Abstract
Ovarian cancer is the most lethal gynecological cancer. Here we show that innate immune agonist, dsRNA, directly induces ovarian cancer cell death and identify biomarkers associated with responsiveness to this targeted treatment. Nuclear staining and MTT assays following dsRNA stimulation revealed two subpopulations, sensitive (OVCAR-3, CAOV-3; patient samples malignant 1 and 2) and resistant (DOV-13, SKOV-3). Microarray analysis identified 75 genes with differential expression that further delineated these two subpopulations. qPCR and immunoblot analyses showed increased dsRNA receptor expression after stimulation as compared to resistant and immortalized ovarian surface epithelial cells (e.g., 70-fold with malignant 2, 43-fold with OVCAR-3). Using agonists, antagonists, and shRNA-mediated knockdown of dsRNA receptors, we show that TLR3, RIG-I, and mda5 coordinated a caspase 8/9- and interferon-dependent cell death. In resistant cells, dsRNA receptor overexpression restored dsRNA sensitivity. When dsRNA was combined with carboplatin or paclitaxel, cell viability significantly decreased over individual treatments (1.5- to 7.5-fold). Isobologram analyses showed synergism in dsRNA combinations (CI=0.4–0.82) vs. an additive effect in carboplatin/paclitaxel treatment (CI=1.5–2). Our data identify a predictive marker, dsRNA receptor expression, to target dsRNA responsive populations and show that, in dsRNA-sensitive cells, dsRNA induces apoptosis and enhances the potency of cytotoxic chemotherapeutics.—Van, D. N., Roberts, C. F., Marion, J. D., Lépine, S., Harikumar, K. B., Schreiter, J., Dumur, C. I., Fang, X., Spiegel, S., Bell, J. K. Innate immune agonist, dsRNA, induces apoptosis in ovarian cancer cells and enhances the potency of cytotoxic chemotherapeutics.
Keywords: pattern recognition receptors, targeted therapy, biomarker, type I interferon, caspase
Ovarian cancer is the most lethal gynecological malignancy (1). Due to the asymptomatic nature of the disease, patients are diagnosed in the latter stages (III and IV), resulting in 5 yr survival rates overall of 44% and for stage III and IV detection ∼27% (2). Standard care involves surgical debulking of the tumor and dual carboplatin/paclitaxel chemotherapy, to which ∼65% of patients respond favorably (3). Following first-line treatment, ∼80% of patients have recurring tumors that, in some cases, have become platinum resistant (3). To address recurrent and chemoresistant disease, new cytotoxic agents and molecularly targeted therapies have been developed, but their patient response rates remain at or below 50% (4), highlighting the need for additional anticancer therapies.
Innate immune agonists trigger tumor-resident innate immune receptors to initiate apoptotic programs in tumor cells, making these ligands attractive targets for chemotherapeutic development. Virally derived double-stranded RNA or its synthetic analogs, polyriboinosinic:polyribocytidylic acid (pI:pC) or polyriboadenylic:polyribouridylic acid (pA:pU), activate at least 4 known innate immune receptors, Toll-like receptor 3 (TLR3; ref. 5), retinoic acid-inducible gene I (RIG-I; ref. 6), melanoma differentiation-associated gene 5 (mda5; ref. 7), and dsRNA-dependent protein kinase receptor (PKR; ref. 8). Once stimulated, these dsRNA receptors initiate signaling pathways that lead to the production of proinflammatory cytokines and type I interferon (IFN), apoptosis and translational inhibition (see Fig. 1 for an overview). Clinical trials for breast (9) and gastric (10) cancer that included dsRNA in combination with standard care showed improved overall survival and progression-free survival. In melanoma (11, 12), breast (13), prostate (14), and hepatoma (15) cancer cell lines, dsRNA has been shown to trigger a caspase-dependent apoptotic response. Each study has implicated a different subset of dsRNA receptors as essential for dsRNA responsiveness and showed that type I IFN (13), increased autophagy (11), and PKC-α activation (14) contributed to dsRNA-induced cell death. Predictive biomarkers for a dsRNA responsive patient population have been difficult to identify due to these varied requirements for dsRNA responsiveness.
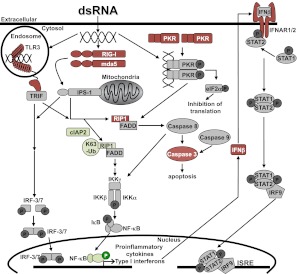
Figure 1.
dsRNA receptor signaling pathways in dsRNA-sensitive and -resistant cells. dsRNA-sensitive cells activate the dsRNA signaling pathways shown. dsRNA-resistant cells lines have altered expression and activation of several pathway components: Red, decreased protein expression or activation; green, increased expression or activation. c-IAP, cellular inhibitor of apoptosis; eIF2α, eukaryotic translation initiation factor 2α; FADD, FAS-associated death domain; IPS-1, interferon-β promoter stimulator 1; IκB, inhibitor of κB; IKK, inhibitor of κB kinase; IFN, interferon; IFNAR, interferon-α/β receptor; IRF, interferon regulatory factor; ISRE, interferon stimulated response element; mda5, melanoma differentiation-associated gene 5; NF-κB, nuclear factor κB; PKR, protein kinase receptor; RIG-I, retinoic acid inducible gene I; RIP1, receptor interacting protein 1 kinase; STAT, signal transducers and activators of transcription; TLR3, Toll-like receptor 3; TRIF, TIR-domain-containing adaptor-inducing interferon-β.
To explore the therapeutic application of dsRNA in ovarian cancer and identify biomarkers to target this treatment to a responsive patient population, we examined the effects of dsRNA treatment on a panel of ovarian cancer cell lines and ascites-derived ovarian cancer cells. We identified two treatment populations, dsRNA sensitive and dsRNA resistant. Using these two subpopulations, we determined gene expression patterns prior to stimulation, and the essential dsRNA receptors and signaling pathways to initiate the dsRNA-induced apoptotic response. We also examined the effect of combining dsRNA with cytotoxic agents on cell viability. Our results suggest that dsRNA is a viable treatment option for ovarian cancer cells with detectable dsRNA receptor expression and, when combined with cytotoxic agents, dsRNA can enhance the chemotherapeutic response.
MATERIALS AND METHODS
Reagents
2-Aminopurine (2-AP), pA:pU, pI:pC, and human IgG were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies for phospho-nuclear factor κB (NF-κB) p65 (Ser536), RIG-I, signal transducer and activator of transcription 1 (STAT1), phospho-STAT1 (Tyr701), caspase 3, cellular inhibitor of apoptosis 2 (c-IAP2), and β-tubulin were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies for NF-κB p65, PKR, and actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies for TLR3 and IFN-α/βR2 were purchased from R&D Systems (Minneapolis, MN, USA). The mda5 antibody was a gift from Dr. Paul Fisher (Virginia Commonwealth University). The keratin 18 antibody was purchased from NeoMarkers (Fremont, CA, USA). The RIP1 kinase antibody was purchased from BD Biosciences (Franklin Lakes, NJ, USA).
Cell culture
OVCAR-3, CAOV-3, SKOV-3, and HEK 293 cells were purchased from American Type Culture Collection (Manassas, VA, USA). DOV-13 cells were provided by X.F. Cells were cultured in complete medium (RPMI 1640 supplemented with 10% low-endotoxin FBS, 20 mM l-glutamine, 100 mM HEPES, 10 mM Na-pyruvate, and 1× nonessential amino acid solution) at 37°C in 5% CO2. Anonymized patient samples (Virginia Commonwealth University Tissue Data Acquisition and Analysis Core) were isolated from ascites fluid and cultured as described previously (16). Immortalized nontumorigenic ovarian surface epithelial (IOSE) cell lines, IOSE 385/6, were a gift from the Canadian Ovarian Cancer Research Culture Bank Core (Vancouver, BC, Canada). Cells were cultured in 50% MCDB 105 (Sigma)/50% M199 medium (Invitrogen, Carlsbad, CA, USA), pH 7.2, 5% low-endotoxin FBS, 2 mM l-glutamine, and 50 μg/ml gentamicin. Epithelial lineage was confirmed via immunoblot of whole-cell lysates for keratin 18 (data not shown). When stimulated with dsRNA, medium was prepared with diluted dsRNA at the indicated concentration, or dsRNA was complexed to jetPEI (Polyplus Transfection, New York, NY, USA) at indicated concentrations, according to the manufacturer's protocol.
Microarray analysis
RNA samples were analyzed on HG-U133A 2.0 arrays following the standardized Affymetrix protocol, as described previously (17). cDNA and cRNA synthesis products were prepared and rigorously evaluated (18). Overall quality was assessed; arrays exhibiting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) 3′/5′ < 3.0 and percentage of present genes > 40% were considered good quality arrays. Statistical analyses were performed using the log-scale robust multiarray analysis method (19). To identify differentially expressed gene probe sets between sensitive and resistant cells, a 2-sample t test was used for each probe set, and statistical significance for multiple comparisons was assessed by estimating the q values to identify probe set-specific false discovery rates (FDRs) using the Bioconductor q value package (20). Identification of altered gene expression in treated vs. untreated cells was assessed by using the significance score (S score) method (21). To account for multiple comparisons, we used the Benjamini-Hochberg (21) correction method and obtained adjusted α levels for each probe set. Data have been deposited into the Gene Expression Omnibuse (GEO) database (U.S. National Center for Biotechnology Information, Bethesda, MD, USA) under accession number GSE33342.
IFN treatment
Human-IFN-β was a gift from Dr. Andrew Larner (Virginia Commonwealth University). IFN-β (1000 U/ml) was added to cells for 0–60 min (immunoblot) or 24-72 h (qPCR, cell death assay). IFN-γ (R&D Systems; 5 ng/ml) was added to cells for 0–60 min.
Immunoblot analysis
Lysates were prepared in 0.02 M Tris, pH 8; 0.15 M NaCl; 1 mM DTT; 1% Nonidet P-40; 1× complete, EDTA-free (Roche Applied Science, Indianapolis, IN, USA), 25 mM NaF, 10× PhosSTOP (Roche) at specified time points following stimulation. Lysates were separated by SDS-PAGE (20–50 μg/lane, determined by Bio-Rad assay), transferred to nitrocellulose membrane, and immunoblotted with specified antibodies. Blots were developed with chemiluminescent reagents, Supersignal West Dura (Thermo Scientific, Rockford, IL, USA) or ECL Plus (GE Healthcare, Piscataway, NJ, USA).
Quantitation of apoptosis
Cells were monitored for apoptotic/necrotic cell death by Bisbenzimide Hoechst 33342 (10 μg/ml, Sigma) and propidium iodide (10 μg/ml, Sigma) staining. DNA was visualized with a Nikon TE300 Eclipse microscope (Nikon, Tokyo, Japan) equipped with an Hg lamp and blue excitation fluorescence filter (λex 330–380 nm, λem 420 nm, long pass). Cells exhibiting blue condensed or fragmented nuclei were considered apoptotic. Red nuclei without signs of condensation or fragmentation were considered necrotic. Counts were taken from 3 fields/well of >100 cells/field. Each experiment contained 3 wells/condition and was repeated 3×.
Quantitative RT-PCR
RNA was extracted from cells using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA, USA), 1 μg of RNA was reverse transcribed. Target and GAPDH mRNA levels were measured using TaqMan Gene Expression assay (Applied Biosystems) on an ABI-7900HT system. Target expression was normalized to GAPDH expression (fold induction) and reported relative to fold induction of a control cell line. Primers (Applied Biosystems): TLR3, Hs00152933_m1; mda5 (IFIH1), Hs00223420_m1; PKR (EIF2AK2), Hs00169345_m1; RIG-I (DDX58), Hs01061434_m1; IFNB1, Hs02621180_s1; GAPDH, Hs99999805_m1.
ELISA and solid-phase multiplex protein assay
Cells were treated with 50 μg/ml pI:pC for 24 h. Supernatants were harvested and precleared by centrifugation. IFN-β levels in the supernatants were determined via ELISA (R&D Systems). Levels of secreted IL-6, IL-8, MCP-1, and CXCL-10 were determined using a multiplex bead immunoassay kit (cytokine human 25-plex panel; Invitrogen). Data were analyzed using a 5-parameter logistic equation (GraphPad Prism; GraphPad, La Jolla, CA, USA).
IFNα/β receptor chain 2 inhibition
CAOV-3 and OVCAR-3 cells were treated with 5 μg/ml anti-IgG or anti-IFN-α/βR2 neutralizing antibody, followed 6 h later by stimulation with 50 μg/ml pI:pC for 24 h.
Caspase inhibition
Cells were treated with 25 μM pan-caspase inhibitor Z-VAD-FMK, caspase 9 inhibitor Z-LEHD-FMK, caspase 8 inhibitor Z-IETD-FMK, or caspase 4 inhibitor Z-YVAD-FMK (R&D Systems), followed 6 h later by 50 μg/ml pI:pC for 24 h.
Second mitochondria-derived activator of caspases (SMAC) mimetic
DOV-13 and SKOV-3 cells were treated with 100 nM SMAC mimetic (22) for 4 h and then treated with 50 μg/ml pI:pC for 48 h.
RIP1 kinase immunoprecipitation
Cell extracts were prepared in 20 mM HEPES, pH 7.4; 150 mM NaCl; 10 mM β-glycerophosphate; 1.5 mM MgCl2; 10 mM NaF; 2 mM dithiothreitol; 1 mM sodium orthovanadate; 2 mM EGTA; 1 mM PMSF; 0.5% Triton X-100; 1:500 protease inhibitor cocktail (Sigma); and 1 mg/ml of N-ethylmaleimide. Lysate (750 μg) was incubated with 1 μg of anti-receptor-interacting protein 1 kinase (RIP1K) antibody overnight at 4°C. Immunocomplexes were captured with protein A/G-plus agarose (Santa Cruz Biotechnology). After washing, bound proteins were released by boiling in SDS-PAGE sample buffer. Ubiquitination state of RIP1K was assessed by immunoblot with anti-RIP1K antibody.
dsRNA receptor overexpression
SKOV-3 cells were transfected with 5, 50, or 500 ng of DNA (pCDNA3.1 HA-mda5, pCDNA3.1 FLAG-RIG-I, or pUNO-TLR3) or 500 ng of empty vector DNA (pCDNA3.1). All transfections were performed using TransIT LT1 (Mirus Bio, Madison, WI, USA). At 24 h post-transfection, cells were treated with 50 μg/ml pI:pC for indicated times.
Lentiviral infections
Lentiviral particle gene silencers (Santa Cruz Biotechnology) targeting hTLR3 were added to CAOV-3 cells at 1:2 multiplicity of infection. Stable cell lines were selected with 4 μg/ml puromycin containing media.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
MTT assays (Invitrogen) were completed 48 h after stimulation. Experiments were performed in triplicate. Mean ± sd cell viability of treated cells relative to untreated control is reported. Drug combination experiments were designed according to Chou (23). ED50 values were determined from dose-response data for individual treatments using SigmaPlot software (Systat Software, Chicago, IL, USA). Isobologram analysis was completed with Calcusyn software (Biosoft, Cambridge, UK).
Data analysis
One-way ANOVA or Student's t test was used for statistical analyses (JMP9; SAS, Cary, NC, USA). Values of P ≤ 0.05 were considered significant.
RESULTS
dsRNA stimulation induces ovarian cancer cell death
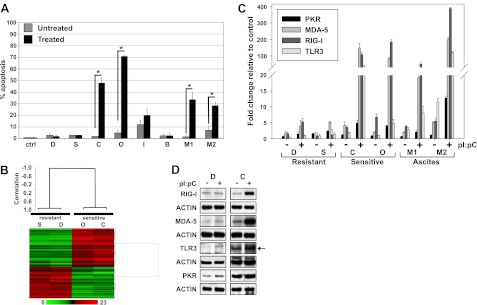
CAOV-3 and OVCAR-3 cells underwent significant levels of apoptosis (P ≤0.05) in response to dsRNA treatment and were classified as sensitive (Fig. 2A). DOV-13 and SKOV-3 cells showed no increased cell death following dsRNA treatment, classified as resistant (Fig. 2A). This pattern of response was not altered when polyethyleneimine (PEI) was used to deliver dsRNA to cells or apoptosis was monitored using annexin V/PI staining (data not shown). The IOSE-385 (normal control) cell line showed a moderate, but not significant, response to dsRNA, suggesting only a low-level response in normal OSE cells (Fig. 2A). We also assessed the effect of dsRNA stimulation on anonymized patient ovarian epithelial cells; two malignant samples (malignant 1, high-grade ovarian serous papillary carcinoma, stage IIIc; malignant 2, ovarian serous papillary carcinoma, stage IIIc) and one benign sample (cyst, not further classifiable). The two malignant samples underwent significant levels of dsRNA-induced apoptosis, whereas the benign sample did not respond (Fig. 2A). These data show that normal or benign ovarian epithelial cells exhibit a minimal response to dsRNA stimulation but that ovarian cancer cells divide into 2 populations, dsRNA sensitive and dsRNA resistant.
Figure 2.
Microarray analysis and dsRNA receptor expression levels identified 2 ovarian cancer cell subpopulations: dsRNA sensitive and dsRNA resistant. A) Ovarian cancer cell lines DOV-13 (D), SKOV-3 (S), CAOV-3 (C), OVCAR-3 (O), IOSE (I) and ascites-derived epithelial cell–benign cyst (B), malignant 1 (M1), and malignant 2 (M2), were treated with 50 μg/ml pI:pC for 48 h. HEK293 (ctrl) cells served as a negative control. Apoptosis was assessed via Hoechst/propidium iodide staining. Data are means ± sd for 3 independent experiments. *P ≤ 0.05. B) Two-dimensional hierarchical supervised clustering of samples and genes using Pearson (centered) correlation and average linkage, based on 75 probe sets that were significantly (P<0.001; FDR<5%) different between resistant and sensitive cells. Each heat map row shows the relative expression for that specific gene in 4 separate cell lines (columns). Relative gene expression levels (0- to 23-fold increase) are plotted as red (overexpressed) and green (underexpressed) areas compared to the median intensity of the samples. C) dsRNA receptor and GAPDH mRNA expression levels were determined by TaqMan qPCR from samples treated with 50 μg/ml pI:pC for 24 h. Receptor expression is reported as fold induction normalized to GAPDH, relative to the normalized receptor expression in HEK293. Data are means ± sd for 3 independent experiments. D) Cells were treated with 50 μg/ml pI:pC for 24 h, and cell lysates were collected. Receptor expression levels were determined by Western blotting. Arrow indicates TLR3 receptor band. Actin was used as a loading control. One representative experiment is shown.
To further characterize the sensitive vs. resistant phenotypes, mRNA expression was examined via microarray analysis. A 2-sample t test identified 75 significantly altered gene probe sets (FDR<5%) between unstimulated sensitive and resistant cell lines (Table S1). A supervised cluster analysis based on this 75-gene probe set showed a clear distinction between the sensitive and resistant subpopulations (Fig. 2B). Ingenuity pathway analysis showed that these genes were involved in development, tissue morphology, cell death and inflammatory disease/response networks. Interestingly, epithelial to mesenchymal transition genes like vimentin (144-fold change) and cancer progression genes such as dihydropyrimidinase-like 3 (24-fold) were more highly expressed in resistant vs. sensitive cells (Table S1). Analysis of unstimulated IOSE cells, which do not show significant dsRNA-induced cell death, showed an mRNA expression pattern for these genes similar to resistant cells (Supplemental Table S1). These data show that ovarian cancer cells can be divided into 2 categories, identifiable by their gene expression pattern prior to dsRNA stimulation, that undergo apoptosis (sensitive) or no altered cell survival (resistant) on dsRNA treatment.
Induced dsRNA receptor expression levels correlate to responsiveness
To determine whether dsRNA receptor expression pattern could discern sensitive vs. resistant phenotype, we examined the effect of dsRNA stimulation on dsRNA receptor mRNA expression levels via qPCR (Fig. 2C). DsRNA receptor mRNA levels in sensitive cells, including patient-derived malignant ovarian cancer cells, increased following pI:pC stimulation. RIG-I like receptors, RIG-I and mda5, were most highly up-regulated, >25-fold in CAOV-3 and OVCAR-3 cells and >10-fold in malignant samples. Resistant cells showed a minimal (2- to 4-fold) increase in only RIG-I-like receptor mRNA expression. Western blot analysis confirmed that receptor mRNA levels corresponded to receptor expression (Fig. 2D; SKOV-3 and OVCAR-3 data not shown). These data show that dsRNA receptor expression increases in sensitive cells and may serve as a predictive marker for dsRNA sensitivity.
dsRNA receptor contribution to cell death response
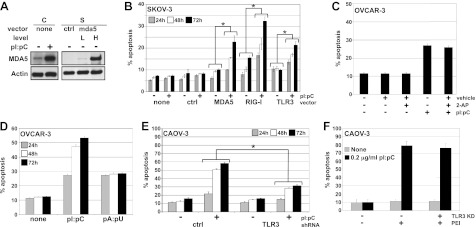
To examine dsRNA receptor expression level as a key determinant in dsRNA responsive status, dsRNA receptors were overexpressed in a resistant cell line, SKOV-3. Optimal receptor DNA concentration to allow a dsRNA-induced response, but limiting cell death by unstimulated overexpression of dsRNA receptors, was determined by titration (data not shown). Overexpression of the dsRNA receptors was similar to basal or dsRNA-stimulated expression levels observed in sensitive cell lines when transfected at low (50 ng) or high (500 ng) plasmid DNA concentration, respectively (Fig. 3A). When the dsRNA receptors were overexpressed in SKOV-3 cells and stimulated with pI:pC for 24, 48, and 72 h, cell death increased significantly (Fig. 3B), indicating that up-regulation of dsRNA receptor expression is required for dsRNA-responsive status.
Figure 3.
TLR3 and RLRs coordinate dsRNA-induced apoptosis. A) SKOV-3 (S) cells were transfected with 50 ng (L) or 500 ng (H) mda5 DNA or empty vector (ctrl), and receptor expression was compared via Western blot to CAOV-3 (C) ± 50 μg/ml dsRNA for 24 h. Actin was used as a loading control. One representative experiment is shown. B) SKOV-3 cells were transfected with 50 ng of receptor DNA or empty vector (control) and treated with 50 μg/ml pI:pC for 24, 48, and 72 h post-transfection. Hoechst and propidium iodide staining were used to measure apoptosis. C) OVCAR-3 cells were treated with vehicle, 5 μg/ml pI:pC, 5 mM 2-AP, or both for 24 h. Hoechst and propidium iodide staining were used to assess apoptosis. D) OVCAR-3 cells were untreated or treated with 5 μg/ml pI:pC or pA:pU, for 24, 48, or 72 h. Hoechst and propidium iodide staining were used to assess apoptosis. E) Stable CAOV-3 TLR3-KD cells were stimulated with 50 μg/ml pI:pC. Cells were stained with Hoechst and propidium iodide to measure apoptosis at 24, 48, and 72 h time points. F) WT or stable CAOV-3 TLR3-KD cells were stimulated with 0.2 μg/ml pI:pC ± PEI. Cell death was assessed after 24, 48, and 72 h with Hoechst and propidium iodide staining. Data are means ± sd for 2 (B) or 3 (C–F) independent experiments. *P ≤ 0.05.
Using 2-AP to inhibit PKR, selective TLR3 agonist, pA:pU (24), and stable shRNA knockdown of TLR3, we examined the role of dsRNA receptors in sensitive cells. OVCAR-3 cells were treated with 2-AP to block PKR activation, as measured by eukaryotic translation initiation factor 2α (eIF2α) phosphorylation (Supplemental Fig. S1A) and then treated with pI:pC (Fig. 3C). DsRNA-induced cell death was unaffected by 2-AP treatment, suggesting that PKR is not required for the early dsRNA-induced cell death response. OVCAR-3 cells were treated with pI:pC or TLR3-selective agonist, pA:pU (Fig. 3D). PI:pC-induced cell death increased to ∼50% at 72 h, whereas pA:pU-induced apoptosis matched pI:pC apoptotic level at 24 h but was unchanged at 48 and 72 h. These data are consistent with dsRNA-induced apoptosis initiated by TLR3 signaling that is subsequently augmented by the RIG-I like receptor signaling. To further investigate this finding, a stable TLR3 shRNA CAOV-3 cell line was generated with an 85% knockdown of TLR3 mRNA and undetectable protein expression (Supplemental Fig. S1B, C). When TLR3-knockdown (TLR3-KD) CAOV-3 cells were treated with pI:pC, dsRNA-induced cell death was significantly reduced compared to wild-type (WT) CAOV-3 or CAOV-3 cells stably expressing control shRNA (Fig. 3E). When TLR3-KD CAOV-3 cells were stimulated with pI:pC complexed to PEI to more efficiently access the RIG-I-like receptors (RLRs; ref. 25), WT CAOV-3, and TLR3-KD CAOV-3 cells showed no significant difference in cell death following stimulation (Fig. 3F). These data indicate that TLR3 initiates the apoptotic response when pI:pC is delivered directly to the cell, but RLRs are sufficient when pI:pC can efficiently access the cytosol. We next examined the signaling pathways activated by these dsRNA receptors and their role in mediating dsRNA-induced apoptosis in the sensitive cells.
Sensitive and resistant cells activate NF-κB
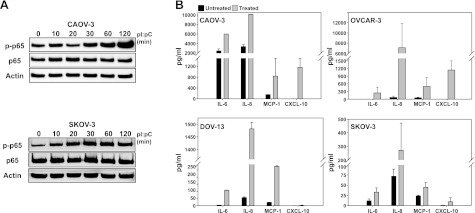
The dsRNA receptor signaling pathways activate NF-κB, which can be monitored by p65 phosphorylation (ref. 26 and Fig. 1). Surprisingly, sensitive CAOV-3 and resistant SKOV-3 cells increased phosphorylated p65 following dsRNA stimulation (Fig. 4A; similar results for OVCAR-3 and DOV-13 cells are not shown). These data indicate that, in the resistant cell lines, the low level of basal dsRNA receptor expression was sufficient to activate downstream signaling pathways. Solid-phase multiplex protein assay of dsRNA treated supernatants for cytokine and chemokine expression detected increased levels of IL-6, IL-8, and MCP-1 (CCL2) in all cell lines, consistent with NF-κB activation (Fig. 4B). However, only sensitive cell lines, CAOV-3 and OVCAR-3, showed significantly increased expression of the NF-κB- and STAT1-regulated chemokine CXCL-10, suggesting that resistant cell lines may have altered STAT1 activation (Fig. 4B).
Figure 4.
dsRNA-sensitive and -resistant cells activate NF-κB. A) Cells were treated with 50 μg/ml pI:pC for indicated times, and cell lysates were collected. Levels of p65 and p-p65 (phospho-S536, p65 subunit) were determined by Western blotting. Actin was used as a loading control. One representative experiment is shown. B) Cells were treated with 50 μg/ml pI:pC for 24 h. Supernatants were assayed for cytokines and chemokine via solid phase multiplex protein assay, as described in Materials and Methods. Data are means ± sd for 2 independent experiments.
Type I IFN response contributes to dsRNA-induced cell death
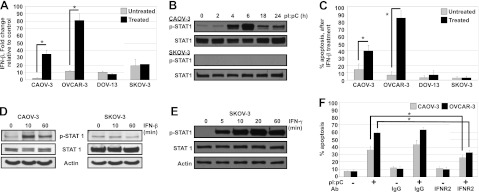
DsRNA receptors activate the transcription factor, IFN regulatory factor 3, leading to the production of IFNβ (27) and subsequent activation of the type I IFN-α/β receptor (IFNAR1/2) pathway (Fig. 1). Microarray data after dsRNA stimulation at 24 h showed that sensitive cell lines have increased levels of several IFN-stimulated gene (ISG) mRNAs, which were confirmed by qRT-PCR (Supplemental Fig. S1D, E and Supplemental Table S2). Although resistant cell lines up-regulated ISG mRNAs after dsRNA treatment, the fold change was blunted in comparison to sensitive ISG mRNA expression (∼10- vs. >1000-fold; Supplemental Fig S1E). To determine whether IFNβ expression levels differed between sensitive and resistant cells, we examined IFNβ mRNA levels (Fig. 5A). IFNβ mRNA production increased only in sensitive cells following dsRNA stimulation. IFNβ binds in an autocrine/paracrine manner to IFNAR1/2, leading to STAT1 activation via phosphorylation (Fig. 1). Sensitive cells phosphorylated STAT1 following dsRNA stimulation but resistant cells did not (Fig. 5B; similar data for OVCAR-3 and DOV-13 cells not shown). As DOV-13 and SKOV-3 cells did not increase IFNβ expression with dsRNA treatment, we stimulated these cell lines with exogenous IFNβ (Fig. 5C, D). Cell survival and STAT1 phosphorylation were unaltered in resistant cells by IFNβ, whereas sensitive cells showed increased apoptosis and increased STAT1 phosphorylation (Fig. 5C, D). To determine whether resistant cells were IFNβ insensitive at the level of type I IFN receptor or downstream signaling components, we treated SKOV-3 cells with exogenous IFNγ, which binds to the IFNγ receptor but also leads to STAT1 phosphorylation. Under these conditions, STAT1 phosphorylation was induced (Fig. 5E). Together, these data indicate that resistant cells have blocked the interferon response at the level of IFNβ production and the IFNAR1/2 receptor. We next examined the contribution of the IFN response to dsRNA-induced apoptosis in sensitive cells. When the IFN response was blocked by an IFNAR2-neutralizing antibody prior to stimulation, dsRNA-induced apoptosis was significantly reduced (Fig. 5F), indicating that sensitive cells utilize type I IFN-mediated apoptosis.
Figure 5.
Activation of the type-I IFN response contributes to dsRNA-induced cell death. A) Total RNA was extracted from cells treated with 50 μg/ml pI:pC for 24 h. IFNβ and GAPDH expression was determined by TaqMan qPCR. Mean ± sd expression is reported as fold induction normalized to GAPDH, relative to the normalized expression in IOSE 385 for 2 independent experiments. *P ≤ 0.05. B) Cells were treated with 50 μg/ml pI:pC for 0–24 h. Levels of phosphorylated (Y701) and total STAT1 were analyzed by Western blot. Actin was used as a loading control. One representative experiment is shown. C) Cells were treated with 1000 U/ml IFN-β for 48 h. Apoptosis was assessed by Hoechst and propidium iodide staining. D) Cells were treated with 1000 U/ml IFN-β for indicated times, and cell lysates were collected. Levels of phosphorylated (Y701) and total STAT1 were determined by Western blot. Actin was used as a loading control. One representative experiment is shown. E) Cells were untreated or treated with IFN-γ for indicated times, and cell lysates were collected. Levels of phosphorylated (Y701) and total STAT1 were determined by Western blot. Actin was used as a loading control. One representative experiment is shown. F) CAOV-3 and OVCAR-3 cells were treated with 5 μg/ml hIgG- or hIFNR2-neutralizing antibody, followed 6 h later by stimulation with 50 μg/ml pI:pC for 24 h. Apoptosis was assessed by Hoechst and propidium iodide staining. Data are means ± sd of 2 independent experiments. *P ≤ 0.05; 1-way ANOVA.
Caspase activation is necessary for dsRNA-induced cell death
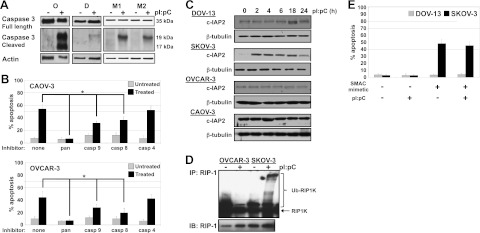
DsRNA receptor signaling pathways activate caspase 8 and 3 through the TIR-domain-containing adapter-inducing interferon-β (TRIF)–RIP1K–FAS-associated death domain (FADD) signaling axis (Fig. 1). After 24 h of dsRNA stimulation, cleaved caspase 3 (Fig. 6A), as well as PARP cleavage (data not shown), was detected in OVCAR-3 and malignant ovarian epithelial cells. Pretreatment with a pan-caspase inhibitor completely abolished the dsRNA-induced apoptotic response (Fig. 6B). Pretreatment with caspase 9- or 8-specific inhibitors decreased dsRNA-induced apoptosis (Fig. 6B), suggesting extrinsic and intrinsic caspase pathway activation. Inhibition of caspase 4, known to cleave caspase 3 in response to ER stress (28), had no effect on dsRNA-induced apoptosis. Interestingly, unlike the NF-κB and IFN response where low-level activation could be detected for resistant cell lines (Fig. 4 and Supplemental S1E), the caspase pathway is completely repressed (Fig. 6A). When TLR3 was overexpressed in SKOV-3 cells, dsRNA-induced caspase 3 cleavage was restored (Supplemental Fig. S2A), suggesting that caspase activation pathways are functional. To further investigate repression of caspase activation in resistant cell lines, we examined c-IAP2 expression. c-IAP2, an E3 ubiquitin ligase, acts on RIP1K in the dsRNA receptor signaling pathway (Fig. 1). When ubiquitinated, RIP1K favors NF-κB activation, whereas unmodified RIP1K activation leads to caspase 8 activation (29). Only resistant cell lines increased c-IAP2 levels, with SKOV-3 increasing c-IAP2 at 2 h poststimulation (Fig. 6C), similar to previous reports (29, 30). Consistent with increased c-IAP2 expression, greater RIP1K ubiquitination was observed for SKOV-3 vs. OVCAR-3 cells (Fig. 6D). When DOV-13 and SKOV-3 cells were treated with a SMAC mimetic to induce c-IAP2 proteasomal degradation (31), cell death increased in SKOV-3 cells, but pI:pC stimulation did not further augment cell death (Fig. 6E). DOV-13 cells were unaffected (Fig. 6E). Together, these data show that caspase activation is required for dsRNA-induced apoptosis. In resistant cells, lack of caspase activation may result from both reduced receptor expression levels, as dsRNA receptor overexpression restores caspase activation, and increased c-IAP2 expression that suppressed caspase 8 activation.
Figure 6.
Caspase activation is required for dsRNA-induced cell death. A) OVCAR-3 (O), DOV-13 (D), and ascites-derived ovarian cancer cells (M1, M2) were treated with 50 μg/ml pI:pC for 24 h, and whole-cell lysates were collected. Expression of full-length (35-kDa) and cleaved (19- and 17-kDa) caspase 3 was determined by Western blot. Actin was used as a loading control. One representative experiment is shown. B) CAOV-3 (top) and OVCAR-3 (bottom) cells were treated with 25 μM of pan-caspase, caspase 9-, caspase 8-, or caspase 4-specific inhibitor. After 6 h, cells were treated with 50 μg/ml pI:pC for 48 h. Apoptosis was assessed by Hoechst and propidium iodide staining. Data are means ± sd for 3 independent experiments. *P ≤ 0.05. C) Ovarian cancer cell lines were treated with 50 μg/ml pI:pC for indicated times, and cell lysates were collected. Expression of c-IAP2 was determined by Western blot. β-Tubulin was used as a loading control. D) Ovarian cancer cell lines were treated with 50 μg/ml pI:pC for 10 min, and whole-cell lysates were collected. RIP1K was immunoprecipitated, and RIP1K ubiquitination level was determined via Western blot analysis. One representative experiment is shown. E) DOV-13 and SKOV-3 cells were treated with 100 nM SMAC mimetic for 4 h, followed by treatment with 50 μg/ml pI:pC for 48 h. Apoptosis was assessed by Hoechst and propidium iodide staining. Data are means ± sd for 2 independent experiments.
dsRNA acts synergistically with cytotoxic agents in dsRNA-sensitive cell line
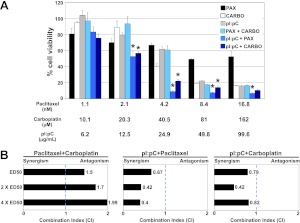
As a single agent, dsRNA induced 30–70% apoptosis in sensitive cell lines, including the malignant ovarian epithelial cells. We next examined the effect of combining dsRNA with standard chemotherapeutic agents, carboplatin and paclitaxel, on sensitive cell lines. We established the effective dose for 50% growth inhibition (ED50) for each of the drugs by titration using an MTT assay. The ED50 values for OVCAR-3 cells were 24.9 ± 8.4 μg/ml for pI:pC, 109.1 ± 7 μM for carboplatin, and 4.9 ± 0.3 nM for paclitaxel (Supplemental Fig. S2B–D). Using the method proposed by Chou (23) to determine drug interactions, OVCAR-3 cells were treated at equipotent ratios equivalent to 0.25-, 0.5-, 1-, 2-, and 4-fold the concentration to inhibit growth 50% for individual drugs. Significantly greater growth inhibition was observed when either cytotoxic agent was combined with pI:pC at concentrations > 50% growth inhibition for individual drugs (Fig. 7A). To determine the type of drug interaction between dsRNA and the cytotoxic agents, isobologram analysis was performed to derive a combination index (CI) value. A value of CI < 1, 1, or > 1 indicates a synergistic, additive or antagonistic effect, respectively. The CI values at drug dosages equivalent to ED50, 2× ED50, and 4× ED50 were calculated (Fig. 7B). At these concentrations, dsRNA acted synergistically with both paclitaxel and carboplatin (Fig. 7B).
Figure 7.
dsRNA potentiates paclitaxel- and carboplatin-mediated cytotoxicity. A) OVCAR-3 cells were treated for 48 h with paclitaxel, carboplatin, and pI:pC at the indicated concentrations, which correspond to 0.25×, 0.5×, 1×, 2×, and 4× the ED50 concentration (left to right). Cell viability was measured via MTT assay. Values are reported relative to untreated control. Data are means ± sd of 3 independent experiments. *P ≤ 0.05 for dual treatment vs. both individual treatments. B) Combination index (CI) values for each drug combination were calculated via isobologram analysis. Dotted line represents additivity, where CI = 1.
DISCUSSION
A major challenge in the treatment of ovarian cancer is overcoming chemoresistance in primary tumors, representing ∼35% of the initial patient population, and arising in recurring disease. DsRNA has been shown to induce cell death in multiple cancer cell types, including breast, prostate, melanoma, and head and neck cancers (11–14, 32). In ovarian cancer, immunogenic, dsRNA-induced tumor cell death has been reported (33, 34). Application of dsRNA in a patient population depends on the ability to target this novel chemotherapeutic to a highly responsive subset of patients. We sought to define the dsRNA receptors and pathways essential for the dsRNA-induced apoptotic response to identify markers for targeting this treatment to a responsive patient population.
Our representative ovarian cancer cell lines, CAOV-3, OVCAR-3, DOV-13, and SKOV-3, were either p53 mutant or p53 null (35), similar to the p53 status seen in >90% of ovarian tumors (36, 37). We also examined patient-derived benign and malignant OSE cells. Within this group, we have identified 2 ovarian cancer cell populations that differ in dsRNA response. DOV-13 and SKOV-3 (resistant cells) do not undergo dsRNA-induced apoptosis and have low dsRNA receptor expression after dsRNA stimulation. CAOV-3, OVCAR-3 and patient-derived malignant ascites cells (sensitive cells) are dsRNA responsive, undergoing significant apoptosis after treatment and showing a marked increase in dsRNA receptor expression levels, especially the RLRs. Unlike a breast cancer retrospective study that showed increased dsRNA receptor expression in dsRNA-responsive tumors prior to treatment (38), our data indicate that prior to treatment dsRNA receptor mRNA and protein expression are equivalent in the two populations. These data suggest that dsRNA expression patterns following dsRNA stimulation may be a predictive factor for dsRNA treatment.
To identify prognostic factors for dsRNA treatment, we examined resistant and sensitive cell mRNA prior to stimulation using microarray analysis. We derived a supervised cluster of 75 gene probes that show differential mRNA expression between these two subpopulations. As IOSE and resistant cells do not undergo dsRNA-induced apoptosis, the unstimulated IOSE mRNA expression pattern for these 75 genes resembled the resistant phenotype. Looking at genes most divergently expressed between the sensitive and resistant groups, we found that dsRNA-sensitive ovarian cancer cells retained higher levels of the epithelial marker, E-cadherin (CDH1), and known ovarian cancer prognostic markers, ERBB3 (EGFR member), RAB25 (RAS-related protein 25), TACSTD2 (tumor-associated calcium signal transducer 2) and ASS1 (arginosuccinate synthase 1), whereas dsRNA-resistant samples increased mRNA expression of genes associated with the epithelial to mesenchymal transition, vimentin, and ZEB1, and cancer cell progression or invasion, dihydropyrimidinase 3, FOSL1, and cavin-1. These genes represent potential prognostic biomarkers for dsRNA-sensitive patient population identification.
In this study, we examined how 4 dsRNA receptors, PKR, TLR3, RIG-I, and mda5, contributed to dsRNA-induced ovarian cancer cell death. In previous studies, Kübler et al. (33, 34) examined lipid-based delivery of dsRNA and reported RIG-I or mda5-dependent responses. Unlike these studies, we observed efficient dsRNA-induced cell death in the absence of an oligonucleotide carrier such as PEI or lipid-based carrier. Our studies showed that inhibition of PKR via 2-AP did not affect dsRNA-induced cell death. Selective TLR3 agonist pA:pU induced apoptosis, but, unlike pI:pC treatment, pA:pU-induced cell death did not further increase at 48 and 72 h. We suggest that pA:pU-activated TLR3 increased expression of the IFN-inducible RLR genes (6, 7) but that the RLRs, in the absence of a suitable ligand, were unable to augment the apoptotic signal. In stable TLR3-KD cells, pI:pC-induced apoptosis was delayed and significantly decreased, which may reflect an inefficient release of pI:pC from TLR3-KD endosomes to the cytosolic RLRs. We could restore dsRNA-induced apoptosis by treating TLR3-KD cells with pI:pC complexed to PEI, which enhanced endosomal release (25). Together, these results show that TLR3 and RLRs coordinate dsRNA-induced apoptosis but that PEI-complexed pI:pC required only the RLR-mediated response.
The dsRNA-sensitive cell lines CAOV-3 and OVCAR-3 underwent caspase- and IFN-dependent dsRNA-induced apoptosis, similar to previous effects observed in breast cancer cells (13). However, transient TLR3 expression in resistant SKOV-3 cells, which are defective in IFNβ production and IFNAR1/2 signaling, restored dsRNA-induced caspase 3 activation. These results suggest that, in the absence of a functional IFN response, direct activation of caspase 8 via the dsRNA receptor activated-RIP1 kinase/FADD signaling axis is sufficient for dsRNA-induced apoptosis.
In the dsRNA-resistant cell lines DOV-13 and SKOV-3, the two main determinants for the nonresponsiveness phenotype were loss of dsRNA-induced increase in dsRNA receptor expression and disrupted type I IFN production and sensing. For SKOV-3 and DOV-13 cells, we did observe NF-κB activation and small increases in mRNA for IFN-stimulated genes following dsRNA treatment, but caspase activation was not detectable. Complete repression of dsRNA receptor-mediated caspase activation may have been a result of the increased c-IAP2 expression, as observed in SKOV-3 cells, to shunt RIP1 kinase to NF-κB activation vs. FADD/caspase 8 activation. When dsRNA receptor expression was restored in SKOV-3 to levels similar to unstimulated sensitive cell lines, cleaved caspase 3 was detected and these cells underwent dsRNA-induced apoptosis, suggesting dsRNA-induced apoptotic signaling pathways remained functional.
To improve the potency of dsRNA-induced apoptosis, we tested dsRNA in combination with the cytotoxic agents, carboplatin and paclitaxel, routinely employed to treat ovarian cancer. The goal was to assess whether dsRNA combinations could increase drug sensitivity that would benefit patient populations with limited response to the current cytotoxic agent regimen. We assessed all dual combinations of carboplatin, paclitaxel, and dsRNA in the sensitive cell line, OVCAR-3. OVCAR-3 is tumorigenic, allowing for future animal model studies on effective drug combinations. When pI:pC was combined with either cytotoxic agent, cell viability was significantly decreased compared to individual drug treatment at concentrations >0.25 ED50 value. Isobologram analysis for the combination carboplatin and paclitaxel indicated an additive interaction, consistent with published reports (39). In contrast, dsRNA:paclitaxel and dsRNA:carboplatin combinations showed synergistic drug interactions. Additional studies are necessary to identify the mechanism by which the synergy between dsRNA and carboplatin/paclitaxel's cytotoxic effects occurs.
In summary, this work shows that dsRNA acts directly to induce caspase- and IFN-dependent cell death. We have identified dsRNA receptor expression as a potential predictive marker for this targeted treatment and have identified a panel of prognostic gene markers. Further comparison to patient-derived samples will be necessary to validate and refine these markers. Moreover, the combination of dsRNA with cytotoxic agents potentiates the action of carboplatin and paclitaxel. These results support further studies to examine dsRNA as part of a combination therapy that would assess dsRNA's chemotherapeutic efficacy.
Supplementary Material
Acknowledgments
The authors thank Drs. Charles Chalfant, Paul Fisher, Andrew Larner [Virginia Commonwealth University (VCU), Richmond, VA, USA] and the Canadian Ovarian Cancer Research Culture Bank for generously sharing reagents. The authors also thank Drs. Paul Dent and Tomasz Kordula for helpful discussions during the development of these experiments.
This work was funded by U.S. National Institutes of Health (NIH) grants CA144083 (D.N.V.), CA102196 (X.F.), CA61774 (S.S.), GM043880 (S.S.), and CA12282801 (J.K.B) and by a V Scholar award (Jimmy V Foundation; J.K.B). Microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility with funding from NIH–National Institute of Neuological Disease and Stroke Center core grant 5P30NS047463-02. VCU Massey Cancer Center Tissue and Data Acquisition and Analysis Core services were supported with funding from NIH–National Cancer Institute Cancer Center support grant P30 CA016059.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 2-AP
- 2-aminopurine
- CI
- combination index
- c-IAP
- cellular inhibitor of apoptosis
- eIF2α
- eukaryotic translation initiation factor 2α
- FADD
- FAS-associated death domain
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- IFN
- interferon
- IFNAR
- interferon-α/β receptor
- IOSE
- immortalized nontumorigenic ovarian surface epithelial
- KD
- knockdown
- mda5
- melanoma differentiation-associated gene 5
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NF-κB
- nuclear factor κB
- OSE
- ovarian surface epithelial
- RIG-I
- retinoic acid-inducible gene I
- RIP1K
- receptor-interacting protein 1 kinase
- RLR
- RIG-I like receptor
- pA:pU
- polyriboadenylic:polyribouridylic acid
- PEI
- polyethyleneimine
- pI:pC
- polyriboinosinic:polyribocytidylic acid
- PKR
- protein kinase receptor
- SMAC
- second mitochondria-derived activator of caspases
- STAT
- signal transducer and activator of transcription
- TLR3
- Toll-like receptor 3
- TRIF
- TIR-domain-containing adapter-inducing interferon-β
- WT
- wild type
REFERENCES
- 1. Jemal A., Siegel R., Xu J., Ward E. (2010) Cancer Statistics, 2010. CA Cancer J. Clin. 60, 277–300 [DOI] [PubMed] [Google Scholar]
- 2. Howlader N., Noone A. M., Krapcho M., Neyman N., Aminou R., Waldron W., Altekruse S. F., Kosary C. L., Ruhl J., Tatalovich Z., Cho H., Mariotto A., Eisner M. P., Lewis D. R., Chen H. S., Feuer E. J., Cronin K. A., Edwards B. K. (2011) SEER Cancer Statistics Review, 1975–2008. Vol. 2011, National Cancer Institute, Bethesda, MD, USA [Google Scholar]
- 3. Pignata S., Cannella L., Leopardo D., Pisano C., Bruni G. S., Facchini G. (2011) Chemotherapy in epithelial ovarian cancer. Cancer Lett. 303, 73–83 [DOI] [PubMed] [Google Scholar]
- 4. Yap T. A., Carden C. P., Kaye S. B. (2009) Beyond chemotherapy: targeted therapies in ovarian cancer. Nat. Rev. Cancer 9, 167–181 [DOI] [PubMed] [Google Scholar]
- 5. Alexopoulou L., Holt A. C., Medzhitov R., Flavell R. A. (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413, 732–738 [DOI] [PubMed] [Google Scholar]
- 6. Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., Taira K., Akira S., Fujita T. (2004) The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 [DOI] [PubMed] [Google Scholar]
- 7. Kang D.-C., Gopalkrishnan R. V., Wu Q., Jankowsky E., Pyle A. M., Fisher P. B. (2002) mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc. Natl. Acad. Sci. U. S. A. 99, 637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levin D., London I. M. (1978) Regulation of protein synthesis: activation by double stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc. Natl. Acad. Sci. U. S. A. 75, 1121–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lacour J., Spira A., Petit J.-Y., Sarrazin D., Lacour F., Michelson M., Delage G., Contesso G., Viguier J. (1980) Adjuvant treatment with polyadenylic-polyuridylic acid (PolyA. PolyU) in operable breast cancer. Lancet 316, 161–164 [DOI] [PubMed] [Google Scholar]
- 10. Jeung H.-C., Moon Y. W., Rha S. Y., Yoo N. C., Roh J. K., Noh S. H., Min J. S., Kim B. S., Chung H. C. (2008) Phase III trial of adjuvant 5-fluorouracil and adriamycin versus 5-fluorouracil, adriamycin, and polyadenylic: polyuridylic acid (poly A:U) for locally advanced gastric cancer after curative surgery: final results of 15-year follow-up. Ann. Oncol. 19, 520–526 [DOI] [PubMed] [Google Scholar]
- 11. Tormo D., Checinska A., Alonso-Curbelo D., Pérez-Guijarro E., Cañón E., Riveiro-Falkenbach E., Calvo T. G., Larribere L., Megías D., Mulero F., Piris M. A., Dash R., Barral P. M., Rodríguez-Peralto J. L., Ortiz-Romero P., Tüting T., Fisher P. B., Soengas M. S. (2009) Targeted activation of innate immunity for therapeutic induction of autophagy and apoptosis in melanoma cells. Cancer Cell 16, 103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Besch R., Poeck H., Hohenauer T., Senft D., Häcker G., Berking C., Hornung V., Endres S., Ruzicka T., Rothenfusser S., Hartmann G. (2009) Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells. J. Clin. Invest. 119, 2399–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salaun B., Coste I., Rissoan M.-C., Lebecque S. J., Renno T. (2006) TLR3 Can directly trigger apoptosis in human cancer cells. J. Immunol. 176, 4894–4901 [DOI] [PubMed] [Google Scholar]
- 14. Paone A., Starace D., Galli R., Padula F., De Cesaris P., Filippini A., Ziparo E., Riccioli A. (2008) Toll-like receptor 3 triggers apoptosis of human prostate cancer cells through a PKC-alpha-dependent mechanism. Carcinogenesis 29, 1334–1342 [DOI] [PubMed] [Google Scholar]
- 15. Peng S., Geng J., Sun R., Tian Z., Wei H. (2009) Polyinosinic-polycytidylic acid liposome induces human hepatoma cells apoptosis which correlates to the up-regulation of RIG-I like receptors. Cancer Sci. 100, 529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shepherd T. G., Theriault B. L., Campbell E. J., Nachtigal M. W. (2007) Primary culture of ovarian surface epithelial cells and ascites-derived ovarian cancer cells from patients. Nat. Protocols 1, 2643–2649 [DOI] [PubMed] [Google Scholar]
- 17. Dumur C. I., Sana S., Ladd A. C., Ferrerira-Gonzalez A., Wilkinson D. S., Powers C. N., Garrett C. T. (2008) Assessing the impact of tissue devitalization time on genome-wide gene expression analysis in ovarian tumor samples. Diagn. Mol. Pathol. 17, 200–206 [DOI] [PubMed] [Google Scholar]
- 18. Dumur C. I., Nasim S., Best A. M., Archer K. J., Ladd A. C., Mas V. R., Wilkinson D. S., Garrett C. T., Ferreira-Gonzalez A. (2004) Evaluation of quality-control criteria for microarray gene expression analysis. Clin. Chem. 50, 1994–2002 [DOI] [PubMed] [Google Scholar]
- 19. Irizarry R. A., Bolstad B. M., Collin F., Cope L. M., Hobbs B., Speed T. P. (2003) Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31, e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Storey J. (2002) A direct approach to false discovery rates. J. R. Statist. Soc. Ser. B (Methodological) 64, 479–498 [Google Scholar]
- 21. Zhang L., Wang L., Ravindranathan A., Miles M. F. (2002) A new algorithm for analysis of oligonucleotide arrays: application to expression profiling in mouse brain regions. J. Mol. Biol. 317, 225–235 [DOI] [PubMed] [Google Scholar]
- 22. Li L., Thomas R. M., Suzuki H., De Brabander J. K., Wang X., Harran P. G. (2004) A small molecule smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science 305, 1471–1474 [DOI] [PubMed] [Google Scholar]
- 23. Chou T.-C. (2006) Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Phamacol. Rev. 58, 621–681 [DOI] [PubMed] [Google Scholar]
- 24. Perrot I., Deauvieau F., Massacrier C., Hughes N., Garrone P., Durand I., Demaria O., Viaud N., Gauthier L., Blery M., Bonnefoy-Berard N., Morel Y., Tschopp J., Alexopoulou L., Trinchieri G., Paturel C., Caux C. (2010) TLR3 and rig-like receptor on myeloid dendritic cells and rig-like receptor on human NK cells are both mandatory for production of IFN-gamma in response to double-stranded RNA. J. Immunol. 185, 2080–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boussif O., Lezoualc'h F., Azanta M. A., Mergny M. D., Scherman D., Demeneix B., Behr J.-P. (1995) A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. U. S. A. 92, 7297–7301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang F., Tang E., Guan K., Wang C.-Y. (2003) IKK-beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J. Immunol. 170, 5630–5635 [DOI] [PubMed] [Google Scholar]
- 27. Yu M., Levine S. J. (2011) Toll-like receptor 3, RIG-I-like receptors and the NLRP3 inflammasome: key modulators of innate immune responses to double-stranded RNA viruses. Cytokine Growth Factor Rev. 22, 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hitomi J., Katayama T., Eguchi Y., Kudo T., Taniguchi M., Koyama Y., Manabe T., Yamagishi S., Bando Y., Imaizumi K., Tsujimoto Y., Tohyama M. (2004) Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J. Cell Biol. 165, 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bertrand M. J. M., Milutinovic S., Dickson K. M., Ho W. C., Boudreault A., Durkin J., Gillard J. W., Jaquith J. B., Morris S. J., Barker P. A. (2008) cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 [DOI] [PubMed] [Google Scholar]
- 30. Friboulet L., Gourzones C., Tsao S., Morel Y., Paturel C., Temam S., Uzan C., Busson P. (2010) Poly(I: C) induces intense expression of c-IAP2 and cooperates with an IAP inhibitor in induction of apoptosis in cancer cells. BMC Cancer 10, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Varfolomeev E., Blankenship J. W., Wayson S. M., Fedorova A. V., Kayagaki N., Garg P., Zobel K., Dynek J. N., Elliott L. O., Wallweber H. J. A., Flygare J. A., Fairbrother W. J., Deshayes K., Dixit V. M., Vucic D. (2007) IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell 131, 669–681 [DOI] [PubMed] [Google Scholar]
- 32. Nomi N., Kodama S., Suzuki M. (2010) Toll-like receptor-3 signaling induces apoptosis in human head and neck cancer via survivin associated pathway. Oncol. Rep. 24, 225–231 [DOI] [PubMed] [Google Scholar]
- 33. Kübler K., Gehrke N., Riemann S., Bohnert V., Zillinger T., Hartmann E., Polcher M., Rudlowski C., Kuhn W., Hartmann G., Barchet W. (2010) Targeted activation of RNA helicase retinoic acid-inducible gene-I induces proimmunogenic apoptosis of human ovarian cancer cells. Cancer Res. 70, 5293–5304 [DOI] [PubMed] [Google Scholar]
- 34. Kübler K., tho Pesch C., Gehrke N., Riemann S., Daßler J., Coch C., Landsberg J., Wimmenauer V., Pölcher M., Rudlowski C., Tüting T., Kuhn W., Hartmann G., Barchet W. (2011) Immunogenic cell death of human ovarian cancer cells induced by cytosolic poly(I: C) leads to myeloid cell maturation and activates NK cells. Eur. J. Immunol. 41, 3028–3039 [DOI] [PubMed] [Google Scholar]
- 35. Yaginuma Y., Westphal H. (1992) Abnormal structure and expression of the p53 gene in human ovarian carcinoma cell lines. Cancer Res. 52, 4196–4199 [PubMed] [Google Scholar]
- 36. Ahmed A. A., Etemadmoghadam D., Temple J., Lynch A. G., Riad M., Sharma R., Stewart C., Fereday S., Caldas C., deFazio A., Bowtell D., Brenton J. D. (2010) Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J. Pathol. 221, 49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. The Cancer Genome Atlas Research Network (2011) Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salaun B., Zitvogel L., Asselin-Paturel C., Morel Y., Chemin K., Dubois C., Massacrier C., Conforti R., Chenard M. P., Sabourin J.-C., Goubar A., Lebecque S., Pierres M., Rimoldi D., Romero P., Andre F. (2011) TLR3 as a biomarker for the therapeutic efficacy of double-stranded RNA in breast cancer. Cancer Res. 71, 1607–1614 [DOI] [PubMed] [Google Scholar]
- 39. Teoh D., Ayeni T. A., Rubatt J. M., Adams D. J., Grace L., Starr M. D., Barry W. T., Berchuck A., Murphy S. K., Secord A. A. (2011) Dasatinib (BMS-35482) has synergistic activity with paclitaxel and carboplatin in ovarian cancer cells. Gynecol. Oncol. 121, 187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.