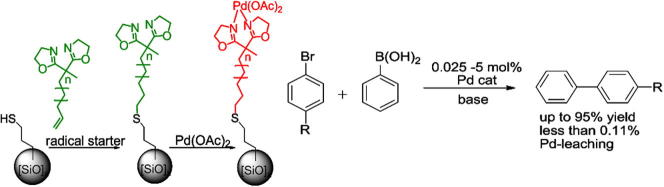
Graphical abstract
A catalytic system including a Pd(OAc)2-bis(oxazoline) complex bonded to 3-mercaptopropyl-functionalized silica gel is presented. The catalyst was tested for Suzuki–Miyaura reactions. The heterogeneity of the catalytic system was studied indicating that there is virtually no Pd-leaching. Furthermore, the catalyst can be reused multiple times without significant loss of stability or structure.
Highlights
► A Pd(OAc)2-bis(oxazoline) complex on functionalized silica gel is presented. ► The catalyst was tested for Suzuki–Miyaura reactions. ► The investigation of the heterogeneity indicated that there is virtually no Pd-leaching. ► The catalyst can be reused multiple times without loss of stability or structure.
Keywords: Supported Pd-catalyst, Suzuki-Miyaura reaction, 3-Mercaptopropyl silica gel, Bis(oxazoline) ligand, Leaching, Proof of heterogeneity
Abstract
The synthesis and characterization of a novel catalytic system including Pd(OAc)2 attached to a bis(oxazoline) (=BOX) ligand that is covalently bonded to 3-mercaptopropyl-functionalized silica gel is presented. The catalyst was tested for Suzuki–Miyaura reactions of different aryl halides with phenylboronic acid. The heterogeneity of the catalytic system was investigated using different approaches, indicating that there is virtually no Pd leaching into the reaction solution under the applied reaction conditions. Furthermore, our results show that the catalytic system can be reused multiple times without significant loss of stability or structure.
1. Introduction
Catalytic C–C, C–O, and C–N couplings using Pd-catalysts are widely employed both in academia and in industry, making Pd the most extensively used metal catalyst for the synthesis of a wide variety of organic compounds [1], [2], [3], including pharmaceuticals [4], [5], heteroarenes [6], liquid crystals [7] as well as natural products prepared by total synthesis [8]. One of the most successful strategies for C–C bond formation is the Suzuki–Miyaura coupling [1], [2], [4], [9], [10], which involves the reaction of organoboron reagents with aryl halides [11], [12]. Due to the high stability of the organoboron reagents, their ease of preparation and low toxicity, as well as the importance of the produced biaryls for pharmaceuticals [4], [5] and liquid crystals [13], this reaction has become part of the standard toolbox of organic chemists. The importance of the reaction is also reflected by the fact that one of the inventors, Akira Suzuki, was awarded with the Nobel Prize in Chemistry in 2010.
A plethora of papers and patents claiming new and highly active catalytic systems for Suzuki–Miyaura couplings has been published. For recent reviews see [9], [14], [15], [16], [17]. The main shortcoming of many of the reported systems is the homogeneous nature of the organo-Pd complexes or colloidal palladium. Homogeneous catalysts are in the same phase as the educts and products (except for phase-transfer catalysts). Thus, complete removal from the reaction mixture to avoid metal carryover is intricate and costly, reducing the potential for industrial implementation, especially in the field of pharmaceutical synthesis. Furthermore, many homogeneous systems usually consist of the metal and the ligands in a particular stoichiometric ratio. Thus, the removal of the ligands makes the purification of the product even more expensive [18].
Immobilization of the catalytic system on solid supports can mitigate these problems, as it allows the straightforward removal of the catalyst from the reaction system. The known heterogeneous Pd-systems include supported Pd-complexes [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], supported Pd-nanoparticles, unsupported Pd-nanoparticles, and encapsulated Pd-complexes (for examples see [29] and references therein). Although heterogenization of the catalysts is advantageous, the majority of heterogeneous catalysts require harsher conditions than well-tuned homogeneous catalysts, which often results in leaching of Pd from the support [30], [31]. Considering the strict guidelines placed on Pd levels in products destined for human consumption [32], there is a clear need for methods to prevent Pd incorporation into organic and pharmaceutical products. These methods can be broadly divided into scavenging and preventative approaches, and the latter being dominated by the use of non-leaching heterogeneous catalysts.
In this study, we report the preparation and testing of a novel heterogeneous Pd-catalyst. In this system, the Pd-complex is attached to functionalized silica gel particles via an organic ligand. Modified silica is by far the most utilized support for immobilizing Pd-complexes [30], [33]. Unfunctionalized mesoporous silica as support was used by Ying et al. [34]. Shimizu et al. [35], Bedford et al. [36], and Crudden et al. [23], [30], [37] have shown that mesoporous silica can be modified with commercially available thiol ligands, resulting in recoverable catalysts for cross-coupling reactions, such as the Suzuki–Miyaura and Heck reactions. However, detailed mechanistic studies have shown that the catalytic reactions using these supports likely occur in solution via leached Pd [23], [38]. For example, the group of Jones et al. have reported that catalysis with Pd-SH-SBA-15 is solely associated with leached metal [39]. Also the group of Crudden et al. found that small amounts of active Pd leach from the surface when using mesoporous molecular sieves modified by mercaptopropyl trimethoxysilane [40].
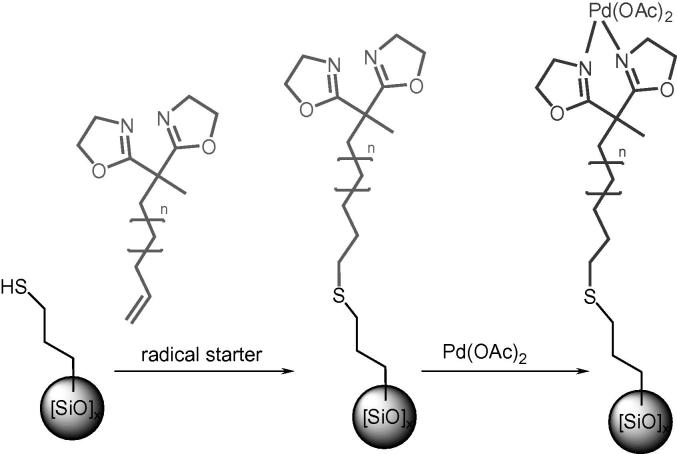
We present a Pd-catalyst that is attached to a tethered bis(oxazoline) (=BOX) ligand that is covalently bonded to functionalized silica gel. In particular, we report here the covalent immobilization of 2,2′-(1-methyl-11-dodecenylidene)bis(4,5-dihydrooxazole) on 3-mercaptopropyl-functionalized silica gel (MPSG). The metalation of the immobilized BOX ligand with Pd(OAc)2 (Scheme 1) yielded the stable and active catalyst Pd(OAc)2–BOX–MPSG. Similar immobilized BOX–M complexes (M = Pd, Cu, Zn, Mg, Rh) have been reported for a large variety of enantioselective reactions [41], [42]. We studied the performance of the Pd(OAc)2–BOX–MPSG system using Suzuki–Miyaura reactions as a test system. Our results show that the complexation of the Pd-compound to the ligand leads to a significant decrease of metal leaching into the solution in comparison with other heterogeneous Pd systems.
Scheme 1.
Two-step preparation of the heterogeneous Pd-complex Pd(OAc)2–BOX–MPSG (n = 7).
2. Materials and methods
2.1. General
1H and13C NMR spectra were recorded on a Varian Inova 500 MHz spectrometer at 499.82 MHz and 125.69 MHz, respectively. Chemical shifts are listed in delta (δ/ppm), employing the residual protio solvent resonance as internal standard (δ = 7.24 ppm in 1H spectra and 77.0 ppm in 13C spectra for CHCl3). GC analysis was carried out on a Perkin–Elmer Clarus 500 using an Optima-5 MS capillary column (Machery-Nagel, 30.0 m × 320 μm × 0.25 μm nominal) with a flame ionization detector and nitrogen as carrier gas. GC/MS analysis: CI mass spectra were recorded with an Agilent 5973N mass selective detector on a Agilent Technologies 6890N gas chromatograph, column: HP-5MS (Agilent 19091S-433), Capillary: 30.0 m × 250 μm × 0.25 μm nominal, carrier gas: helium. SEM/EDX was carried out with a FEI Quanta 600 FEG-ESEM system and a NORAN Vantage system with Si(Li)-detector (10 mm2, PlexUs 0.65 μm window, and energy resolution 132 eV). Transmission electron microscopy (TEM) micrographs were acquired using a Tecnai 12 microscope (FEI Company, 120 kV, LaB6 Cathode). FTIR spectra were recorded on a Bruker VERTEX 70 using a DLaTGS detector and an ATR unit: MVP Pro Star, Diamond crystal, resolution: 4 cm−1, 16 scans, and 4000–600 cm−1. For the IR spectra of the functionalized silica particles, a DRIFT unit was used (EasyDiff, spectral resolution: 4 cm−1, time: 3 min). ICP/OES was carried out using a Varian Vista-MPX CCD Simultaneous ICP/OES device, the used Pd-standard was 10.000 ppm Pd in 5% HNO3. Elemental analysis: The amounts of C, H, N, and S were analyzed using an Elementar Vario El III device with helium as carrier gas. The nitrogen physisorption analysis was carried out on a Micromeritics ASAP 2010. Inner surface area was determined based on the BET method. The pore size distribution was determined using the BJH method applied to the adsorption isotherms.
2.2. Materials
11-Bromo-1-undecene (95%), 1-bromoundecane (98%), diethyl methylmalonate (≥99%), NaH (60% dispersion in mineral oil), thionylchloride (99%), AIBN (2,2′-azobis(2-methylpropionitrile) (98%), ACHN (1,1′-azobis(cyclohexanecarbonitrile) (98%), 3-mercaptopropyl silica gel (=MPSG, 1.2 mmol/g loading, 200–400 mesh, 60 Å pore size, 500 m2/g surface area), Pd(OAc)2 (98%), potassium carbonate (99%), phenylboronic acid (≥97%), 1-bromo-4-methylbenzene (98%), 4-bromobenzoyl chloride (≥97%), 1-bromo-4-(trifluoromethyl)benzene (97%), 4-bromoacetophenone (98%), 3-aminopropyl triethoxysilane, n-dodecane (98%), NMP (1-methyl-2-pyrrolidinone, 99%), DMAc (N,N-dimethylacetamide, 99.8%), 3-aminopropyl silica gel (1 mmol/g loading, 40–63 μm particle size, 60 Å pore size, 550 m2/g surface area), N-methyl morpholine (≥98%), pyridine (99%), poly(vinylpyridine) (2% cross-linked with divinyl benzene), QuadraPure® TU (400–600 μm particle size), and (3-mercaptopropyl)trimethoxysilane (95%) were purchased from Aldrich. The silica gel 60 was purchased from Machery Nagel (0.063–0.2 mm). DMF (>99.8%), CH2Cl2 (≥99.5%), toluene (≥99.5%), and ethanol (99.8%) were purchased from Roth. To prepare degassed and anhydrous THF, CH2Cl2 and toluene, the solvents were purified and degassed using the manual solvent purification system Pure Solve (Innovative Technology). All other chemicals and solvents were used without further treatment. All manipulations of air- and/or moisture-sensitive materials were performed under argon using a dual vacuum/argon line and standard Schlenk techniques.
2.3. Preparation of the heterogeneous Pd-catalyst
2.3.1. Synthesis of the BOX ligand
The tethered bis(oxazoline) ligands, i.e., 2,2′-(1-methyl-11-dodecenylidene)bis(4,5-dihydrooxazole) = BOX and 2,2′-(tridecane-2,2-diyl)bis(4,5-dihydrooxazole) = BOX 2 were synthesized following the synthetic pathway described by Hara et al. [43] 2,2′-(1-methyl-11-dodecenylidene)bis(4,5-dihydrooxazole), BOX: 1H δ (CDCl3) 5.80 (1H, m), 4.96 (2H, 2 dd, J = 1.63, 17.29 Hz), 4.28 (4H, t, J = 9.28 Hz), 3.87 (4H, t, J = 9.52 Hz), 2.01 (2H, m), 1.90 (2H, m), 1.48 (3H, s), 2.24 (14H, m) 13C δ (CDCl3) 169.5, 139.4, 114.2, 67.9, 54.4, 42, 36.6, 33.9, 29.9–29.0 (6Cs), 24.3, 21.5, 11,2 IR: νmax (cm−1) 3076, 2925, 2854, 1655, 1481, 1458, 1351, 1265, 1194, 1139, 1086, 982, 954, 913, 735. 2,2′-(tridecane-2,2-diyl)bis(4,5-dihydrooxazole), BOX 2: 1H δ (CDCl3) 4.20 (4H, t, J = 10 Hz), 3.87 (4H, t, J = 9.52 Hz), 2.01 (2H, m), 1.90 (2H, m), 1.48 (3H, s), 2.24 (14H, m) 13C δ (CDCl3) 169.5, 67.9, 54.4, 42, 36.6, 33.9, 29.9–29.0 (6Cs), 24.3, 21.5, 14.3, 11.2 IR: νmax (cm−1) 2925, 2854, 1655, 1458, 1351, 1265, 1194, 1137, 1091, 982, 955, 914, 735.
2.3.2. Immobilization of the BOX ligand on 3-mercaptopropyl-functionalized silica gel
(=MPSG, according to Sigma 1.2 mmol thiol groups per gram silica gel): The silica gel and catalytic amounts of AIBN (2,2′-azobis(2-methylpropionitrile) or ACHN (1,1′-azobis(cyclohexanecarbonitrile) were added to a solution of the BOX ligand (1 mol equivalent (=equiv.) referring to the thiol groups) in degassed anhydrous CH2Cl2 (or toluene when ACHN was used) under argon. The suspension was continuously stirred and heated under reflux for 18 h. To remove the non-immobilized ligand, the particles were filtered, washed, and sonicated five times for 1 min in CH2Cl2/toluene. Then the material was dried in vacuum at room temperature. After that, the amount of immobilized ligand was determined by elemental analysis.
2.3.3. Metalation
The silica gel particles including the immobilized BOX-ligand were stirred at room temperature in a solution of Pd(OAc)2 (1.5 mol equiv. to the immobilized BOX-ligand) in CH2Cl2 for 2 h. In order to assure that no Pd is adsorbed on the surface, the particles were repeatedly sonicated for several minutes and washed with CH2Cl2. The Pd-amount was determined using ICP/OES.
2.4. General procedure for the Suzuki–Miyaura reactions
2.4.1. Suzuki–Miyaura reactions
The aryl halide (1 mol equiv.) and the phenylboronic acid (1.7 mol equiv.) were dissolved in the reaction solvents (see Table 4, Table 7). Then the base (K2CO3, 1 mol equiv. to the boronic acid), the catalyst (0.025–5 mol% with respect to the aryl halide), and n-dodecane (internal standard, 1 mol equiv.) were added and the reaction mixture was heated to the appropriate reaction temperature (Table 4, Table 7). The reaction progress was monitored using GC analysis. The products were characterized using GC/MS analysis.
Table 4.
Results of the Suzuki–Miyaura reactions.
| Entry | R | Cat[a] | Cat (mol%) | Solvent | Base | T (°C) | Yield[b] (%) |
|---|---|---|---|---|---|---|---|
| 1 | CH3 | Pd(OAc)2 | 5 | Toluene | K2CO3 | 80 | 21 |
| 2 | CF3 | hom | 5 | H2O | NaOH | 80 | – |
| 3 | CF3 | hom | 5 | H2O/MeOH | K2CO3 | 25 | 4 |
| 4 | CF3 | hom | 5 | THF | K2CO3 | 60 | 21 |
| 5 | CF3 | hom | 5 | Toluene | K2CO3 | 80 | 25 |
| 6 | CF3 | in situ | 5 | Toluene | K2CO3 | 60 | 43 |
| 7 | CF3 | het | 5 | Toluene | K2CO3 | 80 | 85 |
| 8 | CH3 | hom | 5 | Toluene | K2CO3 | 80 | 26 |
| 9 | CH3 | in situ | 5 | Toluene | K2CO3 | 80 | 56 |
| 10 | CH3 | het | 5 | Toluene | K2CO3 | 80 | 90 |
| 11 | CH3 | het | 2 | Toluene | K2CO3 | 60 | 27 |
| 12 | CH3 | het | 2 | Toluene | K2CO3 | 100 | 94 |
| 13 | CH3 | het | 2 | Toluene | K2CO3 | 115 | 97 |
| 14 | CH3 | het | 0.025 | Toluene | K2CO3 | 80 | 28 |
| 15 | CH3 | het | 0.075 | Toluene | K2CO3 | 80 | 30 |
| 16 | CH3 | het | 0.125 | Toluene | K2CO3 | 80 | 37 |
| 17 | CH3 | het | 0.25 | Toluene | K2CO3 | 80 | 56 |
| 18 | CH3 | het | 0.75 | Toluene | K2CO3 | 80 | 56 |
| 19 | CH3 | het | 1.25 | Toluene | K2CO3 | 80 | 79 |
| 20 | CH3 | het | 2 | Toluene | K2CO3 | 80 | 90 |
| 21 | COCH3 | het | 2 | Toluene | K2CO3 | 80 | 97 |
| 22 | CH3 | BOX 2, Pd(OAc)2 | 5 | Toluene | K2CO3 | 80 | 54 |
| 23 | CH3 | MPSG, Pd(OAc)2 | 5 | Toluene | K2CO3 | 80 | 41 |
BOX = 2,2′-(1-methyl-11-dodecenylidene)bis(4,5-dihydrooxazole), BOX 2 = 2,2′-(tridecane-2,2-diyl)bis(4,5-dihydrooxazole). [a] hom = Homogeneous system with BOX and Pd(OAc)2, in situ = BOX immobilized on 3-mercaptopropyl silica gel, metalation with Pd(OAc)2 during the Suzuki reactions (in situ), het = heterogeneous system, immobilization of BOX and metalation ex-situ. [b] Yields determined after 4 h with GC using n-dodecane as internal standard.
Table 7.
Effect of the reaction solvent on reaction progress and catalyst poisoning with PVPy of Suzuki–Miyaura reactions of 1-bromo-4-methylbenzene and phenylboronic acid carried out with 2 mol% Pd(OAc)2-BOX-MPSG using K2CO3 as base.
| Solvent | Reaction temperature | Yield after 2 h (%) | Yield after 4 h (%) | Yield after 24 h (%) |
|---|---|---|---|---|
| Toluene | 110 | 43 | 95 | 96 |
| DMAc | 110 | 15 | 17 | 23 |
| NMP | 110 | – | – | – |
| Toluene | 110 | 43 | 95 | 98 |
| DMAc | 150 | 56 | 68 | 75 |
| NMP | 160 | 21 | 55 | 62 |
| DMF | 150 | 34 | 45 | 75 |
| Toluene, with PVPya | 110 | 43 | 65 | 88 |
| DMAc, with PVPya | 150 | 56 | 60 | 70 |
| NMP, with PVPya | 160 | 23 | 59 | 64 |
| DMF, with PVPya | 150 | 22 | 47 | 74 |
Addition of 100 mol equiv. PVPy (referring to Pd) after 2 h.
In the case of the homogeneous (hom in Table 4) reaction, 5 mol% Pd(OAc)2 (with respect to aryl halide) was used and 1 mol equiv. (referring to the catalyst) BOX ligand.
For the “in situ” experiments the BOX-MPSG particles were added to the reactants (amount according to 5 mol% BOX referring to the aryl halide) and 5 mol% Pd(OAc)2 (with respect to aryl halide) was added to the reaction mixture.
2.4.2. Recycling studies
Recycling studies were carried out using the general Suzuki–Miyaura reactions procedure. After the reaction, the catalyst was separated from the reaction mixture by filtration. The catalyst was washed with CH2Cl2 and H2O to remove residual salts and organics. Before reuse, the catalyst was dried under vacuum at room temperature, and the amounts of necessary reactants were recalculated for a 0.75 mol% Pd reaction.
The same cleaning and drying procedure was carried out before the used catalyst was analyzed by nitrogen physisorption.
2.4.3. Three-phase tests
The three-phase tests were carried out in analogy to the procedure published by Crudden et al. [23], [37]. For the immobilization of the aryl halide, 4-bromobenzoyl chloride (219 mg, 1 mmol) was dissolved in dry THF in a round-bottom flask. 3-Aminopropyl-functionalized silica gel (1 g functional silica gel with 1 mmol amino groups) and pyridine (80 μl, 1 mmol) were added under an argon atmosphere. The resulting mixture was stirred at 40 °C for 12 h, then filtered and washed with HCl, followed by K2CO3, distilled water, ethanol, and large amounts of CH2Cl2 to give (after drying under vacuum at room temperature) 0.98 g of BrPhCONH-propyl silica gel I. Elemental analysis: 1.64% (1.17 mmol) N, 15.04% (12.5 mmol) C, and 1.70% (17.0 mmol) H.
4-Bromobenzamide 3-propyltriethoxysilane was prepared also by following the synthesis presented by Crudden et al. [23]: 2 g (9.1 mmol) of 4-bromobenzoyl chloride was mixed with 1 ml (9.1 mmol) N-methyl morpholine in 50 ml THF. This mixture was cooled to −10 °C, and then 3-aminopropyl triethoxysilane (2 ml, 9 mmol) in 10 ml THF was added dropwise to the cooled solution. After warming this mixture to room temperature, the solid was removed by filtration, and the filtrate was concentrated under vacuum yielding a beige solid. This solid was mixed under argon with pyridine (0.9 ml, 11.5 mmol) and was added to a suspension of silica gel 60 (1 g) in anhydrous toluene (3 ml). After refluxing this mixture for 24 h, the suspension was filtered, and the resulting solid was Soxhlet extracted in CH2Cl2 for 24 h. After drying under vacuum at room temperature, we obtained 1.2 g of a beige powder (BrPhCONH-propyl silica gel II). Elemental analysis: 1.28% (0.91 mmol) N, 14.64% (12.2 mmol) C, and 1.54% (15.4 mmol) H.
For the actual three-phase tests, 4-bromoacetophenone (86 mg, 0.5 mmol, 1 mol equiv.), phenylboronic acid (134 mg, 1.1 mmol, 2.22 mol equiv.), and K2CO3 (173 mg, 1.25 mmol, 2.5 mol equiv.) were stirred in 5 ml toluene in the presence of n-dodecane (72 mg, 0.5 mmol, 1 mol equiv.), BrPhCONH-propyl silicagel I or II (500 mg), and Pd(OAc)2-BOX-MPSG (2 mol%) at 115 °C for 24 h. Then the supernatant was analyzed by GC, and the solid was separated by filtration, washed with ethanol, and further extracted with CH2Cl2. The solid was hydrolyzed with KOH in ethanol/water (1.7 g KOH in 10 ml of EtOH plus 5 ml of H2O) at 90 °C for 3 days. The resulting mixture was neutralized with aqueous HCl, extracted with CH2Cl2, followed by ethyl acetate, concentrated, and the resulting mixture was analyzed by 1H and 13C NMR. Further experiments were carried out with 4.4 mol equiv. boronic acid (and 4.3 mol equiv. K2CO3) in order to ensure that the reaction is not starved of boronic acid. The amounts of the other materials were kept constant.
3. Results and discussion
3.1. Synthesis and characterization of the heterogeneous Pd-catalyst
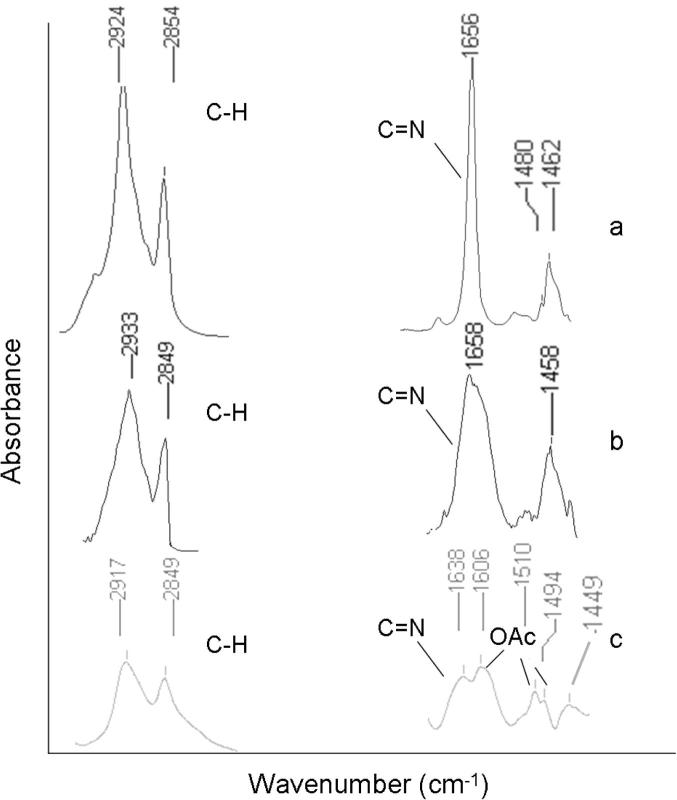
Analogous to the procedure reported by Hara et al., who grafted the tethered BOX ligand (2,2′-(1-methyl-11-dodecenylidene)bis(4,5-dihydrooxazole)) on a H-terminated Si(1 1 1)-wafer followed by complexation with Pd(OAc)2 [43], the preparation of our novel catalytic system was also carried out in two steps (Scheme 1). First, the BOX ligand was immobilized on 3-mercaptopropyl-functionalized silica gel (MPSG) particles using AIBN or ACHN as radical initiator [20], [44], [45], [46]. Then the immobilized ligand (BOX-MPSG) was metalated with Pd(OAc)2 at room temperature in CH2Cl2 to give the complex Pd(OAc)2-BOX-MPSG. The attachment of the ligand and the metal was confirmed using ATR-IR spectroscopy. The spectrum of BOX-MPSG (Fig. 1b) showed the characteristic C N stretching at ∼1660 cm−1 and the C–H stretching modes of the alkyl chain at ∼2940 and 2850 cm−1, similar to the non-immobilized BOX ligand (Fig. 1a). Furthermore, the spectra of Pd-BOX-MPSG showed three additional peaks at 1490, 1510, and 1600 cm−1 (Fig. 1c). According to the IR data reported by Hara et al., we associated these peaks to the signals of the acetato ligands on the Pd atom.
Fig. 1.
Parts of the IR spectra of (a) the BOX ligand (b) BOX–MPSG and (c) Pd(OAc)2–BOX–MPSG. Shown are the regions of the C–H stretching (2950–2830 cm−1) and the peaks in the range of 1670–1430 cm−1.
The heterogeneous catalyst was additionally characterized using elemental analysis (Table 1), SEM/EDX (Table 2), and ICP/OES (Table 3). The results indicate that the mol ratio of S/BOX is about 4:1, and the ratio of S/Pd is 4:1. Thus, the BOX/Pd ratio is 1:1, and we assume that all of the Pd forms a complex with the BOX ligand.1 The nitrogen physisorption analysis of the material showed that the BET surface area of the material is 192 m2/g, and the average pore diameter is 47 Å (Table 6).
Table 1.
Results of the elemental analysis.
| Sample | Element | Wt% | Loading (mmol/g) |
|---|---|---|---|
| 3-Mercaptopropyl silica gel (MPSG) | N | <0.1 | |
| S | 4.6 | 1.4 | |
| BOX-MPSG | N | 1.2 | 0.8 |
| S | 4.6 | 1.4 | |
| Pd(OAc)2-BOX-MPSG | N | 1.2 | 0.8 |
| S | 4.5 | 1.4 |
Table 2.
Results of the SEM/EDX measurements of Pd(OAc)2-BOX-MPSG.
| Element | Wt% | At% |
|---|---|---|
| Si | 55.7 | 75.4 |
| S | 10.6 | 12.6 |
| Pd | 33.7 | 12.0 |
Table 3.
Results of the ICP/OES measurements.
| Sample | Element | Loading (mg/g) | Loading (mmol/g) |
|---|---|---|---|
| 3-Mercaptopropyl silica gel (MPSG) | Si | 325.0 | 11.6 |
| S | 40.8 | 1.3 | |
| Pd(OAc)2-BOX-MPSG | Si | 310.0 | 11.1 |
| S | 39 | 1.2 | |
| Pd | 34 | 0.3 |
Table 6.
Effect of catalyst recycling on BET surface area, total pore volume, and average pore diameter.
| Entry | BET (m2/g) | Total pore volume (cm3/g) | Average pore diameter (Å) |
|---|---|---|---|
| MPSG | 365 | 0.41 | 45 |
| Pd(OAc)2-BOX-MPSG | 192 | 0.23 | 47 |
| Pd(OAc)2-BOX-MPSG after 1st use | 112 | 0.15 | 55 |
3.2. Suzuki reactions
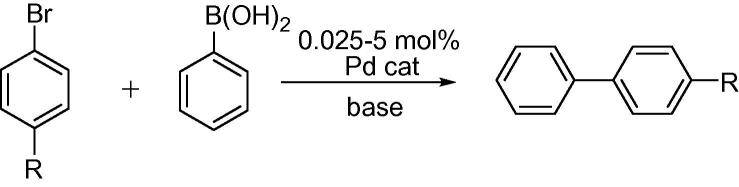
The activity of Pd(OAc)2-BOX-MPSG was tested for Suzuki–Miyaura reactions (Scheme 2) under various conditions (Table 4).
Scheme 2.
Suzuki–Miyaura couplings (R = CH3, CF3, COCH3).
The initial experiments were carried out with the homogeneous catalytic system, i.e., the BOX ligand and Pd(OAc)2, using two different aryl halides (1-bromo-4-methylbenzene and 1-bromo-4-(trifluoromethyl)benzene) as substrates. Bases, solvents, and reaction temperatures were varied in the experiments (Table 4, entry 1–5 and 8). The results show that the nature of the initial precatalyst, the reaction solvent, and the base [48] is critical for the outcome of the reaction. For both aryl halides, the highest yields were obtained with toluene and K2CO3 at a reaction temperature of 80 °C. Furthermore, the results indicate that the catalyst also works in aqueous systems at 25 °C. However, the yield was very low (entry 3 in Table 4).
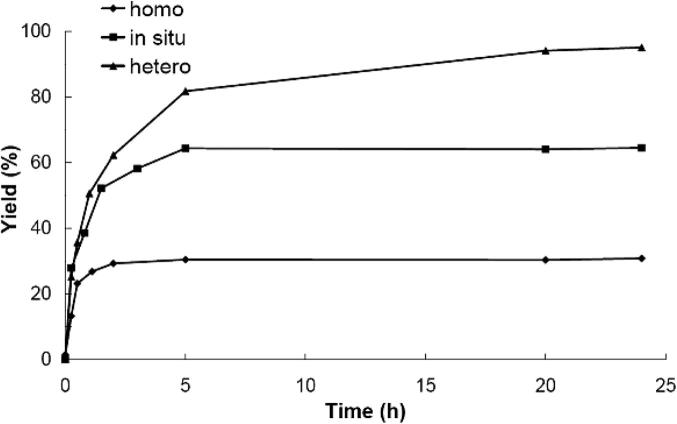
In addition to the homogeneous catalytic system, we also studied the activity of the “in situ” immobilized catalyst (as reported, e.g., by Kuriyama et al. [49] as well as by Yang et al. [50]). For these experiments, the BOX ligand immobilized on MPSG (=BOX-MPSG) was used. The metalation of the ligand was carried out during the Suzuki reaction, i.e., in the presence of the aryl halides, the boronic acid, and the base. The yields using this catalytic system (Table 4, entry 6 and 9 and Fig. 2) are definitely higher than the yields obtained with the homogeneous catalyst.
Fig. 2.
Comparison of the obtained yields of the Suzuki reactions using the homogeneous, the in situ, and the heterogeneous catalytic system. The reactions were carried out with 5 mol% catalyst, 1-bromo-4-methylbenzene, phenylboronic acid, K2CO3, and toluene at 80 °C. Yields were determined with GC using n-dodecane as internal standard. (Curves are drawn to aid the eye.)
Finally, we obtained yields >90% for the Suzuki reactions when the heterogeneous catalytic system was used (Table 4). For these experiments, Pd(OAc)2 was attached to the immobilized ligand in a separate step as described in Scheme 1 before the catalytic system was used for the Suzuki reactions. With this heterogeneous system, 90% yield could be achieved within 4 h; after 24 h yields up to 97% were obtained (Table 4, entries 12, 13 and 20) when 1-bromo-4-methylbenzene was the aryl halide substrate. In addition to that, almost quantitative yields could be achieved after 4 h using 4-bromoacetophenone as starting material (Table 4, entry 21). Comparison of these data with the results reported for other supported Pd-catalysts used for Suzuki couplings2 shows that the activity of our novel system is in the same range.
A reason for the significantly lower yields of the reactions with the homogeneous catalytic system might be the multiple sites of interaction of the palladium(II)acetate with the BOX-ligand. We assume that the low performance can be attributed to the interactions of the Pd-complex with the terminal double bond of the BOX ligand. Since in the heterogeneous case the BOX ligand is covalently attached to the solid support, Pd(OAc)2 can only (beside the possible complexation of Pd with the remaining SH-groups of the solid support) form a complex with the nitrogen atoms of the bis(oxazoline) functionality. This assumption is also supported by the fact that experiments with the analogous ligand without terminal double bond (i.e., 2,2′-(tridecane-2,2-diyl)bis(4,5-dihydrooxazole) = BOX 2, entry 22, Table 4) showed higher yields than the experiments with the BOX ligand. Another reason for the low activity of the homogeneous catalyst can also be self-deactivation of Pd(OAc)2, as it was reported, e.g., by Richardson and Jones [39] and many others.
The lower activity of the in situ immobilized catalyst in comparison with the heterogeneous catalyst can be attributed to the fact that the thiol groups that are not attached to the BOX ligand act as an effective and selective poison [4], [23], [39] of the homogeneous Pd-species that is added to the starting reaction mixture. This poisoning effect is also illustrated by the results of entry 23 in Table 4. When homogeneous Pd(OAc)2 was used in the presence of 3-mercaptopropyl silica gel particles only 41% yield could be achieved (entry 23, Table 4). This result, i.e., the lower yield obtained with the Pd(OAc)2-MPSG system in comparison with the novel Pd(OAc)2–BOX–MPSG system is very important, since it clearly shows the positive influence of the BOX ligand on the reaction. Interestingly, although the thiol groups should lead to a deactivation of Pd(OAc)2, the yield of the Pd(OAc)2-MPSG experiment was higher than the yield that was obtained with homogeneous Pd(OAc)2 (without BOX, entry 1 in Table 4). As mentioned before, a reason for that might be the self-deactivation of the homogeneous Pd(OAc)2 [39].
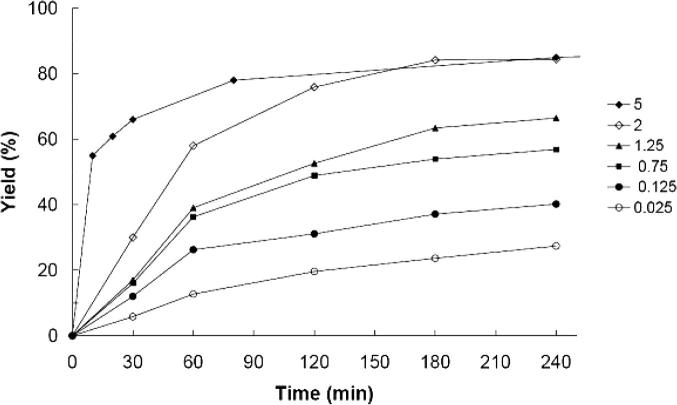
Finally, we tested the influence of the temperature and the catalyst loading on the yield obtained with Pd(OAc)2–BOX–MPSG. The results of entries 10, 11, 12, and 13 in Table 4 demonstrate that an increasing reaction temperature led to increasing yields (when toluene was used as solvent). In addition, the data presented in Fig. 3 and Table 4 show that the yield increased also with increasing catalyst loading. The lowest tested catalyst loading was 0.025 mol%. However, only low yields (28% after 4 h, Table 4, entry 14) were observed with this small catalyst amount.
Fig. 3.
Influence of the catalyst loading on the yield of the Suzuki–Miyaura reaction of 1-bromo-4-methylbenzene and phenylboronic acid carried out with Pd(OAc)2–BOX–MPSG in toluene at 80 °C. Yields were determined with GC using n-dodecane as internal standard. (Curves are drawn to aid the eye.)
3.3. Heterogeneity of the reaction
For the synthesis of active pharmaceutical intermediates, metal contamination of the product is a serious issue [15], [32]. Leaching of the catalyst into the product would implicate a time-consuming and costly cleaning step, which would make the whole process more expensive [52]. Furthermore, leaching of the active metal also leads to a decreasing turn over frequency (TOF) of the catalyst. Thus, the true catalytically active species (i.e., homogeneous or heterogeneous) has been studied systematically for many catalytic systems over the last decades. Several methods have been developed to distinguish between soluble vs. insoluble catalysts, and detailed reviews have been presented by Phan et al. [17] and Lamblin et al. [15]. Some of these methods were also used by us in order to investigate whether the solid catalyst is really heterogeneous or not. The results of these experiments will be presented in the following. However, already the data presented in Table 4 indicate that the catalytic system does not behave like homogeneous Pd(OAc)2. Our results show that an increasing amount of catalyst leads to higher activity (Fig. 3). These data contrast the findings reported, e.g., by Richardson and Jones that a higher loading of homogeneous Pd(OAc)2 leads to lower conversions because of self-deactivation of the homogeneous catalyst [39].
In fact, our catalytic system yielded similar results as the data presented by Richardson and Jones for Heck reactions with Pd-SH-SBA-15 [39]. They also reported an increasing catalyst activity with increasing catalyst loading, as well as higher activities at higher reaction temperatures. This temperature dependency was also found by us (Table 4, entries 10–13). The decrease of activity with decreasing temperature was attributed by Richardson and Jones either due to effects inhibiting the reaction on the surface or to temperature effects on the leaching of the immobilized Pd from supported thiols. We believe that the increasing activity with increasing temperature could be attributed to a standard Arrhenius behavior of a rate-determining reaction step.
The increasing activity at higher catalyst loadings was explained by Richardson and Jones by one of the two possibilities: (i) Pd(0) formed during the Heck cycle cannot aggregate due to its binding to an immobilized organic surface, and thus, increased activity is simply associated with more metal present or (ii) the amount of leached palladium from the surface is low, and thus, the actual concentration of active catalytic species in solution is below levels that promote self-deactivation. In order to evaluate the leaching behavior of the presented Pd(OAc)2-BOX-MPSG system and to compare it with other systems reported in the literature, several tests were carried out by us, which will be presented in the following sections.
3.3.1. Hot filtration test
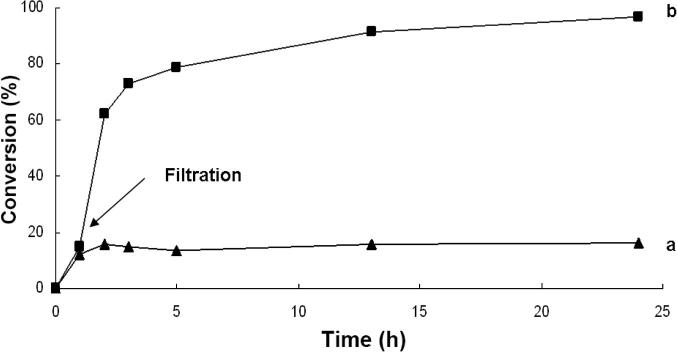
During this test, the catalytically active particles were removed from the reaction by filtration after 50 min using a hot frit, and the filtrate was monitored for continued activity. The results show (Fig. 4) that after removal of the catalyst particles, the reaction did not proceed, indicating that no catalytically active Pd remained in the filtrate. This fact is in contrast to the findings reported by Richardson and Jones [39], Crudden et al. [23], [37], as well as by Davis et al. [38], who also used silica-supported Pd-catalysts for cross-coupling reactions. Their systems continued to react after the catalyst was removed by filtration. However, it is known that the hot filtration test alone cannot prove that the reaction occurs heterogeneously, because the leached Pd can re-deposit so quickly that it may not be detected by a filtration test [15], [17]. Furthermore, disruptions, such as hot filtration, may deactivate an existing active dissolved metal, leading to the incorrect conclusion that there are no active homogeneous catalytic species before the filtration [17], [39], [53]. Therefore, other tests were carried out to investigate the heterogeneity of the Pd(OAc)2-BOX-MPSG system.
Fig. 4.
Hot filtration test for the Pd(OAc)2–BOX–MPSG catalyzed Suzuki–Miyaura reaction of 1-bromo-4-methylbenzene and phenylboronic acid with K2CO3, toluene at 115 °C. In case a, the catalytic active particles were filtered off after 50 min using a hot frit.
3.3.2. ICP/OES analysis
Potential Pd leaching into the reaction mixture was also analyzed with ICP/OES analysis. For this purpose, samples were taken through a syringe filter (Whatman Puradisc 4, 4 mm diameter, 0.45 μm, PTFE) during standard heterogeneous Suzuki–Miyaura reactions (reaction temperature: 115 °C), the solvent was evaporated, and the residue was dissolved in HNO3. The analysis of these samples with ICP/OES showed that the Pd concentration in the reaction solution was less than the detection limit (i.e., 50 ppb), which corresponds to less than 0.11% of the starting Pd-amount. The same result was obtained when the complete reaction mixture of a standard heterogeneous Suzuki–Miyaura reaction was filtered, the solvent was evaporated, and the residue was dissolved in HNO3. Both findings indicate that virtually no Pd leaches from the surface into the solution.
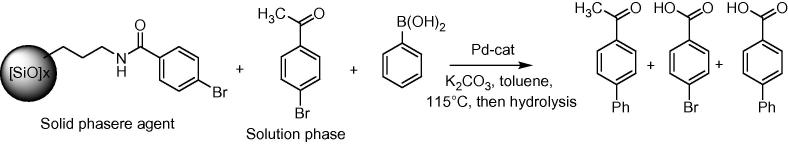
3.3.3. Three-phase test
The three-phase test was carried out in analogy to the procedure reported by Crudden et al. [37]. This test is considered to be a highly useful proof for the presence of catalytically active homogeneous metal species [17], [37], [54]. The test, first developed by Rebek et al. [55], [56], is based on covalently immobilizing one of the reaction partners, in this case an aryl halide, and examining its reaction with a soluble reagent, i.e., the boronic acid. The supported catalyst represents the third phase. If the catalyst remains immobilized, it will be incapable of accessing the supported aryl halide. If homogeneous Pd is released, the covalently immobilized substrate will be converted into the product. To ensure that an active catalyst is present, a soluble aryl halide, i.e., 4-bromoacetophenone, is added. After reaction, the soluble portion is analyzed after filtration while the amide is hydrolyzed from the support and isolated as carboxylic acid (Scheme 5).
Scheme 5.
Reaction scheme of the three-phase test.
To carry out the three-phase test, we immobilized 4-bromobenzoyl chloride on 3-aminopropyl silica gel according to Scheme 3. Since Crudden et al. reported that they found free amines after the immobilization of 4-bromobenzoyl chloride (Scheme 3), we also prepared another supported reagent (BrPhCONH-propyl silicagel II) according to Scheme 4. Both supported reagents were then used for Suzuki reactions together with 4-bromoacetophenone as free available aryl halide (Scheme 5). The reactions were carried out with an excess of boronic acid (2.2 mol equiv. and 4.4 mol equiv., respectively) in order to make sure that the reaction is not starved of boronic acid [23]. The analysis of the supernatant with GC indicated that 4-bromoacetophenone was successfully converted (up to 90% conversion after 24 h). However, in all experiments, the biphenyl carboxylic acid could not be detected using 1H and 13C NMR spectroscopy after the amide was hydrolized from the support. Thus, this test is another important indicator that the catalyst is (at least mostly) heterogeneous under the applied conditions (reactions carried out in toluene, with 2 mol% catalyst, K2CO3 as the base, reaction temperature: 115 °C). When we carried out the same experiment using Pd/C as catalyst, which is known to leach from the surface [23], [31], the NMR spectra showed both, 4-bromo-benzoic acid as well as biphenyl-4-carboxylic acid.
Scheme 3.
Immobilization of 4-bromobenzoyl chloride on 3-aminopropyl silica gel to prepare the solid phase reagent I for the three-phase test.
Scheme 4.
Second way of immobilizing 4-bromobenzoyl chloride on silica gel.
3.3.4. Catalyst reusability
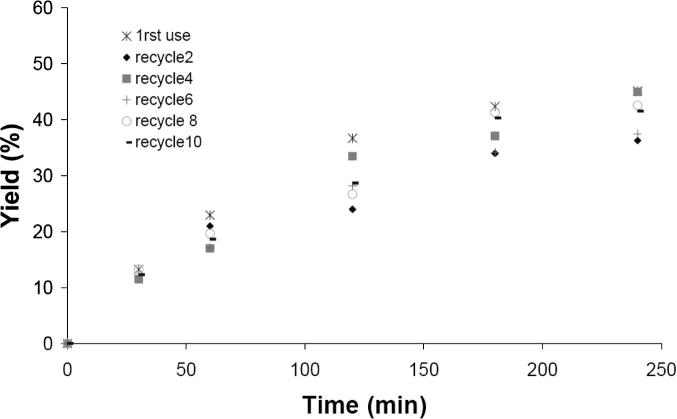
In addition to the total leaching and overall catalytic activity, reusability is a critical feature for supported catalysts [23]. Therefore, we used the same catalytic particles after filtration from the reaction mixture for several reactions under the same conditions. In contrast to the other heterogeneity tests, these experiments were carried out at 80 °C. Since the other tests indicated that higher reaction temperatures do not lead to increased Pd-leaching into the reaction solution, it is assumed that the lower reaction temperature only influences the obtained yields and not the leaching behavior. The results shown in Fig. 5 and Table 5 indicate that there is no apparent loss of catalytic activity even after 10 runs, and the obtained yields of the reusability experiments are within the experimental deviations.
Fig. 5.
Effect of catalyst recycling on the yield. All reactions were carried out with Pd(OAc)2–BOX–MPSG (0.75 mol%), 1-bromo-4-methylbenzene, phenylboronic acid, K2CO3, and toluene at 80 °C. Yields were determined with GC using n-dodecane as internal standard.
Table 5.
Effect of catalyst recycling on yield and turn over frequency (TOF).
| Entry | Yield (%)a | TOF (h−1) |
|---|---|---|
| 1st use | 45 | 14 |
| Recycle 1 | 37 | 12 |
| Recycle 2 | 37 | 12 |
| Recycle 3 | 38 | 12 |
| Recycle 4 | 45 | 14 |
| Recycle 5 | 37 | 12 |
| Recycle 6 | 38 | 12 |
| Recycle 8 | 42 | 13 |
| Recycle 10 | 42 | 13 |
Yields determined after 4 h with GC using n-dodecane as internal standard. All reactions were carried out with 0.75 mol% Pd(OAc)2–BOX–MPSG, 1-bromo-4-methylbenzene, phenylboronic acid, K2CO3, and toluene at 80 °C.
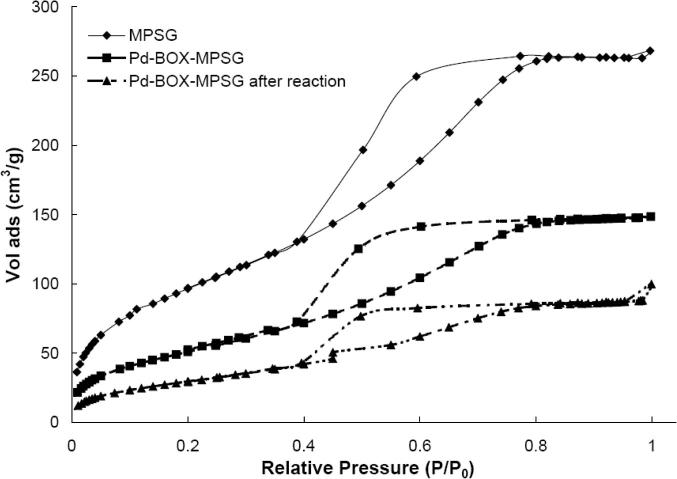
3.3.5. Catalyst texture
Additionally to assessing the loss of Pd, it is crucial to determine whether the catalyst support is affected by the reaction conditions [23]. Therefore, we analyzed the supported catalyst by nitrogen adsorption analysis before and after 24 h of reaction (Fig. 6). The results indicate that although the BET surface area and the pore volume decrease, the mesoporous structure of the solid support is maintained. All samples showed the expected type IV isotherms that are typical for mesoporous materials [57]. Thus, the used solid support shows a higher stability than other mesoporous silica materials, such as SBA-15 or MCM-41 [4], [23], [29]. These materials lost their mesoporous properties after the materials were used at the applied reaction conditions (80 °C, 20:1 DMF:H2O, K2CO3 in [4]).
Fig. 6.
Nitrogen adsorption analysis of the solid support (MPSG), the immobilized catalyst before (Pd–BOX–MPSG) and after use (Pd–BOX–MPSG after reaction).
3.3.6. Effect of the solvent on the activity and stability of the catalyst
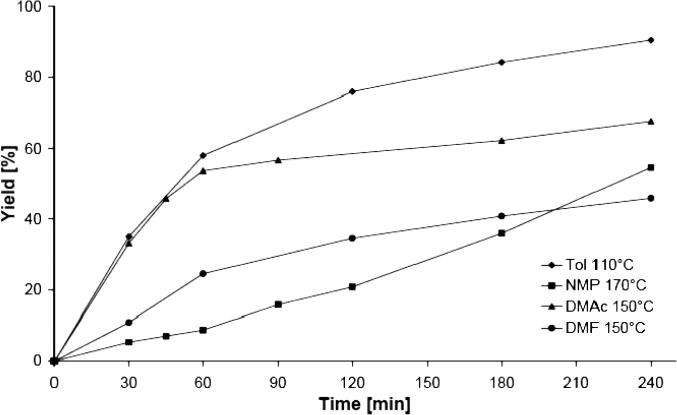
Several groups reported that the nature of the reaction solvent had a dramatic effect on catalytic activity and the extent of Pd leaching [38], [51], [58], [59]. For example, Ji et al. reported that the catalytic activity of supported Pd-catalysts for Heck reactions was much lower in toluene than in DMF. In order to investigate whether the presented catalyst shows a similar behavior, additional experiments were carried out with NMP (1-methyl-2-pyrrolidinone), DMAc (N,N-dimethylacetamide), and DMF (N,N-dimethylformamide) as solvents. The results are presented in Fig. 7 and Table 7.
Fig. 7.
Effect of the solvent on the reaction progress of Suzuki–Miyaura reactions of 1-bromo-4-methylbenzene and phenylboronic acid carried out with 2 mol% Pd(OAc)2–BOX–MPSG.
As can be seen, the polar solvents do not lead to an increase of activity of the catalytic system. In fact, the obtained yields for 4-methylbiphenyl were lower when DMAc, NMP, and DMF were used even when the reactions were carried out at higher temperatures. Additionally, significant by-product was noticed. In all experiments with toluene, this by-product formation was not observed.
Although the influence of the solvent on the catalytic activity is not completely understood [38], it has been shown by other groups that the Pd leaching is more extensive in highly polar solvents (as DMAc, NMP, and DMF) than in non-polar toluene [38], [51], [60].
However, our catalytic system shows a different behavior: ICP/OES analysis of samples taken through a syringe filter from the reactions that were carried out in DMAc (150 °C), NMP (160 °C), and DMF (150 °C) after 2 h and 24 h did not show any Pd. Furthermore, the addition of 100 mol equiv. poly(4-vinylpyridine) (PVPy, 2% cross-linked), a common Pd-scavenger that is known to act as a trap of leached Pd (see below and [38], [39], [52], [61], [62]), did not lead to a catalyst poisoning, i.e., in all solvents, the reaction progress was almost the same as with no poison (Table 7). Thus, we can conclude that highly polar solvents and elevated reaction temperatures do not lead to an extended Pd leaching.
3.3.7. Catalyst poisoning
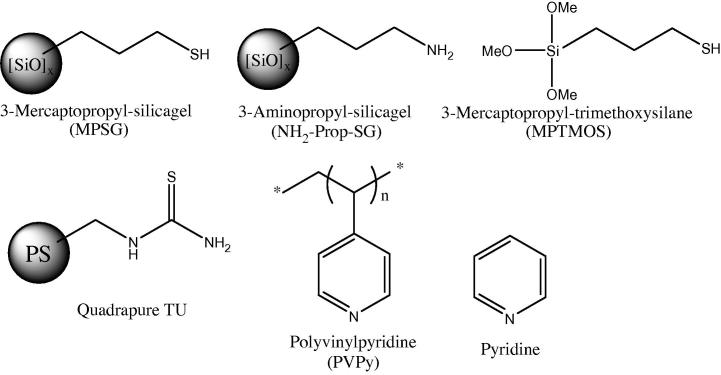
Another method that was used to assess the homogeneity/heterogeneity of our catalytic system was catalyst poisoning. By addition of materials that are known to act as catalyst poisons, soluble reactive Pd can be removed from the solution. The poisons that were used by us are shown in Fig. 8. The first compound was 3-mercaptopropyl-functionalized silica gel (MPSG), as thiol-modified surfaces are known to be very effective Pd-scavengers [4], [39], [40]. In addition, poly(4-vinylpyridine) (PVPy, 2% cross-linked) was used because pyridines are known to bind strongly to Pd(II) [38], [63], and several groups applied insoluble PVPy as a Pd trap to confirm the presence of leached, soluble Pd [38], [39], [52], [61], [62]. Furthermore, Quadrapure TU, an organic polymer resin (polystyrene) with a thiourea functionality, and 3-aminopropyl silica gel were studied as poisons. Since all these compounds are solids, the addition of them can lead to a decrease of reaction progress by a limited mass transfer of the heterogeneous Pd-catalyst to the reactants. Therefore, two homogenous compounds, pyridine and 3-mercaptopropyl-trimethoxysilane, were also utilized as poisons in order to exclude this effect. The results of the experiments are shown in Fig. 9 and Table 8.
Fig. 8.
Names and chemical structures of the used poisons (PS = polystyrene).
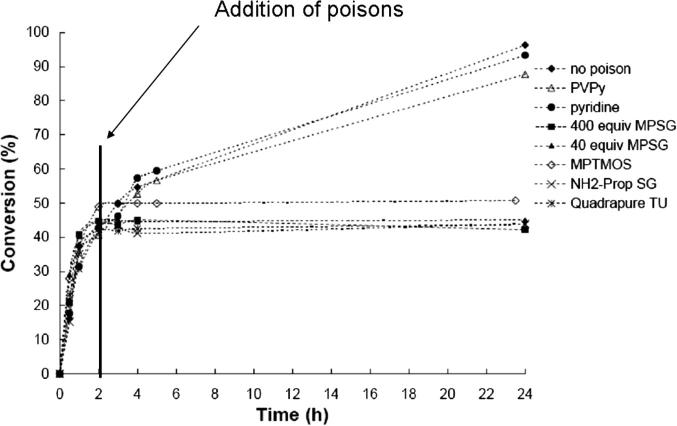
Fig. 9.
Influence of selective catalyst poisons on the reaction progress of Suzuki–Miyaura reactions of 1-bromo-4-methylbenzene and phenylboronic acid carried out with 2 mol% Pd(OAc)2-BOX-MPSG in toluene at 115 °C using K2CO3 as base.
Table 8.
Influence and used amounts of the selective catalyst poisons on the reaction progress of Suzuki–Miyaura reactions of 1-bromo-4-methylbenzene and phenylboronic acid carried out with 2 mol% Pd(OAc)2–BOX–MPSG in toluene at 115 °C using K2CO3 as base. Poisons were added after 2 h.
| Poison | Mol equiv. to Pd | Conversion after 2 h (%) | Conversion after 24 h (%) |
|---|---|---|---|
| No | – | 43 | 96 |
| PVPy | 100 | 43 | 88 |
| Pyridine | 340 | 42 | 93 |
| MPSG | 40 | 45 | 45 |
| MPSG | 400 | 45 | 42 |
| MPTMOS | 40 | 46 | 50 |
| NH2-prop-SG | 40 | 43 | 44 |
| Quadrupure TU | 45 | 42 | 44 |
The results presented in Fig. 9 and Table 8 show that all poisons except for PVPy and pyridine led to a deactivation of the catalyst, which would indicate that Pd leaches from the immobilized BOX ligand and is therefore quenched by the used poisons. The results also show that the addition of (340 mol equiv.) pyridine did not lead to a deactivation of the catalyst. Similar results were found for Heck reactions by the Davis group when homogeneous Pd(OAc)2 and Pd-SiO2(Cab) were the catalysts [38]. Also, Jones et al. reported that the addition of pyridine to a Heck reaction carried out with a homogeneous Pd(II)-SCS-N pincer complex had only little effect on the reaction rate in comparison with a reaction under normal conditions [62]. These findings would indicate that the activity of the Pd(OAc)2-BOX-MPSG catalyst arrives from leached, homogeneous Pd-species and is therefore contradictory to all the other tests that indicated that the catalytic system does not show metal leaching, at least no Pd traces could be detected in the reaction solutions.
Another result that supports the heterogeneous catalytic mechanism is the fact that when 100 mol equiv. PVPy/Pd were used as a poison, the reaction progress was almost the same as with no poison (as already discussed above). However, it is known that due to the equilibrium on–off coordination of the Pd with the pyridine sites and the non-porous nature of the resin, a large excess of pyridine units are required to over-coordinate Pd [39]. Thus, other groups reported that the addition of higher amounts of PVPy was necessary in order to quench the reaction (e.g., Richardson and Jones added up to 350 equiv. PVPy to Pd-SH-SBA 15 [39], and Davis et al. added up to 700 equiv. [38]). Indeed, when we added 500 mol equiv. PVPy, the reaction also stopped to proceed. However, we believe that the addition of such large amounts of solids (in this experiment, 1 g PVPy was added to a reaction with 25 mg of functionalized silica gel including 0.8 mg Pd) can also result in a lower catalytic activity simply by decreasing the accessibility of the reactants to the catalytic sites in the thick slurry multiphase media, reducing diffusion constants and thus creating mass-transfer limited conditions.
In summary, the finding that the activity of our novel catalyst is decreased by selective Pd-poisons is contrary to the results of the other tests that indicated that the activity of the catalytic system can be attributed to a real heterogeneous catalytic system.
3.3.8. Transmission electron microscopy (TEM)
Finally, we analyzed the catalytic system using TEM. The bright-field TEM images (Fig. 10) show that after the catalyst was used once in toluene, nanoparticles with a size of ∼5 nm were formed (Fig. 10c).
Fig. 10.
Bright-field TEM pictures of (a) 3-mercaptopropyl silica gel (MPSG) (b) Pd(OAc)2–BOX–MPSG before use and (c) Pd(OAc)2–BOX–MPSG after 1st use.
In combination with our poisoning studies, this finding stands in contrast to the conclusion by Richardson and Jones [39] who reported that poisoning by SH-SBA-15 implies that the active soluble catalytic species is not from nanoparticle surfaces but rather from molecular and dimeric Pd. This theory is also supported by the work of other groups [13], [64], [65], [66]. One possible explanation for the behavior of our catalytic system, i.e., poisoning by mercapto-functionalized silica gel and nanoparticle formation, could be that small amounts of Pd leach from the immobilized BOX ligand during the reaction. If a poison is present, this Pd is over-coordinated by the poison. Otherwise, Pd nanoparticles are formed, and these particles are trapped by the solid support. Thus, the support would act as the source of the active component and at the same time as sink for Pd, and therefore, constituting a virtually leaching-free system. However, further studies are needed to confirm this theory and to fully understand the reaction mechanism of the investigated catalytic system.
4. Conclusions
A novel catalytic system including a Pd-complex immobilized via a bis(oxazoline) (=BOX) ligand on 3-mercaptopropyl silica gel is presented. The catalytic system was tested for heterogeneous Suzuki–Miyaura reactions of different aryl halides with phenylboronic acid. Using several approaches to test the heterogeneity of the catalytic system, we could show that there is virtually no Pd leaching into the reaction solution under the applied reaction conditions. Furthermore, our results indicate that the catalyst is stable and can be reused for at least 10 times. Such stable heterogeneous catalysts would be a key to implement continuous processes in the preparation of pharmaceutical and fine chemical intermediates. However, the addition of catalyst poisons led to a quenching of the catalytic activity, which indicates the presence of leached homogeneus Pd. Additionally, TEM pictures of the used catalytic material indicate nanoparticles on the surface. Therefore, further studies are needed to fully understand the reaction mechanism of the presented catalytic system.
Acknowledgments
We kindly acknowledge the financial support of the Austrian Science Foundation (Project No. 19410 and Elise Richter Project No. V171-N19).
Footnotes
We tried to quantify the amount of available thiol groups with a spectrophotometric method based on a thiol-exchange reaction with 2,2′-pyridyl thione according to Lindner et al. [47]. However, the presence of Pd influenced the UV-signal which led to implausible results.
(e.g., Crudden et al., Suzuki coupling of bromoacetophenone with phenyl boronic acid using 1% SBA-SH-Pd, 97% yield after 8 h at 80 °C in H2O [37], Corma et al., 100 mg zeolite-supported PdCl2 for Suzuki coupling of 1 mmol PhBr with 1.5 mmol phenyl boronic acid, up to 89% after 24 h at 50 °C in ethanol [51], Richardson and Jones, Suzuki coupling of bromoacetophenone with phenyl boronic acid using 1% Pd-SH-SBA-15, 94% conversion after 6 h in DMF, 80% conversion after 6 h in H2O [39]).
References
- 1.Blaser H.U., Indolese A., Schnyder A., Steiner H., Studer M. J. Mol. Cat. A: Chem. 2001;173:3. [Google Scholar]
- 2.Grasa G.A., Viciu M.S., Huang J., Zhang C., Trudell M.L., Nolan S.P. Organomet. 2002;21:2866. [Google Scholar]
- 3.Kotha S., Lahiri K., Kashinath D. Tetrahedron. 2002;58:9633. [Google Scholar]
- 4.Glasspoole B.W., Webb J.D., Crudden C.M. Journal of Catalysis. 2009;265:148. [Google Scholar]
- 5.Magano J., Dunetz J.R. Chem. Rev. 2011;111:2177. doi: 10.1021/cr100346g. [DOI] [PubMed] [Google Scholar]
- 6.Slagt V.F., de Vries A.ü.H.M., de Vries J.G., Kellogg R.M. Org. Process Res. Dev. 2009;14:30. [Google Scholar]
- 7.Hird M., Gray G.M., Toyne K.L. Mol. Cryst. Liq. Cryst. 1991;206:187. [Google Scholar]
- 8.Nicolaou K.C., Bulger P.G., Sarlah D. Angew. Chem. Int. Ed. 2005;44:4442. doi: 10.1002/anie.200500368. [DOI] [PubMed] [Google Scholar]
- 9.Yin L., Liebscher J. Chem. Rev. 2007;107:133. doi: 10.1021/cr0505674. [DOI] [PubMed] [Google Scholar]
- 10.Felpin F.X., Ayad T., Mitra S. Europ J. Org. Chem. 2006:2679. [Google Scholar]
- 11.Miyaura N., Suzuki A. Chem. Rev. 1995;95:2457. [Google Scholar]
- 12.Suzuki A. Journal of Organometallic Chemistry. 2002;653:83. [Google Scholar]
- 13.Stanforth S.P. Tetrahedron. 1998;54:263. [Google Scholar]
- 14.Slagt V.F., de Vries A.H.M., de Vries J.G., Kellogg R.M. Org. Process Res. Dev. 2009;14:30. [Google Scholar]
- 15.Lamblin M., Nassan-Hardy L., Hierso J.-C., Fouquet E., Felpin F.-X. Adv. Synth. Cat. 2010;352:33. [Google Scholar]
- 16.Corbet J.P., Mignani G. Chem. Rev. 2006;106:2651. doi: 10.1021/cr0505268. [DOI] [PubMed] [Google Scholar]
- 17.Phan N.T.S., Van Der Sluys M., Jones C.W. Adv. Synth. Cat. 2006;348:609. [Google Scholar]
- 18.Luo C., Zhang Y., Wang Y. Journal of Molecular Catalysis A: Chemical. 2005;229:7. [Google Scholar]
- 19.Sayah R., Glegola K., Framery E., Dufaud V. Adv. Synth. Catal. 2007;349:373. [Google Scholar]
- 20.Baleizao C., Corma A., Garcia H., Leyva A. Chem. Commun. 2003:606. doi: 10.1039/b211742h. [DOI] [PubMed] [Google Scholar]
- 21.Schweizer S., Becht J.M., Le Drian C. Org. Lett. 2007;9:3777. doi: 10.1021/ol701460z. [DOI] [PubMed] [Google Scholar]
- 22.Lemo J., Heuze K., Astruc D. Org. Lett. 2005;7:2253. doi: 10.1021/ol050645+. [DOI] [PubMed] [Google Scholar]
- 23.Webb J.D., MacQuarrie S., McEleney K., Crudden C.M. Journal of Catalysis. 2007;252:97. [Google Scholar]
- 24.Qiu H., Sarkar S.M., Lee D.H., Jin M.J. Green Chem. 2008;10:37. [Google Scholar]
- 25.Corma A., Das D., Garcia H., Leyva A. Journal of Catalysis. 2005;229:322. [Google Scholar]
- 26.Garcia-Bernabe A., Tzschucke C., Bannwarth W., Haag R. Adv. Synth. Catal. 2005;347:1389. [Google Scholar]
- 27.Jin M.-J., Lee D.-H. Angew. Chem. 2010;122:1137. doi: 10.1002/anie.200905626. [DOI] [PubMed] [Google Scholar]
- 28.Fan G., Huang J., Li Z., Li T., Li G. Journal of Molecular Catalysis A: Chemical. 2007;267:34. [Google Scholar]
- 29.Lee D.H., Choi M., Yu B.W., Ryoo R., Taher A., Hossain S., Jin M.J. Adv. Synth. Catal. 2009;351:2912. [Google Scholar]
- 30.MacQuarrie S., Nohair B., Horton J.H., Kaliaguine S., Crudden C.M. The Journal of Physical Chemistry C. 2010;114:57. [Google Scholar]
- 31.Glasnov T.N., Findenig S., Kappe C.O. Chem. Eur. J. 2009;15:1001. doi: 10.1002/chem.200802200. [DOI] [PubMed] [Google Scholar]
- 32.Garret C.E., Prasad K. Adv. Synth. Cat. 2004;346:889. [Google Scholar]
- 33.Polshettiwar V., Molnar A. Tetrahedron. 2007;63:6949. [Google Scholar]
- 34.Mehnert C.P., Weaver D.W., Ying J.Y. J. Am. Chem. Soc. 1998;120:12289. [Google Scholar]
- 35.Shimizu K., Koizumi S., Hatamachi T., Yoshida H., Komai S., Kodama T., Kitayama Y. Journal of Catalysis. 2004;228:141. [Google Scholar]
- 36.Bedford R.B., Singh U.G., Walton R.I., Williams R.T., Davis S.A. Chem. Mater. 2005;17:701. [Google Scholar]
- 37.Crudden C.M., Sateesh M., Lewis R. J. Am. Chem. Soc. 2005;127:10045. doi: 10.1021/ja0430954. [DOI] [PubMed] [Google Scholar]
- 38.Ji Y., Jain S., Davis R.J. J. Phys. Chem. B. 2005;109:17232. doi: 10.1021/jp052527+. [DOI] [PubMed] [Google Scholar]
- 39.Richardson J.M., Jones C.W. Journal of Catalysis. 2007;251:80. [Google Scholar]
- 40.Crudden C.M., McEleney K., MacQuarrie S., Sateesh M., Webb J.D. Pure Appl. Chem. 2007;79:247. [Google Scholar]
- 41.Clarke R.J., Shannon I.J. Chem. Commun. 2001:1936. doi: 10.1039/b105655g. [DOI] [PubMed] [Google Scholar]
- 42.Fraile J.M., Garcia J.I., Mayoral J.A. Coordination Chemistry Reviews. 2008;252:624. [Google Scholar]
- 43.Hara K., Tayama S., Kano H., Masuda T., Takakusagi S., Kondo T., Uosaki K., Sawamura M. Chem. Commun. 2007:4280. doi: 10.1039/b710820f. [DOI] [PubMed] [Google Scholar]
- 44.Baleizao C., Gigante B., Das D., Alvaro M., Garcia H., Corma A. J. Catal. 2004;223:106. [Google Scholar]
- 45.Baleizao C., Corma A., Garcia H., Leyva A. J. Org. Chem. 2004;69:439. doi: 10.1021/jo030302u. [DOI] [PubMed] [Google Scholar]
- 46.Sommer W.J., Yu K., Sears J.S., Ji Y., Zheng X., Davis R.J., Sherrill C.D., Jones C.W., Week M. Organometallics. 2005;24:4351. [Google Scholar]
- 47.Nogueira R., Lämmerhofer M., Maier N.M., Lindner W. Analytica Chimica Acta. 2005;533:179. [Google Scholar]
- 48.Amatore C., Jutand A., Le Duc G. Chemistry A European Journal. 2011;17:2492. doi: 10.1002/chem.201001911. [DOI] [PubMed] [Google Scholar]
- 49.Kuriyama M., Shimazawa R., Shirai R. Tetrahedron. 2007;63:9393. [Google Scholar]
- 50.Yang C., Lee H.M., Nolan S.P. Org. Lett. 2001;3:1511. doi: 10.1021/ol015827s. [DOI] [PubMed] [Google Scholar]
- 51.Corma A., Garcia H., Leyva A. Applied Catalysis A: General. 2002;236:179. [Google Scholar]
- 52.Weck M., Jones C.W. Inorg. Chem. 2007;46:1865. doi: 10.1021/ic061898h. [DOI] [PubMed] [Google Scholar]
- 53.Lipshutz B.H., Tasler S., Chrisman W., Spliethoff B., Tesche B. J. Org. Chem. 2002;68:1177. doi: 10.1021/jo020296m. [DOI] [PubMed] [Google Scholar]
- 54.Davies I.W., Matty L., Hughes D.L., Reider P.J. J. Am. Chem. Soc. 2001;123:10139. doi: 10.1021/ja016877v. [DOI] [PubMed] [Google Scholar]
- 55.Rebek J., Gavina F. J. Am. Chem. Soc. 1974;96:7112. [Google Scholar]
- 56.Rebek J., Brown D., Zimmerman S. J. Am. Chem. Soc. 1975;97:454. [Google Scholar]
- 57.Gregg S.J., Sing K.S. Academic Press; London: 1982. Adsorption, Surface Area and Porosity. [Google Scholar]
- 58.Köhler K., Heidenreich R.G., Krauter J.G.E., Pietsch J. Chemistry - A European Journal. 2002;8:622. doi: 10.1002/1521-3765(20020201)8:3<622::AID-CHEM622>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 59.Zhao F., Bhanage B.M., Shirai M., Arai M. Journal of Molecular Catalysis A: Chemical. 1999;142:383. [Google Scholar]
- 60.Dams M., Drijkoningen L., Pauwels B., Van Tendeloo G., De Vos D.E., Jacobs P.A. Journal of Catalysis209. 2002:225. [Google Scholar]
- 61.Chen J.S., Vasiliev A.N., Panarello A.P., Khinast J.G. Appl. Cat. A: General. 2007;325:76. [Google Scholar]
- 62.Yu K., Sommer W., Richardson J., Weck M., Jones C. Adv. Synth. Catal. 2005;347:161. [Google Scholar]
- 63.Hartley F.R. Coordination Chemistry Reviews. 1981;35:143. [Google Scholar]
- 64.Schmidt A.F., Smirnov V.V. Journal of Molecular Catalysis A: Chemical. 2003;203:75. [Google Scholar]
- 65.Alimardanov A., de Vondervoort L., de Vries A.H., deVries J.G. Adv. Synth. Catal. 2004;346:1812. [Google Scholar]
- 66.Fiddy S.G., Evans J., Neisius T., Newton M.A., Tsoureas N., Tulloch A.A.D., Danopoulos A.A. Chem. Eur. J. 2007;13:3652. doi: 10.1002/chem.200601278. [DOI] [PubMed] [Google Scholar]