Abstract
The idea of extending the lifetime of our organs is as old as humankind, fueled by major advances in organ transplantation, novel drugs, and medical devices. However, true regeneration of human tissue has becoming increasingly plausible only in recent years. The human heart has always been a focus of such efforts, given its notorious inability to repair itself following injury or disease. We discuss here the emerging bioengineering approaches to regeneration of heart muscle as a paradigm for regenerative medicine. Our focus is on biologically inspired strategies for heart regeneration, knowledge gained thus far about how to make a “perfect” heart graft, and the challenges that remain to be addressed for tissue-engineered heart regeneration to become a clinical reality. We emphasize the need for interdisciplinary research and training, as recent progress in the field is largely being made at the interfaces between cardiology, stem cell science, and bioengineering.
Keywords: stem cells, tissue engineering, heart regeneration, bioreactor, clinical translation
1. INTRODUCTION
The heart is an organ of unparalleled complexity, a marvel of “engineering by nature” with contractile, conductive, and vascular systems working together to provide vital function. Cardiac myocytes form a three-dimensional synctium that propagates electrical signals across specialized intracellular junctions to produce mechanical contractions and pump the blood forward (1). The heart is also an organ that fails beyond repair, as a result of the only minimal ability of damaged heart tissue to regenerate following injury (2). Upon myocardial infarction, a patient can lose as much as 50 grams of muscle mass, as a result of hypoxia that leads to the release of apoptotic factors and cell death. Heart disease and stroke, the principal components of cardiovascular disease, are the first and the third, respectively, leading causes of death in the United States accounting for nearly 40% of all deaths, more than all of those attributed to cancer. Congenital heart defects, which occur in nearly 14 of every 1000 newborn children (3), are the leading cause of death in the first year of life. The need to re-establish the structural and functional features of native heart tissue, in a predictable way and on a long-term basis, is a major challenge for the field of cardiac tissue engineering (4).
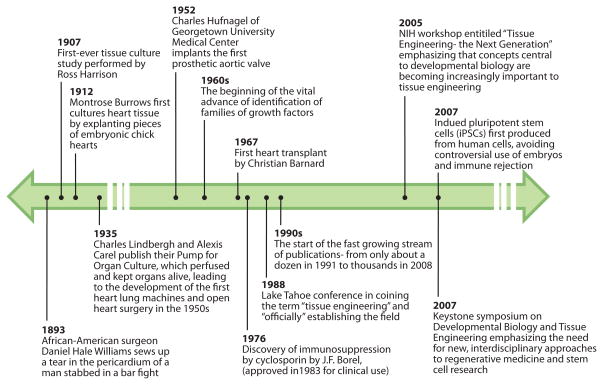
To put the bioengineering of cardiac tissue into perspective, it is instructive to look into the historical development of the concepts and methods that form the basis of this burgeoning new field (Figure 1). Transplantation of cells and organs has its origins in Greek mythology, where we find the Chimera, a dangerous hybrid creature composed of the parts of different animals, a vision that preceded the actual creation of chimeric mice consisting of two or more genetically distinct cell types that are used in today’s research. There is evidence that heart transplantation was tried as early as 300 AD, by the Chinese surgeon Huo T’o, who used a homemade anesthetic (5). In 1905, Alexis Carrell made the first attempts to connect a heart from one dog to the vasculature of another dog (5), leading to the first heart transplant in dog by Shumway and ultimately to more than 75,000 heart transplants routinely done in human patients (6). Major advances in surgery and immunosuppression (with the advent of cyclosporins) have made possible successful heart transplantations, which remains the only effective treatment for end-stage heart failure.
Figure 1.
Some of the milestones leading to current bioengineering approaches to heart regeneration.
The parallel history in the advances within the study of cell and tissue cultures goes back to the 1870s, when Julius Petri invented the glass dishes that he named after himself and the technique of cell cloning, and to the first reported embryonic tissue culture in 1905 (7). In the 1930s, Alexis Carrel and Charles Lindberg jointly developed a perfusion system for keeping explanted adult tissues---including the heart---alive in culture for several weeks. In The Culture of Organs Carrel and Lindberg state that “a new era has opened” with this ability to study organs outside the body “in the fullness of their reality.” Another vital advance came in the 1960s with the identification of families of growth factors that enabled the maintenance of differentiated cells. Tissue engineering was “officially” established only approximately 20 years ago, at a conference in Lake Tahoe, California, in 1988. Tissue engineering was originally defined as “the application of principles and methods of engineering and life sciences toward fundamental understanding of structure-function relationships in normal and pathological mammalian tissues and the development of biological substitutes to restore, maintain, or improve tissue function.” This definition still reflects the unifying concept of the field that remains inseparable from the field of regenerative medicine. The 1990s marked the beginning of rapid advances in tissue engineering and regenerative medicine: The stream of publications grew from approximately one dozen in 1991, including the first review of the field in 1993 (8), to more than 36,000 by October 2010. Recent meetings, such as the National Institutes of Health workshop “Tissue Engineering: The Next Generation” in 2005 and the Keystone Symposium “Developmental Biology and Tissue Engineering” in 2007 have shown that concepts central to developmental biology are becoming increasingly important to tissue engineering and that interdisciplinary approaches are critical to move tissue engineering from observational to mechanistic practices, and from serendipitous to rational approaches.
Over these past two decades, the generation of fully functional human tissue for treating end-stage heart disease has been a central, elusive goal for engineers, stem cell scientists, and physicians. Exponential advances in our ability to generate human cells capable of driving organ regeneration, both of embryonic origin (hESCs) and induced pluripotent stem cells (iPSCs), are now revolutionizing the experimental approaches leading to this goal, as the fields of biomedical engineering, stem cell biology, and regenerative medicine begin to converge. A new discipline termed stem cell bioengineering is on the horizon, offering unprecedented opportunities along with a set of new challenges toward clinical use of lab-made biological “spare parts.” In this regard, a growing body of work at the interface of cardiovascular tissue engineering, heart development, stem cell biology, cardiac pathology, and clinical transplantation serves as a paradigm for understanding the new directions for this emerging field. The identification of a family of human multipotent heart progenitors (9), the pathways that control their renewal, diversification, and differentiation (9–10), and the ability to generate functional ventricular muscle tissue from PSCs (11–12) is beginning to intersect with the fields of device technology, matrix biology, material science, and tissue engineering. At the same time, we have witnessed breathtaking advances in cardiovascular bioengineering with respect to the development of tissue scaffolds (13–23), bioreactor systems (24–29), tissue-engineering technologies (30–34), imaging modalities (35–36), and translation into animal models and human patients (37–38). With several highly meritorious recent reviews on each of the individual areas (39–41), this article focuses on a critical discussion of recent and ongoing work at the intersection of previously distinct fields of cardiovascular stem cell biology and cardiac tissue engineering.
2. CARDIAC DEVELOPMENT
2.1. Cardiac Progenitors
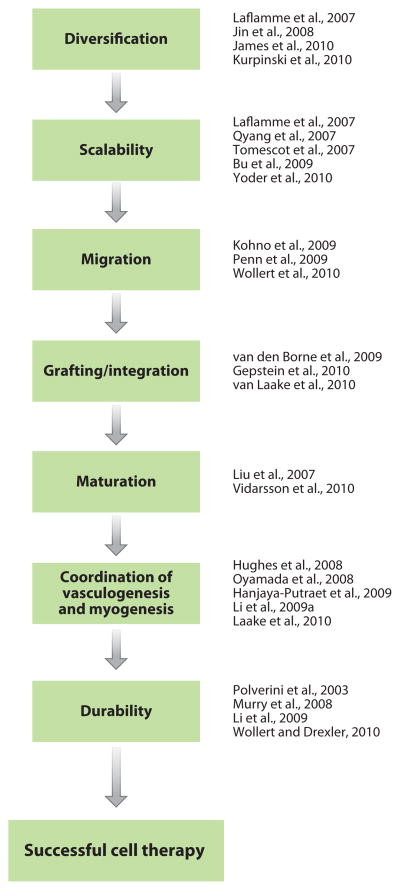
The recent discovery of a family of multipotent cardiac progenitors is one of the major advances in our understanding of mammalian and human cardiogenesis (11). Nature gives rise to the diversity of heart cell lineages, including cardiomyocytes, endothelial, and smooth muscle cells, from a subset of multipotent cardiac progenitors at distinct locations in the embryo [first and second heart fields (FHF and SHF, respectively), epicardium, and neural crest] (42). To build a mammalian heart, these progenitors face the challenge of generating a variety of muscle and nonmuscle cells that form distinct structures---the atrial and ventricular muscle, valves, pacemaker and conduction systems, aortic and pulmonary outflow tract, coronary arterial system, and the endocardium (43). Understanding how these multipotent cardiac progenitors generate specific embryonic heart-cell lineages and form different heart-tissue components serves as a biological template for stem cell engineering of heart parts for cardiovascular regeneration. A growing body of evidence now points to the convergence of cardiogenesis and cardiovascular regeneration, on the basis of the logic of heart-cell diversification (44–47), scalability (9–10, 47–49), migration (50–52), grafting/integration (53–55), maturation (56–57), coordination of vasculogenesis and myogenesis (35, 55, 58–60), and durability (35, 52, 61–62) (Figure 2).
Figure 2.
Stepwise approaches to contemporary challenges in cardiovascular medicine.
2.2. Cardiac Development: Early Events
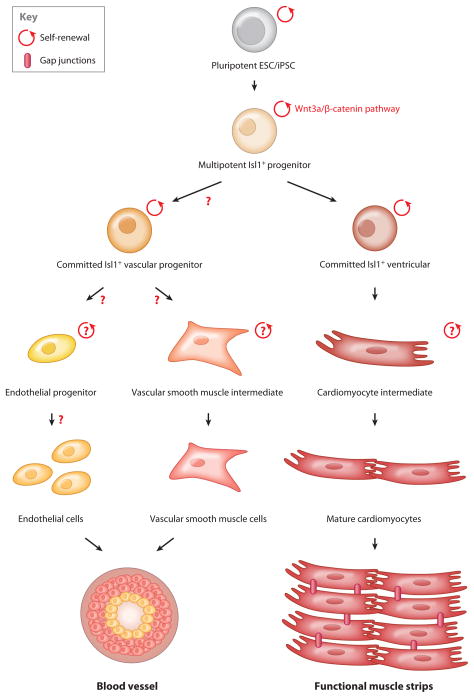
Cardiac development is a dynamic yet tightly orchestrated process with specification of various cardiac progenitors at different developmental stages in distinct embryonic compartments. The first heart cells appear in the anterior splanchnic mesoderm, which gives rise to the cardiac crescent known as the FHF, and migrate out to form the primitive heart tube. Progenitors of the cardiac crescent further extend into the pharyngeal mesoderm, which is known as SHF. At this stage, the heart tube consists of an inner endocardial layer and an outer myocardial layer. Looping and differential growth of the heart tube ultimately generate the multichambered heart (63). Lineage-tracing studies show that the FHF gives rise to the left ventricle and atria, whereas the SHF gives rise to the right side of the heart and outflow tract (64–66). The SHF constitutes a major source of cardiac progenitors, of which the postnatal multipotent progenitor---marked by the expression of the LIM homeodomain transcription factor Islet-1 (Isl1)---gives rise to more than two-thirds of the heart cells. Most of the Isl1 derivatives are located on the right side of the heart, in the conduction system, proximal coronary arterial tree, atrial and right ventricular chambers, and outflow tract myocardium (64–67). Recently, by virtue of the Isl1-cre knockin mouse or human embryonic stem cell (hESC) line, the Isl1+ progenitors were isolated and differentiated into the major three cell types of the heart: cardiomyocytes, smooth muscle, and endothelial cells (9, 65). It is now possible to expand the Isl1+ progenitors through upregulation of the Wnt/β-catenin signaling (9–10). Utilizing insights from murine and human cardiogenesis, a primitive cell-fate map for multipotent heart progenitors has been generated, tracing all the way to the formation of functional ventricular strips (Figure 3). These results suggest the possibility of regenerating the heart by transplanting Isl1+ progenitors derived from human stem cells into patients with cardiovascular disease.
Figure 3.
Pathways to regeneration of heart muscle and blood vessels.
2.3. Emergence of Vasculature
The proepicardial organ also contributes to heart formation. Cells of the proepicardial organ grow over the myocardium of the heart tube to form the outer layer of the epicardium. Some of the epicardial cells undergo epithelial/mesenchymal transition and migrate into the heart to form the coronary smooth muscle cells and cardiac fibroblasts (68). Moreover, cardiac neural crest transits through the posterior pharyngeal arches and enters the anterior part of the heart tube to form the outflow tract (69). Neural crest also takes part in valve formation and septation, the latter contributing to separation of the myocardial base of the aorta and pulmonary trunk. Coronary vessels also form during heart development, with complex coordination between processes in the epicardium, sinus venosus, and myocardium (68, 70). Historical experiments using the chick-quail chimeras documented that progenitors of the coronary vasculature are derived from the proepicardium (71). In the mouse embryo, cells of the subepicardial mesenchyme are thought to generate the coronary endothelial and coronary smooth muscle cells in response to various epicardial and myocardial growth factors such as FGFs, TGFβ, VEGF, and PDGF (68). However, the idea that proepicardium is the origin of coronary arteries has been recently challenged. Although proepicardium gives rise to vascular smooth muscle cells, the apelin-lacZ knockin/lineage-tracing experiments have shown that the majority of endothelial cells of the coronary arteries arise from the endothelial sprouts of the sinus venosus with only a small contribution from the cardiac endothelium (70). Sprouting venous endothelial cells dedifferentiate when they migrate into the myocardium and then differentiate into arteries and capillaries, whereas cells at the surface redifferentiate into veins.
The discovery of specific heart progenitors in murine embryos has led to the identification and empurification of their counterparts in ESCs and iPSCs from both murine and human sources. Because these PSC lines can be expanded almost indefinitely on appropriate feeder layer or defined matrix systems, they can serve as a valuable source of cells for engineering of specific heart parts. In fact, beating cardiomyocytes can be differentiated spontaneously from mouse or human ESCs/iPSCs, suggesting that cardiogenesis may be a default pathway during development, once the issues of cell diversification, scalability, and durability (discussed above) are addressed (Figure 2).
2.4. Recapitulation of Development by Embryonic Stem and Inducible Pluripotent Stem Cells
Although it has been known for almost 25 years that beating cardiomyocytes can be isolated from ESC lines (72), the impact of pluripotent model systems on cardiovascular therapeutics and drug discovery has been minimal. The maturation of the cardiac lineages has been a major issue, for reasons similar to those causing widely accepted limitations of the value of hematopoietic cells derived from PSCs. Elimination of undifferentiated stem cells from differentiated cardiomyocytes has been a major challenge, particularly if the cells are envisioned for therapeutic endpoints. These obstacles could be overcome via the isolation of a committed ventricular heart progenitor in the islet lineage that is capable of limited expansion and subsequent conversion into a fully functional strip of ventricular muscle (Figure 3). These studies could be extended to the generation of a ventricular muscle tissue patch from human PSCs. Toward this goal, the efficiency of cardiac commitment could be enhanced during directed differentiation using growth factors such as BMP4 and activin A (47). Alternatively, BMP-2 and inhibitors of basic fibroblast growth factor (48) could be used for expansion of pure cardiomyocytes derived from hESCs. As is done with pancreatic development, chemical screening can identify further regulatory molecules involved in the commitment, renewal, differentiation, and maturation of specific cardiac progenitors (73). Moreover, new approaches for purification and characterization of progenitors derived from the FHF, which gives rise to the left ventricular chamber, could prove valuable for cell therapy.
3. ENGINEERING A HEART PATCH
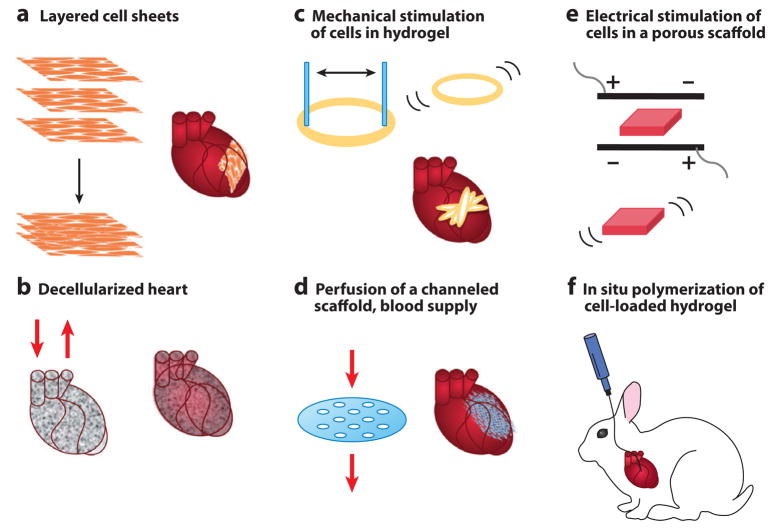
The ultimate goal of tissue-engineered heart regeneration is to (re-)establish normal structure and function on hierarchical levels (4). At the centimeter scale, this overall goal translates into the requirement for a thick and compact tissue that can be maintained only with interstitial flow of culture medium (in vitro: using bioreactors) and blood perfusion (in vivo: by connecting to the blood supply) owing to the high oxygen demand of cardiomyocytes (27.6 nmol/mg protein/min) (74). At the millimeter scale, the structural anisotropy becomes a key requirement, with myofibers aligned in parallel, and the changing spatial arrangement along the thickness of the ventricle. Tight connections between structural properties and the contractile behavior of cardiac muscle necessitate the application of electromechanical stimulation during in vitro culture. At the micrometer scale, the key requirement is vascularization, as the very high cell density in native myocardium (~108 cells/cm3) is supported by rich vasculature with intercapillary distances of only ~20μm (75). Establishment of a vascular tree perfused with blood at the time of implantation remains a major challenge. At the nanometer scale, key requirements are related to the function of gap junctions and cell membrane channels (necessary for the propagation of electrical signals) as well as the sarcomeres inside the cells (necessary for establishing functional excitation-contraction machinery). Different laboratories have approached these requirements in various ways, each with some success, although the integration of multiple efforts into a single and effective cardiac regeneration modality remains to be achieved. We briefly review here the five most extensively used approaches to cardiac tissue engineering: (a) cultivation of scaffold-free stackable cell sheets, (b) repopulation of decellularized native tissue, (c) mechanical stimulation of cells in hydrogels, (d) cell cultivation on perfused channeled scaffolds, (e) electrical stimulation of cells in porous scaffolds, and (f) cell delivery in injectable hydrogels (Figure 4).
Figure 4.
Representative approaches to tissue engineering of heart muscle.
3.1. Scaffold-Free Tissue Constructs
The simplest form of engineered cardiac tissue are scaffold-free cell sheets (33) that are generated by culturing cells on temperature sensitive polymer surfaces and that allow detachment of intact cell monolayers without the use of enzymes (Figure 4a). When such cell sheets are layered one upon another, the cells form junctions and gradually establish signal propagation and contractile function. To enhance vascularization, endothelial layers can be placed between the myocyte layers and implanted in a series of surgeries to allow time for the establishment of blood perfusion. Many other groups adopted this technology and tested it in various animal models (76). Another approach to scaffold-free tissue engineering has been recently explored by the Murry group using ESC-derived cardiomyocytes (77). Notably, cell aggregation alone was sufficient to generate synchronously contracting cardiac tissue. Reporting that greater than 75% of the cells within the graft were cardiomyocytes, with the remaining 25% most likely a mixture of cardiac fibroblasts and endothelial cells, the Murry group demonstrated the feasibility of generating synchronous cell aggregates in vitro (77). Further studies are needed to characterize the mechanical integrity of scaffold-free grafts, their architecture, and cell coupling and, most importantly, the ability of these scaffold-less structures to generate mechanical force.
3.2. Repopulation of Decellularized Native Tissue
By using a platform “made by nature,” the Taylor group (23) demonstrated that decellularized rat hearts can be reseeded with cardiomyocytes and endothelial cells, resulting in the establishment of contractile activity (approximately 2% of normal) (Figure 4b). Decellularization of tissues, pioneered by the Badylak lab (78), removes all cells while leaving the extracellular matrix (ECM) with largely preserved composition, architecture, and mechanical properties. Such decellularized tissues provide a native-like environment for cells to orient, attach, couple with each other, and form a tissue structure while remodeling their environment. Medium perfusion through the heart’s vasculature used in the Taylor approach takes advantage of the natural network and allows exchange of nutrients and, most critically, oxygen throughout the depth of the tissue. Adaptations of this approach may enable the creation of cardiac patches, using pieces of intact decellularized myocardium as a scaffold. Further work in which decellularized tissues are emulsified and reconstituted as extracellular-matrix gel may also prove beneficial.
3.3. Mechanical Stimulation of Cells in Hydrogels
The group of Eschenhagen and Zimmerman established a simple and effective system for mechanical conditioning of cardiomyocytes encapsulated in ring-shaped hydrogels made of a mixture of collagen and Matrigel (Figure 4c). The functional improvements in cell morphology and markedly increased mitochondrial density and force of contraction suggest that mechanical stimulation can drive the constructs to a more mature cardiac muscle structure (79). Notably, force generation in stimulated tissue constructs made using neonatal rat cardiomyocytes approached that of native heart muscle (0.4–0.8 mN twitch force, 0.1–0.3 mN tension) along with stable resting membrane potentials (−66 to −78 mV) and characteristic cardiac waveform kinetics. In their 2006 study (37), the group implanted 1–4-mm-thick, 15-mm-diameter tissue constructs into immunosuppressed rats, and at 28 days post-op, they found significant (400-μm-thick) engrafted muscle layers in the host tissue. These grafts showed undelayed signal propagation without evidence of arrhythmia, which resulted in significant ventricular wall thickening. Further work is needed to better understand the role of mechanical stimulation and to optimize the regimes of mechanical conditioning of cultured tissue constructs. At this time, we are still gleaning insights into how cardiac cells sense and develop mechanical force as well as what the roles of physical factors are in differentiation and maturation of cardiac cell lineages.
3.4. Cell Cultivation in Perfused Channeled Scaffolds
The Vunjak-Novakovic group has developed a “biomimetic” approach to engineer thick and compact cardiac tissue constructs by providing convective-diffusive oxygen transport that is critical for cell survival and function (32, 80). To mimic blood flow in the capillary network, culture medium was perfused through a channeled scaffold seeded with cells at a physiologic density. To mimic the role of hemoglobin and increase the oxygen contents in culture medium, perfluorocarbon (an oxygen carrier) was added (32, 81). Perfusion of culture medium containing perfluorocarbon enabled the maintenance of physiologic density of viable and differentiated cells in millimeters-thick constructs. After only three days, constructs contracted synchronously in response to electrical stimulation, while channels remained open and the pressure drop was as low as 0.1 kPa/mm. Perfusion of medium supplemented with perfluorocarbon improved the construct cellularity, the amounts and distributions of cardiac markers (troponin I, Cx-43), and contractile behavior of cardiac constructs. Notably, improved construct properties correlated with the enhanced supply of oxygen to the cells, a finding consistent with the known dependency of cell viability in engineered cardiac tissue on oxygen concentration (82).
3.5. Electrical Stimulation of Cells in Porous Scaffolds
The Vunjak-Novakovic group also established electrical stimulation as a method for enhancing functional assembly of cardiac cells into a synchronously contracting cardiac patch (26). The method involves cultivation of hydrogel-encapsulated neonatal rat heart cells (a mix of cardiac myocytes, endothelial cells, and fibroblasts) on porous collagen scaffolds. Subsequently, electrical stimulation was extended to a variety of cell types (including hESCs) (83) and scaffold materials (including synthetic elastomers) (4, 28). In all cases, cells were first cultured without stimulation for three days, to allow synthesis and assembly of gap junction and contractile proteins, and then subjected to a regimen of electrical stimulation designed to induce synchronous macroscopic contractions. Electrical stimulation markedly improved the structural organization and contractile properties of engineered cardiac constructs, at all hierarchical levels: marker expression, cell ultrastructure, tissue morphology, and amplitude of contractions. Additional studies (84) investigated various stimulation paradigms that utilized electrical signals (AC or DC, biphasic or monophasic) involved in development and healing. Further understanding electrical regulation of cardiac development may be facilitated by recent developments of microtechnologies that allow precise control of the cellular microenvironment (29).
3.6. Cell Delivery in Injectable Hydrogels
Current cell therapy for cardiovascular disease is largely based on injecting cell suspensions into the heart, either by an intravenous route or by a catheter. Such delivery of the “naked cells” into the ischemic environment of an injured heart, without the ability to form cell-matrix junctions, seriously limits their survival and engraftment. The success of cell therapy thus remains hindered by poor engraftment after intracoronary delivery and low survival rate after intramyocardial injections (52). Clinical trials have revealed extremely low cell-survival rates, for several different approaches to cell delivery: direct intracardiac injection following open chest thoracotomy, intracoronary catheter-based delivery, percutaneous intramyocardial injection via NOGA catheter systems, and intravenous infusion (85). The minimal therapeutic effects may relate to this limitation and/or reflect a predominantly paracrine action of the cells on promoting neoangiogenesis. Control of cell delivery to the injured heart remains a major challenge at this time. One ongoing approach is to use a hydrogel as a cell-delivery vehicle (86) (Figure 4f). Potential advantages of such an approach include providing a cell-friendly microenvironment to engrafted cells to prevent anoikis and shield cells from the infarcted muscle. Also, hydrogels can be tailored to polymerize (chemically, thermally, optically) and deliver cells by minimally invasive catheter-based procedures with high accuracy of cell localization to the area of interest.
4. UNDERSTANDING THE HOST MICROENVIRONMENT
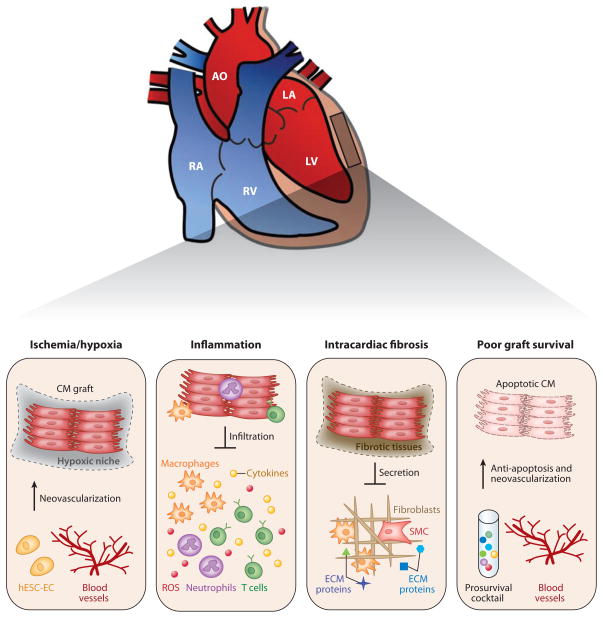
Although the precise mechanistic basis for the poor survival of transplanted cells is not entirely clear, a growing body of evidence has suggested that the microenvironmental niche plays a critical role in the maintenance of multipotency, renewal, and mobilization of progenitors in a wide variety of organ systems (52, 87). Understanding the mechanisms by which the microenvironment regulates the differentiation and maturation of transplanted heart progenitor or stem cells is central to the optimization of protocols for cell-based cardiovascular therapy (85, 88–89). Preclinical studies have suggested that hESC-derived cardiomyocytes (hESC-CM) show poor engraftment and short-term survival in vivo (55). The longest reported survival of hESC-CM after direct injection into murine myocardium may not exceed 12 weeks ( 55). Taken together, several key barriers appear to relate to ischemia/hypoxia, inflammatory signals, intracardiac fibrosis, graft rejection, and vascularization (Figure 5).
Figure 5.
Strategies for tackling major barriers to cardiac regeneration.
4.1. Ischemia and Hypoxia
Myocardial infarction, commonly caused by the blockage of a coronary artery following atherosclerosis, is the leading cause of heart-muscle damage and ensuing heart failure, a result of myocardial ischemia and the subsequent loss of viable cardiomyocytes. Because the adult myocardium has only limited capability to regenerate (90), cell loss after myocardial infarction is irreversible. On the basis of clinical and experimental studies of ischemic heart injury, hypoxia in the host myocardium could be one of the critical factors contributing to the poor engraftment and survival of multiple cell types, including hESC-CM. In humans, new blood vessels form via angiogenesis (the sprouting of new vessels from the existing vasculature), vasculogenesis (de novo formation of blood vessels from endogenous vascular progenitors), or arteriogenesis (the outgrowth of pre-existing arterioles into smaller arteries) (90). Although expansion of endogenous endothelial progenitor cells and neovasculogenesis take place in response to myocardial ischemia, vascular injury, and trauma (85), native angiogenesis is unable to meet the increased requirements of oxygen and nutrients in the hypertrophied infarcted myocardium.
Hypoxia in the failing heart is increasingly recognized as an important barrier to efficient cell grafting. In this regard, the therapeutic potential of hESC-EC for treatment of heart failure in the setting of cardiac ischemia has been examined, by injecting one million hESC-EC into the ischemic SCID murine heart following ligation of left anterior descending artery (35). Two weeks after injection, significant improvement in terms of infarction size, cardiac viability, and left ventricular function were observed in the hESC-EC-treated group compared with the sham (saline injection) group. However, there was no significant difference between the treatment group and control group by 4–8 weeks after injection. Further cell-fate studies demonstrated that such a short-term effect was due to massive cell death within the first few weeks, with less than 2% of injected hESC-EC having survived after 4–8 weeks (35, 91). Importantly, cardiac improvement within the first two weeks could be due to the indirect benefits from paracrine secretion of cytokines, chemokines, and other growth factors by hESC-EC. These studies again suggest the need to better control cell retention and viability to identify and study the factors leading to the functional benefits of therapeutic cells.
4.2. Inflammation
Myocardial ischemia is accompanied by inflammation (7), as the injured myocardium is rich in reactive oxygen species and toxic agents (92) that stimulate infiltration of neutrophils, monocytes, and macrophages. The recruited immune cells can produce proteases and proinflammatory cytokines such as IL-1, IL-6, IL-8, IL-12, IL-18, TNF-a, MIP 1, and MCP1 (88, 93–94). Monocytes can also damage the arterial wall by recruiting smooth muscle cells, connective tissues, and T cells (7, 88). In addition, the high levels of reactive oxygen species in the infarct zone trigger endothelial dysfunction, resulting in the remodeling of vessel walls. Overall, transplantation of unprotected repair cells into such an inflamed and ischemic myocardium results in significant cell death (93). Indeed, acute donor cell death after transplanting various cell types, including fetal, neonatal, adult, and hESC-derived CM into ischemic hearts has been reported (95–97). Laflamme et al. (47) demonstrated that the addition of prosurvival factors was necessary for graft survival in the infarcted heart. Suppressing specific inflammatory pathways or attenuating the over-reactive host environment by expanding regulatory T cells (Treg) at a certain stage of heart failure may also promote graft survival. The numbers of circulating Treg are reduced in patients with chronic heart failure and the immunosuppressive function of these cells is compromised (98). Adoptive transfer of Treg can also ameliorate angiotensin-II-induced cardiac damage with marked reduction of cardiac T cells and infiltrated macrophages (99). Therefore, defective Treg may be a contributor to inflammatory activation in patients with cardiovascular disease and acute cell death following injection. Any therapeutic vaccination that can expand Treg-directed regulation may thus prove effective at promoting graft survival for cell therapy (100).
4.3. Intracardiac Fibrosis
Hypoxia and ischemia following myocardial infarction led to immediate cardiomyocyte apoptosis and replacement of the dying cardiac myocytes with fibrotic tissue. During the initial inflammatory phase, macrophages and other immune cells secrete cytokines and other growth factors to recruit smooth muscle cells into the infarcted myocardium. The fibroblasts and smooth muscle cells then transform into myofibroblasts and contribute to production of the ECM and scar contraction (54). Fibrotic tissues also form around the graft following transplantation of hESC-CM into infarcted hearts (55), and both donor hESC-CM and the host fibroblasts contribute to excessive ECM secretion of collagen I, IV, XVIII, and fibronectin, leading to the separation of the graft from host myocardium and electrophysiological uncoupling. When hESC-CM were transplanted into electrophysiologically silenced guinea pig and swine hearts, they acted as biological pacemakers for the recipient myocardium (101–102), indicating that the donor hESC-CM can indeed functionally couple to host cardiomyocytes. It thus appears that excessive ECM secretion could increase the risk of arrhythmia (103). Among various mediators of ECM homeostasis, transforming growth factor-β1 (TGF-β 1), connective tissue growth factor, platelet-derived growth factor, endothelin I, and angiotensin II play important roles in promoting cardiac fibrosis (104). Understanding how to attenuate ECM secretion from infarcted myocardium could not only reduce the risk of patients developing heart failure, but may also prevent electrophysiological uncoupling of hESC-CM from the host myocardium.
4.4. Promoting Graft Survival via Vasculogenesis
During mammalian development, cardiac myogenesis is closely coordinated with coronary vasculogenesis. Studies of heart regeneration in zebrafish have documented that angiogenesis precedes augmentation of cardiac myogenesis. In terms of three-dimensional tissue engineering, however, vasculogenesis remains a major barrier. In a similar manner, cell engraftment and survival after transplantation is also challenged by ischemia, inflammation-associated oxidative stress, release of cytotoxic cytokines, and apoptosis (52). Recent studies have explored the utility of vascular cells derived from hESC (35, 58, 105) and the enhancement of engraftment and survival of hESC/iPSC-derived CM after cardiac transplantation. In separate studies, cell-free biopolymer scaffolds were shown to support cardiac regeneration. For example, hyaluronan hydrogels improved ejection function, reduced infarct size, and increased vasculogenesis in experimental model systems (106), whereas a porcine-derived myocardial matrix enabled endothelium and myocyte migration and increased arteriole density (107). Biopolymer scaffolds also protected the transplanted cells from apoptosis (108). Importantly, biomaterials can be designed to incorporate growth factors regulating the differentiation, angiogenesis, engraftment, and survival of transplanted cells. Similarly, pretreatment of ESC-CM with heat-shock proteins and prosurvival factors improved graft survival and function in a rat model of myocardial ischemia-reperfusion injury (47). A better understanding of the paracrine pathways of vascular regeneration and cardiac performance would prove useful (52, 109). Identification of paracrine factors from transplanted cells and injured myocardium by secretome analyses and bioinformatics represent other potentially valuable approaches. Combinatorial analysis of vasculogenic factors and subsets of progenitor cells may help coordinate cardiac myogenesis and vasculogenesis in an engineered heart patch and hydrogel-delivered repair cells.
5. MAKING A “PERFECT” GRAFT
A clinically useful tissue-engineered graft needs to be designed to perform many different tasks: re-establish normal structure and function of injured myocardium across different size scales; functionally integrate with the host tissue; and remodel in response to environmental factors, growth, and aging (Figure 6). The three key features of native myocardium: (a) very high density of myocytes and supporting cells, (b) efficient oxygen exchange between the cells and blood, and (c) synchronous contractions orchestrated by electrical signal propagation have provided us with a set of design requirements for engineering cardiac tissue.
Figure 6.
Bioengineering of a clinically useful “patch for a broken heart” is still a puzzle we need to solve. Some of the key pieces include regulating endogenous regeneration; deriving phenotypically mature cardiovascular cells, vascular, and electromechanical integration with the host; optimizing the patch properties and time of implantation; and achieving a durable improvement of heart function (please also see the list of challenges).
5.1. Key Requirements
In spite of the enormous biological complexity of the heart, some success has been achieved with engineering cardiac-like pieces of tissue, using several different approaches (Section 3). Nonetheless, key hurdles to the clinical utility of cardiac tissue engineering are still related to cell selection, localization, and maturation; adaptation of electromechanical function of the graft to that of the host; establishment of vascularization with blood flow; structural and functional integration; and translation into large animal models and eventually human patients. A “perfect” graft would balance these multiple requirements, to provide robust functionality on a long-term basis along with the capacity for vascularization, remodeling, and integration with the host tissue. Lessons learned from heart development tell us that the biophysical signals are present throughout most of the maturation as well as structural and functional development of the heart, which is the first functional organ in the human body, as it starts to beat and pump blood only three weeks into gestation. Therefore, given that most of the heart development and maturation progresses with the presence of hydrodynamic shear in blood vessels and with electrical and mechanical signals orchestrating cell communication, synchronous contractions, and the pumping of blood, it is logical that similar biophysical signals could drive the maturation and functional development of engineered cardiac grafts (32). An obvious question is how much is enough in terms of directing the functional cell assembly in vitro, prior to implantation, and how much of the maturation and functional development can possibly be left in the body.
5.2. Cell Selection, Conditioning, and Localization
Cells are the only “tissue engineers,” as tissue repair does not occur without the cells, either mobilized endogenously or supplied by transplantation of a cardiac graft. It is fair to say that we actually do not engineer tissues, but rather create the environments for the cells to differentiate, couple, and assemble into tissues. To this end, it is critical that the engineered environments can predictably mediate cellular differentiation, maturation, structural specification, and functional assembly. The regeneration of cardiac muscle and supporting vascular compartment depends on the type and developmental stage of the cell populations and their capacity to drive the re-establishment of myocardial structure and function at multiple hierarchical levels. A traditional strategy of cardiac tissue engineering is to generate a functional graft for surgical implantation that may instantly add to the contractile function of the treated heart. From this perspective, potential myogenic cell sources include resident cardiac stem cells, ESCs, and, most recently, iPSCs (4, 61, 110). However, questions remain as to the ideal maturity level for implanted cells as well as the ideal types and ratios of cells to implant. A variety of adult-cell and embryonic-cell sources have emerged as candidates for cell therapy on the basis of their potential for supporting angiogenesis, myogenesis, or both. Some of the most actively studied cell types include bone marrow–derived mesenchymal and hematopoietic stem cells, adipose-derived stem cells, ESCs, iPSCs, and resident cardiac progenitors (85, 89, 110). A “perfect” graft may or may not need to provide exogenous cells. For example, both cellular and acellular patches can contribute to enhanced angiogenesis and preservation of the remaining resident cardiac tissue at the infarct zone after myocardial infarction by delivering angiogenic and cardioprotective factors and chemo-attractants (24). Bone marrow–derived cells, even with minimal engraftment, can serve as sources of paracrine factors, delivered continuously (by the grafted cells) or just once (if the cells are lost from the injury site). However, paracrine signaling had much greater effects on revascularization than on building new muscle mass (111).
5.3. Coordination of Electromechanical Function
Presuming the implantation of an engineered heart patch, electromechanical coupling of the cells with the host myocardium with synchronous contractile activity and generation of contractile force is certainly essential to re-establish contractile function in the heart. Propagation velocities will need to match those in the native heart along with characteristic anisotropic conduction velocities in the direction of propagation with respect to the transverse direction (112). Therefore, spontaneous cell contractions should be abolished, and the construct should respond to the electrical signals in the heart as native tissues does and develop significant contractile forces contributing to the functionality of the repaired heart. In addition to the challenges of establishing the electromechanical coupling, accurate assessment and monitoring of the integration between engineered constructs and host myocardium is critical. Histological identification of proteins involved in establishing cell contacts cannot be considered as evidence of coupling (112). Also, even in cases where undelayed impulse propagation can be demonstrated (e.g., using optical mapping), it is difficult to exclude the possibility that signal propagation is based on electronic contact (37). Given the potential risk for arrhythmogenesis, electrical isolation of the engineered tissue needs to be considered, shifting the task of electrical coordination of contraction from spontaneous electrical activity to pacemaker technology that can insure coordination with host cardiac rhythm.
5.4. Vascularization and Integration
The size and, most critically, the thickness requirements for engineered cardiac tissue grafts imply the necessity of functional integration between the graft and host tissues—both electromechanical and vascular. Tissue-engineering studies show that cardiac myocytes in nonperfused cardiac constructs can survive only within a <100-μm-thick surface layer, i.e., within the penetration depth of oxygen (82). Oxygen availability in the immediate cell microenvironment is the single most important factor limiting the thickness of healthy and viable cardiac tissue that can be engineered in vitro and survive in vivo. It is debatable, however, to what extent vascularization needs to be provided before implantation, for example, by providing channels (32), that can also be lined with vascular cells or by engineering primitive blood vessels (34, 113). Furthermore, it is not entirely clear which aspects of vascularization are most needed early on: structural design that favors the infiltration of host cells and the connection to vascular supply (channels, highly porous structures), coculture of vascular and cardiac cells that is important for paracrine signaling (113–114), or induction of vascularization by immobilization of angiogenic growth factors into scaffolds (115). Although a definite solution for vascularization is still not on the horizon, clues to new approaches may be provided from knowledge of cardiac development. For example, early during embryogenesis, the mammalian heart is largely avascular (116) and the blood vessels form simultaneously with heart development to establish the impressive vascular density in the adult heart (75). This suggests additional strategies that involve the implantation of strands of cardiac muscle small enough to remain viable in nonperfused culture and avascular environments (34) or serial implantations of an increasing number of thin cell sheets (38) done to enhance vascularization.
5.5. Animal Models of Tissue-Engineered Heart Regeneration
Although advanced technologies for three-dimensional culture helped establish the correspondence between in vitro and in vivo studies, several important challenges remain. The choice of animal models is a major one. The rodent models, most frequently used for a variety of practical reasons (for example, the use of human cells in immunocompromised animals, large sample sizes, studies of mechanisms using knockouts, to mention just a few) are not well suited for the studies of electromechanical coupling and the risk of arrhythmia because of the major differences in the electrical properties between the rodent and human cells (79). In addition, there are several problems associated with implanting human cells into nude rodents, including the mismatch of intrinsic beating rates (55) and the size of infract and engineered tissue that impact transport and signaling. The lack of human-like inflammatory response and its associated role in mediating angiogenesis, recruitment of host cells, and the overall process of regeneration has been identified as an additional important deficiency of rodent models (117). The reality is that there is no perfect animal model for studies of cardiac regeneration, as problems with scale are not necessarily alleviated in autologous studies done in large animals, owing to differences in electrical properties between porcine, canine, sheep, and human hearts.
6. SUMMARY
We live in a time of rapid advances toward heart regeneration that are largely driven by collaborative efforts at the interfaces between bioengineering, biological, and medical sciences. Through these collaborations, we are able to pose new questions and to develop new knowledge and tools to tackle these questions. New concepts are being derived through increasingly effective communications between scientific, engineering, and clinical disciplines, which had been progressing independently for a long time. Stem cell biology is transitioning into regenerative medicine, following a path reminiscent of the transition between immunology and transplantation biology. Notably, the approaches to heart regeneration are increasingly moving from being empirical and serendipitous to being biologically inspired and quantitative, again mostly as a result of interdisciplinary research. Although we still do not know which of the approaches will eventually be advanced to an effective clinical therapy, the work being done using controllable bioengineering models of cardiac regeneration is expected to bring some of the answers. Advances in controlling immune tolerance suggest that it may not be necessary to use personalized stem cells and that we may obviate the quality-control issues arising from the high variability of iPSC lines and develop an off-the-shelf heart “patch.” Physician scientists working hand in hand with engineers and stem cell scientists are likely to play a key role in developing the new paradigm for heart regeneration that is looming on the horizon. In this article, we aim to establish a “case study” for the field of regenerative medicine using cardiovascular regeneration as a model.
Summary pointsWe are at the beginning of a promising journey in the development of biologically inspired, clinically sound bioengineering approaches to heart regeneration.
Heart disease and stroke, the principal components of cardiovascular disease, are the first and the third, respectively, leading causes of death in the United States, accounting for nearly 40% of all deaths, more than all cancer deaths combined.
Cells are the only “tissue engineers”: We design only the environments for endogenous or exogenous cells to form functional tissues.
“Biomimetic” environments are essential for unlocking the full biological potential of cells. For cardiac tissue, such an environment involves multiple lineages of cardiac and vascular cells, three-dimensional tissue-like matrix (scaffold), and cascades of molecular and physical (hydrodynamic, electrical, mechanical) regulatory factors
Understanding the heart environment during early development, normal function, and injury is critical for developing effective bioengineering strategies.
The approaches to heart regeneration are moving from being empirical and serendipitous to being biologically inspired and quantitative, largely due to collaborative efforts between the clinicians, scientists, and engineers.
Interdisciplinary research and training a new generation of scientists-bioengineers-clinicians are critical to drive further progress.
-
1
future issuesCardiac regeneration using bioengineering strategies needs to be directed.
-
2
Myogenesis and arteriogenesis during culture and in injured heart need to be coordinated.
-
3
Stem cell diversification, differentiation, and maturation need to be controlled to establish reproducible derivation of cardiogenic and vasculogenic cells.
-
4
The need for immediate function (which requires a mature graft) needs to be reconciled with electromechanical and vascular integration (which requires a developing graft).
Key Terms and Definitions
- Bioreactor
culture system for cells on a biomaterial scaffold designed to provide environmental control, exchange of nutrients and metabolites between the cells and culture medium, and physical signaling
- hESCs
human embryonic stem cells
- Induced pluripotent stem cells (iPSCs)
cells derived from adult human fibroblasts
- Myocardial infarction
a condition that follows interruption of blood flow into a part of the heart muscle and results in the terminal loss of cardiomyocytes
- Regeneration
re-establishment of normal structure and function of a tissue, as opposed to repair that is the immediate response to injury
- Scaffold
biomaterial serving as a template for cell attachment and tissue formation in culture or a delivery vehicle for the transplanted cells
- Tissue engineering
the application of principles and methods toward the development of biological substitutes to restore, maintain, or improve tissue and organ function
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Gordana Vunjak-Novakovic, Email: gv2131@columbia.edu.
Kenneth R. Chien, Email: kchien@partners.org.
LITERATURE CITED
- 1.Severs NJ. The cardiac muscle cell. BioEssays. 2000;22:188–99. doi: 10.1002/(SICI)1521-1878(200002)22:2<188::AID-BIES10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Am. Heart Assoc. American Heart Association Cardiovascular Disease Statistics. Washington, DC: Am. Heart Assoc; 2008. [Google Scholar]
- 4.Vunjak-Novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, et al. Challenges in cardiac tissue engineering. Tissue Eng Part B Rev. 2010;16:169–87. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saltzman WM. Tissue Engineering: Engineering Principles for the Design of Replacement Organs and Tissues. New York: Oxford Univ. Press; 2004. [Google Scholar]
- 6.Chien KR. Regenerative medicine and human models of human disease. Nature. 2008;453:302–5. doi: 10.1038/nature07037. [DOI] [PubMed] [Google Scholar]
- 7.Ross R. Atherosclerosis---an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 8.Langer R, Vacanti J. Tissue engineering. Science. 1993;260:920–26. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 9.Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–17. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 10.Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/β-catenin pathway. Cell Stem Cell. 2007;1:165–79. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Chien KR, Domian IJ, Parker KK. Cardiogenesis and the complex biology of regenerative cardiovascular medicine. Science. 2008;322:1494–97. doi: 10.1126/science.1163267. [DOI] [PubMed] [Google Scholar]
- 12.Domian IJ, Chiravuri M, van der Meer P, Feinberg AW, Shi X, et al. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science. 2009;326:426–29. doi: 10.1126/science.1177350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Au HTH, Cheng I, Chowdhury MF, Radisic M. Interactive effects of surface topography and pulsatile electrical field stimulation on orientation and elongation of fibroblasts and cardiomyocytes. Biomaterials. 2007;28:4277–93. doi: 10.1016/j.biomaterials.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–93. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 15.Chiu LLY, Radisic M, Vunjak-Novakovic G. Bioactive scaffolds for engineering vascularized cardiac tissues. Macromol Biosci. 2010;10:1286–301. doi: 10.1002/mabi.201000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–64. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 17.Engelmayr GC, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater. 2008;7:1003–10. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang H-Y, et al. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:11298–303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerecht S, Townsend SA, Pressler H, Zhu H, Nijst CLE, et al. A porous photocurable elastomer for cell encapsulation and culture. Biomaterials. 2007;28:4826–35. doi: 10.1016/j.biomaterials.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 21.Hunt JA. Regenerative medicine: materials in a cellular world. Nat Mater. 2008;7:617–18. doi: 10.1038/nmat2242. [DOI] [PubMed] [Google Scholar]
- 22.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–41. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 24.Dvir T, Levy O, Shachar M, Granot Y, Cohen S. Activation of the ERK1/2 cascade via pulsatile interstitial fluid flow promotes cardiac tissue assembly. Tissue Eng. 2007;13:2185–93. doi: 10.1089/ten.2006.0364. [DOI] [PubMed] [Google Scholar]
- 25.Radisic M, Marsano A, Maidhof R, Wang Y, Vunjak-Novakovic G. Cardiac tissue engineering using perfusion bioreactor systems. Nat Protocols. 2008;3:719–38. doi: 10.1038/nprot.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radisic M, Park H, Shing H, Consi T, Schoen FJ, et al. From the cover: functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci USA. 2004;101:18129–34. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radisic M, Yang L, Boublik J, Cohen RJ, Langer R, et al. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol Heart Circ Physiol. 2004;286:H507–16. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- 28.Tandon N, Cannizzaro C, Chao P-HG, Maidhof R, Marsano A, et al. Electrical stimulation systems for cardiac tissue engineering. Nat Protocols. 2009;4:155–73. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tandon N, Marsano A, Maidhof R, Numata K, Montouri-Sorrentino C, et al. Surface-patterned electrode bioreactor for electrical stimulation. Lab Chip. 2010;10:692–700. doi: 10.1039/b917743d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J. 1997;11:683–94. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 31.Kofidis T, Lenz A, Boublik J, Akhyari P, Wachsmann B, et al. Pulsatile perfusion and cardiomyocyte viability in a solid three-dimensional matrix. Biomaterials. 2003;24:5009–14. doi: 10.1016/s0142-9612(03)00429-0. [DOI] [PubMed] [Google Scholar]
- 32.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, et al. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–91. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, et al. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res. 2002;90:e40–48. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann W-H, Melnychenko I, Wasmeier G, Didie M, Naito H, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–58. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Wilson KD, Smith B, Kraft DL, Jia F, et al. Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. PLoS ONE. 2009;4:e8443. doi: 10.1371/journal.pone.0008443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ransohoff K, Wu J. Advances in cardiovascular molecular imaging for tracking stem cell therapy. Thromb Haemostasis. 2010;104:13–22. doi: 10.1160/TH09-08-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eschenhagen T, Zimmermann WH, Kleber AG. Electrical coupling of cardiac myocyte cell sheets to the heart. Circ Res. 2006;98:573–75. doi: 10.1161/01.RES.0000215627.13049.5d. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, et al. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006;20:708–10. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 39.Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A. 2009;15:205–19. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–77. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terrovitis JV, Smith RR, Marban E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106:479–94. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vincent SD, Buckingham ME. How to make a heart: the origin and regulation of cardiac progenitor cells. Curr Top Dev Biol. 2010;90:1–41. doi: 10.1016/S0070-2153(10)90001-X. [DOI] [PubMed] [Google Scholar]
- 43.Martin-Puig S, Wang Z, Chien KR. Lives of a heart cell: tracing the origins of cardiac progenitors. Cell Stem Cell. 2008;2:320–31. doi: 10.1016/j.stem.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 44.James D, Nam HS, Seandel M, Nolan D, Janovitz T, et al. Expansion and maintenance of human embryonic stem cell–derived endothelial cells by TGFβ inhibition is Id1 dependent. Nat Biotechnol. 2010;28:161–66. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin S, Hansson EM, Tikka S, Lanner F, Sahlgren C, et al. Notch signaling regulates platelet-derived growth factor receptor-β expression in vascular smooth muscle cells. Circ Res. 2008;102:1483–91. doi: 10.1161/CIRCRESAHA.107.167965. [DOI] [PubMed] [Google Scholar]
- 46.Kurpinski K, Lam H, Chu J, Wang A, Kim A, et al. Transforming growth factor-β and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells. 2010;28:734–42. doi: 10.1002/stem.319. [DOI] [PubMed] [Google Scholar]
- 47.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 48.Tomescot A, Leschik J, Bellamy V, Dubois G, Messas E, et al. Differentiation in vivo of cardiac committed human embryonic stem cells in postmyocardial infarcted rats. Stem Cells. 2007;25:2200–5. doi: 10.1634/stemcells.2007-0133. [DOI] [PubMed] [Google Scholar]
- 49.Yoder MC. Is endothelium the origin of endothelial progenitor cells? Arterioscler Thromb Vasc Biol. 2010;30:1094–103. doi: 10.1161/ATVBAHA.109.191635. [DOI] [PubMed] [Google Scholar]
- 50.Kohno T, Anzai T, Naito K, Miyasho T, Okamoto M, et al. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovasc Res. 2009;81:565–73. doi: 10.1093/cvr/cvn291. [DOI] [PubMed] [Google Scholar]
- 51.Penn MS. Importance of the SDF-1:CXCR4 axis in myocardial repair. Circ Res. 2009;104:1133–35. doi: 10.1161/CIRCRESAHA.109.198929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wollert KC, Drexler H. Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nat Rev Cardiol. 2010;7:204–15. doi: 10.1038/nrcardio.2010.1. [DOI] [PubMed] [Google Scholar]
- 53.Gepstein L, Ding C, Rehemedula D, Wilson EE, Yankelson L, et al. In vivo assessment of the electrophysiological integration and arrhythmogenic risk of myocardial cell transplantation strategies. Stem Cells. 2010;28:2151–61. doi: 10.1002/stem.545. [DOI] [PubMed] [Google Scholar]
- 54.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 55.van Laake LW, van Donselaar EG, Monshouwer-Kloots J, Schreurs C, Passier R, et al. Extracellular matrix formation after transplantation of human embryonic stem cell-derived cardiomyocytes. Cell Mol Life Sci. 2010;67:277–90. doi: 10.1007/s00018-009-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J, Fu JD, Siu CW, Li RA. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells. 2007;25:3038–44. doi: 10.1634/stemcells.2007-0549. [DOI] [PubMed] [Google Scholar]
- 57.Vidarsson H, Hyllner J, Sartipy P. Differentiation of human embryonic stem cells to cardiomyocytes for in vitro and in vivo applications. Stem Cell Rev Rep. 2010;6:108–20. doi: 10.1007/s12015-010-9113-x. [DOI] [PubMed] [Google Scholar]
- 58.Hanjaya-Putra D, Gerecht S. Preview. Mending the failing heart with a vascularized cardiac patch. Cell Stem Cell. 2009;5:575–76. doi: 10.1016/j.stem.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Hughes CC. Endothelial-stromal interactions in angiogenesis. Curr Opin Hematol. 2008;15:204–9. doi: 10.1097/MOH.0b013e3282f97dbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oyamada N, Itoh H, Sone M, Yamahara K, Miyashita K, et al. Transplantation of vascular cells derived from human embryonic stem cells contributes to vascular regeneration after stroke in mice. J Transl Med. 2008;6:54. doi: 10.1186/1479-5876-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–80. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 62.Polverini PJ, Nor JE, Peters MC, Mooney DJ. Growth of human blood vessels in severe combined immunodeficient mice. A new in vivo model system of angiogenesis. Methods Mol Med. 2003;78:161–77. doi: 10.1385/1-59259-332-1:161. [DOI] [PubMed] [Google Scholar]
- 63.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–35. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 64.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–89. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, et al. Multipotent embryonic Isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 66.Sun Y, Liang X, Najafi N, Cass M, Lin L, et al. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev Biol. 2007;304:286–96. doi: 10.1016/j.ydbio.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, et al. Postnatal Isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olivey HE, Svensson EC. Epicardial-myocardial signaling directing coronary vasculogenesis. Circ Res. 106:818–32. doi: 10.1161/CIRCRESAHA.109.209197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hutson MR, Kirby ML. Model systems for the study of heart development and disease: cardiac neural crest and conotruncal malformations. Semin Cell Dev Biol. 2007;18:101–10. doi: 10.1016/j.semcdb.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Red-Horse K, Ueno H, Weissman IL, Krasnow MA. Coronary arteries form by developmental reprogramming of venous cells. Nature. 2010;464:549–53. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manner J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat Rec. 1999;255:212–26. doi: 10.1002/(sici)1097-0185(19990601)255:2<212::aid-ar11>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 72.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 73.Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5:258–65. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- 74.Yamada T, Yang JJ, Ricchiuti NV, Seraydarian MW. Oxygen consumption of mammalian myocardial cells in culture: measurements in beating cells attached to the substrate of the culture dish. Anal Biochem. 1985;145:302–7. doi: 10.1016/0003-2697(85)90365-3. [DOI] [PubMed] [Google Scholar]
- 75.Rakusan K, Flanagan MF, Geva T, Southern J, Van Praagh R. Morphometry of human coronary capillaries during normal growth and the effect of age in left ventricular pressure-overload hypertrophy. Circulation. 1992;86:320–21. doi: 10.1161/01.cir.86.1.38. [DOI] [PubMed] [Google Scholar]
- 76.Bel A, Planat-Bernard V, Saito A, Bonnevie L, Bellamy V, et al. Composite Cell Sheets: a further step toward safe and effective myocardial regeneration by cardiac progenitors derived from embryonic stem cells. Circulation. 2010;122:S118–23. doi: 10.1161/CIRCULATIONAHA.109.927293. [DOI] [PubMed] [Google Scholar]
- 77.Stevens KR, Pabon L, Muskheli V, CEM Scaffold-free human cardiac tissue patch created from embryonic stem cells. Tissue Eng Part A. 2009;15:1211–22. doi: 10.1089/ten.tea.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robinson KA, Li J, Mathison M, Redkar A, Cui J, et al. Extracellular matrix scaffold for cardiac repair. Circulation. 2005;112:I-135–43. doi: 10.1161/CIRCULATIONAHA.104.525436. [DOI] [PubMed] [Google Scholar]
- 79.Zimmermann W-H, Schneiderbanger K, Schubert P, Didie M, Munzel F, et al. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–30. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 80.Radisic M, Park H, Gerecht S, Cannizzaro C, Langer R, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering. Philos Trans R Soc B. 2007;362:1357–68. doi: 10.1098/rstb.2007.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Radisic M, Deen W, Langer R, Vunjak-Novakovic G. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am J Physiol Heart Circ Physiol. 2005;288:H1278–89. doi: 10.1152/ajpheart.00787.2004. [DOI] [PubMed] [Google Scholar]
- 82.Radisic M, Malda J, Epping E, Geng W, Langer R, Vunjak-Novakovic G. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol Bioeng. 2006;93:332–43. doi: 10.1002/bit.20722. [DOI] [PubMed] [Google Scholar]
- 83.Serena E, Figallo E, Tandon N, Cannizzaro C, Gerecht S, et al. Electrical stimulation of human embryonic stem cells: Cardiac differentiation and the generation of reactive oxygen species. Exp Cell Res. 2009;315:3611–19. doi: 10.1016/j.yexcr.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chiu LLY, Iyer RK, King J-P, Radisic M. Biphasic electrical field stimulation aids in tissue engineering of multicell-type cardiac organoids. Tissue Eng Part A. Feature Article. 2010;10(11):1286–1301. doi: 10.1089/ten.tea.2007.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Atluri P, Woo YJ. Pro-angiogenic cytokines as cardiovascular therapeutics: assessing the potential. BioDrugs. 2008;22:209–22. doi: 10.2165/00063030-200822040-00001. [DOI] [PubMed] [Google Scholar]
- 86.Martens T, Godier AFG, Parks JJ, Wan LQ, Koeckert MS, et al. Percutaneous cell delivery into the heart using hydrogels polymerizing in situ. Cell Transplant. 2009;18:297–304. doi: 10.3727/096368909788534915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumar AH, Caplice NM. Clinical potential of adult vascular progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:1080–87. doi: 10.1161/ATVBAHA.109.198895. [DOI] [PubMed] [Google Scholar]
- 88.Apostolakis S, Lip GY, Shantsila E. Monocytes in heart failure: relationship to a deteriorating immune overreaction or a desperate attempt for tissue repair? Cardiovasc Res. 2010;85:649–60. doi: 10.1093/cvr/cvp327. [DOI] [PubMed] [Google Scholar]
- 89.Kuraitis D, Suuronen EJ, Sellke FW, Ruel M. The future of regenerating the myocardium. Curr Opin Cardiol. 2010;25:575–82. doi: 10.1097/HCO.0b013e32833f0318. [DOI] [PubMed] [Google Scholar]
- 90.Kane NM, Xiao Q, Baker AH, Luo Z, Xu Q, Emanueli C. Pluripotent stem cell differentiation into vascular cells: a novel technology with promises for vascular re(generation) Pharmacol Ther. 2011;129:29–49. doi: 10.1016/j.pharmthera.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 91.Li Z, Han Z, Wu JC. Transplantation of human embryonic stem cell-derived endothelial cells for vascular diseases. J Cell Biochem. 2009;106:194–99. doi: 10.1002/jcb.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 93.Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–81. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun J, Li SH, Liu SM, Wu J, Weisel RD, et al. Improvement in cardiac function after bone marrow cell therapy is associated with an increase in myocardial inflammation. Am J Physiol Heart Circ Physiol. 2009;296:H43–50. doi: 10.1152/ajpheart.00613.2008. [DOI] [PubMed] [Google Scholar]
- 95.Dowell JD, Rubart M, Pasumarthi KB, Soonpaa MH, Field LJ. Myocyte and myogenic stem cell transplantation in the heart. Cardiovasc Res. 2003;58:336–50. doi: 10.1016/s0008-6363(03)00254-2. [DOI] [PubMed] [Google Scholar]
- 96.van Laake LW, Passier R, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circ Res. 2008;102:1008–10. doi: 10.1161/CIRCRESAHA.108.175505. [DOI] [PubMed] [Google Scholar]
- 97.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–21. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 98.Tang TT, Ding YJ, Liao YH, Yu X, Xiao H, et al. Defective circulating CD4CD25+Foxp3+CD127low regulatory T-cells in patients with chronic heart failure. Cell Physiol Biochem. 2010;25:451–58. doi: 10.1159/000303050. [DOI] [PubMed] [Google Scholar]
- 99.Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–12. doi: 10.1161/CIRCULATIONAHA.108.832782. [DOI] [PubMed] [Google Scholar]
- 100.Lui KO, Boyd AS, Cobbold SP, Waldmann H, Fairchild PJ. A role for regulatory T cells in acceptance of ESC-derived tissues transplanted across an major histocompatibility complex barrier. Stem Cells. 2010;28:1905–14. doi: 10.1002/stem.506. [DOI] [PubMed] [Google Scholar]
- 101.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–89. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 102.Xue T, Cho HC, Akar FG, Tsang SY, Jones SP, et al. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation. 2005;111:11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- 103.Creemers EE, Pinto YM. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc Res. 2011;89:265–72. doi: 10.1093/cvr/cvq308. [DOI] [PubMed] [Google Scholar]
- 104.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFβ, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–80. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 105.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA. 2003;100:12741–46. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yoon SJ, Fang YH, Lim CH, Kim BS, Son HS, et al. Regeneration of ischemic heart using hyaluronic acid-based injectable hydrogel. J Biomed Mater Res B Appl Biomater. 2009;91:163–71. doi: 10.1002/jbm.b.31386. [DOI] [PubMed] [Google Scholar]
- 107.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–16. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davis ME, Hsieh PC, Grodzinsky AJ, Lee RT. Custom design of the cardiac microenvironment with biomaterials. Circ Res. 2005;97:8–15. doi: 10.1161/01.RES.0000173376.39447.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hansson EM, Lindsay ME, Chien KR. Regeneration next: toward heart stem cell therapeutics. Cell Stem Cell. 2009;5:364–77. doi: 10.1016/j.stem.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 111.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–29. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 112.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–56. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 113.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, et al. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100:263–72. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 114.Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation. 2004;110:962–68. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shen YH, Shoichet MS, Radisic M. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomater. 2008;4:477–89. doi: 10.1016/j.actbio.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 116.Ratajska A, Ciszek B, Sowińska A. Embryonic development of coronary vasculature in rats: Corrosion casting studies. Anat Rec Part A. 2003;270A:109–16. doi: 10.1002/ar.a.10011. [DOI] [PubMed] [Google Scholar]
- 117.Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]