Abstract
The protein kinase liver kinase B1 (LKB1) regulates cell polarity and intercellular junction stability. Also, LKB1 controls the activity of salt-inducible kinase 1 (SIK1). The role and relevance of SIK1 and its downstream effectors in linking the LKB1 signals within these processes are partially understood. We hypothesize that SIK1 may link LKB1 signals to the maintenance of epithelial junction stability by regulating E-cadherin expression. Results from our studies using a mouse lung alveolar epithelial (MLE-12) cell line or human renal proximal tubule (HK2) cell line transiently or stably lacking the expression of SIK1 (using SIK1 siRNAs or shRNAs), or with its expression abrogated (sik1+/+ vs. sik1−/− mice), indicate that suppression of SIK1 (∼40%) increases the expression of the transcriptional repressors Snail2 (∼12-fold), Zeb1 (∼100%), Zeb2 (∼50%), and TWIST (∼20-fold) by activating cAMP-response element binding protein. The lack of SIK1 and activation of transcriptional repressors decreases the availability of E-cadherin (mRNA and protein expression by ∼100 and 80%, respectively) and the stability of intercellular junctions in epithelia (decreases in transepithelial resistance). Furthermore, LKB1-mediated increases in E-cadherin expression are impaired in cells where SIK1 has been disabled. We conclude that SIK1 is a key regulator of E-cadherin expression, and thereby contributes to the stability of intercellular junctions.—Eneling, K., Brion, L., Pinto, V., Pinho, M. J., Sznajder, J. I., Mochizuki, N., Emoto, K., Soares-da-Silva, P., Bertorello, A. M. Salt-inducible kinase 1 regulates E-cadherin expression and intercellular junction stability.
Keywords: epithelial polarity, sodium transport, vectorial transport, transepithelial resistance, metastatic transformation, liver kinase B1
Salt-inducible kinase 1 (SIK1) is a member of the AMPK family of kinases (1) and a downstream target for the liver kinase B1 (LKB1; refs. 2, 3). SIK1 is important for regulation of gluconeogenesis (4), steroid synthesis (5), cardiomyogenesis (6, 7), and sodium transport (8), and lack of SIK1 confers resistance to anoikis (9). Breast and ovarian cancer cells with reduced levels of SIK1 and LKB1 show an invasive phenotype associated with poor outcome due to increased metastatic transformation (9). During tumor invasion and metastasis, cells undergo epithelial to mesenchymal transition (EMT) and acquire a more invasive phenotype; the reduction of E-cadherin expression is a critical determinant in this process (10). Despite being a marker of EMT and of tumor invasiveness, E-cadherin is a key component of the intercellular (adherens) junction during the development and maintenance of epithelial cell polarity (11, 12). LKB1 is known to regulate E-cadherin and cell polarity (13), as well as cell survival (14). Studies in Drosophila suggest that LKB1 probably regulates polarity through the downstream targets SIK1, NUAK, or Par-1 rather than AMPK (15). Against this background, we hypothesize that SIK1 may represent a molecular link between LKB1 and the regulation of intercellular junction stability by controlling the expression and availability of E-cadherin.
MATERIALS AND METHODS
Reagents and plasmids
Monensin and pinacidil was purchased from Sigma-Aldrich (St. Louis, MO, USA). The following antibodies were used: E-cadherin for Western blot (sc-7870; Santa Cruz Biotechnology, Santa Cruz, CA, USA), LKB1 (sc-32245; Santa Cruz Biotechnology), AMPK α1/2 (D-6; Santa Cruz Biotechnology), GSK3β (27C10; Cell Signaling Technology, Danvers, MA, USA), MARK2 (sc-365405; Santa Cruz Biotechnology), phosphoMARK2 (4836; Cell Signaling), β-tubulin (T4026; Sigma-Aldrich), p120CAS and β-catenin (BD, San Jose, CA, USA), ZO1 (Invitrogen, Carlsbad, CA, USA), E-cadherin (microscopy) and vimentin (Takara, Shiga, Japan), and fluorescent secondary antibodies (Alexa 488 and 546) and rhodamine phalloidin (Invitrogen). SIK1 (N-terminal), SIK2, and SIK3 antibodies were previously described, and goat anti-rabbit and goat anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibodies were from Bio-Rad (Hercules, CA, USA). The following gene expression assays were purchased from Applied Biosystems (Foster City, CA, USA): mouse, SIK1 Mm00440317_m1, E-cadherin Mm01247357_m1, RPLP0 Mm00725448_s1, Snail1 Mm00441533_g1, Snail2 Mm00441531_m1, Zeb1 Mm00495564_m1, Zeb2 Mm00497193_m1, MARK2 Mm00433039_m1, TWIST Mm04208233_g1, and pCAS120 Mm01334599_m1; human, SIK1 Hs00545020_m1, E-cadherin Hs01023894_m1, RPLP0 Hs99999902_m1, Snail2 Hs00950344_m1, Zeb1 Hs00232783_m1, Zeb2 Hs00207691_m1, and cAMP-response element (CRE) binding protein 1 (CREB1) Hs00231713_m1. All other reagents were of highest grade available. Stable clones with reduction in SIK1 expression were obtained using pSilencer 3.1-H1-hygro containing shRNA for SIK1 or a negative control containing a scrambled sequence (Invitrogen). For transient silencing experiments, small interfering RNA (siRNA) transfection system from Santa Cruz Biotechnology [SIK1 siRNA (human; sc-91428), scrambled siRNA-A (human; sc-37007); SIK1-siRNA (dog; sc-270182); CREB1 (human; sc-29281)], siRNA transfection medium (sc-36868), siRNA transfection reagent (sc-29528), and siRNA dilution buffer (sc-29527) were used according to manufacturer's protocol. E-cadherin luciferase promoter construct (−108 to +125) plasmid 19290:pGL2Basic-EcadK1was obtained from Addgene (Cambridge, MA, USA; ref.16), pTAL-Cre luciferase vector (17), and Renilla luciferase vector from Promega (Madison, WI, USA). pIRES vectors containing human SIK1 wild-type or SIK1 T322A were previously described (17). Dr. Hiroshi Takemori (National Institute of Biomedical Innovation, Osaka, Japan) provided LKB1, MO, STRAD, and SIK1-T182A plasmids.
Generation of sik1−/− mice
The mice were generated by Lexicon Pharmaceuticals (Woodlands, TX, USA; TF#1350; http://kodatabase.taconic.com/database.php?id=TF1350&geneSearch=1350), and the line was propagated at and purchased from Taconic Farms (Hudson, NJ, USA). The line was generated using the C57BL/6J background mice. The sik1−/− mice do not show any particular phenotype difference from the sik1+/+mice or any difference in their reproduction or life span.
The local ethics committee of Karolinska Institutet and Stockholm City Council approved the experiment. The animals were maintained under standard conditions of light and temperature and had free access to standard chow diet and water ad libitum. Lung tissue for protein determination and gene expression was surgically removed after a midline thorax incision. The procedure was performed in anesthetized animals.
Cell culture and transfection
HK2 cells (kindly provided by Giuseppe Bianchi, San Raffaele Hospital, Milan, Italy) stably expressing the α-adducin variants (α-add.NT: ADD1Gly460 and α-add.HT: ADD1Trp460) were reported elsewhere (18). MDCK [CRL-2935; American Type Culture Collection (ATCC), Manassas, VA, USA] were grown in MEM (Life Technologies/Invitrogen) supplemented with 10% FBS and 1% penicillin/streptomycin. T84 cells (CCL-248; ATCC) were grown in DMEM/F12 (Life Technologies/Invitrogen) supplemented with 10% FBS and 1% penicillin/streptomycin. MLE-12 cells (CRL-2110; ATCC) were grown in DMEM/F12 supplemented with 10% FBS and 1% penicillin/streptomycin, and HK-2 cells were grown in DMEM/F12 supplemented with 5% FBS, 1% penicillin/streptomycin and 1% nonessential amino acids in an atmosphere of 5% CO2. Stable clones were selected and maintained with the addition of 100 μg Hygromycin B (Life Technologies/Invitrogen) to the growth medium. Transient transfections were performed using Lipofectamine 2000 (Invitrogen) in basal DMEM/F12 according to the manufacturer's instructions.
Determination of promoter activity
E-cadherin promoter-derived firefly luciferase and Renilla luciferase activities were analyzed with dual luciferase kit (Promega) according to the manufacturer's protocol. The emitted light of each sample was measured using a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA, USA) from each sample and expressed in arbitrary units or percentage change of the E-cadherin/Renilla luciferase ratio.
Determination of mRNA expression levels
Cells were grown in 6-well plates, harvested, lysed in TRK lysis buffer (Omega Bio-Tek, Norcross, GA, USA), extracted, DNase digested (Omega Bio-Tek E.Z.N.A. RNase free DNase Kit 1), and purified using the Omega Bio-Tek E.Z.N.A. total RNA purification kit, according to the manufacturer's protocol. Total RNA from mouse tissue was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and any genomic DNA was digested using DNaseI (Rnase free; Qiagen) according to the manufacturer's protocols. RNA concentrations were determined using the NanoDrop 1000 Spectrophotometer (Thermo Scientific, Walthman, MA, USA). Reverse transcription of 500 ng total RNA was performed using RevertAID H Minus M-MuLV Reverse Transcriptase, Random Hexamer primer and RiboLock RNase Inhibitor (Fermentas; Life Science, Vilnius, Lithuania). Specific gene expression levels were analyzed using TaqMan with Gene Expression Assays (Applied Biosystems) with the ABI PRISM 7000 Sequence Detection System using the 7000 System SDS 1.2.3 software (Applied Biosystems). In each sample, analyzed in duplicates, the relative amount of the mRNA of interest was normalized to the level of a housekeeping gene, ribosomal protein large P0 (RPLP0), using the standard curve method where applicable or the comparative Ct method.
Protein abundance
Cells grown as described above were washed in ice-cold PBS and then lysed in lysis buffer: 50 mM Tris, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 50 mM NaF, 50 mM β-glycerophosphate, 1% Triton X-100, 30 mM Na4P2O7, 2 mMNa3VO4, and protease inhibitors (1 mM PMSF, 5 μg/ml leupeptin, 5 μg/ml antipain, 5 μg/ml pepstatin A, and 10 μg/ml aprotinin) and mechanically disrupted using needle and syringe and motor pestle. Cell lysates were centrifuged at 1000 g for 5 min at 4°C. Protein concentrations were determined with Bradford (Bio-Rad), and proteins (200 μg) were denatured in Laemmli. The postnuclear supernatant was separated by SDS-PAGE, transferred to PVDF membranes, and blotted with corresponding antibody and HRP-conjugated secondary antibody, and chemiluminescence was detected with ECL plus (GE Healthcare, Little Chalfont, UK).
Fluorescence microscopy
Cells were cultured to confluence on glass-bottomed dishes (Iwaki, Tokyo, Japan) and fixed with 4% paraformaldehyde for 5 min followed by permeabilization with PBS containing 0.025% Triton-X100. Cells were incubated with blocking solution (PBS containing 0.2% albumin) and with the primary antibody at room temperature. Species-matched fluorescence-labeled secondary antibodies were used to visualize the immunoreactivity of the primary antibodies in the cells. F-actin was visualized by rhodamine-labeled phalloidin. The images were obtained with a confocal microscope (Olympus FV-1000; Olympus, Tokyo, Japan). Each XY image shown in the figures represents a typical image obtained from ≥3 independent experiments.
Transepithelial electrical resistance (TER) measurements
MDCK and T84 cells were transiently transfected with the SIK1-siRNA for dog or human (see Reagents and Plasmids), respectively. Thereafter, cells were transferred to polycarbonate filters. The sealing function of the tight junctional belt in cell monolayers cultured onto polycarbonate filters (Transwell; Costar, Corning, NY, USA) was assessed by measurement of the TER with an epithelial volt-ohm meter (EVOM; World Precision Instruments, Sarasota, FL, USA) equipped with chopstick electrodes. TER (Ω·cm2) was measured at 37°C, and background TER of blank filters (±222 Ω·cm2) was subtracted from all samples (19).
Na+/H+ exchanger activity
Na+/H+ exchanger activity was assayed as the initial rate of intracellular pH (pHi) recovery after an acid load imposed by 20 mM NH4Cl followed by removal of Na+ from the Krebs' modified buffer solution (in mM: 140 NaCl, 5.4 KCl, 2.8 CaCl2, 1.2 MgSO4, 0.3 NaH2PO4, 10 HEPES, and 5 glucose, pH 7.4) in the absence of CO2/HCO3. In these experiments, NaCl was replaced by an equimolar concentration of tetramethylammonium chloride. In pHi measurement experiments, cells were grown in 96-well plates, as described previously (19). The cell culture medium was aspirated, and the cell monolayers were incubated for 30 min with 10 μM BCECF/AM, the membrane-permeant acetoxymethyl ester derivative of 2′,7′-bis (carboxyethyl)-5,6-carboxyfluorescein (BCECF), at 37°C in 5% CO2-95% air atmosphere. Cells were placed in the sample compartment of a dual-scanning microplate spectrofluorometer (Spectramax Gemini XS; Molecular Devices, Sunnyvale, CA, USA), and fluorescence was measured every 17 s, alternating between 440 and 490 nm excitation at 535 nm emission, with a cutoff filter of 530 nm. The ratio of intracellular BCECF fluorescence at 490 and 440 nm was converted to pHi values by comparison with values from an intracellular calibration curve using the nigericin (10 μM) and high-K+ method (20, 21).
Cl−/HCO3− exchanger activity
Although there is no specific assay for Cl−/HCO3− exchange-mediated activity, several findings strongly suggest that pHi recovery after removal of CO2/HCO3 in the absence of Na+ reflects the activity of the Cl−/HCO3− exchanger. Thus, the Na+-independent HCO3− transport system activity was assayed as the initial rate of pHi recovery after an alkaline load (CO2/HCO3 removal), in the absence of Na+, as described previously (21). Briefly, cells were loaded in serum-free medium with 10 mM BCECF-AM for 30 min at 37 8C in 5% CO2-95% air atmosphere. The cells were washed free of dye and loaded with Krebs-Hensleit solution (25 mM NaHCO3) for 25 min. Then, the extracellular solution was replaced by a Krebs-Hensleit NaHCO3-free solution for 10 min. In the NaHCO3-free bathing solution, NaHCO3 was replaced by an equimolar concentration of choline. Cells were placed in the sample compartment of a dual-scanning microplate spectrofluoromete, and fluorescence was monitored every 17 s, alternating between 440 and 490 nm excitation at 535 nm of emission, with a cutoff filter of 530 nm.
ATP quantification
Assays were performed using the ATPLite assay (PerkinElmer, Boston, MA, USA) and following the manufacturer's instructions. In brief, 50 μl of cell lysis solution was added per well containing cells in 100 μl culture medium. After 5 min in an orbital shaker, 50 μl of substrate solution was added to each well, and the plate was shaken for another 5 min. The plate was then dark adapted for 10 min, and luminescence was measured on a Microbeta Scintillation Counter (PerkinElmer). In each plate, blanks and a standard curve of ATP (1 μM to 1 mM) were prepared.
Na+-K+-ATPase activity
Na+-K+-ATPase activity was measured by the method of Quigley and Gotterer (22) with minor modifications. Briefly, cells were permeabilized by rapid freezing in liquid nitrogen and thawing. The reaction was initiated by the addition of 4 mM ATP. For determination of ouabain-sensitive ATPase, NaCl and KCl were omitted, and Tris-HCl (150 mM) and ouabain (1 mM) were added to the incubation medium. After incubation at 37°C for 15 min, the reaction was terminated by the addition of 50 μl of ice-cold trichloroacetic acid. Before centrifugation at 3000 rpm at 4°C for 2·min, 1 ml ice-cold Boting's color reagent (560 mM H2SO4, 8.10 mM ammonium molybdate, and 176 mM FeSO4) was added and the color was allowed to develop in a water bath at 37°C for 2 min. The Pi liberated in the supernatant was measured by spectrophotometry at 740 nm. Na+-K+-ATPase activity, determined as the difference between total and ouabain-insensitive ATPase, was expressed as nanomoles of Pi per milligram of protein per minute.
Calcium switch experiments
MLE-12 cells were allowed to establish a confluent monolayer before incubation in low-calcium medium (4 mM EGTA) in both the apical and basal compartments until the TER was reduced to ∼70% of the basal values. Thereafter, the cells were quickly washed 3 times with DMEM-F12 to remove all traces of EGTA and incubated in regular DMEM-F12 containing calcium. TER was measured for the time points indicated before (low calcium) and after switching to normal calcium in the culture medium.
Statistical analysis
Quantitative data were statistically analyzed (means, se, sd, t test, or Mann-Whitney as appropriate) and plotted using Prism software (GraphPad, San Diego, CA, USA). Values of P < 0.05 were considered statistically significant.
RESULTS
SIK1 regulates E-cadherin expression
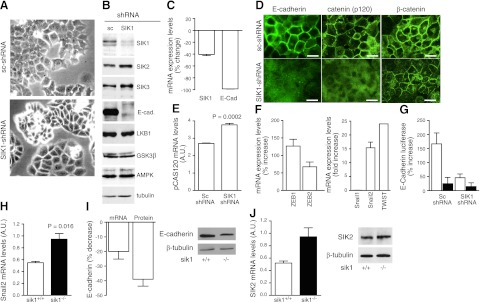
The role of SIK1 in the expression of E-cadherin was examined in an epithelial cell line derived from mouse lung, MLE-12, stably transfected with SIK1-shRNA and compared wtih MLE-12 cells transfected with a scrambled (unrelated sequence) shRNA (sc-shRNA). MLE-12 cells transfected with the SIK1-shRNA expressed a distinct phenotype characterized by acquisition of a rounded shape with fewer lamellipodia, reduced growth rate, the tendency to form islands, and partial loss of ability to form monolayers (Fig. 1A). All experiments were performed with cells grown to 60–80% confluence. Cells lacking SIK1 had a significant reduction in SIK1 protein, but not other SIK isoforms (SIK2 increase ∼6% and SIK3 increase ∼20%; Fig. 1B). Furthermore, the reduction of SIK1 expression was associated with a marked reduction in E-cadherin expression (Fig. 1B). The protein levels of known upstream regulators of SIK1 were not affected by the lack of SIK1, suggesting that the reduction in SIK1 levels alone may selectively influence E-cadherin expression. The mRNA levels of E-cadherin were significantly decreased in MLE-12 cells with reduced SIK1 expression (Fig. 1C). Whereas these data suggest that SIK1 controls E-cadherin synthesis, SIK1 might also affect the rate of E-cadherin degradation. Fluorescent microscopy confirmed the reduction of E-cadherin expression in cells with lower SIK1 expression (Fig. 1D). Reduction of SIK1 was also associated with reduction and abnormal localization to the plasma membrane of pCAS120 (23) but not of β-catenin (Fig. 1D). The misplacement of pCAS120 is consistent with the notion that E-cadherin controls the fate of pCAS120 (pCAS120 binds to the juxtamembrane domain of E-cadherin). In addition, pCAS120 mRNA expression increased ∼40% (Fig. 1E) in cells lacking SIK1 possibly due to a compensatory activation of the pCAS120 gene. The regulation of E-cadherin expression has been extensively studied, and the activation of transcriptional repressors plays a decisive role in this process (24–26). We therefore examined whether the reduction in SIK1 levels resulted in increased expression levels of Zeb1, Zeb2, Snail1, Snail2, and TWIST (known regulators of E-cadherin expression). The results demonstrated a significant increase (∼12- and 20-fold) in Snail2 and TWIST, respectively (Fig. 1F, right panel), a modest increase (∼150 and ∼50% of control) in Zeb1 and Zeb2 (Fig. 1F, left panel), and no significant changes in Snail1. The relevance of SIK1 was further examined by analyzing the regulation of the E-cadherin promoter using a luciferase reporter vector in MLE-12 sc-shRNA cells and MLE-12 SIK1-shRNA cells transiently overexpressing the SIK1 wild-type protein or an inactive mutant, T322A (which lacks the Ca2+-CaMKI phosphorylation site). Basal E-cadherin luciferase activity was significantly lower in cells lacking SIK1 (percentage increase compared with cells having SIK1: 27±2.8, n=3). Overexpression of wild-type SIK1 in MLE-12 sc-shRNA cells resulted in a ∼3-fold increase in E-cadherin promoter activity (Fig. 1G). This effect was not seen in cells overexpressing the inactive SIK1 T322A mutant (Fig. 1G). The fact that this mutant lacks any stimulatory effect on E-cadherin promoter activity is consistent with E-cadherin being a calcium-dependent adhesion molecule and may implicate SIK1 as a calcium-mediatory signaling molecule in this process (8). The ability of overexpressed SIK1 to increase E-cadherin promoter activity was significantly reduced in MLE-12 SIK1-shRNA cells (Fig. 1G). These results excluded that SIK1 exerted direct control over E-cadherin promoter activity.
Figure 1.
Expression of E-cadherin (E-cad) in MLE-12 cells. A) Representative micrographs of MLE-12 cells stably expressing (sc-shRNA) and with reduced levels of SIK1 (SIK1-shRNA) (×100 view). B) Representative Western blot of SIK1 (1:1000), SIK2 (1:2000), SIK3 (1:2000), E-cadherin (1:400), LKB1 (1:500), GSK3β (1:1000), AMPK (1:500), and tubulin (1:1000). Equal amounts of protein (200 μg) were loaded in each lane. C) Expression of SIK1 and E-cadherin mRNA in cells lacking (SIK1-shRNA) vs. cells expressing SIK1 (sc-shRNA); results represent 3 experiments. D) Expression and localization of E-cadherin (1:100), β-catenin (1:100), and p120CAS catenin (1:200) were examined using fluorescent microscopy. Scale bars = 20 μm. E) p120CAS catenin mRNA expression levels in MLE-12 cells expressing- or lacking SIK1. Results represent 3 experiments. F) Right panel: expression of Snail1, Snail2, and TWIST. Left panel: expression of Zeb1 and Zeb2. Data are presented as percentage change or fold increase in cells lacking SIK1 compared wtih those expressing SIK1 (3 experiments). G) MLE-12 cells expressing or lacking SIK1 and cotransfected with the human variant of E-cadherin tagged with luciferase and either the human variant of SIK1 wild-type (SIK1-WT; open bars), the SIK1 T322A mutant (SIK1-T322A; solid bars), or the empty vector (pIRES). Luciferase was determined after 36 h expression. Values are expressed as percentage increase vs. cells expressing the empty vector. Results represents 4 experiments performed in triplicate. H) Expression of Snail2 (mRNA) in lung tissue from sik1+/+ and sik1−/− mice. Data are presented as arbitrary units (A.U.). Data represent 5 mice/group. I) Left panel: E-cadherin expression (mRNA and protein) in sik1+/+ and sik1−/− mice. Changes are expressed as percentage decrease in sik1+/+ vs. sik1−/− mice; n = 5 animals/group. Right panel: representative Western blot. Antibodies were used as in B. J) Left panel: expression of SIK2 (mRNA) in lung tissue from sik1+/+ and sik1−/− mice. Data are presented as arbitrary units; n = 5 animals/group. Right panel: expression of SIK2 (protein) in lung tissue from sik1+/+ and sik1−/− mice. Representative Western blot of 6 mice is shown. Bars represent means ± se.
To further study the role of SIK1 in controlling E-cadherin expression, we generated mice with ablation of the sik1 gene. The mRNA expression levels of Snail2 in lung tissue from sik1−/− were significantly increased when compared wtih sik1+/+(Fig. 1H). Accordingly, E-cadherin mRNA and protein expression was reduced in sik1−/− mice (Fig. 1I). The magnitude of the changes is not dramatic (as in vitro studies). This could be due to the influence of SIK2 isoform. Whereas SIK2 gene expression is elevated (Fig. 1J, left panel), the protein (Fig. 1J, right panel) is not significantly changed (percentage increase in sik1−/−: 12±3.5; n=6). Thus it is not possible to speculate that SIK2 may compensate for the lack of SIK1.
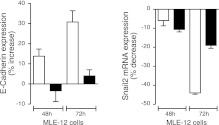
LKB1 activates SIK1, and loss of LKB1 is associated with decreased E-cadherin expression and EMT. MLE-12 cells were used to examine whether SIK1 mediates the effects of LKB1 on E-cadherin. MLE-12 cells transiently overexpressing wild-type SIK1 showed increased E-cadherin expression (Fig. 2A) and this was paralleled by decreases in Snail2 expression (Fig. 2B). Conversely, overexpression of SIK1 lacking the activation site for LKB1 (T182A) had no effect at either the E-cadherin or the Snail2 mRNA level (Fig. 1I). Changes in E-cadherin mRNA expression levels (∼35% increase) are paralleled by changes in protein expression. These results further highlight the role of SIK1 in mediating the effect of LKB1 on E-cadherin. Furthermore, overexpression of active LKB1 (together with STRAD and MO25) elicited an increase in E-cadherin protein expression (percentage increase: 20±1.2; n=3) when compared with cells expressing the empty vector, and this effect occurs only in MLE-12 cells expressing SIK1 and not in those lacking SIK1 expression.
Figure 2.
SIK1 mediates the effect of LKB1 on E-cadherin. A) Expression (mRNA) of E-cadherin in MLE-12 cells transiently (48 and 72 h) expressing SIK1 wild-type (open bars) and SIK1 lacking the LKB1-phosphorylation (T182A) site (solid bars). B) Expression (mRNA) of Snail2 in MLE-12 cells transiently (48 and 72 h) expressing SIK1 wild-type and SIK1 lacking the T182A site. Values are expressed as percentage increase vs. cells expressing the empty vector. Bars represent means ± se of 4-6 experiments.
Fluorescence microscopy indicated that the relative lack of expression and abnormal localization of E-cadherin was not secondary to changes in cytoskeletal proteins or other components of the intercellular junction. Neither the cytoskeleton (actin and vinculin expression and organization; Supplemental Fig. S1A) nor other components of the tight junction, such as ZO-1 protein (Supplemental Fig. S1A), were affected by a reduction in SIK1 levels.
Protein and mRNA levels of another polarity and microtubule regulating kinase (MARK2; ref. 27) were increased (protein levels, percentage increase in SIK1- vs. sc-shRNA: 226±50; n=3; Supplemental Fig. S1B), suggesting that SIK1 may directly and/or indirectly control the expression of MARK2. Thus, the contribution of SIK1 to the establishment of cell polarity via MARK2 could be independent of LKB1 function. The lack of SIK1 and increased levels of MARK2 did not affect the organization of microtubules (Supplemental Fig. S1C).
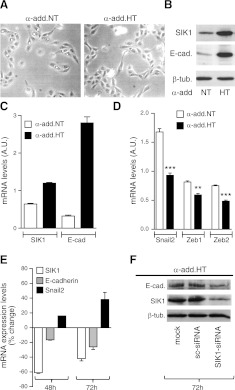
Corresponding experiments were performed in a kidney cell line, HK2, engineered to express high levels of SIK1 through stable transfection with different genetic variants of the cytoskeletal protein α-adducin, a protein associated not only with elevated blood pressure and increased renal sodium reabsorption but also with increased expression and activity of SIK1 (28, 29). HK2 cells stably expressing the different variants of α-adducin (normotensive: α-add.NT; hypertensive: α-add.HT) did not show any distinct phenotype (Fig. 3A). Western blot analysis revealed that the HK2 cells carrying the α-add.HT had significantly higher expression of SIK1 and E-cadherin than cells carrying the α-add.NT (Fig. 3B). The parallelism between SIK1 and E-cadherin is also present at the mRNA level (Fig. 3C). HK2 cells carrying the α-add.HT (higher SIK1 expression) had significantly reduced expression of the transcriptional repressors Snail2, Zeb1, and Zeb2 (Fig. 3D). To examine whether the increase in E-cadherin in HK2 cells expressing α-add.HT was due to higher SIK1 levels, we reduced SIK1 expression by transiently expressing SIK1-siRNA and a scrambled siRNA toward human SIK1 and toward a different sequence than the one used in MLE-12 cells (Fig. 3E). HK2 (α-add.HT) cells that had expressed the SIK1-siRNA for 48 h had a parallel reduction in expression of SIK1 and E-cadherin and an increase (similar magnitude) in the transcriptional repressor Snail2 compared wtih cells expressing the sc-siRNA. The changes in the expression of E-cadherin and Snail2 were even larger after 72 h of SIK1-siRNA expression. Western blot analysis (Fig. 3F) demonstrated a similar pattern of reduction in SIK1 and E-cadherin protein levels (at 72 h) in cells expressing SIK1-siRNA compared wtih cells expressing sc-siRNA. The reduction in E-cadherin protein expression was proportionally larger than the decrease in mRNA levels (∼25%). The mechanism by which α-add.HT increases SIK1 expression is not entirely clear but could involve increased sodium permeability and/or direct protein-protein interactions.
Figure 3.
E-cadherin in cells overexpressing SIK1. A) Representative micrographs of HK2 cell stably expressing the normotensive α-adducin variant (α-add.NT) or the hypertensive variant (α-add.HT; ×100 view). B) Representative Western blot of SIK1 (1:1000), E-cadherin (1:400), and tubulin (1:1000). Equal amounts of protein (200 μg) were loaded in each lane. C) Expression of SIK1 and E-cadherin in α-add.NT and α-add.HT cells. D) Expression of Snail2, Zeb1, and Zeb2. E) Basal expression of SIK1 and E-cadherin in HK2 cells (with high SIK1: expressing (α-add HT) transiently transfected with the SIK1-siRNA or a scrambled sequence at 48 and 72 h post-transfection. Values are expressed as percentage change vs. cells transfected with sc-siRNA. Each bar represents the mean ± se of 3 experiments. F) SIK1 and E-cadherin protein abundance in cells transfected with SIK1-siRNA or a scrambled sequence detected by Western blot as in B. Equal amounts of protein (200 μg) were loaded in each well. Representative Western blot is shown. Bars represent means ± se of 3 experiments.
SIK1 regulates Snail2 expression via CREB
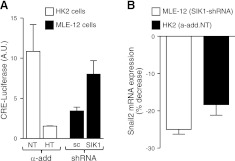
SIK1 has been reported to repress CREB activity both directly and via phosphorylation of TORC (3, 30). Consequently, the lack of SIK1 would result in increased expression of CREB target genes. First, using a CRE-luciferase reporter vector, we determined that basal CREB activity was significantly lower in cells with higher SIK1 expression (HK2 α-add.HT vs. HK2 α-add.NT; Fig. 4A). In line with these results, reduced SIK1 expression was associated with significantly higher CRE activity (MLE-12 SIK1-siRNA vs. MLE-12 sc-siRNA; Fig. 4A). In silico analysis of the human and mouse Snail2 promoter revealed several CREB- and C/EBP-binding sites. To examine the involvement of CREB and C/EBP, we determined the expression of Snail2 in cells that lacked SIK1 and were transiently transfected with CREB1, CREB2, or C/EBPα-siRNAs, and for comparison, cells transfected with a control siRNA (sc-siRNA). The expression levels of Snail2 were reduced in MLE-12 cells transiently expressing the CREB1-siRNA (Fig. 4B), whereas the CREB2-siRNA and C/EBPα-siRNA were ineffective (not shown). In HK2 cells carrying the α-add.NT (expressing lower SIK1/higher CREB activity), transient expression of CREB1-siRNA decreased Snail2 mRNA compared with HK2 cells expressing sc-siRNA (Fig. 4B). CREB1-siRNA did not affect the levels of SIK1 (percentage control, 103.3±2.9; n=3) when compared wtih sc-siRNA expressing cells.
Figure 4.
SIK1 regulates Snail2 expression levels via CREB. A) CRE-activity in stable clones of HK-2 α-add.NT and α-add.HT and MLE-12 SIK1-shRNA and MLE-12 sc-shRNA cells transiently expressing the pTAL-Cre-promoter luciferase and Renilla luciferase constructs. Basal levels of Cre promoter activity were measured with the dual luciferase kit from Promega. B) MLE-12 cells lacking (SIK1-shRNA) SIK1 or HK2 cell expressing lower SIK1 (α-add.NT) were transiently transfected with CREB1 siRNA or a scrambled sequence. Expression of CREB1 siRNA resulted in a reduction of CREB (% decrease, 52±1.5; n=3). Levels of Snail2 are expressed as percentage decrease vs. cells expressing a scrambled siRNA. Bars represent means ± se of 3 (A) or 4 (B) independent experiments.
SIK1 regulates the stability of intercellular junctions
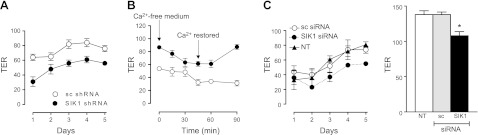
The functional significance of reduced SIK1 and E-cadherin expression was analyzed by examining TER in MLE-12 monolayers. Normal MLE-12 cells form rather tight monolayers (TER of ∼80 Ω·cm2; Fig. 5A); however, the resistance is significantly lower in MLE-12 cells lacking SIK1 than in cells expressing normal levels of SIK1 protein and E-cadherin. The observation that cells with reduced SIK1 expression establish a leaky epithelium is likely to result from improper formation of adherens junctions (due to the lack of E-cadherin/abnormal pCAS120 catenin distribution). The differences in TER were also appreciated when using the calcium-switch technique (Fig. 5B). Furthermore, TER was significantly decreased in two other cell lines (MDCK, dog distal tubules; and T84, human colon) in which SIK1 expression was transiently reduced by using specific SIK1-siRNAs when compared with their respective controls (scrambled siRNA sequences) and nontransfected cells (Fig. 5C). The reduction in TER is not the direct consequence of a reduction in the levels of cellular ATP (a known regulator of intercellular junction stability and polarity; ref. 31) but rather of a reduced SIK1/E-cadherin expression. The plasma membrane Na+-K+-ATPase represents the major energy consumer in animal cells (range 45-60%, depending on the tissue). MLE-12 cells expressing SIK-shRNA had significantly reduced Na+-K+-ATPase catalytic activity (Supplemental Fig. S2A). The levels of intracellular ATP (pmol/mg protein) were higher in MLE-12 cells lacking SIK1 (sc-shRNA: 824±43 vs. SIK1-shRNA: 1442±51; n=12), most likely due to reduced consumption (lower Na+-K+-ATPase activity). The lower Na+-K+-ATPase activity was expected, because SIK1 controls its activity in response to changes in sodium permeability (8), and the reduction was paralleled by a reduction in the Na+/H+-exchanger activity (Supplemental Fig. S2B). Sodium-independent transporters, such as Cl−/HCO3−, remained unchanged (Supplemental Fig. S2C). Similarly, the plasma membrane potential was lower in cells lacking SIK1, and the cells do not depolarize in response to ouabain and also have a reduced response to KCl (Supplemental Fig. S2D). The elevated levels of ATP are also evidenced by the apparent inability of pinacidil to induce changes in plasma membrane potential in cells lacking SIK1 (Supplemental Fig. S3). Pinacidil effects on K+-channels become apparent at low cellular ATP concentrations.
Figure 5.
Effects of SIK1 on transepithelial resistance and ion transporter activities. A) TER was determined in MLE-12 cells expressing (sc-shRNA) and lacking (SIK1-shRNA) SIK1 grown on permeable support for 5 d. Recordings were monitored daily in 6 independent cell preparations in each group. B) TER measured in sc-shRNA (solid circles) and SIK1-shRNA (open circles) MLE-12 cells grown on permeable support. Recordings were monitored in 6 independent cell preparations at time points indicated. Arrows indicate the presence or absence of calcium in the incubation medium. C) TER in MDCK (dog renal distal tubule; right panel) and in T84 (intestinal; left panel) cells with (sc-siRNA) and without a transient reduction (SIK1-siRNA) in SIK1 protein levels. Cells were grown on permeable support for 5 d. Recordings were monitored daily in 6 independent cell preparations in each group. MDCK data represent recordings performed at day 3. *P < 0.05.
DISCUSSION
In this study, we have identified SIK1 as a regulator of E-cadherin expression, i.e., SIK1 activity was necessary to promote and sustain the expression levels of E-cadherin in cells. The effect of SIK1 is mediated by reduction in the expression of transcriptional repressors (mainly Snail2) by repressing CREB1 expression and activity. SIK1 lacking the LKB1 regulatory domain was unable to down-regulate Snail2 and increase E-cadherin expression. This observation is important, because it provides an additional cell mechanism by which LKB1 (by activating SIK1) may contribute to the development of cell polarity as well as tumor invasion by regulating E-cadherin expression. In addition, LKB1 is an activator of CRTC1 (3, 30, 32), and it is likely that this effect is mediated at least in part by activation of SIK1.
The molecular mechanisms by which SIK1 affects E-cadherin expression can be exerted via effects on the expression of transcriptional repressors (Snail2, Zeb1, and Zeb2). Various signaling pathways are known to regulate the expression of Snail and Zeb (see ref. 10 for review). In this study, we provide an alternative regulatory mechanism for the expression of these transcription factors via CREB1 activity. Cells lacking and cells overexpressing SIK1 showed opposite changes in CREB1 activity, and transiently disabling CREB1 expression by siRNA affected Snail2, Zeb1, and Zeb2 expression. The fact that reducing CREB1 does not result in equivalent reduction in Snail2 expression may suggest another, complementary pathway by which SIK1 controls Snail2 expression. However, it is difficult to discern the specific relevance of Snail2 because there are many interactions, known and unknown, between Snail, Zeb, TWIST, and other transcription factors (33).
The lack of SIK1 did not affect the organization and expression of cytoskeletal proteins (actin, vimentin, and β-tubulin) except for pCAS120-catenin, which had reduced expression and abnormal subcellular localization. pCAS120-catenin regulates E-cadherin turnover at the plasma membrane by favoring E-cadherin degradation, but it does not affect its expression (34, 35). Thus, it excludes the possibility that the reduction of E-cadherin gene expression could have been the consequence of reduced pCAS120. However, lower pCAS120 might have independently influenced the stability (by affecting its degradation) of E-cadherin protein. Cells lacking SIK1 express a distinct phenotype. The fact that these cells do not develop lamellipodia may suggest a role of SIK1 protein in polarized motion, a phenomenon that has recently been attributed to its upstream activator LKB1. LKB1 controls polarity and the formation of microvilli as well as ZO1 expression (36). Our finding that lack of SIK1 did not affect ZO1 expression/localization suggests that LKB1 exerts its effects on ZO1 by acting on signaling pathways where SIK1 is not required.
MARK2 belongs to the same family of kinases as SIK1 and plays a key role in establishing cell polarity as well as polarized motion; it is also a direct target for LKB1 (1). We found that the lack of SIK1 increased the expression levels of MARK2 protein. Changes in MARK2 expression could be a compensatory mechanism, or a direct consequence of the lack of SIK1, opening the possibility that SIK1 may suppress MARK2 expression. Along these lines, it can be speculated that the increase in MARK2 results from a potentiated LKB1 effect once a suppressor (SIK1) is absent. In addition, these data suggest that SIK1 may contribute to the establishment of cell polarity via MARK2 but in a LKB1-independent manner. However, given that elevated MARK2 levels did not appear to lead to any morphological alteration in microtubule structures, MARK2 expression might affect only other targets in the cell. An alternative explanation is that the increased expression of MARK2 was not associated with a parallel increase in activation (MARK2 phosphorylation) and thus did not affect microtubule dynamics and organization.
In Drosophila, it has been shown that the lack of LKB1, an activator of SIK1, severely disrupts the organization of intercellular junctions. This phenotype could only be seen (partially) after disruption of downstream kinases like SIK1 but not of AMPK (15). The cited study used siRNA to demonstrate that lack of SIK1 altered the polarize localization of the Na+-K+-ATPase. Our studies performed in Drosophila in which the SIK1 gene has been deleted revealed no abnormality in the expression of abdominal E-cadherin (not shown). Contrasting results could also be seen regarding the effect of AMPK on tight junction stability in vivo (Drosophila) and in vitro (MDCK cells). Lack of AMPK does not affect the development of cell polarity in Drosophila (15), whereas cell biological studies clearly demonstrate a role for AMPK in the deposition of components of the tight junctions (37). On the contrary, the studies performed in mice lacking SIK1 show a significant parallelism with the cell biology studies. These results further highlight the complexity of this function and point to the existence of multiple pathways that may act in synergism and/or serve as back-up compensatory mechanisms that could also be organ specific.
Previous studies indicated that functional SIK1 was necessary for mediating the effects of LKB1 during anoikis (9). Lack of SIK1 is also associated with lung micrometastasis, and decreased expression of SIK1 closely correlates with the development of metastases in breast cancers (9). The mechanisms appear to involve the regulation of p53 protein. Because the metastatic transformation of cells has been the result of (or enhanced by) the lack of E-cadherin expression, the observations from this study may provide the molecular basis for down-regulation of E-cadherin during EMT and highlight the role of SIK1, as a downstream target of LKB1, during the establishment and maintenance of polarized epithelia, independently and/or in combination with the activation of p53 function.
Variations in intracellular sodium concentrations or increases in cell sodium permeability trigger the activation of the plasma membrane Na+-K+-ATPase in order to maintain cellular homeostasis. This is accomplished by activation, in a calcium-calmodulin kinase-dependent manner, of a distinct intracellular signaling network with the SIK1 at its core (8). Consequently, in this study, the lack of SIK1 was associated with reduction in Na+-K+-ATPase activity and protein. Because the integrity of the intercellular junction is also determined in part by the presence of the Na+-K+-ATPase subunits at the plasma membrane (38, 39), it is possible that SIK1 controls the stability of the junctions by regulating both the expression of E-cadherin and the availability of Na+-K+-ATPase molecules at the plasma membrane. It is plausible that once polarity is established, the vectorial transport of sodium across the polarized epithelium could help maintain the stability of the intercellular junction (and polarity) by regulating SIK1 and Na+-K+-ATPase expression levels.
Taken together, these results highlight the potential role of SIK1 in controlling E-cadherin expression and suggest that SIK1 actions might represent an intermediate step (beyond the wide range of LKB1-dependent effects) of importance for the integrity, maintenance, and function of polarized epithelia (e.g., in lung, kidney, and colon), as well as for organogenesis and tumor metastasis.
Supplementary Material
Acknowledgments
The authors thank Hiroshi Takemori (National Institute of Biomedical Innovation, Osaka, Japan), Giuseppe Bianchi (San Raffaele Hospital, Milan, Italy), and Amparo Cano (Universidad Autónoma de Madrid, Madrid, Spain)for providing reagents.
The study was supported by grants from the Swedish Research Council (10860), the Swedish Heart and Lung Foundation, AFA Insurance Sweden, U.S. National Heart, Lung, and Blood Institute grant HL-48129, and Fundação para a Ciência e a Tecnologia grant PIC/IC/83204/2007.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- CRE
- cAMP-response element
- CREB1
- cAMP-response element binding protein 1
- EMT
- epithelial to mesenchymal transition
- HRP
- horseradish peroxidase
- LKB1
- liver kinase B1
- SIK1
- salt-inducible kinase 1
- siRNA
- small interfering RNA
- TER
- transepithelial electrical resistance
REFERENCES
- 1. Lizcano J., M., Göransson O., Toth R., Deak M., Morrice N. A., Boudeau J., Hawley S. A., Udd L., Mäkelä T. P., Hardie D. G., Alessi D. R. (2004) LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23, 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jaleel M., McBride A., Lizcano J. M., Deak M., Toth R., Morrice N. A., Alessi D. R. (2005) Identification of the sucrose non-fermenting related kinase SNRK, as a novel LKB1 substrate. FEBS Lett. 579, 1417–1423 [DOI] [PubMed] [Google Scholar]
- 3. Katoh Y., Takemori H., Lin X. Z., Tamura M., Muraoka M., Satoh T., Tsuchiya Y., Min L., Doi J., Miyauchi A., Witters L. A., Nakamura H., Okamoto M. (2006) Silencing the constitutive active transcription factor CREB by the LKB1-SIK signaling cascade. FEBS J. 273, 2730–2748 [DOI] [PubMed] [Google Scholar]
- 4. Koo S. H., Flechner L., Qi L., Zhang X., Screaton R. A., Jeffries S., Hedrick S., Xu W., Boussouar F., Brindle P., Takemori H., Montminy M. (2005) The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437, 1109–1111 [DOI] [PubMed] [Google Scholar]
- 5. Wang Z., Takemori H., Halder S. K., Nonaka Y., Okamoto M. (1999) Cloning of a novel kinase (SIK) of the SNF1/AMPK family from high salt diet-treated rat adrenal. FEBS Lett. 453, 135–139 [DOI] [PubMed] [Google Scholar]
- 6. Ruiz J. C., Conlon F. L., Robertson E. J. (1994) Identification of novel protein kinases expressed in the myocardium of the developing mouse heart. Mech. Dev. 48, 153–164 [DOI] [PubMed] [Google Scholar]
- 7. Romito A., Lonardo E., Rom G., Minchiotti G., Ballabio A., Cobellis G. (2010) Lack of sik1 in mouse embryonic stem cells impairs cardiomyogenesis by down-regulating the cyclin-dependent kinase inhibitor p57kip2. PLoS One 5, e9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sjöström M., Stenström K., Eneling K., Zwiller J., Katz A. I., Takemori H., Bertorello A. M. (2007) SIK1 is part of a cell sodium-sensing network that regulates active sodium transport through a calcium-dependent process. Proc. Natl. Acad. Sci. U. S. A. 104, 16922–16927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng H., Liu P., Wang Z. C., Zou L., Santiago S., Garbitt V., Gjoerup O. V., Iglehart J. D., Miron A., Richardson A. L., Hahn W. C., Zhao J. J. (2009) SIK1 couples LKB1 to p53-dependent anoikis and suppresses metastasis. Sci. Signal. 2, ra35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nieto M. A. (2011) The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu. Rev. Cell Dev. 27, 347–376 [DOI] [PubMed] [Google Scholar]
- 11. Rodriguez-Boulan E., Nelson W. J. (1989) Morphogenesis of the polarized epithelial cell phenotype. Science 245, 718–725 [DOI] [PubMed] [Google Scholar]
- 12. Marrs J. A., Andersson-Fisone C., Jeong M. C., Cohen-Gould L., Zurzolo C., Nabi I. R., Rodriguez-Boulan E., Nelson W. J. (1995) Plasticity in epithelial cell phenotype: modulation by expression of different cadherin cell adhesion molecules. J. Cell Biol. 129, 507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roy B. C., Khono T., Iwakawa R., Moriguchi T., Kiyono T., Morishita K., Sanchez-Cespedes M., Akiyama T., Yokota J. (2010) Involvement of LKB1 in epithelial-mesenchymal transition (EMT) of human lung cancer cells. Lung Cancer 70, 136–145 [DOI] [PubMed] [Google Scholar]
- 14. Baas A. F., Kuipers J., van der Wel N. N., Batlle E., Koerten H. K., Peters P. J., Clevers H. C. (2004) Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116, 457–466 [DOI] [PubMed] [Google Scholar]
- 15. Amin N., Khan A., St Johnston D., Tomlinson I., Martin S., Brenman J., McNeill H. (2009) LKB1 regulates polarity remodeling and adherens junction formation in the Drosophila eye. Proc. Natl. Acad. Sci. U. S. A. 106, 8941–8946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hajra K. M., Ji X., Fearon E. R. (1999) Extinction of E-cadherin expression in breast cancer via a dominant repression pathway acting on proximal promoter elements. Oncogene 18, 7274–7279 [DOI] [PubMed] [Google Scholar]
- 17. Lin X., Takemori H., Katoh Y., Doi J., Horike N., Makino A., Nonaka Y., Okamoto M. (2001) Salt-inducible kinase is involved in the ACTH/cAMP-dependent protein kinase signaling in Y1 mouse adrenocortical tumor cells. Mol. Endocrinol. 15, 1264–1276 [DOI] [PubMed] [Google Scholar]
- 18. Ferrandi M., Molinari I., Torielli L., Padoani G., Salardi S., Rastaldi M. P., Ferrari P., Bianchi G. (2010) Adducin- and ouabain-related gene variants predict the antihypertensive activity of rostafuroxin, part 1: experimental studies. Sci. Transl. Med. 2: 59ra86. [DOI] [PubMed] [Google Scholar]
- 19. Gomes P., Soares-da-Silva P. (2006) Upregulation of apical NHE3 in renal OK cells overexpressing the rodent alpha(1)-subunit of the Na(+) pump. Am. J. Physiol. 290, R1142–R1150 [DOI] [PubMed] [Google Scholar]
- 20. Pinto V. M. J., Pinho M. J., Hopfer U., Jose P. A., Soares-da-Silva P. (2008) Oxidative stress and the genomic regulation of aldosterone-stimulated NHE1 activity in SHR renal proximal tubular cells. Mol. Cell. Biochem. 310, 191–201 [DOI] [PubMed] [Google Scholar]
- 21. Pedrosa R., Villar V. A., Pascua A. M., Simao S., Hopfer U., Jose P. A., Soares-da-Silva P. (2008) H2O2 stimulation of the Cl−/HCO3- exchanger by angiotensin II and angiotensin II type 1 receptor distribution in membrane microdomains. Hypertension 51, 1332–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quigley J. P., Gotterer G. S. (1969) Distribution of (Na+-K+)-stimulated ATPase activity in rat intestinal mucosa. Biochim. Biophys. Acta 173, 456–468 [DOI] [PubMed] [Google Scholar]
- 23. Anastasiadis P. Z., Reynolds A. B. (2000) The p120 catenin family: complex roles in adhesion, signaling and cancer. J. Cell Sci. 113, 1319–1334 [DOI] [PubMed] [Google Scholar]
- 24. Bolós V., Peinado H., Pérez-Moreno M. A., Fraga M. F., Esteller M., Cano A. (2003) The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell Sci. 116, 499–511 [DOI] [PubMed] [Google Scholar]
- 25. Peinado H., Olmeda D., Cano A. (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer 7, 415–428 [DOI] [PubMed] [Google Scholar]
- 26. Batlle E., Sancho E., Francí C., Domínguez D., Monfar M., Baulida J., García De Herreros A. (2009) The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2, 84–89 [DOI] [PubMed] [Google Scholar]
- 27. Drewes G., Ebneth A., Preuss U., Mandelekow E. M., Mandelekow E. (1997) MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell 89, 297–308 [DOI] [PubMed] [Google Scholar]
- 28. Tripodi G., Valtorta F., Torielli L., Chieregatti E., Salardi S., Trusolino L., Menegon A., Ferrari P., Marchisio P. C., Bianchi G. (1996) Hypertension-associated point mutations in the adducin alpha and beta subunits affect actin cytoskeleton and ion transport. J. Clin. Invest. 97, 2815–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stenström K., Takemori H., Bianchi G., Katz A. I., Bertorello A. M. (2009) Blocking the salt-inducible kinase 1 network prevents the increases in cell sodium transport caused by a hypertension-linked mutation in human α-adducin. J. Hypertens. 27, 2452–2457 [DOI] [PubMed] [Google Scholar]
- 30. Takemori H., Kajimura J., Okamoto M. (2007) TORC-SIK cascade regulates CREB activity through the basic leucine zipper domain. FEBS J. 274, 3202–3209 [DOI] [PubMed] [Google Scholar]
- 31. Fish E. M., Molitoris B. A. (1994) Alterations in epithelial polarity and the pathogenesis of disease states. N. Engl. J. Med. 330, 1580–1588 [DOI] [PubMed] [Google Scholar]
- 32. Gu Y., Lin S., Li J. L., Nakagawa H., Chen Z., Jin B., Tian L., Ucar D. A., Shen H., Lu J., Hochwald S. N., Kaye F. J., Wu L. (2012) Altered LKB1/CREB-regulated transcription co-activator (CRTC) signaling axis promotes esophageal cancer cell migration and invasion. Oncogene 31, 469–479 [DOI] [PubMed] [Google Scholar]
- 33. Dave N., Guaita-Esteruelas S., Gutarra S., Frias A., Beltran M., Peiró S., de Herreros A. G. (2011) Functional cooperation between Snail1 and twist in the regulation of ZEB1 expression during epithelial to mesenchymal transition. J. Biol. Chem. 286, 12024–12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ireton R. C., Davis M. A., van Hengel J., Mariner D. J., Barnes K., Thoreson M. A., Anastasiadis P. Z., Matrisian L., Bundy L. M., Sealy L., Gilbert B., van Roy F., Reynolds A. B. (2002) A novel role for p120 catenin in E-cadherin function. J. Cell Biol. 159, 465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davis M. A., Ireton R. C., Reynolds A. B. (2003) A core function for p120-catenin in cadherin turnover. J. Cell Biol. 163, 525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pearson H. B., McCarthy A., Collins C. M., Ashworth A., Clarke A. R. (2008) Lkb1 deficiency causes prostate neoplasia in the mouse. Cancer Res. 68, 2223–2232 [DOI] [PubMed] [Google Scholar]
- 37. Zhang L., Jouret F., Rinehart J., Sfakianos J., Mellman I., Lifton R. P., Young L. H., Caplan M. J. (2011) AMP-activated protein kinase (AMPK) activation and glycogen synthase kinase-3beta (GSK-3beta) inhibition induce Ca2+-independent deposition of tight junction components at the plasma membrane. J. Biol. Chem. 286, 16879–16890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rajasekaran S. A., Palmer L. G., Quan K., Harper J. F., Ball W. J., Jr., Bander N. H., Peralta Soler A., Rajasekaran A. K. (2005) Na,K-ATPase beta-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol. Biol. Cell 12, 279–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Larre I., Lazaro A., Contreras R. G., Balda M. S., Matter K., Flores-Maldonado C., Ponce A., Flores-Benitez D., Rincon-Heredia R., Padilla-Benavides T., Castillo A., Shoshani L., Cereijido M. (2010) Ouabain modulates epithelial cell tight junction. Proc. Natl. Acad. Sci. U. S. A. 107, 11387–11392 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.