Abstract
G-protein-coupled receptors with dissociable agonists for thyrotropin, parathyroid hormone, and sphingosine-1-phosphate were found to signal persistently hours after agonist withdrawal. Here we show that mouse thyrotropin-releasing hormone (TRH) receptors, subtypes 2 and 1(TRH-R2 and TRH-R1), can signal persistently in HEK-EM293 cells under appropriate conditions, but TRH-R2 exhibits higher persistent signaling activity. Both receptors couple primarily to Gαq/11. To gain insight into the mechanism of persistent signaling, we compared proximal steps of inositolmonophosphate (IP1) signaling by TRH-Rs. Persistent signaling was not caused by slower dissociation of TRH from TRH-R2 (t1/2=77±8.1 min) compared with TRH-R1 (t1/2=82±12 min) and was independent of internalization, as inhibition of internalization did not affect persistent signaling (115% of control), but required continuously activated receptors, as an inverse agonist decreased persistent signaling by 60%. Gαq/11 knockdown decreased persistent signaling by TRH-R2 by 82%, and overexpression of Gαq/11 induced persistent signaling in cells expressing TRH-R1. Lastly, persistent signaling was induced in cells expressing high levels of TRH-R1. We suggest that persistent signaling by TRHRs is exhibited when sufficient levels of agonist/receptor/G-protein complexes are established and maintained and that TRH-R2 forms and maintains these complexes more efficiently than TRH-R1.—Boutin, A., Allen, M. D., Neumann, S., Gershengorn, M. C. Persistent signaling by thyrotropin-releasing hormone receptors correlates with G-protein and receptor levels.
Keywords: TRH receptor, inositolmonophosphate
Traditionally, it is thought that G-protein-coupled receptors (GPCRs) with dissociable agonists signal transiently at the cell surface and that the signaling pathway is rapidly desensitized by several mechanisms including receptor internalization (1). However, recent studies showed that certain GPCRs continue to either stimulate (via Gαs) or inhibit (via Gαi) cAMP signaling (2–6) or stimulate (via Gαq/11) phosphoinositide signaling (7). The mechanism of persistent signaling, however, remains unclear. In one of the studies on the thyrotropin [thyroid-stimulating hormone (TSH)] receptor (TSHR), Calebiro et al. (2) proposed that in mouse thyrocytes receptor internalization is required to ensure sustained cAMP production and that TSHRs internalized in close association with Gαs subunits and adenylyl cyclase; they recently reported that human embryonic kidney 293 (HEK293) cells do not exhibit persistent signaling by TSHR (8). By contrast, our previous findings (5, 7) in HEK-EM293 cells stably expressing TSHRs have shown that TSHR is capable of signaling persistently via both Gαs and Gαq/11 pathways and that this signaling is independent of internalization.
Constitutive (or agonist-independent or basal) signaling has also been found to be exhibited by some, but not all, GPCRs (9) and by mutant GPCRs that cause disease (10). Acknowledgment that constitutive activity required the formation of an agonist-unoccupied activated GPCR/G-protein complex led to development of the extended ternary complex model of GPCR signaling (11).
Thyrotropin-releasing hormone (TRH) receptors, subtypes 1 and 2 (TRH-R1 and TRH-R2), are GPCRs that signal primarily via the Gαq/11 pathway, which activates phospholipase C and leads to generation of inositol 1,4,5-triphosphate that is rapidly degraded to inositolmonophosphate (IP1; ref. 12). TRH-R2, but not TRH-R1, was found to signal constitutively in several cell systems (13–14). TRH-Rs have not been previously studied with regard to persistent signaling.
Here we show that TRH-R2 and TRH-R1 can signal persistently in HEK-EM293 cells under appropriate conditions, but TRH-R2 exhibits higher persistent signaling activity than TRH-R1. In an attempt to elucidate the mechanism of persistent signaling, we compared the proximal steps in signaling by these two subtypes of TRH-Rs and showed that persistent signaling by TRH-Rs correlates with the levels of either Gαq or Gα11 or receptors expressed in the cells.
MATERIALS AND METHODS
Cell culture, transfection, and mutagenesis
HEK-EM293 cells (15) stably expressing mouse TRH-R1 (0.41±0.041×105 sites/cell) or TRH-R2 (1.8±0.12×105 sites/cell) were grown in DMEM supplemented with 10% FBS; 100 U/ml penicillin and 10 μg/ml streptomycin (both from Invitrogen, Carlsbad, CA, USA); and 250 μg/ml hygromycin B (Invitrogen) at 37°C in a humidified 5% CO2 incubator.
HEK-EM293 cells were transiently transfected by plating 7.5 × 104 cells/well in 24-well tissue culture plates or 2.0 × 105 cells on poly-d-lysine-coated coverglass culture dishes (MatTek Corp., Ashland, MA, USA) and using FuGene 6 reagent (Roche, Indianapolis, IN, USA) with 0.025–0.5 μg plasmid/well or 0.8–2.0 μg plasmid/dish. Plasmids containing β-arrestin-2 (βArr2) and dominant-negative dynamin mutant (K44A) were kindly provided by Dr. Marc Caron (Duke University Medical Center, Durham, NC, USA); Gαq–yellow fluorescent protein (YFP) chimera (16) was a gift from Dr. Catherine Berlot (Weis Center for Research, Geisinger Clinic, Danville, PA, USA). The cDNA encoding TRH-R1 and TRH-R2 was in the mammalian cell expression vector pcDNA3.1(+). In TRH-R2, Trp267 was replaced by alanine (TRH-R2-W267A) using the QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA). TRH-R1 and TRH-R2 were also cloned into pAcGFP1-N1 vector (Clontech Laboratories, Inc., Mountain View, CA, USA) to obtain receptor-GFP or -mCherry [red fluorescent protein (RFP)] fusions used for fluorescent microscopy. mCherry plasmid was kindly provided by Dr. Jennifer Lippincott-Schwartz (National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, Bethesda, MD, USA; ref. 17). Plasmids encoding G proteins αs, αq, and α11 with an internal Glu-Glu (EE) epitope tag were purchased from Missouri S&T cDNA Resource Center (Rolla, MO, USA). Empty vector pcDNA3.1(+) was used as a mock-transfection control. Experiments were performed 48 or 72 h after transfection.
Infection with AdCMVmTRH-R1
The virus AdCMVmTRH-R1 was constructed by overlap recombination of pAdCMVmTRH-R1 in HEK-EM293 cells as described previously (18). We used AdCMVmTRH-R1 to transiently express TRH-R1 to very high levels. For infection, 1.5 × 105 HEK-EM293 cells were seeded in 24-well-plates in DMEM supplemented with 10% FBS and were incubated in a humidified atmosphere of 5% CO at 37°C. After 24 h, the medium was aspirated and replaced with 2% FBS DMEM containing 0, 1, 3, or 10 multiplicity of infection (MOI)/cell, and the cells were incubated at 37°C overnight.
IP1 production
The protocol to measure persistent IP1 production, which is defined as continued IP1 production above basal after washout of agonist, was described previously (5, 7). Briefly, cells seeded into 24-well plates at a density of 2.2 × 105 cell/well were cultured for 24 h (details for the experiments that required transfections are described above). Then, they were washed with 37°C HBSS and incubated in HBSS/10 mM HEPES, pH 7.4, for 30 min. Thereafter, acute TRH stimulation was measured as IP1 production in cells incubated in a humidified incubator at 37°C for 1 h in HBSS/HEPES containing 1 μM TRH (Sigma, St. Louis, MO, USA) and 50 mM LiCl. To determine the effects of persistent TRH stimulation, we measured IP1 production in cells pretreated without (control) or with 1 μM TRH (or with 0, 0.01, 0.03, 0.1, 0.3, 1, 3, 10, 30, 100, 300, and 1000 nM TRH for the dose-response curve) for 30 min (pretreatment), washed 3 times with cold HBSS followed by 1 wash with HBSS at room temperature, and incubated in HBSS/HEPES at 37°C for 1 h (washout). After 1 h or at the designated times (1, 2, 4, 6 h, and overnight to determine the time course of loss of persistent signaling after the washes), cells were treated with HBSS/HEPES containing 50 mM LiCl with or without 1 μM TRH at 37°C for 30–90 min (treatment). To determine the effects of TRH-R antagonist 2 (19), the washout was performed in 2 steps: 45 min of HBSS/HEPES incubation (first phase) and 15 min with no addition or with 10 or 30 μM 2 (second phase). After both washout phases, the buffers were aspirated, and the cells were incubated in HBSS/HEPES with LiCl and without or with 10 or 30 μM antagonist 2. To determine the time of acquisition of persistent signaling, the TRH pretreatment was performed for 0, 1, 2.5, 10, or 30 min. Basal IP1 signaling of the receptors was measured after incubation of the cells in HBSS/HEPES supplemented with 50 mM LiCl for 30, 60, 90, 120, 180, and 240 min. IP1 production was stopped by addition 0.05 ml lysis buffer (IP-One ELISA kit; CIS Bio International, Gif-Sur-Yvette, France). IP1 content in the samples was determined according to the manufacturer's protocol. Optical density was measured in SpectraMax Plus384 (Molecular Devices, Sunnyvale, CA, USA).
Radioligand binding
To determine the rate of dissociation of the radiolabeled TRH, cells were incubated in binding buffer (HBSS/HEPES) containing 10 nM [3H]TRH (American Radiolabeled Chemicals, St. Louis, MO, USA) for 1 h at 37°C and then washed 3 times with ice-cold acetic acid/Na acetate buffer, pH 2.8. After the washes, cells were incubated in HBSS/HEPES at 37°C for 0, 15, 30, 60, and 90 min and then washed with ice-cold HBSS and solubilized in 0.4 N NaOH. Total binding was measured in the absence of unlabeled TRH and nonspecific binding in the presence of 10 μM unlabeled TRH or 1 μM MeTRH. Specific binding (total minus nonspecific binding) was calculated as a percentage of time 0. For cells transiently expressing TRH-Rs, [3H]MeTRH (Perkin Elmer, Waltham, MA, USA) binding was measured 48 h after transfection with plasmid containing genes for TRH-R1 or TRH-R2. To measure loss of cell surface binding, cells were incubated with 20 nM TRH for 30 min at 37°C, washed 3 times with ice-cold acetic acid/Na acetate buffer (pH 2.8), and washed 3 times with ice-cold HBSS. Next, cells were incubated with 2 nM [3H]MeTRH alone or with 10 μM unlabeled MeTRH for nonspecific binding for 2 h at 4°C, washed 3 times with ice-cold HBSS, solubilized, and counted. After subtraction of nonspecific binding, the down-regulation of cell surface binding was calculated as a percentage of control.
Live-cell imaging
To monitor receptor internalization, TRH-R1 or TRH-R2 tagged with GFP was cotransfected with Mock, βArr2, or K44A in HEK-EM293 cells. After 24 h, the cells were incubated without or with TRH in HBSS-HEPES for 30 min at 37°C and visualized. Cells were imaged on a Zeiss 510 NLO/Meta system, using a Plan-Neofluor ×40/N.A. 1.3 objective (Carl Zeiss, Oberkochen, Germany). The pinhole was completely open to generate epifluorescent images. GFP was excited with a 477-nm laser at 75% transmission and detected using a 510/20 emission filter. Images were acquired before addition of TRH and after 30 min incubation with the ligand.
To detect localizations of TRH-R subtypes and Gαq, cells were transiently transfected with plasmids carrying the genes for βArr2, Gαq tagged with YFP (Citrine) plus either TRH-R1 or TRH-R2 tagged with mCherry (RFP). Cells were transfected for 24 h before growth medium was replaced by HBSS/HEPES. Cells were visualized on a Zeiss Observer. Z1 microscope with a Yokogawa spinning disc module using an Apochromat ×40/N.A. 1.3 objective (Carl Zeiss). YFP was excited with a 488-nm laser at 90% transmission. YFP emission was detected through a 525/50 bandpass emission filter. RFP was excited with a 560-nm laser at 80% transmission before emission passed through a 629/62 bandpass filter. Exposure times for both channels were set to 500 ms. Microscope parameters remained unchanged throughout all experiments.
IP1 assay was used to confirm the functionality of GFP- and RFP-labeled TRH-Rs and Gαq-YFP.
Western blot analysis
Cell lysates (15–20 μg of protein) were separated on 4–12% NuPAGE Bis-Tris gels and electrophoretically transferred to nitrocellulose membranes (both Invitrogen). Blots were blocked with 5% milk in PBS containing 0.1% Tween 20 and then incubated with primary Glu-Glu monoclonal antibody (Covance, Emeryville, CA, USA) or polyclonal antibody to GNAQ + GNA11 (Abcam, Cambridge, MA, USA). After being washed and secondary antibody incubation, membranes were developed with Pierce SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL, USA) to detect the protein of interest.
RNA interference
siRNA duplexes with sequences specifically targeting Gαq and Gα11 mRNA were purchased from Dharmacon (Lafayette, CO, USA). A day before siRNA treatment, cells were seeded in 24-well plates at 7.5 × 104 cells/well. siRNA duplexes at a concentration of 50 nM were then transfected into TRH-R2 cells using DharmaFECT (Dharmacon) for 72 h before experiments. siRNA-mediated mRNA silencing was evaluated by qPCR and Western blot. Percentage of knockdown was calculated using the ΔΔCT method normalized to GAPDH reference gene and the controls.
Statistical analysis
The data were analyzed by Student's t test or 1-way ANOVA; values of P < 0.05 were considered significant.
RESULTS
Persistent phosphoinositide signaling By TRH-Rs
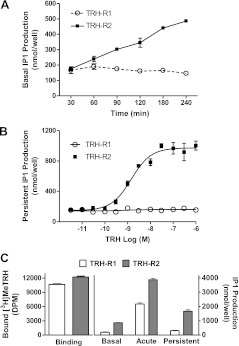
It is known that TRH-R2, but not TRH-R1 (14), exhibits basal signaling activity. With the use of IP1 production as a readout of signaling, HEK-EM293 cells stably expressing TRH-R2 exhibited measurable basal signaling when the incubation time was longer than 60 min, whereas basal IP1 production by cells stably expressing TRH-R1 was not measurable (Fig. 1A).
Figure 1.
TRH-stimulated IP1 persistent signaling in HEK-EM293 cells expressing TRH-R2 or TRH-R1. A) Basal signaling activity of TRH-R1 and TRH-R2. Basal IP1 production was measured in cells stably expressing TRH-R1 or TRH-R2 in the presence of 50 mM LiCl. At the time points indicated, cells were lysed, and IP1 levels were measured as described in Materials and Methods. Data are expressed as means ± se of assays performed in triplicate in 3 experiments. B) Persistent signaling dose response to TRH in cells stably expressing TRH-R1 or TRH-R2. For the pretreatment phase, TRH-R1- or TRH-R2-expressing cells were treated with the noted concentrations of TRH for 30 min, and persistent IP1 production was measured as described in Materials and Methods. Data are from 1 of 3 experiments with duplicate samples and are presented as means ± range. C) Comparison of signaling by TRH-R1 and TRH-R2 expressed transiently at similar levels in HEK-EM293 cells. Cells were transfected with 0.45 and 0.065 μg of plasmids containing genes for TRH-R1 and TRH-R2, respectively. After 48 h, binding and signaling experiments were performed as described in Materials and Methods with 90 min production phase. Figure represents data from 1 of 2 experiments. Bars are means ± sd of triplicate measurements. DPM, disintegrations per minute.
Using our experimental protocol to measure persistent IP1 production, we found that cells stably expressing TRH-R2, but not cells stably expressing TRH-R1, were stimulated in a dose-dependent manner to exhibit persistent signaling for ≥1 h after TRH removal (Fig. 1B). The determined dose dependency for persistent signaling was similar to that of acute stimulation of TRH-R2 by TRH (20).
Since TRH-R2 expression levels in our HEK-EM293 stable cell lines are 4 times higher than those of TRH-R1, we next determined whether different receptor numbers accounted for the differences observed in persistent signaling by TRH-Rs. We performed a series of transient transfections to determine the amounts of plasmid required for equal TRH-R1 and TRH-R2 expression levels. We found that TRH-R2 was more highly expressed than TRH-R1 at equal amounts of transfected plasmid (data not shown). We were able to achieve similar expression levels using 0.45 and 0.065 μg of plasmid containing cDNA for TRH-R1 and TRH-R2, respectively (Fig. 1C). After 90 min incubation with LiCl, we observed 4.5-fold increase over background in basal IP1 production by cells expressing TRH-R2, whereas there was no measurable basal IP1 production in cells expressing TRH-R1. Acute TRH stimulation by TRH-R1 and TRH-R2 was 11- and 20-fold above background, respectively. TRH-R2 exhibited persistent signaling 8.6-fold over background following removal of TRH, whereas persistent IP1 production in cells expressing TRH-R1 was only up to 1.6-fold over background (Fig. 1C). Thus, we showed that at similar levels of receptor expression in HEK-EM293 cells, TRH-R2 exhibited higher levels of persistent signaling than TRH-R1 although cells expressing TRH-R1 could be made to signal persistently (see below also).
Next, we sought to examine the time courses of acquisition and loss of persistent signaling stimulated by 1 μM TRH. We found that activation of persistent IP1 production by cells expressing TRH-R2 reached its maximum after 1 min of stimulation and then remained constant for up to 30 min (Supplemental Fig. S1A) and that the level of persistent IP1 production fell progressively after a peak at 1 h, declining at a rate of ∼10%/h, and had returned to basal levels after 23 h (Supplemental Fig. S1B).
We compared dissociation rates of TRH from TRH-R1 and TRH-R2 to determine whether the differences in persistent signaling were caused by different rates of TRH dissociation. We found that the rates of dissociation of [3H]-labeled TRH from cells stably expressing TRH-R1 or TRH-R2 were similar (t1/2=77±8.1 min for TRH-R2 compared with t1/2=82±12 min for TRH-R1; Supplemental Fig. S2) and therefore did not explain the differences in signaling.
TRH-R2 persistent IP1 production does not depend on internalization
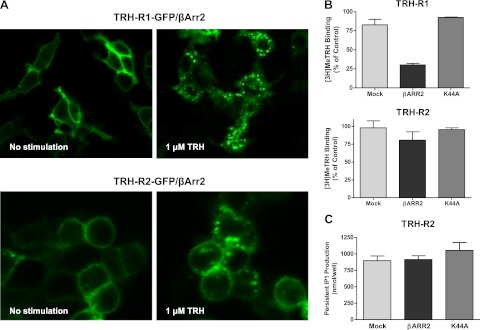
We have previously shown that persistent cAMP and IP1 production by TSHR was not affected by receptor internalization (5, 7). In the present study, we attempted to determine the effects of internalization on persistent IP1 signaling by TRH-Rs. We fused TRH-R1 and TRH-R2 with GFP and transiently coexpressed them without or with β-arrestin-2, which is known to promote internalization of both TRH-Rs (21), or K44A to inhibit internalization. There was little or no TRH-R internalization in these cells or in cells expressing K44A (data not shown), as we reported previously for TSHR (5, 7). In cells overexpressing exogenous β-arrestin-2, we observed robust internalization of TRH-R1 after 30 min of 1 μM TRH stimulation, whereas TRH-R2 exhibited less internalization with its cell-surface membrane association still distinct after 30 min exposure to TRH (Fig. 2A).
Figure 2.
TRH-R2 persistent signaling does not depend on internalization. A) Visualization of agonist- and β-arrestin-2-induced TRH-R1 and TRH-R2 internalization. HEK-EM293 cells were transfected to transiently express TRH-R1-GFP or TRH-R2-GFP and βARR2. After 24 h, the cells were incubated without or with TRH for 30 min at 37°C. Images were acquired as described in Materials and Methods. Left panels: control, no stimulation. Right panel: 1 μM TRH stimulation for 30 min. B) Loss of cell surface [3H]MeTRH binding. HEK-EM293 cells stably expressing TRH-R1 or TRH-R2 were transiently transfected with pcDNA3.1, βARR2, or K44A. At 48 h after transfection, the cells were incubated without or with 20 nM TRH for 30 min at 37°C, washed, incubated with 2 nM [3H]MeTRH for 2 h at 4°C, and then washed and solubilized. Bars are means ± se of duplicate measurements in 3 experiments. C) Persistent IP1 production. HEK-EM293 cells stably expressing TRH-R2 were transfected with pcDNA3.1, βArr2, or K44A. After 48 h, the cells were incubated without (control) or with 1 μM TRH at 37°C for 30 min. After pretreatment and 1 h washout, the cells were incubated in HBSS/HEPES with LiCl at 37°C for 60 min (production) and lysed, and IP1 content was measured in the cell lysates. Bars are means ± se of duplicate measurements in 3 experiments.
These findings were also supported by the results of binding experiments showing loss of cell surface [3H]MeTRH binding sites. Although it was traditionally thought that all acid-resistant receptor-radioligand binding represents receptor internalization, it has recently been shown that some acid-resistant TRH binding is present even when internalization is completely inhibited (5, 7, 22). Therefore, we used a different approach to monitor the loss of binding sites from the surface of cells stably expressing TRH-Rs. Cells were pretreated with unlabeled TRH and incubated at 37°C to allow receptor internalization. After 30 min, the cells were washed with ice-cold acid, followed by ice-cold HBSS, and then incubated with [3H]MeTRH for 2 h at 4°C so [3H]MeTRH could only bind to receptors that had remained on the plasma membrane. Figure 2B shows that >70% of TRH-R1 binding sites were lost from the cell surface, whereas only 20% of TRH-R2 sites were lost. Thus, TRH-R2 is internalized less effectively than TRH-R1, which is contrary to what would be predicted if persistent signaling were dependent on internalization.
We measured persistent IP1 production in cells stably expressing TRH-R2 and overexpressing β-arrestin-2 to enhance or K44A to inhibit internalization. Neither β-arrestin-2 nor K44A affected persistent IP1 production (Fig. 2C). Thus, we have shown that even though TRH-R1 robustly internalizes, it does not signal persistently in HEK-EM293 cells under these conditions. The level of TRH-R2 internalization, in contrast, is lower than that of TRH-R1, but TRH-R2 exhibits persistent signaling. These findings allow a conclusion that internalization is not required for persistent signaling by TRH-R2.
Persistent signaling depends on a continuously activated TRH-R2
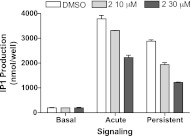
To distinguish whether the ability of TRH-R2 to signal persistently required continuous receptor activation, we used a TRH-R inverse antagonist 2 (19) added after initiation of persistent signaling. The pretreatment step and washes were performed according to our usual experimental protocol for persistent signaling, but the 60-min washout was divided into 2 steps: 45 min with buffer alone, followed by 15 min with antagonist 2. The production phase was also carried out with antagonist 2. The results showed that in addition to inhibiting acute stimulation, antagonist 2 inhibited persistent signaling even though it was added 45 min after TRH was washed out (Fig. 3). Thus, persistent signaling by TRH-R2 is depended on a persistently activated receptor (see Discussion).
Figure 3.
Antagonist 2 inhibits persistent signaling by TRH-R2. Cells stably expressing TRH-R2 were incubated without or with 1 μM TRH at 37°C to measure basal, acute, or persistent IP1 production, as described in Materials and Methods. To measure persistent signaling, after a 30-min pretreatment with TRH, the cells were washed and then incubated in HBSS/HEPES for 45 min; then 10 or 30 μM of the antagonist 2 was added to half of the wells for 15 min. After this 1 h washout, the medium was replaced by HBSS/HEPES without or with 10 or 30 μM antagonist 2 and LiCl for 1 h (persistent). Incubations were stopped, and IP1 levels were measured. Data are means ± range of duplicate measurements in a representative experiment.
Since antagonist 2 is an inverse agonist, our data could not allow the determination of whether TRH was still present in the complex, because an inverse agonist may inhibit signaling by competition with agonist for binding to receptors or by inhibiting signaling by unbound receptors. Binding assays failed to show an effect of antagonist 2 on the rate of dissociation of [3H]TRH (data not shown). However, the dissociation data cannot be interpreted conclusively, since there are at least 2 possible scenarios that may underlie these findings. First, persistent signaling occurs when receptors are unbound by TRH, and second, when receptors are bound to TRH, antagonist 2 competes for them but previously bound TRH is not lost from the cells.
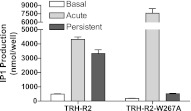
TRH-R2-W267A mutant loses basal and persistent activity
It is known that persistently active TSHR (2, 5, 7) and TRH-R2, but not TRH-R1 (13, 14), exhibit basal signaling activity. We next determined whether there is a correlation between constitutive and persistent signaling in TRH-R2. We used a TRH-R2 mutant with a mutation within transmembrane helix-6: Trp-267 replaced by Ala (TRH-R2-W267A; ref. 20). This Trp is highly conserved among GPCRs, is part of a hydrophobic cluster composed of aromatic residues in transmembrane helices 5 and 6 (23), appears critical for maintaining the common topological features of GPCRs (24) and their activities (25), and may function as a key switch in the receptor activation mechanism (26). TRH-R2-W267A was shown previously to lose its basal activity (20). TRH-R2-W267A exhibited parallel losses in basal and persistent IP1 production (Fig. 4).
Figure 4.
TRH-R2 mutant W267A exhibits parallel loss of basal and persistent activity. HEK-EM293 cells were transiently transfected with TRH-R2 or TRH-R2-W267A. Basal and persistent IP1 production was measured as described in Materials and Methods. Bars are means ± se of triplicate measurements in 2 experiments.
Persistent IP1 production by TRH-R2 can be inhibited by knockdown of Gαq or Gα11
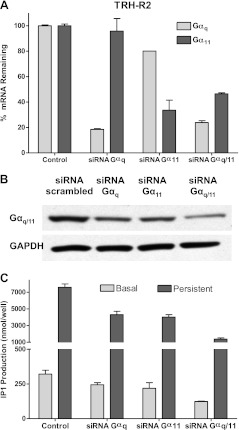
TRH-R2 presumably exhibits a stronger association with Gαq or Gα11, and its constitutive activity is caused in part by the continuous presence of a threshold level of unoccupied TRH-R2/Gαq/11 complexes. Indeed, in fluorescence resonance energy transfer (FRET) experiments with chimeric receptors in which the carboxyl tails of TRH-R1 or TRH-R2 were fused with mCherry and coexpressed with Gαq-YFP, the cells expressing TRH-R2 exhibited higher FRET ratios than cells expressing TRH-R1 (data not shown). We reasoned that persistent signaling, like constitutive signaling, was in part dependent on attaining a threshold level of TRH/TRH-R/Gαq/11 complexes. If this hypothesis were correct, decreasing Gαq or Gα11 would be predicted to decrease persistent (and constitutive) signaling and overexpression of Gαq or Gα11 to induce persistent (and constitutive) signaling. We used siRNAs to knock down expression of Gαq and Gα11 in HEK-EM293 cells stably expressing TRH-R2 to determine its effects on basal and persistent signaling. Figure 5A, B illustrates the specific down-regulation of the mRNAs and protein for Gαq and Gα11. As predicted, knockdown of Gαq or/and Gα11 resulted in decreases of both persistent and constitutive IP1 production in cells stably expressing TRH-R2; combined knockdown decreased persistent signaling by 82% (Fig. 5C).
Figure 5.
Effects of siRNA knockdown of Gαq and/or Gα11 in HEK-EM293 cells stably expressing TRH-R2. Cells were transfected with 50 nM of siRNA Gαq and/or Gα11. Experiments were performed 72 h after transfection. A) Percentage siRNA knockdown of Gαq and/or Gα11 mRNA. B) Effects of siRNA knockdown on Gαq and/or Gα11 protein levels. Cell lysates were probed for G-protein content using antibody against GNAQ + GNA11 in Western blot analysis. C) Effects of siRNA silencing of Gαq and/or Gα11 mRNA on basal and persistent signaling by TRH-R2. Data are from 1 of 3 experiments with duplicate samples and are presented as means ± range.
Persistent IP1 production by TRH-R1 can be induced by Gαq or Gα11
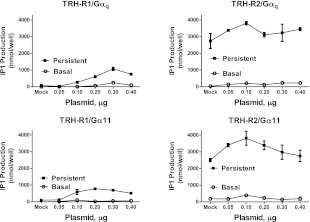
To further test the above-noted hypothesis, we overexpressed Glu-Glu internal epitope-tagged Gαq or Gα11 in cells stably expressing TRH-R1 or TRH-R2 and then monitored basal and persistent IP1 production as well as G-protein expression in the cells. To obtain uniform data on G-protein expression and avoid complications due to possible different affinities of different antibodies, we used a single Glu-Glu antibody to probe G proteins containing an internal Glu-Glu epitope tag (Supplemental Fig. S3). The overexpression of Gαq or Gα11 had a small effect on the levels of persistent signaling in cells expressing TRH-R2 (Fig. 6, right panels). By contrast, TRH-R1-expressing cells exhibited persistent signaling that increased with increasing amounts of Gαq or Gα11 plasmids introduced into the cells (Fig. 6, left panels). It should be noted that TRH-R1 never stimulated persistent IP1 production to the same levels as TRH-R2. There was no effect of Gαq or Gα11 on TRH-mediated persistent signaling in cells not expressing TRH-Rs (data not shown). HEK-EM293 cells not expressing TRH-Rs as well as both TRH-R1- and TRH-R2-expressing cells showed increases in their agonist-independent IP1 production that in the case of TRH-R1-expressing cells was indistinguishable from the parental HEK-EM293 cells (data not shown). The effects of Gαq and Gα11 were specific since overexpression of Gαs did not affect either basal or persistent activities of TRH-Rs (data not shown).
Figure 6.
Exogenous expression of Gαq or Gα11 induces persistent signaling by TRH-R1. HEK-EM293 cells stably expressing TRH-R1 or TRH-R2 were transfected with increasing concentrations of either Gαq or Gα11. Experiments were performed 48 h after transfection. IP1 production in parental HEK-EM293 cells was subtracted from values in TRH-R-expressing cells. Gαq (top panels) and Gα11 (bottom panels) effects in TRH-R1-expressing (left panels) and TRH-R2-expressing cells (right panels). Values are means ± range from duplicate samples in a representative experiment.
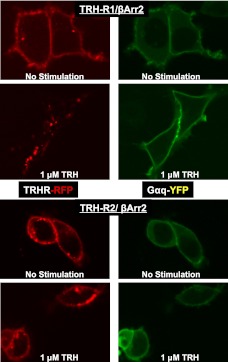
As persistent signaling was mediated by Gαq, we determined whether Gαq internalized along with TRH-Rs after TRH addition. Figure 7 illustrates that Gαq remained on the cell surface when TRH caused TRH-R1 or TRH-R2 to internalize. The lack of internalization of Gαq is more apparent in cells expressing TRH-R1 than TRH-R2 because TRH-R1 is internalized to a greater extent (Figs. 2 and 7).
Figure 7.
TRH-Rs and Gαq do not cointernalize after TRH Stimulation. HEK-EM293 cells were transfected to transiently express TRH-R1-RFP or TRH-R2-RFP, Gαq-YFP, and βARR2. After 24 h, the cells were incubated without or with TRH for 30 min at 37°C. Images were acquired as described in Materials and Methods. Left panels: control, no stimulation. Right panels: 1 μM TRH stimulation for 30 min.
Persistent IP1 production by TRH-R1 can be induced by increasing the level of receptor expression
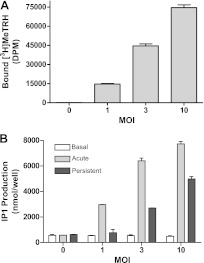
In the view of the fact that increasing levels of Gαq/11 increases persistent signaling in TRH-R1-expressing cells, we hypothesized that increasing the number of TRH-R1s, the other component of the continually activated TRH-R1/Gαq/11 complex required for persistent signaling, would also elevate levels of persistent signaling. To test this hypothesis, we used adenoviral-mediated gene transfer to produce cells with markedly increased levels of TRH-R1 (18). Figure 8 illustrates that increasing the number of TRH-R1s does not affect basal signaling but markedly enhances persistent signaling, as does increasing the levels of Gαq/11 (Fig. 6).
Figure 8.
Transient, marked overexpression by adenovirus-mediated gene transfer induces persistent signaling by TRH-R1. A) [3H]MeTRH binding. After 24 h, HEK-EM293 cells infected with 0, 1, 3, or 10 MOI of AdCMVmTRH-R1 were incubated with 2 nM [3H]MeTRH for 4 h at 4°C, washed with ice-cold HBSS, and solubilized. B) Effects of increasing levels of TRH-R1 expression on basal, acute, and persistent IP1 signaling. HEK-EM293 cells were infected with 0 (nonspecific), 1, 3, or 10 MOI of AdCMVmTRH-R1. After 24 h, basal, acute, and persistent IP1 production was determined as described in Materials and Methods. Figure represents data from 1 of 2 experiments. Data are means ± range of duplicate measurements.
DISCUSSION
We found that cells expressing TRH-R2 and TRH-R1 could be made to exhibit persistently stimulated IP1 production for hours after TRH had been washed out of cultures of HEK-EM293 cells expressing one or the other of these receptors. However, under all conditions tested, TRH-R2 was more effective than TRH-R1 in stimulating persistent signaling. We confirmed that TRH-R2, but not TRH-R1, signals constitutively also. To gain insight into the mechanism of persistent signaling, we compared proximal steps in TRH-R2 and TRH-R1 signaling. We found that persistent signaling was not caused by slower dissociation of TRH from TRH-R2 compared with TRH-R1 and was not dependent on receptor internalization as TRH-R1 could be made to internalize to a greater extent than TRH-R2 by overexpressing β-arrestin-2. Thus, these aspects of TRH receptor biology did not explain the differences in persistent signaling exhibited by TRH-R2 and TRH-R1. Importantly, we found that knockdown of Gαq and Gα11 by siRNA decreased persistent and constitutive signaling by TRH-R2 and that persistent and constitutive signaling were induced in cells expressing TRH-R1 by overexpression of Gαq or Gα11. Furthermore, persistent (and acute) signaling but not basal signaling could be induced by overexpressing TRH-R1. Thus, we showed that persistent signaling was dependent on the level of G-protein and TRH receptor expression.
Some GPCRs exhibited persistent and constitutive signaling at endogenous levels of G proteins: TRH-R2 (this study), thyrotropin receptor (2, 5, 7), and sphingosine-1-phosphate receptor (4, 27). By contrast, parathyroid hormone receptor type 1 exhibited persistent signaling (3, 6) but not measurable constitutive signaling at endogenous G-protein levels (28) and others, such as TRH-R1 (this study), do not exhibit persistent or constitutive signaling with endogenous G proteins but could be induced to exhibit persistent signaling in cells overexpressing Gαq and Gα11. It has been previously shown that overexpression of cognate G proteins may also lead to increased basal levels of second messengers. For example, cotransfection of G protein into cells containing dopamine receptors has been shown to produce constitutive signaling activity (29), and high levels of Gαq cotransfected with various muscarinic receptor subtypes in NIH3T3 cells resulted in increased basal activities of the receptors (30). In our experiments, we did not observe an increase in basal activity of TRH-R1 when we induced persistent IP1 production by this receptor (Figs. 6 and 8). Nevertheless, we showed that mutation in TRH-R2 resulting in a loss of basal signaling also virtually abolished persistent signaling. We think there is a correlation between the levels of basal and persistent signaling, but it is not perfect. This can be explained because TRH-bound activated receptors may have a different conformation than unbound activated receptors, leading to different states of G-protein activation and thereby different levels of signaling.
We have presented data that show that persistent signaling by TRH-R2 (Fig. 1) and thyrotropin receptor (5) can be inhibited by adding a receptor antagonist after the agonist has been washed out. These data show that a continually activated receptor/G-protein complex is required for persistent signaling, suggesting that persistent signaling is not caused by an activated G-protein uncoupled from the receptor, but do not allow us to conclude that the receptor is continuously occupied by the agonist, because the antagonists used were inverse agonists that could have inhibited signaling by unoccupied receptors. However, we did not observe inhibition of TRH-R2 basal activity by antagonist 2 over the course of 4 h using IP1 ELISA in these experiments (data not shown). We suggest that these data are most consistent with the idea that persistent and constitutive signaling by GPCRs are exhibited when levels of agonist/activated receptor/G-protein and receptor/G-protein complexes, respectively, needed for signaling are maintained.
There are well-documented examples of GPCRs that signal persistently via β-arrestin-mediated pathways, and evidence has been provided that some of these have been found to be important in vivo (31). However, to our knowledge, all studies to date that have reported G-protein-mediated persistent signaling by GPCRs have been performed in cells in tissue culture. One could argue that persistent signaling might be a phenomenon only observed in artificial expression systems. Yet, endogenous TSHR has been shown to signal persistently via Gαs in mouse (2) and human (our unpublished data) thyroid follicles in culture. It is likely that because of differences in levels of expression of endogenous GPCRs and G proteins that persistent and constitutive signaling will vary in different cell types in culture. More than 60 wild-type GPCRs from humans, mice, and rats have been shown to exhibit constitutive activity, and many of them have been found to be linked to human diseases (9). Therefore, it is likely that constitutively active and, perhaps, persistently active GPCRs are important in both normal physiology and disease states. Unfortunately, an experimental protocol to distinguish between persistent and constitutive signaling in vivo is not apparent, and thus the pathophysiological significance of G-protein-mediated persistent signaling by GPCRs remains unclear.
TRH-R1 has been conserved throughout all searched vertebrate genomes, whereas TRH-R2 is lacking in primates, chickens, and fugu, suggesting that it has been lost independently in 3 lineages. Phylogenetic analysis of the molecular evolution of TRH receptors in vertebrates, including a third putative receptor (32), reveals that the TRH-R2 gene is the most ancient among TRH receptors and diverged first from the ancestral gene (33). It is difficult to envision why in some cases several species lack a certain receptor while the same receptor has been retained during evolution in other lineages and species. One of the most likely explanations is that the gene has been lost because another gene has taken over its functions (34). It appears plausible that TRH-R2, with its ability to signal in both TRH-independent and persistent fashions, might have been replaced by TRH-R1, which is under more stringent control by TRH.
In summary, we have shown that TRH-R2 signals persistently via the Gαq/11, phospholipase C, inositol-1,4,5-trisphosphate pathway in HEK-EM293 cells by coupling to endogenous G proteins and that TRH-R1, which does not signal persistently in these cells, can be induced to exhibit persistent signaling when Gαq or Gα11 are overexpressed and when TRH-R1 is expressed at even higher levels. We suggest that the critical step in both constitutive and persistent signaling is the continuing maintenance of a level of receptor/G-protein complexes that, on G-protein activation, exceeds a threshold needed to activate phospholipase C. Since the list of G-protein regulatory proteins is ever growing, including proteins capable of coupling G proteins to GPCR in unconventional ways, it is reasonable to hypothesize that such complexes are important and dependent on regulators or adaptors not yet identified. Future studies will focus on elucidating such contributors to TRHR signaling.
Supplementary Material
Acknowledgments
The authors thank Dr. Marc Caron (Duke University Medical Center, Durham, NC, USA) for the plasmids expressing βArr2 and K44A, Dr. Catherine Berlot (Weis Center for Research, Geisinger Clinic, Danville, PA, USA) for the plasmid expressing Gαq-YFP chimera, and Dr. Jennifer Lippincott-Schwartz (National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, Bethesda, MD, USA) for the mCherry plasmid.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- βArr2
- β-arrestin-2
- GPCR
- G-protein-coupled receptor
- HEK
- human embryonic kidney
- IP1
- inositolmonophosphate
- K44A
- dominant-negative dynamin mutant
- RFP
- red fluorescent protein
- TRH
- thyrotropin-releasing hormone
- TRH-R1
- thyrotropin-releasing hormone receptor, subtype 1
- TRH-R2
- thyrotropin-releasing hormone receptor, subtype 2
- TSH
- thyroid-stimulating hormone
- TSHR
- thyroid-stimulating hormone receptor
- YFP
- yellow fluorescent protein
REFERENCES
- 1. Hausdorff W. P., Caron M. G., Lefkowitz R. J. (1990) Turning off the signal: desensitization of β-adrenergic receptor function. FASEB J. 4, 2881–2889 [PubMed] [Google Scholar]
- 2. Calebiro D., Nikolaev V. O., Gagliani M. C., de Filippis T., Dees C., Tacchetti C., Persani L., Lohse M. J. (2009) Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 7, e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferrandon S., Feinstein T. N., Castro M., Wang B., Bouley R., Potts J. T., Gardella T. J., Vilardaga J. P. (2009) Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat. Chem. Biol. 5, 734–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mullershausen F., Zecri F., Cetin C., Billich A., Guerini D., Seuwen K. (2009) Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat. Chem. Biol. 5, 428–434 [DOI] [PubMed] [Google Scholar]
- 5. Neumann S., Geras-Raaka E., Marcus-Samuels B., Gershengorn M. C. (2010) Persistent cAMP signaling by thyrotropin (TSH) receptors is not dependent on internalization. FASEB J. 24, 2347–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feinstein T. N., Wehbi V. L., Ardura J. A., Wheeler D. S., Ferrandon S., Gardella T. J., Vilardaga J. P. (2011) Retromer terminates the generation of cAMP by internalized PTH receptors. Nat. Chem. Biol. 7, 278–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boutin A., Allen M. D., Geras-Raaka E., Huang W., Neumann S., Gershengorn M. C. (2011) Thyrotropin receptor stimulates internalization-independent persistent phosphoinositide signaling. Mol. Pharmacol. 80, 240–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Werthmann R. C., Volpe S., Lohse M. J., Calebiro D. (2012) Persistent cAMP signaling by internalized TSH receptors occurs in thyroid but not in HEK293 cells. FASEB J. 26, 2043–2048 [DOI] [PubMed] [Google Scholar]
- 9. Seifert R., Wenzel-Seifert K. (2002) Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch. Pharmacol. 366, 381–416 [DOI] [PubMed] [Google Scholar]
- 10. Tao Y. X. (2008) Constitutive activation of G protein-coupled receptors and diseases: insights into mechanisms of activation and therapeutics. Pharmacol. Ther. 120, 129–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samama P., Cotecchia S., Costa T., Lefkowitz R. J. (1993) A mutation-induced activated state of the β2-adrenergic receptor. Extending the ternary complex model. J. Biol. Chem. 268, 4625–4636 [PubMed] [Google Scholar]
- 12. Sun Y., Lu X., Gershengorn M. C. (2003) Thyrotropin-releasing hormone receptors–similarities and differences. J. Mol. Endocrinol. 30, 87–97 [DOI] [PubMed] [Google Scholar]
- 13. Neumann S., Raaka B. M., Gershengorn M. C. (2010) Constitutively active thyrotropin and thyrotropin-releasing hormone receptors and their inverse agonists. Methods Enzymol. 485, 147–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang W., Gershengorn M. C. (1999) Rat TRH receptor type 2 exhibits higher basal signaling activity than TRH receptor type 1. Endocrinology 140, 4916–4919 [DOI] [PubMed] [Google Scholar]
- 15. Robbins A. K., Horlick R. A. (1998) Macrophage scavenger receptor confers an adherent phenotype to cells in culture. Biotechniques 25, 240–244 [DOI] [PubMed] [Google Scholar]
- 16. Hughes T. E., Zhang H. L., Logothetis D. E., Berlot C. H. (2001) Visualization of a functional Gαq-green fluorescent protein fusion in living cells–association with the plasma membrane is disrupted by mutational activation and by elimination of palmitoylation sites, but not by activation mediated by receptors or Alf4-*S. J. Biol. Chem. 276, 4227–4235 [DOI] [PubMed] [Google Scholar]
- 17. Shaner N. C., Campbell R. E., Steinbach P. A., Giepmans B. N., Palmer A. E., Tsien R. Y. (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 [DOI] [PubMed] [Google Scholar]
- 18. Gershengorn M. C., Heinflink M., Nussenzveig D. R., Hinkle P. M., Falck-Pedersen E. (1994) Thyrotropin-releasing hormone (TRH) receptor number determines the size of the TRH-responsive phosphoinositide pool. Demonstration using controlled expression of TRH receptors by adenovirus mediated gene transfer. J. Biol. Chem. 269, 6779–6783 [PubMed] [Google Scholar]
- 19. Engel S., Skoumbourdis A. P., Childress J., Neumann S., Deschamps J. R., Thomas C. J., Colson A. O., Costanzi S., Gershengorn M. C. (2008) A virtual screen for diverse ligands: discovery of selective G protein-coupled receptor antagonists. J. Am. Chem. Soc. 130, 5115–5123 [DOI] [PubMed] [Google Scholar]
- 20. Sun Y., Gershengorn M. C. (2002) Correlation between basal signaling and internalization of thyrotropin-releasing hormone receptors: evidence for involvement of similar receptor conformations. Endocrinology 143, 2886–2892 [DOI] [PubMed] [Google Scholar]
- 21. Hanyaloglu A. C., Seeber R. M., Kohout T. A., Lefkowitz R. J., Eidne K. A. (2002) Homo- and hetero-oligomerization of thyrotropin-releasing hormone (TRH) receptor subtypes–differential regulation of β-arrestins 1 and 2. J. Biol. Chem. 277, 50422–50430 [DOI] [PubMed] [Google Scholar]
- 22. Jones B. W., Hinkle P. M. (2008) Arrestin binds to different phosphorylated regions of the thyrotropin-releasing hormone receptor with distinct functional consequences. Mol. Pharmacol. 74, 195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gershengorn M. C., Osman R. (1996) Molecular and cellular biology of thyrotropin-releasing hormone (TRH) receptors. Physiol. Rev. 76, 175–191 [DOI] [PubMed] [Google Scholar]
- 24. Rosenbaum D. M., Rasmussen S. G., Kobilka B. K. (2009) The structure and function of G-protein-coupled receptors. Nature 459, 356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wess J., Nanavati S., Vogel Z., Maggio R. (1993) Functional role of proline and tryptophan residues highly conserved among G protein-coupled receptors studied by mutational analysis of the m3 muscarinic receptor. EMBO J. 12, 331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwartz T. W., Frimurer T. M., Holst B., Rosenkilde M. M., Elling C. E. (2006) Molecular mechanism of 7TM receptor activation–a global toggle switch model. Annu. Rev. Pharmacol. Toxicol. 46, 481–519 [DOI] [PubMed] [Google Scholar]
- 27. Waters C. M., Long J., Gorshkova I., Fujiwara Y., Connell M., Belmonte K. E., Tigyi G., Natarajan V., Pyne S., Pyne N. J. (2006) Cell migration activated by platelet-derived growth factor receptor is blocked by an inverse agonist of the sphingosine 1-phosphate receptor-1. FASEB J. 20, 509–511 [DOI] [PubMed] [Google Scholar]
- 28. Carter P. H., Petroni B. D., Gensure R. C., Schipani E., Potts J. T., Jr., Gardella T. J. (2001) Selective and nonselective inverse agonists for constitutively active type-1 parathyroid hormone receptors: evidence for altered receptor conformations. Endocrinology 142, 1534–1545 [DOI] [PubMed] [Google Scholar]
- 29. Senogles S. E., Spiegel A. M., Padrell E., Iyengar R., Caron M. G. (1990) Specificity of receptor-G protein interactions. Discrimination of Gi subtypes by the D2 dopamine receptor in a reconstituted system. J. Biol. Chem. 265, 4507–4514 [PubMed] [Google Scholar]
- 30. Burstein E. S., Spalding T. A., Brann M. R. (1997) Pharmacology of muscarinic receptor subtypes constitutively activated by G proteins. Mol. Pharmacol. 51, 312–319 [DOI] [PubMed] [Google Scholar]
- 31. Whalen E. J., Rajagopal S., Lefkowitz R. J. (2011) Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol. Med. 17, 126–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lu X. P., Bidaud I., Ladram A., Gershengorn M. C. (2003) Pharmacological studies of thyrotropin-releasing hormone (TRH) receptors from Xenopus laevis: is xTRHR3 a TRH receptor? Endocrinology 144, 1842–1846 [DOI] [PubMed] [Google Scholar]
- 33. Mekuchi M., Saito Y., Aoki Y., Masuda T., Iigo M., Yanagisawa T. (2011) Molecular cloning, gene structure, molecular evolution and expression analyses of thyrotropin-releasing hormone receptors from medaka (Oryzias latipes). Gen. Comp. Endocrinol. 170, 374–380 [DOI] [PubMed] [Google Scholar]
- 34. Gloriam D. E., Fredriksson R., Schioth H. B. (2007) The G protein-coupled receptor subset of the rat genome. BMC Genomics 8, 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.