Abstract
Neuropeptide Y (NPY) mediates stress-induced obesity in adult male mice by activating its Y2 receptor (Y2R) in visceral adipose tissue (VAT). Here, we studied whether the NPY-Y2R system is also activated by maternal low-protein diet (LPD) and linked to obesity in offspring. Prenatal LPD offspring had lower birth weights compared to normal-protein diet (NPD) offspring. Female prenatal and lactation stress (PLS) offspring from mothers fed an LPD developed abdominal adiposity and glucose intolerance associated with a 5-fold up-regulation of NPY mRNA and a 6-fold up-regulation of Y2R mRNA specifically in VAT, in addition to elevated platelet-rich-plasma (PRP) NPY, compared to control females fed a high-fat diet (HFD). Conversely, PLS male offspring showed lower NPY in PRP, a 10-fold decrease of Y2R mRNA in VAT, lower adiposity, and improved glucose tolerance compared to control males. Interestingly, prenatal LPD offspring cross-fostered to control lactating mothers had completely inverse metabolic and NPY phenotypes. Taken together, these findings suggested that maternal LPD activates the VAT NPY-Y2R system and increases abdominal adiposity and glucose intolerance in a sex- and time-specific fashion, suggesting that the peripheral NPY system is a potential mediator of programming for the offspring's vulnerability to obesity and metabolic syndrome.—Han, R., Li, A., Li, L., Kitlinska, J. B., Zukowska, Z. Maternal low-protein diet up-regulates the neuropeptide Y system in visceral fat and leads to abdominal obesity and glucose intolerance in a sex- and time-specific manner.
Keywords: NPY-Y2R, sex differences, metabolic syndrome
Obesity is a major problem in the Western world and an emerging concern in developing countries. In the United States, 32.2% of adults are obese [body mass index (BMI)>30 kg/m2] (1). Perhaps even more alarming is the fact that 17.1% of children are now obese (1), and overall, up to 2/3 of children and adolescents in the United States are overweight or obese. This surge in obesity has dramatically increased the prevalence of type 2 diabetes, now also detectable in children, as well as metabolic syndrome and cardiovascular diseases (1). While human obesity is unquestionably a multifactorial, heritable disease, it is the environment, including sedentary lifestyle and diet, that play the major role in adulthood. This finding raises the question of whether environmental factors operating in mothers and early after birth may contribute to the recent epidemic of obesity.
Increasing numbers of epidemiological and animal studies have elucidated a relationship between poor fetal growth with the subsequent development of obesity, type 2 diabetes, and metabolic syndrome (2–4). Studies of the Dutch Famine (1944–1945) (5) showed that children of pregnant women exposed to famine (i.e., stressed nutritionally and psychologically) were more susceptible to diabetes, obesity, and cardiovascular disease. Such developmental programming has been explained by the “thrifty phenotype ” hypothesis, which proposes that poor fetal nutrition can result in reprogramming of the fetus, which allows the offspring to maximize the body's capacity for energy storage under conditions of poor nutrition once out of the womb. However, this phenotype would be detrimental under conditions where normal or excessive nutrition are present and would thus promote obesity (4). Indeed, studies in humans indicated that offspring of mothers underfed during pregnancy have increased body fat (6) and, in particular, central adiposity in adulthood (7). Similar vulnerability for metabolic as well as mental and cognitive disorders occurs in response to maternal stress as well as with the administration of stress hormones (8). The stress-conferred risk for obesity and diabetes appears to be sex-specific and transmitted transgenerationally (3).
In humans and animals, maternal stress, psychosocially or metabolically [e.g., low-protein diet (LPD); 8% protein], increases the risk of obesity and diabetes in the progeny (5, 8, 9). Stress-induced programming can be mimicked by maternal exposure to an excess of glucocorticoids in rodents and humans (10) and has been linked to epigenetic regulation of the hypothalamic-pituitary-adrenal (HPA) axis (11). Neuropeptide Y (NPY) is a major determinant of the body's stress response, synergizing with the actions of glucocorticoids, and activated by psychosocial and physical stress and nutritional cues, such as starvation (12–14). Environmental cues appear to epigenetically regulate the NPY gene, which has been linked to stem cell pluripotency (15), differentiation (16), and development of mental disorders (17). Centrally, NPY is anxiolytic and also potently orexigenic (18). Thus, increased activity of NPY and its receptors, Y1R, Y2R, and Y5R, has been found to be involved in many forms of experimental obesity (18, 19).
NPY also promotes obesity through peripheral actions (12). Circulating NPY levels can be derived from at least two peripheral sources: platelets [measured as platelet-rich-plasma (PRP)] and the sympathetic nerves (14). Both sources appear to contribute to the stress-induced elevation of NPY in adult mice (12). The sympathetic source of NPY is released from postganglionic sympathetic nerve terminals in combination with norepinephrine (20). The platelet pool of NPY can reflect both NPY synthesis by its precursors, megakaryocytes, as well as uptake from the plasma of nerve-released NPY. Similarly, elevation of platelet NPY was recently found by us (21) and others (22) in human patients with chronic stress-related conditions, such as depression and peripheral artery disease. Since platelets can acquire NPY over their lifetime of 10–14 d, this led us to suspect that this particular pool of NPY can serve as a better marker of chronicity and intensity of stress than its plasma level alone (21).
NPY is highly angiogenic and adipogenic when signaling through Y2R and on activation of dipeptidyl peptidase IV (DPPIV) (12). This NPY-Y2R system plays an important role in neovascularization of ischemic tissues (23), retinopathy (24), wound healing (23), tumors (14), and adipose tissue (12, 25). Recently, we discovered that chronic stress in adult male mice, when combined with high-fat diet (HFD), led to up-regulation of NPY, systemically and specifically, in visceral adipose tissue (VAT) where Y2R expression was also elevated (12). The activation of the VAT NPY-Y2R system was in part glucocorticoid dependent, and it directly stimulated adipogenesis and angiogenesis, leading to abdominal obesity and metabolic-like syndrome. Fat accretion and metabolic consequences of stress and HFD were completely prevented by intrafat Y2R inactivation (12).
In the current study, we sought to determine whether the NPY system also plays a role in the programming of obesity induced by maternal LPD. The maternal LPD has been used extensively in rats to demonstrate the importance of the early environment in determining susceptibility to future development of obesity and diabetes (26), particularly in male offspring. We used two paradigms: pregnancy and lactation stress (PLS), with maternal LPD during pregnancy and lactation; or pregnancy stress (PS), with maternal LPD only during pregnancy, and cross-fostering of offspring after birth to control mothers fed a normal-protein diet (NPD). We believe that the first paradigm best resembles the human condition, where undernutrition during pregnancy is often extended through the lactation period and is associated with increased levels of stress in mothers living in poverty.
MATERIALS AND METHODS
Diets
The 20% protein NPD and 8% protein LPD given to the pregnant and lactating mice were purchased from Research Diet (New Brunswick, NJ, USA). These two diets were isocaloric, and their composition is as described previously (26). The HFD was also purchased from Research Diets (D12451).
LPD during pregnancy and lactation
All animal protocols used here were approved by the Georgetown University animal care and use committee, which conforms to the U.S. National Institutes of Health and U.S. Department of Agriculture guidelines for the care and use of animals. All mice were housed in microisolator cages. Mice were maintained on a 12-h light-dark cycle with lights on from 6:00 AM to 6:00 PM at 22–23°C. Virgin wild-type 129/ SvImJ female mice were housed 2–3 mice/cage. They were mated with 129/ SvImJ male at 8 wk of age, and day 0 of gestation was taken as the day on which vaginal plugs were expelled. Groups of 10 pregnant mice were maintained. Every pregnant mouse was housed in a single cage and fed either the control NPD (20% protein, 68% carbohydrate, 12% fat) or the isocaloric LPD (8% protein, 80% carbohydrate, 12% fat) during pregnancy and lactation. To exclude the influences of litter size in our experiments, only a litter size of 6–7 pups was used to measure body weights and kept for further experiments. If the litter size was 7, we reduced the number to 6 after birth. Six offspring were kept with 1 dam in experimental and control groups in order to let pups have equal access to milk and maternal care. At weaning (21 d), offspring from each group were put onto HFD (20% protein, 35% carbohydrate, 45% fat) for 18 wk. A diagram for the prenatal and lactating LPD study design is shown in Fig. 1A.
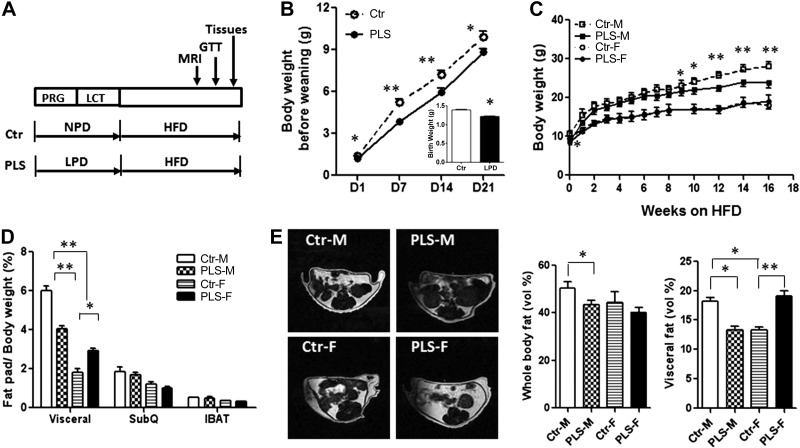
Figure 1.
PLS female offspring developed abdominal obesity, while male offspring were protected. A) Diagram for the prenatal and lactating LPD study design (PRG, pregnancy; LCT, lactation; GTT, glucose tolerance test; Ctl, control offspring). B) Prenatal LPD offspring had lower body weight before weaning when kept on LPD during lactation (PLS) compared to control offspring (Ctr). Inset: birth weight. C) Body weight of offspring weaned on HFD (Ctr-M, control male offspring; PLS-M, PLS male offspring; Ctr-F, control female offspring; PLS-F, PLS female offspring). D) Fat pads (SubQ, subcutaneous abdominal fat pad; IBAT, interscapular brown adipose tissue). E) Whole-body fat content and abdominal fat content after 12 wk of HFD, measured by MRI. n = 36 mice/group (B); 7–10 mice/group (C–E). Results represent means ± se. *P < 0.05, **P < 0.01.
LPD during pregnancy
All the procedures were the identical to those described above, except that only pregnant mice were fed either NPD (20% protein) or isocaloric LPD (8% protein). To exclude the influences of litter size in our experiments, only a litter size of 5–7 pups was used to measure body weights and kept for further experiments. During the cross-fostering after birth, 6 pups were kept for each mother to let pups have equal access to milk and maternal care. Offspring were fostered by control dams, which were fed NPD during pregnancy and lactation. A diagram for the prenatal LPD study design is shown in Fig. 4A.
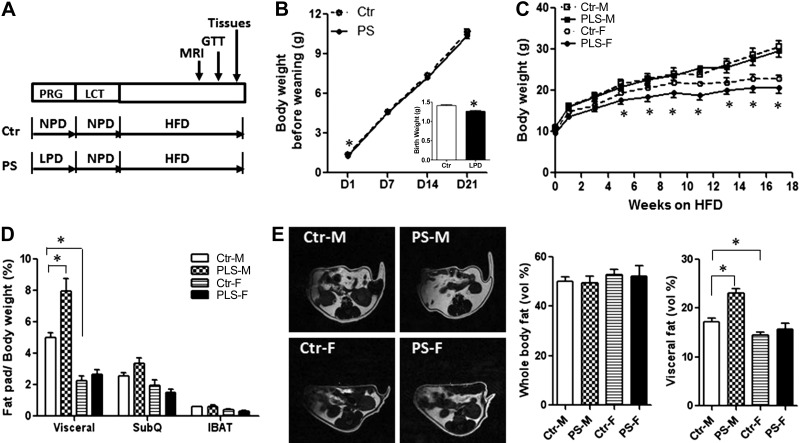
Figure 4.
PS male offspring developed abdominal obesity, while female offspring had normal adiposity. A) Diagram for the prenatal LPD study design. B) Catch-up growth before weaning in prenatal LPD offspring cross-fostered to control lactating mothers (Ctr, control offspring, cross-fostered to control lactating mothers; PS, prenatal LPD offspring, cross-fostered to control lactating mothers). Inset: birth weight. C) Body weight of offspring weaned onto HFD. D) Fat pad weight after sacrifice (SubQ, subcutaneous abdominal fat pad IBAT, interscapular brown adipose tissue). E) Whole-body fat content and abdominal fat content, measured by MRI after 12 wk of HFD. n = 36 mice/group (B); 7–10 mice/group (C–E). Results represent means ± se. *P < 0.05.
Body weight
Pups were weighed weekly beginning on postnatal day 1. Pups were weaned on postnatal day 21 and housed in groups of 2–3 by sex and weighed once per week.
Magnetic resonance imaging (MRI)
Mice fed the HFD for 12 wk were evaluated for their fat deposition using MRI. A Bruker 7-T small-animal magnetic resonance imager coil (Bruker Corp., Billerica, MA, USA)was used to visualize and noninvasively quantify various fat depots, using a 3-dimensional T1/T2-weighted imaging protocol optimized for high-contrast fat imaging, as described previously (12). Quantification of the total body fat in addition to separate specific fat depots was calculated using thresholding and voxel count plugins, as described previously (12).
Glucose tolerance tests
Mice fed HFD for 13 wk (7–10 mice/group) were unfed for 3 h from 9:00 to 12:00 AM and then injected intraperitoneally with glucose (2 g/kg body weight) (27). Blood glucose levels were monitored before and at 30, 60, and 120 min after injection using a glucometer (FreeStyle; TheraSense, Alameda, CA, USA).
Euthanasia
Mice (21 wk old) fed HFD for 18 wk were sacrificed in the morning (7–10 mice/group). White fat pads and brown fat pads were unilaterally dissected and weighed. Blood samples were collected from the abdominal vena cava of anesthetized mice into EDTA-treated tubes. PRP was prepared by sequential centrifugation, as described previously (21). The NPY levels in the PRP were measured using ELISA (S-1145; Bachem Laboratories, King of Prussia, PA, USA).
Reverse transcription and real-time PCR
RNA was isolated using Tri Reagent (Sigma-Aldrich, St. Louis, MO, USA), and cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). Real-time PCR was performed using the ICycler iQ Detection System (Bio-Rad) and the TaqMan PCR Reagent Kit (Applied Biosystems, Foster City, CA, USA) with predesigned primers and fluorescent-labeled probes from Applied Biosystems with duplicates, as described previously (12). Applied Biosystems primer ID numbers are as follows: β-actin, 16403392; NPY, 16403380; Y1R, 16403381; Y2R, 14381272; Y5R, Mm02620267_s1, DDPIV, 16403393. Expression levels were calculated by the comparative CT method using β-actin as an endogenous reference gene, as described previously (12).
Statistical analysis
Data were analyzed by 2-way ANOVA and subsequently by post hoc test (Prism 3.0;GraphPad, San Diego, CA, USA). Statistical significance was inferred at P < 0.05.
RESULTS
Female offspring of mothers fed LPD during pregnancy and lactation developed abdominal obesity and glucose intolerance
No difference was found in litter size between prenatal LPD mice and control mice (6.6±0.5 for prenatal LPD mice and 6.3±0.7 for control mice, n=10/group). Sex ratio was also unaffected by prenatal LPD. Pregnancy and lactation LPD revealed major sex differences in the offspring's propensity for obesity and metabolic disorders. Offspring of mice fed LPD were significantly lighter (1.24±0.02 g) at birth compared to progeny of mice fed NPD (1.47±0.01 g). The body weight of the LPD offspring remained lower until weaning if their mothers continued to be fed LPD during lactation (PLS group; Fig. 1B inset and Supplemental Table S1) compared to control offspring. At weaning, control and PLS offspring were separated by sex and subjected to HFD for 18 wk. Surprisingly, male and female PLS offspring responded differently. Female PLS offspring gained weight at a significantly higher rate when fed HFD (Supplemental Fig. S1) and, after 4 wk, their weight did not differ from that of control females (Fig. 1C).
To determine whether the increased body weight was due to higher adiposity and, if so, where the fat had accrued, we measured body fat deposition by MRI. As shown in Fig. 1E, female PLS offspring had normal whole-body fat volume but significantly increased visceral fat depots compared to control females. Consistent with these observations, perigonadal fat pad weight in female PLS offspring was significantly larger than in control females, whereas no differences were found in subcutaneous abdominal fat pad weight and interscapular brown adipose tissue weight (Fig. 1D). In addition, female PLS offspring also developed glucose intolerance (Fig. 2) compared to control females. These abnormalities occurred in the absence of changes in food intake between the LPD group and control groups (Table 1).
Figure 2.
PLS female offspring had impaired glucose tolerance, while male offspring had improved glucose tolerance. A) Glucose tolerance tests after 13 wk of HFD in PLS offspring and control offspring. B) Quantification of data by area under the curve (AUC). n = 7–10 mice/group. Results represent means ± se. *P < 0.05, **P < 0.01.
Table 1.
Food intake in offspring of mice fed LPD during pregnancy only (PS group) and during pregnancy and lactation (PLS group) and their respective controls
| Offspring sex | Food intake (g/d) |
|||
|---|---|---|---|---|
| PLS-ctr | PLS | PS-ctr | PS | |
| Male | 2.13 ± 0.18 | 2.09 ± 0.19 | 1.89 ± 0.11 | 2.11 ± 0.16** |
| Female | 1.50 ± 0.30 | 1.53 ± 0.34 | 1.84 ± 0.17 | 1.79 ± 0.21 |
Food intake in PS offspring, PLS offspring, and their respective controls (PS-ctr, PLS-ctr). n = 7–10 mice/group. Results represent means ± se.
P < 0.05,
P < 0.01.
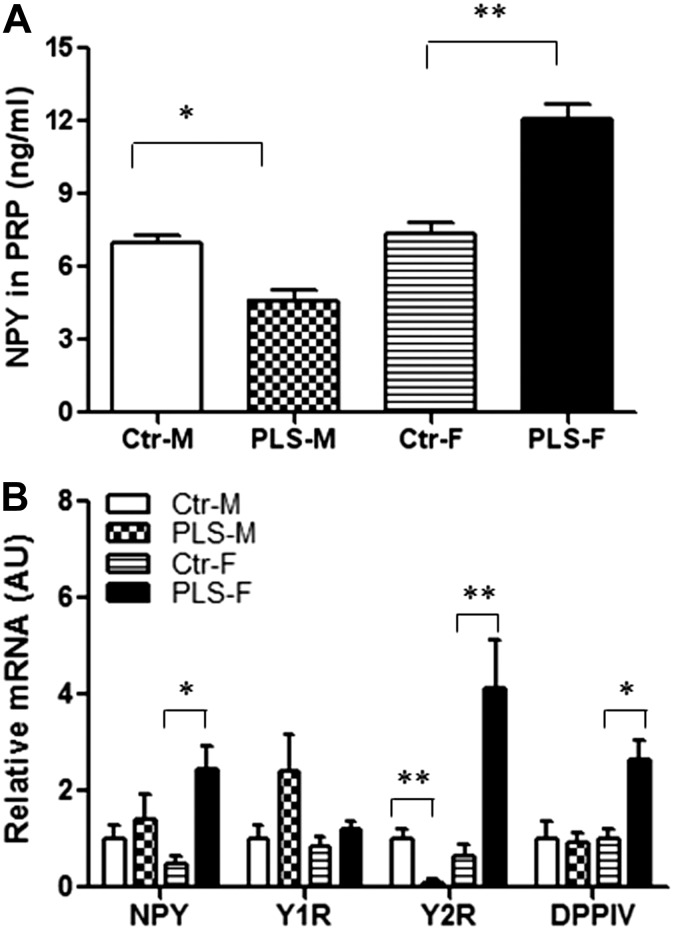
Since previous data from our laboratory has shown that stress accelerates diet-induced obesity and metabolic syndrome in adult mice, we examined whether the NPY system plays a role in abdominal obesity related to maternal LPD as well. Female PLS offspring had significantly higher NPY levels in PRP (Fig. 3A) compared to control females. Female PLS offspring also had a 5-fold increase in NPY expression, a 6-fold increase in Y2R expression, and a 3-fold increase in DPPIV expression specifically in the abdominal fat compared to control females (Fig. 3B). The expression of Y5R was not detectable in the abdominal fat of PLS females and control females (data not shown). However, no differences in NPY, Y1R, Y2R, and DPPIV expression were detected in muscle, liver, and subcutaneous adipose tissue of PLS females when compared to controls (Supplemental Fig. S2).
Figure 3.
Up-regulation of the NPY system was associated with maternal LPD-induced abdominal obesity and glucose intolerance in PLS offspring. A) Levels of NPY-IR in PRP from PLS offspring and control after 18 wk of HFD. B) NPY, Y1R, Y2R, and DPPIV mRNA levels in visceral fat pad by real-time PCR. n = 7–10 mice/group. Results represent means ± se. *P < 0.05, **P < 0.01.
Male offspring of mice fed LPD during pregnancy and lactation were protected from obesity and glucose intolerance
In contrast to females, male offspring of mice fed LPD during pregnancy and lactation had lower body weights after 9 wk of HFD (Fig. 1C). MRI results show that in the male PLS offspring, the whole-body fat content was slightly decreased compared to control males, but the abdominal fat volume was markedly reduced (Fig. 1E). Consistent with these observations, the epididymal fat pad weight in male PLS offspring was significantly lighter compared to control males. However, no differences were found in the subcutaneous abdominal fat pad weight and interscapular brown adipose tissue weight (Fig. 1D) between PLS males and control males. Male PLS offspring also had a faster glucose clearance during glucose tolerance tests compared to control males (Fig. 2A, B). The improved glucose tolerance test was associated with significantly lower NPY levels in PRP (Fig. 3A) and a 10-fold lower expression of the Y2R in the abdominal fat (Fig. 3B) compared to control males. The expression of Y5R was not detectable in the abdominal fat of PLS males and control males (data not shown).
Cross-fostering to control mothers during lactation inverses sex differences on obesity and glucose intolerance
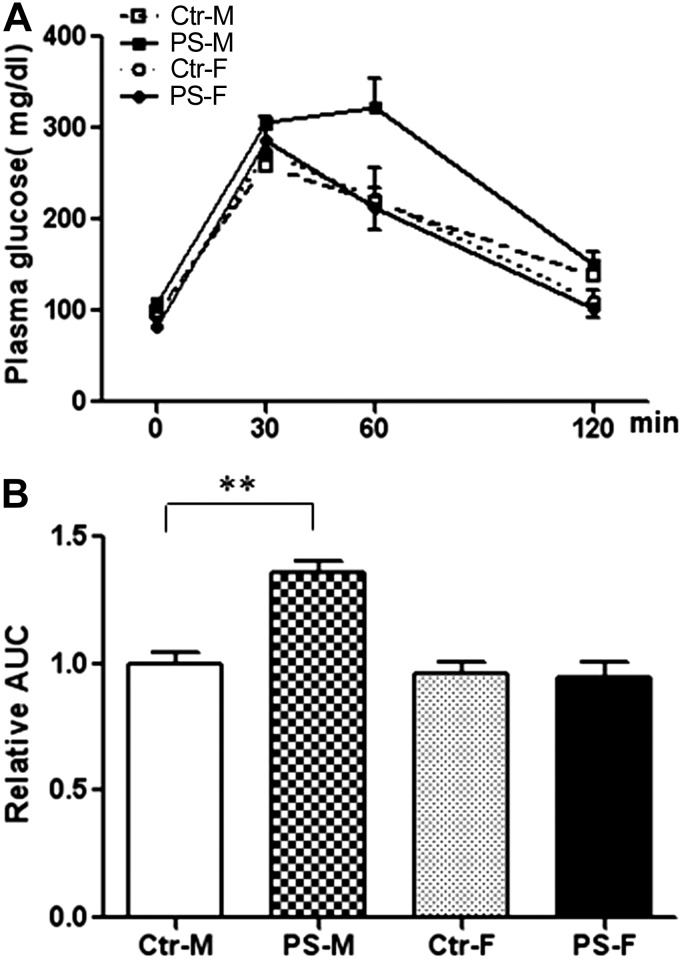
To test the importance of lactation in this phenotype, both prenatal LPD offspring and control offspring were cross-fostered to mothers fed NPD (Fig. 4A). Prenatal LPD offspring were significantly smaller at birth, but their body weight quickly caught up before weaning when they were cross-fostered to NPD-fed mothers (Fig. 4B and Supplemental Table S1). Male offspring of mice fed LPD during pregnancy, cross-fostered to control mothers during lactation (PS), had higher food intake as compared to controls (Table 1), but no difference in body weight for up to 18 wk on HFD (Fig. 4C and Supplemental Table S1). Male PS offspring also showed normal whole-body fat content compared to control males but significantly higher abdominal fat content by MRI (Fig. 4E). Consistent with these observations, the epididymal fat pad weight in male PS offspring was significantly higher than in control males (Fig. 4D). However, no differences in subcutaneous abdominal fat pad weight and interscapular brown adipose tissue weight were found (Fig. 4D) between PS males and control males. In addition, the PS male offspring also developed glucose intolerance (Fig. 5A, B) compared to control males. These metabolic abnormalities in PS males were accompanied by significantly higher NPY levels in PRP (Fig. 6A) and a 6-fold up-regulation of Y2R mRNA in the abdominal fat (Fig. 6B) compared to control males.
Figure 5.
PS male offspring had impaired glucose tolerance. A) Glucose tolerance tests after 13 wk of HFD in PS and control offspring. B) Quantification of data by area under the curve (AUC). n = 7–10 mice/group. Results represent means ± se. **P < 0.01.
Figure 6.
NPY system was associated with prenatal LPD-induced abdominal obesity and glucose tolerance in the PS offspring. A) Levels of NPY-IR in PRP from PS and control offspring measured after 18 wk of HFD. B) NPY, Y1R, Y2R, and DPPIV mRNA levels in visceral fat pad by real-time PCR. n = 7–10 mice/group. Results represent means ± se. **P < 0.01.
In contrast, female offspring of mice fed LPD during pregnancy, cross-fostered to control mothers during lactation, had significantly lower body weight while on HFD, starting at 5 wk on HFD (Fig. 4C), but displayed normal adiposity (Fig. 4E) and glucose tolerance (Fig. 5A, B) compared to control females. Moreover, NPY levels in PRP (Fig. 6A) and Y2R expression in the abdominal fat (Fig. 6B) of PS females were also not different when compared to control female offspring.
DISCUSSION
Our data indicate that prenatal poor-protein diet induced marked changes in body weight, fat accretion, and metabolism in male but not in female mice. The sex-specific response to prenatal protein restriction identified in our study are important in relation to earlier studies showing increased vulnerability for the development of obesity (28, 29), hypercholesterolaemia (30), triacylglycerolaemia (30) and insulin resistance (29, 31) in male rats as a consequence of prenatal protein restriction. At birth, prenatal LPD offspring had lower birth weight but quickly caught up with the control males when weaned to NPD mothers. Epidemiological data also showed that catch-up growth subsequent to low weight at birth or in infancy increases the susceptibility for central obesity, type 2 diabetes, and cardiovascular diseases (32). Litter size is known to affect metabolic neonatal programming in mice and rats (9, 29, 33). It is reported that plasma levels of cholesterol and insulin were elevated later in life in those raised in small litters (33). To exclude the influences of litter size in our experiments, only litters of 5–7 pups were maintained and used to measure body weight in our experiments. No differences in litter size and sex ratio were found between prenatal LPD group and control group by us and others (35). In support of sex differences in the NPY system in the periphery, we have shown previously that stress causes greater NPY release from sympathetic nerves and pressor responses in male than female rats (13) due to testosterone-induced strong up-regulation of the NPY gene expression. Other researchers (34) have shown similar effects of androgens on NPY mRNA in the arcuate nucleus, the site of the orexigenic action of the peptide.
Striking sex differences were also found in the accretion of abdominal fat and glucose intolerance in offspring of mice fed LPD during pregnancy and lactation. In previous rat studies, female offspring of rats fed LPD during pregnancy and lactation had similar glucose tolerance compared to control (31); whereas young male offspring rats had improved glucose tolerance (35) and enhanced insulin sensitivity (26) when weaned to normal chow. In the present study in mice, we also found that male offspring had reduced adiposity and improved glucose tolerance when challenged with HFD after weaning. However, the results in female offspring differed from those obtained in rats, and in our experiment, female offspring developed abdominal obesity and glucose intolerance. This is likely due to challenging the offspring to HFD, since HFD may enhance the phenotype on obesity and glucose intolerance.
Considering the fact that the major adipose tissue expansion occurs early postnatally (36), it is conceivable that the nutrition during lactation has important effects on the phenotype of offspring's adiposity. In rodents, offspring from lactating LPD mothers (NPD-fed offspring fostered by LPD-fed mothers) had lower body weight and lower fat content in adulthood compared to control littermates, both in males and females (9, 29). In addition, male offspring from lactating LPD mothers have shown increased longevity (37). Whether the metabolic phenotype in postnatal LPD offspring is related to the NPY system in abdominal fat still needs to be determined. These data suggested that affective interventions during lactation may prevent the increased risk of developing adulthood obesity. In contrast to low birth weight in prenatal LPD offspring, infants of diabetic mothers are heavier than normal at birth (38). Interestingly, infants of mothers with diabetes fed their own mother's milk, rather than lower-nutrient banked donor breast milk, have a greater risk developing obesity later in life; whereas those fed banked milk have less risk of developing impaired glucose tolerance compared to those fed maternal breast milk (39).
NPY is widely expressed in the central nervous systems and is an important regulator of obesity in adulthood. Intrahypothalamic administration of NPY in rodents leads to accelerated body weight gain, massive hyperphagia, and increased adiposity (18). Y1R, Y2R, and Y5R mRNA have been detected in numerous hypothalamic nuclei, including paraventricular, arcuate, and perifornical nucleus (40). Y1R and Y5R are thought to be involved in the control of feeding behavior (41, 42). In our study, the food intake in PLS male and female and PS female groups did not change compared to their relative controls (Table 1). In rats, it was reported that relative food intake of maternal LPD offspring correlated inversely with weight (29). Taken together, these data suggest that maternal LPD-induced obesity in our study was not due to the orexigenic effects of NPY in hypothalamus. However, in the pilot study, we found that prenatal LPD stress increased anxiety in male offspring, with lower NPY levels in the amygdala. Thus, understanding the role of NPY mechanisms in brain areas related to anxiety will be an important direction of our future research.
NPY also promotes obesity through peripheral actions (12). Peripheral-specific Y2R-knockdown mice are protected from HFD-induced obesity (43). Recent studies from our laboratory showed that NPY and Y2R in visceral fat mediate stress-induced obesity and metabolic syndrome in adult male mice (12). In the present study, we found that high NPY in PRP and Y2R specifically in abdominal fat were associated with increased abdominal adiposity and glucose intolerance in maternal LPD offspring.
DPPIV mRNA was up-regulated in the PLS female offspring compared with female controls. DPPIV is a membrane-bound serine protease, which cleaves proline in the N-terminal penultimate position and modifies the activity of various regulatory peptides and chemokines (44). The substrates of DPPIV include glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic peptide (GIP), substance P, bradykinin, endomorphine, polypeptide Y (PYY) and NPY, with NPY1–36 possessing the highest affinity for DPP-IV (44, 45). The protease converts full-length NPY1–36 to a shorter form, NPY3–36, and stimulates angiogenesis and adipogenesis in the fat tissue (12).
The mechanisms behind the long-lasting effects of nutritional maternal stress remain unknown, but epigenetic processes have been implicated in the developmental origins of the adult disease (46). It is reported that perturbation of the fetal environment by poor nutrition or increases in glucocorticoids could lead to obesity and diabetes in both rodents and humans, which are associated with epigenetic abnormalities (46). A recent study from Weaver et al. (47) suggests that epigenetic reprogramming can also be influenced by the early postnatal environment. The NPY system contributes critically to the accretion and conservation of energy reserves under conditions threatening survival (14). Adipose tissue, like all peripheral tissues except sweat glands, has both sympathetic and parasympathetic innervation (48). We have shown previously that stress-induced obesity is mediated by the NPY-Y2R system in VAT in adult male mice (12). Here we also show that high expression levels of NPY and its Y2R were associated with increased abdominal adiposity and glucose intolerance induced by maternal LPD. Whether increased expression of NPY and Y2R is due to maternal LPD-induced alteration in methylation of these genes is unknown. The NPY gene is rich in CpG islands and is highly hypermethylated in the majority of available human embryonic stem cell lines (15). In contrast, the NPY gene is hypomethylated in somatic tissues and many differentiated cells (15). Thus, in the future studies, we will further investigate how the epigenetic patterns of the VAT NPY system are influenced by maternal LPD and how epigenetic modifications of the NPY system will promote obesity and diabetes in offspring.
In summary, we propose that the peripheral NPY system is a potential mediator and a marker for adult obesity and metabolic dysfunction induced by maternal stress. In our study, female offspring of mice fed LPD during pregnancy and lactation had lower birth weight but grew faster on HFD and developed abdominal adiposity and glucose intolerance compared to control females. This impaired metabolic phenotype was associated with elevated circulating NPY levels and up-regulation of NPY-Y2R adipogenic pathway in the visceral fat. In contrast, male offspring from LPD mothers also had lower birth weight but accumulated less fat and had improved glucose tolerance and lower systemic levels of NPY and intrafat Y2R expression compared to control males. Interestingly, prenatal LPD offspring cross-fostered to control lactating mothers had completely inverse metabolic and NPY phenotypes (Table 2). Thus, we conclude that maternal LPD may induce gender-specific regulation on the peripheral NPY system, specifically NPY in the PRP and Y2R in the visceral fat, and therefore program for future development of abdominal obesity and metabolic syndrome.
Table 2.
High NPY in PRP and Y2R in abdominal fat predicted and was associated with increased abdominal adiposity and glucose intolerance in offspring from maternal LPD stress
| Treatment | Offspring sex | Whole-body fat | Abdominal adiposity | Glucose tolerance | PRP NPY | Abdominal fat Y2R mRNA |
|---|---|---|---|---|---|---|
| PLS: LPD stress in pregnancy and lactation | Female | − | ↑ | ↓ | ↑ | ↑ |
| Male | ↓ | ↓ | ↑ | ↓ | ↓ | |
| PS: LPD stress in pregnancy and cross-fostering to control mothers | Female | − | − | − | − | − |
| Male | − | ↑ | ↓ | ↑ | ↑ |
Parameters were measured in PS offspring, PLS offspring and their respective controls as described in Materials and Methods (n=7–10 mice/group). Vertical arrows, increased or reduced; horizontal line, no difference compared to their relative controls.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health grants R01HL067357 and R37HL055310 to Z.Z.
The authors thank Allison Gurney, Amrutesh Puranik, Dalay Hirsch, Jana Strakova, Jixia Liu, Shuangwei Li, and Allan V. Kalueff for their insightful comments and editing. The authors thank Naz Moaddab for the pilot study reporting that prenatal LPD stress increased anxiety in male offspring, with lower NPY levels in the amygdala. The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BMI
- body mass index
- DPPIV
- dipeptidyl peptidase IV
- HFD
- high-fat diet
- HPA
- hypothalamic-pituitary-adrenal
- LPD
- low-protein diet
- MRI
- magnetic resonance imaging
- NPD
- normal-protein diet
- NPY
- neuropeptide Y
- PLS
- pregnancy and lactation stress
- PS
- pregnancy stress
- PRP
- platelet-rich plasma
- VAT
- visceral adipose tissue
- Y1R
- Y1 receptor
- Y2R
- Y2 receptor
- Y5R
- Y5 receptor
REFERENCES
- 1. Ogden C. L., Carroll M. D., Curtin L. R., McDowell M. A., Tabak C. J., Flegal K. M. (2006) Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295, 1549–1555 [DOI] [PubMed] [Google Scholar]
- 2. Painter R. C., Osmond C., Gluckman P., Hanson M., Phillips D. I., Roseboom T. J. (2008) Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG 115, 1243–1249 [DOI] [PubMed] [Google Scholar]
- 3. Pembrey M. E. (2010) Male-line transgenerational responses in humans. Hum. Fertil. (Camb.) 13, 268–271 [DOI] [PubMed] [Google Scholar]
- 4. Hales C. N., Ozanne S. E. (2003) For debate: fetal and early postnatal growth restriction lead to diabetes, the metabolic syndrome and renal failure. Diabetologia 46, 1013–1019 [DOI] [PubMed] [Google Scholar]
- 5. Ravelli G. P., Stein Z. A., Susser M. W. (1976) Obesity in young men after famine exposure in utero and early infancy. N. Engl. J. Med. 295, 349–353 [DOI] [PubMed] [Google Scholar]
- 6. Jaquet D., Gaboriau A., Czernichow P., Levy-Marchal C. (2000) Insulin resistance early in adulthood in subjects born with intrauterine growth retardation. J. Clin. Endocrinol. Metab. 85, 1401–1406 [DOI] [PubMed] [Google Scholar]
- 7. Law C. M., Barker D. J., Osmond C., Fall C. H., Simmonds S. J. (1992) Early growth and abdominal fatness in adult life. J. Epidemiol. Community Health 46, 184–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lindsay R. S., Lindsay R. M., Waddell B. J., Seckl J. R. (1996) Prenatal glucocorticoid exposure leads to offspring hyperglycaemia in the rat: studies with the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone. Diabetologia 39, 1299–1305 [DOI] [PubMed] [Google Scholar]
- 9. Ozanne S. E., Lewis R., Jennings B. J., Hales C. N. (2004) Early programming of weight gain in mice prevents the induction of obesity by a highly palatable diet. Clin. Sci. (Lond.) 106, 141–145 [DOI] [PubMed] [Google Scholar]
- 10. Seckl J. R. (2004) Prenatal glucocorticoids and long-term programming. Eur. J. Endocrinol. 151(Suppl. 3), U49–U62 [DOI] [PubMed] [Google Scholar]
- 11. Dunn G. A., Bale T. L. (2009) Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology 150, 4999–5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuo L. E., Kitlinska J. B., Tilan J. U., Li L., Baker S. B., Johnson M. D., Lee E. W., Burnett M. S., Fricke S. T., Kvetnansky R., Herzog H., Zukowska Z. (2007) Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 13, 803–811 [DOI] [PubMed] [Google Scholar]
- 13. Zukowska-Grojec Z. (1995) Neuropeptide Y. A novel sympathetic stress hormone and more. Ann. N. Y. Acad. Sci. 771, 219–233 [DOI] [PubMed] [Google Scholar]
- 14. Abe K., Kuo L., Zukowska Z. (2010) Neuropeptide Y is a mediator of chronic vascular and metabolic maladaptations to stress and hypernutrition. Exp. Biol. Med. (Maywood) 235, 1179–1184 [DOI] [PubMed] [Google Scholar]
- 15. Bibikova M., Chudin E., Wu B., Zhou L., Garcia E. W., Liu Y., Shin S., Plaia T. W., Auerbach J. M., Arking D. E., Gonzalez R., Crook J., Davidson B., Schulz T. C., Robins A., Khanna A., Sartipy P., Hyllner J., Vanguri P., Savant-Bhonsale S., Smith A. K., Chakravarti A., Maitra A., Rao M., Barker D. L., Loring J. F., Fan J. B. (2006) Human embryonic stem cells have a unique epigenetic signature. Genome Res. 16, 1075–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Son M. Y., Kim M. J., Yu K., Koo D. B., Cho Y. S. Involvement of neuropeptide Y and its Y1 and Y5 receptors in maintaining self-renewal and proliferation of human embryonic stem cells. J. Cell. Mol. Med. 15, 152–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Costa E., Chen Y., Dong E., Grayson D. R., Kundakovic M., Maloku E., Ruzicka W., Satta R., Veldic M., Zhubi A., Guidotti A. (2009) GABAergic promoter hypermethylation as a model to study the neurochemistry of schizophrenia vulnerability. Expert Rev. Neurother. 9, 87–98 [DOI] [PubMed] [Google Scholar]
- 18. Kalra S. P., Kalra P. S. (2004) NPY and cohorts in regulating appetite, obesity and metabolic syndrome: beneficial effects of gene therapy. Neuropeptides 38, 201–211 [DOI] [PubMed] [Google Scholar]
- 19. Sainsbury A., Bergen H. T., Boey D., Bamming D., Cooney G. J., Lin S., Couzens M., Stroth N., Lee N. J., Lindner D., Singewald N., Karl T., Duffy L., Enriquez R., Slack K., Sperk G., Herzog H. (2006) Y2Y4 receptor double knockout protects against obesity due to a high-fat diet or Y1 receptor deficiency in mice. Diabetes 55, 19–26 [PubMed] [Google Scholar]
- 20. Lundberg J. M., Franco-Cereceda A., Hemsen A., Lacroix J. S., Pernow J. (1990) Pharmacology of noradrenaline and neuropeptide tyrosine (NPY)-mediated sympathetic cotransmission. Fundam. Clin. Pharmacol. 4, 373–391 [DOI] [PubMed] [Google Scholar]
- 21. Li L., Najafi A. H., Kitlinska J. B., Neville R., Laredo J., Epstein S. E., Burnett M. S., Zukowska Z. (2011) Of mice and men: neuropeptide Y and its receptors are associated with atherosclerotic lesion burden and vulnerability. J. Cardiovasc. Transl. Res. 4, 351–362 [DOI] [PubMed] [Google Scholar]
- 22. Nilsson C., Karlsson G., Blennow K., Heilig M., Ekman R. (1996) Differences in the neuropeptide Y-like immunoreactivity of the plasma and platelets of human volunteers and depressed patients. Peptides 17, 359–362 [DOI] [PubMed] [Google Scholar]
- 23. Lee E. W., Michalkiewicz M., Kitlinska J., Kalezic I., Switalska H., Yoo P., Sangkharat A., Ji H., Li L., Michalkiewicz T., Ljubisavljevic M., Johansson H., Grant D. S., Zukowska Z. (2003) Neuropeptide Y induces ischemic angiogenesis and restores function of ischemic skeletal muscles. J. Clin. Invest. 111, 1853–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koulu M., Movafagh S., Tuohimaa J., Jaakkola U., Kallio J., Pesonen U., Geng Y., Karvonen M. K., Vainio-Jylha E., Pollonen M., Kaipio-Salmi K., Seppala H., Lee E. W., Higgins R. D., Zukowska Z. (2004) Neuropeptide Y and Y2-receptor are involved in development of diabetic retinopathy and retinal neovascularization. Ann. Med. 36, 232–240 [DOI] [PubMed] [Google Scholar]
- 25. Yang K., Guan H., Arany E., Hill D. J., Cao X. (2008) Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J. 22, 2452–2464 [DOI] [PubMed] [Google Scholar]
- 26. Ozanne S. E., Smith G. D., Tikerpae J., Hales C. N. (1996) Altered regulation of hepatic glucose output in the male offspring of protein-malnourished rat dams. Am. J. Physiol. 270, E559–E564 [DOI] [PubMed] [Google Scholar]
- 27. Han R., Lai R., Ding Q., Wang Z., Luo X., Zhang Y., Cui G., He J., Liu W., Chen Y. (2007) Apolipoprotein A-I stimulates AMP-activated protein kinase and improves glucose metabolism. Diabetologia 50, 1960–1968 [DOI] [PubMed] [Google Scholar]
- 28. Anguita R. M., Sigulem D. M., Sawaya A. L. (1993) Intrauterine food restriction is associated with obesity in young rats. J. Nutr. 123, 1421–1428 [DOI] [PubMed] [Google Scholar]
- 29. Zambrano E., Bautista C. J., Deas M., Martinez-Samayoa P. M., Gonzalez-Zamorano M., Ledesma H., Morales J., Larrea F., Nathanielsz P. W. (2006) A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J. Physiol. 571, 221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lucas A., Baker B. A., Desai M., Hales C. N. (1996) Nutrition in pregnant or lactating rats programs lipid metabolism in the offspring. Br. J. Nutr. 76, 605–612 [DOI] [PubMed] [Google Scholar]
- 31. Zambrano E., Martinez-Samayoa P. M., Bautista C. J., Deas M., Guillen L., Rodriguez-Gonzalez G. L., Guzman C., Larrea F., Nathanielsz P. W. (2005) Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J. Physiol. 566, 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eriksson J. G., Forsen T., Tuomilehto J., Winter P. D., Osmond C., Barker D. J. (1999) Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ 318, 427–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hahn P. (1984) Effect of litter size on plasma cholesterol and insulin and some liver and adipose tissue enzymes in adult rodents. J. Nutr. 114, 1231–1234 [DOI] [PubMed] [Google Scholar]
- 34. Urban J. H., Bauer-Dantoin A. C., Levine J. E. (1993) Neuropeptide Y gene expression in the arcuate nucleus: sexual dimorphism and modulation by testosterone. Endocrinology 132, 139–145 [DOI] [PubMed] [Google Scholar]
- 35. Shepherd P. R., Crowther N. J., Desai M., Hales C. N., Ozanne S. E. (1997) Altered adipocyte properties in the offspring of protein malnourished rats. Br. J. Nutr. 78, 121–129 [DOI] [PubMed] [Google Scholar]
- 36. Tang W., Zeve D., Suh J. M., Bosnakovski D., Kyba M., Hammer R. E., Tallquist M. D., Graff J. M. (2008) White fat progenitor cells reside in the adipose vasculature. Science 322, 583–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ozanne S. E., Hales C. N. (2004) Lifespan: catch-up growth and obesity in male mice. Nature 427, 411–412 [DOI] [PubMed] [Google Scholar]
- 38. Casson I. F., Clarke C. A., Howard C. V., McKendrick O., Pennycook S., Pharoah P. O., Platt M. J., Stanisstreet M., van Velszen D., Walkinshaw S. (1997) Outcomes of pregnancy in insulin dependent diabetic women: results of a five year population cohort study. BMJ 315, 275–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Plagemann A., Harder T., Franke K., Kohlhoff R. (2002) Long-term impact of neonatal breast-feeding on body weight and glucose tolerance in children of diabetic mothers. Diabetes Care 25, 16–22 [DOI] [PubMed] [Google Scholar]
- 40. Parker R. M., Herzog H. (1999) Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur. J. Neurosci. 11, 1431–1448 [DOI] [PubMed] [Google Scholar]
- 41. Stanley B. G., Magdalin W., Seirafi A., Nguyen M. M., Leibowitz S. F. (1992) Evidence for neuropeptide Y mediation of eating produced by food deprivation and for a variant of the Y1 receptor mediating this peptide's effect. Peptides 13, 581–587 [DOI] [PubMed] [Google Scholar]
- 42. Mashiko S., Ishihara A., Iwaasa H., Sano H., Ito J., Gomori A., Oda Z., Moriya R., Matsushita H., Jitsuoka M., Okamoto O., MacNeil D. J., Van der Ploeg L. H., Fukami T., Kanatani A. (2007) A pair-feeding study reveals that a Y5 antagonist causes weight loss in diet-induced obese mice by modulating food intake and energy expenditure. Mol. Pharmacol. 71, 602–608 [DOI] [PubMed] [Google Scholar]
- 43. Shi Y. C., Lin S., Castillo L., Aljanova A., Enriquez R. F., Nguyen A. D., Baldock P. A., Zhang L., Bijker M. S., Macia L., Yulyaningsih E., Zhang H., Lau J., Sainsbury A., Herzog H. (2011) Peripheral-specific y2 receptor knockdown protects mice from high-fat diet-induced obesity. Obesity 19, 2137–2148 [DOI] [PubMed] [Google Scholar]
- 44. Heymann E., Mentlein R. (1984) [Has dipeptidyl peptidase IV an effect on blood pressure and coagulation?]. Klin. Wochenschr. 62, 2–10 [DOI] [PubMed] [Google Scholar]
- 45. Mentlein R. (1999) Dipeptidyl-peptidase IV (CD26)–role in the inactivation of regulatory peptides. Regul. Pept. 85, 9–24 [DOI] [PubMed] [Google Scholar]
- 46. Whitelaw N. C., Whitelaw E. (2008) Transgenerational epigenetic inheritance in health and disease. Curr. Opin. Genet. Dev. 18, 273–279 [DOI] [PubMed] [Google Scholar]
- 47. Weaver I. C., Cervoni N., Champagne F. A., D'Alessio A. C., Sharma S., Seckl J. R., Dymov S., Szyf M., Meaney M. J. (2004) Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854 [DOI] [PubMed] [Google Scholar]
- 48. Bartness T. J. (2002) Dual innervation of white adipose tissue: some evidence for parasympathetic nervous system involvement. J. Clin. Invest. 110, 1235–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.