Abstract
Ascites in epithelial ovarian cancer (EOC) promotes tumor development by mechanisms that are incompletely understood. Lysophosphatidic acid (LPA), a major tumor-promoting factor in EOC ascites, is an enzymatic product of autotaxin (ATX) and phospholipase A2 (PLA2)enzymes. The contribution of PLA2 activities to ovarian tumorigenesis was investigated. The quantitative measurement of PLA2 activities in ascites and tissues, as well as assay conditions selective for PLA2 subtypes, were optimized and validated. PLA2 activities correlated with tumor-promoting activates in cell-based and in vivo assays. High activities consistent with both cytosolic and calcium-independent PLA2 were found in human EOC ascites for the first time. Elevated PLA2 and ATX activities were also observed in EOC compared to benign tumors and normal tissues. Cell-free and vesicle-free (S4) human EOC ascites potently promoted proliferation, migration, and invasion of human EOC cells in a PLA2-dependent manner. LPA mediated a significant part of the cell-stimulating effects of ascites. S4 ascites stimulated tumorigenesis/metastasis in vivo, and methyl arachidonyl fluorophosphonate was highly effective in inhibiting EOC metastasis in mouse xenograft models. PLA2 activity was found in conditioned media from both EOC cells and macrophages. Collectively, our work implies that PLA2 activity is a potential marker and therapeutic target in EOC.— Cai, Q., Zhao, Z., Antalis, C., Yan, L., Del Priore, G., Hamed, A. H., Stehman, F. B., Schilder, J. M., Xu, Y. Elevated and secreted phospholipase A2 activities as new potential therapeutic targets in human epithelial ovarian cancer.
Keywords: ascites, migration, autotaxin, methyl arachidonyl fluorophosphonate
The majority of patients with epithelial ovarian cancer (EOC) present with late-stage metastatic disease, often accompanied by significant peritoneal ascites, for which no curative treatment exists. Hence, an urgent need remains for improved detection and new targets for more effective modalities to treat late-stage EOC. We originally identified lysophosphatidic acid (LPA) as a major tumor-promoting factor in ovarian cancer ascites, showed its effects in EOC, and conducted extensive work in vitro and in vivo (1, 2). Overexpression or down-regulation of LPA receptors 1–3 (LPA1–3) in several human EOC cell lines and in vivo mouse studies further demonstrated that LPA is involved in EOC development (3).
LPA is produced by the action of autotaxin (ATX), phospholipase A1 (PLA1), and PLA2 (4). More than 30 enzymes that possess PLA2 or related activity have been identified in mammals (5), and they are divided into four groups based on their cellular localization, substrate specificity, and calcium-dependence (6), including cytosolic PLA2 (cPLA2), calcium-independent PLA2 (iPLA2), secreted PLA2 (sPLA2), and lipoprotein-associated PLA2 (Lp-PLA2). sPLA2 and Lp-PLA2 are secreted enzymes. In contrast, both cPLA2 and iPLA2 are cytosolic enzymes, and their cell-free presentation has been shown to be related to exosomes from only RBL-2H3 cells (a mast and basophil cell line; ref. 7). Exosomes are 40- to 100-nm-diameter membrane vesicles released from multivesicular bodies by intact cells that participate in intercellular signaling (8).
Only in recent years have PLA2s emerged as cancer targets (9), and most if not all of these studies focus on sPLA2 and cPLA2 (6). Aberrant expression of various PLA2s has been shown in >10 different cancer types, including breast, lung, and prostate cancers. However, up-regulation of PLA2s in EOC has not been detected clearly in any of several studies (10–12). However, we and others have shown that iPLA2β is involved functionally in promoting EOC development in vitro and in vivo (1, 2, 13–15).
Since the protein activities are related directly to the biological effects, we focused on activities rather than expression of PLA2s in the current work and examined PLA2 activities in human EOC tissues, including ascites specimens. The activities from different subgroups of PLA2s were distinguished using selective inhibitors and/or other reagents. The quantitative fluorescent PLA2 assays were optimized and validated using human samples. Bioactive lipid concentrations in cell-free ascites samples, and different fractions of ascitic samples were measured. The cell-stimulating activities of human EOC ascites samples were tested in cell-based assays, and the mechanisms involved were investigated. Moreover, the efficacy of an iPLA2 and cPLA2 dual inhibitor was examined in a mouse xenograft model.
MATERIALS AND METHODS
Human sample collection and processing
Ascites and tissue samples were obtained from the Department of Obstetrics and Gynecology, Indiana University School of Medicine (IUSM) and the Cleveland Clinic (Cleveland, OH, USA) or through the Cooperative Human Tissue Network (CHTN), a U.S. National Institutes of Health–sponsored organization providing human tissues to researchers under approved institutional review board (IRB) protocols. Ascites from patients with EOC were kept at 4°C throughout processing, and fractions were portioned into aliquots and stored at −80°C. Samples were centrifuged on the day of collection at 3000 g for 20 min to sediment cells and debris. The supernatant (S1) was fractionated further by centrifuging at 20,000 g for 20 min, which resulted in S2 and pellet 2 (P2; cell fragments and large vesicles). S2 was ultracentrifuged at 110,000 g for 2 h, which resulted in S3 and P3 (exosomes). A final centrifugation of S3 at 200,000 g for 2 h resulted in S4 and P4 (other microvesicles). P3 and P4 fractions were resuspended in cold PBS and subjected to another ultracentrifugation before final suspension in PBS. Snap-frozen tissues were collected from surgically removed malignant (from both primary and metastatic sites) ovarian tumors or benign tumors, along with adjacent normal tissues for both. The demographic data for human ascites and tissue samples are shown in Tables 1 and 2.
Table 1.
Demographic data for human ascites samples
| ID | Age | Race | Histopathology | Grade | Stage | Previous chemo |
|---|---|---|---|---|---|---|
| A1 | 70 | Caucasian | Papillary serous adenocarcinoma | 3 | III-C | Yes |
| A2 | 73 | Caucasian | Papillary serous adenocarcinoma, clear cell carcinoma | 3 | IIIC | Yes |
| A3 | 51 | Caucasian | Papillary serous adenocarcinoma | 3 | IV | Yes |
| A4 | 66 | Caucasian | Peritoneal carcinomatosis | NG | IV | Yes |
| A5 | 50 | Caucasian | Papillary serous adenocarcinoma | 3 | IIIC | Yes |
| A6 | 63 | Caucasian | Endometroid adenocarcinoma | 2 | III | Yes |
| A7 | 54 | Caucasian | Papillary serous adenocarcinoma | 3 | IIIC | No |
| A8 | 65 | Caucasian | Papillary serous adenocarcinoma | 3 | IIC | Yes |
| A9 | 47 | Caucasian | Mixed endometrioid and mucinous adenocarcinoma | 1 | IIIC | No |
| A10 | 72 | African American | Papillary serous adenocarcinoma | NG | IV | No |
Chemo, chemotherapy; NG, not graded.
Table 2.
Demographic data for human tissue samples
| ID | Age | Race | Histopathology | Stage | Grade |
|---|---|---|---|---|---|
| N1 | 49 | Caucasian | Normal ovary | ||
| N2 | 46 | African American | Normal ovary | ||
| N3 | 51 | Caucasian | Normal ovary | ||
| N4 | 62 | Caucasian | Normal ovary | ||
| N5 | 47 | Caucasian | Normal ovary | ||
| N6 | 59 | Caucasian | Normal ovary | ||
| N7 | 69 | African American | Normal ovary | ||
| N8 | 54 | Caucasian | Normal ovary | ||
| N9 | 50 | Caucasian | Normal ovary | ||
| N10 | 45 | Caucasian | Normal ovary | ||
| B1 | 44 | Caucasian | Benign ovary | ||
| B2 | 80 | Caucasian | Benign ovarian cystadenoma | ||
| B3 | 49 | Caucasian | Benign ovarian cystadenoma | ||
| B4 | 42 | Caucasian | Benign ovarian fibroma | ||
| B5 | 27 | Other | Benign ovary | ||
| B6 | 34 | Caucasian | Benign ovary | ||
| B7 | 36 | Caucasian | Benign ovary | ||
| B8 | 37 | Caucasian | Benign ovary | ||
| B9 | 42 | Caucasian | Benign ovary | ||
| B10 | 31 | Caucasian | Benign ovary | ||
| O1 | 76 | Caucasian | Serous adenocarcinoma | II | III |
| O2 | 55 | Caucasian | Mucinous adenocarcinoma | I | I |
| O3 | 70 | Caucasian | Papillary serous adenocarcinoma | II | III |
Reagents and inhibitors
The PLA2 substrate 1-O-(6-dabcyl-aminohexanoyl)-2-O-(6-(12-BODIPY-dodecanoyl) aminohexanoyl)-sn-3-glyceryl phosphatidylcholine (DBPC) and the ATX substrate FS-3 were from Echelon Bioscience (Salt Lake City, UT, USA). The radio-labeled substrate 1-palmitoyl-2-[1-14C] palmitoyl-sn-glycero-3-phosphocholine was from PerkinElmer (Boston, MA, USA). siRNAs were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Bromoenol lactone (BEL) and methyl arachidonyl fluorophosphonate (MAFP) were from Santa Cruz Biotechnology; arachidonyl trifluoromethyl ketone (AACOCF3) was from EMD Chemicals (Philadelphia, PA, USA); thioetheramide-phosphatidylcholine (TAPC) was from Cayman Chemical Co. (Ann Arbor, MI, USA); and BrP-LPA was from Echelon Bioscience. Antibody to cPLA2 was from Cell Signaling (Danvers, MA, USA); antibody to iPLA2 was from Cayman Chemical.
PLA2 enzymatic activity analyses
To prepare tissue homogenate, pulverized tissue (100–150 mg) was mixed with 500 μl lysis buffer (10 mM hepes, 0.34 M sucrose, pH 7.5) containing protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). Samples were homogenized with an Omni Tissue Homogenizer (Kennesaw, GA, USA) and centrifuged at 16,000 g at 4°C for 40 min. The supernatant was removed to a fresh tube, and a small aliquot was retained for protein assay (BCA assay; Thermo-Fisher Scientific, Rockford, IL, USA).
PLA2 activities were analyzed using the fluorescent substrate DBPC, a fluorogenic phosphatidylcholine (PC) substrate (16). We also used a radiolabeled PC substrate to validate the DBPC-based method, as described previously (16). For the DBPC-based assays, tissue homogenate (0.02 mg protein) or human EOC ascites (1.0 μl) were mixed with DBPC (0.2 μg in 200 μl of PBS). The PLA2 activities were expressed as change in fluorescence intensity per minute per milligram of protein or per microliter of body fluid.
For frozen tissue section PLA2 assays, the snap-frozen tumors were embedded in optimal cutting temperature (OCT) compound (Electron Microscopy Sciences, Hatfield, PA, USA) and sliced into 10-μm sections on glass slides. The sections were fixed with cold methanol for 20 min and then incubated with cold PBS for 5 min. The sections were incubated with DBPC (0.1 μg in 100 μl 1% DMSO in PBS) for 1 h at room temperature and then washed with PBS. Cell nuclei were costained by the use of Vectashield Mounting Medium with DAPI (Vector Laboratories Inc., Burlingame, CA, USA). Inhibitors (40 μM BEL; 40 μM MAFP, a dual inhibitor of cPLA2 and iPLA2; or 250 μM TAPC in PBS/DMSO, 100:1, v/v), were preincubated with the frozen sections for 5 min prior to adding DBPC. The fluorescence was imaged using a Nikon fluorescent microscope (EclipseTE2000-S; Nikon Melville, NY, USA). The quantification of the activity was performed using the Show Region Statistics function in the MetaMorph software (Molecular Devices Corp., Sunnyvale, CA, USA) and expressed as fluorescence intensity per cell.
PLA2 activity in conditioned medium (CM)
For detection of secreted PLA2 activity, a near-confluent monolayer of RAW 264.7 mouse macrophages was rinsed with PBS and covered with 0.5 vol of serum-free (SF) supportive medium (DMEM/F12 with 1% ITS, 0.1% BSA, and antibiotics). Samples of the CM were taken and analyzed for PLA2 activity using the fluorescent DBPC method. To test inhibitors of cell secretion, the inhibitor was added to the cells in SF medium and incubated for 4 h. Inhibitors were removed, and fresh medium was added. After 15–18 h incubation, samples of CM were assayed for PLA2 activity as described. Amiloride, 5-(N,N-dimethyl)-hydrochloride (DMA), brefeldin A (BFA), cyclosporine A (CsA), and glyburide were obtained from Santa Cruz Biotechnology. Methyl-β-cyclodextrin (MCD) was obtained from Sigma-Aldrich (St. Louis, MO, USA). MK571 was obtained from EMD Chemicals (Philadelphia, PA, USA).
Cell culture
SKOV3 and CaOV3 cell lines were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA). HEY cells were obtained from Dr. Gordon Mills (MD Anderson Cancer Center, Houston, TX, USA) and were certified by Biosynthesis (Lewisville, TX, USA). SKOV/VLB, a SKOV3-derived multidrug-resistant cell line expressing high levels of P-glycoprotein (17) was obtained from Dr. Jian-ting Zhang, IUSM, and certified by Biosynthesis. SKOV3-Luc cells (a gift from Dr. Melissa Fishel, IUSM), which express both green fluorescent protein and luciferase, were used for the mouse models of EOC. EOC cells were cultured in RPMI with 5% FBS and antibiotics. RAW 264.7 mouse macrophages were obtained from ATCC and cultured in DMEM with 10% FBS and antibiotics. All cell lines were maintained in a humidified incubator at 37°C and 5% CO2 and were used within 20 passages of receipt or certification.
ATX enzymatic activity analyses
Tissue homogenate (0.02 mg protein) or human EOC ascites (1.0 μl) per assay were mixed with FS-3 (0.5 μg in 200 μl of PBS) in a 96-well plate and incubated at 37°C. The fluorescence was read at intervals over several hours on a Victor3V plate reader (Perkin Elmer, Waltham, MA, USA). ATX activity was expressed as change in fluorescence intensity per minute per milligram of protein or per microliter of body fluid.
Proliferation, migration and invasion, and lipid analyses
Proliferation assays using MTT, cell migration, and invasion assays were conducted as we described previously (2). Proliferation data are presented as means ± sd of the OD555 for 4–6 wells. Migration and invasion data are presented as means ± sd of cells per field per membrane for ≥3 membranes. Lipids were extracted with our simple lysophospholipid (LPL) extraction method (the MeOH method; ref. 18), and mass spectrometry analyses were performed using API-4000 (Applied Biosystems /MDS SCIEX, Carlsbad, CA, USA) with the Analyst data acquisition system, as described previously (18, 19).
Quantitative real-time PCR
SKOV3 cells in 6-well plates were incubated with 33 nM control siRNA or one of the LPA1–4 siRNAs for 60 h. Then SKOV3 cells were collected in the Qiagen RLT lysis buffer (Qiagen, Valencia, CA, USA). RNA was extracted with an RNeasy mini kit (Qiagen) and reverse transcribed by M-MLV reverse transcriptase. Quantitative real-time PCR was performed on a Light Cycler 480 (Roche, Indianapolis, IN, USA). Primer sequences were: GAPDH-F, 5′-GAAGGTGAAGGTCGGAGT-3′, GAPDH-R, 5′-GAAGATGGTGATGGGATTTC-3′; LPA1-F, 5′-AATCGAGAGGCACATTACGG-3′, LPA1-R, 5′-GTTGAAAATGGCCCAGAAGA-3′; LPA2-F, 5′-TTGTCTTCCTGCTCATGGTG-3′, LPA2-R, 5′-TCAGCATCTCGGCAAGAGTA-3′; LPA3-F, 5′-TGCTCATTTTGCTTGTCTGG-3′, LPA3-R, 5′-GCCATACATGTCCTCGTCCT-3′; LPA4-F, 5′-CTTCGCAAGCCTGCTACTCT-3′, and LPA4-R, 5′-GGCTTTGTGGTCAAAGGTGT-3′.
Mouse xenograft model of EOC
Female NOD/SCID mice were obtained from the IUSM In Vivo Therapeutics Core at 6 to 8 wk of age. All animal protocols were approved by the IUSM Animal Care and Use Committee. The xenograft models used were essentially the same as we described previously (2). In the first study, SKOV3-Luc cells (107 in 500 μl PBS) were injected i.p. into mice (n=12/group) on day 0. Starting at day 10, the mice were injected i.p. with MAFP (0.22 mg/kg) or vehicle 3×/wk for 4 wk. At 38–40 d after tumor cell injections, mice were sacrificed, and tumor development was assessed. Tumors were counted at each metastatic location, and tumor diameters and volume of ascites were measured. For testing the potential toxic effects on tissues, fixed paraffin-embedded tissue slices from the kidney, liver, small intestine, lung, and brain were subjected to hematoxylin and eosin staining and pathological examination. For the second study, NOD/SCID mice (n=7) were injected i.p. with 106 HEY cells in 500 μl PBS. After 2 d, 0.5 ml S4 ascites (mixture of 3 specimens) or 0.5 ml PBS was injected i.p. into the mice, and this was continued 3×/wk for 3 wk. Mice were sacrificed, and tumor and ascites development was assessed as described above. In a third study, 107 SKOV3-Luc cells were injected i.p. into NOD/SCID mice (n=6). After 10 d, a pooled P3 fraction (containing 500 μg protein) was injected i.p., and this was continued 2×/wk for 3 wk. Mice were sacrificed, and tumor and ascites development were assessed.
Statistical analyses
Data are presented as mean ± sd. For differences between 2 groups, the Student's t test was employed. For differences between multiple groups or treatments, 1-way ANOVA was performed with Dunnett's test to compare the treatments to the control. The statistical program GraphPad Prism 5.04 (GraphPad Software, Inc., La Jolla, CA, USA) was used for all analyses. The significance level was set at α = 0.05.
RESULTS
Lipid products of PLA2 in human EOC ascites were high
We have shown that EOC ascites contained high levels of oncogenic lipid growth factors, such as LPA, that stimulate EOC cell proliferation, adhesion, migration, and invasion (20, 21). Compared to ascites from patients with benign liver cirrhosis, EOC ascites also contained higher levels of lysophosphatidylcholine (LPC), the lipid product of PLA2 (22). We now analyzed arachidonic acid (AA, another product of PLA2 activity) and found that it was also high in EOC ascites (3.2±1.4 and 0.90±0.43 μM for EOC and liver cirrhosis ascites samples, respectively, n=10 in each category, P<0.001). The combined elevation of LPC/LPA and AA supports high PLA2 activities in EOC ascites.
Development, optimization, and validation of DBPC-based quantitative PLA2 assays in human tissue and ascites
DBPC has been used in cell-based PLA2 assays (2, 4). However, whether it can be used for quantitative assays in tissues or biological fluids had not been tested. We optimized and validated DBPC-based quantitative PLA2 assays in human samples (tumor tissues and ascites). We first compared PLA2 activities in tissue homogenates (ovarian tumors vs. benign gynecologic tumors, n=3 each) using both the DBPC and the classical radiometric method (6, 16, 23). The results were highly correlative (Fig. 1) despite the different arbitrary units used for activity, supporting the quantitative nature of the DBPC method.
Figure 1.
Comparison of radiometric and fluorometric methods for PLA2 activity assay. A, B) PLA2 activities in 6 tissue homogenates [ovarian tumors (O1–O3) vs. benign gynecologic tumors (B1–B3)] using both the classic radiometric method (A) and the DBPC-based fluorometric method (B). C) Results obtained from the two methods were highly correlative (R2=0.918, P=0.0026).
Next, we optimized the conditions to be used for DBPC assays. We found that the increase in fluorescence was linear for ≥4 h incubation at 37°C. We chose a 2 h end point for most assays reported here. We also found that DBPC was sensitive to freeze-thaw cycles with decreased fluorescence. However, after 3 freeze-thaw cycles, it became stable when stored at −80°C. Hence, we used freeze-thaw-treated aliquots of DBPC substrate for the assays. We examined the precision and reproducibility of the assays using 3 human EOC ascites samples. The intraday coefficient of variation (CV) was between 3.7 to 5%. Similarly, the interday CVs were <3.2% for all samples tested. Using two different ascites samples, we found that 1–3 freeze-thaw cycles did not significantly change the PLA2 activity. In addition, the PLA2 activity was not changed in samples stored at −80°C for ≤4 mo, which suggests that the activity was relatively stable. We also optimized the amount of substrate to be used. Using a fixed amount of ascites (10 μl), we determined that the increase in fluorescence was linear at least in the range of 0.1 to 0.6 μg DBPC. We chose to use 0.2 μg DBPC substrate in our assays and identified the linear range for each type of biological sample to ensure that the substrate would not be rate limiting. The linear ranges were 0.2–1 μl and 0.002–0.02 mg protein for ascites and tissues homogenates, respectively. Thus, the optimized conditions for the work presented here were: 0.2 μg of DBPC and 1 μl of ascites or 0.02 mg protein of tissue homogenate in 200 μl PBS for 2 h incubation at 37°C. The PLA2 activities were expressed as change in fluorescence intensity per minute per microliter or as change in fluorescence intensity per minute per microgram of protein. Although the protein concentrations of 10 human EOC ascites specimens that we used in this work ranged from 39 to 64 mg/ml, no correlation was found between PLA2 activity and the total protein concentration in ascites (R2<0.01, P>0.5), which suggests that PLA2 activity is an independent parameter of ascites (Table 3).
Table 3.
Protein concentration and PLA2 and ATX activities of ascites samples
| ID | Protein (mg/ml) | Natural PLA2 | iPLA2 | sPLA2 | cPLA2 | ATX | ATX/BrP-LPA |
|---|---|---|---|---|---|---|---|
| A1 | 61 | 378 | 434 | 0.9 | 269 | 27.0 | 7.9 |
| A2 | 45 | 466 | 558 | 5.1 | 407 | 31.4 | 6.8 |
| A3 | 42 | 213 | 294 | 2.0 | 188 | 31.4 | 8.0 |
| A4 | 39 | 319 | 452 | 5.4 | 184 | 28.6 | 5.2 |
| A5 | 48 | 213 | 331 | 0 | 132 | 29.4 | 5.2 |
| A6 | 55 | 247 | 382 | 7.4 | 181 | 35.7 | 6.6 |
| A7 | 54 | 384 | 595 | 3.7 | 300 | 39.2 | 7.6 |
| A8 | 47 | 381 | 495 | 5.2 | 273 | 30.0 | 9.3 |
| A9 | 48 | 225 | 369 | 2.2 | 166 | 43.0 | 9.8 |
| A10 | 64 | 302 | 463 | 6.3 | 207 | 33.5 | 5.6 |
cPLA2- and iPLA2-like activities accounted for the majority of the PLA2 activities detected in cell- and vesicle-free human EOC ascites
Lucas and Dennis (24) have summarized the inhibitors and assay conditions for distinguishing the different PLA2 subtypes. We similarly selected a set of conditions for our assay to distinguish PLA2 activity derived from different subtypes: the “natural PLA2 activity” was detected in the samples without any exogenous additives; the iPLA2 activity was detected in the presence of 5 mM EDTA (a divalent cation chelator to block all PLA2s requiring calcium, including sPLA2 and cPLA2); the sPLA2 activity was detected in the presence of 1.2 mM calcium chloride (the natural ionized calcium concentration in blood; ref. 25) and MAFP (10 μM, a dual inhibitor of cPLA2 and iPLA2); and the cPLA2 activity was detected in the presence of 100 μM calcium chloride and BEL (10 μM, a selective inhibitor for iPLA2).
Strong PLA2 activity was detected in all 10 human EOC cell-free ascites specimens tested. While sPLA2 has been described in cell-free biological fluids (6), we surprisingly found that cPLA2- and iPLA2-like activities (MAFP sensitive) accounted for >98% of the natural PLA2 activity detected in cell-free ascites (Table 3). We noted that the sum of defined cPLA2 and iPLA2 activities were higher than the natural PLA2 activity. This is likely due to enhanced iPLA2 activity beyond its natural activity in biological samples under the conditions used. In addition, cautions were taken in interpretation of the results with inhibitors, since their efficacies and specificities may not be 100%, and they may have off-target effects. Additional reagents, such as TAPC, an sPLA2-selective inhibitor (26), dithiothreitol (reduces disulfide bonds to denature protein structures of sPLA2s), or LY311727 (a selective inhibitor for sPLA2 II) did not inhibit the ascitic PLA2 activity, confirming the low sPLA2 activity in ascites.
cPLA2 and iPLA2 are cytosolic enzymes and not known to be secreted by classical secretion pathways. It has been reported that PLA2 activity is associated with reticulocyte and mast cell exosomes (7). To test whether EOC ascitic PLA2 activities were associated with exosomes, we used stepwise centrifugation to obtain exosomes (27). As shown in Fig. 2A, we found that >50% of the natural PLA2, iPLA2, and cPLA2 activities remained in the S3 and S4 fractions of the ascites, and <3% of these activities was associated with the P3 (exosome) fraction when the activities were adjusted to the original volumes. The P2 fraction (cell fragments and some larger vesicles) contained <3% of PLA2 activities as well (Fig. 2A). Highly purified exosomes can be obtained from P3 using a sucrose gradient (27). However, since minimal PLA2 activities were associated with P3, additional work with P3 and more purified exosomes were not the major focus of the current work.
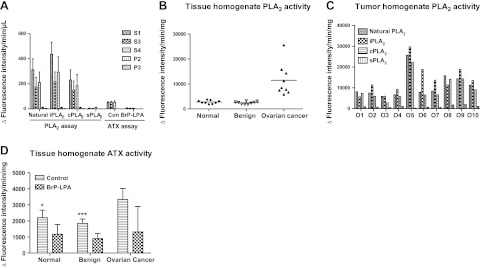
Figure 2.
PLA2 and ATX activity in ascites and tissue homogenates. A) PLA2 and ATX activities in ascites fractions. Ascites fractions (S1, S3, S4, P2, and P3, n=10/group) were analyzed for PLA2 and ATX activities as described in Materials and Methods. Data are means ± sd from 2–4 analyses. B) Summary of PLA2 activity in tissue homogenates (n=10/group). C) Tumor PLA2 subgroup determination using specific inhibitors. D) Summary of ATX activity in tissue homogenates (n=10/group). *P < 0.05, ***P < 0.001 vs. ovarian cancer.
We and others have demonstrated that LPA is a major lipid growth factor for EOC (1, 28) and ATX has been considered the main or even the sole enzyme producing LPA in biological samples (4, 29). We detected strong ATX activity in all 10 EOC ascites samples tested (Table 3). Minimal or no ATX activity was associated with P2 or P3 fractions. All ATX activity detected in the S1 fraction was maintained in the S3 and S4 fractions and >85% was inhibited by the ATX selective inhibitor BrP-LPA (100 μM; Fig. 2A).
cPLA2 and iPLA2, as well as ATX, activities were elevated in EOC tissues
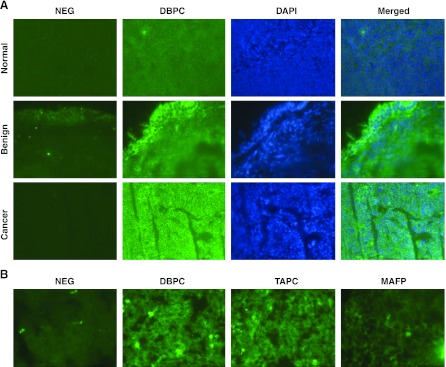
For tissue PLA2 activities, we tested both frozen tissue sections (in situ detection) and tissue homogenates (microplate assay). The former has the advantage of preserving tissue morphology and the cellular location of the PLA2 activity, but it was relatively difficult to quantify. Representative fluorescent images of PLA2 activities in frozen tissue sections tissues from normal ovaries, benign gynecologic tumors, and ovarian cancer tissues are shown in Fig. 3. The total PLA2 activities in EOC tissues (881±395 fluorescence intensity units/cell) were significantly increased compared to those in either benign (276±58) or normal (55±16) tissues (n=6 samples/group). High levels of cytosolic PLA2 activity throughout the section was observed in the ovarian and endometrial tumor specimens (representative data are shown in Fig. 3A). For benign tumors, the activity was limited to the superficial cell layers (Fig. 3A). In contrast, very little or no cytosolic PLA2 activity was observed in normal ovarian tissues (Fig. 3A). In addition, tumor PLA2 activity was sensitive to a dual inhibitor of cPLA2 and iPLA2 (MAFP), but not to an inhibitor of sPLA2 (TAPC) (Fig. 3B), suggesting that the majority if not all total PLA2 activity in tumor tissues was from cPLA2 and/or iPLA2.
Figure 3.
Tissue PLA2 activity and expression. A) Representative tissue PLA2 activities in normal ovaries, benign tumors, and EOC tumors. Images represent PLA2 activity without DBPC (negative control), PLA2 activity with DBPC, staining of nucleus (DAPI), and merged image. B) Tumor PLA2 activity in the presence of inhibitors TAPC (250 μM) and MAFP (40 μM).
For more quantitative PLA2 assays, we used tissue homogenates (Fig. 2B–D). As in the tissue sections, the natural PLA2 activity was elevated in EOC tumor tissue homogenates (Fig. 2B) and the effects of inhibitors support that the activities were consistent with iPLA2 and cPLA2 (Fig. 2C). ATX activity was also significantly different among normal/benign vs. malignant EOC tissues (Fig. 2D). Taken together, our preliminary studies suggest that EOC tissue has elevated PLA2 and ATX activities when compared to benign gynecological and normal tissues.
S1, S3, and S4, but not P3, ascites fractions stimulated proliferation of EOC cells
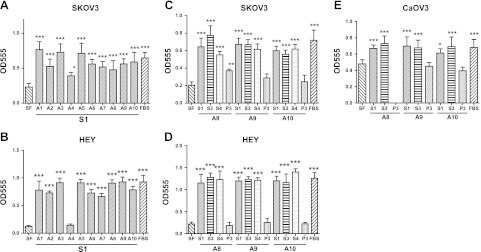
We and others have shown the proliferation-stimulating activity of human EOC ascites on EOC cells >20 yr ago (20). Here we tested a set of 10 EOC ascites specimens, fractionated as described, for their effects on proliferation in EOC cells. SKOV3 and HEY cells demonstrated a dose-responsive increase in proliferation over the range of 0.1 to 10% S1 in SF medium, with maximum effect at 5% S1 (detailed data not shown). All 10 S1 fractions (at 5%) stimulated proliferation with similar or higher potency as compared to FBS (5%) in SKOV3 cells (Fig. 4A). These effects were similar in HEY cells, except that one sample had low activity (Fig. 4B). The S3 fraction (at 5%) from different ascites specimens had almost identical proliferation-stimulating activity when compared to S1 in all three EOC cells lines tested (SKOV3, HEY, and CaOV3; Fig. 4C–E), while the P3 fraction had much lower or no activity. The S4 fraction (at 5%) had very similar activity as S1 and S3, suggesting the majority of human EOC ascites proliferation-stimulating activity is not associated with exosomes. Proliferation of SKOV3 cells was reduced by 28.8 ± 9.98% (P<0.001) by 100 μM MAFP, by 24.6 ± 13.2% by 25 μM BrP-LPA (P=0.006), and by 12.4 ± 7.44% (P=0.016) by 10 μM Ki16425, showing involvement of PLA2 and ATX enzymes and LPA receptors, respectively, in the stimulation of proliferation by S1.
Figure 4.
Human EOC ascites fractions stimulated proliferation of human EOC cells. Cells in 96-well plates were treated with 5% S1, S3, or S4 ascites fractions in SF medium or an amount of the P3 fraction equal to 2.5× the concentration in the original ascites. SF medium and SF containing 5% FBS were used as controls. After 72 h incubation, the MTT assay was conducted, and absorbance at 555 nm was recorded. A, B) Effect of 10 patient ascites on proliferation of SKOV3 and HEY cells. C–E) Effect of 3 patient ascites and respective fractions on proliferation of SKOV3 (C), HEY (D), and CaOV3 (E) cells. Data are means ± sd of 4–6 replicates. Experiments were repeated at least twice.*P < 0.05, **P < 0.01, ***P < 0.001 vs. SF medium.
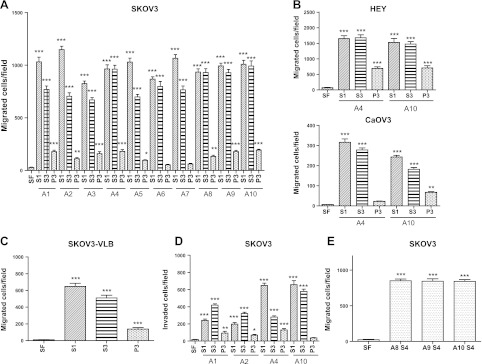
S1, S3, and S4 but not P3 ascites fractions potently stimulated migration and invasion of EOC cells
Human S1 and S3 ascites fractions dose-dependently stimulated migration of SKOV3 cells, reaching a maximum effect at 1% ascites in SF medium (not shown). All 10 S1 and S3 ascites fractions potently stimulated migration of SKOV3 cells, with the S3 fraction having ∼60–90% of the activity of the S1 fraction. In contrast, the P3 fraction had only ∼10% of the activity of S1 (Fig. 5A). Migration of HEY and CaOV3 cells was also stimulated by S1, S3, and P3 ascites fractions (Fig. 5B). In HEY and CaOV3 cells, S3 fractions had 80–100% of the activity of S1, and P3 had 0–30% of the activity of S1 in stimulating cell migration. In addition, the migration-stimulating effects of ascites fractions on the drug-resistant cell line SKOV3-VLB (30) was very similar to the effects seen in SKOV3 cells, showing that the stimulatory effect of ascites was maintained in this multidrug resistant cell line (Fig. 5C). S1, S3, and P3 fractions also stimulated cell invasion (Fig. 5D), with the S4 fraction having very similar activity as compared to S3 (Fig. 5E).
Figure 5.
Human EOC ascites fractions stimulated migration and invasion of EOC cells. A) Effects of ascites fractions S1, S3, and P3 from 10 patients on migration of SKOV3 cells. B) Effect of ascites fractions S1, S3, and P3 on migration of HEY and CaOV3 cells. C) Effect of ascites fractions S1, S3, and P3 on migration of drug-resistant SKOV3-VLB cells. D) Effect of ascites fractions S1, S3, and P3 on invasion of SKOV3 cells. E) Effect of ascites fraction S4 on SKOV3 cell migration. Data are means ± sd of ≥3 experiments. .*P < 0.05, **P < 0.01, ***P < 0.001 vs. SF medium.
LPA was a major mediator of the tumor-promoting activity of ascites fractions
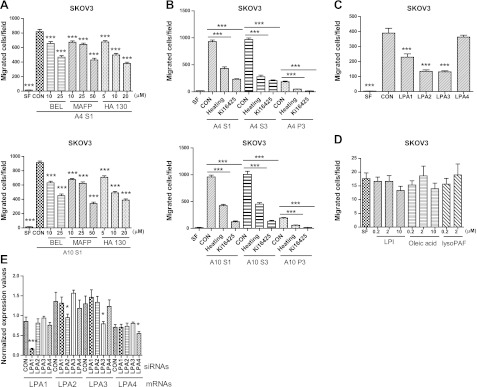
To determine whether PLA2 and ATX were functionally involved in ascites-induced migration of EOC cells, we added the inhibitors MAFP, BEL, and HA130 to the lower chambers during the assay. All three inhibitors dose-dependently reduced migration of SKOV3 cells induced by two different S1 fractions (Fig. 6A). The effect of each inhibitor was almost identical whether using S1, S3, or S4 fractions (Fig. 6 and data not shown).
Figure 6.
LPA mediated the tumor-promoting activity of ascites fractions. A) Effect of PLA2 and ATX inhibitors on ascites S1-induced migration. B) Effect of heat treatment of ascites fractions or pretreatment of SKOV3 cells with Ki16425 (10 μM) on EOC cell migration. C) Effect of siRNA-mediated knockdown of LPA1–4 in SKOV3 cells on S1-induced cell migration. D) Effect of LPI, oleic acid or lyso-PAF on SKOV3 cell migration. E) Normalized LPA receptor mRNA levels from siRNA-treated SKOV3 cells determined by quantitative real-time PCR. Data are means ± sd of 3 experiments. *P < 0.05, ***P < 0.001 vs. control (CON).
While we and others have reported the effect of LPA on EOC cell migration and invasion (2, 16, 21, 31), we tested whether LPA was a component of the effects of S1 and S3 ascites fractions. First, we treated S1 and S3 fractions at 95°C for 10 min and then determined their effect on migration (Fig. 6B). Approximately 40% and 50% of the migration-stimulating activity was retained using SKOV3 (Fig. 6B) and HEY cells (data not shown), respectively, suggesting that a significant portion of the activity is likely mediated by heat-resistant lipid factors. Second, we used Ki16425, a selective blocker of LPA1 and LPA3 receptors, and found that it inhibited >70% of the migratory activity induced by S1, S3, and P3 fractions (Fig. 6B). Third, we used specific siRNAs for LPA1–4. These siRNAs effectively and specifically down-regulated each of these receptors at the mRNA level (Fig. 6E). siRNAs against LPA1–3, but not LPA4, significantly reduced S1-induced cell migration (Fig. 6C).
We have shown that one of the major PLA2 products, LPC, does not stimulate EOC cell migration (32, 33). We also tested lysophosphatidylinositol (LPI), another major LPL that is elevated in human EOC ascites (22), oleic acid, and lyso-platelet activating factor (lyso-PAF), and found that they did not stimulate migration or invasion (Fig. 6D). Collectively, our data support that LPA, present and produced in EOC ascites fractions, is a major mediator of the migration-promoting effects.
In summary, all 10 S1 and S3 specimens stimulated proliferation (1.4- to 2.6-fold), migration (3- to 40-fold), and invasion (4- to 35-fold) of EOC cells. These functions were sensitive to MAFP (15–40% inhibition), BEL (20–40%), and the ATX inhibitor HA 130 (10–45% inhibition), supporting the involvement of PLA2 and ATX activities in EOC cell migration.
Ex vivo lipid generation in S1 and S4 ascites fractions is time-dependent and sensitive to MAFP, BEL, and BrP-LPA
Active enzymatic lipid generation in EOC ascites was examined using the mass spectrometry-based lipidomics approach developed in our laboratory (18, 19). We measured lipids in S1 and S4 ascites fractions incubated at 37°C at 0 and 48 h time points. We found that incubation of S1 ascites fractions (n=3) produced a 6- to 9-fold increase in total LPA concentration over 48 h, as well as increases in other LPLs and fatty acids, including AA (3- to 5-fold increase; Table 4). In addition, lipid generation was highly sensitive to MAFP (74–80% inhibition) and much less sensitive to BrP-LPA (13–28% inhibition) (Table 4). The S1 and S4 fractions had very similar patterns of lipid generation (Table 4). In contrast, P3 did not generate LPA under similar conditions (not shown), indicating that the majority, if not all, of the LPA-producing activity in ascites is soluble and not associated with vesicles (including exosomes).
Table 4.
Lipid generation in ascites samples ex vivo
| ID, fraction, and condition | Total LPC | AA | Total LPA |
|---|---|---|---|
| A5 S1 | |||
| 0 h | 73.4 | 1.1 | 7.5 |
| 48 h | 49.3 | 3.2 | 43.2 |
| MAFP | 65.8 | 3.1 | 14.2 |
| BrP-LPA | 63.7 | 2.8 | 34.4 |
| A6 S1 | |||
| 0 h | 51.3 | 1.9 | 4.3 |
| 48 h | 49.1 | 6.7 | 34.9 |
| MAFP | 55.3 | 5.4 | 6.9 |
| BrP-LPA | 57.2 | 4.8 | 24.5 |
| A7 S1 | |||
| 0 h | 39.2 | 2.1 | 4.9 |
| 48 h | 28.2 | 5.4 | 42.5 |
| MAFP | 46.2 | 4.3 | 12.0 |
| BrP-LPA | 56.2 | 4.8 | 39.2 |
| A5 S4 | |||
| 0 h | 68.5 | 8.0 | |
| 48 h | 45.7 | 44.6 | |
| MAFP | 64.6 | 14.2 | |
| BrP-LPA | 64.3 | 33.5 | |
| A6 S4 | |||
| 0 h | 56.4 | 5.8 | |
| 48 h | 41.7 | 36.8 | |
| MAFP | 54.8 | 12.3 | |
| BrP-LPA | 58.6 | 29.1 | |
| A7 S4 | |||
| 0 h | 44.6 | 6.8 | |
| 48 h | 31.8 | 47.3 | |
| MAFP | 49.4 | 16.1 | |
| BrP-LPA | 58.3 | 45.1 |
S1 and S4 ascites fractions were incubated at 37°C for 48 h in the presence or absence of inhibitors (100 μM). Lipids were analyzed by LC-MS and are expressed as micromolar concentration in ascites.
Further analysis of changes in individual LPC and LPA species support the role of PLA2 in LPA production (Table 5). During the incubation, the levels of LPC, the product of PLA2 and the substrate of ATX, expectedly decreased, supporting the conversion of LPC to LPA by the ascites ATX activity. However, note that in the S1 fractions of 3 individual ascites tested after 48 h incubation, the total LPC levels decreased by 24, 2, and 11 μM, and the corresponding total LPA levels increased by 36, 30, and 38 μM. Where the yield of LPA was higher than the loss of LPC, a reasonable assumption is that PLA2 was actively providing LPC substrate for ATX (Table 4). This notion was supported further by a closer look at the individual LPC and LPA species (Table 5). For the 16:0, 18:2, and 18:1 species, the decreased amounts of LPC correlated well with concomitant increase in LPA, suggesting a near stochiometric conversion of LPC to LPA by ATX. However, for other species (18:0, 20:4, and 22:6), the molar increases in LPAs were 2- to 5-fold higher than the decreases in corresponding LPCs, suggesting the action of PLA2s in regenerating these LPC species (from PC), which were actively being hydrolyzed by ATX. Following ex vivo incubation, the 48 h changes in LPC and LPA species when comparing S1 and S4 fractions were strikingly similar (correlation coefficient r=0.936, n=72, P<0.0001), which validates the methodology and further supports that PLA2 activity is associated with a vesicle-free fraction of ascites.
Table 5.
Individual LPC and LPA lipid species in control and incubated ascites fractions from 3 patients with EOC
| ID, fraction, and condition | LPC |
LPA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16:0 | 18:2 | 18:1 | 18:0 | 20:4 | 22:6 | 16:0 | 18:2 | 18:1 | 18:0 | 20:4 | 22:6 | |
| A5 S1 | ||||||||||||
| Control | 43.9 | 7.05 | 8.96 | 9.80 | 2.34 | 0.77 | 2.10 | 1.77 | 0.90 | 0.30 | 2.04 | 0.31 |
| 37°C, 48 h | 31.7 | 2.37 | 4.51 | 8.88 | 1.03 | 0.48 | 18.1 | 6.72 | 6.25 | 2.60 | 7.62 | 1.59 |
| 37°C, 48 h + MAFP | 39.3 | 5.82 | 7.90 | 9.13 | 2.13 | 0.89 | 3.88 | 3.46 | 1.72 | 0.61 | 3.83 | 0.63 |
| 37°C, 48 h + BrP-LPA | 39.8 | 3.58 | 6.84 | 10.9 | 1.50 | 0.78 | 13.2 | 6.39 | 4.55 | 1.95 | 6.76 | 1.27 |
| A5 S4 | ||||||||||||
| Control | 41.4 | 6.04 | 8.37 | 9.30 | 2.21 | 0.66 | 2.20 | 1.99 | 0.94 | 0.34 | 2.15 | 0.34 |
| 37°C, 48 h | 28.9 | 2.52 | 4.55 | 7.96 | 1.05 | 0.44 | 17.5 | 7.49 | 6.68 | 2.71 | 8.13 | 1.69 |
| 37°C, 48 h + MAFP | 38.2 | 5.96 | 7.91 | 8.85 | 2.28 | 0.90 | 3.95 | 3.49 | 1.79 | 0.61 | 3.57 | 0.61 |
| 37°C, 48 h + BrP-LPA | 38.5 | 3.95 | 7.79 | 11.1 | 1.80 | 0.81 | 12.8 | 6.26 | 4.51 | 1.89 | 6.61 | 1.17 |
| A6 S1 | ||||||||||||
| Control | 33.0 | 2.37 | 5.40 | 6.96 | 2.67 | 0.58 | 1.13 | 0.50 | 0.39 | 0.20 | 1.80 | 0.22 |
| 37°C, 48 h | 32.7 | 1.44 | 3.99 | 9.00 | 1.28 | 0.39 | 14.9 | 2.85 | 4.61 | 2.23 | 8.76 | 1.33 |
| 37°C, 48 h + MAFP | 33.7 | 3.17 | 6.15 | 7.69 | 3.27 | 0.84 | 1.70 | 0.83 | 0.66 | 0.31 | 2.94 | 0.34 |
| 37°C, 48 h + BrP-LPA | 36.1 | 2.12 | 5.99 | 9.60 | 2.33 | 0.70 | 9.65 | 2.36 | 2.89 | 1.38 | 7.11 | 0.90 |
| A6 S4 | ||||||||||||
| Control | 35.8 | 2.98 | 5.92 | 7.47 | 3.13 | 0.66 | 1.72 | 0.62 | 0.59 | 0.26 | 2.32 | 0.26 |
| 37°C, 48 h | 26.9 | 1.62 | 3.62 | 7.27 | 1.61 | 0.46 | 14.3 | 3.34 | 4.49 | 2.21 | 10.70 | 1.44 |
| 37°C, 48 h + MAFP | 33.7 | 3.23 | 6.12 | 7.52 | 3.18 | 0.73 | ||||||
| 37°C, 48 h + BrP-LPA | 35.0 | 2.36 | 6.64 | 10.5 | 2.87 | 0.88 | 10.3 | 3.10 | 3.08 | 1.57 | 9.73 | 1.07 |
| A7 S1 | ||||||||||||
| Control | 20.8 | 4.45 | 5.36 | 4.95 | 2.88 | 0.48 | 0.70 | 1.07 | 0.48 | 0.20 | 2.21 | 0.25 |
| 37°C, 48 h | 16.9 | 1.51 | 2.34 | 6.03 | 1.04 | 0.23 | 16.2 | 4.98 | 6.17 | 2.85 | 10.54 | 1.49 |
| 37°C, 48 h + MAFP | 26.1 | 4.19 | 5.89 | 6.52 | 2.60 | 0.57 | 2.00 | 2.40 | 1.31 | 0.48 | 5.06 | 0.61 |
| 37°C, 48 h + BrP-LPA | 33.5 | 2.62 | 6.17 | 11.2 | 1.91 | 0.50 | 13.3 | 5.22 | 5.61 | 2.50 | 11.01 | 1.33 |
| A7 S4 | ||||||||||||
| Control | 24.0 | 4.50 | 6.11 | 6.26 | 3.04 | 0.48 | 1.25 | 1.38 | 0.72 | 0.29 | 2.82 | 0.30 |
| 37°C, 48 h | 18.7 | 1.78 | 2.61 | 7.10 | 1.13 | 0.25 | 17.3 | 5.84 | 7.25 | 3.01 | 11.82 | 1.65 |
| 37°C, 48 h + MAFP | 27.8 | 4.62 | 6.30 | 6.98 | 2.78 | 0.60 | 2.95 | 3.24 | 1.83 | 0.66 | 6.57 | 0.72 |
| 37°C, 48 h + BrP-LPA | 34.2 | 2.91 | 6.47 | 11.8 | 2.08 | 0.56 | 15.9 | 6.16 | 6.35 | 2.92 | 12.09 | 1.29 |
S1 and S4 ascites fractions were incubated at 37°C for 48 h, and lipids were analyzed by LC-MS. Lipids are expressed as micromolar concentration in ascites. Inhibitors were added for the 48 h incubation (100 μM).
Effect of MAFP in EOC models in vivo
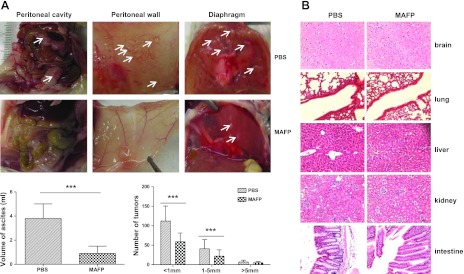
We have shown that BEL, an iPLA2 inhibitor, has a combinational effect with low and nontoxic doses of paclitaxel in inhibiting EOC metastasis in a mouse model (34). Since our data suggest that MAFP is more effective than BEL in blocking PLA2 activity and migration- and invasion-inducing activity of ascites S1 and S3 fractions, we tested whether MAFP would be effective in our mouse model of EOC (2, 31, 34, 35). NOD/SCID mice were injected i.p. with SKOV3-Luc cells, and treatment with MAFP or vehicle was initiated 10 d later. Tumor numbers and sizes were significantly reduced in the MAFP-treated vs. the vehicle-treated group (Fig. 7A), and MAFP caused no detectable toxicity in the major tissues, including the brain, lung, liver, kidney, and intestine (Fig. 7B). We performed two additional mouse studies to confirm that soluble factors in S4 (and not the exosome fraction P3) were tumor-promoting in vivo. In one study, we tested whether HEY ovarian cancer cells, which have low tumorigenicity (36), would be more tumorigenic in the presence of S4. Beginning 2 d after i.p. tumor cell injection, 0.5 ml pooled S4 or PBS was injected i.p. 3×/wk. At 3 wk, all mice in the S4 group (n=7) had many tumors throughout the peritoneum; while in the PBS group (n=5), only one mouse had many tumors, one mouse had few tumors, and 3 mice had a small tumor only at the injection site. In another study, we injected SKOV3-Luc cells i.p., and 10 d later began injection of a pooled P3 fraction (containing 500 μg protein; n=3) or PBS (n=3). This treatment was continued 2×/wk for 3 wk. No difference was found in tumor or ascites development between the two groups.
Figure 7.
MAFP treatment decreased tumor formation in a mouse xenograft model of EOC. Human SKOV3 cells were injected i.p. into NOD/SCID mice. After 10 d, i.p. injection of MAFP or PBS was initiated. Mice were sacrificed at 38–40 d. A) Representative images of tumors in peritoneal cavity, peritoneal wall and diaphragm. Tumors are indicated by arrows. Bottom left panel: volume of ascites development in each group. Bottom right panel: total tumors developed in each group by size (n=8/group). ***P < 0.001. B) Hematoxylin- and eosin-stained sections of various organs from representative mice in each treatment group.
EOC cells or macrophages secrete cPLA2 and iPLA2 in an ABC-transporter-dependent, but exosome secretion-independent, manner
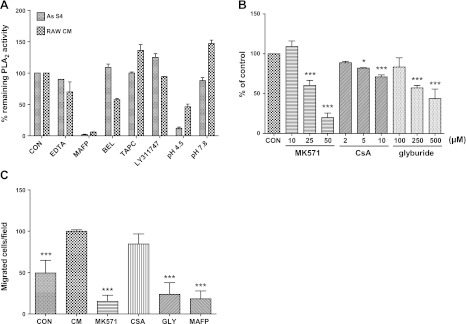
The findings described above are the first to indicate that the cytosolic enzymes cPLA2 and iPLA2 may be associated with microvesicle-free biological fluids. If the enzymes originate in live cells, they could come from tumor cells, immune system cells, peritoneal mesothelial cells, and/or the interaction of two or more cell types. We tested for the release of cPLA2- and/or iPLA2-like activity in CM from EOC cell lines SKOV3 and HEY. We found a small amount of activity but not enough to account for the PLA2 activity of EOC ascites (data not shown). In CM from RAW 264.7 mouse macrophages, however, we found a high level of PLA2 activity. This activity was sensitive to PLA2 inhibitors in a manner that was similar to the EOC ascites fractions (Fig. 8A), suggesting similar PLA2 enzymes were present. The assays were also run at a very low pH (4.5) and a higher pH (7.8) to test whether lysosomal PLA2 (LPLA2) activity, which is maximal at low pH, was present. The data indicate that LPLA2 activity is not significant in ascites S4 or in RAW 264.7 macrophage CM.
Figure 8.
Conditioned medium from RAW 246.7 macrophages contains PLA2 and cell stimulatory activity. A) Sensitivity of PLA2 activity of CM from RAW 246.7 macrophages to inhibitors, EDTA or pH, was compared to that of ascites S4 fraction. Inhibitor concentrations: EDTA, 5 mM; MAFP, 10 μM; BEL, 10 μM; TAPC, 50 μM; and LY311747, 50 μM. B) PLA2 activity of 17-h CM from cells treated for 4 h with inhibitors of ABC transporters, normalized to cell protein and expressed as percentage of untreated cells. *P < 0.05, ***P < 0.001 vs. control (CON, medium alone). Data are means ± sd of duplicate treatments, experiment repeated 3 times with similar results. C) Migration of SKOV3 cells to 17-h CM from untreated cells and cells treated with inhibitors (MK571, 50 μM; CsA, 10 μM; glyburide, 500 μM; and MAFP, 20 μM). ***P < 0.001 vs. CM. Data are means ± sd of 3 membranes; experiment repeated twice.
To understand the mechanisms of PLA2 secretion from RAW 264.7 mouse macrophages, we tested two inhibitors known to inhibit exosome secretion: DMA (5 and 12.5 nM) and methyl-β-cyclodextrin (MBC), a lipid-raft pathway inhibitor that reduces exosome secretion (1 and 2 mM) (37). We found neither had any significant effect on the PLA2 activity detected in CM of RAW 264.7 mouse macrophages (not shown). These results are consistent with our findings that human ascitic PLA2 activity was not associated with P3 (the exosome-containing fraction), supporting the idea that PLA2s are secreted in a vesicle-independent pathway. We then collected CM from RAW macrophages treated with selective inhibitors of different ATP-binding cassette (ABC) transporters: MK571, an ABCC1 inhibitor (10–50 μM); CsA, an ABCB1 inhibitor (2–50 μM); and glyburide (Gly), an ABCA1 inhibitor (0.1–2 mM) (38). RAW CM PLA2 activities, normalized to micrograms of cell protein, are presented in Fig. 8B. All inhibitors dose-responsively reduced PLA2 activity of CM, and the order of effectiveness was MK571 > glyburide > CsA.
We tested whether CM from RAW 264.7 mouse macrophages would stimulate migration of SKOV3 cells (Fig. 8C). We found an ∼2-fold increase in migration with the CM compared to the medium control in the bottom well of the migration chamber. CM generated from RAW 264.7 macrophages treated with MK571 or glyburide did not stimulate migration. Addition of MAFP to the CM also abrogated migration. These results further support a PLA2-ABC transporter system operating in the tumor ascites microenvironment.
DISCUSSION
This is the first study to investigate EOC ascitic and tissue cPLA2- and iPLA2-like enzymatic activities. We validated the quantitative nature of the DBPC-based PLA2 assays via extensive studies presented in this work. The presence of microvesicle-free cPLA2 and iPLA2 in human EOC ascites is evidenced by PLA2 enzymatic activity assays, by measuring the sensitivities of ascitic tumor-promoting activities to PLA2-selective inhibitors, and by measuring lipid products produced via their activities. We are fully aware that all pharmacological inhibitors have potential off-target effects. BEL has been shown to have noniPLA2 targets (39). While we cannot use genetic tools such as RNAi techniques in cell-free fluids to confirm our results, several lines of evidence support our conclusions: 1) the DBPC-based PLA2 assays eliminate most off-target effects, since other known BEL targets do not use DBPC as a substrate; 2) lack of divalent cations in the presence of EDTA further specifies iPLA2 activity; and 3) our cell-based assays and lipid production assays consistently support the cPLA2 and iPLA2 activities. When it was applicable, we indeed employed genetic methods, such as siRNAs against LPA receptors, to confirm our results.
We found that the ascites exosome fraction (P3) had low or no tumor-promoting activity in vitro. In contrast, we found that the ultracentrifuged, exosome-free S4 fraction, similar to the cell-free S1 fraction, had strong tumor promoting activity in vitro and in vivo, suggesting that the majority of the tumor-promoting activities of ascites are in the exosome-free form. The ascitic tumor promoting activities were correlated with and functionally related to PLA2 activity. We found that inhibitors against ABCC1 and ABCB1 (Pgp) reduced secreted MAFP-sensitive, EDTA-partially insensitive PLA2 activity in CM from RAW 264.7 mouse macrophages, suggesting that these transporters are involved in the secretion pathway of PLA2 enzymes from these cells. Interestingly, we also found that the drug-resistant SKOV3-VLB cells (overexpressing ABCB1) responded similarly to the parental cells to the migration- and invasion-promoting activity of ascites fractions, suggesting that the same targets and signaling pathways may also be effective in drug-resistant EOC cells. These novel findings warrant further study to characterize the cellular source of the cPLA2- and iPLA2-like activity in EOC ascites and the secretion mechanisms involved. We noticed that MAFP inhibition could effectively block the decrease in LPC and the increase in LPA, but not the increase in AA. cPLA2s and iPLA2s also use substrates (mainly PCs) containing other fatty acids. It is possible that the alterations of one and more other fatty acids are more correlated with LPC changes. In addition, the levels of AA can be regulated by many other enzymes, and our results only suggest that its levels in ascites are not affected by MAFP.
We showed that LPA present and produced by ATX and PLA2 in S1 and S4 ascites fractions mediates a significant part of the tumor-promoting effects of ascites, as evidenced by ex vivo LPA production in S1 and S4 ascites fractions and by our inhibitor and siRNA work. We have noted that while 40–50% of the effect of ascites fractions on EOC cell migration is heat stable, an LPA antagonist inhibits >70% of the effect. The heat-stable effect was likely due to the lipid signaling molecules (LPA in particular) that are already present in the ascites. Our current work implies that lipids continuously synthesized in ascites via PLA2s and ATX are functionally involved in the biological effects and the enzymes are heat-sensitive. In addition, protein factors (likely to be heat-sensitive) can modulate LPA signaling. For example, LPA in biological fluids binds to serum albumin and other proteins that enhance its effects on cellular functions (40). EGF and angiotensin II modulate LPA1 receptor function and phosphorylation state (41). EGF increases LPA production in human EOC cells (42). Therefore, LPA receptor blocking resulted in a higher percentage of inhibition. Published data on catabolic loss of LPA via lysolipid phosphatases and other enzymes, as well as local and temporal supplies of signaling LPA generated by ATX (43–45), strongly support the notion that dynamic processes are involved in both production and degradation of bioactive lipids in the tumor microenvironment. Interestingly, while we have shown that each of LPA1–3 play important roles, LPA4 is not likely to be involved in S1- or S4-induced cell migration, consistent with the reported inhibitory effect of LPA4 on migration of mouse embryonic fibroblasts, colon cancer cells, and neuroblastoma cells (46).
We show for the first time that PLA2 activities are elevated in EOC tumor tissues. We indeed have preliminary data to show that PLA2 activity is also elevated in the blood of EOC patients (data not shown). Our data imply that PLA2 activity may represent a useful marker for diagnosis, prognosis and/or disease monitoring of EOC. However, large numbers of clinical samples with complete follow-up data are required to test the significance of PLA2 activity as either a diagnostic or a prognostic marker for EOC (work in progress). Regardless of whether PLA2 enzymes contribute indirectly (by providing substrate for ATX) or directly (by using phosphatidic acid as their substrate), our data show that PLA2 enzymes play an important role in producing oncogenic LPA and hence are an important target in EOC. Taken together, our studies have provided provocative and exciting directions for developing new therapeutic modalities for EOC.
Acknowledgments
Lipid analytical work was performed by the Clinical Pharmacology Analytical Core laboratory, a core laboratory of the Indiana University Melvin and Bren Simon Cancer Center, supported by the National Cancer Institute, grant P30 CA082709. This work was supported in part by the U.S. National Institutes of Health, grant R01CA95042 to Y.X., and by the Mary Fendrich Hulman Charitable Trust.
The authors report no conflicts of interest.
Footnotes
- AA
- arachidonic acid
- ABC
- ATP-binding cassette
- ATX
- autotaxin
- BEL
- bromoenol lactone
- CM
- conditioned medium
- CV
- coefficient of variation
- cPLA2
- cytosolic phospholipase A2
- CsA
- cyclosporine A
- DBPC
- 1-O-(6-dabcyl-aminohexanoyl)-2-O-(6-(12-BODIPY-dodecanoyl) aminohexanoyl)-sn-3-glyceryl phosphatidylcholine
- DMA
- 5-(N,N-dimethyl)-hydrochloride
- EOC
- epithelial ovarian cancer
- iPLA2
- calcium-independent phospholipase A2
- LPA
- lysophosphatidic acid
- LPC
- lysophosphatidyl choline
- LPI
- lysophosphatidylinositol
- LPL
- lysophospholipid
- Lp-PLA2
- lipoprotein-associated phospholipase A2
- MAFP
- methyl arachidonyl fluorophosphonate
- PAF
- platelet activating factor
- PC
- phosphatidylcholine
- PLA1
- phospholipase A1
- PLA2
- phospholipase A2
- SF
- serum-free
- sPLA2
- secreted phospholipase A2
- TAPC
- thioetheramide-phosphatidylcholine
REFERENCES
- 1. Xu Y., Xiao Y. J., Zhu K., Baudhuin L. M., Lu J., Hong G., Kim K. S., Cristina K. L., Song L., F. S. W., Elson P., Markman M., Belinson J. (2003) Unfolding the pathophysiological role of bioactive lysophospholipids. Curr. Drug Targets Immune Endocr. Metabol. Disord. 3, 23–32 [PubMed] [Google Scholar]
- 2. Sengupta S., Kim K. S., Berk M. P., Oates R., Escobar P., Belinson J., Li W., Lindner D. J., Williams B., Xu Y. (2007) Lysophosphatidic acid downregulates tissue inhibitor of metalloproteinases, which are negatively involved in lysophosphatidic acid-induced cell invasion. Oncogene 26, 2894–2901 [DOI] [PubMed] [Google Scholar]
- 3. Murph M. M., Liu W., Yu S., Lu Y., Hall H., Hennessy B. T., Lahad J., Schaner M., Helland A., Kristensen G., Borresen-Dale A. L., Mills G. B. (2009) Lysophosphatidic acid-induced transcriptional profile represents serous epithelial ovarian carcinoma and worsened prognosis. PLoS ONE 4, e5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aoki J., Inoue A., Okudaira S. (2008) Two pathways for lysophosphatidic acid production. Biochim. Biophys. Acta 1781, 513–518 [DOI] [PubMed] [Google Scholar]
- 5. Murakami M., Taketomi Y., Sato H., Yamamoto K. (2011) Secreted phospholipase A2 revisited. J. Biochem. 150, 233–255 [DOI] [PubMed] [Google Scholar]
- 6. Kudo I., Murakami M. (2002) Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 68-69, 3-58 [DOI] [PubMed] [Google Scholar]
- 7. Subra C., Grand D., Laulagnier K., Stella A., Lambeau G., Paillasse M., De Medina P., Monsarrat B., Perret B., Silvente-Poirot S., Poirot M., Record M. (2010) Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid Res. 51, 2105–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iguchi H., Kosaka N., Ochiya T. (2010) Secretory microRNAs as a versatile communication tool. Commun. Integr. Biol. 3, 478–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cummings B. S. (2007) Phospholipase A2 as targets for anti-cancer drugs. Biochem. Pharmacol. 74, 949–959 [DOI] [PubMed] [Google Scholar]
- 10. Scott K. F., Sajinovic M., Hein J., Nixdorf S., Galettis P., Liauw W., de Souza P., Dong Q., Graham G. G., Russell P. J. (2010) Emerging roles for phospholipase A2 enzymes in cancer. Biochimie (Paris) 92, 601–610 [DOI] [PubMed] [Google Scholar]
- 11. Iorio E., Ricci A., Bagnoli M., Pisanu M. E., Castellano G., Di Vito M., Venturini E., Glunde K., Bhujwalla Z. M., Mezzanzanica D., Canevari S., Podo F. (2010) Activation of phosphatidylcholine cycle enzymes in human epithelial ovarian cancer cells. Cancer Res. 70, 2126–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hendrix N. D., Wu R., Kuick R., Schwartz D. R., Fearon E. R., Cho K. R. (2006) Fibroblast growth factor 9 has oncogenic activity and is a downstream target of Wnt signaling in ovarian endometrioid adenocarcinomas. Cancer Res. 66, 1354–1362 [DOI] [PubMed] [Google Scholar]
- 13. Xu Y., Wang D., Wang Z. (2009) Lipid generation and signaling in ovarian cancer. In Cancer Treatment and Research-Ovarian Cancer (Stack M. S., Fishman D. A., eds) pp. 241–268, Springer, New York, Dordrecht, Heidelberg, London: [DOI] [PubMed] [Google Scholar]
- 14. Song Y., Wilkins P., Hu W., Murthy K. S., Chen J., Lee Z., Oyesanya R., Wu J., Barbour S. E., Fang X. (2007) Inhibition of calcium-independent phospholipase A2 suppresses proliferation and tumorigenicity of ovarian carcinoma cells. Biochem. J. 406, 427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li H., Zhao Z., Wei G., Yan L., Wang D., Zhang H., Sandusky G. E., Turk J., Xu Y. (2010) Group VIA phospholipase A2 in both host and tumor cells is involved in ovarian cancer development. FASEB J. 24, 4103–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao X., Wang D., Zhao Z., Xiao Y., Sengupta S., Xiao Y., Zhang R., Lauber K., Wesselborg S., Feng L., Rose T. M., Shen Y., Zhang J., Prestwich G., Xu Y. (2006) Caspase-3-dependent activation of calcium-independent phospholipase A2 enhances cell migration in non-apoptotic ovarian cancer cells. J. Biol. Chem. 281, 29357–29368 [DOI] [PubMed] [Google Scholar]
- 17. Wang G., Pincheira R., Zhang M., Zhang J. T. (1997) Conformational changes of P-glycoprotein by nucleotide binding. Biochem. J. 328(Pt. 3), 897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao Z., Xu Y. (2010) An extremely simple method for extraction of lysophospholipids and phospholipids from blood samples. J. Lipid Res. 51, 652–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao Z., Xu Y. (2009) Measurement of endogenous lysophosphatidic acid by ESI-MS/MS in plasma samples requires pre-separation of lysophosphatidylcholine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877, 3739–3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu Y., Fang X. J., Casey G., Mills G. B. (1995) Lysophospholipids activate ovarian and breast cancer cells. Biochem. J. 309(Pt. 3), 933–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sengupta S., Xiao Y. J., Xu Y. (2003) A novel laminin-induced LPA autocrine loop in the migration of ovarian cancer cells. FASEB J. 17, 1570–1572 [DOI] [PubMed] [Google Scholar]
- 22. Xiao Y. J., Schwartz B., Washington M., Kennedy A., Webster K., Belinson J., Xu Y. (2001) Electrospray ionization mass spectrometry analysis of lysophospholipids in human ascitic fluids: comparison of the lysophospholipid contents in malignant vs nonmalignant ascitic fluids. Anal. Biochem. 290, 302–313 [DOI] [PubMed] [Google Scholar]
- 23. Burke J. E., Dennis E. A. (2009) Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 50(Suppl.), S237–S242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lucas K. K., Dennis E. A. (2005) Distinguishing phospholipase A2 types in biological samples by employing group-specific assays in the presence of inhibitors. Prostaglandins Other Lipid Mediat. 77, 235–248 [DOI] [PubMed] [Google Scholar]
- 25. Murakami M., Kudo I. (2001) Diversity and regulatory functions of mammalian secretory phospholipase A2s. Adv. Immunol. 77, 163–194 [DOI] [PubMed] [Google Scholar]
- 26. Yu L., Deems R. A., Hajdu J., Dennis E. A. (1990) The interaction of phospholipase A2 with phospholipid analogues and inhibitors. J. Biol. Chem. 265, 2657–2664 [PubMed] [Google Scholar]
- 27. Liu C., Yu S., Zinn K., Wang J., Zhang L., Jia Y., Kappes J. C., Barnes S., Kimberly R. P., Grizzle W. E., Zhang H. G. (2006) Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J. Immunol. 176, 1375–1385 [DOI] [PubMed] [Google Scholar]
- 28. Mills G. B., Moolenaar W. H. (2003) The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer 3, 582–591 [DOI] [PubMed] [Google Scholar]
- 29. Houben A. J., Moolenaar W. H. (2011) Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev. 30, 557–565 [DOI] [PubMed] [Google Scholar]
- 30. Brown D. P., Gokmen-Polar Y., Jiang L., Tan J., Ringham H., Janecki D. J., Qi G., Witzmann F. A., Sledge G. W., Jr., Wang M. (2007) A comparative proteomic study to characterize the vinblastine resistance in human ovarian cancer cells. Proteomics Clin. Appl. 1, 18–31 [DOI] [PubMed] [Google Scholar]
- 31. Kim K. S., Sengupta S., Berk M., Kwak Y. G., Escobar P. F., Belinson J., Mok S. C., Xu Y. (2006) Hypoxia enhances lysophosphatidic acid responsiveness in ovarian cancer cells and lysophosphatidic acid induces ovarian tumor metastasis in vivo. Cancer Res. 66, 7983–7990 [DOI] [PubMed] [Google Scholar]
- 32. Li H., Wang D., Zhang H., Kirmani K., Zhao Z., Steinmetz R., Xu Y. (2009) Lysophosphatidic acid stimulates cell migration, invasion, and colony formation as well as tumorigenesis/metastasis of mouse ovarian cancer in immunocompetent mice. Mol. Cancer Ther. 8, 1692–1701 [DOI] [PubMed] [Google Scholar]
- 33. Xu Y., Sengupta S., Singh S., Steinmetz R. (2006) Novel lipid signaling pathways in ovarian cancer cells. Cell Sci. Rev. 3, 168–197 [Google Scholar]
- 34. Li H., Zhao Z., Antalis C., Emerson R., Wei G., Zhang S., Zhang Z. Y., Xu Y. (2011) Combination therapy of an inhibitor of group via phospholipase A(2) with paclitaxel is highly effective in blocking ovarian cancer development. Am. J. Pathol. 179, 452–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ren J., Xiao Y. J., Singh L. S., Zhao X., Zhao Z., Feng L., Rose T. M., Prestwich G. D., Xu Y. (2006) Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells. Cancer Res. 66, 3006–3014 [DOI] [PubMed] [Google Scholar]
- 36. Mills G. B., May C., Hill M., Campbell S., Shaw P., Marks A. (1990) Ascitic fluid from human ovarian cancer patients contains growth factors necessary for intraperitoneal growth of human ovarian adenocarcinoma cells. J. Clin. Invest. 86, 851–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Merendino A. M., Bucchieri F., Campanella C., Marciano V., Ribbene A., David S., Zummo G., Burgio G., Corona D. F., Conway de Macario E., Macario A. J., Cappello F. (2010) Hsp60 is actively secreted by human tumor cells. PLoS One 5, e9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kobayashi N., Yamaguchi A., Nishi T. (2009) Characterization of the ATP-dependent sphingosine 1-phosphate transporter in rat erythrocytes. J. Biol. Chem. 284, 21192–21200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burke J. E., Dennis E. A. (2009) Phospholipase A2 biochemistry. Cardiovasc. Drugs Ther. 23, 49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goetzl E. J., Lee H., Azuma T., Stossel T. P., Turck C. W., Karliner J. S. (2000) Gelsolin binding and cellular presentation of lysophosphatidic acid. J. Biol. Chem. 275, 14573–14578 [DOI] [PubMed] [Google Scholar]
- 41. Colin-Santana C. C., Avendano-Vazquez S. E., Alcantara-Hernandez R., Garcia-Sainz J. A. (2011) EGF and angiotensin II modulate lysophosphatidic acid LPA(1) receptor function and phosphorylation state. Biochim. Biophys. Acta 1810, 1170–1177 [DOI] [PubMed] [Google Scholar]
- 42. Snider A. J., Zhang Z., Xie Y., Meier K. E. (2010) Epidermal growth factor increases lysophosphatidic acid production in human ovarian cancer cells: roles for phospholipase D2 and receptor transactivation. Am. J. Physiol. Cell Physiol. 298, C163–C170 [DOI] [PubMed] [Google Scholar]
- 43. Goetzl E. J., Lee H., Dolezalova H., Kalli K. R., Conover C. A., Hu Y. L., Azuma T., Stossel T. P., Karliner J. S., Jaffe R. B. (2000) Mechanisms of lysolipid phosphate effects on cellular survival and proliferation. Ann. N. Y. Acad. Sci. 905, 177–187 [DOI] [PubMed] [Google Scholar]
- 44. Samadi N., Bekele R., Capatos D., Venkatraman G., Sariahmetoglu M., Brindley D. N. (2011) Regulation of lysophosphatidate signaling by autotaxin and lipid phosphate phosphatases with respect to tumor progression, angiogenesis, metastasis and chemo-resistance. Biochimie (Paris) 93, 61–70 [DOI] [PubMed] [Google Scholar]
- 45. Inoue S., Iida T., Tanikawa T., Maruyama T., Morita C. (1991) Isolation of Listeria monocytogenes from roof rats (Rattus rattus) in buildings in Tokyo. J. Vet. Med. Sci./Japan Soc. Vet. Sci. 53, 521–522 [DOI] [PubMed] [Google Scholar]
- 46. Lee Z., Cheng C. T., Zhang H., Subler M. A., Wu J., Mukherjee A., Windle J. J., Chen C. K., Fang X. (2008) Role of LPA4/p2y9/GPR23 in negative regulation of cell motility. Mol. Biol. Cell 19, 5435–5445 [DOI] [PMC free article] [PubMed] [Google Scholar]