Abstract
Adult neurogenesis occurs throughout life in the mammalian hippocampus and is essential for memory and mood control. There is significant interest in identifying ways to promote neurogenesis and ensure maintenance of these hippocampal functions. Previous work with a synthetic small molecule, isoxazole 9 (Isx-9), highlighted its neuronal-differentiating properties in vitro. However, the ability of Isx-9 to drive neurogenesis in vivo or improve hippocampal function was unknown. Here we show that Isx-9 promotes neurogenesis in vivo, enhancing the proliferation and differentiation of hippocampal subgranular zone (SGZ) neuroblasts, and the dendritic arborization of adult-generated dentate gyrus neurons. Isx-9 also improves hippocampal function, enhancing memory in the Morris water maze. Notably, Isx-9 enhances neurogenesis and memory without detectable increases in cellular or animal activity or vascularization. Molecular exploration of Isx-9-induced regulation of neurogenesis (via FACS and microarray of SGZ stem and progenitor cells) suggested the involvement of the myocyte-enhancer family of proteins (Mef2). Indeed, transgenic-mediated inducible knockout of all brain-enriched Mef2 isoforms (Mef2a/c/d) specifically from neural stem cells and their progeny confirmed Mef2's requirement for Isx-9-induced increase in hippocampal neurogenesis. Thus, Isx-9 enhances hippocampal neurogenesis and memory in vivo, and its effects are reliant on Mef2, revealing a novel cell-intrinsic molecular pathway regulating adult neurogenesis.—Petrik, D., Jiang, Y., Birnbaum, S. G., Powell, C. M., Kim, M.-S., Hsieh, J., Eisch, A. J. Functional and mechanistic exploration of an adult neurogenesis-promoting small molecule.
Keywords: neural stem cells, dendrites, chemical biology, Morris water maze, Mef2
Once controversial, it is now accepted that neurons are generated in the hippocampal dentate gyrus (DG) subgranular zone (SGZ) of the mammalian brain throughout life (1, 2). Adult-generated hippocampal granule cell (GC) neurons incorporate into hippocampal circuitry (3, 4) and are implicated in many hippocampal functions, including learning and memory (5) and mood control (6). Therefore, it is of great interest to identify approaches, both physiological (e.g., running) and pharmaceutical (e.g., antidepressants), that preserve or enhance neurogenesis and hippocampal function (6, 7). Such manipulations could be useful in combating the diminished hippocampal neurogenesis and function that occur with aging and in certain pathological situations (8, 9). In nonaged healthy animals, pharmaceutical manipulations enhancing neurogenesis might consist of candidate cognitive enhancers or “smart drugs,” since increased neurogenesis improves hippocampal function (10, 11). While several drugs have been found to increase neurogenesis (12, 13), given the variety of situations where neurogenesis-increasing compounds might be employed, a large arsenal of novel candidate compounds will be needed.
A promising route for identification of novel neurogenesis-increasing compounds is the screening of high-throughput chemical libraries in stem cell-based assays (14, 15). Via this route, several small molecules have been reported that increase the number or differentiation of neurons (16–18). Of these, the small molecule isoxazole 9 [Isx-9; N-cyclopropyl-5-(thiophen-2-yl)isoxazole-3-carboxamide] was particularly intriguing (18). In vitro, Isx-9 robustly induced neuronal differentiation. In addition, Isx-9's effects appeared to involve myocyte-enhancer factor 2 (Mef2), a family of transcription factors (19) appreciated in the embryo and early postnatal period as proneuronal and prosurvival factors (20) but never before linked to adult neurogenesis in vivo.
This seminal in vitro work with Isx-9 (18) raised 3 questions that are the focus of this current work. First, does Isx-9 drive adult neurogenesis in vivo as the in vitro data suggest it might? In vivo, cells exist in many stages of adult DG neurogenesis. These stages include neural stem cells (NSCs or Type-1 cells), rapidly proliferating progenitors (Type-2 cells), neuronally committed neuroblasts (Type-3 cells), and surviving, fully differentiated, adult-generated DG neurons. Correct progression through these stages of adult DG neurogenesis depends on the complex environment of the neurogenic niche, which consists of embryonic-generated neurons, astrocytes, and vasculature, among other components. As this niche is largely absent from in vitro preparations, it is critical to assess whether the promising in vitro work with Isx-9 translates to increasing neurogenesis in vivo well. Here we address whether Isx-9 crosses the blood-brain barrier (BBB) in vivo, how cells in the diverse stages of neurogenesis are influenced by single or repeated systemic injections of Isx-9, and whether gross alterations in hippocampal activity or niche components contribute to Isx-9's effects. Second, does Isx-9 improve hippocampal function? Prior work has shown that pharmacologically induced increases in hippocampal neurogenesis are linked to improvement in hippocampal function (16, 21). Here we address whether Isx-9 improves learning and memory using two hippocampal-dependent spatial tasks, the Morris water maze (MWM) and fear conditioning (FC). Third, does Isx-9's ability to increase neurogenesis in vivo rely on intrinsic Mef2 signaling in adult-generated neurons as the in vitro data suggest it might? Here we assess whether Mef2 is required to mediate Isx-9's in vivo effects and use inducible transgenic approaches to inducibly delete three brain-enriched Mef2 isoforms (Mef2a/c/d) specifically from NSCs and their progeny. Taken together, our results reveal the potent in vivo effects of Isx-9 on hippocampal neurogenesis and function and define a cell intrinsic pathway on which Isx-9's neurogenesis-promoting effects rely.
MATERIALS AND METHODS
Animals
All experiments in this study were approved by the Institutional Animal Use and Care Committee at the University of Texas Southwestern (UTSW) Medical Center. Mice were housed at UTSW in a vivarium accredited by the Association for Assessment and Accreditation of Laboratory Animal Care and were kept on a 12-h light-dark cycle. Several different strains of mice were used. Adult (8- to 11-wk-old) nestin–green fluorescent protein (GFP) mice (22) bred at UTSW were used for most experiments [including immunohistochemistry (IHC), behavioral studies, and microarrays]. The remaining experiments used adult C57BL6/J mice (Jackson Laboratory, Bar Harbor, ME, USA), nestin-CreERT2/R26R-YFP mice (23), and Mef2a/dflox/flox and Mef2a/c/dflox/flox mice (generated at UTSW; refs. 24, 25) as described below. Unless otherwise noted, 6-11 male and female mice/group were used.
The transgenic nestin-CreERT2/R26R-YFP mice developed by the A.J.E. laboratory allow temporal and tissue-specific control of yellow fluorescent protein (YFP) expression in nestin-expressing cells induced by tamoxifen (Tam) administration (23). To test the role of intrinsic Mef2 in adult hippocampal neurogenesis in vivo, mice carrying transgenes for nestin-CreERT2, R26R-YFP, Mef2aflox/flox (unpublished results), Mef2cflox/flox, and Mef2dflox/flox (24, 25) were used. When these mice are given Tam, CreERT2 in nestin-expressing NSCs and their progeny translocates to the nucleus, allowing excision of coding exons in 2 (Mef2a/d) or 3 (Mef2a/c/d) isoforms of Mef2. Mice were generated as Mef2a/d-knockout (KO) mice (genotype of nestin-CreERT2+/−, R26R-YFP+/−, and Mef2a/dflox/flox) or Mef2a/c/d-KO mice (genotype of nestin-CreERT2+/−, R26R-YFP+/−, and Mef2a/c/dflox/flox). As the breeding strategy did not allow production of offspring carrying wild-type (WT) alleles for Mef2a/c/d, mice with the genotype nestin-CreERT2+/−, R26R-YFP+/− were used as controls and referred to as Mef2-WT. To account for this potential caveat, statistical analysis was done within the same genotype in mice that received Veh vs. Isx-9 (see Fig. 3E–H). Transgenic mice (Mef2-WT, Mef2a/d-KO, and Mef2a/c/d-KO) were genotyped using PCR for Cre (26), YFP (27), Mef2a (M.-S.K., personal communication), Mef2c (24, 25), and Mef2d (25).
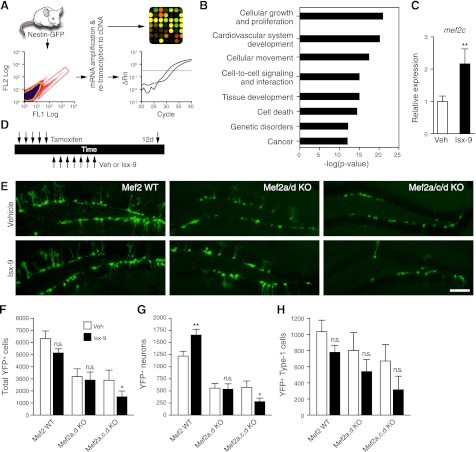
Figure 3.
Isx-9 up-regulates adult neurognesis through a cell-intrinsic Mef2-dependent mechanism. A–C) Cells from nestin-GFP mice were sorted (FACS), and their RNA was amplified, reverse-transcribed, and used for a gene chip (A, B) or qPCR (C). Data analysis identified several biofunctions where most transcripts showed altered relative expression levels by Isx-9 (B). Isx-9-induced up-regulation of Mef2c was detected via microarray (data not shown) and confirmed via qPCR (C). D) To assess whether Mef2 was necessary for Isx-9-induced enhancement of adult hippocampal neurogenesis, nestin-CreERT2/R26R-YFP-WT, Mef2a/dflox/flox or Mef2a/c/dflox/flox transgenic mice were given Tam to induce Cre-mediated recombination and then given Veh or Isx-9. E) Representative photomicrographs show YFP+ DG cells in mice that are Mef2-WT (left column), Mef2a/d-KO (middle column), and Mef2a/c/d-KO (right column) 12 d after 7 d administration of Veh (top panels) or Isx-9 (bottom panels). Scale bars = 50 μm. F) While Isx-9 did not change YFP+ cell numbers in Mef2-WT mice, it significantly decreased YFP+ cell numbers in Mef2a/c/d-KO mice. G, H) Isx-9 increased YFP+ neuron numbers in Mef2-WT mice, but decreased YFP+ neuron numbers in Mef2a/c/d-KO mice (G) without changing YFP+ Type-1 cell numbers in any group (H). n.s., not significant (P>0.05). *P < 0.05, **P < 0.01; paired t test.
Drug administration
Mice were given a disubstituted isoxazole, Isx-9, or its vehicle [Veh; (2-hydroxypropyl)-β-cyclodextrin; Sigma, St. Louis, MO, USA]. As previously reported (18), Isx-9 was discovered from a NeuroD/GluR2-luciferase in vitro screening of a small molecule library owned by UTSW. After several isoforms of disubstituted isoxazoles were identified, side-chain modification showed that an isoxazole with N-cyclopropyl and 5-thiopen (Isx-9) had fast pharmacokinetics and increased activity in transgenic Nkx2.5-luciferase reporter mice. The Isx-9 used for this present work was synthesized by Omm Scientific (Dallas, TX, USA). For in vivo administration, Isx-9 was prepared as 2 mg/ml of 30% Veh in sterile milliQ-purified H2O (Millipore Corp., Billerica, MA, USA). Veh (v/v) or Isx-9 (20 mg/kg) was injected intraperitoneally in 3 different in vivo paradigms to measure different aspects of Isx-9's effects (paradigms are depicted in figures that accompany data). To assess acute (1 d) effects of Isx-9 (see Fig. 1D and Supplemental Fig. S2I), mice were given a single injection of Veh or Isx-9 and killed 10, 30, or 60 min later for mass spectrometry (MS) or 12 d later for IHC. To assess the effects of repeated (7 d) Isx-9 (see Figs.1C, E, K, L; 2; and 3D and Supplemental Figs. S2A and S3), mice were given a single daily injection (∼5 PM) of Veh or Isx-9 for 7 d and killed 1, 12, 30, or 60 d after the last injection. This was used for IHC, locomotion, fluorescent-activated cell sorting (FACS), microarray, quantitative PCR (qPCR), and inducible transgenic analyses. To assess the effects of extended (12 d) Isx-9 on weight and learning and memory (see Fig. 2K and Supplemental Fig. S1), mice were given a daily injection of Veh or Isx-9 (∼5 PM) and a second injection every other day (∼9 AM) for a total of 18 intraperitoneal injections over 12 d.
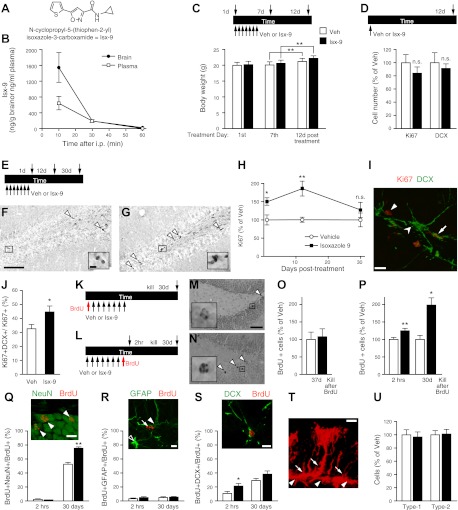
Figure 1.
Isx-9 crosses the BBB and increases proliferation of neuroblasts and neurogenesis in the hippocampal SGZ. A, B) Isx-9 (A) crossed the BBB, as shown by the concentration of Isx-9 (ng/g) in plasma or whole brain 10, 30, and 60 min (B) after 1 i.p. injection of Isx-9 (n=3). C) Seven days of Isx-9 or Veh (arrows under timeline) did not alter body weight in nestin-GFP mice during treatment (1st or 7th d) or after treatment (12th d post-treatment). D) One Isx-9 injection did not change the number of proliferating (Ki67+) cells or DCX+ cells vs. Veh. E) Timeline (applies to F–J) of 7 d Veh or Isx-9 administration to nestin-GFP mice (arrows under timeline) and neurogenesis analysis 1, 12, or 30 d later (arrows above timeline). F–H) Qualitative analysis of Ki67+ cells 12 d after Veh (F) or Isx-9 (G) suggested Isx9-induced increase in proliferation, and quantification at 1, 12, and 30 d post-treatment confirmed this (H). I, J) Twelve days post-treatment, Isx-9 also increased the proportion of Ki67+ cells that were also DCX+. Representative photomicrograph (I) shows cells that are Ki67+DCX− (simple arrowhead), Ki67−DCX+ (split arrowhead), and Ki67+DCX+ (arrow). K–P) Timelines in panels K and L show 3 paradigms of BrdU injection used in panels M–P to test the influence of Isx-9 on neurogenesis. K, M, O) BrdU given before treatment used to examine survival of BrdU+ cells [mice killed 37d later (30 d post-Veh or Isx-9). L, N, P) BrdU given after treatment (red arrow 1 d after 7 d of Veh or Isx-9) used to examine proliferation (mice killed 2 h later) or survival (mice killed 30 d later) of BrdU+ cells. Isx-9-treated mice (N) had more BrdU+ cells than Veh (M) when BrdU is given after but not before Veh or Isx-9 (P). Q–S) Confocal phenotyping revealed an Isx-9-induced increased in the proportion of BrdU+NeuN+/BrdU+ neurons (Q; BrdU, red; NeuN, green) at 30 d post-BrdU, no change in proportion of BrdU+GFAP+/BrdU+ at either time point (R; BrdU, red; GFAP, green; open arrowhead, GFAP+ astrocyte; closed arrowhead, GFAP+ Type-1/NSC; arrow, BrdU+ cell), but increased proportion of BrdU+DCX+/BrdU+ cells at 2 h, not 30 d (S; BrdU, red; DCX, green). T, U) Proportion of nestin-GFP+ Type-1 (arrows) and Type-2 (arrowheads) cells (T) was also not changed after Isx-9 (U). Scale bars = 100 μm (F); 10 μm (F, inset; I, Q–T); 50 μm (M). *P < 0.05, **P < 0.01; unpaired t test.
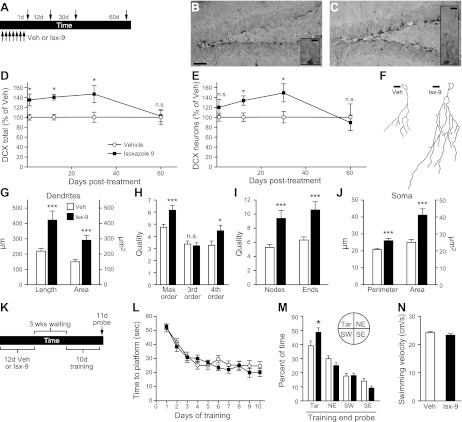
Figure 2.
Isx-9 increases differentiation and dendritic complexity of immature neurons and improves memory. A) Timeline for panels B–J. Nestin-GFP mice were given Veh or Isx-9 for 7d (arrows under timeline) and DCX+ SGZ cells were quantified at 1, 12, 30, and 60 d post-treatment (arrows above timeline). B–J) At 12 d post-treatment (B, Veh; C, Isx-9), Isx-9 mice had more DCX+ cells (D), and DCX+ immature neurons (E); Isx-9 also increased dendritic arborization (insets in B, C; sample tracings in F), including dendritic length and branching (G–I), as well as soma size (J). K–M) MWM training after 12 d of Veh or Isx-9 (K) revealed no difference in learning (L, repeated measure 2-way ANOVA; Bonferroni posttest); however, Isx-9-treated mice spent more time in the target quadrant during the probe test (M, 11 d) when the platform was removed. N) There was no difference in swim speed between Veh and Isx-9. Scale bars = 50 μm (B, C); 10 μm (B, C insets; F). n.s., not significant (P>0.05). *P < 0.05, **P < 0.01, ***P < 0.001; unpaired t test.
To label proliferating cells, mice received intraperitoneal 5-bromo-2-deoxyuridine (BrdU; Roche Applied Science, Indianapolis, IN, USA; 150 ml/kg, 10 mg/ml of 0.9% NaCl, pH 7.4; ref. 28) in 1 of 2 injection paradigms. To assess the effect of Isx-9 on survival of proliferating cells, mice received 2 injections of BrdU (6 h apart) 1 d before Veh or Isx-9 and were killed 30 d later (e.g., 37 d post-BrdU; Fig. 1K). To assess the ability of Isx-9 to influence proliferation and differentiation, other mice received 1 injection of BrdU 1 d after receiving 7 d of daily Veh or Isx-9 and were killed 2 h or 30 d later (Fig. 1L).
To induce Cre-mediated recombination in transgenic mice (see Fig. 3D and ref. 23), mice were given Tam (Sigma) for 5 d (150 mg/kg i.p.; 30 mg/ml of 10% ethanol and 90% sunflower oil) before 7 d of Veh or Isx-9. Mice were then killed 12 d after Isx-9 or Veh treatment.
MS
The concentration of Isx-9 in plasma, hippocampus, or whole-brain tissue was measured via MS (e.g., 16). Mice were perfused via ice-cold 0.1 M PBS, with the nondiluted, exsanguinated blood from the body and brain collected into a citrate buffer (pH 5.0). Plasma was separated from hematocrit by centrifugation. The whole brains (n=3) or microdissected hippocampi (n=1) were weighed and snap-frozen. Samples were homogenized and run on sonic spray MS to estimate amount of Isx-9 (ng) per weight unit (g tissue or ml plasma).
FACS, microarray, and qPCR
One day after 7 d of Veh or Isx-9 injections, the hippocampi of adult nestin-GFP mice (4 mice/group) were prepared for cell sorting (29). Briefly, the hippocampi were microdissected and enzymatically digested into a single-cell suspension (29). Cell sorting was performed using a MoFlo machine (Dako, Carpinteria, CA, USA). GFP-expressing, live cells were separated from GFP-negative, dead cells using standard parameters of forward- and side-scattering (e.g., ref. 30). Approximately 8000 live, GFP-expressing cells per group were sorted directly into TriZol (Invitrogen, Carlsbad, CA, USA), with multiple, separate groups of mice being run via FACS.
The RNA was isolated from DNA and proteins using a standard phenol-chlorophorm extraction. The isolated RNA was amplified in a linear fashion to cDNA using 2 rounds of amplification of the MessageAmp II aRNA kit (Ambion, Austin, TX, USA). Amplified cDNA was used for a single microarray analysis (Affymetrix GeneChip Mouse Genome 430 2.0 array; Affymetrix, Santa Clara, CA, USA) using standardized protocols of the U.S. National Institutes of Health Neuroscience Microarray Consortium (Duke University Medical Center, Durham, NC, USA), which included loading via nanodrop and use of GAPDH as an internal control. Data analysis was performed with Partek Genomics Suite (Partek, St. Louis, MO, USA) and Ingenuity software (Ingenuity Systems, Redwood City, CA, USA). When compared with Veh-treated mice, only genes with 2-fold higher or 0.5-fold lower expression were considered significantly up- or down-regulated. Comparative analyses on biofunctions were performed using Ingenuity Pathway Analysis (Ingenuity Systems) using Fisher's exact test to calculate the P values (31).
With the use of the amplified cDNA from sorted nestin-GFP live cells, qPCR was performed on an ABI Prism 7900 HT detection machine (Applied Biosystems, Foster City, CA, USA) to determine relative expression of Mef2c mRNA. Amplification conditions consisted of 10 min denaturation at 95°C followed by 40 cycles of 95°C denaturation (15 s) and 60°C annealing and elongation. The results were analyzed using SDS2.1.1 software (Applied Biosystems) following the 2−ΔΔCT method and normalized by the expression levels of GAPDH. The 50-μl qPCR reactions used the SYBR Green dye system (Applied Biosystems) with 300 nM primers for Mef2c or GAPDH. The sequences of the used primers were as follows: GAPDH, forward TGCCAAGTATGATGACATCAAGAAG and reverse AGCCCAGGATGCCCTTTAGT; Mef2c, forward GTGCTGTGCGACTGTGAGATT and reverse CGGTGTACTTGAGCAACACCTT.
IHC
Mice were anesthetized and perfused as described previously (23, 32). Thirty-micrometer coronal sections spanning the entire hippocampus were collected on a freezing microtome in serial sets of 9 to allow stereological evaluation. Slide-mounted IHC was performed, and immunoreactive (+) SGZ cells were quantified (23, 32). The following primary antibodies were used: rabbit polyclonal anti-Arc (1:2000; Paul Worley laboratory, Johns Hopkins University, Baltimore, MD, USA); rat monoclonal anti-BrdU (1:300; Accurate, Westbury, NY, USA); rabbit polyclonal anti-cFos [1:300; Santa Cruz (SC) Biotechnology, Santa Cruz, CA, USA]; goat polyclonal anti-doublecortin (DCX; 1:1000; SC Biotechnology); rat anti-endoglin (1:500; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA; ref. 33); mouse polyclonal anti-glial fibrillary acidic protein (GFAP; 1:400; Millipore, Billerica, MA, USA) or rabbit polyclonal anti-GFP (1:3000; Invitrogen, Eugene, OR, USA); rabbit polyclonal anti-Ki67 (1:500; Novocastra Laboratories, Norwell, MA, USA); rabbit polyclonal anti-Ki67 (1:200; Thermo Scientific, Rockford, IL, USA); and mouse monoclonal anti-neuronal nuclei (NeuN; 1:500; Millipore). All secondary antibodies (1:200) were from Jackson ImmunoResearch (West Grove, PA, USA).
For single labeling with primary antibodies against Ki67, BrdU, DCX, and cFos, primary antibody incubation was followed by 60 min in biotin-conjugated secondary antibody, and 60 min in ABC (1:50; Vector Laboratories, Burlingame, CA, USA). For Ki67, BrdU, and cFos, the signal was visualized with DAB/metal concentrate (10×; Thermo Scientific). For DCX, the signal was visualized with Vector SG peroxidase substrate (Vector Laboratories). For GFP, YFP, and endoglin, visualization was performed by incubation with Tyramide-Plus signal amplification (1:50; PerkinElmer, Boston, MA, USA). For DCX and Ki67 double labeling, tissue was incubated with both primary antibodies overnight. For double labeling with BrdU, tissue was incubated with the antibody against NeuN, GFAP, or Ki67 (Thermo Scientific) overnight, followed by anti-mouse Cy2-conjugated antibody, full pretreatment for antigen unmasking (32), and then incubation with the anti-BrdU primary and Cy3-conjugated secondary antibody. For brightfield microscopy, slides were counterstained with fast red (Vector Laboratories). For epifluorescent microscopy, slides were counterstained with DAPI (1:5000; Roche). All slides were dehydrated and coverslipped using DPX (Fluka, Steinheim, Germany).
Cell, dendrite, and vasculature quantification
All cell quantification was performed according to previously published stereological principles (34). Arc+ and cFos+ cells were quantified in the GC layer (GCL; e.g., ref. 35), while Ki67+, BrdU+, DCX+, and YFP+ cells, YFP+ neurons, and nestin-GFP+ Type-1 and Type-2 cells were quantified in the SGZ (32, 33). While main figures present data as percentages, the absolute values for all counts are provided in Supplemental Fig. S3C. Unpaired t tests were used for statistical analysis.
Dendritic and somatic analysis of DCX+ neurons was performed using the Neurolucida software (MBF Bioscience, Williston, VT, USA) with unbiased systematic random sampling of the visual field (75-μm lateral steps; e.g., ref. 36). Eighteen DCX+ neurons from both groups (3 neurons/brain) were selected for analysis of the dendritic trees and neuronal soma (×40 objective). Data were analyzed with unpaired t tests.
Estimation of the volume of the endoglin+ vascular bed (33) as a fraction of the DG volume was performed with Stereoinvestigator software (MBF Bioscience) using the Cavalieri stereological method. The results were analyzed via unpaired t tests.
Phenotypic analyses
Phenotypic analyses were performed using scanning confocal microscopy with optical sectioning in the Z plane using a Zeiss LSM-510 (Carl Zeiss, Oberkochen, Germany; refs. 28, 37) to determine the proportion of Ki67+ cells that were Ki67+DCX+ or the proportion of BrdU+ cells that were BrdU+NeuN+, BrdU+GFAP+, or BrdU+DCX+. For phenotyping with BrdU, both solid and speckled BrdU+ profiles were assessed for colocalization (38). Results were analyzed with unpaired t tests.
Open field locomotion
Locomotor activity was collected in 5-min data bins by photocell beams linked to computer data acquisition (San Diego Instruments, San Diego, CA, USA; Supplemental Fig. S2A–C and ref. 34). Data were collected both during the Veh or Isx-9 treatment (30 min before and 2.5 h after each daily injection, for total of 180 min; Supplemental Fig. S2B) and during the 3 d after the completion of the 7-d treatment (180 min/d; Supplemental Fig. S2C). Results were analyzed via repeated-measure 2-way ANOVA with Bonferroni post hoc test.
MWM and FC
To assess the effects of Isx-9 on learning and memory, 2 tests were used: MWM and FC. For MWM (see Fig. 2K), at 3 wk after the last injection of Veh or Isx-9, mice (15/group) were trained to find a submerged platform in a pool of opaque water (diameter 150 cm; ref 39). Mice received 4 training trials/d (intertrial interval 30–45 min) for 10 d. In all trials, mice were allowed to swim until they landed on the platform or 60 s had elapsed. On d 11, a probe test was conducted in the morning with the platform removed from the pool. The swim path, time to platform, time in each quadrant, and total distance were recorded during the training days. Results are presented as means ± se. For the training days, repeated-measure 2-way ANOVA (GraphPad Prism, GraphPad Software, San Diego, CA, USA) with Bonferroni post hoc test was used with treatment as a within-subject factor and time as a between-subject factor. For the probe test, an unpaired t test was used.
For FC (Supplemental Fig. S1D), 3 wk after the last injection of Veh or Isx-9, mice (15/group) were trained and then tested in contextual and cue FC. For training, mice were placed into FC chambers (Med Associates, St. Albans, VT, USA) for 6 min. Two minutes into the 6-min training session, a tone was presented (80 dB white noise, 30 s), which coterminated with a footshock (0.5 mA, 2 s). The tone-shock pairing was repeated for a total of 3 shocks, with 1 min interval between tone presentations. Twenty-four hours after training, mice were returned to the same context and scored for freezing behavior for 5 min. Forty-eight hours after training, mice were tested for cue conditioning by being placed in a modified environment (vanilla scent, plastic floor over the grid bars, and a triangle roof) for 6 min with the tone presented continuously for the last 3 min and freezing behavior monitored. Scoring of freezing behavior was automatically performed by the Med Associates software using a threshold of 20 arbitrary units and minimum freeze duration of 0.5 s. Data are presented as percentage of total time that freezing behavior was presented and analyzed via unpaired t test.
Field recordings
Acute coronal brain slices and DG GCL recordings were performed as described previously (40). Briefly, recordings were performed on 400-μm brain slices kept at 32°C in artificial cerebrospinal fluid (ACSF) containing 124 mM NaCl, 2 mM KCl, 2 mM MgSO4, 1.25 mM NaH2PO4, 26 mM NaHCO3, 2 mM CaCl2, 10 mM glucose, and a gas mixture of 95% O2 and 5% CO2. The stimulation (0.1 Hz) in the medial perforant path used a paired-pulse stimulation paradigm (2 stimuli separated by 50 ms). The baseline recording from the dorsal GCL was performed for 20 min. Slices were then perfused for 20 min with 20 μM Isx-9 and ACSF prepared as 100 mM stock in DMSO or with DMSO (v/v) as the Veh control, followed by 20 min washout with ACSF. The fEPSP amplitude and slope were measured from the first and second peak with no statistical difference observed. The amplitude and slope values of the drug wash-in and washout were normalized to the baseline values and expressed as percentage of the baseline. Repeated-measure 2-way ANOVA with Bonferroni post hoc test was used.
RESULTS
Isx-9 crosses the BBB and does not have gross adverse health effects
In prior in vitro studies (18), a chemical compound library screening showed that Isx-9 (Fig. 1A) induced neuronal differentiation in cultured adult hippocampal neural progenitor cells. To assess whether Isx-9 influenced neurogenesis in vivo, we had to first assess whether a systemic (intraperitoneal) injection of Isx-9 crossed into adult brain tissue. MS analysis revealed that intraperitoneal Isx-9 passes the BBB into the brain (Fig. 1B) and even into the hippocampus (e.g., 1332 ng Isx-9/g hippocampal tissue). Isx-9 had a brain tissue half-life of ∼25 min (Fig. 1B). Isx-9 was well tolerated, as there was no difference in weight or weight gain vs. Veh after either 7 d (Fig. 1C) or 12 d (Supplemental Fig. S1A) of injections and no evidence of diminished overall health after Isx-9 in any of the administration paradigms used in this study.
Isx-9 increases the proliferation of hippocampal neuroblasts and adult neurogenesis
Given the prior work with Isx-9 in vitro (18), we hypothesized that in vivo Isx-9 would predominantly enhance proneuronal differentiation. Specifically, we hypothesized that Isx-9 would not change the number of Ki67+ SGZ progenitors (28) but would enhance the number of immature neurons immunopositive for DCX, an intermediate filament expressed in neuroblasts and immature SGZ neurons (2, 41). While a single intraperitoneal injection of Isx-9 was not capable of increasing the number of Ki67+ or DCX+ cells 12 d later (Fig. 1D), repeated Isx-9 injections did alter neurogenesis (Fig. 1E–J). Specifically, Isx-9 transiently increased the number of SGZ Ki67+ cells 1 and 12 d after Isx-9 by ∼50 and 86% relative to Veh (Fig. 1F–H), but returned to Veh levels by 30 d.
As Ki67+ cells represent both uncommitted Type-2a cells (Ki67+/DCX−) and neuronally committed Type-2b/3 cells (Ki67+/DCX+; refs. 42, 43), we next analyzed cellular colocalization of Ki67 with DCX (Fig. 1I). Confocal phenotyping revealed a statistically significant increase in the proportion of Ki67+DCX+/Ki67+ SGZ cells 12 d post-treatment (Fig. 1J). These data suggest that Isx-9 increases the proportion of proliferating neuroblasts. To further analyze how Isx-9 affects cells in these early stages of adult neurogenesis, mice received an injection of the thymidine analog BrdU to label dividing cells either before (Fig. 1K) or after (Fig. 1L) Veh or Isx-9 and were killed 2 h or 30 d later. BrdU+ cells were then visualized and counted (Fig. 1M–P and refs. 44, 45). While Isx-9 did not change the number of BrdU+ cells when BrdU was given before treatment (Fig. 1O), Isx-9 increased the number of BrdU+ cells 2 h and 30 d later when BrdU was given after treatment (Fig. 1P). Taken together with Fig. 1J, these data suggest that Isx-9 increases proliferation of SGZ neuroblasts.
While BrdU labels dividing cells, and most surviving BrdU+ cells in the adult DG GCL become neurons, a small proportion of them also become astrocytes (46). Therefore, to assess the phenotype of the surviving BrdU+ cells, we used confocal microscopy to determine the colocalization of BrdU+ cells with the neuronal protein NeuN, the astrocytic/NSC protein GFAP, and the neuroblast/immature neuron protein DCX (Fig. 1Q–S). There were almost no BrdU+NeuN+ cells 2 h post-BrdU in Veh or Isx-9 treated mice, as expected (Fig. 1Q and ref. 41). However, there was a statistically significant increase in proportion of BrdU+NeuN+/BrdU+ cells in Isx-9 vs. Veh 30 d post-BrdU, and in proportional amounts similar to those seen in the adult mouse (35). In contrast, there was no change in proportion of BrdU+GFAP+/BrdU+ cells at either time point (Fig. 1R), in keeping with no obvious change in DG GFAP+ cell number (data not shown). Isx-9 also enhanced the proportion of BrdU+DCX+/BrdU+ cells 2 h but not 30 d post-BrdU (Fig. 1S). These results suggest that Isx-9 increases the proliferation of neuronally committed SGZ cells and leads to a subsequent enhancement in the number of adult-generated GCL neurons.
A key facet of the SGZ neurogenesis cellular cascade is the NSC/Type-1 cell, which is the putative source of adult-generated GCL neurons (47). NSCs rarely or slowly divide, so they are rarely labeled by either Ki67 IHC or an injection of BrdU (48, 49). Therefore, to assess whether Isx-9 influenced NSCs and to confirm the lack of influence of Isx-9 on Type-2a cells, we exploited the ability of nestin-GFP reporter mice to reveal the number of radial-glial-like Type-1 NSCs and dividing Type-2 progenitors labeled with GFP under control of the intermediate filament nestin (22). Morphological dissection of GFP+ cells into Type-1 and Type-2 GFP+ cells via stereology (Fig. 1T) revealed no change in either cell type number at the 12 d time point (Fig. 1U). Furthermore, we did not observe any difference between the total number of GFP+ cells (Type-1 and Type-2 combined) in the SGZ at multiple time points after Isx-9 or Veh treatment (data not shown). Our findings allowed us to verify our in vitro-inspired hypothesis of proneuronal differentiation and notably expand it within the in vivo context: Isx-9 enhances the proliferation of neuroblasts and adult neurogenesis without exhausting the NSC pool.
Isx-9 increases the number of newborn neurons and their dendritic complexity
Based on our in vivo finding that Isx-9 enhanced neuroblast proliferation, we next assessed whether Isx-9 influences the number or maturation of immature adult-born neurons via DCX+ cell quantification. There was a transient increase in the total number of SGZ DCX+ cells of mice given Isx-9 vs. Veh at the 1, 12, and 30 d time points (Fig. 2A–C), with a return to Veh levels by 60 d (Fig. 2D). Isx-9 also resulted in a greater proportion of DCX+ neurons 12 and 30 d post-treatment (Fig. 2E) but returned to Veh levels by 60 d. During DCX+ neuron quantification, we noted a striking qualitative difference in DCX dendrite length in mice given Isx-9 vs. Veh (12 d; Fig. 2B, C, insets). To quantify this, we performed a detailed analysis of the dendritic trees of DCX+ cells 12 d post-treatment (Fig. 2F–J). Indeed, Isx-9 increased the total length and area of the dendritic trees of DCX+ immature neurons (Fig. 2G). Isx-9 also increased dendritic complexity, marked by increased branching above the third order of branching (Fig. 2H), almost doubled the number of branching nodes and ends of branches (Fig. 2I), and increased soma size (Fig. 2J). Taken together, Isx-9 increased the number of DCX+ neurons and the complexity of their dendritic trees.
Isx-9 improves spatial memory in MWM but has no influence in FC
Since both adult neurogenesis and immature neuron dendritic complexity are linked with hippocampal function (50, 51), we explored the functional effect of the Isx-9-induced increase in neurogenesis and dendritic complexity. We first evaluated the effect of Isx-9 on the MWM, a test that allows assessment of hippocampus-dependent learning and memory (39). To maximize the response to Isx-9 and to allow the adult-generated neurons sufficient time to incorporate into hippocampal circuitry (e.g., ref. 4), mice were given 12 d of Isx-9 or Veh and were trained on the MWM 3 wk later (Fig. 2K). There was no overall effect of Isx-9 on learning during training (Fig. 2L). However, during the probe test, mice given Isx-9 spent significantly more time in the target quadrant (Fig. 2M), showing a magnitude of change and pattern of probe results similar to the published literature (52, 53). The Isx-9-induced enhancement of spatial memory in the MWM was also evident in a second, separate cohort of mice (Supplemental Fig. S1B, C). A possible explanation for improved water maze performance (and for increased neurogenesis) might be enhanced locomotion (54). However, mice did not show any change in swim speed (Fig. 2N) or in locomotion during and after administration of Isx-9 vs. Veh (Supplemental Fig. S2A–C). In contrast to the Isx-9-induced enhancement of spatial memory in the MWM, Isx-9 did not have any influence on another hippocampal-dependent task, contextual FC (Supplemental Fig. S1D), or on its hippocampal-independent component, cue FC (Supplemental Fig. S1D).
Isx-9 does not promote hippocampal neurogenesis through common global mechanisms, including cellular activation or altered DG vascularization
To understand the cellular basis of Isx-9-mediated neurogenesis, we considered common global or nonspecific mechanisms known to enhance neurogenesis. First, as noted above, we found no Isx9-induced increase in locomotion or exploration (Supplemental Fig. S2A–C). Second, while the Isx-9-induced neurogenesis might be indirectly due to increased vascularization (55, 56), we found no difference in the volume of the vascular bed in mice after Isx-9 vs. Veh (Supplemental Fig. S2D–F). Third, while Isx-9-induced neurogenesis might be due to increased DG excitation (57), we found no difference in hippocampal extracellular field potential after stimulation of the medial perforant path (Supplemental Fig. S2J–L) or expression of immediate early genes (Supplemental Fig. S2G–I) as markers of polysynaptic neuronal activation (58, 59). Taken together, these data show that Isx-9 does not up-regulate adult neurogenesis through conventional, globally activating mechanisms.
Isx-9 effects on adult neurogenesis are Mef2-dependent
To gain molecular insight regarding Isx-9's ability to increase memory and neurogenesis in vivo, we used microarray and qPCR to identify Isx-9-induced alterations in gene expression in SGZ progenitors (Fig. 3A). We used nestin-GFP mice for this endeavor, since Isx-9-induced changes in gene expression in earlier stages of neurogenesis might precede or contribute to Isx-9-induced changes in neurogenesis we see in the later stages of neurogenesis (e.g., Figs. 1J and 2A–J). GFP+ cells from nestin-GFP hippocampi were sorted (FACS), and their mRNAs were amplified and subjected to genome-wide Affymetrix gene chip analysis. There were 874 genes with ≥2-fold expression level changes after Isx-9 vs. Veh, and 121 genes with ≥3-fold expression level changes after Isx-9 vs. Veh. Many target genes (Table 1) encoded transcription factors critical for neuronal progenitors (e.g., Zic3; ref. 60) or molecules important for neural stem cells and progenitors (e.g., Smad7; ref. 61). Ingenuity software analysis (Fig. 3B) showed that Isx-9 regulates biofunction pathways involved in cellular growth and development as well as intercellular signaling and movement, which collectively may suggest, among other factors, involvement of transcription factors, such as Mef2. Indeed, closer analysis of the array results showed that Mef2c was up-regulated 2.05-fold in the GFP+ cells from mice given Isx-9 vs. Veh. While in vitro studies showed Mef2c up-regulated in response to Isx-9 (18), this was the first in vivo link among adult neurogenesis, Mef2, and Isx-9. To further understand this link, we first confirmed the Isx-9-induced increase in Mef2c by qPCR in a new set of hippocampi from nestin-GFP mice (Fig. 3C). We then set out to determine whether the Isx-9-induced up-regulation of neurogenesis involved Mef2 function.
Table 1.
Data analysis of the microarray results reveals genes that are most up- and down-regulated by Isx-9
| Gene | Symbol | Function | Fold change, Isx-9 vs. control |
|---|---|---|---|
| Actin, α2, aorta | Acta2 | Structural protein; cytoskeleton, cell motility | +4.96 |
| Friend leukemia integration 1 | Fli1 | Transcription factor; epithelia, heart development | +4.95 |
| Zinc finger protein of the cerebellum 3 | Zic3 | Transcription factor; CNS and muscle development | +4.95 |
| Interleukin 2 receptor, γ chain | Il2rg | Internalization receptor; increases cyclin D2 expression, cell proliferation | +4.35 |
| Cadherin 5 | Cdh5 | Adhesion protein; Ca2+-dependent adherence junction | +4.35 |
| Synapsin II | Syn2 | Synaptic vesicle release | −2.33 |
| Cyclin M4 | Cnnm4 | Membrane metal ion transport | −2.36 |
| Transforming growth factor β3 | Tgfb3 | Cell signaling; antiproliferative, stops cell cycle at G1 | −2.65 |
| Zinc finger, H2C2 domain containing | Zh2c2 | DNA binding protein; gene expression in neurons | −2.87 |
| Kinesin family member 2C | Kif2c | Motor protein; dendritic growth | −3.81 |
Table shows the fold change in gene expression, gene name, and abbreviation, and some important gene functions.
To explore whether Isx-9 requires Mef2c for its ability to increase adult neurogenesis, we had to take into account that the adult DG also expresses other family members, including Mef2a and Mef2d (62, 63). We used our Tam-sensitive nestin-CreERT2 driver mouse to conditionally and inducibly delete genes flanked by a floxed allele in nestin-expressing NSCs and their progeny. Crossing nestin-CreERT2 mice with R26R-YFP reporter mice (23) allowed us to visualize the recombined cells by YFP expression. We then employed a quintuple transgenic mouse strategy that allowed comparison of 3 groups of mice: Mef2-WT (nestin-CreERT2/R26R-YFP/Mef2a/c/dwt/wt), Mef2a/d-KO (nestin-CreERT2/R26R-YFP/Mef2a/dflox/flox), and Mef2a/c/d-KO (nestin-CreERT2/R26R-YFP/Mef2a/c/dflox/flox). YFP expression in KO mice indicated conditional deletion of Mef2a/d or Mef2a/c/d in nestin-expressing NSCs and their progeny. As expected, based on the effects of Isx-9 on neurogenesis, in Mef2-WT mice Isx-9 increased the number of YFP+ neurons when examined 12 d later (∼35%; Fig. 3G). We hypothesized that loss of Mef2 expression would prevent the Isx-9-induced increase in number of immature neurons in vivo (Fig. 3D–H). Interestingly, in Mef2a/d-KO mice, Isx-9 did not increase the number of YFP+ neurons (Fig. 3G) or Type-1 NSCs (Fig. 3H). This suggested that Mef2a and Mef2d are required for the proneuronal actions of Isx-9. Surprisingly, Isx-9 given to Mef2a/c/d-KO mice resulted in significantly fewer YFP+ neurons (Fig. 3G), suggesting that in the absence of Mef2c, Isx-9 is antineurogenic. Moreover, Mef2a/d-KO and Mef2a/c/d-KO mice had half the number of YFP+ SGZ cells (Fig. 3F) and YFP+ SGZ neurons (Fig. 3G) relative to Mef2-WT mice but no difference in the number of YFP+ NSC Type-1 cells (Fig. 3H). These effects on YFP+ neuron number took place in the absence of any significant effects of Tam on body weight in Veh- or Isx-9-treated mice (Supplemental Fig. S3). These results show that Isx-9-induced increase in adult hippocampal neurogenesis requires Mef2 transcription factors in nestin-expressing NSCs and their progeny. However, these results also show that baseline adult hippocampal neurogenesis requires intrinsic intact Mef2 transcription factor signaling (Fig. 4), highlighting a completely novel role for Mef2 in the brain.
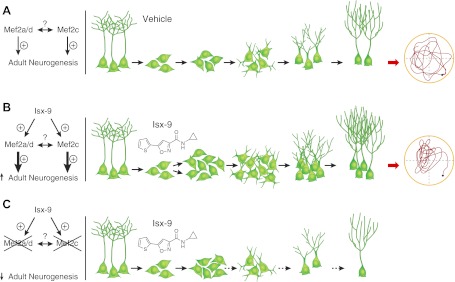
Figure 4.
Model of how Isx-9 may positively influence adult hippocampal neurogenesis via Mef2-specific mechanisms to improve spatial memory. A) In WT Mef2 mice given Veh, both Mef2a/d and Mef2c support basal levels of adult neurogenesis. Their reciprocal relationship is yet to be identified. Adult neurogenesis is a process where NSCs (nestin+ Type-1 cells) with triangular cell body and tufted process produce transiently amplifying progenitors (nestin+ Type-2 and DCX+ Type-3 cells), which differentiate into immature DCX+ neurons (with short dendrites) that finally mature into DG GC (with long dendrites). Adult-generated hippocampal neurons are thought to contribute to hippocampal function, as represented by the activity trace of a mouse finding a hidden platform in the MWM (circular schematic at right). B) WT Mef2 mice given Isx-9 have increased proliferation of DCX+ SGZ progenitors and enhanced differentiation and dendritic arborization of DCX+ neurons, but no change in the number of Type-1 or Type-2 nestin+ cells. The improved adult hippocampal neurogenesis is linked to improved memory in the MWM, as represented by the more concentrated search pattern for a hidden platform (circular schematic at right). C) We propose that Isx-9 mediates enhanced neurogenesis via Mef2a/d and Mef2c. The absence of Mef2 appears to be antineurogenic, and Isx-9 exacerbates that effect. However, the precise relationship between Mef2 and basal neurogenesis and between the different Mef2 isoforms remains to be thoroughly tested.
DISCUSSION
Adult neurogenesis is critical for key aspects of hippocampal function, including specific types of learning and memory, the hippocampal-dependent stress response, and mood regulation (e.g., refs. 6, 34, 51, 64, 65). Dysregulated neurogenesis is proposed to contribute to a range of disorders, from disease- or age-induced memory deficits to depression and addiction (e.g., refs. 8, 66–69). Up-regulating or maintaining adult neurogenesis is expected to improve neurogenesis-dependent brain functions, and studies with transgenic mice show the benefit of enhancing neurogenesis even in normal, nondiseased animals (10). Thus, there is great interest in finding ways to enhance neurogenesis, for example, by physiological stimuli such as running (70) or environmental enrichment (71). However, such manipulations may not always be easily employed to enhance neurogenesis and hippocampal function in aged or diseased individuals. Thus, for optimal versatility in enhancing neurogenesis in the normal and diseased brain, it would be strategic to also have a wide variety of pharmacological agents at our disposal.
Here we used a disubstituted isoxazole, Isx-9, to promote hippocampal neurogenesis in adult mice in vivo. We found that Isx-9 boosts proliferation and differentiation of SGZ neuroblasts and adult neurogenesis in a cell-intrinsic Mef2-dependent manner, which ultimately correlates with improved spatial memory. Thus, in addition to discovering the novel in vivo effects of Isx-9, our studies indicate a completely new molecular pathway governing the production of adult-generated neurons.
Isx-9-induced increase in adult hippocampal neurogenesis
There is a growing number of pharmacological agents in the neurogenesis-enhancing toolkit. In addition to the relatively well-known drugs (e.g., antidepressants, anticonvulsants; refs. 21, 72, 73), small molecules that promote adult neurogenesis have more recently been discovered via a chemical biology approach. These small molecules have been shown, for example, to increase the number of BrdU+ cells and decrease the number of apoptotic cells in the SGZ (16) or decrease SGZ proliferation but increase the number of BrdU+NeuN+ cells (17). While these recent studies were notable in their use of novel small molecules to enhance neurogenesis, in most cases it remains unclear whether these compounds modulate adult neurogenesis via global effects (increasing locomotion, neurovascularization, metabolic capacity; e.g., ref. 74), or how they alter the complex cascade of neurogenesis that occurs in vivo.
We present Isx-9 as a new neurogenesis inducer in the toolkit to enhance adult neurogenesis in vivo. Isx-9 promoted the proliferation of hippocampal neuroblasts, leading to enhanced dendritic complexity of immature neurons and more adult-generated neurons. Isx-9, however, did not change the number of NSCs. This is an important feature, since only an unexpended pool of NSCs ensures continuing adult neurogenesis (32, 75). This profile of Isx-9-induced alterations in the stages of neurogenesis makes Isx-9 a promising preclinical candidate drug for situations when neuroblasts in neurodegenerative diseases (38, 76) and/or adult-generated neurons are reduced and traditional physiological stimulation of adult neurogenesis fails (77) or may be otherwise untenable or impractical.
A main goal of this work was to identify Isx-9's effect on adult hippocampal neurogenesis in vivo. We found that Isx-9 crossed the BBB and reached the hippocampus. We cannot assume that Isx-9 directly binds to cells in the process of adult hippocampal neurogenesis. However, the data we have collected support the stance that Isx-9 increases (directly or indirectly) neuroblast proliferation, which consequently results in more immature and mature adult-generated DG GCL neurons. This speculation is supported by the vast literature showing the sensitivity of proliferating cells to physiological and pharmacological stimuli (66). To determine whether Isx-9's effects are mediated primarily via an initial effect on proliferating neuroblasts, future studies might employ ablation strategies (e.g., irradiation) combined with Isx-9 administration. This would allow us to clarify whether Isx-9 in vivo influences later stages of adult neurogenesis, such as survival and maturation of adult-generated neurons, or whether as we hypothesize it merely stimulates proliferation.
Link between Isx-9-induced dendritic arborization and hippocampal-dependent memory
In addition to Isx-9 increasing the number of neuroblasts and consequently adult neurogenesis, Isx-9 also increased the dendritic complexity of immature DCX+ neurons. Dendritic complexity is positively correlated with learning and memory (e.g., refs. 50, 78), and immature adult-generated DG GC neurons show unique membrane properties important for hippocampal learning and memory (e.g., ref. 79). Thus, it is possible that the Isx-9-enhanced complexity of the dendritic trees contributes to Isx-9-induced improved MWM memory. Of course, the Isx-9-induced increase in absolute number of adult-generated neurons is also likely to contribute to improved hippocampal-dependent memory (78, 80). Notably, Isx-9 did not change performance in another hippocampal-dependent test, FC. This dissociation between MWM and FC is common in the neurogenesis literature (e.g., refs. 81–84). Our data may reflect Isx-9's influence on spatial memory in particular rather than on hippocampal-dependent function in general, and warrants further assessment of how other factors, such as stress, may be involved (65).
Our data suggest that the increased neurogenesis seen after Isx-9 is a direct consequence of increased neuroblast proliferation: more dividing cells leads to more surviving cells (Fig. 1P), and Isx-9 has no obvious effect on survival alone (Fig. 1O). However, the mechanism of how Isx-9 enhances dendritic complexity remains more speculative. Isx-9 increases proliferation and differentiation, but these effects do not necessarily result in enhanced dendritic complexity unless the increased complexity is only transient and results from accelerated transition from the precursor stage into immature neurons. We think this is unlikely, since we also observed increased dendritic complexity 30 d post-Isx-9 (data not shown). Another possibility is that the Isx-9-induced enhancement of dendritic arborization and improved memory are due to Isx-9-induced up-regulation of Mef2 in putative neuroblasts. Despite prior work showing that Mef2 is important in embryonic neurite outgrowth (85), in neural plasticity (86), and in learning and memory (86, 87), our work is the first to suggest a cell-intrinsic role of Mef2 transcriptional factors in adult neurogenesis-dependent memory. While this cell-intrinsic possibility is supported by our data presented here, it also remains possible that Isx-9 enhances dendritic complexity via an as-yet unidentified cell intrinsic or circuit-level change, and this should be a focus of future work.
Mef-2 plays a key role in adult neurogenesis as shown by Isx-9
Our search for new molecular pathways that control adult mouse SGZ revealed Isx-9-dependent regulation of gene expression networks critical for cell proliferation, development, and movement. From these networks, Mef2 emerged as a prime candidate involved with Isx-9's effects on adult neurogenesis. Mef2 was robustly up-regulated in nestin-expressing stem and progenitor cells by Isx-9 in vivo (data presented here), similar to prior in vitro studies with cultured hippocampal progenitor cells (18). Mef2 family members are expressed in DG (62) and are known for their roles in the developing nervous system in regulating neurite growth of Tuj1-positive neurons in vitro (85) and embryonic neural stem cell differentiation in vivo (20). However, prior to our data here, Mef2 had never been assessed for a cell-intrinsic role in adult-generated hippocampal neurons. Here we genetically deleted brain-enriched Mef2 isoforms (Mef2a/c/d) specifically in NSCs and their progeny in vivo and showed that this deletion prevents Isx-9-induced enhancement of adult neurogenesis. These results suggest that Isx-9 acts, at least in part, through increasing Mef2 expression in nestin-expressing cells and their progeny.
We propose that cell-intrinsic Mef2a, Mef2c, and Mef2d are necessary for Isx-9-mediated enhancement of adult neurogenesis (Fig. 4). When Mef2a and Mef2d are removed from nestin-expressing stem cells and their progeny, the presence of Mef2c is not sufficient to allow the proneurogenic effects of Isx-9. When Mef2c is additionally removed, this is antineurogenic, and Isx-9 exacerbates that effect. It remains unclear whether Mef2c is particularly important in these cells, whether it is the first Mef2 family member to be expressed in most tissues during development (19), or whether it is just the order in which we deleted the Mef2 family members. Also, it remains unclear where Mef2 is expressed during the stages of adult hippocampal neurogenesis basally; this will be critical to understand in order to fully appreciate the role of Mef2 in adult hippocampal neurogenesis, and to fully interpret the inducible transgenic work we present here. However, the fact that there is no increase in YFP+ neurons after Isx-9 in Mef2a/d-KO suggests that Mef2 is critical for neuronal differentiation in the adult hippocampus. In addition, our data with Isx-9 suggest a feed-forward mechanism: changes in Mef2 in cells in earlier stages of neurogenesis likely influence those cells as well as cells in later stages of neurogenesis. However, better comprehension of Mef2's role in adult neurogenesis as well as clarification of Isx-9's binding sites and cellular sites of action are warranted to fully understand and support this model.
Future directions
There are several future directions that emerge from our discovery that Isx-9 promotes memory and increases adult hippocampal neurogenesis in a Mef2-dependent manner. First, as Isx-9 promotes the later stages of adult proliferation and enhances neurogenesis without exhausting the NSC pool, Isx-9 might be useful in conditions when proliferation is reduced but NSCs are still present, such as during disease or aging. However, since neurogenesis is evident in other brain regions, such as the more anterior subventricular zone, and since neurogenesis in these regions could have functional consequences, future studies should clarify if and how Isx-9 alters neurogenesis outside of the SGZ. Second, as Isx-9 also promotes spatial memory without significantly influencing learning, it joins the growing list of agents or manipulations that can promote memory but not learning (88–90). Given that there are manipulations that enhance learning but not memory (e.g., running; ref. 75), future studies on Isx-9's specific binding sites and cellular targets might help expand our understanding of how memory vs. learning is regulated. In addition, even though Isx-9 has no obvious effects on global measures of hippocampal activity or vasculature (Supplemental Fig. S2), it also remains to be thoroughly tested whether Isx-9 promotes the proliferation of other cells types, such as astrocytes. Third, Isx-9 promotes neurogenesis in part via up-regulation of Mef2 in nestin-expressing NSCs and their progeny. This might suggest specific application of Isx-9 to adult neurogenesis-dependent memory, a type of memory that recently been shown to be linked to the Mef2 molecular cascade (e.g., ref. 91). Finally, while our study focused on Isx-9-induced enhancement of hippocampal neurogenesis and function and its reliance on Mef2, our data show that Isx-9 has a broad effect on genes. When combined with the fact that Isx-9 has positive effects on other cell types and systems (92, 93), additional work is warranted to clarify whether Isx-9 serves as a proliferation/differentiation agent in many stem-cell driven tissues. In summary, our study illustrates that Isx-9, a neurogenesis-enhancing small molecule, can reveal new cell-intrinsic in vivo modulators and can serve as a platform for development of new pharmacology for adult neurogenesis.
Supplementary Material
Acknowledgments
The authors thank Eric N. Olson (University of Texas Southwestern Medical Center) for providing the Mef2a/c/dflox/flox mice and Paul Worley (Johns Hopkins University, Baltimore, MD, USA) for the anti-Arc antibody. The authors also thank Masahiro Yamaguchi (University of Tokyo, Tokyo, Japan) for providing the initial nestin-GFP breeders that helped establish the colony at University of Texas Southwestern. The authors also thank Haley Speed, Noelle Williams, and Kerstin Ure for helpful comments on the experiments, and the U.S. National Institutes of Health (NIH) Neuroscience Consortium for the microarray help.
This work was supported in part by grants from the NIH (R01 DA016765, K02 DA023555, and R21 DA023701 to A.J.E.; R01 AG032383 to J.H.), the National Alliance for Research on Schizophrenia and Depression (Independent Investigator Award to A.J.E.), the Welch Foundation (I-1660 to J.H.), and the Cancer Prevention and Research Institute of Texas (RP100674 to J.H.).
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies. D.P. designed and performed experiments, analyzed data, made figures, and cowrote the manuscript; Y.J. performed experiments and analyzed data; S.B. performed experiments; C.M.P. provided electrophysiological equipment and expertise; M.-S.K. made Mef2aflox/flox mice;. J.H. and A.J.E. designed experiments and cowrote the manuscript. The authors thank Ami Pettersen, Junie Leblanc, Sohail Kamrudin, and Sean Goetsch for technical assistance and Jose Cabrera for artwork.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BBB
- blood brain barrier
- BrdU
- 5-bromo-2-deoxyuridine
- DCX
- doublecortin
- DG
- dentate gyrus
- FACS
- fluorescent activated cell sorting
- FC
- fear conditioning
- GC
- granule cell
- GCL
- granule cell layer
- GFAP
- glial fibrillary acidic protein
- GFP
- green fluorescent protein
- IHC
- immunohistochemistry
- Isx-9
- isoxazole 9 [N-cyclopropyl-5-(thiophen-2-yl)isoxazole-3-carboxamide]
- KO
- knockout
- MS
- mass spectrometry
- Mef2
- myocyte-enhancer family 2
- MWM
- Morris water maze
- NeuN
- neuronal nuclei
- NSC
- neural stem cell
- qPCR
- quantitative PCR
- SGZ
- subgranular zone
- Tam
- tamoxifen
- WT
- wild type
- YFP
- yellow fluorescent protein
REFERENCES
- 1. Altman J., Das G. D. (1965) Post-natal origin of microneurones in the rat brain. Nature 207, 953–956 [DOI] [PubMed] [Google Scholar]
- 2. Duan X., Kang E., Liu C. Y., Ming G. L., Song H. (2008) Development of neural stem cell in the adult brain. Curr. Opin. Neurobiol. 18, 108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ge S., Pradhan D. A., Ming G. L., Song H. (2007) GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 30, 1–8 [DOI] [PubMed] [Google Scholar]
- 4. Kee N., Teixeira C. M., Wang A. H., Frankland P. W. (2007) Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 10, 355–362 [DOI] [PubMed] [Google Scholar]
- 5. Aimone J. B., Gage F. H. (2011) Modeling new neuron function: a history of using computational neuroscience to study adult neurogenesis. Eur. J. Neurosci. 33, 1160–1169 [DOI] [PubMed] [Google Scholar]
- 6. Petrik D., Lagace D. C., Eisch A. J. (2012) The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building? Neuropharmacology 62, 21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fabel K., Kempermann G. (2008) Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromolecular Med. 10, 59–66 [DOI] [PubMed] [Google Scholar]
- 8. Lazarov O., Mattson M. P., Peterson D. A., Pimplikar S. W., van Praag H. When neurogenesis encounters aging and disease. Trends Neurosci. 33, 569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mandyam C. D., Koob G. F. (2012) The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci. 35, 250–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sahay A., Scobie K. N., Hill A. S., O'Carroll C. M., Kheirbek M. A., Burghardt N. S., Fenton A. A., Dranovsky A., Hen R. (2011) Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rose S. P. (2002) “Smart drugs”: do they work? Are they ethical? Will they be legal? Nat. Rev. Neurosci. 3, 975–979 [DOI] [PubMed] [Google Scholar]
- 12. Takahashi J., Palmer T. D., Gage F. H. (1999) Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures. J. Neurobiol. 38, 65–81 [PubMed] [Google Scholar]
- 13. Palmer T. D., Takahashi J., Gage F. H. (1997) The adult rat hippocampus contains primordial neural stem cells. Mol. Cell. Neurosci. 8, 389–404 [DOI] [PubMed] [Google Scholar]
- 14. Ding S., Schultz P. G. (2004) A role for chemistry in stem cell biology. Nat. Biotechnol. 22, 833–840 [DOI] [PubMed] [Google Scholar]
- 15. Lipinski C., Hopkins A. (2004) Navigating chemical space for biology and medicine. Nature 432, 855–861 [DOI] [PubMed] [Google Scholar]
- 16. Pieper A. A., Xie S., Capota E., Estill S. J., Zhong J., Long J. M., Becker G. L., Huntington P., Goldman S. E., Shen C. H., Capota M., Britt J. K., Kotti T., Ure K., Brat D. J., Williams N. S., MacMillan K. S., Naidoo J., Melito L., Hsieh J., De Brabander J., Ready J. M., McKnight S. L. (2010) Discovery of a proneurogenic, neuroprotective chemical. Cell 142, 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wurdak H., Zhu S., Min K. H., Aimone L., Lairson L. L., Watson J., Chopiuk G., Demas J., Charette B., Weerapana E., Cravatt B. F., Cline H. T., Peters E. C., Zhang J., Walker J. R., Wu C., Chang J., Tuntland T., Cho C. Y., Schultz P. G. A small molecule accelerates neuronal differentiation in the adult rat. Proc. Natl. Acad. Sci. U. S. A. 107, 16542–16547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schneider J. W., Gao Z., Li S., Farooqi M., Tang T. S., Bezprozvanny I., Frantz D. E., Hsieh J. (2008) Small-molecule activation of neuronal cell fate. Nat. Chem. Biol. 4, 408–410 [DOI] [PubMed] [Google Scholar]
- 19. Potthoff M. J., Olson E. N. (2007) MEF2: a central regulator of diverse developmental programs. Development 134, 4131–4140 [DOI] [PubMed] [Google Scholar]
- 20. Li H., Radford J. C., Ragusa M. J., Shea K. L., McKercher S. R., Zaremba J. D., Soussou W., Nie Z., Kang Y. J., Nakanishi N., Okamoto S., Roberts A. J., Schwarz J. J., Lipton S. A. (2008) Transcription factor MEF2C influences neural stem/progenitor cell differentiation and maturation in vivo. Proc. Natl. Acad. Sci. U. S. A. 105, 9397–9402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., Belzung C., Hen R. (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809 [DOI] [PubMed] [Google Scholar]
- 22. Yamaguchi M., Saito H., Suzuki M., Mori K. (2000) Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport 11, 1991–1996 [DOI] [PubMed] [Google Scholar]
- 23. Lagace D. C., Whitman M. C., Noonan M. A., Ables J. L., DeCarolis N. A., Arguello A. A., Donovan M. H., Fischer S. J., Farnbauch L. A., Beech R. D., DiLeone R. J., Greer C. A., Mandyam C. D., Eisch A. J. (2007) Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J. Neurosci. 27, 12623–12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arnold M. A., Kim Y., Czubryt M. P., Phan D., McAnally J., Qi X., Shelton J. M., Richardson J. A., Bassel-Duby R., Olson E. N. (2007) MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev. Cell 12, 377–389 [DOI] [PubMed] [Google Scholar]
- 25. Kim Y., Phan D., van Rooij E., Wang D. Z., McAnally J., Qi X., Richardson J. A., Hill J. A., Bassel-Duby R., Olson E. N. (2008) The MEF2D transcription factor mediates stress-dependent cardiac remodeling in mice. J. Clin. Invest. 118, 124–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Indra A. K., Warot X., Brocard J., Bornert J. M., Xiao J. H., Chambon P., Metzger D. (1999) Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 27, 4324–4327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soriano P. (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 [DOI] [PubMed] [Google Scholar]
- 28. Mandyam C. D., Harburg G. C., Eisch A. J. (2007) Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience 146, 108–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brewer G. J., Torricelli J. R. (2007) Isolation and culture of adult neurons and neurospheres. Nat. Protoc. 2, 1490–1498 [DOI] [PubMed] [Google Scholar]
- 30. Gilley J. A., Yang C. P., Kernie S. G. (2009) Developmental profiling of postnatal dentate gyrus progenitors provides evidence for dynamic cell-autonomous regulation. Hippocampus 21, 33–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang X., Bao X., Pal R., Agbas A., Michaelis E. K. (2010) Transcriptomic responses in mouse brain exposed to chronic excess of the neurotransmitter glutamate. BMC Genomics 11, 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ables J. L., Decarolis N. A., Johnson M. A., Rivera P. D., Gao Z., Cooper D. C., Radtke F., Hsieh J., Eisch A. J. (2010) Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J. Neurosci. 30, 10484–10492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arguello A. A., Fischer S. J., Schonborn J. R., Markus R. W., Brekken R. A., Eisch A. J. (2009) Effect of chronic morphine on the dentate gyrus neurogenic microenvironment. Neuroscience 159, 1003–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lagace D. C., Donovan M. H., DeCarolis N. A., Farnbauch L. A., Malhotra S., Berton O., Nestler E. J., Krishnan V., Eisch A. J. (2010) Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc. Natl. Acad. Sci. U. S. A. 107, 4436–4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Snyder J. S., Choe J. S., Clifford M. A., Jeurling S. I., Hurley P., Brown A., Kamhi J. F., Cameron H. A. (2009) Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J. Neurosci. 29, 14484–14495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arisi G. M., Garcia-Cairasco N. (2007) Doublecortin-positive newly born granule cells of hippocampus have abnormal apical dendritic morphology in the pilocarpine model of temporal lobe epilepsy. Brain Res. 1165, 126–134 [DOI] [PubMed] [Google Scholar]
- 37. Mandyam C. D., Norris R. D., Eisch A. J. (2004) Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. J. Neurosci. Res. 76, 783–794 [DOI] [PubMed] [Google Scholar]
- 38. Donovan M. H., Yazdani U., Norris R. D., Games D., German D. C., Eisch A. J. (2006) Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer's disease. J. Comp. Neurol. 495, 70–83 [DOI] [PubMed] [Google Scholar]
- 39. Morris R. G., Garrud P., Rawlins J. N., O'Keefe J. (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683 [DOI] [PubMed] [Google Scholar]
- 40. Hawasli A. H., Koovakkattu D., Hayashi K., Anderson A. E., Powell C. M., Sinton C. M., Bibb J. A., Cooper D. C. (2009) Regulation of hippocampal and behavioral excitability by cyclin-dependent kinase 5. PLoS One 4, e5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brown J. P., Couillard-Despres S., Cooper-Kuhn C. M., Winkler J., Aigner L., Kuhn H. G. (2003) Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 467, 1–10 [DOI] [PubMed] [Google Scholar]
- 42. Kempermann G., Jessberger S., Steiner B., Kronenberg G. (2004) Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 27, 447–452 [DOI] [PubMed] [Google Scholar]
- 43. Encinas J. M., Enikolopov G. (2008) Identifying and quantitating neural stem and progenitor cells in the adult brain. Methods Cell Biol. 85, 243–272 [DOI] [PubMed] [Google Scholar]
- 44. Miller M. W., Nowakowski R. S. (1988) Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 457, 44–52 [DOI] [PubMed] [Google Scholar]
- 45. Kuhn H. G., Cooper-Kuhn C. M. (2007) Bromodeoxyuridine and the detection of neurogenesis. Curr. Pharm. Biotechnol. 8, 127–131 [DOI] [PubMed] [Google Scholar]
- 46. Cameron H. A., McKay R. D. (2001) Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 435, 406–417 [DOI] [PubMed] [Google Scholar]
- 47. Seri B., Garcia-Verdugo J. M., McEwen B. S., Alvarez-Buylla A. (2001) Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 21, 7153–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kronenberg G., Reuter K., Steiner B., Brandt M. D., Jessberger S., Yamaguchi M., Kempermann G. (2003) Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 467, 455–463 [DOI] [PubMed] [Google Scholar]
- 49. Seki T., Namba T., Mochizuki H., Onodera M. (2007) Clustering, migration, and neurite formation of neural precursor cells in the adult rat hippocampus. J. Comp. Neurol. 502, 275–290 [DOI] [PubMed] [Google Scholar]
- 50. Tronel S., Fabre A., Charrier V., Oliet S. H., Gage F. H., Abrous D. N. (2010) Spatial learning sculpts the dendritic arbor of adult-born hippocampal neurons. Proc. Natl. Acad. Sci. U. S. A. 107, 7963–7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Deng W., Aimone J. B., Gage F. H. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci 11, 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Halt A. R., Dallapiazza R. F., Zhou Y., Stein I. S., Qian H., Juntti S., Wojcik S., Brose N., Silva A. J., Hell J. W. CaMKII binding to GluN2B is critical during memory consolidation. EMBO J. 31, 1203–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Murphy G. G., Fedorov N. B., Giese K. P., Ohno M., Friedman E., Chen R., Silva A. J. (2004) Increased neuronal excitability, synaptic plasticity, and learning in aged Kvbeta1.1 knockout mice. Curr. Biol. 14, 1907–1915 [DOI] [PubMed] [Google Scholar]
- 54. Van Praag H., Christie B. R., Sejnowski T. J., Gage F. H. (1999) Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. U. S. A. 96, 13427–13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Udo H., Yoshida Y., Kino T., Ohnuki K., Mizunoya W., Mukuda T., Sugiyama H. (2008) Enhanced adult neurogenesis and angiogenesis and altered affective behaviors in mice overexpressing vascular endothelial growth factor 120. J. Neurosci. 28, 14522–14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van der Borght K., Kobor-Nyakas D. E., Klauke K., Eggen B. J., Nyakas C., Van der Zee E. A., Meerlo P. (2009) Physical exercise leads to rapid adaptations in hippocampal vasculature: Temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus 19, 928–936 [DOI] [PubMed] [Google Scholar]
- 57. Deisseroth K., Singla S., Toda H., Monje M., Palmer T. D., Malenka R. C. (2004) Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron 42, 535–552 [DOI] [PubMed] [Google Scholar]
- 58. Sagar S. M., Sharp F. R., Curran T. (1988) Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science 240, 1328–1331 [DOI] [PubMed] [Google Scholar]
- 59. Guzowski J. F., McNaughton B. L., Barnes C. A., Worley P. F. (1999) Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 2, 1120–1124 [DOI] [PubMed] [Google Scholar]
- 60. Inoue T., Ota M., Ogawa M., Mikoshiba K., Aruga J. (2007) Zic1 and Zic3 regulate medial forebrain development through expansion of neuronal progenitors. J. Neurosci. 27, 5461–5473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Krampert M., Chirasani S. R., Wachs F. P., Aigner R., Bogdahn U., Yingling J. M., Heldin C. H., Aigner L., Heuchel R. (2010) Smad7 regulates the adult neural stem/progenitor cell pool in a transforming growth factor beta- and bone morphogenetic protein-independent manner. Mol. Cell. Biol. 30, 3685–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lyons G. E., Micales B. K., Schwarz J., Martin J. F., Olson E. N. (1995) Expression of mef2 genes in the mouse central nervous system suggests a role in neuronal maturation. J. Neurosci. 15, 5727–5738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lin X., Shah S., Bulleit R. F. (1996) The expression of MEF2 genes is implicated in CNS neuronal differentiation. Brain Res. Mol. Brain Res. 42, 307–316 [DOI] [PubMed] [Google Scholar]
- 64. Perera T. D., Coplan J. D., Lisanby S. H., Lipira C. M., Arif M., Carpio C., Spitzer G., Santarelli L., Scharf B., Hen R., Rosoklija G., Sackeim H. A., Dwork A. J. (2007) Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci. 27, 4894–4901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Snyder J. S., Soumier A., Brewer M., Pickel J., Cameron H. A. (2011) Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Abrous D. N., Koehl M., Le Moal M. (2005) Adult neurogenesis: from precursors to network and physiology. Physiol. Rev. 85, 523–569 [DOI] [PubMed] [Google Scholar]
- 67. Noonan M. A., Bulin S. E., Fuller D. C., Eisch A. J. (2010) Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J. Neurosci. 30, 304–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. DeCarolis N. A., Eisch A. J. (2010) Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology 58, 884–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Samuels B. A., Hen R. (2011) Neurogenesis and affective disorders. Eur. J. Neurosci. 33, 1152–1159 [DOI] [PubMed] [Google Scholar]
- 70. van Praag H., Kempermann G., Gage F. H. (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270 [DOI] [PubMed] [Google Scholar]
- 71. Kempermann G., Brandon E. P., Gage F. H. (1998) Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr. Biol. 8, 939–942 [DOI] [PubMed] [Google Scholar]
- 72. Jessberger S., Zhao C., Toni N., Clemenson G. D., Jr., Li Y., Gage F. H. (2007) Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J. Neurosci. 27, 9400–9407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang J. W., David D. J., Monckton J. E., Battaglia F., Hen R. (2008) Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J. Neurosci. 28, 1374–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Clark P. J., Brzezinska W. J., Puchalski E. K., Krone D. A., Rhodes J. S. (2009) Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus 19, 937–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Encinas J. M., Sierra A. (2012) Neural stem cell deforestation as the main force driving the age-related decline in adult hippocampal neurogenesis. Behav. Brain Res. 227, 433–439 [DOI] [PubMed] [Google Scholar]
- 76. Ermini F. V., Grathwohl S., Radde R., Yamaguchi M., Staufenbiel M., Palmer T. D., Jucker M. (2008) Neurogenesis and alterations of neural stem cells in mouse models of cerebral amyloidosis. Am. J. Pathol. 172, 1520–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kohl Z., Kandasamy M., Winner B., Aigner R., Gross C., Couillard-Despres S., Bogdahn U., Aigner L., Winkler J. (2007) Physical activity fails to rescue hippocampal neurogenesis deficits in the R6/2 mouse model of Huntington's disease. Brain Res. 1155, 24–33 [DOI] [PubMed] [Google Scholar]
- 78. Farioli-Vecchioli S., Saraulli D., Costanzi M., Pacioni S., Cina I., Aceti M., Micheli L., Bacci A., Cestari V., Tirone F. (2008) The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS Biol. 6, e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schmidt-Hieber C., Jonas P., Bischofberger J. (2004) Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429, 184–187 [DOI] [PubMed] [Google Scholar]
- 80. Chohan M. O., Li B., Blanchard J., Tung Y. C., Heaney A. T., Rabe A., Iqbal K., Grundke-Iqbal I. (2011) Enhancement of dentate gyrus neurogenesis, dendritic and synaptic plasticity and memory by a neurotrophic peptide. Neurobiol. Aging 32, 1420–1434 [DOI] [PubMed] [Google Scholar]
- 81. Shors T. J., Townsend D. A., Zhao M., Kozorovitskiy Y., Gould E. (2002) Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12, 578–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Saxe M. D., Battaglia F., Wang J. W., Malleret G., David D. J., Monckton J. E., Garcia A. D., Sofroniew M. V., Kandel E. R., Santarelli L., Hen R., Drew M. R. (2006) Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. U. S. A. 103, 17501–17506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wojtowicz J. M., Askew M. L., Winocur G. (2008) The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur. J. Neurosci. 27, 1494–1502 [DOI] [PubMed] [Google Scholar]
- 84. Dupret D., Revest J. M., Koehl M., Ichas F., De Giorgi F., Costet P., Abrous D. N., Piazza P. V. (2008) Spatial relational memory requires hippocampal adult neurogenesis. PLoS One 3, e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lam B. Y., Chawla S. (2007) MEF2D expression increases during neuronal differentiation of neural progenitor cells and correlates with neurite length. Neurosci. Lett. 427, 153–158 [DOI] [PubMed] [Google Scholar]
- 86. Barbosa A. C., Kim M. S., Ertunc M., Adachi M., Nelson E. D., McAnally J., Richardson J. A., Kavalali E. T., Monteggia L. M., Bassel-Duby R., Olson E. N. (2008) MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function. Proc. Natl. Acad. Sci. U. S. A. 105, 9391–9396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vetere G., Restivo L., Cole C. J., Ross P. J., Ammassari-Teule M., Josselyn S. A., Frankland P. W. (2011) Spine growth in the anterior cingulate cortex is necessary for the consolidation of contextual fear memory. Proc. Natl. Acad. Sci. U. S. A. 108, 8456–8460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ross A. P., Bartness T. J., Mielke J. G., Parent M. B. (2009) A high fructose diet impairs spatial memory in male rats. Neurobiol. Learn. Mem. 92, 410–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Aboulkassim T., Tong X. K., Chung Tse Y., Wong T. P., Woo S. B., Neet K. E., Brahimi F., Hamel E., Saragovi H. U. (2011) Ligand-dependent TrkA activity in brain differentially affects spatial learning and long-term memory. Mol. Pharmacol. 80, 498–508 [DOI] [PubMed] [Google Scholar]
- 90. John J. P., Sunyer B., Hoger H., Pollak A., Lubec G. (2009) Hippocampal synapsin isoform levels are linked to spatial memory enhancement by SGS742. Hippocampus 19, 731–738 [DOI] [PubMed] [Google Scholar]
- 91. Guo W., Allan A. M., Zong R., Zhang L., Johnson E. B., Schaller E. G., Murthy A. C., Goggin S. L., Eisch A. J., Oostra B. A., Nelson D. L., Jin P., Zhao X. (2011) Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nat. Med. 17, 559–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang L., Li P., Hsu T., Aguilar H. R., Frantz D. E., Schneider J. W., Bachoo R. M., Hsieh J. (2011) Small-molecule blocks malignant astrocyte proliferation and induces neuronal gene expression. Differentiation 81, 233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dioum E. M., Osborne J. K., Goetsch S., Russell J., Schneider J. W., Cobb M. H. (2011) A small molecule differentiation inducer increases insulin production by pancreatic beta cells. Proc. Natl. Acad. Sci. U. S. A. 108, 20713–20718 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.