scholar]
- 16.Ganesan V, Prengler M, Wade A, Kirkham FJ. Clinical and radiological recurrence after childhood arterial ischemic stroke. Circulation 2006; 114: 2170– 2177 [DOI] [PubMed] [Google Scholar]
- 17.Beslow LA, Licht DJ, Smith SE, et al. Predictors of outcome in childhood intracerebral hemorrhage: a prospective consecutive cohort study. Stroke 2010; 41: 313– 318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo WD, Lee J, Rusin J, Perkins E, Roach ES. Intracranial hemorrhage in children: an evolving spectrum. Arch Neurol 2008; 65: 1629– 1633 [DOI] [PubMed] [Google Scholar]
- 19.Grotta J, Pasteur W, Khwaja G, Hamel T, Fisher M, Ramirez A. Elective intubation for neurologic deterioration after stroke. Neurology 1995; 45: 640– 644 [DOI] [PubMed] [Google Scholar]
- 20.Bushnell CD, Phillips-Bute BG, Laskowitz DT, Lynch JR, Chilukuri V, Borel CO. Survival and outcome after endotracheal intubation for acute stroke. Neurology 1999; 52: 1374– 1381 [DOI] [PubMed] [Google Scholar]
- 21.Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol 2007; 6: 215– 222 [DOI] [PubMed] [Google Scholar]
- 22.Gupta R, Connolly ES, Mayer S, Elkind MS. Hemicraniectomy for massive middle cerebral artery territory infarction: a systematic review. Stroke 2004; 35: 539– 543 [DOI] [PubMed] [Google Scholar]
- 23.Diringer MN, Edwards DF. Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med 2001; 29: 635– 640 [DOI] [PubMed] [Google Scholar]
- 24.Bershad EM, Feen ES, Hernandez OH, Suri MF, Suarez JI. Impact of a specialized neurointensive care team on outcomes of critically ill acute ischemic stroke patients. Neurocrit Care 2008; 9: 287– 292 [DOI] [PubMed] [Google Scholar]
- 25.Hayden EC. Neuroscience: the most vulnerable brains. Nature 2010; 463: 154– 156 [DOI] [PubMed] [Google Scholar]
- 26.Bell MJ, Carpenter J, Au AK, et al. Development of a pediatric neurocritical care service. Neurocrit Care 2009; 10: 4– 10 [DOI] [PubMed] [Google Scholar]
- 27.Scher M. Proposed cross-disciplinary training in pediatric neurointensive care. Pediatr Neurol 2008; 39: 1– 5 [DOI] [PubMed] [Google Scholar]
- 28.Tasker RC. Pediatric neurocritical care: Is it time to come of age? Curr Opin Pediatr 2009; 21: 724– 730 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.