Abstract
Waldenström’s Macroglobulinemia (WM) is a B-cell disorder characterized by the infiltration of the bone marrow (BM) with lymphoplasmacytic cells, as well as detection of an IgM monoclonal gammopathy in the serum. WM is considered an incurable disease, with an overall median survival of only 5-6 years. The success of targeted therapy in multiple myeloma (MM) has led to the development and investigation of more than 30 new compounds in this disease and in other plasma cell dyscrasias WM, both in the preclinical settings and as part of clinical trials. Among therapeutical options, first-line therapies have been based on single-agent or combination regimens with alkylator agents, nucleoside analogues, and the monoclonal antibody anti-CD20. Based on the understanding of the complex interaction between tumor cells and bone marrow microenvironment and the signaling pathways that are deregulated in WM pathogenesis, a number of novel therapeutic agents are now available; and demonstrated significant efficacy in WM. The range of the ORR to these novel agents is between 25-80%. Ongoing and planned future clinical trials include those using PKC inhibitors such as enzastaurin, new proteasome inhibitors such as carfilzomib, histone deacetylase inhibitors such as LBH589, humanized CD20 antibodies such as Ofatumumab, and additional alkylating agents such as bendamustine. These agents, when compared to traditional chemotherapeutic agents, may lead in the future to higher responses, longer remissions and better quality of life for patients with WM. This review will mainly focus on those novel agent that entered clinical trial for the treatment of WM.
INTRODUCTION
Waldenstrom Macroglobulinemia (WM) is a B-cell disorder characterized by the infiltration of the bone marrow (BM) with lymphoplasmacytic cells, as well as demonstration of an IgM monoclonal gammopathy.[1-4] WM is classified, according to the Revised European American Lymphoma (REAL) and World Health Organization (WHO) systems, as a lymphoplasmacytic lymphoma.[3,4] The overall incidence of WM is about 3 per million persons per year, equating to 1,500 new cases diagnosed each year in the United States.[5-7] The incidence rates are higher in Caucasians compared with African-Americans and when looking at the age-adjusted rates for men and women within the US, men have a higher incidence with 3.4 per million compared to 1.7 per million.[5-7] The most recognized risk factor for developing WM is IgM-monoclonal gammopathy of undetermined significance (IgM-MGUS), which confers a 46 fold higher relative risk.[8] There is also a higher risk among individuals who have a first degree relative with a B- cell neoplasm; which is about 18.7% of patients in various studies.[9,10] WM remains an incurable disease with an overall median survival of only 5-6 years and a median disease specific survival of 11.2 years[7]. Factors associated with poor prognosis include advanced age, high β2-microglobulin (β2M), cytopenias, low albumin, serum IgM monoclonal protein, and organomegaly.[7-11] Patients with low risk disease had a 5-year survival of 87%, while patients with high-risk disease had a 5-year survival of only 36%. This is now accepted as the uniform prognostic staging system for WM.[12]
After almost forty years, the paradigm for the treatment of monoclonal gammopathies has dramatically changed: for example, therapeutic options in MM have evolved from the introduction of melphalan and prednisone in the 1960s, high-dose chemotherapy and stem cell transplantation in the late 1980s and 1990s, to the rapid introduction of small novel molecules within the last seven years. Similar advances have been reached in the treatment of WM: based on the understanding of the complex interaction of WM cells with the bone marrow (BM) microenvironment and the signaling pathways that are dysregulated in this process, a number of novel therapeutic agents are now available. Novel agents including immunomodulatory drugs, proteasome inhibitors, PI3K/Akt and mTOR inhibitors are now all playing a key role in the preclinical settings and/or as part of clinical trials for the treatment of WM.
TIMING AND CHOICE OF TREATMENT
The treatment of patients with WM depends on the presence of symptoms or signs of disease progression. Patients with asymptomatic disease should not be treated independent of the monoclonal protein level. Some of the clinical signs or symptoms that indicate time to initiate therapy include: platelet count <100×109/L, hemoglobin level <10 g/dL, symptomatic hyperviscosity, amyloidosis, bulky adenopathy or organomegaly, moderate, severe or advancing peripheral neuropathy, cryoglobulinemia, or cold-agglutinin disease. Furthermore, important considerations to keep in mind when discussing treatment include: age, occurrence of cytopenias, necessity of rapid disease control, as well as the patient’s eligibility for autologous transplant therapy. Certain therapeutic options should be avoided in patients eligible for autologous transplant therapy such as alkylating agents and nucleoside analogues. A recent study has shown nucleoside analogue treatment leads to difficulty collecting stem cells, developing myeodysplasia or acute myelogenous leukemia, as well as increasing disease transformation risk, and therefore should be used judiciously with WM patients.[13]
STANDARD THERAPEUTIC OPTIONS
Current therapies used in the upfront or relapsed settings include alkylator agents (e.g. chlorambucil or cyclophasphamide), nucleoside analogues (cladribine or fludarabine), and the monoclonal antibody rituximab.[14-17] The overall response rates (ORR) in the upfront setting varies from 30-70%; this includes complete response (CR), partial response (PR), and minimal response (MR), with CR rates < 10%, and median durations of response averaging 2-3 years.[15,18] In the salvage setting, the ORR is between 30-40%, with a median response duration≤ 1 year. [15,19] The use of fludarabine or alkylating agents in combination therapy will stimulate high responses, however the toxicities are often significant, especially in the elderly.[20,21] Rituximab (anti-CD20 monoclonal antibody) is the most widely used therapeutic agent in WM and standard treatment yielded response rates of 35-48% (4 weekly infusions of 375 mg/m2 or extended treatment involving 4 weekly rituximab treatments repeated at 3 months).[16,22-24] Another important note involving rituximab treatment is the initial increase in the IgM level; this is termed IgM flare and is seen in about 54% of patients.[25,26] Although these levels may remain elevated for 3-4 months, they do not indicate treatment failure.
In a recent study by the Waldenstrom’s Macroglobulinemia Clinical Trials Group (WMCTG), the use of fludarabine with rituximab has been tested, with an the overall response rate of 96%, median progression-free survival of 51.2 months. Because of the significant and prolonged myelosuppression observed in the WMCTG study, which used 6 cycles of fludarabine, each with 5-day courses at 25 mg/m2, a more condensed course (ie, 4 days of fludarabine at 25 mg/m2 per cycle for 4 cycles) may be preferable in more indolent patients.[27]
Rituximab has been also evaluated in combination with cladribine and cyclophosphamide in 18 previously untreated patients. The ORR (CR+PR) was 94%, including CR in 17% of patients. Median time to response was 2.4 months, and median duration of response was 58.6 months.[28] Rituximab has been tested in combination with drugs other than nucleoside analogs. For example, Deamethasone (20mg) followed by Rituximab (375 mg/m2) on day 1 and cyclophosphamide (100 mg/m2) orally bid on days 1 to 5, has been administered in 72 previously untreated patients. Objective response was reported in 83% of patients, including 7% with CR, 67% with PR and 9% with MR. The median time to response was 4.1 months and 2-year progression-free survival rate was 90%.[29]
Alemtuzumab has also been tested in 28 patients with WM, 5 were untreated and 23 were treated. The ORR was 76% with 32% partial responses. In addition, the combinations of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) or R-CVP or R-CP have shown high responses with over 80% ORR in patients with WM in small prospective or retrospective studies.[30-32] The combination of bendamustine (nitrogen mustard) and rituximab (BR) has recently been compared to R-CHOP in a large cohort of newly diagnosed untreated low-grade lymphomas that include 42 patients with WM.[33,34] The overall response rate in 40 evaluable patients was 96% for BR versus 94% for R-CHOP. BR was associated with lower incidences of grade 3 and 4 cytopenias, infectious complications and alopecia. Another Study has evaluated the efficacy of rituximanb + bendamustine in low-grade non-Hodgking lymphoma patients; and demonstrated an ORR of 90% with a median PFS time of 24 months, and a CR rate of 60%. The safety profile showed a relatively low incidence of grade 3 or 4 hematologic and non-hematologic adverse events.[35]
NOVEL THERAPEUTIC AGENTS
Novel therapeutic agents that have demonstrated efficacy in WM include bortezomib (proteasome inhibitor), thalidomide (immunomodulatory agent), perifosine (Akt inhibitor), enzastaurin (PKC inhibitor) and everolimus (mTOr inhibitor). The range of the ORR to these agents is between 25-80%.[15,36-38]
Bortezomib
The use of bortezomib as a single agent in WM has been tested in two phase II clinical trials in relapsed WM. The agent was used in the standard dose of 1.3 mg/m2 twice a week on days 1, 4, 8 and 11. To determine the effectiveness in the general WM patient population, Chen et al[39] administered bortezomib to 27 WM patients, 44% of whom were previously untreated and 56% were previously treated with bortezomib. The ORR was 78% and major responses (PR or better) were seen in 44% of patients; there were no complete responses observed in these studies. Sensory neuropathy was the primary toxicity affecting 20/27 patients. A recent study using the combination of bortezomib, rituximab and dexamethasone (BDR) in newly diagnosed patients with WM exhibited an exciting overall response rate of 96% including 83% achieving partial response.[40] However, neuropathy was again a major toxicity with this regimen. Therefore, treatment of bortezomib in current clinical trials has been reduced to once a week at 1.6 mg/m2 in an attempt to reduce the occurrence of peripheral neuropathy.[340] Recent clinical trials have been conducted using weekly bortezomib in combination with rituximab in either newly diagnosed or relapsed WM. The rationale for these studies were preclinical studies that demonstrated synergistic activity of bortezomib with the anti-CD20 antibody rituximab in WM cell lines and patients samples. A phase II trial of weekly bortezomib in combination with rituximab has been conducted in untreated patients with Waldenstrom’s Macroglobulinemia.[41] This study aimed to determine activity and safety of weekly bortezomib and rituximab in this disease. Patients who had symptomatic WM and were not previously treated were eligible. All patients received bortezomib IV weekly at 1.6 mg/m2 on days 1, 8, 15, q 28 days × 6 cycles, and rituximab 375 mg/m2 weekly on cycles 1 and 4. Dexamethasone was not added.
Twenty-six patients were treated. At least minimal response or better was observed, assessed using serum protein electropheresis, in 24/26 cases (92%) with 2 patients (8%) in complete remission (CR)/near CR, 15 (54%) in partial response (PR), and 7(27%) in minimal response (MR). Two patients (8%) had stable disease. By assessing IgM using nephlometry, all 26 patients (100%) had at least a minor response, with 2 (8%) CR, 15 (58%) in PR and 9 (35%) with minor response. The median time of follow up wass 11.2 months (range, 3-18.6). To date, six (23%) patients have developed progressive disease or required a new therapy. A single patient died due to disease progression. The median progression-free survival and overall survival have not been reached. The most common grade 3 and 4 therapy related adverse events included anemia in 3 patients, lymphopenia in 2 patients; neutropenia, leucopenia, thrombocytopenia, pneumonia, fatigue, allergic reaction and nausea and vomiting in 1 patient for each. Five patients developed grade 2 peripheral neuropathy including 4 who did not have neuropathy at baseline. It required dose reductions in cycles 4 and 5 and these neuropathies resolved to grade 1 or less with follow up. One case developed grade 1 herpes zoster reactivation in cycle 1. The combination of weekly bortezomib and rituximab showed significant activity and minimal neurological toxicity in patients with untreated WM.
A phase II trial of weekly bortezomib in combination with Rituximab was conducted in patients with relapsed or relapsed/refractory WM.[42] Patients who had at least one previous therapy were eligible. All patients received bortezomib IV weekly at 1.6 mg/m2 on days 1, 8, 15, q 28 days x 6 cycles, and rituximab 375 mg/m2 weekly on cycles 1 and 4. Primary endpoint was the percent of patients with at least a minor response. Thirty-seven patients were treated. The majority of patients (78%) completed treatment per protocol. At least minimal response (MR) or better was observed in 81% (95% CI: [65,92]) with 2 patients (5%) in complete remission (CR)/near CR, 17 (46%) in partial response (PR), and 11(30%) in MR. The median time to progression was 16.4 months (95% CI, 11.4–21.1). Death occurred in 1 patient due to viral pneumonia. The most common grade 3 and 4 therapy related adverse events included reversible neutropenia in 16%, anemia in 11%, and thrombocytopenia in 14%. Grade-3 peripheral neuropathy occurred in only 2 patients (5%). The median event-free survival (EFS) is 12 months (95% CI, 11–20) with estimated 12 month and 18 month EFS of 49% (95% CI: [31, 67%]) and 38% (95% CI: [20, 56%]). The combination of weekly bortezomib and rituximab showed significant activity and minimal neurological toxicity in patients with relapsed WM.
Immunomodulatory drugs
The combination of thalidomide and rituximab has been tested in WM, using thalidomide 200 mg daily for the first 2 weeks followed by 400 mg daily for a total of one year. Twenty-three patients were evaluable for this study and showed an ORR of 78% with 65% partial responses. Dose reductions of thalidomide occurred in all patients and led to discontinuation of therapy in 11 patients.[43] Lenaldiomide 25 mg/day in combination with rituximab has been tested in 16 patients. Of these, 12 were evaluable for response with an ORR of 67% including 4 partial responses. Acute decreases in hematocrit were observed during the first 2 weeks of lenalidomide therapy in 13/16 (81%) patients with a median hematocrit decrease of 4.4% (1.7-7.2%), resulting in 4 patient hospitalizations.[44] Despite dose reductions, lenalidomide-related anemia persisted in many patients. As such, the use of lenalidomide should be avoided in WM patients.
Perifosine
We have previously shown that primary WM cells present with a constitutive activation of Akt, providing the preclinical rational for testing Perifosine in this disease. Perifosine is a novel Akt inhibitor that belongs to a class of lipidrelated compounds called alkylphospholipids.[45] It has shown activity in phase II trials in MM. Our previous studies have shown that the activity of the survival protein Akt is upregulated in patients with WM compared with normal B cells, and that downregulation of Akt leads to significant inhibition of proliferation and induction of apoptosis in WM cells in vitro. In vivo studies of perifosine have shown significant cytotoxicity and inhibition of tumor growth in a xenograft mouse model.[46] Subsequently, perifosine was shown to induce synergistic cytotoxicity with rituximab and bortezomib as well as with other conventional agents, including fludarabine and cyclophosphamide.[47] Based on this preclinical activity, a phase II clinical trials using Perifosine involved 37 patients (27 men and 10 women; median age; 65 years; range, 44-82 years).[48] Of these patients, 65% were relapsed, and 35% were relapsed and refractory to previous therapy. The median number of lines of previous treatment was 3 (range, 1-5 lines), with 35% of the patients having > 3 lines of previous therapy. Previous therapy included rituximab, nucleoside analogues (fludarabine and cladribine), combination chemotherapy (eg, CHOP, CVP), chlorambucil, and bortezomib. A total of 36 patients are evaluable for response after 2 cycles of therapy. The overall response rate (PR + MR) was 36%. Partial response occurred in 2 patients (6%), with a median duration of response of 9+ and 18+ months; MR occurred in 11 patients (30%), with a median duration of response of 7 months (range, 2-21+ months). Stable disease occurred in 21 patients (58%) and progressive disease in 2 patients (6%) at 2 and 4 months. The most common adverse events were gastrointestinal toxicities (nausea, vomiting, and diarrhea) with grade 1/2 in 36% of the patients. Grade 3/4 events included anemia (9%) and leukopenia (9%). Grade 3 arthritis occurred in 9% of the patients, was considered likely related to therapy (especially in rapidly responding patients), and reversed with symptomatic treatment as well as dose reduction. Dose reductions to 100 mg occurred in a total of 36% of the patients and were otherwise due to gastrointestinal toxicity or cytopenias. Responses were maintained despite dose reductions in patients. As of August 2008, the progression-free survival (PFS) and time to progression (TTP) are similar at a median of 10.7 months (7.2- not reached). Therefore, perifosine alone induces a prolonged TTP in relapsed/refractory WM, with a promising response rate of 36%, stabilization of disease in 58% of patients, and manageable toxicity, as well as the convenience of oral administration.
Enzastaurin
Protein kinase Cbeta (PKCbeta) regulates cell survival and growth in many B-cell malignancies. We have previously demonstrated up-regulation of PKCbeta protein in primary WM cells, using either protein array techniques or immunohistochemistry, providing the rational for testing Enzastaurin, a PKCbeta inhibitor, in this disease. Enzastaurin. Enzastaurin is an oral serine/threonine kinase inhibitor that targets the PKC and PI3K/AKT pathways. Enzastaurin has demonstrated activity in preclinical models of multiple myeloma (MM) and Waldenstrom’s macroglobulinemia (WM),[49,50] and clinical studies suggest encouraging activity and a well-tolerated safety profile in a variety of hematologic cancers. A multicenter phase II trial is on-going to determine whether further study of single-agent enzastaurin is warranted in patients with previously treated WM or MM.[51] The primary objective is to assess the response rate (RR); secondary objectives include assessment of time to progression (TTP), safety, biomarkers, and the impact of adding dexamethasone to enzastaurin in patients with progressive disease (PD). Eligible patients with WM treated with 1-5 prior therapies were enrolled and treated with 250 mg oral enzastaurin twice daily (1125-mg loading dose on day 1) in 28-day cycles. Patients continued for 8 cycles or until PD or unacceptable toxicity occurred. At the investigator’s discretion, dexamethasone (20-40 mg po QD, days 1-4, 9-12, and 17-20 for 4 cycles; days 1-4 of each cycle thereafter) was added to enzastaurin in patients with PD. The median age was 65.6 years. Patients had a median of 2 prior systemic therapies and 26 patients (89.7%) had prior rituximab. Patients completed a median of 4 cycles. Six patients received ≥6 cycles of enzastaurin treatment. None of the patients had a complete response (CR). One patient had a PR and 7 patients had a MR, for a RR (CR+PR+MR) of 27.6%. Immunoglobulin M decreased by ≥25% in 11 patients. Three (10.3%) patients had a PD. One patient had a drug-related grade 3 wound complication; there were no other drug-related grade ≥3 toxicities. Although the results are preliminary, enzastaurin appears to have activity and is well tolerated in patients with previously treated WM. The WM cohort was expanded to allow up to 50 patients to be treated on study.
Everolimus (RAD001)
Based on the preclinical data showing increased activity of the PI3K/mTOR pathway in WM, rapamycin (mTOR inhibitor) has been studied in vitro in WM and showed significant cytotoxicity in WM cell lines, specifically when combined with bortezomib.[52]
A phase II trial of single-agent everolimus was initiated in patients with WM with relapsed or relapsed/refractory disease.[53] This study was conducted in a collaborative effort between Dana-Farber Cancer Institute (DFCI) and Mayo Clinic College of Medicine. Eligible patients had measurable disease (IgM monoclonal protein >1000 mg/dL with >10% marrow involvement or nodal masses >2 cm), a platelet count >75,000 × 106/L, a neutrophil count >1,000 × 106/L, and a creatinine and bilirubin <2x laboratory upper limit of normal. Patients received everolimus 10 mg PO daily and were evaluated monthly. Tumor response was assessed after cycles 2 and 6 and then every 3 cycles until progression. 50 pts were treated. The median age was 63 years (range, 43-85). The overall response rate (CR+PR+MR) was 70% (95% CI: 55-82%), with a PR of 42% and 28% MR. The median duration of response and median progression-free survival (PFS) has not been reached. The estimated PFS at 6 and 12 months is 75% (95%CI: 64-89%) and 62% (95%CI: 48-80%), respectively. Grade 3 or higher related toxicities were observed in 56% of patients. The most common were hematological toxicities with cytopenias. Pulmonary toxicity occurred in 10% of patients. Dose reductions due to toxicity occurred in 52% of patients.
Everolimus has high single-agent activity with an overall response rate of 70% and manageable toxicity in patients with relapsed WM, and offers a potential new therapeutic strategy for this patient group. Future studies of combination of this agent with rituximab and bortezomib are currently being planned.
Preclinical efficacy of new small molecules and epigenetic-based therapies in WM
Preclinical work has demonstrated anti-WM activity of new proteasome inhibitors (NPI-0052; PR-047); histone deacetylase inhibitors (LBH589); PI3K/Akt/mTOR inhibitor (NVP-BEZ235),[54-56] either in vitro and in vivo, providing the rational for testing these compounds in WM. Up-regulation of microRNA155 in primary WM cells has recently been demonstrated, providing in vitro and in vivo preclinical evidence for testing anti-microRNA155-based therapy in this disease.[57]
CONCLUSION
In summary, the last decade has marked a new era in the treatment of monoclonal gammopathies. Indeed, a new paradigm shift has evolved utilizing novel therapeutic agents targeting the malignant clone and its bone marrow microenvironment. The combination of novel agents with chemotherapeutic drugs and/or glucocorticoids has demonstrated high response rates with complete remission rates comparable to those achieved in the stem cell transplant setting. This has been supported by in vitro and in vivo evidence showing the antitumor activity of those novel agents in WM, as well as in other B cell malignancies (Figure 1). Based on the update on treatment recommendations from the 4th International Workshop on Waldenstrom’s Macroglobulinemia, patients with WM may be treated as summarized in Table I. In the salvage setting, use of different regimens, including novel agents should be considered, along with bortezomib-based therapies, alemtuzumab and autologous stem cell transplantation. The future holds many more challenges for the treatment of WM. These include combination of agents that achieve higher response rates, more durable duration of response, less toxicity and prolonged survival for patients. The desired outcome will be to make Wm an increasingly chronic and treatable disease.
Figure 1. Mechanisms of action of novel agents.
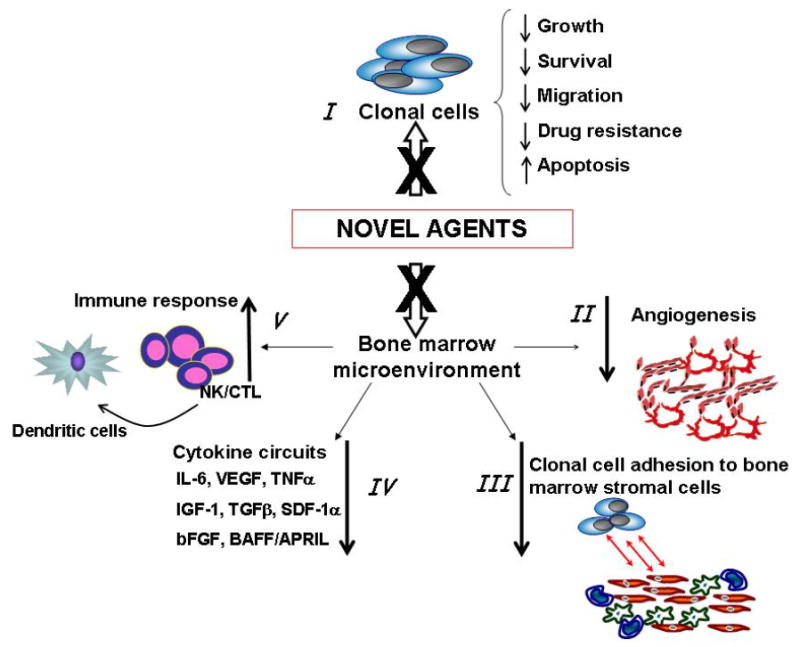
Novel agents target both tumor lymphoplasmacytic cells, leading to reduced WM cells proliferation and increased apoptosis. Moreover, they also inhibit WM cells growth by inhibiting their interaction with bone marrow (BM) milieu, leading to reduced adhesion and migration. In addition, new drugs target BM stromal cells directly, resulting in reduced cytokine secretion; and enhanced immune response. (IL-6: interleukin 6; VEGF: Vascular Endothelial Growth Factor; TNFα: tumor necrosis factor alpha; TGFβ: transforming growth factor beta; SDF-1α: stromal cell derived factor -1 alpha; bFGF: basic fibroblast growth factor; BAFF: B-cell activating factor; APRIL: proliferation inducing ligand)
Table I.
Recommendations for newly diagnosed sympthomatic WM patients, based on specific conditions (as per 4th International Workshop on WM)
| TRANSPLANT CANDIDATE
|
NON-TRANSPLANT CANDIDATE
|
||
|---|---|---|---|
| Cytopenia | DRC | Cytopenia | DRC |
| R+ Thal | R+ Thal | ||
| High M-protein | R-CHOP | High M-protein | R + NA |
| DRC | R + C + NA | ||
| DRC | |||
DRC: Dexamethasone, Rituximab, Cyclophosphamide; R: Rituximab; CHOP: cyclophosphamide, doxorubicine, vincristine, prednisone; Thal: Thalidomide; NA: nucleoside analogs
Acknowledgments
Supported in part by the Kirsch Laboratory for WM, The Heje Fellowship of WM, International Waldenstrom Macroglobulinemia Foundation. Ms. Jennifer Stedman for reviewing the manuscript.
Footnotes
Publisher's Disclaimer: ROSS Open Access articles will be distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided that the original work will always be cited properly.
References
- 1.Dimopoulos MA, Panayiotidis P, Moulopoulos LA, Sfikakis P, Dalakas M. Waldenstrom’s macroglobulinemia: clinical features, complications, and management. J Clin Oncol. 2000;18:214–226. doi: 10.1200/JCO.2000.18.1.214. [DOI] [PubMed] [Google Scholar]
- 2.Ghobrial IM, Witzig TE. Waldenstrom macroglobulinemia. Curr Treat Options Oncol. 2004;5:239–247. doi: 10.1007/s11864-004-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimopoulos MA, Kyle RA, Anagnostopoulos A, Treon SP. Diagnosis and management of Waldenstrom’s macroglobulinemia. J Clin Oncol. 2005;23:1564–1577. doi: 10.1200/JCO.2005.03.144. [DOI] [PubMed] [Google Scholar]
- 4.Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML, Morra E, Pangalis GA, San Miguel JF, Branagan AR, Dimopoulos MA. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003;30:110–115. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 6.Herrinton L, Weiss NS. Incidence of Waldenstrom’s macroglobulinemia. Blood. 1993;82:3148–3150. [PubMed] [Google Scholar]
- 7.Ghobrial IM, Fonseca R, Gertz MA, Plevak MF, Larson DR, Therneau TM, Wolf RC, Hoffmann RJ, Lust JA, Witzig TE, Lacy MQ, Dispenzieri A, Vincent Rajkumar S, Zeldenrust SR, Greipp PR, Kyle RA. Prognostic model for disease-specific and overall mortality in newly diagnosed symptomatic patients with Waldenstrom macroglobulinaemia. Br J Haematol. 2006;133:158–164. doi: 10.1111/j.1365-2141.2006.06003.x. [DOI] [PubMed] [Google Scholar]
- 8.Kyle RA, Therneau TM, Rajkumar SV, Remstein ED, Offord JR, Larson DR, Plevak MF, Melton LJ., 3rd Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood. 2003;102:3759–3764. doi: 10.1182/blood-2003-03-0801. [DOI] [PubMed] [Google Scholar]
- 9.McMaster M. Familial Waldenstrom’s macroglobulinemia. Semin Oncol. 2003;30:146–152. doi: 10.1053/sonc.2003.50063. [DOI] [PubMed] [Google Scholar]
- 10.Treon SP, Hunter ZR, Aggarwal A, Ewen EP, Masota S, Lee C, Santos DD, Hatjiharissi E, Xu L, Leleu X, Tournilhac O, Patterson CJ, Manning R, Branagan AR, Morton CC. Characterization of familial Waldenstrom’s macroglobulinemia. Ann Oncol. 2006;17:488–494. doi: 10.1093/annonc/mdj111. [DOI] [PubMed] [Google Scholar]
- 11.Schnitzler L, Schubert B, Boasson M, Gardais J, Tourmen A. Urticaire chronique, lésions osseuses, macroglobulinémie IgM: maladie de Waldenström? Bull Soc Fr Derm Syph. 1974;81:363–367. [Google Scholar]
- 12.Morel P, Duhamel A, Gobbi P, Dimopoulos MA, Dhodapkar MV, McCoy J, Crowley J, Ocio EM, Garcia-Sanz R, Treon SP, Leblond V, Kyle RA, Barlogie B, Merlini G. International prognostic scoring system for Waldenstrom macroglobulinemia. Blood. 2009;113:4163–70. doi: 10.1182/blood-2008-08-174961. [DOI] [PubMed] [Google Scholar]
- 13.Leleu X, Soumerai J, Roccaro A, Hatjiharissi E, Hunter ZR, Manning R, Ciccarelli BT, Sacco A, Ioakimidis L, Adamia S, Moreau AS, Patterson CJ, Ghobrial IM, Treon SP. Increased incidence of transformation and myelodysplasia/acute leukemia in patients with Waldenstrom macroglobulinemia treated with nucleoside analogs. J Clin Oncol. 2009;27:250–255. doi: 10.1200/JCO.2007.15.1530. [DOI] [PubMed] [Google Scholar]
- 14.Dimopoulos MA, Gertz MA, Kastritis E, Garcia-Sanz R, Kimby EK, Leblond V, Fermand JP, Merlini G, Morel P, Morra E, Ocio EM, Owen R, Ghobrial IM, Seymour J, Kyle RA, Treon SP. Update on treatment recommendations from the fourth International Workshop on Waldenstrom’s Macroglobulinemia. J Clin Oncol. 2009;27:120–6. doi: 10.1200/JCO.2008.17.7865. [DOI] [PubMed] [Google Scholar]
- 15.Treon SP, Morel P, Leblond V, Fermand JP. Report of the Third International Workshop on Waldenstrom’s macroglobulinemia. Clin Lymphoma. 2005;5:215–216. doi: 10.3816/clm.2005.n.001. [DOI] [PubMed] [Google Scholar]
- 16.Treon SP, Emmanouilides C, Kimby E, Kelliher A, Preffer F, Branagan AR, Anderson KC, Frankel SR Waldenström’s Macroglobulinemia Clinical Trials Group. Extended rituximab therapy in Waldenstrom’s macroglobulinemia. Ann Oncol. 2005;16:132–138. doi: 10.1093/annonc/mdi022. [DOI] [PubMed] [Google Scholar]
- 17.Dimopoulos MA, O’Brien S, Kantarjian H, Pierce S, Delasalle K, Barlogie B, Alexanian R, Keating MJ. Fludarabine therapy in Waldenstrom’s macroglobulinemia. Am J Med. 1993;95:49–52. doi: 10.1016/0002-9343(93)90231-d. [DOI] [PubMed] [Google Scholar]
- 18.Weber DM, Dimopoulos MA, Delasalle K, Rankin K, Gavino M, Alexanian R. 2-Chlorodeoxyadenosine alone and in combination for previously untreated Waldenstrom’s macroglobulinemia. Semin Oncol. 2003;30:243–247. doi: 10.1053/sonc.2003.50070. [DOI] [PubMed] [Google Scholar]
- 19.Dimopoulos MA, Weber D, Delasalle KB, Keating M, Alexanian R. Treatment of Waldenstrom’s macroglobulinemia resistant to standard therapy with 2-chlorodeoxyadenosine: identification of prognostic factors. Ann Oncol. 1995;6:49–52. doi: 10.1093/oxfordjournals.annonc.a059040. [DOI] [PubMed] [Google Scholar]
- 20.McLaughlin P, Estey E, Glassman A, Romaguera J, Samaniego F, Ayala A, Hayes K, Maddox AM, Preti HA, Hagemeister FB. Myelodysplasia and acute myeloid leukemia following therapy for indolent lymphoma with fludarabine, mitoxantrone, and dexamethasone (FND) plus rituximab and interferon alpha. Blood. 2005;105:4573–4575. doi: 10.1182/blood-2004-08-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treon SP, Gertz MA, Dimopoulos M, Anagnostopoulos A, Blade J, Branagan AR, Garcia-Sanz R, Johnson S, Kimby E, Leblond V, Fermand JP, Maloney DG, Merlini G, Morel P, Morra E, Nichols G, Ocio EM, Owen R, Stone MJ. Update on treatment recommendations from the Third International Workshop on Waldenstrom’s macroglobulinemia. Blood. 2006;107:3442–3446. doi: 10.1182/blood-2005-02-0833. [DOI] [PubMed] [Google Scholar]
- 22.Dimopoulos MA, Zervas C, Zomas A, Hamilos G, Gika D, Efstathiou E, Panayiotidis P, Vervessou E, Anagnostopoulos N, Christakis J. Extended rituximab therapy for previously untreated patients with Waldenstrom’s macroglobulinemia. Clin Lymphoma. 2002;3:163–166. doi: 10.3816/clm.2002.n.022. [DOI] [PubMed] [Google Scholar]
- 23.Dimopoulos MA, Zervas C, Zomas A, Kiamouris C, Viniou NA, Grigoraki V, Karkantaris C, Mitsouli C, Gika D, Christakis J, Anagnostopoulos N. Treatment of Waldenstrom’s macroglobulinemia with rituximab. J Clin Oncol. 2002;20:2327–2333. doi: 10.1200/JCO.2002.09.039. [DOI] [PubMed] [Google Scholar]
- 24.Gertz MA, Rue M, Blood E, Kaminer LS, Vesole DH, Greipp PR. Multicenter phase 2 trial of rituximab for Waldenstrom macroglobulinemia (WM): an Eastern Cooperative Oncology Group Study (E3A98) Leuk Lymphoma. 2004;45:2047–2055. doi: 10.1080/10428190410001714043. [DOI] [PubMed] [Google Scholar]
- 25.Ghobrial IM, Fonseca R, Greipp PR, Blood E, Rue M, Vesole DH, Gertz MA Eastern Cooperative Oncology Group. Initial immunoglobulin M ‘flare’ after rituximab therapy in patients diagnosed with Waldenström macroglobulinemia: an Eastern Cooperative Oncology Group Study. Cancer. 2004;101:2593–2598. doi: 10.1002/cncr.20658. [DOI] [PubMed] [Google Scholar]
- 26.Treon S, Branagan AR, Hunter Z, Santos D, Tournhilac O, Anderson KC. Paradoxical increases in serum IgM and viscosity levels following rituximab in Waldenström’s macroglobulinemia. Ann Oncol. 2004;15:1481–1483. doi: 10.1093/annonc/mdh403. [DOI] [PubMed] [Google Scholar]
- 27.Treon SP, Branagan AR, Ioakimidis L, Soumerai JD, Patterson CJ, Turnbull B, Wasi P, Emmanouilides C, Frankel SR, Lister A, Morel P, Matous J, Gregory SA, Kimby E. Long-term outcomes to fludarabine and rituximab in Waldenström macroglobulinemia. Blood. 2009;113:3673–3678. doi: 10.1182/blood-2008-09-177329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas SK, Delasalle KB, Gavino M, Wang M, Alexanian R, Weber DM. 2-CDA-cyclophosphamide +/- rituximab for symptomatic WM. Haematologica. 2007;92:1227. abstract. [Google Scholar]
- 29.Dimopoulos MA, Anagnostopoulos A, Kyrtsonis MC, Zervas K, Tsatalas C, Kokkinis G, Repoussis P, Symeonidis A, Delimpasi S, Katodritou E, Vervessou E, Michali E, Pouli A, Gika D, Vassou A, Terpos E, Anagnostopoulos N, Economopoulos T, Pangalis G. Primary treatment of Waldenstrom macroglobulinemia with dexamethasone, rituximab and cyclophosphamide. J Clin Oncol. 2007;25:3344–3349. doi: 10.1200/JCO.2007.10.9926. [DOI] [PubMed] [Google Scholar]
- 30.Treon SP, Hunter Z, Barnagan AR. CHOP plus rituximab therapy in Waldenstrom’s macroglobulinemia. Clin Lymphoma. 2005;5:273–277. doi: 10.3816/clm.2005.n.015. [DOI] [PubMed] [Google Scholar]
- 31.Vijay A, Gertz MA. Current treatment options for Waldenstrom macroglobulinemia. Clin Lymphoma Myeloma. 2008;8:219–229. doi: 10.3816/CLM.2008.n.029. [DOI] [PubMed] [Google Scholar]
- 32.Treon SP, Hatjiharissi E, Leleu X, Moreau AS, Roccaro A, Hunter ZR, Soumerai JD, Ciccarelli B, Xu L, Sacco A, Ngo HT, Jia X, Yang C, Adamia S, Branagan AR, Ho AW, Santos DD, Tournilhac O, Manning RJ, Leduc R, O’Connor K, Nelson M, Patterson CJ, Ghobrial I. Novel agents in the treatment of Waldenstrom’s macroglobulinemia. Clin Lymphoma Myeloma. 2007;7(Suppl 5):S199–206. doi: 10.3816/clm.2007.s.023. [DOI] [PubMed] [Google Scholar]
- 33.Kalaycio M. Bendamustine: a new look at an old drug. Cancer. 2009;115:473–479. doi: 10.1002/cncr.24057. [DOI] [PubMed] [Google Scholar]
- 34.Treon SP. How I treat Waldenstrom’s macroglobulinemia. Blood. 2009;114:2375–85. doi: 10.1182/blood-2009-05-174359. [DOI] [PubMed] [Google Scholar]
- 35.Rummel MJ, Al-Batran SE, Kim SZ, Welslau M, Hecker R, Kofahl-Krause D, Josten KM, Dürk H, Rost A, Neise M, von Grünhagen U, Chow KU, Hansmann ML, Hoelzer D, Mitrou PS. Bendamustine plus rituximab is effective and has a favourable tosicity profile in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:3383–3389. doi: 10.1200/JCO.2005.08.100. [DOI] [PubMed] [Google Scholar]
- 36.Dimopoulos MA, Zomas A, Viniou NA, Grigoraki V, Galani E, Matsouka C, Economou O, Anagnostopoulos N, Panayiotidis P. Treatment of Waldenstrom’s macroglobulinemia with thalidomide. J Clin Oncol. 2001;19:3596–3601. doi: 10.1200/JCO.2001.19.16.3596. [DOI] [PubMed] [Google Scholar]
- 37.Hunter Z, Branagan A, Treon ST. Campath-1H in Waldenstrom’s Macroglobulinemia. Blood (ASH Annual Meeting Abstracts) 2004;104 Abstract 4924. [Google Scholar]
- 38.Treon S, Hunter Z, Matous J, Badros A, Joyce RM, Mannion B, Advani R, Cook D, Songer J, Hill J, Kaden BR, Sharon D, Steiss R, Branagan AR, Patterson CJ. Phase II Study of Bortezomib in Waldenstrom’s Macroglobulinemia: Results of WMCTG Trial 03-248. Blood. 2005;106 Abstract. [Google Scholar]
- 39.Chen CI, Kouroukis CT, White D, Voralia M, Stadtmauer E, Stewart AK, Wright JJ, Powers J, Walsh W, Eisenhauer E National Cancer Institute of Canada Clinical Trials Group. Bortezomib is Active in Patients With Untreated or Relapsed Waldenstrom’s Macroglobulinemia: A Phase II Study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1570–1575. doi: 10.1200/JCO.2006.07.8659. [DOI] [PubMed] [Google Scholar]
- 40.Treon SP, Ioakimidis L, Soumerai JD, Patterson CJ, Sheehy P, Nelson M, Willen M, Matous J, Mattern J, 2nd, Diener JG, Keogh GP, Myers TJ, Boral A, Birner A, Esseltine DL, Ghobrial IM. Primary Therapy of Waldenstrom Macroglobulinemia With Bortezomib, Dexamethasone, and Rituximab: WMCTG Clinical Trial 05-180. J Clin Oncol. 2009;27:3830–3835. doi: 10.1200/JCO.2008.20.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.I M, Padmanabhan S, Badros AZ, Leduc R, Rourke M, Chuma S, Kunsman J, Warren D, Harris B, Sam A, Anderson KC, Richardson PG, Treon SP, Matous J. Phase II Trial of Weekly Bortezomib in Combination with Rituximab in Untreated Patients with Waldenstrom’s Macroglobulinemia. Blood (ASH Annual Meeting Abstracts) 2009 Nov; doi: 10.1002/ajh.21788. Abstract 3752. [DOI] [PubMed] [Google Scholar]
- 42.Ghobrial IM, Hong F, Padmanabhan S, Badros A, Rourke M, Leduc R, Chuma S, Kunsman J, Warren D, Harris B, Sam A, Anderson KC, Richardson PG, Treon SP, Weller E, Matous J. Phase II Trial of Weekly Bortezomib in Combination with Rituximab in Relapsed or Relapsed/Refractory Waldenstrom’s Macroglobulinemia. J Clin Oncol. 2010;28:1422–1428. doi: 10.1200/JCO.2009.25.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Treon SP, Soumerai JD, Branagan AR, Hunter ZR, Patterson CJ, Ioakimidis L, Briccetti FM, Pasmantier M, Zimbler H, Cooper RB, Moore M, Hill J, 2nd, Rauch A, Garbo L, Chu L, Chua C, Nantel SH, Lovett DR, Boedeker H, Sonneborn H, Howard J, Musto P, Ciccarelli BT, Hatjiharissi E, Anderson KC. Thalidomide and rituximab in Waldenstrom macroglobulinemia. Blood. 2008;112:4452–4457. doi: 10.1182/blood-2008-04-150854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treon SP, Soumerai JD, Branagan AR, Hunter ZR, Patterson CJ, Ioakimidis L, Chu L, Musto P, Baron AD, Nunnink JC, Kash JJ, Terjanian TO, Hyman PM, Nawfel EL, Sharon DJ, Munshi NC, Anderson KC. Lenalidomide and rituximab in Waldenstrom’s macroglobulinemia. Clin Cancer Res. 2009;15:355–360. doi: 10.1158/1078-0432.CCR-08-0862. [DOI] [PubMed] [Google Scholar]
- 45.Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, Podar K, Munshi NC, Chauhan D, Richardson PG, Anderson KC. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–62. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leleu X, Jia X, Runnels J, Ngo HT, Moreau AS, Farag M, Spencer JA, Pitsillides CM, Hatjiharissi E, Roccaro A, O’Sullivan G, McMillin DW, Moreno D, Kiziltepe T, Carrasco R, Treon SP, Hideshima T, Anderson KC, Lin CP, Ghobrial IM. The Akt pathway regulates survival and homing in Waldenstrom macroglobulinemia. Blood. 2007;110:4417–26. doi: 10.1182/blood-2007-05-092098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leleu X, Eeckhoute J, Jia X, Roccaro AM, Moreau AS, Farag M, Sacco A, Ngo HT, Runnels J, Melhem MR, Burwick N, Azab A, Azab F, Hunter Z, Hatjiharissi E, Carrasco DR, Treon SP, Witzig TE, Hideshima T, Brown M, Anderson KC, Ghobrial IM. Targeting NF-kappaB in Waldenstrom macroglobulinemia. Blood. 2008;111:5068–77. doi: 10.1182/blood-2007-09-115170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghobrial IM, Roccaro A, Hong F, Weller E, Rubin N, Leduc R, Rourke M, Chuma S, Sacco A, Jia X, Azab F, Azab AK, Rodig S, Warren D, Harris B, Varticovski L, Sportelli P, Leleu X, Anderson KC, Richardson PG. Clinical and Translational Studies of a Phase II Trial of the Novel Oral Akt Inhibitor Perifosine in Relapsed or Relapsed/Refractory Waldenstrom’s Macroglobulinemia. Clin Cancer Res. 2010;16:1033–1041. doi: 10.1158/1078-0432.CCR-09-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Podar K, Raab MS, Zhang J, McMillin D, Breitkreutz I, Tai YT, Lin BK, Munshi N, Hideshima T, Chauhan D, Anderson KC. Targeting PKC in multiple myeloma: in vitro and in vivo effects of the novel, orally available small-molecule inhibitor enzastaurin (LY317615.HCl) Blood. 2007;109:1669–77. doi: 10.1182/blood-2006-08-042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreau AS, Jia X, Ngo HT, Leleu X, O’Sullivan G, Alsayed Y, Leontovich A, Podar K, Kutok J, Daley J, Lazo-Kallanian S, Hatjiharissi E, Raab MS, Xu L, Treon SP, Hideshima T, Anderson KC, Ghobrial IM. Protein kinase C inhibitor enzastaurin induces in vitro and in vivo antitumor activity in Waldenstrom macroglobulinemia. Blood. 2007;109:4964–72. doi: 10.1182/blood-2006-10-054577. [DOI] [PubMed] [Google Scholar]
- 51.Ghobrial IM, Harousseau J, Treon SP, Harris B, Lin CE, Tuan Z, Benhadji K, Wooldridge JE, Leblond V. Enzastaurin in Previously Treated Waldenstrom’s Macroglobulinemia: An Open-Label, Multicenter, Phase II Study. Blood (ASH Annual Meeting Abstracts) 2009 Nov;114:3867. [Google Scholar]
- 52.Vesole S, Jia X, Roccaro AM, Sacco A, Ngo HT, Azab F, Azab AK, Melhem MR, Runnels JM, Quang P, Anderson KC, Ghobrial IM. RAD001 Exerts Anti-Tumor Activity in Waldenstrom Macroglobulinemia. Blood (ASH Annual Meeting Abstracts) 2009 Nov;114:3732. [Google Scholar]
- 53.Ghobrial IM, Gertz MA, LaPlant B, Camoriano J, Haymen SR, Lacy MQ, Chuma S, Sheehy P, Harris B, Leduc R, Rourke M, Ansell SM, DeAngelo DJ, Dispenzieri A, Bergsagel L, Reeder CB, Anderson KC, Richardson PG, Treon SP, Witzig TE. A Phase II Trial of the Oral mTOR Inhibitor Everolimus (RAD001) in Relapsed or Refractory Waldenstrom’s Macroglobulinemia. Blood (ASH Annual Meeting Abstracts) 2009 Nov;114:587. [Google Scholar]
- 54.Roccaro AM, Leleu X, Sacco A, Jia X, Melhem M, Moreau AS, Ngo HT, Runnels J, Azab A, Azab F, Burwick N, Farag M, Treon SP, Palladino MA, Hideshima T, Chauhan D, Anderson KC, Ghobrial IM. Dual targeting of the proteasome regulates survival and homing in Waldenstrom macroglobulinemia. Blood. 2008;111:4752–63. doi: 10.1182/blood-2007-11-120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.X, Roccaro AM, Azab AK, Ngo HT, Sacco A, Melhem MR, Azab F, Runnels J, Burwick N, Quang P, Husu EN, Leleu X, Anderson KC, Bradner J, Ghobrial IM. Regulation of Histone Deacetylase in Waldenström Macroglobulinemia. Blood (ASH Annual Meeting Abstracts) 2008 Nov;112:2617. [Google Scholar]
- 56.Roccaro AM, Sacco A, Husu EN, Pitsillides C, Vesole S, Azab AK, Azab F, Melhem M, Ngo HT, Quang P, Maiso P, Runnels J, Liang MC, Wong KK, Lin C, Ghobrial IM. Dual targeting of the PI3K/Akt/mTOR pathway as an antitumor strategy in Waldenstrom macroglobulinemia. Blood. 2010;115:559–569. doi: 10.1182/blood-2009-07-235747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roccaro AM, Sacco A, Chen C, Runnels J, Leleu X, Azab F, Azab AK, Jia X, Ngo HT, Melhem MR, Burwick N, Varticovski L, Novina CD, Rollins BJ, Anderson KC, Ghobrial IM. microRNA expression in the biology, prognosis, and therapy of Waldenström macroglobulinemia. Blood. 2009;113:4391–402. doi: 10.1182/blood-2008-09-178228. [DOI] [PMC free article] [PubMed] [Google Scholar]


