Abstract
Pharmacological manipulation of opioid receptors alters feeding behavior. However, the individual contributions of each opioid receptor subtype on energy balance remain largely unknown. Herein, we investigated whether genetic disruption of the δ-opioid receptor (DOR) also controls energy homeostasis. Mice lacking DOR and wild-type mice were fed with standard diet and high-energy diet (HED). Mice were analyzed in vivo with the indirect calorimetry system, and tissues were analyzed by real-time PCR and Western blot analysis. DOR-knockout (KO) mice gained less weight (P<0.01) and had lower fat mass (P<0.01) when compared to WT mice fed an HED. Although DOR-KO mice were hyperphagic, they showed higher energy expenditure (P<0.05), which was the result of an increased activation of the thermogenic program in brown adipose tissue. The increased nonshivering thermogenesis involved the stimulation of uncoupling protein 1 (UCP1; P<0.01), peroxisome proliferator-activated receptor γ coactivator (PGC1α; P<0.05), and fibroblast growth factor 21 (FGF21; P<0.01). DOR deficiency also led to an attenuation of triglyceride content in the liver (P<0.05) in response to an HED. These findings reveal a novel role of DOR in the control of thermogenic markers and energy expenditure, and they provide a potential new therapeutic approach for the treatment of obesity.—Czyzyk, T. A., Romero-Picó, A., Pintar, J., McKinzie, J. H., Tschöp, M. H., Statnick, M. A., Nogueiras, R. Mice lacking δ-opioid receptors resist the development of diet-induced obesity.
Keywords: body weight, fat mass, energy expenditure, brown adipose tissue
The involvement of the opioid system in energy balance has been known for several decades (1, 2). Numerous studies have established that both systemic and intracerebroventricular administration of general opioid receptor antagonists reduce food intake and body weight in rodent models, including genetically obese Zucker rats and rats with diet-induced obesity (3–7). Accordingly, agonists of the opioid receptors increase food intake (8).
The classic opioid receptor system includes 3 Gi-coupled receptors, μ-, κ-, and δ-opioid receptors (MOR, KOR, and DOR), as well as their endogenous ligands (β-endorphin, dynorphins, and enkephalins). The opioid receptors are widely distributed throughout the central nervous system, and they are located in several brain areas related to the regulation of energy homeostasis. Within the hypothalamus, the blockade of both μ and κ receptors suppressed agouti-related peptide (AgRP)- and neuropeptide Y (NPY)-induced food intake (9, 10). Orexin-induced feeding behavior was also blunted by naltrexone (11). In addition, endogenous opioids regulate the mesolimbic dopamine pathway (12), and activation of opioid receptors in this area stimulates food consumption, whereas their blockade suppresses feeding behavior (13–15). Thus, the endogenous opioid system likely interacts with both homeostatic and hedonic signals to control energy balance.
Although the behavioral effects of opioid receptor agonists and antagonists were largely known, only recently genetic studies have demonstrated a key role of the endogenous opioid system in energy homeostasis. MOR-deficient mice were resistant to diet-induced obesity and showed an improved glucose tolerance compared to wild-type mice (16, 17) and showed a decreased motivation to eat both normal diet and sucrose pellets (18). Similarly to mice lacking MOR, KOR-deficient mice were resistant to diet-induced obesity, and this was driven by maintenance of energy expenditure and locomotor activity levels (19). However, to our knowledge, the potential endogenous role of the DOR in the regulation of energy balance and metabolism has not been unmasked.
In the present work, we sought to investigate whether genetic ablation of DOR in mice alters energy, glucose, or lipid metabolism in response to a calorically dense high-energy diet (HED). We found that DOR-knockout (KO) mice were resistant to weight gain after prolonged exposure to HED that was driven by maintenance of energy expenditure and increased thermogenic activity in the brown adipose tissue (BAT).
MATERIALS AND METHODS
Animals
All procedures were approved by the Institutional Animal Care and Use Committees of Eli Lilly and Co. (Indianapolis, IN, USA) and the University of Cincinnati and were in accordance with the U.S. National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals. The principles of laboratory animal care were followed, as well as specific national laws where applicable.
Wild-type (WT) and DOR-KO male mice (20) were bred on the 129S6 background for 12 generations at Taconic Farms (Germantown, NY, USA) until shipment. Mice were fed a high-fat HED (RD12451; 45% fat, 20% protein, 35% carbohydrate, 4.73 kcal/g; Research Diets, New Brunswick, NJ, USA) beginning at 7–8 wk of age. Age-matched controls were maintained on a standard rodent chow diet (NIH-31M; 5% fat, 4.02 kcal/g; Taconic Farms). All mice were maintained on a 12-h light-dark cycle (lights on, 7 AM; lights off, 7 PM).
Determination of body composition and energy balance
Whole-body composition was measured using NMR imaging (Whole Body Composition Analyzer; EchoMRI, Houston, TX, USA). Animals were monitored in a custom 32-cage indirect calorimetry, food intake, and locomotor activity monitoring system (TSE LabMaster; TSE Systems, Bad Homburg, Germany), as described previously (19, 21). Mice were acclimated for 48 h to the test chambers and then were monitored for an additional 48 h. Data collected from the last 48 h were used to calculate all parameters for which results are reported.
Quantitative RT-PCR analysis
Total RNA was extracted from frozen tissue samples homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and purified with the MagAttract RNA Universal Tissue M48 kit (Qiagen, Germantown, MD, USA) with DNase treatment. cDNAs were synthesized using 1 μg of total RNA (high-capacity cDNA reverse transcription kit; Applied Biosystems, Carlsbad, CA, USA). Real-time quantitative PCR was performed using TaqMan probe and primer sets (Applied Biosystems; Supplemental Table S1). Reactions were cycled in an ABI Prism 7900HT Sequence Detection System (Applied Biosystems), and data were analyzed with Sequence Detection Software (SDS) 2.2.2 (Applied Biosystems). Samples were measured in triplicate. Data were normalized to acidic ribosomal binding protein (ARBP) or hypoxanthine guanine phosphoribosyltransferase (HPRT).
Western blot analysis
Western blots were performed as described previously (21, 22). Briefly, total protein lysates from BAT (15 μg) were subjected to SDS-PAGE, electrotransferred onto a polyvinylidene difluoride membrane, and probed with the indicated antibodies: acetyl coenzyme A (CoA) carboxylase (ACC), AMP-activated protein kinase α1 (AMPKα1) and AMPKα2 (Upstate, Lake Placid, NY, USA); phospho-AMPK-Thr172 (pAMPK; Cell Signaling, Danvers, MA, USA); uncoupling protein 1 (UCP1), UCP3, and β-actin in liver or β-tubulin in BAT (Abcam, Cambridge, UK); and fatty acid synthase (FAS; Santa Cruz Biotechnology, Santa Cruz, CA, USA). For protein detection, we used horseradish peroxidase-conjugated secondary antibodies and chemiluminescence (Amersham Biosciences, Little Chalfont, UK). We used 8 mice/group, and the protein levels were normalized to β-actin or β-tubulin for each sample.
Measurement of hepatic malonyl CoA and triglyceride (TG) levels
Hepatic malonyl CoA levels were measured by liquid chromatography–tandem mass spectrometry (LC-MS/MS) using a modified method of Minkler et al. (23), as described previously (19, 23). Total TG content was measured with Infinity TG reagent (Thermo Fisher Scientific, Middletown, VA, USA) following manufacturer's instructions (19, 21).
Cold adaptation studies
Animals were placed in a cold-temperature environment of 4°C, and body core temperature was measured using a rectal thermometer (Physitemp, Clifton, NJ, USA). Heat production was visualized using a high-resolution infrared camera (FLIR PM280; FLIR Systems, Portland, OR, USA). Images of the backs of the mice were captured before exposure and at 2, 4, and 6 h after exposure to the 4°C environment. Infrared thermography images were taken from the upper half of the body to specifically visualize heat production from the BAT (24).
Plasma collection and hormonal analysis
Mice were sacrificed 4 h after the start of the light cycle. Whole trunk blood was collected in tubes containing 20 μl 0.5 M EDTA and then were spun for 15 min at 3000 g and 4°C. Plasma was collected and stored at −80°C until being shipped to Millipore (St. Charles, MI, USA) for radioimmunoassay analysis of leptin, corticosterone, T4, and IGF-1. Plasma TG and free fatty acid (FFA) levels were assessed using a commercial kit based on a colorimetric method (glucose and triglyceride; Spinreact, Girona, Spain).
Glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs)
Blood glucose levels were measured with an Accucheck glucometer (Roche, Minneapolis, MN, USA) after an intraperitoneal injection of either 2 mg/g d-glucose (Sigma, St. Louis, MO, USA) or 0.075 U/kg insulin (Humalin; Eli Lilly; ref. 24). Area under the curve (AUC) values were determined, and data were analyzed with 2-way ANOVA and post hoc analysis, as described previously (19).
Data analysis and statistics
Values are plotted as the means ± se for each genotype. Statistical analyses were conducted with GraphPad Prism 4 (GraphPad Software, San Diego, CA, USA). Comparisons among genotype and diet were performed with 2-way ANOVA and Bonferroni posttests. Unpaired t tests were also used to compare 2 groups as indicated. Growth curves were analyzed with 2-way repeated-measures ANOVA. Analysis of covariance (ANCOVA) was used to test the effects of lean mass on energy expenditure. Statistical significance was set at P ≤ 0.05 for all experiments.
RESULTS
DOR-KO mice fed chow diet do not have alterations in body weight or adiposity
No differences in body weight (Fig. 1A), fat mass (Fig. 1B), fat-free mass (Fig. 1C) or food intake (Fig. 1D) were found between DOR-KO and WT mice when they were fed the chow diet.
Figure 1.
DOR-KO mice fed the chow diet have a normal phenotype. Body weight (A), fat mass (B), fat-free mass (C), and cumulative food intake (D) over 48 h normalized to body weight of WT and DOR-KO mice after 8 wk of free access to chow diet. n = 7–8/group.
DOR-KO mice fed HED have reduced body weight and adiposity but increased energy expenditure
Age-matched male WT and DOR-KO 129S6 mice were maintained on an HED from 8 wk of age (45% kcal fat, 4.73 kcal/g) for 13 wk to assess their metabolic phenotypes. DOR-KO mice gained significantly less body weight than WT mice when fed the HED (Fig. 2A, B). Weekly body weight measurements demonstrated that WT mice gained 13.9 ± 0.5 g during HED exposure, compared to DOR-KO mice, which gained only 8.1 ± 1.1 g (Fig. 2A). Body composition analysis with quantitative NMR revealed that DOR-KO mice fed HED accrued less fat mass compared to WT mice after 13 wk of HED consumption (Fig. 2C), with no changes in fat-free mass (Fig. 2D). Changes in body weight and composition were not likely due to daily reductions in total caloric intake, since total HED intake in DOR-KO mice was slightly increased relative to WT mice during a 24-h period (Fig. 2E), and this increase became significant when food intake was corrected per gram of body weight (Fig. 2F). Food efficiency was significantly lower in DOR-KO mice when compared to WT mice fed the HED (Fig. 2G) and is consistent with the reductions in body weight and fat mass in DOR-KO mice fed HED. Energy expenditure of DOR-KO mice was higher than in WT mice in both the light and dark phases of the circadian cycle after 13 wk of HED consumption (Fig. 2H, I). Similar results were found when energy expenditure was corrected per gram of fat-free mass (Fig. 2J). In addition, an ANCOVA test was used to explore the effects of lean mass and energy expenditure; results indicated that the increased energy expenditure is independent of lean mass (Table 1). Despite increased energy expenditure, spontaneous locomotor activity levels remained unchanged between WT and DOR-KO mice fed an HED (Fig. 2K), suggesting that other mechanisms rather than increased locomotor activity were stimulating energy expenditure in DOR-KO mice.
Figure 2.
Diet-dependent alterations in body weight, adiposity, food intake, and energy expenditure in DOR-KO mice. A) Weekly body weights in KO and WT mice fed an HED. B) Body weight after 13 wk of free access to an HED. Mice were ∼6 mo of age at time of measurement. C–F) Fat mass (C), fat-free mass (D), cumulative food intake over 24 h (E), and cumulative food intake over 24 h normalized to body weight (F). Food intake was measured over a 24-h period in 5-mo-old DOR-KO and WT mice fed chow or HED. G) Food efficiency was calculated as the ratio between body weight gain over the experimental period and cumulative food intake and was expressed as a percentage. H) Forty-eight-hour total energy expenditure determined by indirect calorimetry. I) Real-time measurements of energy expenditure in mice after 13 wk of HED consumption. Measurements were taken every 45 min. Shaded areas indicate lights off. J) Energy expenditure corrected by grams of lean mass in mice after 13 wk of HED consumption. K) Locomotor activity during a 48-h period. *P < 0.005, **P < 0.01, ***P < 0.001; n = 7–8/group.
Table 1.
ANCOVA of lean mass effects on energy expenditure
| Measure | Df | Sum of squares | Mean square | F | P |
|---|---|---|---|---|---|
| Lean mass | 1 | 9,153 | 9,153 | 4.54 | 0.05645 |
| F1 | 1 | 45,660 | 45,660 | 22.65 | 0.00059 |
| Lean mass:F1 | 1 | 5,038 | 5,038 | 2.50 | 0.14215 |
| Residuals | 11 | 22,166 | 2,015 |
Deletion of DOR activates thermogenesis
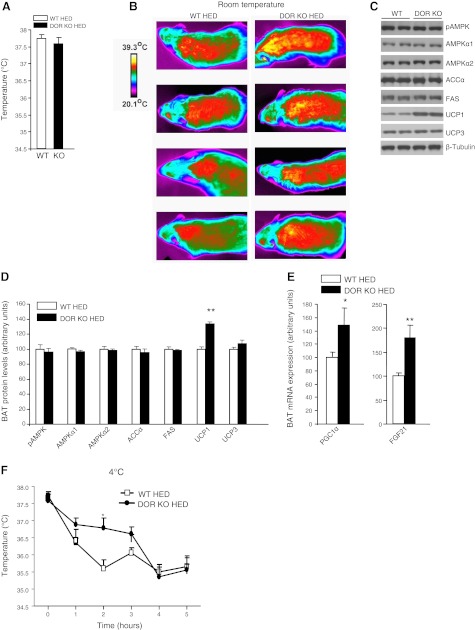
To investigate the potential mechanism responsible for increased energy expenditure in DOR-KO mice, we measured core body temperature and the expression of several thermogenic markers in BAT. There were no differences in basal core body temperatures between DOR-KO and WT control mice fed an HED (Fig. 3A). However, DOR-KO mice showed increased body surface temperatures compared with that of wild-type mice when they were kept at an ambient temperature of 22°C (Fig. 3B). In agreement with the higher intrascapular surface temperatures, the levels of UCP1 in BAT were increased in DOR-KO mice in comparison to WT mice fed an HED (Fig. 3C, D). However, the protein levels of several key enzymes involved in lipid metabolism remained unchanged in the BAT between WT and DOR-KO mice, including phosphorylated AMPK and ACCα (Fig. 3C, D). DOR deficiency stimulated the expression of genes promoting thermogenesis, including peroxisome proliferator-activated receptor γ coactivator-1-α (PGC1α) and fibroblast growth factor (FGF21) (Fig. 3E). It is also important to note that we detected DOR mRNA expression in BAT, in comparison to the lack of expression of KOR or MOR in this tissue (Table 2). When we carried out a cold tolerance test, we found that DOR-KO mice were more tolerant to cold than WT mice fed an HED, particularly after 2 h (WT vs. DOR-KO: 35.6±0.25 vs. 36.7±0.28°C; P<0.05, n=7; Fig. 3F). Overall, our findings indicate that DOR deficiency up-regulates thermogenic markers in BAT.
Figure 3.
BAT metabolism in DOR-KO mice fed an HED. A) Basal core body temperature. B) Body surface temperature at 22°C. Images are representative from 4 of 6 total mice per genotype that were analyzed. C, D) Protein levels of pAMPK, AMPKα1, AMPKα2, ACCα, FAS, UCP1, and UCP3 in brown adipose tissue of WT and DOR-KO mice after 13 wk of HED consumption. C) Representative blot. D) Quantification of data. E) mRNA expression levels of PGC1α and FGF21 in BAT of WT and DOR-KO mice. F) Body core temperature measurements over a 5-h period at 4°C. *P < 0.05, **P < 0.01; n = 7–8/group.
Table 2.
Summary of peripheral opioid receptor and ligand expression
| Receptor or ligand | Liver | WAT | Adrenals | Pituitary | Skeletal muscle | BAT |
|---|---|---|---|---|---|---|
| MOR | − | − | + | + | − | − |
| KOR | − | − | − | + | − | − |
| DOR | − | − | + | − | − | ++ |
| proENK | ++ | ++ | + | + | ++ | ++ |
| proDYN | − | − | − | + | − | − |
| POMC | − | − | +++ | ++++ | ND | ND |
++++, Very high expression; +++, high expression; ++, moderate expression; +, weak evidence of expression; −, no expression; ND, no data.
DOR-KO mice fed an HED have reduced hepatic fat storage
It is well known that diet-induced obesity causes liver steatosis. Thus, we aimed to investigate whether DOR deficiency was also affecting hepatic lipid metabolism. We found that total hepatic TG content was lower in DOR-KO mice after 13 wk of HED consumption (Fig. 4A). Lower liver TGs could be due to either an increase in hepatic fatty acid oxidation or a reduction in TG synthesis. Since malonyl CoA is the primary negative regulator of fatty acid β-oxidation, we measured malonyl CoA in the liver using LC-MS/MS methods and found that malonyl CoA levels were similar in WT and DOR-KO mice (Fig. 4B). However, the gene expression of adipose triglyceride lipase (ATGL), a major hepatic lipase that regulates TG turnover (25), was increased in DOR-KO mice fed HED in comparison to WT mice (Fig. 4C), whereas no changes were found in the gene expression of other enzymes involved in lipid metabolism (Fig. 4C). Taken together, these data suggest that DOR-KO mice fed an HED have reduced hepatic fat storage.
Figure 4.
Reductions in hepatic TGs in DOR-KO mice fed an HED. A) Total liver TG content after HED exposure. B) Liver malonyl CoA levels in WT and DOR-KO mice after 13 wk of HED. C) Quantitative PCR analysis of ACCα, CPT1, FAS, PPARα, SCD1, and ATGL in liver after 13 wk of HED. *P < 0.05; n = 7–8/group.
DOR-KO mice fed an HED have increased fatty acid turnover in white adipose tissue (WAT)
The lower amount of fat mass in DOR-KO mice fed an HED in comparison to their WT controls suggests that the lack of DOR could inhibit lipid uptake, synthesis, and/or storage in WAT. To test that hypothesis, we analyzed the expression profile of key enzymes involved in lipid metabolism in this tissue. DOR deficiency markedly increased mRNA levels of genes promoting lipogenesis and TG storage in adipocytes, including stearoyl-CoA desaturase-1 (SCD1), lipoprotein lipase (LPL), ACCα, and FAS (Fig. 5). mRNA levels of lipolytic enzymes, including ATGL, were also significantly stimulated in DOR-KO mice, whereas carnitine palmitoyl transferase-1a (CPT-1a) was unmodified (Fig. 5). These results suggest that fatty acid turnover is elevated in the WAT of DOR-KO mice fed an HED.
Figure 5.
Increased lipid turnover in WAT in DOR-KO mice fed an HED. mRNA expression of ACCα, SCD1, LPL, ATGL, CPT1, and FAS in WAT after 13 wk of HED. **P < 0.01, ***P < 0.001; n = 7–8/group.
Glucose homeostasis in DOR-KO mice
Next, we assessed key parameters of glucose homeostasis in WT and DOR-KO mice. This analysis revealed unaltered fasting blood glucose concentrations in DOR-KO mice compared to controls fed the chow diet (Fig. 6A). However, when we subjected DOR-KO mice fed the chow diet to intraperitoneal GTTs (ipGTTs), we found a higher tolerance to glucose (Fig. 6A), and the area under the curve was significantly lower in DOR-KO mice than in WT mice (Fig. 6B). When we subjected DOR-KO mice fed on HED to ipGTTs, these mice did not exhibit alterations in glucose tolerance compared to their control littermates (Fig. 6C, D). Furthermore, we subjected DOR-KO mice to ITTs and failed to find any difference between DOR-KO mice compared to controls fed with chow diet (Fig. 6E, F) or HED (Fig. 6G, H).
Figure 6.
Glucose tolerance and insulin sensitivity in DOR-KO mice. A) IpGTT in mice fed the chow diet. B) Area under the curve after the ipGTT in mice fed the chow diet. C) ipGTT after 13 wk of HED. D) Area under the curve after the ipGTT in mice fed the HED. E) ITT in mice fed the chow diet. F) Area under the curve after the ITT in mice fed the chow diet. G) ITT after 14 wk of HED. H) Area under the curve after the ITT in mice fed the HED. n = 7–8/group.
DOR-KO mice have increased plasma thyroid hormone and decreased leptin and TG levels
We measured plasma leptin, FFA, corticosterone, and insulin-like growth factor-1 (IGF-1) as a surrogate marker for growth hormone, TG, and thyroxine T4 in WT and DOR-KO mice maintained on HED, since these hormones can profoundly alter metabolism. Plasma leptin levels were lower in DOR-KO mice compared to WT controls fed the HED (Fig. 7A) and were consistent with reduced adiposity in DOR-KO mice. FFA (Fig. 7B), corticosterone (Fig. 7C), and IGF-1 (Fig. 7D) levels were not significantly different in DOR-KO mice compared to WT mice fed an HED. Plasma TG levels were decreased consistent with the reductions in liver (Fig. 7E). However, levels of circulating thyroxine T4 were increased (Fig. 7F) in DOR-KO mice.
Figure 7.
Circulating parameters in plasma: leptin (A), FFAs (B), corticosterone (C), IGF-1 (D), TGs (E), and thyroid hormone T4 (F) levels in DOR-KO mice. *P < 0.05, **P < 0.01; n = 7–8/group.
DISCUSSION
Mice deficient for DOR show anxiogenic- and depressive-like responses (26), impaired place conditioning (27), and increased chronic pain (28). Pharmacological studies have indicated that manipulation of the DOR evokes changes in food intake and body weight (29). However, there have been no studies identifying the metabolic phenotypes in mice lacking DOR. We found that DOR-KO mice fed a high-fat, energy-dense diet for 13 wk gained significantly less body weight than WT controls. The higher energy expenditure, increased thermogenic program, and reduction in hepatic TGs are consistent with reduced body weight and fat mass in DOR-KO mice after HED treatment. These findings support the hypothesis that overactivation of opioid receptors contribute to the development of obesity when there is prolonged consumption of high-fat, calorically dense diets (30).
Previous reports have described an interaction between glucose metabolism and the DOR. In humans, plasma enkephalin levels were significantly higher in patients with diabetes than in controls (31). In animals, the results are controversial, since enkephalin analogues stimulated deoxyglucose uptake in obese diabetic mice (32) and increased glucose and insulin concentrations in lean and ob/ob mice (33). However, others showed that the administration of enkephalin inhibits insulin secretion induced by glucose (34, 35). Despite changes in fat mass, body weight, and increased hepatic proENK mRNA levels (data not shown), DOR-KO mice did not show improved glycemic control or insulin sensitivity. This suggests that, relative to fat mass, DOR-KO mice fed an HED already have impaired glycemic control without obesity. On the contrary, when mice were fed a chow diet and had similar amounts of fat, we found that DOR-deficient mice showed improved glucose tolerance. Overall, our results suggest that, depending on the diet, there is a shift in the glycemic control of DOR-KO mice.
The opioid receptors are widely distributed throughout the central nervous system, and they are located in several brain areas related to the regulation of energy homeostasis (1, 36). The first work reporting that the blockade of opioid receptors decreases food intake used naloxone, a general opioid receptor antagonist (37). Since then, numerous studies have established that both systemic and intracerebroventricular administration of general opioid receptor antagonists reduce food intake and body weight in rodent models, including genetically obese Zucker and diet-induced obesity model rats (3–7). Consistently, specific antagonists for each receptor significantly reduced body weight and food intake in obese rats (38). Chronic central administration of δ1 [[D-Ala2,Leu5,Cys6]-enkephalin (DALCE)] and δ2 [naltrindole isothiocyanate (NTII)] opioid receptor subtype antagonists reduced food intake and body weight in obese rats (38). Our results, however, indicated that mice lacking DOR gained less weight, even though they were hyperphagic when fed an HED. The hyperphagia found in DOR-KO mice is likely a compensatory response driven by the energy demands to support the increase in energy expenditure observed in many lean KO mouse models (19). Indeed, further studies are necessary to elucidate whether this hyperphagia is due to homeostatic or hedonic mechanisms.
In addition to its effects on food intake, the administration of DOR antagonists in the paraventricular nucleus significantly increased the metabolic response to cold in obese rats during thermoneutrality (39). Our findings are also consistent with this study, supporting an important role of DOR in the regulation of the thermogenic program. For instance, we found that mice lacking DOR showed higher energy expenditure than WT mice when fed an HED. The increased energy expenditure cannot be explained by changes in locomotor activity, since those remained unaltered between DOR-KO and WT mice. Previous studies have clearly described the key role of BAT in energy expenditure through thermogenesis in the form of heat dissipation (40, 41). DOR-deficient mice had an increase in body surface temperature and were more tolerant to cold when they were acutely exposed to 4°C. At a molecular level, we found that the levels of the thermogenic protein UCP1 were increased in the BAT of DOR-KO mice fed the HED. Consistent with activation of BAT, the gene expression of FGF21, which contributes to thermogenic activation (42), was stimulated in the BAT of DOR-KO mice fed the HED. The transcriptional coactivator, PGC1α, which activates mitochondrial biogenesis and oxidative metabolism (43), was also up-regulated in the BAT of DOR-KO mice fed an HED. To test whether deletion of DOR might produce changes in thermogenesis directly in BAT, we assessed DOR and its ligand enkephalin gene expression in several metabolic peripheral tissues. We found that in addition to its wide expression throughout the central nervous system, both DOR and ligand are located in BAT. Thus, the enkephalin/DOR system might modulate thermogenic markers directly through the central nervous system/BAT axis. Further studies should elucidate which specific neuronal populations are responsible for these actions. Overall, the increase in body surface temperature, together with the up-regulation of thermogenic markers in BAT, suggests an increased resting thermogenesis in DOR-KO mice fed an HED.
One of the main risks for developing nonalcoholic fatty liver disease is diet-induced obesity. Because we found that DOR-KO mice gain less weight than their WT littermates when fed an HED, we hypothesized that the lack of DOR might affect liver metabolism. Depletion of DOR decreased the amount of TG accumulation in the liver in comparison with controls. Consistently, we detected higher hepatic levels of the lipolytic enzyme ATGL (44), indicating that the reduction of hepatic TG appears to involve mechanisms of lipid mobilization. However, in the WAT of DOR-KO mice fed an HED, we detected higher levels of several lipogenic enzymes and also increased expression of ATGL. These findings suggest that futile cycling in adipocytes might contribute to increased energy expenditure and decreased body weight in DOR-KO mice fed an HED. Overall, our data support the view that these alterations in lipid metabolism in liver and WAT results in the protection against hepatic steatosis and a reduction of fat mass that accumulates with energy-dense diets.
Circulating thyroid hormone levels were significantly higher in DOR-KO mice fed HED. Hyperthyroidism is a hypermetabolic disorder characterized by increased energy expenditure and weight loss despite marked hyperphagia (45). Furthermore, hyperthyroidism up-regulates thermogenic markers in BAT (46, 47). Our findings showing that DOR-deficient mice are hyperthyroid are in agreement with previous reports demonstrating that enkephalin decreased TRH release (48, 49). Thus, it is plausible to hypothesize that the high levels of thyroid hormones in DOR-KO mice fed HED are, at least partially, responsible for the phenotype observed in these mice.
In summary, this study documents the prominent role of DOR in modulating energy metabolism and sensitivity to diet-induced obesity. The resistance of DOR-KO mice to HED is mainly caused by the increased thermogenic markers in BAT that trigger energy expenditure, facilitating the decreased gain of body weight and fat mass. In view of the important central effects caused by the opioid system on analgesia, addiction, and other psychosocial behaviors, these data are of importance, as they suggest that the specific inactivation of DOR populations in metabolic peripheral tissues might be effective avoiding side effects associated with centrally acting pharmacotherapies.
Supplementary Material
Acknowledgments
This work has been supported by grants from Ministerio de Educacion y Ciencia (R.N.: RYC-2008-02219 and SAF2009-07049), Xunta de Galicia (R.N.: 2010/14), and the European Union (R.N.: Health-F2-2008-223713, Reprobesity; FP7/2007-2013 under grant agreement no. 245009, NeuroFAST; and ERC-2011-StG-OBESITY53-281408). Centro de Investigación Biomédica en Red de Fisiopatología de la Obesidad y Nutrición is an initiative of Instituto de Salud Carlos III. Research at the University of Cincinnati was funded by the U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants NIDDK59630, NIDDK69987, and NIDDK56863 (M.H.T.).
Author contributions: T.A.C., conception and design of data and drafting the article; A.R., analysis and interpretation of data; J.P., analysis and interpretation of data; J.H.M., analysis and interpretation of data; M.H.T. and M.A.S., critical revision for important intellectual content; R.N., conception and design of data, drafting the article or critical revision for important intellectual content. During the course of these studies, T.A.C., J.H.M, and M.A.S. were employees of Lilly Research Laboratories, Eli Lilly and Company. Sponsorship was indirect through personnel and generation of some of the data. No direct funds were granted.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ACC
- acetyl coenzyme A carboxylase
- AgRP
- agouti related peptide
- AMPK
- AMP-activated protein kinase
- ANCOVA
- analysis of covariance
- ARBP
- acidic ribosomal binding protein
- ATGL
- adipose triglyceride lipase
- BAT
- brown adipose tissue
- CoA
- coenzyme A
- DOR
- δ-opioid receptor
- FAS
- fatty acid synthase
- FFA
- free fatty acid
- FGF21
- fibroblast growth factor 21
- GTT
- glucose tolerance test
- HED
- high-energy diet
- HPRT
- hypoxanthine guanine phosphoribosyltransferase
- IGF-1
- insulin-like growth factor-1
- ipGTT
- intraperitoneal glucose tolerance test
- ITT
- insulin tolerance test
- KO
- knockout
- KOR
- κ-opioid receptor
- LC-MS/MS
- liquid chromatography–tandem mass spectrometry
- MOR
- μ-opioid receptor
- NPY
- neuropeptide Y
- PGC1α
- peroxisome proliferator-activated receptor γ coactivator 1α
- SCD1
- stearoyl-CoA desaturase-1
- TG
- triglyceride
- UCP
- uncoupling protein
- WAT
- white adipose tissue
- WT
- wild type
REFERENCES
- 1. Yeomans M. R., Gray R. W. (2002) Opioid peptides and the control of human ingestive behaviour. Neurosci. Biobehav. Rev. 26, 713–728 [DOI] [PubMed] [Google Scholar]
- 2. Martin W. R. (1983) Pharmacology of opioids. Pharmacol. Rev. 35, 283–323 [PubMed] [Google Scholar]
- 3. Levine A. S., Grace M., Billington C. J. (1991) Beta-funaltrexamine (beta-FNA) decreases deprivation and opioid-induced feeding. Brain Res. 562, 281–284 [DOI] [PubMed] [Google Scholar]
- 4. Shaw W. N., Mitch C. H., Leander J. D., Mendelsohn L. G., Zimmerman D. M. (1991) The effect of the opioid antagonist LY255582 on body weight of the obese Zucker rat. Int. J. Obes. 15, 387–395 [PubMed] [Google Scholar]
- 5. Shaw W. N. (1993) Long-term treatment of obese Zucker rats with LY255582 and other appetite suppressants. Pharmacol. Biochem. Behav. 46, 653–659 [DOI] [PubMed] [Google Scholar]
- 6. Statnick M. A., Tinsley F. C., Eastwood B. J., Suter T. M., Mitch C. H., Heiman M. L. (2003) Peptides that regulate food intake: antagonism of opioid receptors reduces body fat in obese rats by decreasing food intake and stimulating lipid utilization. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R1399–R1408 [DOI] [PubMed] [Google Scholar]
- 7. Sahr A. E., Sindelar D. K., Alexander-Chacko J. T., Eastwood B. J., Mitch C. H., Statnick M. A. (2008) Activation of mesolimbic dopamine neurons during novel and daily limited access to palatable food is blocked by the opioid antagonist LY255582. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R463–R471 [DOI] [PubMed] [Google Scholar]
- 8. Gosnell B. A., Levine A. S., Morley J. E. (1986) The stimulation of food intake by selective agonists of μ, κ and δ opioid receptors. Life Sci. 38, 1081–1088 [DOI] [PubMed] [Google Scholar]
- 9. Brugman S., Clegg D. J., Woods S. C., Seeley R. J. (2002) Combined blockade of both μ- and κ-opioid receptors prevents the acute orexigenic action of Agouti-related protein. Endocrinology 143, 4265–4270 [DOI] [PubMed] [Google Scholar]
- 10. Kotz C. M., Grace M. K., Billington C. J., Levine A. S. (1993) The effect of norbinaltorphimine, β-funaltrexamine and naltrindole on NPY-induced feeding. Brain Res. 631, 325–328 [DOI] [PubMed] [Google Scholar]
- 11. Sweet D. C., Levine A. S., Kotz C. M. (2004) Functional opioid pathways are necessary for hypocretin-1 (orexin-A)-induced feeding. Peptides 25, 307–314 [DOI] [PubMed] [Google Scholar]
- 12. Spanagel R., Herz A., Shippenberg T. S. (1992) Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc. Natl. Acad. Sci. U. S. A. 89, 2046–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herz A. (1998) Opioid reward mechanisms: a key role in drug abuse? Can. J. Physiol. Pharmacol. 76, 252–258 [DOI] [PubMed] [Google Scholar]
- 14. Reid L. D. (1985) Endogenous opioid peptides and regulation of drinking and feeding. Am. J. Clin. Nutr. 42, 1099–1132 [DOI] [PubMed] [Google Scholar]
- 15. Levine A. S., Billington C. J. (1997) Why do we eat? A neural systems approach. Annu. Rev. Nutr. 17, 597–619 [DOI] [PubMed] [Google Scholar]
- 16. Tabarin A., Diz-Chaves Y., Carmona Mdel C., Catargi B., Zorrilla E. P., Roberts A. J., Coscina D. V., Rousset S., Redonnet A., Parker G. C., Inoue K., Ricquier D., Penicaud L., Kieffer B. L., Koob G. F. (2005) Resistance to diet-induced obesity in mu-opioid receptor-deficient mice: evidence for a “thrifty gene”. Diabetes 54, 3510–3516 [DOI] [PubMed] [Google Scholar]
- 17. Zuberi A. R., Townsend L., Patterson L., Zheng H., Berthoud H. R. (2008) Increased adiposity on normal diet, but decreased susceptibility to diet-induced obesity in mu-opioid receptor-deficient mice. Eur. J. Pharmacol. 585, 14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Papaleo F., Kieffer B. L., Tabarin A., Contarino A. (2007) Decreased motivation to eat in mu-opioid receptor-deficient mice. Eur. J. Neurosci. 25, 3398–3405 [DOI] [PubMed] [Google Scholar]
- 19. Czyzyk T. A., Nogueiras R., Lockwood J. F., McKinzie J. H., Coskun T., Pintar J. E., Hammond C., Tschop M. H., Statnick M. A. κ-Opioid receptors control the metabolic response to a high-energy diet in mice. FASEB J. 24, 1151–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu Y., King M. A., Schuller A. G., Nitsche J. F., Reidl M., Elde R. P., Unterwald E., Pasternak G. W., Pintar J. E. (1999) Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron 24, 243–252 [DOI] [PubMed] [Google Scholar]
- 21. Nogueiras R., Perez-Tilve D., Veyrat-Durebex C., Morgan D. A., Varela L., Haynes W. G., Patterson J. T., Disse E., Pfluger P. T., Lopez M., Woods S. C., DiMarchi R., Dieguez C., Rahmouni K., Rohner-Jeanrenaud F., Tschop M. H. (2009) Direct control of peripheral lipid deposition by CNS GLP-1 receptor signaling is mediated by the sympathetic nervous system and blunted in diet-induced obesity. J. Neurosci. 29, 5916–5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Velasquez D. A., Martinez G., Romero A., Vazquez M. J., Boit K. D., Dopeso-Reyes I. G., Lopez M., Vidal A., Nogueiras R., Dieguez C. The central Sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin. Diabetes 60, 1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Minkler P. E., Kerner J., Kasumov T., Parland W., Hoppel C. L. (2006) Quantification of malonyl-coenzyme A in tissue specimens by high-performance liquid chromatography/mass spectrometry. Anal. Biochem. 352, 24–32 [DOI] [PubMed] [Google Scholar]
- 24. Lee S. J., Pfluger P. T., Kim J. Y., Nogueiras R., Duran A., Pages G., Pouyssegur J., Tschop M. H., Diaz-Meco M. T., Moscat J. A. functional role for the p62-ERK1 axis in the control of energy homeostasis and adipogenesis. EMBO Rep 11, 226–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ong K. T., Mashek M. T., Bu S. Y., Greenberg A. S., Mashek D. G. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology 53, 116–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Filliol D., Ghozland S., Chluba J., Martin M., Matthes H. W., Simonin F., Befort K., Gaveriaux-Ruff C., Dierich A., LeMeur M., Valverde O., Maldonado R., Kieffer B. L. (2000) Mice deficient for δ- and μ-opioid receptors exhibit opposing alterations of emotional responses. Nat. Genet. 25, 195–200 [DOI] [PubMed] [Google Scholar]
- 27. Le Merrer J., Plaza-Zabala A., Boca C. D., Matifas A., Maldonado R., Kieffer B. L. (2011) Deletion of the delta opioid receptor gene impairs place conditioning but preserves morphine reinforcement. Biol. Psychiatry 69, 700–703 [DOI] [PubMed] [Google Scholar]
- 28. Gaveriaux-Ruff C., Nozaki C., Nadal X., Hever X. C., Weibel R., Matifas A., Reiss D., Filliol D., Nassar M. A., Wood J. N., Maldonado R., Kieffer B. L. Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain 152, 1238–1248 [DOI] [PubMed] [Google Scholar]
- 29. Vuong C., Van Uum S. H., O'Dell L. E., Lutfy K., Friedman T. C. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr. Rev. 31, 98–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Welch C. C., Kim E. M., Grace M. K., Billington C. J., Levine A. S. (1996) Palatability-induced hyperphagia increases hypothalamic Dynorphin peptide and mRNA levels. Brain Res. 721, 126–131 [DOI] [PubMed] [Google Scholar]
- 31. Negri M., Tonnarini G., D'Alessandro M., Fallucca F. (1992) Plasma met-enkephalin in type I diabetes. Metabolism 41, 460–461 [DOI] [PubMed] [Google Scholar]
- 32. Evans A. A., Tunnicliffe G., Knights P., Bailey C. J., Smith M. E. (2001) Delta opioid receptors mediate glucose uptake in skeletal muscles of lean and obese-diabetic (ob/ob) mice. Metabolism 50, 1402–1408 [DOI] [PubMed] [Google Scholar]
- 33. Bailey C. J., Flatt P. R. (1987) Increased responsiveness to glucoregulatory effect of opiates in obese-diabetic ob/ob mice. Diabetologia 30, 33–37 [DOI] [PubMed] [Google Scholar]
- 34. Giugliano D., Quatraro A., Consoli G., Ceriello A., Torella R., D'Onofrio F. (1987) Inhibitory effect of enkephalin on insulin secretion in healthy subjects and in non insulin-dependent diabetic subjects. Metabolism 36, 286–289 [DOI] [PubMed] [Google Scholar]
- 35. Ahren B. (1989) Effects of beta-endorphin, met-enkephalin, and dynorphin A on basal and stimulated insulin secretion in the mouse. Int. J. Pancreatol. 5, 165–178 [DOI] [PubMed] [Google Scholar]
- 36. Bodnar R. J. (2009) Endogenous opiates and behavior: 2008. Peptides 30, 2432–2479 [DOI] [PubMed] [Google Scholar]
- 37. Holtzman S. G. (1979) Suppression of appetitive behavior in the rat by naloxone: lack of effect of prior morphine dependence. Life Sci. 24, 219–226 [DOI] [PubMed] [Google Scholar]
- 38. Cole J. L., Berman N., Bodnar R. J. (1997) Evaluation of chronic opioid receptor antagonist effects upon weight and intake measures in lean and obese Zucker rats. Peptides 18, 1201–1207 [DOI] [PubMed] [Google Scholar]
- 39. Rodd D. W., Loomis J. L., Farrell P. A. (1994) Influence of opioids in hypothalamic nuclei on cold thermogenesis of lean and obese LA/N-cp rats. Obes. Res. 2, 246–254 [DOI] [PubMed] [Google Scholar]
- 40. Himms-Hagen J. (1984) Thermogenesis in brown adipose tissue as an energy buffer. Implications for obesity. N. Engl. J. Med. 311, 1549–1558 [DOI] [PubMed] [Google Scholar]
- 41. Lowell B. B., Spiegelman B. M. (2000) Towards a molecular understanding of adaptive thermogenesis. Nature 404, 652–660 [DOI] [PubMed] [Google Scholar]
- 42. Hondares E., Rosell M., Gonzalez F. J., Giralt M., Iglesias R., Villarroya F. Hepatic FGF21 expression is induced at birth via PPARα in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab 11, 206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Handschin C., Spiegelman B. M. (2006) Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr. Rev. 27, 728–735 [DOI] [PubMed] [Google Scholar]
- 44. Zechner R., Kienesberger P. C., Haemmerle G., Zimmermann R., Lass A. (2009) Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J. Lipid Res. 50, 3–21 [DOI] [PubMed] [Google Scholar]
- 45. Silva J. E. (2006) Thermogenic mechanisms and their hormonal regulation. Physiol. Rev. 86, 435–464 [DOI] [PubMed] [Google Scholar]
- 46. Coppola A., Liu Z. W., Andrews Z. B., Paradis E., Roy M. C., Friedman J. M., Ricquier D., Richard D., Horvath T. L., Gao X. B., Diano S. (2007) A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab. 5, 21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lopez M., Varela L., Vazquez M. J., Rodriguez-Cuenca S., Gonzalez C. R., Velagapudi V. R., Morgan D. A., Schoenmakers E., Agassandian K., Lage R., Martinez de Morentin P. B., Tovar S., Nogueiras R., Carling D., Lelliott C., Gallego R., Oresic M., Chatterjee K., Saha A. K., Rahmouni K., Dieguez C., Vidal-Puig A. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat. Med. 16, 1001–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jordan D., Veisseire M., Borson-Chazot F., Mornex R. (1986) In vitro effects of endogenous opiate peptides on thyrotropin function: inhibition of thyrotropin-releasing hormone release and absence of effect on thyrotropin release. Neurosci. Lett. 67, 289–294 [DOI] [PubMed] [Google Scholar]
- 49. Mitsuma T., Nogimori T. (1983) Effects of leucine-enkephalin on hypothalamic-pituitary-thyroid axis in rats. Life Sci. 32, 241–248 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.