Abstract
Objective:
To present a new family with tyrosine hydroxylase deficiency (THD) that presented with a new phenotype of predominant, levodopa-responsive myoclonus with dystonia due to compound heterozygosity of one previously reported mutation in the promoter region and a novel nonsynonymous mutation in the other allele, thus expanding the clinical and genetic spectrum of this disorder.
Methods:
We performed detailed clinical examination of the family and electrophysiology to characterize the myoclonus. We performed analysis of the TH gene and in silico prediction of the possible effect of nonsynonymous substitutions on protein structure.
Results:
Electrophysiology suggested that the myoclonus was of subcortical origin. Genetic analysis of the TH gene revealed compound heterozygosity of a point mutation in the promoter region (c.1-71 C>T) and a novel nonsynonymous substitution in exon 12 (c.1282G>A, p.Gly428Arg). The latter is a novel variant, predicted to have a deleterious effect on the TH protein function and is the first pathogenic TH mutation in patients of African ancestry.
Conclusion:
We presented a THD family with predominant myoclonus-dystonia and a new genotype. It is important to consider THD in the differential diagnosis of myoclonus-dystonia, because early treatment with levodopa is crucial for these patients.
Tyrosine hydroxylase deficiency (THD) is a rare autosomal recessive, dopa-responsive dystonia due to mutations in the TH gene.1 The phenotype of THD is thought to be a spectrum with overlap of clinical features between 2 main phenotypes2: one with onset of symptoms in the first year presenting as progressive hypokinetic-rigid syndrome, generalized dystonia, and good response to levodopa and the other with earlier onset presenting as a more complex encephalopathy, perinatal abnormalities, diurnal fluctuations, autonomic disturbances, and less response to levodopa.3–7 Here we present a new family with THD and a new phenotype of prominent levodopa-responsive myoclonus-dystonia (M-D) due to compound heterozygosity of one previously reported mutation in the promoter region and a novel nonsynonymous mutation in the other allele, thus expanding the clinical and genetic spectrum of this disorder.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was approved by the University College London Hospitals/University College London ethics committee (University College London Hospitals project ID number 06/N076), and informed consent was given by all family members.
Patients.
The clinical characteristics of the patients and the family pedigree are described in the Results.
Electrophysiology.
EMG and back-averaged EEG.
Surface EMG was recorded from the involved muscles, chosen based on clinical observation of the most affected body parts. The muscles chosen were on the right side: sternocleidomastoid, biceps, flexor carpi radialis, first dorsal interosseus, rectus femoris, and tibialis anterior. EEG was recorded from an electrode positioned on the frontocentral areas (International 10–20 system) with Fz as reference. Recordings were obtained at rest, with both arms and legs held outstretched.
Median nerve somatosensory evoked potential and C reflex.
The median nerve was stimulated at the left wrist with square wave pulses of 200 μsec pulse width at just above the motor threshold at 2 Hz. Silver-silver chloride recording electrodes were placed on C30 (2 cm behind C3), C40 (2 cm behind C4) contralateral to the side of stimulation, and Fz (midline frontal). The filter bandpass was 0.5 Hz to 1 kHz, and 1,000 sweeps were averaged. The amplitude of the N20 and P25 and N35 peaks were determined from the C30-Fz or C40-Fz recordings. The long loop reflex (C reflex) was simultaneously recorded on the thenar muscles from the median nerve stimulation at the wrist.
Genetic analysis of the TH gene.
Genomic DNA of all family members was extracted from peripheral blood leukocytes using standard methods. A set of 10 of primer pairs was designed using Gene Runner software (Hastings Software, Inc.). All 14 coding exons, splice sites, and the promoter region of the TH gene were amplified by touch-down PCR. Each reaction was performed in a 20-μL volume containing 10 μL of FastStart PCR master mix (Roche), 5 μL of water, 2 μL of each primer (5 pmol/μL), and 30 ng of genomic DNA. PCR products were purified using a Millipore PCR purification plate (Millipore, County Cork, Ireland) and sequenced in both forward and reverse directions using BigDye Terminator v3.1 sequencing chemistry and then were loaded on the ABI3730xl genetic analyzer (Applied Biosystems, Foster City, CA).
The sequences were analyzed with Sequencher software (version 4.9; Gene Codes). In silico prediction of the possible effect of nonsynonymous substitutions on protein structure and function was obtained with PolyPhen (http://genetics.bwh.harvard.edu/pph/) and SIFT (http://sift.jcvi.org/) software. Evolutionary conservation of the amino acid implicated by the identified missense mutation was tested with ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/). Nucleotides were numbered according to the cDNA numbering with +1 corresponding to the A of the ATG translation initiation codon of the Ensemble reference sequence ENST00000381178.
RESULTS
Index case description.
Patient II:2 is the index case, a 27-year-old woman, who had normal birth (figure 1). At the age of 6 months, she was floppy with poor head control and subsequently never achieved milestones, not being able to sit up, crawl, stand, or talk. She developed prominent and violent myoclonic jerks in all 4 limbs, the trunk, and the neck, making it impossible for her to hold objects or use the limbs. Dystonia was most prominent on the upper limbs and face without diurnal fluctuation. She had intermittently painful spasms, with extension of all 4 limbs and the trunk, which occurred several times daily, lasted up to several hours, were accompanied by oculogyric crises and double incontinence, and were exacerbated by fever, infections, or tiredness. At that stage, cerebral palsy was diagnosed.
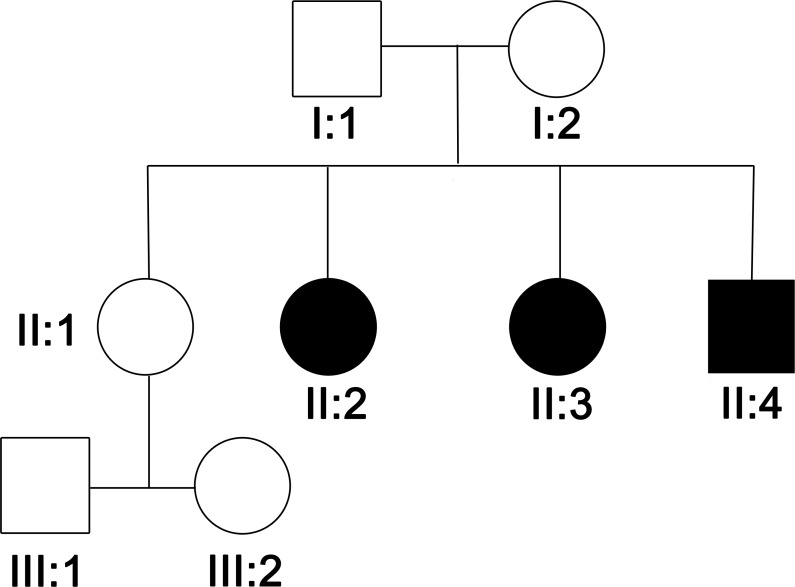
Figure 1.
Family pedigree
At the age of 13 years, levodopa treatment was started, initially at 100 mg and subsequently increased up to 800 mg over about 5 years. This led to a gradual but significant improvement of dystonia and myoclonic jerks; she started speaking and was able to control the electric wheelchair with her left hand, but she could still not use her right hand because of myoclonic jerks. The spasms improved in frequency and severity (5–7 times a week). In the past, she had also tried anticholinergic drugs with no effect and ropinirole with side effects in the past (peripheral edema and weight gain). After the introduction of levodopa, cognition improved and she was able to attend a special school and later college.
On examination at the age of 18 years, eye movements were normal, and she had dystonic grimacing and dysarthria. She had generalized dystonia that was more prominent on the upper limbs with occasional choreoathetoid movements, and generalized nonstimulus-sensitive, spontaneous, and action-induced myoclonus more prominent on the right side, mostly the right leg. There was slowing in finger-tapping but not true bradykinesia. She could walk some steps with help. The rest of the examination was normal. Follow-up over the next 9 years revealed no progression of the symptoms (video 1 on the Neurology® Web site at www.neurology.org). She is still receiving 800 mg levodopa daily and occasionally up to 1,000 mg, when the spasms are exacerbated. She has no dyskinesias or further side effects from levodopa.
Because of the response to levodopa, a dopamine synthesis pathway disorder was suspected. Results of MRI brain and dopamine transporter imaging were normal. Lumbar puncture was performed under treatment with levodopa because the patient refused to stop treatment for investigations, and, consequently, the results are not reliable. CSF showed normal pterins, homovanillic acid (HVA) was in the lower normal range (71 nmol/L; normal range 71–565 nmol/L), 5-hydroxyindoleacetic acid (5-HIAA) was lower than normal (28 nmol/L; normal range 58–220 nmol/L), and 3-methyldopa was markedly elevated as a result of levodopa treatment. Because of treatment, prolactin levels were not tested. A phenylalanine loading test was normal. Results of genetic tests for DYT1, DYT11, GTP cyclohydrolase 1 (GTPCH1), sepiapterin reductase (SR), and l-amino acid decarboxylase (AADC) mutations were negative.
Family pedigree.
The father is from Nigeria; the mother is Caucasian British. The parents and their ancestors are unaffected, and no neurologic disorder is reported in the family history. Three children are affected, and there is an older unaffected sister aged 29, who has 2 healthy children aged 6 and 8 (figure 1).
Patient II:3 is a 24-year-old woman with a picture identical to that of patient II:2. She started levodopa treatment at age 8 years with improvement similar to that of patient II:2 but had significantly better improvement of the jerks. Additional treatment with 10 mg selegiline was discontinued because of side effects. At the age of 9 years, she had a spinal fusion to treat scoliosis due to the trunk dystonia. On examination, she had findings similar to those of the index case, but fewer myoclonic jerks, although these were occasionally evident mostly on the right side (video 2). Patient II:4 is now 19 years old and is the youngest sibling. He started developing similar symptoms at the same age as II:2 and II:3 with generalized dystonia and prominent myoclonus, but he was treated earlier with levodopa at the age of 4 years. The jerks nearly disappeared (video 3). Both patients II:2 and II:3 are being treated with 800 mg levodopa daily (occasionally up to 1,000 mg), with no dyskinesias and no further side effects.
Electrophysiology results.
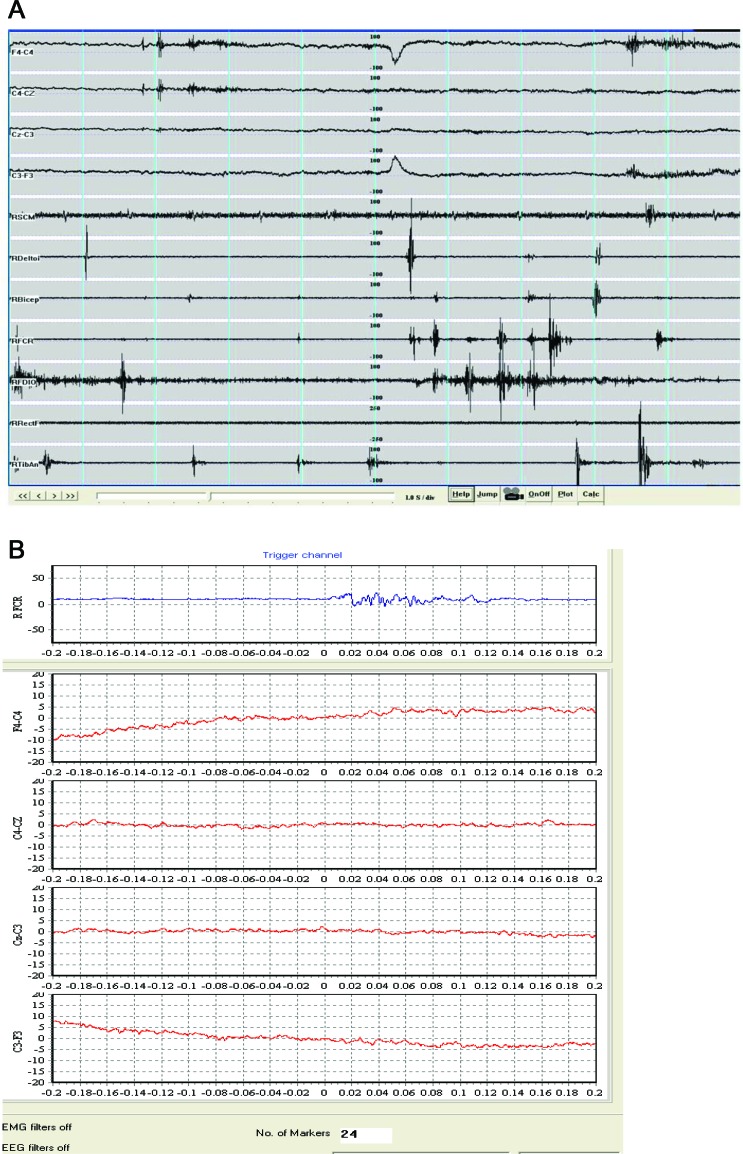
EMG recordings showed irregular, myoclonic bursts, increased with maintenance of a posture (arm or legs outstretched) and with action. The jerk durations ranged from 20 to >100 msec (maximum 200 msec) (figure 2A), they were multifocal, and there was no consistent sequence of rostrocaudal recruitment of muscles to suggest a cortical origin. There was underlying muscle overactivity due to dystonia. EEG back-averaging of short jerks did not reveal a cortical correlate (24 epochs were averaged) (figure 2B). There were no giant somatosensory evoked potentials and the C reflex was absent. These findings are more compatible with jerks being of subcortical origin.
Figure 2. EMG recordings and back-averaged EEG.
(A) EMG recordings from patient II:2 showing irregular, myoclonic bursts with duration range from 20 to >100 msec. (B) Back-averaging shows no premyoclonic cortical potential.
Genetic results for the TH gene.
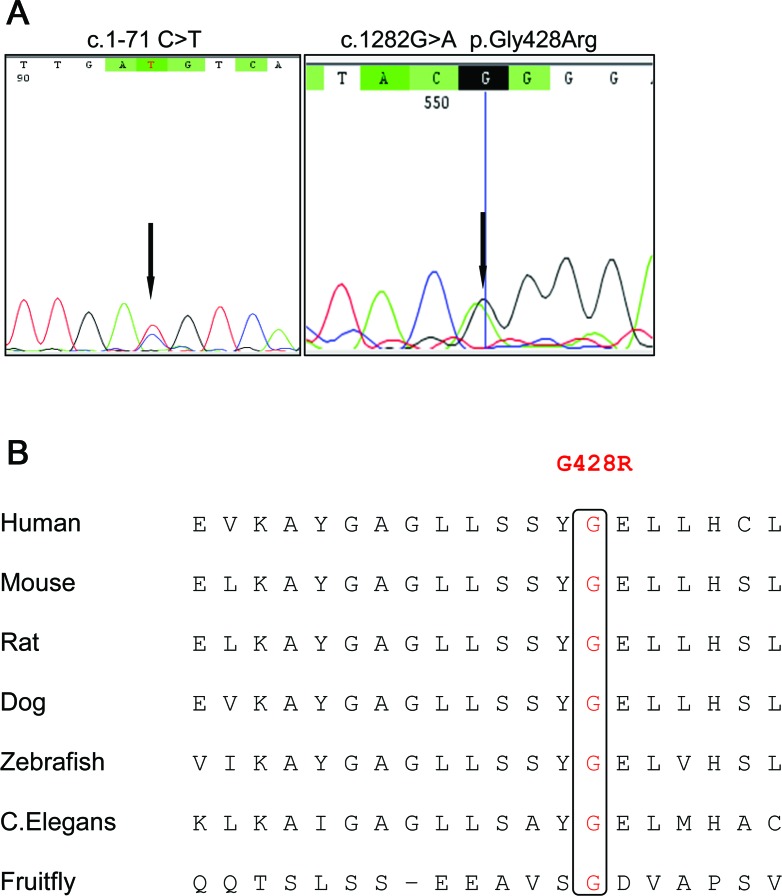
Direct sequencing of all exons, splice sites, and the promoter revealed that the 3 affected siblings carried 2 mutations in the TH gene: a previously described point mutation (c.1-71 C>T) in the promoter region8,9 and a novel nonsynonymous substitution in exon 12 (c.1282G>A, p.Gly428Arg) (figure 3A). Segregation analysis showed that the patients were compound heterozygotes, with the c.1-71 C>T mutation of maternal origin and the c.1282G>A inherited from the father. The unaffected sister was a heterozygote for the latter mutation. The mutation identified in the gene promoter lies in the cyclic adenosine monophosphate response element (CRE), a highly conserved octomer (TGACGTCA→TGATGTCA) located at −74 to −68 upstream of the transcription initial codon.
Figure 3. Genetic studies.
(A) Chromatograms of TH mutations identified in the present study. (B) Amino acid alignment of TH protein showing conservation of the p.G428R residue in different species.
The novel variant identified in exon12 p.Gly428Arg is not reported in the 1000 Genomes project (http://www.1000genomes.org/) or in the National Heart, Lung, and Blood Institute Exome Sequencing Project (http://evs.gs.washington.edu/EVS/) data, and it affects an amino acid highly conserved throughout species (figure 3B). It is predicted by both SIFT and PolyPhen to have a deleterious effect on the TH protein function (table).
Table.
In silico prediction of the novel potential pathogenic missense mutation in TH exon 12 identified in our study
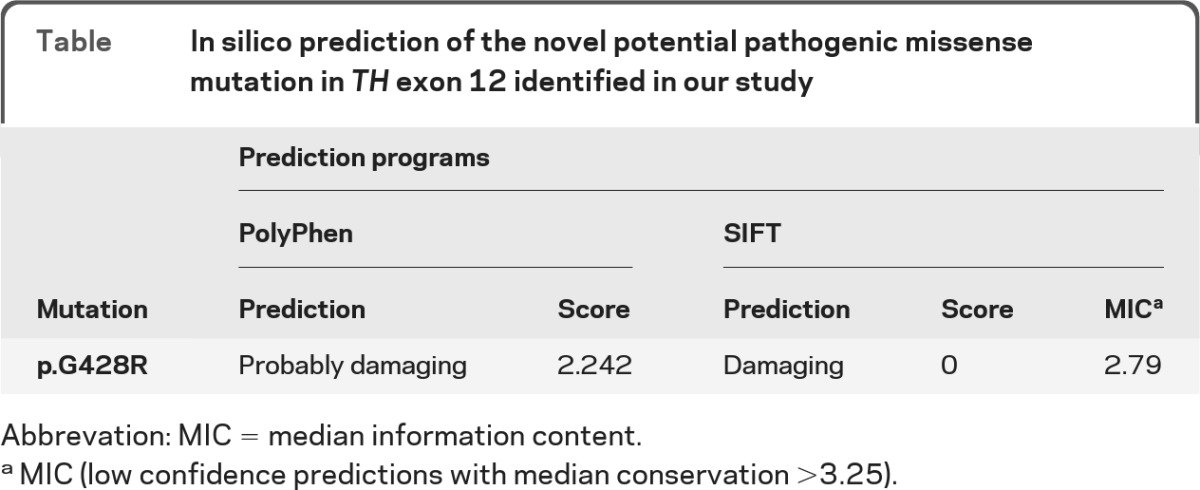
Abbrevation: MIC = median information content.
MIC (low confidence predictions with median conservation >3.25).
DISCUSSION
We describe here 3 members of a THD family who presented with severe myoclonus as the predominant feature accompanied by dystonia. Despite the large phenotypical variability of THD,2 prominent myoclonus is not considered classically to belong to the THD phenotype. There has been one case with jerks reported; however, those presented in the concept of severe encephalopathy, rigidity, spasticity, no response to levodopa, and death at age 9 years.10 It is important that this unusual THD phenotype is recognized and considered in the differential diagnosis of early-onset M-D, because early treatment with levodopa is crucial for the final motor outcome in THD.2 This may be reflected here by the fact that although all 3 patients were affected equally before the introduction of levodopa and were subsequently treated with the same levodopa dosage, the motor outcome differs: the patient who was treated earliest (II:4, 4 years old) showed better response with regard to both the myoclonus and the dystonia than the one who was treated at age 9 years (II:3), and both of them showed a better outcome than the index case (II:2), who was first treated at the age of 13 years.
The differential diagnosis of early-onset M-D includes a variety of autosomal dominant and autosomal recessive disorders. Initially, the term M-D was used to describe an autosomal dominant disorder without further signs.11–13 DYT11 M-D due to ϵ-sarcoglycan gene mutations and DYT15 (only locus identified)14 belong to this category, whereas in many families no locus or gene has been identified.15,16 Despite the initial definition requiring no further signs, more complex manifestations of DYT11 with developmental delay, skeletal abnormalities, and facial dysmorphism due to exonic deletion mutations and other larger deletions have been described.17,18 Other autosomal dominant M-D syndromes include some cases of benign hereditary chorea due to thyroid transcription factor-1 mutations, with additional signs such as delayed motor milestones and later chorea12,19 and one family with GTPCH1 mutations, which presented with levodopa-responsive M-D and later developed parkinsonism.20 With regard to autosomal recessive disorders, M-D was described in one patient with vitamin E deficiency,21 who later developed ataxia, and has been rarely reported in other disorders affecting the dopamine synthesis pathway such as SR or AADC deficiency.22,23 When myoclonus and dystonia are only part of the phenotype, a number of mitochondrial24 or neurometabolic disorders (e.g., Lafora body disease, GM2 gangliosidosis, and Niemann-Pick disease)25 should be considered in the differential diagnosis.
The myoclonus in our patients was asymmetrical but generalized, most probably of subcortical origin as shown by electrophysiology. Electrophysiologic data in our family showed findings similar to those described in DYT11 M-D, but the typical distribution in DYT11 of predominantly cervical involvement with the classic retrocollic brief lightening jerks, was not seen in our patients.12,17,26,27 Similar to that in the patients with THD described here, the myoclonus in DYT11 tends to increase with posture and action and is not stimulus sensitive. The duration of myoclonic bursts in DYT11 has a range from 25 to 256 msec (whereas cortical myoclonus is in the range of 20 to 50 msec), the C reflex is negative, and back-averaging shows no premyoclonic cortical potential, suggesting subcortical myoclonus.26 Of interest, rest and action tremor in THD has been described, in which, however, electrophysiology showed rhythmic bursting at 4 Hz, which was not the case in our patients with arrhythmic and irregular myoclonic jerks.28
Pretreatment CSF analysis in THD typically shows reduced HVA, 3-methoxy-4-hydroxyphenylglucol, and HVA/5-HIAA ratio that correlates with clinical severity, whereas 5-HIAA is normal.2,3,29 After levodopa treatment, CSF results to monitor treatment effects at the biochemical level are not frequently reported in the literature and when reported they refer mostly to HVA and not 5-HIAA values.2,29 At 5 years after initiation of levodopa treatment, our patient showed HVA in the lower normal range and lower than normal 5-HIAA in the CSF, and these posttreatment results have been reported before in THD.9,30,31 Low 5-HIAA under chronic levodopa treatment has been suggested to be due to competitive levodopa uptake in serotonergic neurons in preference to tryptophan, which is then converted to dopamine by AADC.32–35
A recent review of all genetically confirmed cases of THD revealed no phenotype-genotype correlations.2 Our family showed compound heterozygosity of a point mutation in the promoter region (c.1-71 C>T) and a novel nonsynonymous substitution in exon 12 (c.1282G>A, p.Gly428Arg). Mutations in the promoter region of the TH gene have been reported previously; mutagenesis studies in rats and cell lines clearly point out that each nucleotide substitution in the CRE of the TH gene results in a drastic reduction of the basal transcription.36,37 The c.1-71 C>T mutation has been found to cause a reduction of 90% in basal transcription of the TH promotor,38 allowing residual activity, which explains the response to levodopa. It has been reported previously in 2 patients, one in the homozygous state and one in the compound heterozygous state,9 the latter having a much milder phenotype than our patients.
The novel variant identified in exon12 p.Gly428Arg is not likely to be a benign polymorphism: it is not present in the sets of variants reported in publicly available databases (more than 12,000 chromosomes studied), it affects an amino acid highly conserved throughout different species down to invertebrates, and it is predicted in silico to have a deleterious effect on the TH protein function. TH pathogenic variants reported up to now are often private mutations confined to a single or a few pedigrees and, except for a common mutation (c.698G>A, p.Arg233His) in the Dutch population,39 and one reported in 3 Greek families (c.707T>C, p.L236P),40 no other founder effects have been described. Consequently, despite most THD being reported in Western Europe, the disease is expected to occur worldwide2 and the novel mutation described here is the first pathogenic TH mutation in patients of African ancestry.
We presented here a new THD family with predominant, levodopa-responsive M-D and a new genotype and suggest that THD should be included in the differential diagnosis of M-D syndromes. This is crucial for the initiation of early treatment that may influence the outcome.
Supplementary Material
GLOSSARY
- CRE
cyclic adenosine monophosphate response element
- 5-HIAA
5-hydroxyindoleacetic acid
- HVA
homovanillic acid
- M-D
myoclonus-dystonia
- THD
tyrosine hydroxylase deficiency
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
M. Stamelou, N.E. Mencacci: drafting/revising the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data; acquisition of data. C. Cordivari, A. Batla: drafting/revising the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data; acquisition of data. N.W. Wood, H. Houlden, J. Hardy: drafting/revising the manuscript for content, including medical writing for content; supervision. K.P. Bhatia: drafting/revising the manuscript for content, including medical writing for content; supervision.
DISCLOSURE
M. Stamelou and N. Mencacci report no disclosures. C. Cordivari received honoraria from Ipsen. A. Batla and N. Wood report no disclosures. H. Houlden received funding from the Medical Research Council (MRC), Wellcome Trust and NORD, DMRF, NORD and the UCL/UCLH Biomedical Research Centre. J. Hardy reports no disclosures. K.P. Bhatia received speaker honoraria and travel from GlaxoSmithKline, Ipsen, Merz Pharmaceuticals, LLC, Novartis, Sun Pharmaceutical Industries Ltd.; received personal compensation for scientific advisory boards from GSK and Boehringer Ingelheim; and received research support from Ipsen and from the Halley Stewart Trust through Dystonia Society UK, and the Wellcome Trust MRC strategic neurodegenerative disease initiative award (Ref. number WT089698), a grant from the Dystonia Coalition, and a grant from Parkinson's UK (Ref. number G-1009). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Brautigam C, Wevers RA, Jansen RJT, et al. Biochemical hallmarks of tyrosine hydroxylase deficiency. Clin Chem 1998; 44: 1897– 1904 [PubMed] [Google Scholar]
- 2.Willemsen MA, Verbeek MM, Kamsteeg EJ, et al. Tyrosine hydroxylase deficiency: a treatable disorder of brain catecholamine biosynthesis. Brain 2010; 133: 1810– 1822 [DOI] [PubMed] [Google Scholar]
- 3.Brautigam C, Steenbergen-Spanjers GCH, Hoffmann GF, et al. Biochemical and molecular genetic characteristics of the severe form of tyrosine hydroxylase deficiency. Clin Chem 1999; 45: 2073– 2078 [PubMed] [Google Scholar]
- 4.De Lonlay P, Nassogne MC, van Gennip AH, et al. Tyrosine hydroxylase deficiency unresponsive to L-dopa treatment with unusual clinical and biochemical presentation. J Inherit Metab Dis 2000; 23: 819– 825 [DOI] [PubMed] [Google Scholar]
- 5.de Rijk-van Andel JF, Gabreels FJM, Geurtz B, et al. L-dopa-responsive infantile hypokinetic rigid parkinsonism due to tyrosine hydroxylase deficiency. Neurology 2000; 55: 1926– 1928 [DOI] [PubMed] [Google Scholar]
- 6.Diepold K, Schutz B, Rostasy K, et al. Levodopa-responsive infantile parkinsonism due to a novel mutation in the tyrosine hydroxylase gene and exacerbation by viral infections. Mov Disord 2005; 20: 764– 767 [DOI] [PubMed] [Google Scholar]
- 7.Zafeiriou DI, Willemsen MA, Verbeek MM, Vargiami E, Ververi A, Wevers R. Tyrosine hydroxylase deficiency with severe clinical course. Mol Genet Metab 2009; 97: 18– 20 [DOI] [PubMed] [Google Scholar]
- 8.Ribases M, Serrano M, Fernandez-Alvarez E, et al. A homozygous tyrosine hydroxylase gene promoter mutation in a patient with dopa-responsive encephalopathy: clinical, biochemical and genetic analysis. Mol Genet Metab 2007; 92: 274– 277 [DOI] [PubMed] [Google Scholar]
- 9.Verbeek MM, Steenbergen-Spanjers GC, Willemsen MA, et al. Mutations in the cyclic adenosine monophosphate response element of the tyrosine hydroxylase gene. Ann Neurol 2007; 62: 422– 426 [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann GF, Assmann B, Brautigam C, et al. Tyrosine hydroxylase deficiency causes progressive encephalopathy and dopa-nonresponsive dystonia. Ann Neurol 2003; 54: S56– S65 [DOI] [PubMed] [Google Scholar]
- 11.Saunders-Pullman R, Shriberg J, Heiman G, et al. Myoclonus dystonia: possible association with obsessive-compulsive disorder and alcohol dependence. Neurology 2002; 58: 242– 245 [DOI] [PubMed] [Google Scholar]
- 12.Asmus F, Langseth A, Doherty E, et al. “Jerky” dystonia in children: spectrum of phenotypes and genetic testing. Mov Disord 2009; 24: 702– 709 [DOI] [PubMed] [Google Scholar]
- 13.Hess CW, Raymond D, Aguiar Pde C, et al. Myoclonus-dystonia, obsessive-compulsive disorder, and alcohol dependence in SGCE mutation carriers. Neurology 2007; 68: 522– 524 [DOI] [PubMed] [Google Scholar]
- 14.Han F, Racacho L, Lang AE, Bulman DE, Grimes DA. Refinement of the DYT15 locus in myoclonus dystonia. Mov Disord 2007; 22: 888– 892 [DOI] [PubMed] [Google Scholar]
- 15.Schule B, Kock N, Svetel M, et al. Genetic heterogeneity in ten families with myoclonus-dystonia. J Neurol Neurosurg Psychiatry 2004; 75: 1181– 1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nardocci N, Zorzi G, Barzaghi C, et al. Myoclonus-dystonia syndrome: clinical presentation, disease course, and genetic features in 11 families. Mov Disord 2008; 23: 28– 34 [DOI] [PubMed] [Google Scholar]
- 17.Asmus F, Salih F, Hjermind LE, et al. Myoclonus-dystonia due to genomic deletions in the epsilon-sarcoglycan gene. Ann Neurol 2005; 58: 792– 797 [DOI] [PubMed] [Google Scholar]
- 18.Marinoni JC, Stevenson RE, Evans JP, Geshuri D, Phelan MC, Schwartz CE. Split foot and developmental retardation associated with a deletion of three microsatellite markers in 7q21.2-q22.1. Clin Genet 1995; 47: 90– 95 [DOI] [PubMed] [Google Scholar]
- 19.Armstrong MJ, Shah BB, Chen R, Angel MJ, Lang AE. Expanding the phenomenology of benign hereditary chorea: evolution from chorea to myoclonus and dystonia. Mov Disord 2011; 26: 2296– 2297 [DOI] [PubMed] [Google Scholar]
- 20.Leuzzi V, Carducci C, Cardona F, Artiola C, Antonozzi I. Autosomal dominant GTP-CH deficiency presenting as a dopa-responsive myoclonus-dystonia syndrome. Neurology 2002; 59: 1241– 1243 [DOI] [PubMed] [Google Scholar]
- 21.Angelini L, Erba A, Mariotti C, Gellera C, Ciano C, Nardocci N. Myoclonic dystonia as unique presentation of isolated vitamin E deficiency in a young patient. Mov Disord 2002; 17: 612– 614 [DOI] [PubMed] [Google Scholar]
- 22.Clot F, Grabli D, Cazeneuve C, et al. Exhaustive analysis of BH4 and dopamine biosynthesis genes in patients with dopa-responsive dystonia. Brain 2009; 132: 1753– 1763 [DOI] [PubMed] [Google Scholar]
- 23.Longo N. Disorders of biopterin metabolism. J Inherit Metab Dis 2009; 32: 333– 342 [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Cazorla A, Duarte S, Serrano M, et al. Mitochondrial diseases mimicking neurotransmitter defects. Mitochondrion 2008; 8: 273– 278 [DOI] [PubMed] [Google Scholar]
- 25.Gouider-Khouja N, Kraoua I, Benrhouma H, Fraj N, Rouissi A. Movement disorders in neuro-metabolic diseases. Eur J Paediatr Neurol 2010; 14: 304– 307 [DOI] [PubMed] [Google Scholar]
- 26.Roze E, Apartis E, Clot F, et al. Myoclonus-dystonia: clinical and electrophysiologic pattern related to SGCE mutations. Neurology 2008; 70: 1010– 1016 [DOI] [PubMed] [Google Scholar]
- 27.Tezenas du Montcel S, Clot F, Vidailhet M, et al. Epsilon sarcoglycan mutations and phenotype in French patients with myoclonic syndromes. J Med Genet 2006; 43: 394– 400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grattan-Smith PJ, Wevers RA, Steenbergen-Spanjers GC, Fung VSC, Earl J, Wilcken B. Tyrosine hydroxylase deficiency: clinical manifestations of catecholamine insufficiency in infancy. Mov Disord 2002; 17: 354– 359 [DOI] [PubMed] [Google Scholar]
- 29.Wevers RA, de Rijk-van Andel JF, Brautigam C, et al. A review of biochemical and molecular genetic aspects of tyrosine hydroxylase deficiency including a novel mutation (291delC). J Inherit Metab Dis 1999; 22: 364– 373 [DOI] [PubMed] [Google Scholar]
- 30.Yeung WL, Wong VC, Chan KY, et al. Expanding phenotype and clinical analysis of tyrosine hydroxylase deficiency. J Child Neurol 2011; 26: 179– 187 [DOI] [PubMed] [Google Scholar]
- 31.Ludecke B, Knappskog PM, Clayton PT, et al. Recessively inherited L-DOPA-responsive parkinsonism in infancy caused by a point mutation (L205P) in the tyrosine hydroxylase gene. Hum Mol Genet 1996; 5: 1023– 1028 [DOI] [PubMed] [Google Scholar]
- 32.Arai R, Karasawa N, Geffard M, Nagatsu I. L-DOPA is converted to dopamine in serotonergic fibers of the striatum of the rat: a double-labeling immunofluorescence study. Neurosci Lett 1995; 195: 195– 198 [DOI] [PubMed] [Google Scholar]
- 33.Arai R, Karasawa N, Nagatsu I. Aromatic L-amino acid decarboxylase is present in serotonergic fibers of the striatum of the rat: a double-labeling immunofluorescence study. Brain Res 1996; 706: 177– 179 [DOI] [PubMed] [Google Scholar]
- 34.Borah A, Mohanakumar KP. Long-term L-DOPA treatment causes indiscriminate increase in dopamine levels at the cost of serotonin synthesis in discrete brain regions of rats. Cell Mol Neurobiol 2007; 27: 985– 996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navailles S, Bioulac B, Gross C, De Deurwaerdere P. Chronic L-DOPA therapy alters central serotonergic function and L-DOPA-induced dopamine release in a region-dependent manner in a rat model of Parkinson's disease. Neurobiol Dis 2011; 41: 585– 590 [DOI] [PubMed] [Google Scholar]
- 36.Nagamoto-Combs K, Piech KM, Best JA, Sun B, Tank AW. Tyrosine hydroxylase gene promoter activity is regulated by both cyclic AMP-responsive element and AP1 sites following calcium influx: evidence for cyclic AMP-responsive element binding protein-independent regulation. J Biol Chem 1997; 272: 6051– 6058 [DOI] [PubMed] [Google Scholar]
- 37.Lazaroff M, Patankar S, Yoon SO, Chikaraishi DM. The cyclic AMP response element directs tyrosine hydroxylase expression in catecholaminergic central and peripheral nervous system cell lines from transgenic mice. J Biol Chem 1995; 270: 21579– 21589 [DOI] [PubMed] [Google Scholar]
- 38.Tinti C, Yang C, Seo H, et al. Structure/function relationship of the cAMP response element in tyrosine hydroxylase gene transcription. J Biol Chem 1997; 272: 19158– 19164 [DOI] [PubMed] [Google Scholar]
- 39.van den Heuvel L, Luiten B, Smeitink JAM, et al. A common point mutation in the tyrosine hydroxylase gene in autosomal recessive L-DOPA-responsive dystonia in the Dutch population. Hum Genet 1998; 102: 644– 646 [DOI] [PubMed] [Google Scholar]
- 40.Pons R, Serrano M, Ormazabal A, et al. Tyrosine hydroxylase deficiency in three greek patients with a common ancestral mutation. Mov Disord 2010; 25: 1086– 1090 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.