Abstract
Energy production in mitochondria is a multistep process that requires coordination of several subsystems. While reversible phosphorylation is emerging as the principal tool, it is still unclear how this signal network senses the workloads of processes as different as fuel procurement, catabolism in the Krebs cycle, and stepwise oxidation of reducing equivalents in the electron transfer chain. We previously proposed that mitochondria use oxidized cytochrome c in concert with retinol to activate protein kinase Cδ, thereby linking a prominent kinase network to the redox balance of the ETC. Here, we show that activation of PKCε in mitochondria also requires retinol as a cofactor, implying a redox–mechanism. Whereas activated PKCδ transmits a stimulatory signal to the pyruvate dehdyrogenase complex (PDHC), PKCε opposes this signal and inhibits the PDHC. Our results suggest that the balance between PKCδ and ε is of paramount importance not only for flux of fuel entering the Krebs cycle but for overall energy homeostasis. We observed that the synthetic retinoid fenretinide substituted for the retinol cofactor function but, on chronic use, distorted this signal balance, leading to predominance of PKCε over PKCδ. The suppression of the PDHC might explain the proapoptotic effect of fenretinide on tumor cells, as well as the diminished adiposity observed in experimental animals and humans. Furthermore, a disturbed balance between PKCδ and PKCε might underlie the injury inflicted on the ischemic myocardium during reperfusion.—Gong, J., Hoyos, B., Acin-Perez, R., Vinogradov, V., Shabrova, E., Zhao, F., Leitges, M., Fischman, D., Manfredi, G., Hammerling, U. Two protein kinase C isoforms, δ and ε, regulate energy homeostasis in mitochondria by transmitting opposing signals to the pyruvate dehydrogenase complex.
Keywords: mitochondrial energy metabolism, retinoids, vitamin A physiology, redox biology, respiration, signal transduction
During energy generation, mitochondria perform several tasks in parallel. They produce acetyl-coenzyme A (CoA) from a variety of fuels; they convert acetyl-CoA in the tricarboxylic acid (TCA) cycle into nicotine adenine dinucleotide (reduced form; NADH); they feed these reducing equivalents into the electron transfer chain (ETC) for stepwise oxidation and establishment of an electrochemical gradient, and they use the proton-motive force to drive ATP generation. These diverse processes require extensive intramitochondrial coordination. This task falls on a number of conventional signal pathways based on reversible phosphorylations. Best studied among these are sets of pyruvate dehydrogenase kinases (PDKs) and pyruvate dehydrogenase phosphatases (PDPs) that control the pyruvate dehydrogenase complex (PDHC) (1–3) and protein kinase A that regulates the cytochrome-c oxidase (COX) complex of the ETC (COXIV) (4, 5). While the latter pathway senses the CO2 emanating from the PDHC and TCA and thus monitors the biochemical workload, the provenance of signals operating upstream of the PDHC are still unclear. We defined the protein kinase Cδ (PKCδ) signalosome as a positive regulator of the PDHC, working via PDK2 (6). Essential components of the PKCδ signal complex are the adapter protein, p66Shc, cytochrome c, and vitamin A (retinol) (7). By combining biochemical and mutational analyses with functional studies of the PDHC, respiration, and ATP generation, we learned that the PKCδ signalosome was indispensible for mitochondrial regulation and depended on the concerted action of all four components. Of special interest was the finding that PKCδ was activated in situ by a novel redox mechanism. Activation was driven by cytochrome c and was critically dependent on retinol bound to the activation domain of PKCδ. We surmised that because the oxidation of PKCδ by Fe3+ cytochrome c proceeded by 1-electron chemistry, it required the participation of an electron transfer agent. This agent is retinol, functioning in precise analogy to ubiquinol in the ETC. We further surmised that the PKCδ signalosome sensed the electrochemical potential of cytochrome c, thereby monitoring the workload of the ETC and accordingly adjusting the flux of fuel entering the TCA cycle (8).
A second PKC isoform, PKCε, has been reported to oppose the action of PKCδ in mitochondria (9–11). Although a number of targets were proposed on the basis of genetic, biochemical, and proteomic analyses (12), there is still no consensus on the signaling mechanism of PKCε and on the biochemical basis of the antagonism toward PKCδ. Our study was undertaken to resolve this question. We confirmed the mutual antagonism between PKCδ and ε and identified the PDHC as the common downstream target that was alternately stimulated by PKCδ or inhibited by PKCε signaling. Like PKCδ, the activation of the PKCε isoform occurred in situ and required retinol as a coactivator, bound to the εC1B activation domain, and implying redox activation, although the upstream signal providing the oxidative stimulus remains unknown. Given the importance of retinoids, such as anhydroretinol, for the regulation of intermediary metabolism (13–15), we were interested in the extent to which retinol was interchangeable with synthetic retinoids that display proapototic properties, such as anhydroretinol. We found that in short-term applications, 4-hydroxyphenyl retinamide (4-HPR; p-hydroxyanilide; fenretinide) unexpectedly was equal to retinol in its capacity to coactivate both PKCδ and ε isoforms and to stimulate respiration. However, long-term exposure of cells to fenretinide led to the predominance of the inhibitory PKCε signal, causing severe PDHC suppression. The ensuing dearth of ATP production was a major cause of necrotic cell death. Fenretinide was proposed for clinical exploration for the treatments of cancer (16, 17) and metabolic disease (18, 19). The disruption of intermediary metabolism by this drug might furnish a mechanistic explanation of its anticancer properties. Our results also call attention to the importance of the fledgling PKC signal network in mitochondria, the crosstalk between PKC isoforms, and the homeostasis of their partners, including vitamin A. If perturbed, this PKC signal network might contribute to cardiac and cerebral reperfusion injury and impact on the etiology of the metabolic syndrome.
MATERIALS AND METHODS
Biological reagents and expression vectors
All-trans-retinol, retinoic acid, fenretinide, doxycycline, phorbol myristoyl acetate (PMA), and horse heart cytochrome c were purchased from Sigma-Aldrich (St. Louis, MO, USA). The following Western blot antibodies were used: anti-PDHE1, anti-COXIV, and anti-VDAC (Invitrogen, Carlsbad, CA, USA); antihsp60 (Stressgen, Ann Arbor, MI, USA), anti-GAPDH (Abcam, Cambridge, MA, USA) anti-PKCδ, anti-PKCε, anti-p66Shc, anti-cytochrome c, and anti-Tim23 (BD Biosciences, San Jose, CA, USA); anti-phospho-PKCδ (Thr505; Cell Signaling, Boston, MA, USA); anti-phospho-PDHE1 (Ser293; Novus Biologicals Littleton, CO, USA). The pBABE-puro and MigR1 retroviral and pLVPT-tTR-KRAB lentiviral mammalian expression vectors were purchased from Addgene (Cambridge, MA, USA). The expression vector encoding mutant PKCδ Y332F was generously donated by U. Kikkawa (Biosignal Research Centre, Kobe University, Japan; ref. 20). Mutations at E132Q and E133Q of p66Shc, to generate a cytochrome-c nonbinding p66Shc, were carried out by QuickChange (Stratagene, Santa Clara, CA, USA), as suggested by Giorgio et al. (21).
Construction of retinol nonbinding mutants
PKCε/2C1B retinoid nonbinding mutant was generated by exchanging both PKCε C1A and C1B domains in the full-length gene with PKCα C1B domain (22). The following primers were used for PCR amplification of the substituting domains using human PKCα as the template: εC1A, forward 5′-GTCAGGCGCAGGGTCCACCAGGTAAATGGCCCAAAGTTCAAAATCCACAC, reverse 5′-GTCAGGGGTTTCCTGTTTCTTCAGCCCAGCGCAGAGGCTGGGGACATTGA; and εC1B, forward 5′-GGCTCCCAACGGTTCAGCGTCAACATGCCCCACAAGTTCAAAATCCACAC, reverse 5′-CACTTTGGCAATTCCTCTGGCGTCTACCCCGCAGAGGCTGGGGACATTGA. The PCR products were then used in site-directed mutagenesis of the pBABE-mouse PKCε vector using QuickChange. The construct was confirmed by sequencing, expressed in PKCε−/− MEFs, and verified by Western blot.
PKCε C1B retinoid nonbinding mutant (N249T, W264Y) was created by site-directed mutagenesis using the following primers: forward 5′-CGGGATTCACACCTACAAGGTCCCC and reverse 5′-GCCGCAAGAGGCCGTAGAGCAGGGACCC. The construct was sequenced, expressed in PKCε−/− MEFs, and verified by Western blot analysis.
The GST-mut εC1B required a tryptophane residue at the C terminus for fluorescence energy transfer to the bound ligand. It was produced by PCR using the following primers: forward 5′-TAGGATCCAACATGCCCCACAAGTTCGG and reverse 5′-TCGAATTCCGTCTACCCAACAGTTGGGAGCCAC. The PCR fragment was cloned into BamH1-EcoRI sites of GEX2T (Amersham Pharmacia Biotech, Piscataway, NJ, USA), expressed in DH5α (Invitrogen), and purified by affinity chromatography on glutathione-agarose matrix (Sigma-Aldrich) to >90% purity, as determined by Coomassie blue staining of sodium dodecyl sulfate (SDS)-polyacrylamide gel.
Measurement of retinoid binding affinity for PKCε zinc-finger domains
PKCε C1A and C1B zinc-finger domains were expressed as glutathione-S-transferase (GST)-fusion proteins, purified by affinity chromatography, and used to measure retinol and fenretinide binding by the protein-fluorescence quench method of Norris et al. (23). Qualitative binding specificity was also demonstrated by the ELISA method of Yin et al. (24).
Mice
p66Shc−/− mice were maintained at Sloan-Kettering Institute from founders originally donated by G. Pelicci (European Institute of Oncology, Milan, Italy). C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA).
Cell lines
MEFs were derived from 13.5-d-old mouse embryos of C57BL/6 mice or p66Shc−/− mice. The PKCδ−/−; PKCε+/+ and PKCδ−/−; PKCε−/− MEF cell lines were provided by M.L. For cytochrome-c (somatic) knockdown, the lentiviral vector pLKO.1-puro with the target sequence: CCGGGCAGACCTAATAGCTTATCTTCTCGAGAAGATAAGCTATTGGTCTGCTTTTTG was used (7). PKCε−/− and transgenic PKCε overexpressing MEFs (PKCεHI) were generous gifts of P. Parker (Cancer Research UK London Institute, London, UK; ref. 25). The fragment encoding the catalytic domain of PKCδ (26) or full-length PKCε were inserted into the pLVPT-tTR-KRAB vector, containing an expression cassette downstream of a tetracycline-inducible promoter (27). Virus was generated in 293T cells. The conditional PKCδ catalytic domain (PKCδ cat) gene was transfected into PKCδ−/− MEFs and the conditional PKCε allele into PKCε−/− MEFs. Protein expression was induced 3 d after transduction with 5 μM doxycycline for 6 h in the presence or absence of 2 μM fenretinide.
Cell culture and transfection
Mouse embryonic fibroblasts (MEFs) were grown in Dulbecco modified Eagle's medium (DMEM) supplemented with 10% FBS, l-glutamine, 1 mM pyruvate, and 4.5 mg/ml glucose. For vitamin A depletion, cells were incubated for 18 h in serum-free TLB medium (DMEM supplemented with 4.5 mg/ml glucose, 0.05% BSA, 5 μg/ml transferrin, 1 μM linoleic acid, and 2 mM glutamine). Reintroduction of the full-length wild-type (WT) PKCδ gene and the retinol nonbinding mutant PKCδ was previously described (6). Reintroductions of the mutant PKCδ Y332F, p66Shc WT and the double mutant E132Q;E133Q p66Shc were performed using the pBABE retroviral vector, essentially as described for WT PKCδ (7). Recipient cells were the respective knockout cell lines.
Cell death assay
Cell death assay was performed as described previously (15).
Measurements of oxidative phosphorylation in cells and isolated mitochondria
Intact cells (1.5×106) were used for O2 consumption measurements in an oxygraph equipped with a Clark electrode (Hansatech Instruments, Norfolk, UK). Mouse liver mitochondria were isolated as described previously (28), and state III O2 consumption driven by specific respiratory chain complexes was measured on 75–100 μg of mitochondrial protein as described previously (29). All reagents were purchased from Sigma-Aldrich. ATP synthesis in isolated mitochondria (15–25 μg of protein) or in cells permeabilized with digitonin (1×106 cells) was measured using the kinetic luminescence assay as described by Vives-Bauza (30).
Titration of retinoids
Dose-response relations of retinol and fenretinide were determined by incubating MEFs with graded concentrations of retinol or fenretinide for 30 min at 37°C, using albumin as carrier. ATP synthase activity was measured as above.
Mitoplast preparation for PKCε localization within mitochondria
Mitoplasts from crude liver mitochondria were prepared as described previously (6). Briefly, mitochondria 500 mg (1 mg/ml) were resuspended in MS-EGTA (225 mM mannitol, 75 mM sucrose, 5 mM HEPES, and 1 mM EGTA, pH 7.4). Water (0.1 vol) and digitonin (1 mg digitonin/5 mg mitochondrial protein) were added, and the mixture was incubated on ice for 45 min. KCl (150 mM) was then added, followed by incubation for 2 min on ice, and centrifugation at 18,000 g for 20 min at 4°C. The pellet containing the mitoplast fraction was resuspended at 1 mg/ml in 300 mM Tris-HCl and 10 mM CaCl2 at pH 7.4. The supernatant containing the postmitoplast fraction was precipitated with 12% TCA and centrifuged at 18,000 g for 15 min at 4°C. The pellet was resuspended in 500 ml acetone and centrifuged at 18,000 g for 15 min at 4°C. The final pellet was then suspended in 50 μl of 2× Laemmli sample buffer.
Mitoplast preparation for cytochrome c import
Mitoplasts for cytochrome c import were prepared from MEFs that were cultured for 18 h in serum-free TLB medium. Mitochondria were isolated by homogenization of the cell pellets in buffer A (0.32 M sucrose, 1 mM EDTA, and 10 mM Tris-HCl, pH 7.4) followed by centrifugation at 1000 g for 5 min. Supernatant containing mitochondria was then centrifuged at 20,000 g for 10 min, and mitochondria were resuspended at 1 mg/ml in MAITE buffer (10 mM Tris-HCl, pH 7.4; 25 mM sucrose; 75 mM sorbitol; 100 mM KCl; 10 mM K2HPO4; 0.05 mM EDTA; 5 mM MgCl2; and 1 mg/ml BSA). Digitonin (1 mg digitonin/5 mg mitochondrial protein) was added, and the mixture was incubated on ice for 45 min. Then, cytochrome c (20 mM) or the combination of cytochrome c plus retinol (20 mM+2 μM) was added to the mitoplasts and incubated at 37°C for 10 min. Pyruvate + malate-driven respiration was measured using 50 μg of the mitoplast preparation.
Immunoblot analyses
To determine the phosphorylation levels of PKCδ and PDH E1 in mitochondria and mitoplasts, 10 μg of protein was separated by 12.5% SDS-polyacrylamide gel electrophoresis (PAGE), electroblotted onto PVDF filters (Bio-Rad, Hercules, CA, USA), and immunoblotted with the appropriate antibodies. To determine the levels of cytochrome c after silencing, 50 μg of total cell lysate were electrophoretically separated under the same conditions as described above, electroblotted, and detected using the appropriate antibodies.
Statistical analyses
Comparisons between groups were made using 1-way ANOVA. Pair-wise comparisons were made by post hoc Fisher PLSD test. Differences were considered statistically significant at P < 0.05. Data analyses were performed using the statistical program StatView (Adept Scientific, Bethesda, MD, USA). In all experiments, error bars indicate sd.
RESULTS
The PKCδ signalosome is defined by 4 components that act in unison: PKCδ associates in the mitochondrial intermembrane space with the adapter protein, p66Shc, with retinol, and with cytochrome c. PKCδ is activated by oxidized cytochrome c with mandatory assistance by retinol (7). Activated PKCδ signals the PDHC to increase the generation of acetyl-CoA from pyruvate, resulting in increased tricarboxylic acid cycle (TCA) activity and ATP synthesis by oxidative phosphorylation. Redox activation of PKCδ requires that retinol occupy a specific binding pocket in its activation domain, using the β-ionone ring as a contact (6, 22). Because fenretinide shares the β-ionone ring with retinol, we asked whether fenretinide could functionally substitute for retinol. As shown in Fig. 1A, fenretinide was as effective as retinol in coactivating the PKCδ signaling pathway. Within minutes, isolated liver mitochondria exhibited a 28% increase in the rate of ATP production (P<0.001).
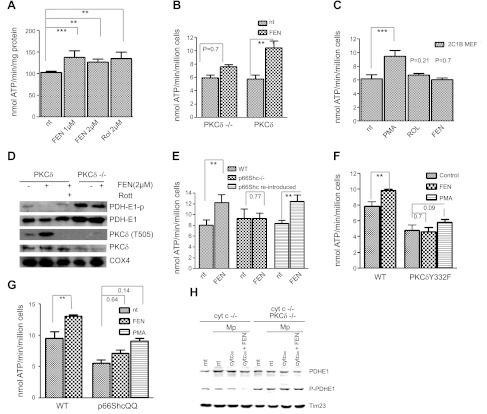
Figure 1.
Fenretinide as coactivator of PKCδ. A) Capacity of fenretinide to accelerate the ATP synthase rate of WT MEFs equals that of retinol. WT MEFs were treated for 30 min with either 1 or 2 μM fenretinide (FEN) or 2 μM retinol (Rol), or left untreated. Pyruvate/malate-driven rates of ATP synthesis were measured as described in Materials and Methods and expressed as nanomoles ATP per minute per milligram protein or per million cells. ATP synthesis increased by 28, 25, or 28%, respectively (n=6). One of 3 independent experiments is shown. **P < 0.01, ***P < 0.001. B) Failure to stimulate ATP synthesis in PKCδ−/− MEFs indicates dependence on PKCδ; reintroduction of PKCδ restores responsiveness to fenretinide. PKCδ−/− MEFs, and PKCδ−/− MEFs reconstituted with full-length PKCδ by transfection, were treated with fenretinide and analyzed for ATP production as in A. The response of PKCδ−/− MEFs was statistically insignificant, whereas reintroduction of PKCδ reestablished response to fenretinide (45% increase, n=3). One of 4 independent experiments is shown. **P < 0.01. C) Expression of a mutant PKCδ gene deficient in retinoid-binding sites fails to rescue the PKCδ phenotype. This block is bypassed by phorbol ester (PMA). PKCδ was converted to a retinol-nonbinding form by genetic exchange of both endogenous zinc-finger domains for the PKCα C1B domain. The reengineered full-length gene was expressed in PKCδ−/− MEFs. Responsiveness to retinoids (2 μM), or PMA (100 nM) was determined as described in A. Neither retinol nor fenretinide elicited a cofactor response commensurate with WT PKCδ responses shown in A and B. A 35% increase in ATP synthesis (n=4) was observed with PMA, which bypasses the requirement for retinoid cofactor. ***P < 0.001. D) Fenretinide stimulates autophosphorylation of threonine-505 of PKCδ, indicating conversion to active enzyme. The E1 subunit of PDH is dephosphorylated, indicating PDH activation. WT and PKCδ−/− MEFs were stimulated with 2 μM fenretinide as in A. Mitochondrial proteins were separated by SDS-PAGE and analyzed by immunoblotting for the proteins indicated. Phosphorylation of T 505 of PKCδ and dephosphorylation of PDH E1 indicated activation of both enzymes, and this was prevented by rottlerin (Rott). PDH was not dephosphorylated in PKCδ−/− MEFs. One of 3 independent experiments is shown. Direct immunoblotting of PKCδ stained a contaminating band. This was eliminated by prior enrichment of PKCδ with immunoprecipitation (not shown). E) Fenretinide-mediated up-regulation of oxidative phosphorylation is ablated by p66Shc gene knockout, and it is restored by reintroduction of the intact gene. WT MEFs, MEFs with a defective p66Shc gene, and p66Shc−/−MEFs reconstituted with intact p66Shc gene were treated with fenretinide and analyzed as in A. ATP synthesis increased by 30% in WT MEFs, was not enhanced in the knockout MEFs, but was enhanced by 30% after reintroduction of p66Shc (P < 0.002 for both responders, n=4). One of 3 independent experiments is shown. **P < 0.01. F) Fenretinide-mediated up-regulation of oxidative phosphorylation is abolished in MEFs expressing the Y332F mutant PKCδ, defective in its ability to bind p66Shc. WT MEFs and PKCδ−/− MEFs reconstituted with the mutated PKCδ Y332F gene were treated with 2 μM fenretinide and analyzed as in A. WT cells responded by a 25% increase in ATP synthesis (P < 0.01, n=4), but MEFs carrying the defective PKCδ did not. The block was partially overridden by stimulation with PMA. One of 6 independent experiments is shown. **P < 0.01. G) Fenretinide-mediated up-regulation of oxidative phosphorylation is abolished in MEFs expressing the E132Q/E133Q mutant of p66Shc incapable of binding cytochrome c. WT MEFs and p66Shc−/− MEFs reconstituted with the mutated p66ShcQQ gene were treated with 2 μM fenretinide and analyzed as in A. WT cells responded by a 25% increase in ATP synthesis (P < 0.01), but responses of MEFs carrying the mutated QQ gene were attenuated. PMA partially overrode this block (35% increase, P < 0.14). One of 6 independent experiments is shown. **P < 0.01. H) PKCδ signaling is defective in mitoplasts deprived of cytochome c, and is restored by introduction of oxidized cytochrome c protein in combination with fenretinide (left panel); mitoplasts of PKCδ−/− MEFs fail to respond to cytochrome c and fenretinide (right panel). Mitochondria (mt) and mitoplasts (Mp) of cytochrome c-knockdown (cyt c−/−) MEFs or cytochrome c-knockdown/PKCδ-deficient (cyt c−/− PKCδ−/−) cells were prepared as described in Materials and Methods. Mitoplasts were reconstituted with 25 mM oxidized cytochrome c (cyt cox) with and without fenretinide for 10 min at 37°C, or left untreated (nt). Mitochondrial proteins were separated by SDS-PAGE and analyzed by immunoblot for PDH E1 content and phosphorylation status. Time 23 was used as a loading control. Dephosphorylation was observed solely in mitoplasts treated with the combination of cytochrome c and fenretinide (6-fold reduction by densitometry). All other preparations, including the mitochondria and mitoplasts devoid of PKCδ, did not yield dephosphorylated PDH E1 species. One of two repeat experiments is shown.
To confirm that fenretinide action was mediated through PKCδ signaling, we analyzed MEFs carrying an inactive PKCδ gene (31). These cells exhibited a statistically insignificant response (P=0.7) to 2 μM fenretinide in short-term stimulation assays, similar to our earlier results with retinol (6). When full-length PKCδ was reintroduced into the PKCδ−/− cells, up-regulation of ATP synthase activity by fenretinide was restored (80% increase; P < 0.01; Fig. 1B). PKCδ has one retinoid-binding site in each of its two zinc-finger domains (32). To test whether these sites were important for fenretinide action, we swapped both PKCδ C1A and C1B domains for the PKCα C1B domain, which naturally does not bind retinol (22). We previously showed that the dual C1B exchange-mutant of PKCδ was signaling-defective in mitochondria (6). When expressed in PKCδ−/− cells, this retinol nonbinding mutant PKCδ failed to respond to fenretinide and retinol (Fig. 1C). It was, however, activated by phorbol ester (30% increase, P < 0.001), indicating that kinase activity and forward signaling were intact. We conclude that binding of fenretinide is required for kinase activation, not for signal propagation. To confirm that fenretinide action results in increased WT PKCδ kinase activity we examined the phosphorylation status of threonine-505 by Western blot analysis. Phosphorylation at that site serves as an indicator for the active enzyme (33, 34). Fenretinide treatment increased phospho-T505 intensity, and this correlated with augmented ATP synthase activity, indicating that PKCδ phosphotransferase activity was responsible for the up-regulation of oxidative phosphorylation (compare Fig. 1D with A, B). As expected, phosphorylation of T-505 was blocked by rottlerin, an inhibitor of PKCδ (35).
PDHC is regulated by reversible phosphorylation of its E1 regulatory domain by 4 pyruvate dehydrogenase kinases (PDKs), which impart a negative signal (1, 2, 36–40) and two phosphatases (PDPs), which reverse PDK-mediated inhibition of PDH (3, 41). A forward target of the PKCδ pathway in mitochondria is the PDHC, presumably through regulation of the PDK and PDP systems (6). To test whether short-term fenretinide stimulation results in PDHC activation, we analyzed its phosphorylation state with phospho-specific PDHC antibodies by Western blot (42). The E1 subunit was substantially dephosphorylated after fenretinide treatment, but it retained phosphorylation in the presence of rottlerin (Fig. 1D), indicating that fenretinide, like retinol, acts as coactivator of PKCδ in mitochondria.
The adapter protein p66Shc partners with PKCδ at several cellular sites, including mitochondria (20, 43). Since we knew that p66Shc was required for retinol-mediated up-regulation of oxidative phosphorylation (7), we asked whether it was required for fenretinide costimulation as well. We found that genetic ablation of p66Shc blocked the fenretinide cofactor effect. Reintroduction of p66Shc restored responsiveness to fenretinide, yielding a 48% increase in the ATP synthetic rate (P < 0.01; Fig. 1E). Physical interaction of PKCδ with p66Shc is required for retinol-dependent signaling and is accomplished by binding phospho-Y332 on PKCδ by the p66Shc SH2 domain (20). Y to F mutations of this docking site disrupted complex formation between p66Shc and PKCδ and diminished fenretinide-mediated up-regulation of ATP synthesis. Therefore, proper association of p66Shc with PKCδ is mandatory for fenretinide signaling (Fig. 1F). P66Shc also partners with cytochrome c (21), raising the additional question of whether cytochrome c plays a role in fenretinide action. Since the docking site of cytochrome c on p66Shc is known, we produced a binding-defective cell line by expression of the mutant E132Q, E133Q p66Shc allele in p66Shc-knockout MEFs. Earlier studies in our laboratory indicated that these cells did not respond to retinol costimulation (7). This defect was also evident for fenretinide costimulation but was partially overridden by phorbol stimulation (Fig. 1G).
Cytochrome c 3+ was previously shown to drive PKCδ activation (7). To explore whether cytochrome c was required for fenretinide-dependent PKCδ activation, we tested mitoplasts, which were supplemented with exogenous cytochrome c. We found that fenretinide cooperated with cytochrome c 3+, suggesting that fenretinide, like retinol, facilitates the redox-activation of PKCδ (Fig. 1H). These results affirm that fenretinide substitutes effectively for retinol. At the same time, the likelihood appears remote that fenretinide converts to an activated metabolite in isolated mitoplasts.
Despite these demonstrably positive effects on energy balance, comparable to those of retinol, fenretinide exhibits strongly proapoptotic properties, the basis for its use as a tumoricidal agent (17). In this regard, fenretinide is reminiscent of anhydroretinol (14, 44). We previously showed that long-term exposure of cultured cells to AR produces, after a lag period of 1 h, a phase of progressive decline in ATP synthesis, resulting in programmed cell death. If added prior to a point of no return, retinol is capable of restoring cell viability, reflecting the mutual reversible inhibition characteristic of AR and retinol (15). To investigate whether prolonged fenretinide treatment would similarly create a delayed energy crisis and induce cell death, we examined the time course of ATP production in the presence of fenretinide. We observed that fenretinide (2 μM) initiated a phase of augmented ATP synthesis lasting 1 h, followed by a progressive decline, eventually falling below basal levels. In comparison, exposure to retinol produced a sustained elevation of ATP production over 2 to 4 h and declined moderately thereafter (Fig. 2A). Since the ATP synthase rates are affected by PDHC activity, which is inversely correlated with E1 phosphorylation (1, 41), we analyzed the time course of E1 subunit phosphorylation. Our results show that fenretinide causes a decline in the ratio of phospho-PDHC:PDHC at 30 min, but phosphorylation increases thereafter to reach a level comparable to that of untreated cells. By contrast, dephosphorylation of the E1 subunit of retinol-treated cells was sustained over the entire observation time (Figs. 2B and 4D). Thus, the kinetics of PDHC activity, as reflected by its dephosphorylated state, parallel those of ATP synthesis.
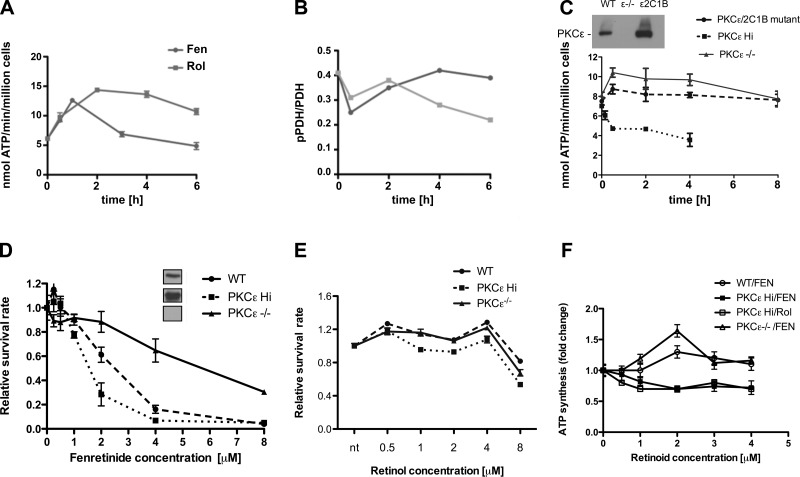
Figure 2.
The inhibitory effects of late-acting fenretinide are mediated by PKCε. A, B) Fenretinide elicits biphasic responses, as shown for ATP synthesis (A) and PDH phosphorylation status (B). WT MEFs were stimulated with 2 μM retinol (Rol) or fenretinide (Fen) for indicated time periods and analyzed for ATP synthesis as described in Fig. 1A. In parallel, mitochondrial proteins were separated by SDS gel electrophoresis and analyzed for PDH E1 content and phosphorylation status by immunoblotting. Ratios of PDH: phosphoPDH E1 were determined densitometrically (B). The monophasic retinol response contrasts with the biphasic response to fenretinide (A). The late-phase decline is characteristic of fenretinide and is less pronounced with retinol. One of 3 independent experiments is shown. C) PKCε phenotype influences the changes in ATP synthesis rates elicited by fenretinide. MEFs expressing elevated levels of PKCε (PKCεHi), MEFs expressing a defective PKCε gene (PKCε−/−) and PKCε−/− cells reconstituted with a mutated PKCε gene lacking intact retinoid binding sites (PKCε/2C1B domain-exchange mutant) were treated and analyzed as in A. Expression levels were monitored by immunoblot (inset). PKCε gene ablation abolished the late-phase inhibition but permitted the short-term up-regulation of ATP synthesis. Conversely, PKCε overexpression (6-fold over WT levels) abolished the short-term ATP up-regulation and accelerated fenretinide-mediated ATP suppression. Expression of mutant PKCε, defective in the ability to bind retinoids, in PKCε−/− MEFs did not restore fenretinide mediated suppression. One of 3 independent experiments is shown. D, E) Fenretinide-mediated cell death is influenced by the PKCε phenotype. WT, PKCε Hi, and PKCε−/− MEFs were cultured in serum-free medium and treated with indicated concentrations of fenretinide (D) or retinol (E) for 24 h. Insets show expression levels by immunoblotting. Relative survival of cells was determined as described in ref. 15. Ablation of PKCε conferred resistance to fenretinide, whereas overexpression of PKCε conferred increased sensitivity. Up to 4 μM retinol preserved the viability of all three cell types. Above 4 μM, cell viability declined due to toxicity. One of 6 independent experiments is shown. ***P < 0.01. F) Optimal dose-responses of fenretinide and retinol are in the range of 1 to 2 μM. WT, PKCε−/−, and PKCε overexpressing (PKCε Hi) MEFs were stimulated for 30 min with the doses of retinoids shown, and rates of ATP synthesis were determined. Fold changes ± se relative to cells treated with vehicle are shown. In all 4 dose-response curves, differences between treatments with vehicle and 2-μM retinoids reached statistical significance (P < 0.014 or better).
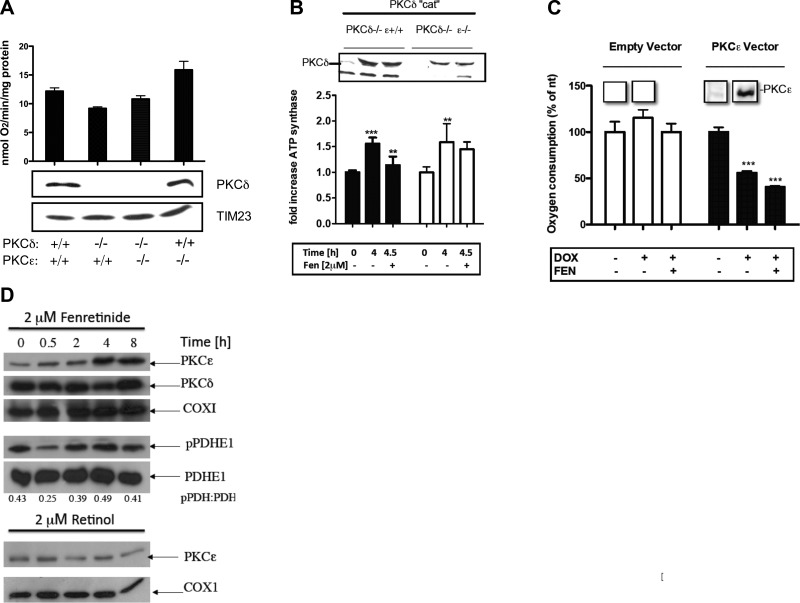
Figure 4.
Respiration is controlled by the balance between PKCδ and PKCε. A) Baseline levels of respiration are influenced by PKCδ and PKCε phenotypes. WT, PKCδ−/−, PKCε−/−, and PKCδ−/− PKCε−/− MEFs were rendered quiescent by culturing in serum-free medium and analyzed for baseline oxygen consumption without reactivation. MEFs lacking a functional PKCδ gene displayed depressed baseline level of respiration compared to WT MEFs. Conversely, ablation of PKCε resulted in elevated respiration. The dual δ,ε-knockout MEFs equaled WT cells in oxygen consumption. Tim23 levels were comparable in all 4 cell types, suggesting equal amounts of mitochondria. This was confirmed by mitotracker staining (data not shown). PKCε-knockout MEFs had no more PKCδ in their mitochondria than WT cells, implicating the missing PKCε gene in negative regulation of respiration. B) Constitutively active PKCδ stimulates respiration, which is attenuated by fenretinide-assisted PKCε activation. WT PKCδ cat was placed under a tetracycline-inducible promoter and transfected into either PKCδ−/− PKCε+/+ or PKCδ−/− PKCε−/− MEFs by a lentiviral vector (26, 27). Expression was induced by doxycyclin. Presence of PKCδ cat in mitochondria was monitored by immunoblot (inset). Increased ATP synthesis (1.5-fold) coincided with PKCδ-cat appearance after 4 h of induction in both cell lines. The addition of 2 μM fenretinide for the last 30 min decreased ATP synthesis to near baseline level in the former but not PKCε-knockout MEFs, indicating that suppression of ATP synthesis was dependent on PKCε. **P < 0.01, ***P < 0.001. C) Activation of PKCε leads to diminished respiration. The full-length PKCε gene under inducible promoter was expressed in PKCε−/− cells by lentiviral transfection. Induction of PKCε expression by doxocycline was observed after 4 h (see inset showing PKCε immunoblot of mitochondrial proteins) and caused 50% reduction of oxygen consumption. The addition of fenretinide elicited further 15% reduction. MEFs transfected with empty vector did not display suppression of ATP synthesis. ***P < 0.001. D) Fenretinide causes selective accumulation of PKCε in mitochondria. WT MEFs were stimulated with 2 μM fenretinide for the indicated periods of time. Mitochondria were isolated, and their proteins were separated by SDS-PAGE and analyzed by immunoblotting for the indicated proteins. ATP synthesis rates and phosphorylation status of PDHE1 were determined as described in Fig. 1A. Scans of similar immunoblots displayed here are presented in Fig. 2A, B. Fenretinide stimulated a progressive increase in the amount of PKCε present in mitochondria. PKCδ remained at a constant level (top panels). The late-phase PKCε accumulation correlated with PDH E1 hyperphosphorylation, indicating loss of PDHC activity. The ratios of phospho-PDH to PDH E1 were determined by densitometry. COXI was used as a loading control (middle panels). The treatment of cells with 2 μM retinol did not result in accumulation of PKCε in mitochondria over the 8-h period (bottom panels). Experiment shown is representative of 3 repeats.
Why fenretinide, but not retinol, initially stimulates ATP synthesis, then reverses course after 1 h, and subsequently inhibits oxidative phosphorylation, is unclear. That irreversible commitment to cell death was preceded by a period of fairly normal metabolism (15) suggested to us that fenretinide might generate inhibitory signals that accumulate in the late phase and eventually create a negative energy balance. A second PKC isoform, PKCε, is present in mitochondria and reportedly inhibits PKCδ signaling (9, 11, 45). Therefore, we explored the possibility that activation of PKCε by fenretinide was responsible for PDHC inhibition and for the progressive decline in ATP synthesis. The PKCεHi cell line (originally named C5) expresses 6-fold higher levels of PKCε protein (25). It was constructed by introducing the full-length PKCε transgene into PKCε−/− MEFs. Stimulation of these cells with fenretinide produced a prompt and irreversible suppression of ATP synthesis. Cells appeared moribund after 4 h (Fig. 2C). The parental PKCε−/− MEFs yielded the converse: ATP synthesis was robustly and durably coactivated by fenretinide. Notably, the biphasic response to fenretinide seen in WT cells with its characteristic late-phase suppression (Fig. 2A) was no longer apparent. To confirm the hypothesis that fenretinide acted as coactivator of PKCε, we genetically modified PKCε−/− cells with a mutant form of PKCε in which both retinoid-binding sites were eliminated by replacing the endogenous ones with retinol nonbinding PKCα C1B zinc fingers. These cells were inured against the negative influence of fenretinide on ATP generation (Fig. 2C).
Since declining ATP synthesis is a key factor in cell death, we asked whether PKCε was responsible. As expected, the PKCεHi cell line was remarkably sensitive to fenretinide (IC50=1.5 μM for PKCεHi cells compared to IC50=2.5 μM for WT cells), while the PKCε−/− MEFs were relatively resistant to fenretinide (IC50=6 μM) (Fig. 2D). Retinol did not share death-inducing properties with fenretinide, nor were these related to differential PKCε expression, since WT, PKCεHi, and PKCε−/− MEFs survived in serum-free medium supplemented with retinol up to 4 μM (Fig. 2E). The maximum effective fenretinide doses for PKCε-mediated ATP suppression (using PKCε-overexpressing MEFs) and PKCδ-mediated ATP up-regulation (using PKCε-deficient cells) were in the range of 1 to 2 μM, comparable to that of retinol and previous determinations (ref. 6 and Fig. 2F).
Taken together, these results indicated that the inhibition of energy generation, including extreme ATP depletion leading to cell death, was genetically linked to PKCε. While the literature refers to fenretinide-mediated cell death as apoptosis (17), our interpretation is in tune with necrosis, i.e., with the consequences of negative energy balance (15). Caspase activation was not a factor (Supplemental Fig. S1).
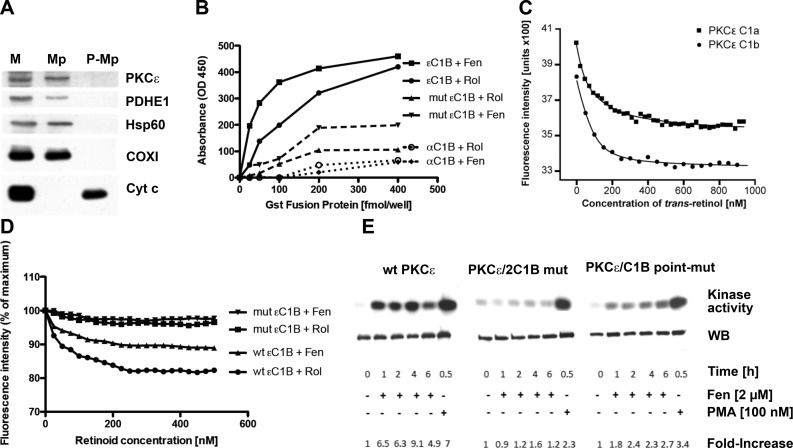
Using the protease protection assay, we determined that PKCε is localized in the mitochondrial matrix. This rests on the evidence that PKCε resists proteolysis of mitoplasts, i.e., mitochondria where the outer membrane is perforated, is, however, degraded when the inner membrane is breached (Fig. 3A). The close structural and functional similarity with PKCδ suggested that PKCε contains retinoid-binding sites in the activation domains. We generated recombinant GST-fusion proteins containing intact εC1A or C1B domains, as well as mutant versions of the latter where retinol contact sites in the binding pocket (N249T, W264Y) were mutated (22). We applied 2 methods, the qualitative ELISA assay developed by Yin et al. (24) and the fluorimetric method of Norris et al. (23) and Noy and Xu (46). Both yielded concordant results. ELISA provided evidence for specific binding of GST-εC1B fusion protein to retinol and fenretinide. Binding to double point-mutated εC1B, or to the natural retinol nonbinding αC1B (32) was attenuated (Fig. 3B). Apparent binding affinities of retinol, determined by fluorimetric methods, were 87 nM for εC1A and 24 nM for εC1B. Owing to the small quench effect elicited by fenretinide, the fluorimetric estimation of the Kd for εC1B at 60 nM was at the limit of resolution (Fig. 3C, D).
Figure 3.
PKCε residing in the mitochondrial matrix binds retinol and fenretinide; cofactor binding to the activation domain is required for in situ conversion to active enzyme. A) PKCε resides in the matrix. Mouse liver mitochondria were treated with digitonin to permeabilize the outer membrane to generate mitoplasts (Mp; ref. 28). Proteins of mitochondria (M), mitoplasts, and postmitoplast supernates (P-Mp) were separated by SDS-PAGE and analyzed by immunoblotting. Western blots reveal the presence of PKCε in intact mitochondria and mitoplasts along with matrix proteins: PDH E1, Hsp60, and COXI. Mitoplasts released proteins associated with the intermembrane space, including cytochrome c (Cyt c). PKCε was notably absent from postmitoplast supernates. B) ELISA assays using solid-phase retinol (Rol) or fenretinide (Fen) indicate specific interaction of GST-PKCεC1B fusion protein with both retinoids. Ablation of 2 contact sites in the retinol-binding pocket attenuates binding. Control GST-PKCαC1B fusion protein did not bind retinoids as expected (32). One of 3 independent experiments is shown. C, D) PKCε contains 2 retinoid-binding sites associated with zinc-finger domains. Retinoid-binding assays were based on quenching of tryptophane fluorescence (23, 71). Both εC1A and εC1B recombinant zinc-finger proteins bound retinol with high affinity (Kd=87 and 24 nM; C). Although fenretinide quenched tryptophane fluorescence emission with lower amplitude, its nominal binding affinity for εC1B (Kd=60 nM) is in range with that of retinol. Fluorescence quenching is abolished in the double-mutant GST-PKCεC1B fusion protein, affirming specificity of retinoid binding (D). E) Activation of PKCε in mitochondria depends on binding of retinoid cofactor. MEFs expressing the WT (left panel), the domain-exchange mutated (middle panel), or the double point-mutated PKCε gene (right panel) were rendered quiescent in serum-free and retinoid-free medium. They were reactivated by FBS in the presence of fenretinide. PKCε was immunoprecipitated from mitochondrial lysates and assayed for phosphotransferase activity for the histone substrate. Note that in the presence of FBS 2 μM fenretinide does not kill MEFs (data not shown). Blots show the autoradiographs of histone phosphorylation, and immunoblots of PKCε levels below. The fold increases are presented as the ratios of phospho-histone of activated vs. quiescent cells, normalized for the PKCε amounts recovered. WT PKCε was on average 4 times as active compared to two retinoid binding-deficient PKCε constructs.
To obtain direct functional evidence for coactivator function of fenretinide, we measured PKCε phosphotransferase activity following stimulation of cells with fenretinide (2 μM). Using immunoprecipitation/kinase assays, we found consistently elevated PKCε enzyme activity in fenretinide stimulated cells. Control cells expressing the PKCε dual domain-exchange mutation (2C1B mut), or PKCε with dual-point mutations in the C1B domain (C1B point-mut), displayed attenuated kinase activities (Fig. 3E).
To determine the influence of the PKC isoforms on respiratory capacity, we measured the baseline levels of oxygen consumption of MEFs of different genotypes. We observed that cells devoid of PKCδ (PKCδ−/− mutants) consumed less oxygen than WT cells, consistent with absence of the stimulatory PKCδ isoform. Conversely, PKCε−/− cells that lack the inhibitory influence of PKCε displayed higher cell oxygen consumption than WT cells. Cells that lacked both PKC isoforms (i.e., double knockouts) equaled the oxygen consumption of WT MEFs (Fig. 4A). These differences do not reflect changes in mitochondria content, since all 4 cell types displayed equal Tim23 amounts.
To formally prove that activation of PKCε by fenretinide causes suppression of respiration, we expressed PKCδ cat under a tetracycline-inducible promoter in PKCδ−/− cells. The PKCδ cat is known to translocate to mitochondria and, since it lacks the regulatory domain, it is constitutively active at that location (26, 47). As expected, 4 h after TET-On, when PKCδ cat was expressed, the rate of ATP synthesis increased by 70% (P < 0.001). Fenretinide added at this point resulted in a significant drop (20%, P < 0.01) in ATP synthesis within 30 min (Fig. 4B). The fenretinide-mediated reduction of ATP synthesis depended on the presence of PKCε, since expression of PKCδ cat in PKC δ−/−; ε−/− cells elicited a 1.5-fold increase in ATP synthesis (P < 0.01), but on fenretinide addition, no subsequent reduction. To obtain further evidence for its inhibitory nature, full-length PKCε under control of an inducible promoter was cloned into PKCε−/− cells. After 6 h of induced expression, PKCε elicited a 50% reduction in oxygen consumption, and this was further reduced within 30 min by fenretinide addition. PKCε−/− cells transfected with an empty vector showed no changes in the rates of oxygen consumption (Fig. 4C).
These results are consistent with negative regulation of oxidative phosphorylation by activated PKCε, but they do not explain the biphasic nature of the ATP time course observed in fenretinide-activated WT cells, nor the proapoptotic properties of fenretinide. We monitored PKCε accumulation over time in mitochondria of fenretinide-treated cells and observed that PKCε levels were constant for the first hour but increased dramatically thereafter (Fig. 4D, top panel). A similar PKCε increase was not evident after retinol treatment (Fig. 4D, bottom panel). By comparison, PKCδ concentrations did not change over the 8-h time course (Fig. 4D, next to top panel). The accumulation of inhibitory PKCε in fenretinide-treated mitochondria is consistent with the decline in oxidative phosphorylation capacity. Whether the mitochondrial accumulation of PKCε results from increased influx from the cytoplasm or from delayed clearance from mitochondria remains to be determined.
DISCUSSION
The most significant finding from this study is that two opposing retinoid-regulated PKC isoforms, PKCδ and PKCε, have a profound influence on the activity of PDHC. Because the PDHC is the portal of entry of the glycolytic end product, pyruvate, into mitochondria, it is of fundamental importance to fuel flux and is regulated at many levels, including allosteric feedback by pyruvate substrate, the ADP to ATP ratios, ion gradients (e.g., Ca2+), and reversible phosphorylation (48–50, reviewed in ref. 51). We have recently uncovered a phosphorylation cascade upstream of the PDHC that is based on the intramitochondrial PKCδ signalosome. Novel insight that this PKCδ signal pathway is driven by cytochrome c has uncovered a potential feedback link to the cytochrome c reductase/oxidase system, suggesting a mechanism for coordinating the workload of the electron transfer chain with the flux of fuel entering the TCA cycle (7). In the present study we demonstrate how the stimulatory effects of PKCδ on the PDHC are counteracted by mitochondrial PKCε signaling, forming a yin/yang control system. Several reports previously described the mutually opposing effects of these two kinases in operational terms (reviewed in ref. 52).
Retinol was recognized as an essential cofactor for PKCδ activation in mitochondria, and on the basis of current results, this requirement extends to PKCε. Both PKC isoforms harbor high-affinity retinoid binding sites in their C1A and C1B activation domains. Mutational analyses indicate that the binding sites associated with the δ and ε C1B domains are relevant and need to be occupied by retinol for kinase activation (Figs. 2 and 3). It is not clear how retinol is delivered to PKCs, but since they are imported from the cytoplasm, PKCs are likely loaded with retinol by interaction with holo-cellular retinol binding protein (CRBP). The dissociation constants of retinol-CRBP (20 nM; ref. 53) and PKC (10–20 nM; ref. 32) are at equilibrium, ensuring in normal nutritional circumstances that PKCs are loaded with retinol. It follows that disturbances in the RBP-CRBP equilibrium, as might occur during times of vitamin A overabundance or deficiency, affect the distribution of retinol between CRBP and PKC. This could alter the functional capacity of PKC and its downstream target, the PDHC. These considerations are relevant to the longstanding mystery of why vitamin A concentrations in plasma are tightly regulated by the liver, and why upward or downward deviation from the norm leads to illness.
It should be emphasized that retinol possesses no innate ability to activate PKC but serves as an enabler of redox activation. The crucial first step in the PKC activation process, initiated either by diacyl-glycerol or by oxidation, appears to be a restructuring of the zinc-finger domain, followed by large-scale unfolding of the molecule and loss of autoinhibition (54). Consistent with the second model, we found that PKCδ activation in mitochondria is mediated by oxidized cytochrome c, suggesting a redox mechanism. Retinol was required to facilitate the transfer of electrons from the zinc-finger-like PKCδ C1B activation domain to the iron core of cytochrome c, in a manner reminiscent of ubiquinol in the ETC (7). The fact that PKCε activation is dependent on retinol bound to the εC1B activation domain implies a similar redox mechanism, but neither companion proteins nor the oxidizing agent are presently known. Since PKCε predominantly resides in the mitochondrial matrix (Fig. 3B), the putative PKCε signalosome likely differs in composition and primary substrate from that of the PKCδ signalosome.
We previously reported that PKCδ inactivates PDK2 via an intermediary, yet unidentified phosphatase (6). In so doing, it permits the dephosphorylation of the PDH E1 regulatory subunit by PDP. This leads to PDHC activation. The substrate of PKCε and the downstream signal path to PDHC is unknown, but it is likely to intersect with the PKCδ pathway at a point upstream of the PDHC.
It was long known that a number of natural and synthetic retinoids are functional antagonists of retinol. In cell cultures, anhydroretinol produced a bewildering array of adverse effects, including depolymerization of the cytoskeleton, depolarization of mitochondrial membrane, blebbing of the plasma membrane, depletion of ATP synthesis, and programmed cell death (13, 14, 44, 55, 56). If supplied in time, retinol reversed these effects. Fenretinide shares cell death-promoting properties with anhydroretinol and reportedly negatively affects the ETC (17). Because these retinoids possess similar binding affinity as retinol (binding being mediated by the conserved β-ionone ring), we correctly concluded that they displace retinol from PKC, but erred in our assumption that they are ineffective as coactivators. Our new results that fenretinide is, in fact, a potent PKC coactivator requires us to rethink the mechanism of action. We propose that instead of mutual pharmacological inhibition, the net effect sensed by the PDHC is determined by the relative strength of its two opposing upstream signals. Results of experiments designed to alter the expression levels are consistent with this model. Genetic ablation of one of the two PKC isoforms permits the other to dominate. Thus, MEFs with defective PKCε alleles have increased oxygen consumption rates in their resting state. Conversely, PKCδ-knockout MEFs or liver cell mitochondria that lack the stimulatory branch display reduced baseline levels of respiration (Fig. 4A). Similarly, overexpression of a PKCε transgene curtails respiratory capacity of resting cells by down-regulating PDHC capacity (Fig. 2C). When PKCε-overexpressing cells are stimulated with growth factors, such as FCS, increased energy demands may no longer be satisfied, explaining, in part, their heightened sensitivity to fenretinide. The ablation of PKCε yields the converse phenotype: eliminating the suppressive influence on the PDHC confers resistance to programmed cell death (Figs. 2D). Although these results might reflect fixed metabolic set-points in genetically modified cells, our studies using conditional PKCδ and ε genes demonstrate the dynamic nature of PDHC regulation. For instance, when constitutively active PKCδ accumulates in mitochondria, oxidative phosphorylation increases markedly, but is diminished soon after PKCε activation is enabled by fenretinide addition. In the same vein, expression and activation of a conditional full-length PKCε gene result in PDHC suppression, and this effect is amplified when fenretinide is added. In summary, our model is consistent with the result of genetic manipulations.
It remains to be explained why retinol and fenretinide are qualitatively different from one another, the former supporting cell survival, and the latter inducing cell death. If the balance between PKCδ and PKCε were the decisive factor in the regulation of cell viability, as we propose, the question can be rephrased: what are the biochemical and/or pharmacological differences that set fenretinide apart from retinol? One preliminary answer might be found in the biphasic nature of the fenretinide response, where fenretinide stimulation over time results in the selective accumulation of PKCε in mitochondria, thus shifting the balance from an initial stimulatory, PKCδ-mediated, signal to a dominant, inhibitory, PKCε-mediated signal (Fig. 4D). It is unclear whether fenretinide accelerates the import into mitochondria, or prolongs the half-life of PKCε at that location, thereby promoting the inactivation of the PDHC. Methods are currently unavailable to measure the half-life of PKCε in mitochondria in the presence of very large pools in the cytoplasm.
The intramitochondrial interplay between PKCε and δ has been reported by others (57, 58). Most of the research has addressed their function within mitochondria of the heart (11), although similar data have been obtained in the brain (10) and kidney (45). This should come as no surprise, considering that ATP synthetic pathways are conserved in mitochondria of most tissues. Activation of PKCδ has been found to retard the recovery of the ischemic myocardium during reperfusion injury, whereas PKCε activation has been shown to be protective (52). However, in the absence of firm evidence defining the primary targets of the two PKC isoforms in mitochondria, the distinctions between one as cardioprotective (PKCε) and the other as damaging (PKCδ) must be considered preliminary until molecular details of the PKC signal network are clarified. Experiments with PKC-knockout mice have revealed a strong interdependence between the isoforms. With multiple molecular associations and the likelihood of many regulatory targets in mitochondria (12, 59, 60), PKCδ and PKCε are probably integrated into a larger metabolic network that finely tunes energy flow in a balanced manner. In support of this concept, PKCε has been shown to accumulate in mitochondria of cardiomyocytes subjected to hypoxia and to increase the activity of COX by phosphorylating its subunit COX-IV-1 (61). The finding that PKA also phosphorylates COX-IV-1 (5) emphasizes the importance of crosstalk among diverse signal systems for energy homeostasis.
During trials with fenretinide, to prevent second breast tumors in premenopausal women, a marked risk reduction was noted. However, this beneficial effect unexpectedly correlated with weight loss induced by the drug, not its hoped-for antitumor effect. Thus the reduction in breast cancer risk by chronic fenretinide treatment was attributable to reduced adiposity, with increased insulin sensitivity and markedly improved diabetic conditions afflicting these overweight women as secondary benefits (18). These provocative findings suggested that retinoids themselves were involved in the regulation of intermediary metabolism. Opposite observations, but no less relevant to an understanding of the metabolic syndrome, were raised in animal studies of type II diabetes, where it was noted that overexpression of the vitamin A transporter, RBP4, was causally related to insulin resistance and glucose intolerance (62). Remarkably, fenretinide treatment of obese mice lowered circulatory holo-RBP4 levels and led to increased glucose tolerance, reduced insulin resistance and adiposity (19).
CONCLUSIONS
Obesity and the accompanying metabolic syndrome now occur in epidemic proportions that impose a considerable medical, financial, and social burden on society. A better understanding of the pathways dysregulated in the metabolic syndrome must be considered a high medical priority. PKCδ emerged as an important factor as overabundance of this kinase in C57BL/6 mice and obese patients correlated with increased risk for type 2 diabetes, whereas inactivation of PKCδ resulted in improved glucose tolerance (63). Remarkably, inactivation of p66Shc, the adapter protein associated with PKCδ in mitochondria, resulted in a metabolic phenotype resembling that of PKCδ-knockout mice, characterized by improved glucose tolerance (64). The third component of the PKCδ signalosome, vitamin A, has also been implicated in the metabolic syndrome, since overexpression of the serum transporter, holo-RBP4, is a factor in progression to type 2 diabetes (62). While binding of holo-RBP4 to the cell surface receptor, STRA6 (65–67) was shown to activate a transcriptional program affecting energy homeostasis and insulin responsiveness (68), it seems appropriate to ask whether the cargo carried by RBP4, namely vitamin A, has an additional, independent impact on the genesis and/or progression of the metabolic syndrome. Our finding supports the view that vitamin A is a potent coactivator of the PKCδ signalosome that drives respiration. Chronic acceleration of fuel flux in the PDHC and of electron flow beyond the capacity of the respiratory chain might result in the release of harmful oxygen radicals. Many investigators consider these as prime factors in the etiology of the metabolic syndrome and coincidentally also as strong risk enhancers for ischemia reperfusion injury and coronary heart disease in which PKCδ has been implicated (69, 70).
Supplementary Material
Acknowledgments
The authors acknowledge the generous gifts by Dr. P. Parker (Cancer Research UK London Institute, London, UK) of the PKCε−/− and the PKCε-overexpressing cell lines, and by Dr. U. Kikkawa (Biosignal Research Centre, Kobe University, Kobe, Japan) of the PKCδ Y332F mutant cell line. The authors also thank Dr. Gavril Pasternak and Dr. Richard Kolesnick for their advice on binding affinity measurements.
This work was supported by grants from the U.S. National Institutes of Health, DK-089348 (to U.H.) and GM-088999 (to G.M.), and from the Muscular Dystrophy Association.
Author contributions: G.M. and U.H. designed research; J.G., B.H., R.A-P., F.Z., E.S., and V.V. performed research; M.L. provided research materials; D.F., G.M., and UH wrote the article. The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- CoA
- coenzyme A
- COX
- cytochrome c oxidase
- CRBP
- cellular retinol binding protein
- ETC
- electron transfer chain
- MEF
- mouse embryo fibroblast
- Fen
- fenretinide
- NADH
- nicotine adenine dinucleotide (reduced form)
- GST
- glutathione-S-transferase
- PAGE
- polyacrylamide gel electrophoresis
- PDHC
- pyruvate dehydrogenase complex
- PDK
- pyruvate dehydrogenase kinase
- PDP
- pyruvate dehydrogenase phosphatase
- PKC
- protein kinase C
- PKCδ cat
- PKCδ catalytic domain
- PMA
- phorbol-myristoyl-acetate
- RBP
- retinol binding protein
- Rol
- retinol
- SDS
- sodium dodecyl sulfate
- TCA
- tricarboxylic acid
- WT
- wild type
REFERENCES
- 1. Holness M. J., Sugden M. C. (2003) Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem. Soc. Trans. 31, 1143–1151 [DOI] [PubMed] [Google Scholar]
- 2. Bowker-Kinley M. M., Davis W. I., Wu P., Harris R. A., Popov K. M. (1998) Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem. J. 329, 191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang B., Gudi R., Wu P., Harris R. A., Hamilton J., Popov K. M. (1998) Isoenzymes of pyruvate dehydrogenase phosphatase. DNA-derived amino acid sequences, expression, and regulation. J. Biol. Chem. 273, 17680–17688 [DOI] [PubMed] [Google Scholar]
- 4. D'Aurelio M., Pallotti F., Barrientos A., Gajewski C. D., Kwong J. Q., Bruno C., Beal M. F., Manfredi G. (2001) In vivo regulation of oxidative phosphorylation in cells harboring a stop-codon mutation in mitochondrial DNA-encoded cytochrome c oxidase subunit I. J. Biol. Chem. 276, 46925–46932 [DOI] [PubMed] [Google Scholar]
- 5. Acin-Perez R., Salazar E., Kamenetsky M., Buck J., Levin L. R., Manfredi G. (2009) Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 9, 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Acin-Perez R., Hoyos B., Zhao F., Vinogradov V., Fischman D. A., Harris R. A., Leitges M., Wongsiriroy N., Blaner W. S., Manfredi G., Hammerling U. (2010) Control of oxidative phosphorylation by vitamin A illuminates a fundamental role in mitochondrial energy homoeostasis. FASEB J. 24, 627–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Acin-Perez R., Hoyos B., Gong J., Vinogradov V., Fischman D. A., Leitges M., Borhan B., Starkov A., Manfredi G., Hammerling U. (2010) Regulation of intermediary metabolism by the PKCdelta signalosome in mitochondria. FASEB J. 24, 5033–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoyos B., Acin-Perez R., Fischman D. A., Manfredi G., Hammerling U. (2011) Hiding in plain sight: uncovering a new function of vitamin A in redox signaling. Biochim. Biophys. Acta 1821, 241–247 [DOI] [PubMed] [Google Scholar]
- 9. Chen L., Hahn H., Wu G., Chen C. H., Liron T., Schechtman D., Cavallaro G., Banci L., Guo Y., Bolli R., Dorn G. W., 2nd, Mochly-Rosen D. (2001) Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc. Natl. Acad. Sci. U. S. A. 98, 11114–11119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bright R., Mochley-Rosen D. (2005) The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke 36, 2781–2790 [DOI] [PubMed] [Google Scholar]
- 11. Budas G. R., Churchil E. N., Mochley-Rosen D. (2007) Cardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharm. Res. 55, 523–536 [DOI] [PubMed] [Google Scholar]
- 12. Ping P., Zhang J., Pierce W. M., Jr., Bolli R. (2001) Functional proteomic analysis of protein kinase C epsilon signaling complexes in the normal heart and during cardioprotection. Circ. Res. 88, 59–62 [DOI] [PubMed] [Google Scholar]
- 13. Buck J., Grun F., Kimura S., Noy N., Derguini F., Hammerling U. (1993) Anhydroretinol: A naturally occurring inhibitor of lymphocyte physiology. J. Exp. Med. 178, 675–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen Y., Buck J., Derguini F. (1999) Anhydroretinol induces oxidative stress and cell death. Cancer Res. 59, 3985–3990 [PubMed] [Google Scholar]
- 15. Chiu H.-J., Fischman D. A., Hammerling U. (2008) Vitamin A-depletion causes oxidative stress, mitochondrial dysfunction and PARP-1-dependent energy deprivation. FASEB J. 22, 3738–3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kurie J., Lee J. S., Khuri F. R., Mao L., Morice R., Lee J. R., Walsh G. L., Broxson A., Lippman S. M., Ro J. Y., Kemp B. L., Liu D., Fritsche H., Xu X., Lotan R., Hong W.K. (2000) N-(4-hydroxyphenyl)retinamide in the chemoprevention of squamous metaplasia and dysplasia of the bronchial epithelium 1. Clin. Cancer Res. 6, 2973–2979 [PubMed] [Google Scholar]
- 17. Hail N. J., Kim H. J., Lotan R. (2006) Mechanisms of fenretinide-induced apoptosis. Apoptosis 11, 1677–1694 [DOI] [PubMed] [Google Scholar]
- 18. Johansson H., Gandini S., Guerrieri-Gonzaga A., Iodice S., Ruscica M., Bonanni B., Gulisano M., Magni P., Formelli F., Decensi A. (2008) Effect of fenretinide and low-dose tamoxifen on insulin sensitivity in premenopausal women at high risk for breast cancer. Cancer Res. 68, 9512–9518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Preitner F., Mody N., Graham T. E., Peroni O. D., Kahn B. B. (2009) Long-term fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis. Am. J. Physiol. Endocrinol. Metab. 297, E1420–E1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morita M., Matsuzaki H., Yamamoto T., Fukami Y., Kikkawa U. (2008) Epidermal growth factor receptor phosphorylates protein kinase Cδ at Tyr332 to form a trimeric complex with p66Shc in the H2O2-stimulated cells. J. Biol. Chem. 143, 31–38 [DOI] [PubMed] [Google Scholar]
- 21. Giorgio M., Migliaccio E., Orsini F., Paolucci D., Moroni M., Contursi C., Pellicia G., Luzi L., Minucci S., Marcaccio M., Pinton P., Rizzuto R., Bernardi P., Paolucci F., Pelicci P. G. (2005) Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122, 221–233 [DOI] [PubMed] [Google Scholar]
- 22. Hoyos B., Jiang S., Hammerling U. (2005) Location and functional significance of retinol binding sites on the serine/threonine kinase, cRaf. J. Biol. Chem. 280, 6872–6878 [DOI] [PubMed] [Google Scholar]
- 23. Norris A. W., Cheng L., Giguere V., Rosenberger M., Li E. (1994) Measurement of subnanomolar retinoic acid binding affinities for cellular retinoic acid binding proteins by fluorometric titration. Biochim. Biophys. Acta 1209, 10–18 [DOI] [PubMed] [Google Scholar]
- 24. Yin X., Zafrullah M., Lee H., Haimovitz-Friedman A., Fuks Z., Kolesnick R. (2009) A ceramide-binding C1 domain mediates kinase suppressor of ras membrane translocation. Cell. Physiol. Biochem. 24, 219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ivaska J., Whelan R. D., Watson R., Parker P. J. (2002) PKC epsilon controls the traffic of beta1 integrins in motile cells. EMBO J. 21, 3608–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ghayur T., Hugunin M., Talanian R. V., Ratnofsky S., Quinlan C., Emoto Y., Pandey P., Datta R., Huang Y., Kharbanda S., Allen H., Kamen R., Wong W., Kufe D. (1996) Proteolytic activation of protein kinase Cδ by an ICE/CED3-like protease induces characteristics of apoptosis. J. Exp. Med. 184, 2399–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Szulc J., Wiznerowicz M., Sauvain M. O., Trono D., Aebischer P. (2006) A versatile tool for conditional gene expression and knockdown. Nat. Methods 3, 109–116 [DOI] [PubMed] [Google Scholar]
- 28. Fernandez-Vizarra E., Lopez-Perez M. J., Enriquez J. A. (2002) Isolation of biogenetically competent mitochondria from mammalian tissues and cultured cells. Methods 26, 292–297 [DOI] [PubMed] [Google Scholar]
- 29. Hofhaus G., Johns D. R., Hurko O., Attardi G., Chomyn A. (1996) Respiration and growth defects in transmitochondrial cell lines carrying the 11778 mutation associated with Leber's hereditary optic neuropathy. J. Biol. Chem. 271, 13155–13161 [DOI] [PubMed] [Google Scholar]
- 30. Vives-Bauza C., Yang L., Manfredi G. (2007) Assay of mitochondrial ATP synthesis in animal cells and tissues. Methods Cell Biol. 80, 155–171 [DOI] [PubMed] [Google Scholar]
- 31. Leitges M., Mayr M., Braun U., Mayr U., Li C., Pfister G., Ghaffari-Tabrizi N., Baier G., Hu Y., Xu Q. (2001) Exacerbated vein graft arteriosclerosis in protein kinase Cdelta-null mice. J. Clin. Invest. 108, 1505–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Imam A., Hoyos B., Swenson C., Chua R., Levi E., Viriya E., Hammerling U. (2001) Retinoids as ligands and coactivators of protein kinase C alpha. FASEB J. 15, 28–30 [DOI] [PubMed] [Google Scholar]
- 33. Kikkawa U., Matsuzaki H., Yamamoto T. (2002) Protein kinase Cδ (PKCδ): activation mechanisms and functions. J. Biochem. 132, 831–839 [DOI] [PubMed] [Google Scholar]
- 34. Le Good J. A., Ziegler W. H., Parekh D. B., Alessi D. R., Cohen P., Parker P. J. (1998) Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281, 2042–2045 [DOI] [PubMed] [Google Scholar]
- 35. Gschwendt M., Muller H. J., Kielbassa K., Zang R., Kittstein W., Rincke G., Marks F. (1994) Rottlerin, a novel protein kinase inhibitor. Biochem. Biophys. Res. Commun. 199, 93–98 [DOI] [PubMed] [Google Scholar]
- 36. Gudi R., Bowker-Kinkley M. M., Kedishvili N. Y., Zhao Y., Popov K. M. (1995) Diversity of the pyruvate dehydrogenase kinase gene family in humans. J. Biol. Chem. 270, 28989–28994 [DOI] [PubMed] [Google Scholar]
- 37. Patel M. S., Roche T. E. (1990) Molecular biology and biochemistry of pyruvate dehydrogenase complexes. FASEB J. 4, 3224–3233 [DOI] [PubMed] [Google Scholar]
- 38. Patel M. S., Korochkina L. G. (2001) Regulation of mammalian pyruvate dehydrogenase complex by phosphorylation: complexity of multiple phosphorylation sites and kinases. Exp. Mol. Med. 33, 191–197 [DOI] [PubMed] [Google Scholar]
- 39. Kolobova E., Tuganova A., Boulatnikov I., Popov K. M. (2001) Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites. Biochem. J. 358, 69–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patel M. S., Korotchkina L. G. (2006) Regulation of the pyruvate dehydrogenase complex. Biochem. Soc. Trans. 34, 217–222 [DOI] [PubMed] [Google Scholar]
- 41. Harris R. A., Bowker-Kinley M. M., Huang B., Wu P. (2002) Regulation of the activity of the pyruvate dehydrogenase complex. Adv. Enzyme Regul. 42, 249–259 [DOI] [PubMed] [Google Scholar]
- 42. Halim N. D., McFate T., Mohyeldin A., Okagaki P., Korotchkina L. G., Patel M. S., Jeoung N. H., Harris R. A., Schell M. J., Verma A. (2010) Phosphorylation status of pyruvate dehydrogenase distinguishes metabolic phenotypes of cultured rat brain astrocytes and neurons. Glia 58, 1168–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hu Y., Kang C., Philp R., Li B. (2007) PKCδ phosphorylates p52ShcA at Ser29 to regulate ERK activation in response to H2O2. Cell. Signal. 19, 410–418 [DOI] [PubMed] [Google Scholar]
- 44. O'Connell M., Chua R., Hoyod B., Buck J., Chen C. Q., Derguini F., Hammerling U. (1996) Retro-retinoids in regulated cell growth and death. J. Exp. Med. 184, 549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nowak G., Bakajsova D., Samarel A. M. (2011) Protein kinase C-ε activation induces mitochondrial dysfunction and mitochondrial fragmentation in renal proximal tubules. Am. J. Physiol. Renal Physiol. 301, F197–F208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Noy N., Xu Z. J. (1990) Thermodynamic parameters of the binding of retinol to binding proteins and to membranes. Biochemistry 29, 3888–3892 [DOI] [PubMed] [Google Scholar]
- 47. Majumder P. K., Mishra N. C., Sun X., Bharti A., Kharbanda S., Saxena S., Kufe D. (2001) Targeting of protein kinase C delta to mitochondria in the oxidative stress response. Cell Growth Differ. 12, 465–470 [PubMed] [Google Scholar]
- 48. Vander Heiden M. G., Chandel N. S., Li X. X., Schumacker P. T., Colombini M., Thompson C. B. (2000) Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc. Natl. Acad. Sci. U. S. A. 97, 4666–4671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Walsh D. A., Cooper R. H., Denton R. M., Bridges B. J., Randle P. J. (1976) The elementary reactions of the pig heart pyruvate dehydrogenase complex. A study of the inhibition by phosphorylation. Biochem. J. 157, 41–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Salvi M., Brunati A. M., Toninello A. (2005) Tyrosine phosphorylation in mitochondria: a new frontier in mitochondrial signaling. Free Radic. Biol. Med. 38, 1267–1277 [DOI] [PubMed] [Google Scholar]
- 51. Pagliarini D. J., Wiley S. E., Kimple M. E., Dixon J. R., Kelly P., Worby C. A., Casey P. J., Dixon J. E. (2005) Involvement of a mitochondrial phosphatase in the regulation of ATP production and insulin secretion in pancreatic beta cells. Mol. Cell. 19, 197–207 [DOI] [PubMed] [Google Scholar]
- 52. Palaniyandi S. S., Sun L., Ferreira J. C., Mochly-Rosen D. (2009) Protein kinase C in heart failure: a therapeutic target? Cardiovasc. Res. 82, 229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Noy N., Blaner W. S. (1991) Interactions of retinol with binding proteins: studies with rat cellular retinol-binding protein and with rat retinol-binding protein. Biochemistry 30, 6380–6386 [DOI] [PubMed] [Google Scholar]
- 54. Zhao F., Ilbert M., Varadan R., Cremers C. M., Hoyos B., Acin-Perez R., Vinogradov V., Cowburn D., Jakob U., Hammerling U. (2011) Are zinc-finger domains of protein kinase C dynamic structures that unfold by lipid or redox activation? Antioxid. Redox Signal. 14, 757–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Korichneva I., Hammerling U. (1999) F-actin as a functional target for retro-retinoids: a potential role in anhydroretinol-triggered cell death. J. Cell Sci. 112, 2521–2528 [DOI] [PubMed] [Google Scholar]
- 56. Korichneva I., Waka J., Hammerling U. (2003) Regulation of the cardiac mitochondrial membrane potential by retinoids. J. Pharmacol. Exp. Ther. 305, 426–433 [DOI] [PubMed] [Google Scholar]
- 57. Mayr M., Liem D., Zhang J., Li X., Avliyakulov N. K., Yang J. I., Young G., Vondriska T. M., Ladroue C., Madhu B., Griffiths J. R., Gomes A., Xu Q., Ping P. (2009) Proteomic and metabolomic analysis of cardioprotection: Interplay between protein kinase C epsilon and delta in regulating glucose metabolism of murine hearts. J. Mol. Cell. Cardiol. 46, 268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Frangioudakis G., Burchfield J. G., Narasimhan S., Cooney G. J., Leitges M., Biden T. J., Schmitz-Peiffer C. (2009) Diverse roles for protein kinase C delta and protein kinase C epsilon in the generation of high-fat-diet-induced glucose intolerance in mice: regulation of lipogenesis by protein kinase C delta. Diabetologia 52, 2616–2620 [DOI] [PubMed] [Google Scholar]
- 59. Guo D., Nguyen T., Ogbi M., Tawfik H., Ma G., Yu Q., Caldwell R. W., Johnson J. A. (2007) Protein kinase C-epsilon coimmunoprecipitates with cytochrome oxidase subunit IV and is associated with improved cytochrome-c oxidase activity and cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 293, H2219–H2230 [DOI] [PubMed] [Google Scholar]
- 60. Barnett M., Lin D., Akoyev V., Willard L., Takemoto D. (2008) Protein kinase C epsilon activates lens mitochondrial cytochrome c oxidase subunit IV during hypoxia. Exp. Eye Res. 86, 226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ogbi M., Johnson J. A. (2006) Protein kinase C epsilon interacts with cytochrome c oxidase subunit IV and enhances cytochrome c oxidase activity in neonatal cardiac myocyte preconditioning. Biochem. J. 393, 191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang Q., Graham T. E., Mody N., Pretriner F., Peroni O. D., Zabolotny J. M., Kotani K., Kahn B. B. (2005) Serum retinol binding protein 4 contributes to insulin resistance in obesity and type II diabetes. Nature 436, 356–362 [DOI] [PubMed] [Google Scholar]
- 63. Bezy O., Tran T. T., Pihlajamaki J., Suzuki R., Emanuelli B., Winnay J., Mori M. A., Haas J., Biddinger S. B., Leitges M., Goldfine A. B., Patti M. E., King G. L., Kahn C. R. (2011) PKCdelta regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans. J. Clin. Invest. 121, 2504–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ranieri S. C., Fusco S., Panieri E., Labate V., Mele M., Tesori V., Ferrara A. M., Maulucci G., De Spirito M., Martorana G. E., Galeotti T., Pani G. (2010) Mammalian life-span determinant p66shcA mediates obesity-induced insulin resistance. Proc. Natl. Acad. Sci. U. S. A. 107, 13420–13425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bouillet P., Sapin V., Chazaud C., Messaddeq N., Decimo D., Dolle P., Chambon P. (1997) Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech. Dev. 63, 173–186 [DOI] [PubMed] [Google Scholar]
- 66. Sapin V., Bouillet P., Oulad-Abdelghani M., Dastugue B., Chambon P., Dolle P. (2000) Differential expression of retinoic acid-inducible (Stra) genes during mouse placentation. Mech. Dev. 92, 295–299 [DOI] [PubMed] [Google Scholar]
- 67. Blaner W. S. (2007) STRA6, a cell-surface receptor for retinol-binding protein: the plot thickens. Cell Metab. 5, 164–166 [DOI] [PubMed] [Google Scholar]
- 68. Berry D. C., Jin H., Majumdar A., Noy N. (2011) Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc. Natl. Acad. Sci. U. S. A. 108, 4340–4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hayden M. R., Tyagi S. C. (2004) Homocysteine and reactive oxygen species in metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: the pleiotropic effects of folate supplementation. Nutr. J. 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Churchill E. N., Murriel C. L., Chen C. H., Mochley-Rosen D., Szweda L. I. (2005) Reperfusion-induced translocation of delta-PKC to cardiac mitochondria prevents pyruvate dehydrogenase reactivation. Circ. Res. 97, 78–85 [DOI] [PubMed] [Google Scholar]
- 71. Hoyos B., Imam A., Chua R., Swenson C., Tong G.-X., Levi E., Noy N., Hammerling U. (2000) The cysteine-rich regions of the regulatory domains of Raf and protein kinase C as retinoid receptors. J. Exp. Med. 192, 835–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.