Abstract
Objective:
To investigate the effects of baseline white matter hyperintensity (WMH) and rates of WMH extension and emergence on rate of change in cognition (episodic memory and executive function).
Methods:
A total of 150 individuals including cognitively normal elderly individuals and those with Alzheimer disease and mild cognitive impairment completed serial episodic memory and executive function evaluations and serial MRI scans sufficient for longitudinal measurement of WMH (mean delay 4.0 years). Incident WMH voxels were categorized as extended (baseline WMH that grew larger) or emergent (newly formed WMH). We used a stepwise regression approach to investigate the effects of baseline WMH and rates of WMH extension and emergence on rate of change in cognition (episodic memory and executive function).
Results:
WMH burden significantly increased over time, and approximately 80% of incident WMH voxels represented extensions of existing lesions. Each 1 mL/y increase in WMH extension was associated with an additional 0.70 SD/y of subsequent episodic memory decrease (p = 0.0053) and an additional 0.55 SD/y of subsequent executive function decrease (p = 0.022). Emergent WMHs were not found to be associated with a change in cognitive measures.
Conclusions:
Aging-associated WMHs evolve significantly over a 4-year period. Most of this evolution represents worsening injury to the already compromised surround of existing lesions. Increasing WMH was also significantly associated with declining episodic memory and executive function. This finding supports the view that white matter disease is an insidious and continuously evolving process whose progression has clinically relevant cognitive consequences.
Characterizing the impact of cerebral white matter (WM) damage on age-related cognitive decline is of growing interest.1–3 White matter hyperintensities (WMHs), seen on fluid-attenuated inversion recovery (FLAIR) sequences, feature prominently as a marker of WM injury, increasing with age and vascular risk factors.4–6 WMHs have multiple histopathologic correlates including ependymal loss, cerebral ischemia, demyelination, venous collagenosis, and microcystic infarcts.7 Because cross-sectional studies have widely and consistently reported associations between WMHs and decreased cognitive performance,8–10 there is a pressing need to better characterize the emergence of WMHs, their progression over time, and the time course of their effects on cognition.
The goal of this study, therefore, was to explore the strength of association between longitudinally measured WMHs and longitudinally measured cognition in a group of 150 individuals who spanned a broad and continuous range of cognitive function, including cognitively normal (CN) participants and those with the clinical diagnosis of Alzheimer disease (AD) and mild cognitive impairment (MCI). To achieve this goal, individual WMHs were spatially tracked between baseline and follow-up to distinguish between extension of existing WMHs and the emergence of new WMHs. The longitudinal cognitive measures included psychometrically matched measures of executive function and episodic memory.
METHODS
Sample.
Subjects included 150 individuals enrolled in the University of California, Davis Alzheimer's Disease Center longitudinal cohort (table 1). Approximately 73% of participants were recruited through community-based recruitment protocols designed to enhance racial and ethnic diversity and the spectrum of cognitive dysfunction with an emphasis on normal cognition and MCI.11 The other 27% of the sample initially sought a clinical evaluation at the University of California, Davis Alzheimer's Disease Center.
Table 1.
Summary of subject characteristics, broken down by diagnostic categorya
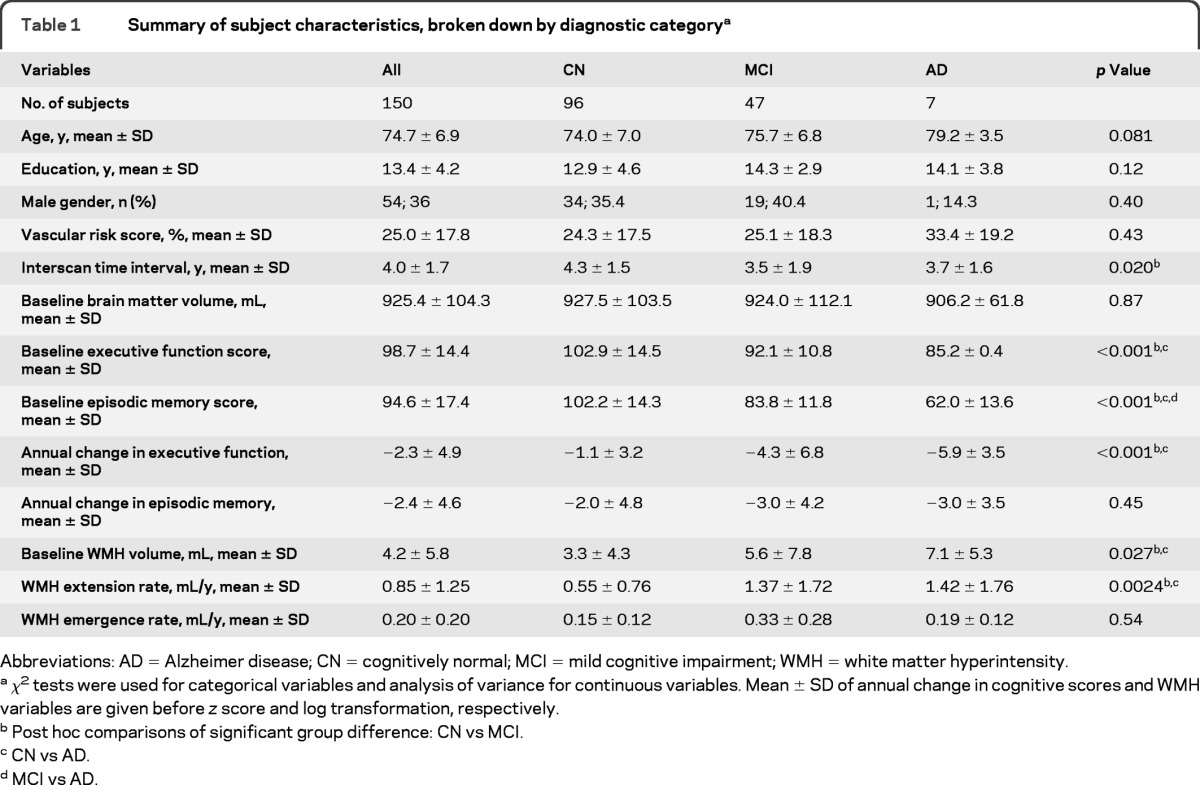
Abbreviations: AD = Alzheimer disease; CN = cognitively normal; MCI = mild cognitive impairment; WMH = white matter hyperintensity.
χ2 tests were used for categorical variables and analysis of variance for continuous variables. Mean ± SD of annual change in cognitive scores and WMH variables are given before z score and log transformation, respectively.
Post hoc comparisons of significant group difference: CN vs MCI.
CN vs AD.
MCI vs AD.
Regardless of recruitment source, inclusion criteria were age older than 60 years and the ability to speak English or Spanish. Exclusion criteria included unstable major medical illness, major primary psychiatric disorder, and substance abuse or dependence in the last 5 years.
Standard protocol approvals, registrations, and patient consents.
The institutional review boards at all participating institutions approved this study, and subjects or their legal representatives gave written informed consent.
Clinical diagnosis and cognitive assessment.
For all participants, determination of clinical syndrome was made at baseline. The diagnosis of AD was made according to the National Institute of Neurological and Communication Disorders and Stroke−Alzheimer's Disease and Related Disorders Association criteria.12 MCI was diagnosed according to current consensus criteria.13 Individuals were declared CN if there was no clinically significant cognitive impairment, defined as performance on any clinical cognitive tests greater than 1.5 SDs below age- and education-adjusted means.
As part of the clinical evaluation, the presence or absence of stroke, diabetes, hyperlipidemia, TIA, hypertension, and coronary artery disease was systematically assessed to create a composite vascular risk score (VRS), which was the sum of the factors present, ranging from 0 to 6, reported as a percentage.14
All participants received a comprehensive clinical evaluation and neuropsychological testing from a standardized test battery on a yearly basis. This study used a subset of the Spanish and English Neuropsychological Assessment Scales,15–17 tests that were averaged within domains to create composite measures of executive function and episodic memory, 2 cognitive measures widely reported to be associated with WMH.1,2 The executive function composite was created from a set of fluency and working memory measures. The episodic memory composite was created from Word List Learning I and Word List Learning II. The cognitive measures are psychometrically matched across scales and across English and Spanish versions,15,18,19 lack appreciable floor or ceiling effects, have linear measurement properties across a broad ability range, and have equivalent reliability and sensitivity. The measures are also continuously distributed across individuals with diagnoses of CN, MCI, and AD, thus justifying the inclusion of all 3 diagnostic groups into unified models of WMH effects on cognition. Rate of change in the measures served, independently of clinical diagnosis, as a measure of cognitive decline and was obtained by subtracting the baseline from the follow-up score and dividing by the interassessment interval. These rates were converted to z scores in the statistical analysis.
Image acquisition and processing.
All subjects received a standardized MRI scan of the brain on 2 different dates (mean delay ± SD 4.0 ± 1.7 years). All brain imaging was performed at the University of California, Davis Imaging Research Center on a 1.5-T GE Signa Horizon LX-Echospeed system. Two sequences were used: a 3-dimensional T1-weighted coronal spoiled gradient-recalled echo acquisition and a FLAIR sequence. The segmentation of WMHs was performed at baseline and follow-up by a semiautomated procedure that was described previously20 and that demonstrates high interrater reliability.11 With use of a previously described image registration method,14 baseline and follow-up WMH maps were linearly aligned to the corresponding T1-weighted scan and then were linearly aligned and nonlinearly deformed to a minimal deformation template21 with a 0.98 × 1.5 × 0.98 mm3 voxel size. Baseline brain matter volume (BV), intracranial volume (ICV), and hippocampal volume (HV) were quantified. BV and ICV were obtained using the Quanta package of software routines.20 HV was obtained from manual segmentation of hippocampus performed by trained analysts according to a strict anatomical protocol as described previously.22 Such volumes were used in statistical analyses to adjust for neuroanatomic degeneration and individual differences in ICV.
Tracking WMHs between baseline and follow-up.
For each participant, distinct WMHs were labeled on their baseline and follow-up scans in template space. Superimposing corresponding baseline and follow-up WMH maps allowed identification of incident WMH voxels. Incident WMH voxels were categorized into 2 classes: voxels that could trace a path through other WMH voxels to any voxel that was labeled WMH at baseline were labeled as WMH extension voxels; otherwise, they were labeled as WMH emergence voxels (figure e-1 on the Neurology® Web site at www.neurology.org).
We computed the volumes of the 3 types of WMH voxels: baseline, extension, and emergence. Volumes were log-transformed to normalize population variance. Individual rates of WMH extension and emergence were finally obtained by dividing the corresponding volumes by the interscan interval.
Statistical analyses.
We first explored associations among baseline WMH volume, rates of WMH extension and emergence, and traditional WMH risk factors. Relations among the 3 WMH measures were assessed by Pearson correlation.
Because baseline WMH volume is associated with ICV and greater accrual of subsequent WMHs,23 we adjusted all WMH measures by ICV and adjusted both emergence and extension by baseline WMH volume and ICV. We did so by performing linear regressions with the variable to adjust as the dependent variable and the potential mediators as independent variables; the resulting residual values were used instead of the original variable in all the following statistical analyses.
Predictors of WMH measures.
We used multivariate linear regression models to identify individual risk factors that may be associated with the adjusted WMH variables: each model included one adjusted WMH measure as the dependent variable and age, gender, years of education, ethnicity, VRS, and BV as independent variables.
Relationship between WMH measures and change in cognitive performance.
We pursued a model building approach to assess the 3 WMH measures as independent predictors of rate of change in episodic memory and executive function. First, reference models were constructed with clinical diagnosis, gender, years of education, ethnicity, BV, VRS, HV, and one of the baseline cognitive scores as independent variables and rate of change in the same cognitive score as the dependent variable. Then each of the 3 WMH measures was separately added to the reference model, and the resulting increase in goodness of fit compared with the reference model was calculated using a likelihood ratio test. Each WMH measure whose likelihood ratio test showed a trend toward significance (p < 0.10) was retained for inclusion in the final model, which was computed if 2 or more WMH measures were significant in the primary models. At first, subject age was not a fixed effect in these models because of earlier indications that age adjustment may attenuate relationships between MRI variables and cognition without providing specific explanatory value about biological mechanisms,24 particularly in this cohort.25 However, to examine the influence of age inclusion on our models of WMH effects on cognition, we repeated the same analyses including age as a covariate.
Continuous variables were mean-centered in all analyses. Statistical analyses were performed using R (version 2.13.0, 2009; R Development Core Team, Vienna, Austria).
Structural equation modeling was also conducted to ensure that our reported WMH-cognition relationships were not confounded by correlations among predictors (see e-Methods).
RESULTS
Demographics.
Table 1 summarizes participant characteristics. The majority of subjects were CN. The 3 clinical groups did not differ significantly in terms of age, education level, gender distribution, VRS, and BV. The interscan interval was significantly longer among CN participants than among those with MCI (4.3 ± 1.5 vs 3.5 ± 1.9 years; p = 0.015). As expected, baseline clinical diagnoses of MCI and AD were associated with greater baseline WMH volumes (p = 0.040 and p = 0.033, respectively) and a higher rate of WMH extension (p = 0.0028 and p = 0.045, respectively) compared with baseline CN. WMH emergence rate, however, was not significantly different among the clinical groups (p = 0.54). Although mean cognitive performance differed significantly among CN, MCI, and AD groups as expected, there was substantial overlap in distributions of these scores among groups, suggesting that the cohort spans a continuous and broad range of cognitive ability.
Relationships between WMH measures.
Mean baseline WMH volume was 4.2 mL (SD 5.8 mL). Over a mean delay of 4 years, extension of existing WMHs accounted for 2.5 mL (SD 3.1) of incident WMH volume and emergence of new WMHs accounted for 0.6 mL (SD 0.5). Greater baseline WMH was strongly associated with a greater extension rate (R = 0.81, p < 0.001) and was more weakly associated with a greater emergence rate (R = 0.34, p < 0.001). The rates of extension and emergence were strongly and positively correlated (R = 0.50, p < 0.001).
Predictors of WMH measures.
In multivariate analyses, a 1-year increase in baseline age was associated with a 0.051 mL/y increase in baseline WMH (p < 0.001). No predictors were significantly associated with WMH extension rate or WMH emergence rate.
Relationships between WMH measures and cognitive change.
Primary model.
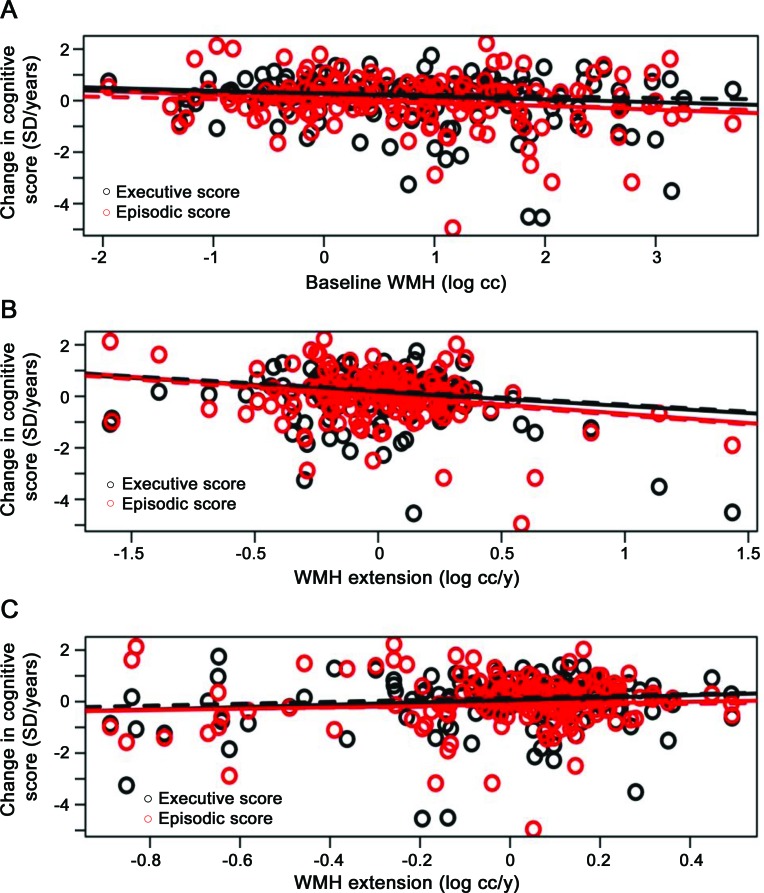
The WMH extension rate, but not WMH emergence rate, added significant explanatory power to reference models of episodic memory and executive function (table 2). Baseline WMH tended to add significant explanatory power to reference models of episodic memory only. Each 1 mL/y increase in WMH extension rate was associated with an additional 0.72 SD/y of subsequent episodic memory decrease (p = 0.0057) and an additional 0.55 SD/y of subsequent executive function decrease (p = 0.027). Each 1 mL increase in baseline WMH volume was associated with an additional 0.14 SD/y of subsequent decrease in episodic memory (p = 0.058).
Table 2.
Summary of regression models of cognitive trajectories using each of the WMH measuresa
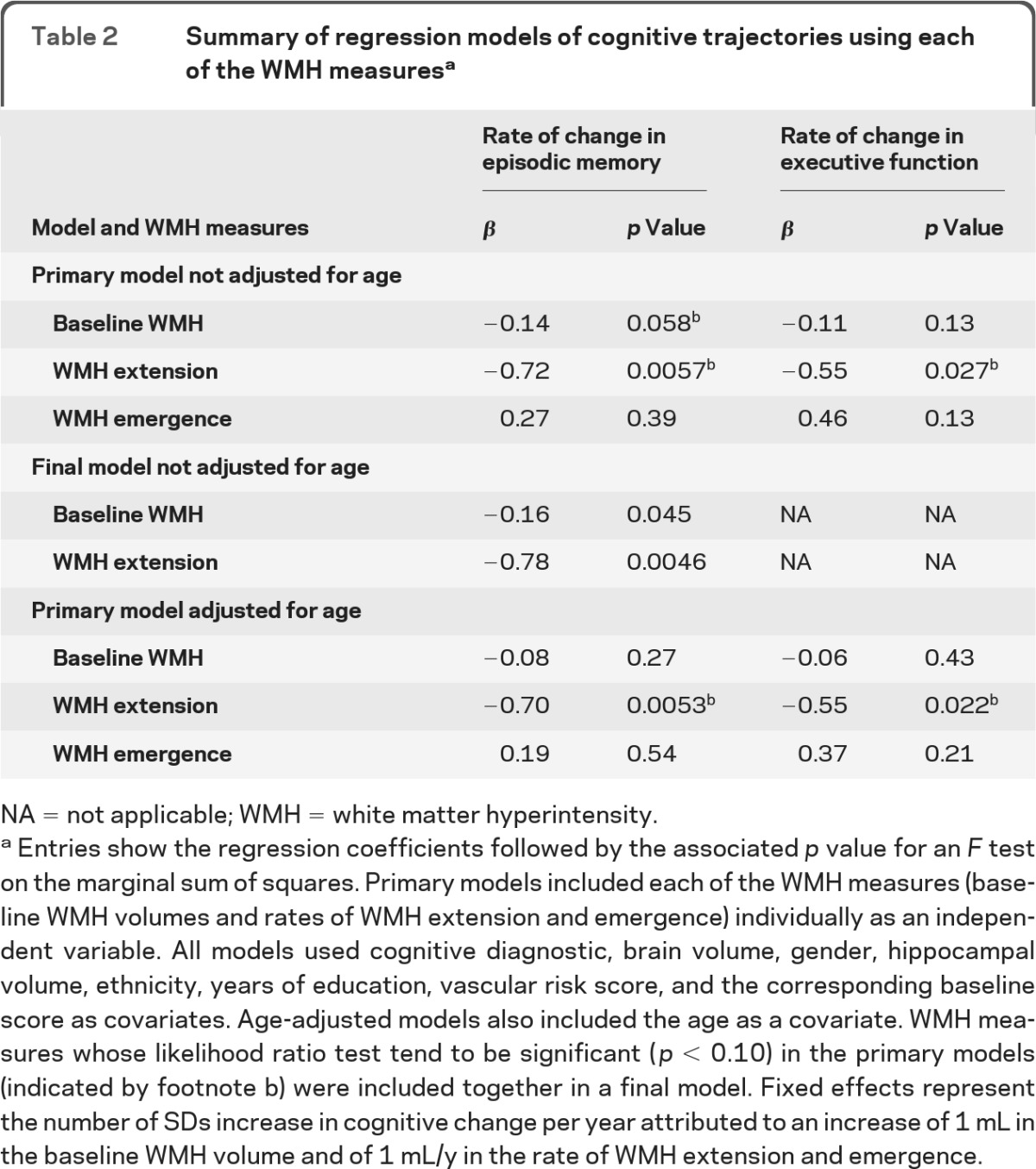
NA = not applicable; WMH = white matter hyperintensity.
Entries show the regression coefficients followed by the associated p value for an F test on the marginal sum of squares. Primary models included each of the WMH measures (baseline WMH volumes and rates of WMH extension and emergence) individually as an independent variable. All models used cognitive diagnostic, brain volume, gender, hippocampal volume, ethnicity, years of education, vascular risk score, and the corresponding baseline score as covariates. Age-adjusted models also included the age as a covariate. WMH measures whose likelihood ratio test tend to be significant (p < 0.10) in the primary models (indicated by footnote b) were included together in a final model. Fixed effects represent the number of SDs increase in cognitive change per year attributed to an increase of 1 mL in the baseline WMH volume and of 1 mL/y in the rate of WMH extension and emergence.
Both baseline WMH and rate of WMH extension were included in the final model on change in episodic memory. The associations observed in separate models were largely unchanged in the final models (table 2).
In addition, structural equation modeling largely recapitulated these same observations (see e-Methods).
Age-adjusted model.
When age was added as a covariate to the primary models, WMH extension rate, but neither WMH emergence rate nor baseline WMH, added significant explanatory power to reference models of episodic memory and executive function (table 2). Each 1 mL/y increase in WMH extension was associated with an additional 0.70 SD/y of subsequent episodic memory decrease (p = 0.0053) and an additional 0.55 SD/y of subsequent executive function decrease (p = 0.022).
Figure 1 illustrates the relationships between each WMH measure and change in cognitive scores for models in the presence or absence of age adjustment as a covariate.
Figure 1. Regression lines relating changes in episodic memory (red) and executive function measures (black) as a function of baseline white matter hyperintensity (WMH) volumes (A) and rates of WMH extension (B) and emergence (C), adjusting (plain lines) or not (dashed lines) by age.
Spatial distribution of WMHs.
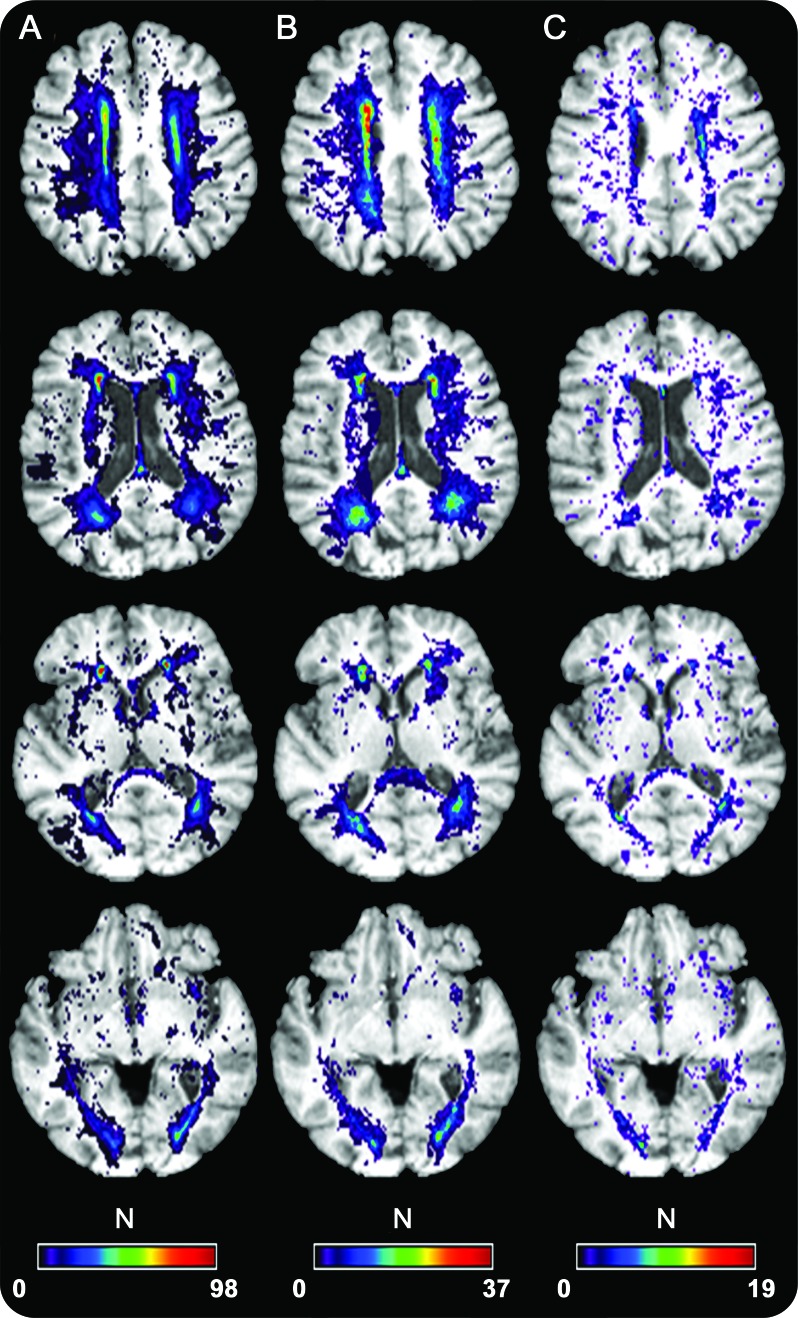
Figure 2 illustrates the spatial distribution of baseline WMHs, WMH extension voxels, and WMH emergence voxels. Baseline WMH voxels and WMH extension voxels were mostly distributed in the vascular watershed area. Although the spatial distribution of emerging WMH voxels was more dispersed, these voxels also occurred mostly in watershed territories.
Figure 2. Spatial frequency of (A) baseline white matter hyperintensities (WMHs), (B) WMH extension, and (C) WMH emergence.
DISCUSSION
The 2 key findings of this study were that WMH accrual largely amounts to growth of existing lesions rather than development of new lesions and that greater cognitive decline is associated with greater lesion growth but not with greater emergence of new lesions. We discuss the significance of each finding in turn.
Approximately 80% of incident WMH volume over time was due to growth of existing lesions. This finding suggests that the bulk of aging-associated WM degeneration may represent a continuous process of gradually spreading injury rather than a series of abrupt and isolated anatomic events. Our previous study suggested that WMHs are surrounded by regions of milder WM injury26; the current study and others27 extend this finding by suggesting that this same mildly injured tissue is differentially vulnerable to subsequent worsening of the injury to such a severity that it is labeled as WMH. The histopathologic origins of age-related WM degeneration include gliosis, degeneration in myelinated axons, and, importantly, small vessel changes whose linear effects on WM integrity may lead to the emergence/development of WMH. Hypertension, diabetes, and other small-vessel disease risk factors, from their earliest phases in life, are likely to be accompanied by progressive and subtle cerebral WM degeneration and whose long-term expression is WMH.28,29 This finding has relevance for the clinical interpretation of WMHs, which should be considered merely the most extreme foci of much more diffuse and incipient brain injury. In addition, the vulnerable surround of existing WMHs may represent a novel treatment target that, if salvaged, might modify the time course of progressive WM degeneration and its cognitive consequences.
The finding that WMH extension paralleled episodic memory and executive function losses supports a model in which WMHs injure cortical connections critical to higher-order cognition30 and WMH extension further exacerbates the same injury. Results from previous cross-sectional studies linked the presence of WMH in specific white matter tracts to poorer executive function and episodic memory.5,30 Our finding further reinforces the importance of understanding the gradual spatial spreading of WM degeneration as the mode of aging or vascular disease-related WM injury that carries the most substantial cognitive consequences. Our findings also question whether disruption of connection between gray matter regions may provide complementary or better information for gray matter volumes in certain regions to explain worse cognitive performances in specific domains. Further validation of this model, for example, by linking WMH growth in specific WM tracts to tract-specific progressive cognitive losses is required.
In contrast to prior observations that different categories of WMHs carry different cognitive repercussions,31 our findings suggest that emergent WMHs might not be immediately relevant to changes in cognitive performance. Emergent WMHs increase in number with increases in baseline WMH burden but are not strongly associated with age and clinical diagnosis, 2 traditionally strong predictors of WMH accrual6; this observation suggests that emergent WMH may involve different pathologic substrates than the far more numerous extending WMHs.7 However, the emergent WMHs constitute a very small proportion of the volume of new WMH, which may provide insufficient power to detect associations with change in cognitive measures. Further studies with greater representation of emerging WMHs are needed to determine this conclusively.
In the present study, age adjustment mitigated the significant effect of baseline WMH, but not of WMH extension, on the rates of change in both cognitive scores. Previous studies suggested that because brain injury (such as WMHs) become more common with age, the correlation between age and cognition, which is the basis of age corrections, is partly due to an underlying effect of brain injury on cognition.17,24 Thus, to correct for “age” is to correct for both age and brain injury, and this correction reduces the ability to associate brain injury with cognition. Therefore, whereas adjustments for age might be justified in the context of extremely healthy brains, such adjustments among populations representing substantial aging-related lesions may have lessened justification. Although age adjustment remains controversial, we emphasize that the key findings of predominant WMH extension and relation of WMH extension to cognitive change were observed irrespective of this methodologic detail. Because the theoretical and clinical implications of age adjustment are not entirely clear, additional investigations are needed to clarify this question.
These results have, nonetheless, to be considered with some caution with regard to the size sample of clinical groups, notably the AD group. That annual episodic memory change did not differ according to clinical group is consistent with findings of 2 previous studies that included larger number of individuals.11,25 These studies suggested that boundaries for differentiating normal cognition, MCI, and dementia are by nature arbitrary cut points in a continuum of pathologic changes, and none of the 3 diagnoses may strictly guarantee a particular predetermined level of cognitive ability in a particular domain.
The present study provides new evidence of a continuous process of WM degeneration over time that parallels concurrent cognitive decline. Additional studies are needed to clarify the distinct pathologic substrates and consequences of growing and emerging WMHs.
Supplementary Material
GLOSSARY
- AD
Alzheimer disease
- BV
brain matter volume
- CN
cognitively normal
- FLAIR
fluid-attenuated inversion recovery
- HV
hippocampal volume
- ICV
intracranial volume
- MCI
mild cognitive impairment
- VRS
vascular risk score
- WM
white matter
- WMH
white matter hyperintensity
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
P. Maillard: drafted the manuscript, participated in study concept and design, conducted the statistical analyses, analyzed and interpreted the data. O. Carmichael: revised the manuscript, participated in study concept and design, analyzed and interpreted data. E. Fletcher: interpreted the data. B. Reed: participated in study concept and design. D. Mungas: participated in study concept and design. C. Decarli: revised the manuscript, participated in study concept and design, analyzed and interpreted the data.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.DeCarli C, Murphy DG, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology 1995; 45: 2077– 2084 [DOI] [PubMed] [Google Scholar]
- 2.O'Brien J, Desmond P, Ames D, Schweitzer I, Harrigan S, Tress B. A magnetic resonance imaging study of white matter lesions in depression and Alzheimer's disease. Br J Psychiatry 1996; 168: 477– 485 [DOI] [PubMed] [Google Scholar]
- 3.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron 2004; 44: 195– 208 [DOI] [PubMed] [Google Scholar]
- 4.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Carmelli D. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol 2001; 58: 643– 647 [DOI] [PubMed] [Google Scholar]
- 5.Nordahl CW, Ranganath C, Yonelinas AP, Decarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci 2006; 18: 418– 429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshita M, Fletcher E, Harvey D, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology 2006; 67: 2192– 2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim K, Macfall J, Payne M. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiatry 2008; 64: 273– 280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmichael O, Schwarz C, Drucker D, et al. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol 2010; 67: 1370– 1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Groot JC, De Leeuw FE, Oudkerk M, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 2002; 52: 335– 341 [DOI] [PubMed] [Google Scholar]
- 10.Stewart R, Dufouil C, Godin O, et al. Neuroimaging correlates of subjective memory deficits in a community population. Neurology 2008; 70: 1601– 1607 [DOI] [PubMed] [Google Scholar]
- 11.Carmichael O, Mungas D, Beckett L, et al. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiol Aging 2012: 33: 85– 95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34: 939– 944 [DOI] [PubMed] [Google Scholar]
- 13.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment: beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256: 240– 246 [DOI] [PubMed] [Google Scholar]
- 14.Lee DY, Fletcher E, Martinez O, et al. Regional pattern of white matter microstructural changes in normal aging, MCI, and AD. Neurology 2009; 73: 1722– 1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mungas D, Reed BR, Crane PK, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess 2004; 16: 347– 359 [DOI] [PubMed] [Google Scholar]
- 16.Mungas D, Reed BR, Tomaszewski Farias S, DeCarli C. Criterion-referenced validity of a neuropsychological test battery: equivalent performance in elderly Hispanics and non-Hispanic whites. J Int Neuropsychol Soc 2005; 11: 620– 630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mungas D, Reed BR, Farias ST, Decarli C. Age and education effects on relationships of cognitive test scores with brain structure in demographically diverse older persons. Psychol Aging 2009; 24: 116– 128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mungas D, Reed BR, Haan MN, Gonzalez H. Spanish and English neuropsychological assessment scales: relationship to demographics, language, cognition, and independent function. Neuropsychology 2005; 19: 466– 475 [DOI] [PubMed] [Google Scholar]
- 19.Mungas D, Reed BR, Marshall SC, Gonzalez HM. Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology 2000; 14: 209– 223 [DOI] [PubMed] [Google Scholar]
- 20.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke 2005; 36: 50– 55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochunov P, Lancaster JL, Thompson P, et al. Regional spatial normalization: toward an optimal target. J Comput Assist Tomogr 2001; 25: 805– 816 [DOI] [PubMed] [Google Scholar]
- 22.DeCarli C, Reed BR, Jagust W, Martinez O, Ortega M, Mungas D. Brain behavior relationships among African Americans, whites, and Hispanics. Alzheimer Dis Assoc Disord 2008; 22: 382– 391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt R, Enzinger C, Ropele S, Schmidt H, Fazekas F. Progression of cerebral white matter lesions: 6-year results of the Austrian Stroke Prevention Study. Lancet 2003; 361: 2046– 2048 [DOI] [PubMed] [Google Scholar]
- 24.Reitan RM, Wolfson D. Clinical and forensic issues regarding age, education, and the validity of neuropsychological test results. J Forensic Neuropsychol 2004; 4: 1– 32 [Google Scholar]
- 25.Mungas D, Beckett L, Harvey D, et al. Heterogeneity of cognitive trajectories in diverse older persons. Psychol Aging 2010; 25: 606– 619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maillard P, Fletcher E, Harvey D, et al. White matter hyperintensity penumbra. Stroke 2011; 42: 1917– 1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt R, Petrovic K, Ropele S, Enzinger C, Fazekas F. Progression of leukoaraiosis and cognition. Stroke 2007; 38: 2619– 2625 [DOI] [PubMed] [Google Scholar]
- 28.de Leeuw FE, de Groot JC, Oudkerk M, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain 2002; 125: 765– 772 [DOI] [PubMed] [Google Scholar]
- 29.van Dijk EJ, Breteler MM, Schmidt R, et al. The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension 2004; 44: 625– 630 [DOI] [PubMed] [Google Scholar]
- 30.Smith EE, Salat DH, Jeng J, et al. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology 2011; 76: 1492– 1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gold G, Giannakopoulos P, Herrmann FR, Bouras C, Kovari E. Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain 2007; 130: 2830– 2836 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.