Abstract
Insulin resistance is a prominent feature in heart failure, while hyperglycemia impairs cardiac contraction. We propose that decreased insulin-mediated glucose uptake by the heart preserves cardiac function in response to metabolic and hemodynamic stress. To test this hypothesis, we fed rats a high-sucrose diet (HSD). Energy substrate metabolism and cardiac work were determined ex vivo in a sequential protocol simulating metabolic and hemodynamic stress. Compared to chow-fed, control rats, HSD impaired myocardial insulin responsiveness and induced profound metabolic changes in the heart, characterized by reduced rates of glucose uptake (7.91±0.30 vs. 10.73±0.67 μmol/min/g dry weight; P<0.001) but increased rates of glucose oxidation (2.38±0.17 vs. 1.50±0.15 μmol/min/g dry weight; P<0.001) and oleate oxidation (2.29±0.11 vs. 1.96±0.12 μmol/min/g dry weight; P<0.05). Tight coupling of glucose uptake and oxidation and improved cardiac efficiency were associated with a reduction in glucose 6-phosphate and oleoyl-CoA levels, as well as a reduction in the content of uncoupling protein 3. Our results suggest that insulin resistance lessens fuel toxicity in the stressed heart. This calls for a new exploration of the mechanisms regulating substrate uptake and oxidation in the insulin-resistant heart.—Harmancey, R., Lam, T. N., Lubrano, G. M., Guthrie, P. H., Vela, D., Taegtmeyer, H. Insulin resistance improves metabolic and contractile efficiency in stressed rat heart.
Keywords: high-sucrose diet, metabolism, hyperglycemia, glucotoxicity
Insulin resistance is an independent predictor of heart failure (1). Impaired insulin stimulation of glucose transport is a defining feature of type 2 diabetes and is thought to be the cause of contractile dysfunction in the heart, especially when the heart is stressed (2). In fact, the incidence of heart failure is 5-fold higher in patients with diabetes than in patients without diabetes (3).
While hyperglycemia may compensate for impaired insulin-stimulated glucose transport in muscle, there is mounting evidence that supraphysiologic concentrations of glucose are deleterious to cardiac function (4). Akin to the molecular derangements induced by excess glucose entry into the β-cells of the pancreas and skeletal myotubes, cardiac glucotoxicity may also develop in the heart by rerouting of hexose 6-phosphates into the hexosamine biosynthetic and pentose phosphate pathways (5). In support of this hypothesis, extensive experimental work performed on animal models suggests that hyperglycemia per se is associated with a modification of contractile proteins and altered calcium homeostasis in cardiac myocytes, and impaired diastolic and systolic function (6–8).
In light of these observations, there is still uncertainty about whether reduced glucose uptake has deleterious or protective effects for the heart subjected to hyperglycemia. We propose that myocardial insulin resistance may preserve the heart's function when glucose supply is abnormally elevated. To address this question, we fed rats a high-sucrose diet (HSD), a dietary manipulation known to rapidly impair systemic and myocardial insulin sensitivity (9). We then tested our hypothesis in working hearts perfused ex vivo using a sequential protocol simulating metabolic and hemodynamic stress.
MATERIALS AND METHODS
Animals and diets
Male Sprague Dawley rats (200–224 g) were obtained from Harlan Laboratories (Indianapolis, IN, USA) and housed as described previously (10). Rats were fed an HSD (sucrose 67% of total kilocalories; diet D11725; Research Diets, Inc., New Brunswick, NJ, USA) or maintained on standard laboratory chow (LabDiet Laboratory Rodent Diet 5001; PMI Nutrition International, St. Louis, MO, USA) for up to 8 wk. Heparinized plasma samples were obtained from the tail vein of conscious rats maintained in the fed state or following 18 h of food withdrawal. The protocol was approved by the Animal Welfare Committee of the University of Texas Health Science Center at Houston.
Histology
Hearts were rinsed with saline and sectioned into 2-mm-thick slices from apex to base. Two equatorial slices were taken from each heart. One slice was fresh-frozen, embedded in optimal cutting temperature compound, and stored at −20°C. The other slice was fixed in 10% neutral buffered formalin. Frozen tissue was sliced into 5-μm-thick sections and stained with oil red O for triglyceride detection. Formalin-fixed tissue was embedded in paraffin and serially cut into 5-μm-thick sections. Sections were stained with hematoxylin and eosin (H&E), Masson's trichrome, and periodic acid-Schiff (PAS) for morphometric analysis and for detection of fibrosis and glycogen, respectively.
Working heart perfusions
Hearts were perfused as working heart ex vivo (11). In brief, hearts were perfused at 37°C with nonrecirculating Krebs-Henseleit buffer equilibrated with 95% O2-5% CO2 and supplemented with glucose and sodium oleate bound to 1% BSA (Fraction V, fatty acid free; Millipore, Billerica, MA, USA). The filling pressure was 15 cmH2O, with an initial afterload pressure of 100 cmH2O. Cardiac power (watts) was calculated as the product of cardiac output (coronary plus aortic flow, m3/s) times the afterload (pascals). Myocardial oxygen consumption (MVo2; μmol/min) was measured with a YSI 5300A biological oxygen monitor (YSI, Yellow Springs, OH, USA), using 1.06 mM for the concentration of dissolved O2 at 100% saturation (12). Cardiac efficiency was expressed as the ratio of cardiac power to MVo2. Glucose transport and phosphorylation were determined by [3H]2O production from [2-3H]glucose (0.05 μCi/ml) (13). In parallel experiments, glucose oxidation and oleate oxidation were determined by quantitative collection of [14C]O2 and [3H]2O released in the coronary effluent using [U-14C]glucose (0.08 μCi/ml) and [9,10-3H]oleate (0.1 μCi/ml), respectively (14). Samples of the coronary effluent were assayed for lactate levels using a YSI 2300 STAT Plus glucose and lactate analyzer. At the end of perfusion, the hearts were freeze-clamped on their cannula with aluminum tongs cooled in liquid N2 and then stored at −80°C.
Tissue metabolite analyses
The extraction of glycogen and total lipids was performed as reported previously (10). Lipids were emulsified by sonication for 30 s in buffer containing 28.75 mM 1,4-piperazinediethanesulfonic acid, 57.76 mM MgCl2·6H2O, 8.76 μM BSA (fatty acid free), and 0.1% SDS (15), and triacylglycerol content was quantified using the L-Type TG H assay (Wako Chemicals, Richmond, VA, USA). Oleyl-CoA levels were measured by HPLC (10). Glucose 6-phosphate was measured according to Lang and Michal (16).
Immunoblot analyses
Tissue was homogenized in the presence of a cocktail of phosphatase and protease inhibitors. Levels of total and/or phosphorylated proteins were detected by immunoblotting using horseradish peroxidase-conjugated secondary antibodies and chemiluminescence (Santa Cruz Biotechnology, Santa Cruz, CA, USA). A list of the primary antibodies used is given in Supplemental Table S1.
Quantitative real-time PCR
Total RNA was extracted from heart muscle with an RNeasy fibrous tissue kit (Qiagen, Valencia, CA, USA) and treated with DNA-free (Life Technologies, Grand Island, NY, USA). Quantification of transcripts was based on known amounts of synthetic DNA standard using TaqMan probes (Sigma-Aldrich, St. Louis, MO, USA). All mRNA expression levels were normalized to cyclophilin A and expressed in fold change vs. chow-fed animals. Primer and probe sequences are given in Supplemental Table S2.
Statistical analysis
The statistical significance between means was assessed by unpaired Student's t test or by ANOVA followed by a Newman-Keuls or Bonferroni test.
RESULTS
HSD induces hyperlipidemia and systemic insulin resistance
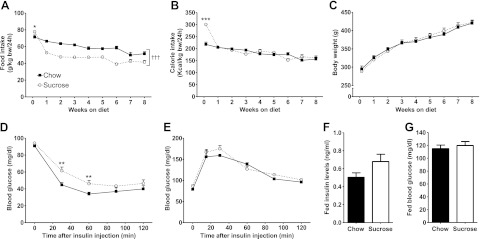
On the first day of HSD feeding, rats ate significantly more than the control group (Fig. 1A). However, the HSD-fed rats subsequently reduced their food intake to levels lower than the chow-fed animals, resulting in a normalization of calorie intake (Fig. 1B). Weight gain was similar between chow- and HSD-fed rats (Fig. 1C). After 3 wk of HSD consumption, plasma total cholesterol, triglyceride, and free fatty acid levels rose by 77, 178, and 36%, respectively (Table 1). Fasting insulin levels were also higher, although euglycemia was maintained (Table 1). At the time of sacrifice for the heart perfusion, systemic insulin sensitivity was significantly impaired with HSD (Fig. 1D), while glucose tolerance was not altered (Fig. 1E). Fed plasma insulin levels and fed blood glucose levels remained similar to controls at the time of sacrifice (Fig. 1F, G).
Figure 1.
The HSD-fed rat as a nonobese rodent model of insulin resistance. A) Food intake of chow-fed (solid squares with solid trace) and HSD-fed (open circles with dotted trace) rats was recorded over the first 24 h of feeding, and then weekly until the end of the protocol. *P < 0.05 and †††P < 0.001 vs. chow from 1 wk until 8 wk. B) Calorie intake was determined according to the energy content of the standard rodent chow (3.04 kcal/g) and of the HSD (3.90 kcal/g). ***P < 0.001 vs. chow at d 1. C) Body weight was measured for the same time points. Data are means ± se from 12 animals/group. D) An insulin tolerance test was performed starting 5 wk after the beginning of the feeding protocol by performing a subscapular subcutaneous injection of 0.5 U insulin/kg body weight in rats that were unfed for 18 h. Data are means ± se from 11 chow- and 9 HSD-fed rats. **P < 0.01 vs. chow. E) A glucose tolerance test was performed on rats that were unfed for 18 h after 5 wk on diet by administering 1g glucose/kg body weight by oral gavage of a 50% (w/v) glucose solution. Data are means ± se from 4 chow-fed and 5 HSD-fed rats. F, G) Fed plasma insulin levels (F) and fed blood glucose levels (G) were measured at time of sacrifice prior to heart perfusion. Data represent means ± se from 6 animals/group.
Table 1.
Fasting plasma parameters after 3 wk of HSD
| Parameter | Chow | HSD | P |
|---|---|---|---|
| Cholesterol (mg/dl) | 66.1 ± 1.9 | 116.9 ± 7.9 | <0.001 |
| Triacylglycerol (mg/dl) | 38.1 ± 10.1 | 105.8 ± 10.6 | <0.001 |
| Free fatty acids (mM) | 0.14 ± 0.01 | 0.19 ± 0.02 | N.S. |
| Glucose (mg/dl) | 99.8 ± 3.5 | 107.2 ± 7.5 | N.S. |
| Insulin (ng/ml) | 0.40 ± 0.04 | 1.05 ± 0.19 | <0.01 |
Data represent means ± se of 6 animals/group. N.S., not significant.
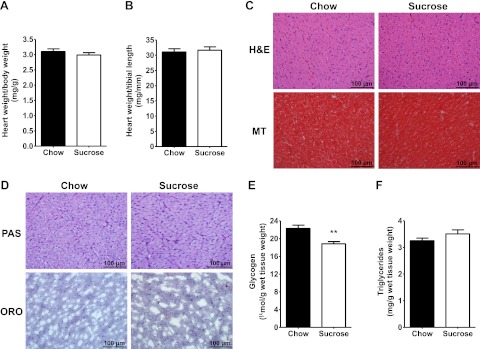
HSD reduces cardiac glycogen stores but has no effect on cardiac structure
Heart weights did not differ (Fig. 2A, B), and histological analyses did not reveal any difference in cardiac cell size or fibrosis (Fig. 2C). Glycogen staining patterns were similar for the hearts of chow- and HSD-fed animals (Fig. 2D), although subsequent enzymatic quantification revealed a reduction in tissue glycogen content with sucrose feeding (Fig. 2E). Myocardial triglyceride levels were the same in both groups (Fig. 2D, F).
Figure 2.
Effects of HSD on tissue structure and substrate storage in the heart. A, B) Cardiac size of chow-fed (solid bars) and HSD-fed (open bars) rats was assessed by normalizing heart weight to body weight (A) or heart weight to tibial length (B). C) Cardiac cell size and extracellular fibrosis were analyzed on H&E- and Masson's trichrome (MT)-stained cardiac tissue paraffin sections, respectively. D) Intracardiac glycogen and triglyceride levels were compared by PAS and oil red O (ORO) staining, respectively. E, F) Intracardiac glycogen (E) and triglycerides (F) were further quantified by enzymatic methods. Data represent means ± se from 6 animals/group. **P < 0.01 vs. chow.
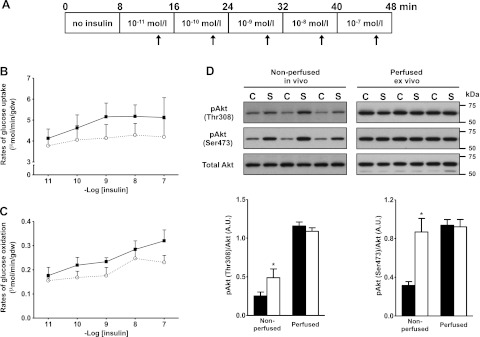
Insulin signaling through Akt is blunted in the hearts of HSD-fed rats
The insulin responsiveness of the isolated working hearts was assessed by adding escalating doses of insulin to the perfusion buffer (Fig. 3A). In the hearts of chow-fed rats, rates of glucose uptake gradually increased with subphysiologic and physiological concentrations of the hormone, and then leveled out at 1 nM insulin (Fig. 3B). In addition, the increase in insulin gradually stimulated glucose oxidation (Fig. 3C). This response to insulin was blunted in the hearts of HSD rats. During the perfusion protocol, cardiac power was steady and identical in both groups (data not shown). We next investigated the effect of HSD on cardiac insulin signaling at the level of Akt. As expected, Akt phosphorylation at Thr308 and Ser473 was increased in the hearts of chow-fed rats with insulin in the perfusion medium (Fig. 3D). Basal Akt phosphorylation at both Thr308 and Ser473 was higher in the nonperfused hearts of HSD-fed rats. Following insulin stimulation ex vivo, the phosphorylation of Akt at Thr308 increased to a level similar to controls, but the amplitude of the response was consequently reduced. Because Akt phosphorylation at Ser473 was maximal in nonperfused hearts, the stimulatory effect of insulin on that phosphorylation site was completely lost. There was no change in total Akt levels in the hearts of HSD-fed rats.
Figure 3.
Impaired insulin responsiveness in the working heart of HSD-fed rats. A) Isolated hearts were perfused in the working mode for 48 min in Krebs-Henseleit buffer supplemented with 5 mM glucose and 0.8 mM oleate. After allowing for stabilization of baseline parameters, the hearts were perfused in presence of 10−11 M insulin. The insulin concentration was then increased 10-fold every 8 min and up to 10−7 M. Cardiac function and myocardial glucose utilization rates were monitored 5 min after each change in the insulin concentration (arrows). B, C) Rates of glucose uptake (B) and glucose oxidation (C) for the perfused hearts of chow-fed (solid squares with solid trace; n=5) or HSD-fed (open circles with dotted trace; n=7) rats were measured for each concentration of insulin tested. D) Immunoblot analysis of phospho-Akt in hearts from chow-fed (C; solid bars) and sucrose-fed (S; open bars) rats. Akt phosphorylation at Thr308 and Ser473 was assessed at baseline in nonperfused hearts and in hearts that were perfused ex vivo with insulin with the protocol described in A. Data are shown as means ± se. *P < 0.05 vs. chow.
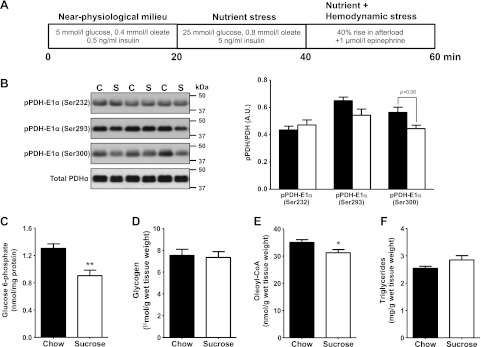
Reduced glucose uptake is associated with improved contractile function in HSD-fed rat hearts subjected to metabolic and hemodynamic stress
Hearts were first perfused at a normal workload for 20 min with near-physiological concentrations of glucose, oleate, and insulin (Fig. 4A). Rates of glucose uptake were lower, but not statistically significant, in hearts from HSD-fed rats. This small decrease in glucose uptake may partly account for the decrease in glycolytic flux (decreased lactate release). However, rates of glucose oxidation and oleate oxidation, as well as cardiac performance, were similar in both groups (Table 2). For the next 20 min, hearts were subjected to a metabolic stress simulating hyperglycemic, hyperlipidemic, and hyperinsulinemic conditions (Fig. 4A). This intervention resulted in relatively higher rates of myocardial glucose uptake in both groups, although the increase was significantly lower for the insulin-resistant hearts (+65% with HSD vs. +81% with chow). Rates of glucose and fatty acid oxidation, as well as lactate release, all increased similarly in both groups. Overall, the amount of glucose going through the glycolytic pathway was identical in both groups, and cardiac power and efficiency remained steady under metabolic stress. Last, we added a hemodynamic stress to metabolic stress for the last 20 min of perfusion (Fig. 4A). The new stress condition led to an additional increase in the rates of glucose uptake by the hearts of chow-fed animals (Table 2). Conversely, rates of glucose uptake did not increase further for the hearts of HSD-fed rats. However, rates of glucose and fatty acid oxidation increased in both groups. Unexpectedly, the increase was greater in the hearts of HSD-fed rats, for rates of oleate oxidation (+45% with HSD vs. +28% with chow), but especially for rates of glucose oxidation (+164% with HSD vs. +43% with chow). The amount of glucose going through the glycolytic pathway was still identical in both groups, suggesting an increased flux of pyruvate into mitochondria in the hearts of HSD-fed rats. Interestingly, cardiac contractile performance of control animals failed to increase in response to the acute increase in workload. However, cardiac power increased by 8%, and cardiac efficiency was better preserved, for the hearts of HSD-fed rats (Table 2).
Figure 4.
Increased flux through PDH and reduced levels of intermediary metabolites in the stressed hearts of HSD-fed rats. A) Isolated hearts from chow-fed (C; solid bars; n=11) or sucrose-fed (S; open bars; n=9) rats were perfused successively in 3 different conditions for a total of 60 min. The hearts were perfused with 5 mM glucose, 0.4 mM oleate, and 0.5 ng/ml insulin (near-physiological milieu) during the first 20 min, before being perfused with a buffer containing 25 mM glucose, 0.8 mM oleate, and 5 ng/ml insulin (metabolic stress) for the next 20 min. A hemodynamic stress (afterload raised from 100 to 140 cmH2O; 1 μM epinephrine) was superimposed to the metabolic stress for the last 20 min of perfusion. Cardiac function and myocardial rates of glucose and fatty acid oxidation were monitored every 5 min and averaged for each perfusion condition. Data are reported in Table 2. B) At the end of the perfusion protocol, the levels of inhibitory phosphorylation of PDH on serine residues 232, 293, and 300 were determined by immunoblot. C–F) Intracardiac levels of the glycolytic intermediate glucose 6-phosphate (C), glycogen (D), the fatty acid intermediate oleoyl-CoA (E), and triglycerides (F) were quantified from freeze-clamped, perfused heart tissue. All data are presented as means ± se. *P < 0.05, **P < 0.01 vs. chow.
Table 2.
Improved metabolic and contractile efficiency for working hearts of HSD-fed rats subjected to metabolic and hemodynamic stress
| Parameter | Near-physiological milieu: 5 mM glucose, 0.4 mM oleate, 0.5 ng/ml insulin |
Metabolic stress: 25 mM glucose, 0.8 mM oleate, 5 ng/ml insulin |
Metabolic and hemodynamic stress: 40% rise in afterload + 1 μM epinephrine |
|||
|---|---|---|---|---|---|---|
| Chow | HSD | Chow | HSD | Chow | HSD | |
| Glucose uptake (μmol/min/gdw) | 5.24 ± 0.14 | 4.81 ± 0.24 | 9.51 ± 0.36 | 7.96 ± 0.39** | 10.73 ± 0.67 | 7.91 ± 0.30*** |
| Glucose oxidation (μmol/min/gdw) | 0.40 ± 0.04 | 0.43 ± 0.03 | 1.05 ± 0.12 | 0.90 ± 0.10 | 1.50 ± 0.15 | 2.38 ± 0.17*** |
| Lactate release (μmol/min/gdw) | 5.20 ± 0.43 | 3.52 ± 0.21** | 9.68 ± 0.41 | 8.81 ± 0.51 | 13.11 ± 0.53 | 12.40 ± 0.39 |
| Glucose utilized (μmol/min/gdw) | 5.60 ± 0.46 | 3.94 ± 0.22** | 10.73 ± 0.44 | 9.71 ± 0.56 | 14.61 ± 0.57 | 14.79 ± 0.48 |
| Oleate oxidation (μmol/min/gdw) | 1.13 ± 0.06 | 1.13 ± 0.06 | 1.52 ± 0.07 | 1.58 ± 0.07 | 1.96 ± 0.12 | 2.29 ± 0.11* |
| Cardiac power (mW) | 8.0 ± 0.4 | 8.8 ± 0.4 | 8.2 ± 0.4 | 9.1 ± 0.3 | 8.3 ± 0.5 | 9.8 ± 0.4* |
| Cardiac efficiency | 0.83 ± 0.03 | 0.83 ± 0.04 | 0.82 ± 0.03 | 0.85 ± 0.04 | 0.54 ± 0.02 | 0.61 ± 0.03* |
Isolated hearts were perfused as described in Fig. 4A. Lactate release is given in micromole glucose equivalents. Glucose utilized represents the sum of glucose released as lactate and being oxidized. Data are presented as means ± se from 5 chow-fed and 6 HSD-fed animals/group for rate of glucose uptake; 11 chow-fed and 9 HSD-fed animals/group for all other parameters. gdw, grams dry weight.
P < 0.05,
P < 0.01,
P < 0.001 vs. chow.
The stressed hearts of HSD-fed rats display increased flux of glucose through the pyruvate dehydrogenase complex and reduced accumulation of intermediary metabolites
We next investigated the cause and consequence of this increased coupling between glucose uptake and oxidation at the molecular level. We observed that HSD was associated with the reduced inhibitory phosphorylation of the pyruvate dehydrogenase (PDH) E1α subunit on Ser300 (Fig. 4B), thereby explaining the higher capacity of the insulin-resistant heart to oxidize glucose. Intracellular levels of glucose 6-phosphate were significantly lower (Fig. 4C). However, glycogen content did not differ between groups at the end of the perfusion protocol (Fig. 4D), suggesting that the increase of pyruvate oxidation, rather than an increase in glucose storage as glycogen, causes the reduction in intracellular glucose 6-phosphate levels. The intracellular levels of the fatty acid intermediate oleoyl-CoA also decreased (Fig. 4E), and this decrease was independent of intracellular triglyceride content (Fig. 4F).
Uncoupling protein 3 (UCP3) is post-transcriptionally down-regulated in the hearts of HSD-fed rats
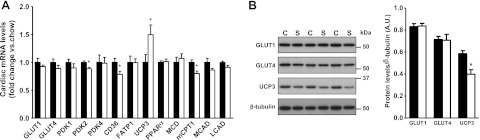
To gain insight into the molecular basis of enhanced efficiency of the stressed hearts of HSD-fed rats, we investigated potential changes in the expression levels of enzymes involved in substrate partitioning. The mRNA and protein levels of GLUT1 and GLUT4 were similar in both groups (Fig. 5A, B). However, the transcript levels of the fatty acid transporter CD36, the pyruvate dehydrogenase kinase (PDK) 2, and of the muscle type carnitine palmitoyl transferase-1 were decreased. Transcript levels of the other fatty acid oxidation-related enzymes were not altered, with the notable exception of UCP3, the expression of which was markedly increased (Fig. 5A). Surprisingly, UCP3 protein levels were decreased (Fig. 5B), suggesting the activation of specific post-transcriptional regulatory mechanisms associated with HSD.
Figure 5.
Post-transcriptional down-regulation of UCP3 in the heart of HSD-fed rats. A) Total RNA was prepared from the hearts of chow-fed (solid bars) or HSD-fed (open bars) rats and analyzed by real-time PCR to determine the abundance of transcripts encoding metabolic enzymes. The proteins analyzed included the glucose transporters GLUT1 and GLUT4; the PDK isoforms 1, 2, and 4; the fatty acid transporters CD36 and fatty acid transporter 1 (FATP1); the uncoupling protein UCP3; peroxisome proliferator-activated receptor α (PPARα); malonyl-CoA decarboxylase (MCD); muscle carnitine palmitoyl transferase-1 (mCPT1); and the medium-chain and long-chain acyl-CoA dehydrogenases (MCAD and LCAD, respectively). B) Protein levels of GLUT1, GLUT4, and UCP3 were measured by immunoblot and normalized to β-tubulin levels. Data represent means ± se from 6 animals/group. *P < 0.05 vs. chow.
DISCUSSION
We have shown that reduced glucose uptake and increased rates of glucose oxidation are associated with improved contractile function of the heart subjected to metabolic and hemodynamic stress. The improvement of contractile efficiency for the insulin-resistant heart of HSD-fed rats can be explained as follows. First, glucose uptake is reduced in response to excess fuel supply, which may limit the rerouting of glycolytic intermediates into nonoxidative pathways. Second, the coupling between glycolysis and pyruvate oxidation is improved. Third, cardiac efficiency is improved because there is increased utilization of glucose for oxidative phosphorylation in a state of increased energy demand.
A key element of the present study was to utilize an animal model where myocardial glucose uptake would be impaired in response to insulin. The HSD rapidly induces systemic insulin resistance in rats. The impairment of insulin action in skeletal muscle and liver following short-term high-sucrose feeding has already been studied (17). However, the effects on the heart are less documented and sometimes conflicting (9). In our study, we observed a mild reduction in cardiac glycogen levels, which may reflect an increased inhibition of glycogen synthase due to reduced myocardial insulin sensitivity. Interestingly, basal Akt activity was increased and corresponded to a blunted response to insulin stimulation in the isolated working hearts. This observation is in accordance with previous findings made by others in the liver of rats fed with the same HSD for 3 wk, where increased basal activity of the PI3K/Akt axis was proposed to contribute to the maintenance of normal rates of basal glucose production (18). The alteration of Akt activity had a modest inhibitory effect on myocardial glucose uptake when the hearts were perfused with normal glucose levels (Fig. 3B). This observation (made ex vivo) and the maintenance of normoglycemia in vivo suggest that the animals were in a phase of compensated insulin resistance at the time the experiments were performed.
The reduction in myocardial glucose uptake became significant when simulating a phase of decompensated insulin resistance by raising insulin and glucose to supraphysiologic concentrations ex vivo. The role of impaired insulin signaling through Akt in this decrease is supported by the fact that GLUT1 and GLUT4 transporter levels were not altered by the HSD. Interestingly, the quantities of glucose oxidized or released as lactate remained the same, which demonstrates that, in presence of hyperglycemia, the flux of glucose through the glycolytic and oxidation pathways can be maintained despite a decrease in glucose transport. Even more surprising is the fact that substrate oxidation was higher in the hearts of HSD-fed rats when a hemodynamic stress was superimposed to the metabolic stress. Increased oxidation of glucose by the insulin-resistant, stressed hearts was an unpredicted finding since rats fed the HSD rapidly developed hyperlipidemia, a condition that usually inhibits myocardial glucose utilization due to increased lipid delivery to the heart (Randle cycle; ref. 19). It is of note that we observed a small, but significant, reduction in the intracellular levels of the fatty acid intermediate oleoyl-CoA. Moreover, the expression of the fatty acid transporter CD36 was also decreased at the mRNA level. Insulin has been shown to stimulate fatty acid uptake in cardiac myocytes (20), and although we did not measure the rates of myocardial fatty acid uptake, the data suggest that these rates may also be reduced in the hearts of HSD-fed rats. Therefore, glucose oxidation may be stimulated by a reduction of fatty acid-mediated inhibition of glucose oxidation, and fatty acid oxidation may have been, in turn, stimulated by a lesser accumulation of fatty acid intermediates. The reduced inhibitory phosphorylation of PDH at high workload is consistent with a switch toward preponderant glucose utilization, increased cardiac efficiency, and improved contraction of the stressed heart. This rapid increase in pyruvate oxidation was associated with a decrease in glucose 6-phosphate levels. Conversely to the hearts of HSD-fed rats, heart work failed to increase for control rats in response to the increase in workload, and this despite higher rates of glucose uptake. Reduced coupling of glucose metabolism is linked to poor mechanical function of the heart in diabetes, most likely because the spillover of excess glucose in nonoxidative pathways reduces the activity of the sarco(endo)plasmic reticulum Ca2+-ATPase and impairs calcium homeostasis (7, 8). Therefore, the increase in contractile performance in control hearts may have been hampered by glucotoxic effects.
We acknowledge that further evidence is needed to demonstrate that reduced glucose uptake is directly linked to the improvement of efficiency of the stressed heart, as the decreased expression of UCP3 was also likely to increase cardiac efficiency in our model. The striking discordance between UCP3 mRNA and protein levels has been reported in the gastrocnemius muscle of rodents (21, 22). While the UCP3 protein turns over rapidly, the factors regulating its turnover are unknown (23). However, both plasma insulin and glucose levels are negatively correlated to UCP3 protein level in human skeletal muscle (24). Therefore, it is not impossible that both synthesis and degradation of UCP3 in the heart is under the control of insulin signaling. Additional models of myocardial insulin resistance will have to be studied in order to confirm our hypothesis, but interestingly, impaired myocardial glucose uptake induced by high-fat feeding is associated with preserved contractile function in rats undergoing coronary artery ligation (25).
In summary, the results suggest an adaptive role for impaired myocardial insulin sensitivity in a state of “fuel overload. ” The novelty of the findings is 2-fold. First, we demonstrate that in hyperglycemic, hyperlipidemic, and hyperinsulinemic conditions, a moderate impairment in glucose uptake does not reduce the glycolytic flux or the rates of glucose oxidation. Second, a normal rat heart fails to respond to an acute increase in workload when subjected to this metabolic stress, whereas cardiac performance increases for the hearts of HSD-fed rats. The preserved contractile response of the insulin-resistant heart is likely the result of reduced accumulation of intracellular metabolites and of increased contractile efficiency (Fig. 6). Taken together, our data call for a new exploration of the mechanisms regulating substrate uptake and oxidation in the insulin-resistant heart.
Figure 6.
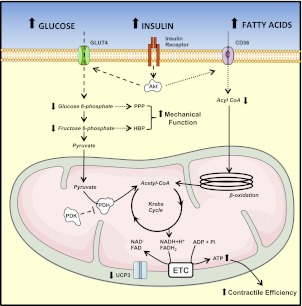
Proposed mechanisms leading to increased contractile performance for the insulin-resistant, stressed hearts of HSD-fed rats. In presence of hyperglycemia and hyperinsulinemia, impaired insulin-stimulated phosphorylation of Akt limits glucose transport into cardiac myocytes. Because insulin is known to stimulate the translocation of the fatty acid transporter CD36 at the sarcolemma (20), insulin resistance is also likely to limit fatty acid uptake in the heart of HSD-fed rats. Both rates of glucose and fatty acid oxidation are increased in response to an acute increase in workload. However, glucose oxidation increases more than fatty acid oxidation, and combines to an improved mitochondrial energy coupling (reduced UCP3 levels) to increase contractile efficiency. The energetic response of the stressed heart may have been preserved by the impairment of insulin-stimulated fatty acid uptake, and the reduction of fatty acid-mediated inhibition of glucose oxidation. The increased coupling between glucose uptake and glucose oxidation limits the accumulation of glycolytic intermediates and their rerouting into the pentose phosphate pathway (PPP) and the hexosamine biosynthetic pathway (HBP). This could increase the mechanical function of the heart by preserving the activity of contractile proteins and calcium homeostasis in cardiac myocytes. Dashed lines represent pathways with a reduced activity. Dotted lines represent possible consequences of insulin resistance that have not been tested directly in the present model.
Supplementary Material
Acknowledgments
The authors thank the Mouse Metabolism Core (Baylor College of Medicine, Houston, TX, USA) for the plasma analyses and Roxy Tate for editorial assistance. Figure 6 was produced using Servier Medical Art (http://www.servier.com/servier-medical-art).
This work was supported by grants from the U.S. National Heart, Lung, and Blood Institute and the American Heart Association.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- H&E
- hematoxylin and eosin
- HSD
- high-sucrose diet
- MVo2
- myocardial oxygen consumption
- PAS
- periodic acid-Schiff
- PDH
- pyruvate dehydrogenase
- PDK
- pyruvate dehydrogenase kinase
- UCP
- uncoupling protein
REFERENCES
- 1. Ingelsson E., Sundstrom J., Arnlov J., Zethelius B., Lind L. (2005) Insulin resistance and risk of congestive heart failure. JAMA 294, 334–341 [DOI] [PubMed] [Google Scholar]
- 2. Garvey W. T., Hardin D., Juhaszova M., Dominguez J. H. (1993) Effects of diabetes on myocardial glucose transport system in rats: implications for diabetic cardiomyopathy. Am. J. Physiol. 264, H837–H844 [DOI] [PubMed] [Google Scholar]
- 3. Kannel W. B., Hjortland M., Castelli W. P. (1974) Role of diabetes in congestive heart failure: the Framingham study. Am. J. Cardiol. 34, 29–34 [DOI] [PubMed] [Google Scholar]
- 4. Rubin J., Matsushita K., Ballantyne C. M., Hoogeveen R., Coresh J., Selvin E. (2012) Chronic hyperglycemia and subclinical myocardial injury. J. Am. Coll. Cardiol. 59, 484–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rossetti L. (2004) Glucose toxicity: effect of chronic hyperglycemia on insulin action. In Diabetes Mellitus: A Fundamental and Clinical Text (LeRoith D., Taylor S. I., Olefsky J. M., eds) pp. 939–951, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 6. Chess D. J., Stanley W. C. (2008) Role of diet and fuel overabundance in the development and progression of heart failure. Cardiovasc. Res. 79, 269–278 [DOI] [PubMed] [Google Scholar]
- 7. Clark R. J., McDonough P. M., Swanson E., Trost S. U., Suzuki M., Fukuda M., Dillmann W. H. (2003) Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J. Biol. Chem. 278, 44230–44237 [DOI] [PubMed] [Google Scholar]
- 8. Tang W. H., Cheng W. T., Kravtsov G. M., Tong X. Y., Hou X. Y., Chung S. K., Chung S. S. (2010) Cardiac contractile dysfunction during acute hyperglycemia due to impairment of SERCA by polyol pathway-mediated oxidative stress. Am. J. Physiol. Cell Physiol. 299, C643–C653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mellor K. M., Ritchie R. H., Davidoff A. J., Delbridge L. M. (2010) Elevated dietary sugar and the heart: experimental models and myocardial remodeling. Can. J. Physiol. Pharmacol. 88, 525–540 [DOI] [PubMed] [Google Scholar]
- 10. Harmancey R., Wilson C. R., Wright N. R., Taegtmeyer H. (2010) Western diet changes cardiac acyl-CoA composition in obese rats: a potential role for hepatic lipogenesis. J. Lipid Res. 51, 1380–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taegtmeyer H., Hems R., Krebs H. A. (1980) Utilization of energy-providing substrates in the isolated working rat heart. Biochem. J. 186, 701–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Starnes J. W., Wilson D. F., Erecinska M. (1985) Substrate dependence of metabolic state and coronary flow in perfused rat heart. Am. J. Physiol. 249, H799–H806 [DOI] [PubMed] [Google Scholar]
- 13. Katz J., Dunn A. (1967) Glucose-2-t as a tracer for glucose metabolism. Biochemistry 6, 1–5 [DOI] [PubMed] [Google Scholar]
- 14. Goodwin G. W., Ahmad F., Doenst T., Taegtmeyer H. (1998) Energy provision from glycogen, glucose, and fatty acids on adrenergic stimulation of isolated working rat hearts. Am. J. Physiol. 274, H1239–H1247 [DOI] [PubMed] [Google Scholar]
- 15. Rodriguez-Sureda V., Peinado-Onsurbe J. (2005) A procedure for measuring triacylglyceride and cholesterol content using a small amount of tissue. Anal. Biochem. 343, 277–282 [DOI] [PubMed] [Google Scholar]
- 16. Lang G., Michal G. (1981) D-glucose-6-phosphate and D-fructose-6-phosphate. In Methods of Enzymatic Analysis (Bergmeyer H., ed) pp. 1238–1242, Verlag Chemie International, Deerfield Beach, FL, USA [Google Scholar]
- 17. Pagliassotti M. J., Shahrokhi K. A., Moscarello M. (1994) Involvement of liver and skeletal muscle in sucrose-induced insulin resistance: dose-response studies. Am. J. Physiol. 266, R1637–R1644 [DOI] [PubMed] [Google Scholar]
- 18. Pagliassotti M. J., Kang J., Thresher J. S., Sung C. K., Bizeau M. E. (2002) Elevated basal PI 3-kinase activity and reduced insulin signaling in sucrose-induced hepatic insulin resistance. Am. J. Physiol. Endocrin. Metab. 282, E170–E176 [DOI] [PubMed] [Google Scholar]
- 19. Randle P. J., Garland P. B., Hales C. N., Newsholme E. A. (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1, 785–789 [DOI] [PubMed] [Google Scholar]
- 20. Luiken J. J., Koonen D. P., Willems J., Zorzano A., Becker C., Fischer Y., Tandon N. N., Van Der Vusse G. J., Bonen A., Glatz J. F. (2002) Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes 51, 3113–3119 [DOI] [PubMed] [Google Scholar]
- 21. Jimenez M., Yvon C., Lehr L., Leger B., Keller P., Russell A., Kuhne F., Flandin P., Giacobino J. P., Muzzin P. (2002) Expression of uncoupling protein-3 in subsarcolemmal and intermyofibrillar mitochondria of various mouse muscle types and its modulation by fasting. Eur. J. Biochem. 269, 2878–2884 [DOI] [PubMed] [Google Scholar]
- 22. Kontani Y., Wang Z., Furuyama T., Sato Y., Mori N., Yamashita H. (2002) Effects of aging and denervation on the expression of uncoupling proteins in slow- and fast-twitch muscles of rats. J. Biochem. 132, 309–315 [DOI] [PubMed] [Google Scholar]
- 23. Azzu V., Jastroch M., Divakaruni A. S., Brand M. D. (2010) The regulation and turnover of mitochondrial uncoupling proteins. Biochim. Biophys. Acta 1797, 785–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schrauwen P., Hesselink M. K., Blaak E. E., Borghouts L. B., Schaart G., Saris W. H., Keizer H. A. (2001) Uncoupling protein 3 content is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 50, 2870–2873 [DOI] [PubMed] [Google Scholar]
- 25. Christopher B. A., Huang H. M., Berthiaume J. M., McElfresh T. A., Chen X., Croniger C. M., Muzic R. F., Jr., Chandler M. P. (2010) Myocardial insulin resistance induced by high fat feeding in heart failure is associated with preserved contractile function Am. J. Physiol. Heart Circ. Physiol. 299, H1917–H1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.