Summary
The antifungal plant defensin RsAFP2 isolated from radish interacts with fungal glucosylceramides and induces apoptosis in Candida albicans. To further unravel the mechanism of RsAFP2 antifungal action and tolerance mechanisms, we screened a library of 2,868 heterozygous C. albicans deletion mutants and identified 30 RsAFP2-hypersensitive mutants. The most prominent group of RsAFP2 tolerance genes was involved in cell wall integrity and hyphal growth/septin ring formation. Consistent with these genetic data, we demonstrated that RsAFP2 interacts with the cell wall of C. albicans, which also contains glucosylceramides, and activates the cell wall integrity pathway. Moreover, we found that RsAFP2 induces mislocalization of septins and blocks the yeast-to-hypha transition in C. albicans. Increased ceramide levels have previously been shown to result in apoptosis and septin mislocalization. Therefore, ceramide levels in C. albicans membranes were analyzed following RsAFP2 treatment and, as expected, increased accumulation of phytoC24-ceramides in membranes of RsAFP2-treated C. albicans cells was detected. This is the first report on the interaction of a plant defensin with glucosylceramides in the fungal cell wall, causing cell wall stress, and on the effects of a defensin on septin localization and ceramide accumulation.
Keywords: Candida albicans, plant defensin, mode of action, cell wall, septin, ceramide
Introduction
Plant defensins are small, basic, cysteine-rich peptides, which possess antifungal and in some cases also antibacterial activities (reviewed by Aerts et al., 2008). They are postulated to be part of the innate immune system of plants, and provide protection against invading fungal and bacterial pathogens. Plant defensins are not only active against phytopathogenic fungi (e.g. Fusarium culmorum and Botrytis cinerea), but also against baker’s yeast and human pathogenic fungi including Candida albicans. The latter is an opportunistic pathogen that can cause superficial and invasive infections in immunocompromised patients. Mortality associated with invasive Candida infection is high (Mavor et al., 2005). Besides their anti-Candida activity, plant defensins are nontoxic to human cells (Thevissen et al., 2007b; Tavares et al., 2008), highlighting their therapeutic potential as novel antimycotics. We recently demonstrated that RsAFP2, a plant defensin isolated from seeds of radish (Raphanus sativus) (Terras et al., 1992), was active in a prophylactic murine model of candidiasis, and was at least as effective as the frequently used drug fluconazole (Tavares et al., 2008).
RsAFP2 interacts with the sphingolipid glucosylceramide (GlcCer) present in membranes and cell walls of susceptible fungal species, but not with structurally related GlcCer from humans, explaining their selective antifungal activity (Thevissen et al., 2004). GlcCer is produced by most fungal pathogens (Barreto-Bergter et al., 2004), and was recently described to be required for virulence in C. albicans (Noble et al., 2010). The RsAFP2-GlcCer interaction leads to a subsequent permeabilization of the cell, Ca2+ influx, and growth arrest (Thevissen et al., 1996, Thevissen et al., 1999). This RsAFP2-induced permeabilization is not mediated by pore-formation via direct insertion of RsAFP2 into the fungal membrane (Aerts et al., 2007). But instead, it is the result of the induction of specific signaling pathway(s), involving the induction of reactive oxygen species (ROS) and programmed cell death or apoptosis, with concomitant activation of caspases (Aerts et al., 2009). Strains with membranes lacking GlcCer, either due to a non-functional glucosylceramide synthase (GCS/HSX11) gene or due to the absence of GCS (as in Saccharomyces cerevisiae or Candida glabrata) (Leipelt et al., 2001), are resistant to RsAFP2-induced membrane permeabilization and cell death (Thevissen et al., 2004). This points to GlcCer as a prerequisite for RsAFP2 antifungal activity. Moreover, strains with a membrane composed of GlcCer with a structurally altered ceramide moiety are also resistant to RsAFP2-induced cell death (Ferket et al., 2003; Park et al., 2005), emphasizing the importance of the ceramide part of GlcCer in this process.
In the present study, we tested RsAFP2 in a C. albicans fitness test (CaFT) to further unravel its mechanism of action (MOA). This CaFT assay relies on chemically-induced haploinsufficiency by treating a collection of C. albicans heterozygotes (currently consisting of approx. 5,400 heterozygotes, covering ~90% of the C. albicans genome) with sublethal concentrations of an antifungal agent, and subsequent identification of fitness variations of the treated heterozygotes (Xu et al., 2007). The C. albicans heterozygotes displaying fitness variations upon treatment with sublethal RsAFP2 concentrations could be grouped in three classes. Two classes represented RsAFP2-hypersensitive heterozygotes involved in cell wall (glucan synthesis) or bud/septin formation, and one class represented RsAFP2-resistant heterozygotes involved in sphingolipid/ceramide biosynthesis. Consistent with these data, we demonstrated that RsAFP2 interacts primarily with the cell wall of C. albicans, and induces cell wall abnormalities and aberrant budding. As cell wall defects affect septin localization in C. albicans (Blankenship et al., 2010), we further investigated a putative effect of RsAFP2 on the yeast-to-hypha transition, on septin localization itself, and on ceramide accumulation in C. albicans.
Experimental procedures
Strains and chemicals
The following yeast strains were used: C. albicans strain 78 (Tavares et al., 2008), C. albicans CAI4 (Ura-) (Fonzi and Irwin, 1993), the homozygous Δgcs (homozygous deletion of HSX11/orf19.4592) C. albicans (Ura-) (Leipelt et al., 2001), and a previously described C. glabrata isolate (Tavares et al., 2008). Media used were YPD (1% Yeast Extract, Difco; 2% Peptone, Difco; 2% dextrose) or potato dextrose broth/yeast peptone dextrose (PDB/YPD 80/20 with PDB = 2.4% PDB, Sigma, St. Louis, MO, US; supplemented with 50 mM HEPES, pH 7.0). Inhibitor 235236 (Toenjes et al., 2005) was obtained from ChemBridge (San Diego, CA, USA). Caspofungin was obtained from Merck Sharpe & Dohme (Whitehouse Station, NY, USA) as the pharmaceutical formulation Cancidas (containing caspofungin acetate). RsAFP2 was isolated from radish seed as described (Terras et al., 1992).
Statistical analysis
Statistical analysis was performed using unpaired student t test; differences were considered significant if p<0.05.
C. albicans fitness test
The C. albicans fitness test (CaFT) was performed as described (Xu et al., 2007). C. albicans heterozygous mutants were treated with 10 µg/ml, 13 µg/ml or 16 µg/ml RsAFP2 in YPD/PDB. The CaFT results were analyzed by hierarchical clustering with a cut-off value as indicated in the figure legend.
Transmission electron microscopy (TEM)
Morphological changes caused by RsAFP2 treatment were evaluated by TEM. Strain 78 of C. albicans (105 yeast cells) was treated with 50 µg/ml RsAFP2 in PDB/YPD for 16 h, the cells were fixed and prepared for TEM as described (Franzen et al., 2006). Ultrathin sections were examined with a Zeiss 900 transmission electron microscope.
Immunofluorescence and FACS analysis
The localization of RsAFP2 in treated C. albicans cultures was analyzed using a polyclonal antibody preparation from rabbits immunized with RsAFP2 (François et al., 2002). C. albicans (strains CAI4 and 78, and Δgcs (negative control)) and S. cerevisiae were treated with 50 RsAFP2 in PDB/YPD for 3h and fixed with 4% paraformaldehyde in PBS. Heat-inactivated (autoclaving) RsAFP2 was used as a control. The cells were washed and incubated with anti-RsAFP2 rabbit serum (1:200) for 1 h at room temperature. To block non-specific direct binding of rabbit antibodies to C. albicans, the serum preparation was incubated with 107 paraformaldehyde-fixed C. albicans cells for 2 h at room temperature, before exposure to peptide-treated cells. Different dilutions of serum were tested, and controls included cells that were not treated with RsAFP2. After washing with PBS, the cells were incubated with a fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit IgG for 1 h at room temperature. Then, cells were incubated for 15 min with a 10 µg/ml solution of Uvitex 2B to detect chitin at the fungal cell wall (Polysciences Inc., Warrington, PA, US). Cells were observed using an Observer Z1 (Zeiss, Germany) fluorescence microscope. Images were acquired with a Color View AxioCam MRm digital camera. Epifluorescent or deconvolved z-stacks were analyzed with AxioVision software (Zeiss). Samples were also analyzed by flow cytometry to determine the percentage of RsAFP2 positive cells. Fluorescent cells were measured by FACSCalibur flow cytometer (BD Biosciences), and 10,000 events were analyzed with winMDI software (NHI).
Cell wall glucan and mannan
For total cell wall glucan (and mannan) determination, cell walls were isolated (De Groot et al., 2004), and wall carbohydrates were hydrolyzed to monomers using sulfuric acid hydrolysis as described (Pardini et al., 2006). Amounts of glucose (and mannose) in the samples were determined by HPLC (Agilent series 1200, Bio-Rad), using an Aminex HPX-87H precolumn coupled with two Aminex HPX-87H ion exclusion columns of 300 mm by 7.8 mm, thermostatically controlled at 65°C (Agilent G1316A). Sugars were detected by a G1314B variable-wavelength detector (Agilent) set to 210 nm and a refractive index detector (Agilent G1362A) in series, and compared to glucose and mannose standards. Given values are the mean of duplicate measurements from two independent experiments.
Determination of glucosylceramide (GlcCer)
Cell wall and membrane fractions of C. albicans CAI4 and strain 78 were collected for GlcCer quantitation according to a previously established protocol (Fontaine et al., 2003). Briefly, cells were washed with distilled water and then mechanically disrupted with glass beads (0.5–0.75 mm diameter; 1:1 v/v) in lysis buffer (in 200 mM Tris–HCl, 20 mM EDTA, pH 8.0, 1 mM phenylmethylsulfonyl fluoride) using a cell homogeneizer (B. Braun Biotech International). Ten cycles of 1 minute, alternating with intervals of 1 minute on ice, were used. Cell disruption was visualized microscopically. The cell wall fraction was collected after centrifugation of the lysate at 10,000 × g for 10 min at 4°C. The total membrane content was obtained after ultracentrifugation of the supernatant at 125,000 × g for 1 h at 4°C. GlcCer extraction and quantification was performed according to a method routinely used in our laboratory (Barreto-Bergter et al., 2004). Pellets were then extracted with mixtures of successively chloroform/methanol 2:1, 1:1 and 1:2 (v/v) at room temperature. The crude lipids were pooled, dried under vacuum, and partitioned as described (Folch et al., 1957). The lipids recovered from the lower phase were analyzed on high-performance thin layer chromatography (HPTLC) plates developed with chloroform/methanol/water 65:25:4 (v/v/v). A GlcCer fraction purified from C. neoformans was used as standard (Rodrigues et al., 2000). The spots were visualized by transient reactivity of the bands with iodine vapor and by charring with orcinol/H2SO4. Quantification of GSL was performed using Scion Image software (2000 Scion Corporation, NIH).
Intracellular RsAFP2 accumulation
Overnight C. albicans cultures in YPD (2×108 cells/ml) were washed and resuspended in PDB/YPD at 2×107 cells/ml. 30 µg/ml RsAFP2 was added to 500 µl of these cultures. After 2.5 h of incubation at 30°C with shaking, 20 µl of the cultures was used for determination of the number of colony forming units, whereafter the spent medium of the cultures was collected. The cell pellet was washed three times with PBS, and the wash fractions were pooled and added to the supernatant. Five hundred µl 20% acetonitrile/80% PBS was added to the cell pellet, whereafter the cells were lysed using a Fastprep (BIO101/Savant, Toronto) reciprocal shaker, followed by centrifugation (5 min at 3000 rpm). The cell pellet was washed 2 times with 500 µl PBS and aliquots were used for cell counting in a Thoma counting chamber (Marienfeld GmbH & Co, Germany). The cell pellet was resuspended in 300 µl acetonitrile/water 20/80 (V/V), after which the RsAFP2 concentration in both supernatant and cell lysates was determined using HPLC (Agilent Series 1100, Agilent Technologies, Santa Clara, CA, US). Values were normalised to the number of cells. UV signals (λ = 214 nm) were monitored and peaks were integrated using the Agilent Chemstation software (Agilent Technologies). Separation of RsAFP2 was performed on a Microsorb-MV 300 C8 (250×4.6mm) column with a particle size of 5 µm (Varian Medical Systems, Palo Alto, CA, US) (equilibrated with acetonitrile/water 20/80 (V/V)). The column was eluted with a linear gradient of 20 to 100% acetonitrile at 1 ml/min. Experiments were repeated at least three times, each measured in duplo.
Antifungal activity assays
C. albicans CAI4 cultures (2×107 cells/ml) were incubated with various concentrations of RsAFP2 or water in the presence or absence of 2 or 4 ng/ml caspofungin in PDB/YPD for 2.5h. Percentage survival was calculated as described previously (Aerts et al., 2009). To assess growth of the C. albicans cultures in the absence or presence of different RsAFP2 concentrations, the cultures were incubated in an automated OD plate reader (Bioscreen C, Thermo Fisher Scientific Inc., Waltham, MA, US).
Mkc1p phosphorylation assay
A single colony of Candida albicans (strain CAI4) was inoculated into 15mL PDB/YPD supplemented with uridine and grown overnight at room temperature. Cells were collected by centrifugation, resuspended in 40mL fresh media (PDB/YPD + uridine) and allowed to grow for 4 hours at 30°C. Cells were then treated with RsAFP2 (50 µg/ml), Nikkomycin Z (10 µg/mL), or water as a control for 1 hour. Cells were collected over ice and total protein was extracted as described previously (Kumamoto 2005). A total of 50 µg (phospho-Mkc1p) or 10 µg (actin) total protein was loaded per sample on an 8.5% (Mkc1p) or 10% (actin) SDS-PAGE. Gels were transferred onto 0.2um nitrocellulose membranes using standard protocols and probed with anti-dually phosphorylated p42/44 MAPK rabbit monoclonal antibody (Cell Signaling CS-4370) or with rabbit anti-actin (Sigma A5060). Alexa 647-conjugated mouse anti-rabbit (Jackson Labs 211-605-109) was used as a secondary antibody, and signal was detected and quantified using a fluorescence imager. The experiment was performed twice.
Yeast-to-hypha transition assay using solid phase cytometry (SPC)
The fraction of hyphae in C. albicans cultures, taking only viable cells into account, was determined as described (Nailis et al., 2009). C. albicans CAI4 cultures, grown overnight in YPD (2×108 cells/ml), were diluted in YPD supplemented with 10% foetal calf serum (FCS) (8×104 cells/ml), and incubated with different concentrations of RsAFP2 or inhibitor 235236 for 4 h at 37°C. Cell suspensions (30 µl) were diluted in 1 ml sterile and particle free 0.9% (w/v) NaCl. Further processing of the diluted suspensions and analysis of the fluorescent signals was performed as previously described (Nailis et al., 2009). To test whether the inhibition of yeast-to-hypha transition after RsAFP2 treatment can be cured, C. albicans CAI4 cultures were treated with 0 and 50 µg/ml RsAFP2 for 2h, washed three times with physiological saline, and further incubated for 2h at 37°C in YPD supplemented with 10% FCS. Data are means ± SEM of at least sixtuple measurements, experiments have been repeated at least twice.
Septin localization assay
The SEP7-GFP allele was transformed into C. albicans CAI4 (resulting in JRB247) as described previously (Blankenship et al., 2010). An overnight culture of JRB247 was diluted 100 fold in YPD/PDB, and allowed to grow to an OD600 of ~1. This culture was split, and one half was treated with 50 µg/ml RsAFP2, while the other half was mock treated. After 2 h of incubation, 200 µl of these cultures were pipeted onto glass bottom dishes (MatTek Corporation, Ashland, MA) coated with concanavalin A. The dishes were washed twice with SC and cells were resuspended in SC for immediate visualization. For the washout experiment, cells were treated as above, however, treated cells were imaged 1.5 h after treatment with RsAFP2. The remaining cells were washed 3 times with fresh YPD/PDB, allowed to recover for 2 h in fresh YPD/PDB, and then imaged. Approximately 220 yeast cells for each experimental condition were visualized with a Zeiss Axio Observer Z.1 fluorescence microscope and a 100X NA 1.4 objective. Fluorescent images were acquired with an exposure time of 750 ms using a Coolsnap HQ2 (Photometrics) camera and Axiovision (Zeiss) software. DIC images are representative of a single image slice. GFP images were created by merging seven Z-stack slices (0.5 µm depth) in NIH ImageJ. DIC/GFP overlay images were created on ImageJ. Bud-neck width and the diameter of mother cells was measured using the Axiovision software. Because C. albicans cells normally are not totally round, two measurements were taken for the diameter: one across the length of the cell and the other approximately 90° from this measurement across the width of the cell. These measurements were averaged and a neck width/cell diameter ratio was calculated each cell independently.
RT-PCR expression analysis
Following treatment with RsAFP2 (50 µg/ml) for 2.5 h at 30°C, cells were collected and washed with physiological saline. Cell disruption, RNA purification, DNase treatment, and RT-PCR were performed as described (Nailis et al., 2006). Primers were developed for GIN4 (forward: AGTGGTACGCAGTGGGTCCAAA – reverse: TGTTGCACCAGCGCCAGGAT). After testing their specificity, real-time PCR (CFX96 Real Time System, Bio-Rad, Nazareth, Belgium) was performed using a Mesa Green qPCR kit (Eurogentec, Seraing, Belgium). The expression levels of GIN4 in both conditions were normalized using four reference genes (RPP2B, RIP, PMA1 and LSC2) (Nailis et al., 2006). Experiments were repeated three times.
Sphingolipidomics
Following treatment with RsAFP2 (25 µg/ml) for 5h at 30°C, analysis of sphingolipid metabolites of total membrane preparations of C. albicans was performed as described previously using a sphingolipidomics approach (Bielawski et al., 2006). Data represent duplicates of two independent experiments.
Results
C. albicans fitness test profiling of RsAFP2
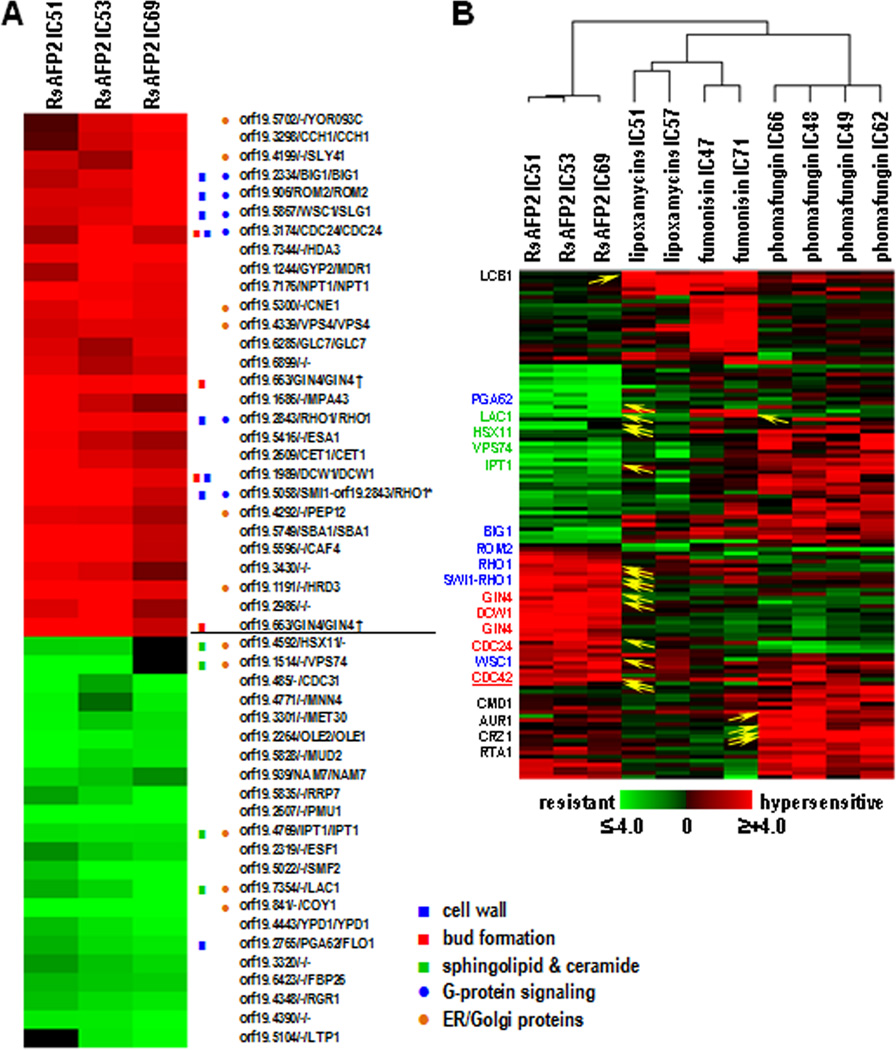
RsAFP2 was tested in the CaFT at three concentrations (10, 13, and 16 µg/ml, IC51, 53 and 69, respectively). As the profile bore no relatedness to over 4,000 antifungal agents tested in the same assay (data not shown), we selected strains whose absolute values of the normalized z-scores (positive indicating RsAFP2-hypersensitivity, negative RsAFP2-resistance (Xu et al., 2007)) were ≥ 3.0 in at least two experiments. Of the 50 selected strains, 28 were hypersensitive to RsAFP2, and 22 resistant (Fig. 1A; Table S1). At least three groups of genes with clear functional connections were represented in these strains, including 1) those involved in cell wall structure, especially glucan synthesis: orf19.2843/RHO1, encoding the regulatory subunit of the β-1,3-glucan synthase; orf19.906/ROM2, encoding the GTP/GDP exchange factor of Rho1p and Rho2p; orf19.2334/BIG1, encoding a modulator of Rho1p, orf19.5867/WSC1, encoding a mechanosensor protein of the PKC-MPK signaling pathway; orf19.2765/PGA62 encoding a GPI-anchored protein potentially contributing to cell wall stability and structure; a strain containing two heterozygous deletions of both RHO1 and orf19.5058/SMI1; and orf19.1989/DCW1, encoding a GPI-anchored mannosidase required for cell wall biosynthesis during bud formation. With the exception of PGA62, all heterozygotes were RsAFP2-hypersensitive; 2) those involved in bud/septin ring formation: orf19.663/GIN4, encoding a protein kinase required for bud and septin ring formation, represented by two independently constructed heterozygotes; and orf19.3174/CDC24, encoding a GTP/GDP exchange factor for Cdc42p, required for cellular polarity. All heterozygotes were RsAFP2-hypersensitive; 3) those involved in sphingolipid/ceramide biosynthesis: orf194592/HSX11, encoding glucosylceramide synthase, GCS; orf19.1514/VPS74, encoding a protein required for Golgi localization of GCS; orf19.4769/IPT1, encoding inositol phosphoryl transferase, involved in the synthesis of mannosylinositolphosphoryl-containing sphingolipids; and orf1919.7354/LAC1, encoding a ceramide synthase component. All heterozygotes were RsAFP2-resistant (Fig. 1A). The resistance of heterozygous deletions of HSX11, VPS74 and LAC1 reiterates the previous conclusion that the antifungal activity of RsAFP2 is mediated via its interaction with GlcCer (Thevissen et al., 2004), thus validating our approach of using the fitness test to study its MOA. Note that 11 genes identified in the CaFT RsAFP2 profile correspond to proteins that are located in the ER and/or Golgi apparatus (Fig. 1A), which is not surprising as sphingolipids and ceramides are synthesized in the ER and Golgi apparatus (Funato and Riezman, 2001). Within groups 1) and 2), various genes encode proteins that are regulated by or are part of G-protein signaling systems, namely RHO1, ROM2, BIG1, WSC1, CDC24 and SMI1 (Fig. 1A). In S. cerevisiae, the corresponding proteins have been implicated in governing tolerance against or signaling of cell wall stress. Recently, cell wall integrity and septin localization were found to be linked processes (Blankenship et al., 2010). The hypersensitivity of heterozygotes for genes involved in these two related processes in the RsAFP2 CaFT further suggests that RsAFP2, upon interaction with GlcCer, perturbs the fungal cell wall integrity and/or activates cell wall stress signaling pathways. Next, we compared the data of the RsAFP2 CaFT with CaFT data obtained with other antifungal compounds that affect sphingolipid/ceramide biosynthesis (Fig. 1B), namely the mycotoxins lipoxamycins and fumonisin and the cyclic lipodepsipeptide phomafungin (Mandala et al., 1994; Cowart and Obeid, 2007; Herath et al., 2009). The CaFT profiles of these antifungal compounds were distinct from that of RsAFP2 (Fig. 1B), pointing to a unique MOA of RsAFP2.
Fig. 1. CaFT profile of RsAFP2.
A, The plant defensin was tested at three different concentrations (10, 13 and 16 µg/ml, equivalent IC51, IC53 and IC69 in the CaFT using YPD/PDB medium. In the hierarchical clustering, heterozygotes with significant fitness variations were selected based on the absolute values of their z-scores (positive indicating hypersensitivity, and negative resistance) being ≥3 in at least two experiments. Gene annotations were adopted from the Candida Genome Database in following order: orf19 designation, C. albicans gene name, S. cerevisiae ortholog/homolog. Highlighted are those described in the text, with the functional group indicated. B, Comparison of the CaFT profiles of RsAFP2 and other antifungal natural products that affect sphingolipid/ceramide biosynthesis, by hierarchical clustering. Heterozygotes were selected as described in A. Yellow arrows indicate the corresponding genes that are related to the Mode of Action (MOA) of compounds in question, with gene names given on the left. The three functional groups (reflecting the MOA of RsAFP2) described in the text are highlighted in color, with those in black reflecting MOAs of other natural products. Note that the same heat map scale is used in both A and B, with black indicating data not available (most likely) due to low hybridization signals (and thus excluded from z-score calculation).
To validate the above genetic data, we assessed the effect of RsAFP2 on C. albicans cell wall morphology and integrity and on septin localization.
RsAFP2 interacts with the cell wall
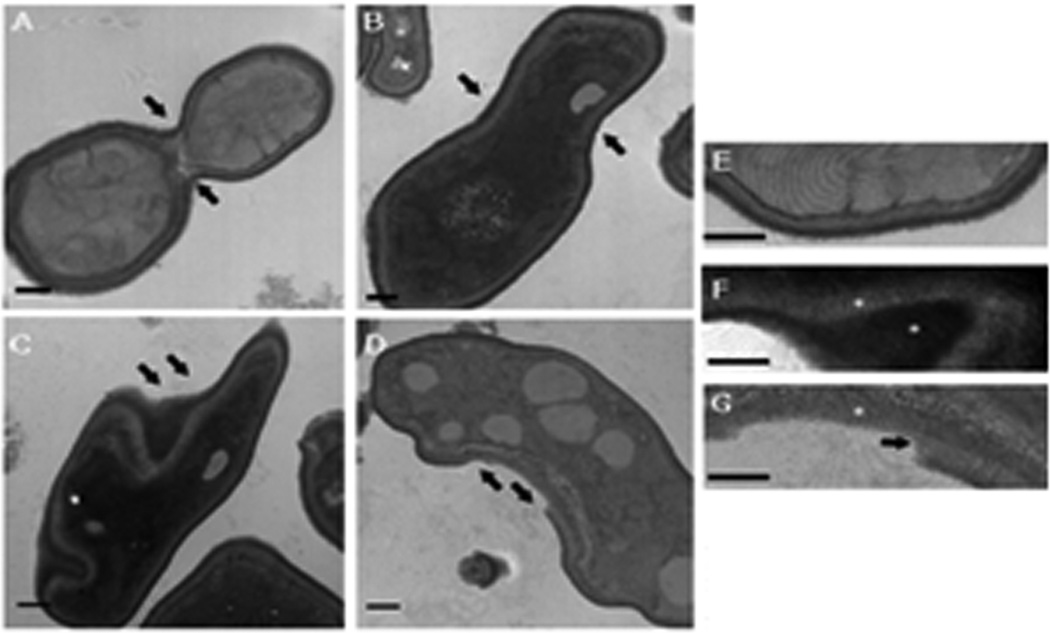
First, we assessed whether RsAFP2 induces morphological changes in C. albicans. Cells were incubated with 50 µg/ml RsAFP2 for 16h and analysed by transmission electron microscopy (TEM) (Fig. 2). Incubation of C. albicans with 25–50 µg/ml RsAFP2 for 5–24 h typically resulted in 45% to 65% survival as compared to the control treatment (water). RsAFP2 induced clear alterations in cell wall morphology and septum formation. Whereas control cells displayed a normal ultrastructural profile with a continuous cytoplasmic membrane, a compact cell wall with an external fibrilar layer and short-sized bud necks (Fig. 2A, panels A and E), RsAFP2-treated C. albicans cells were characterized by an aberrant budding profile with large bud neck size (Fig. 2A, panel B), altered cell shape with abnormal cell wall structure (Fig. 2A, panels C and F); and by the absence of the outermost wall layer at some loci (Fig. 2A, panel D). All the above phenotypes appeared in more than 90% of the RsAFP2-treated cells. Similar observations were made on other Candida species including C. krusei and C. parapsilosis (data not shown). These data point to the induction of cell wall alterations and aberrant septin formation in C. albicans by RsAFP2. However, it remains uncertain whether the fungal cell wall is the primary target of RsAFP2, or whether cell wall disruption is an indirect effect. Moreover, it can not be ruled out that the above described alterations result from factors other than RsAFP2-induced cell wall stress, implying that RsAFP2 may act on multiple targets.
Fig. 2. Effect of RsAFP2 on cell wall morphology in C. albicans.
Transmission electron micrographs (TEM) of Candida albicans strain 78 incubated with 50 µg/ml RsAFP2 for 16 h at room temperature. Control cells have normal ultrastructural profiles with a continuous cytoplasmic membrane, a compact cell wall with an external fibrilar layer, and budding cells with short-sized necks (arrows in panel A). RsAFP2-treated cells show ultrastructural alterations, such as an aberrant budding profile with a large neck size of the buds (arrows in panel B), altered cell shape with abnormal cell wall structure (arrows in panel C), and absence of the outermost wall layer in some areas (arrows in panel D). Panels E–G represent large magnification images of B, C and D, respectively. Bars: 0.3 µm.
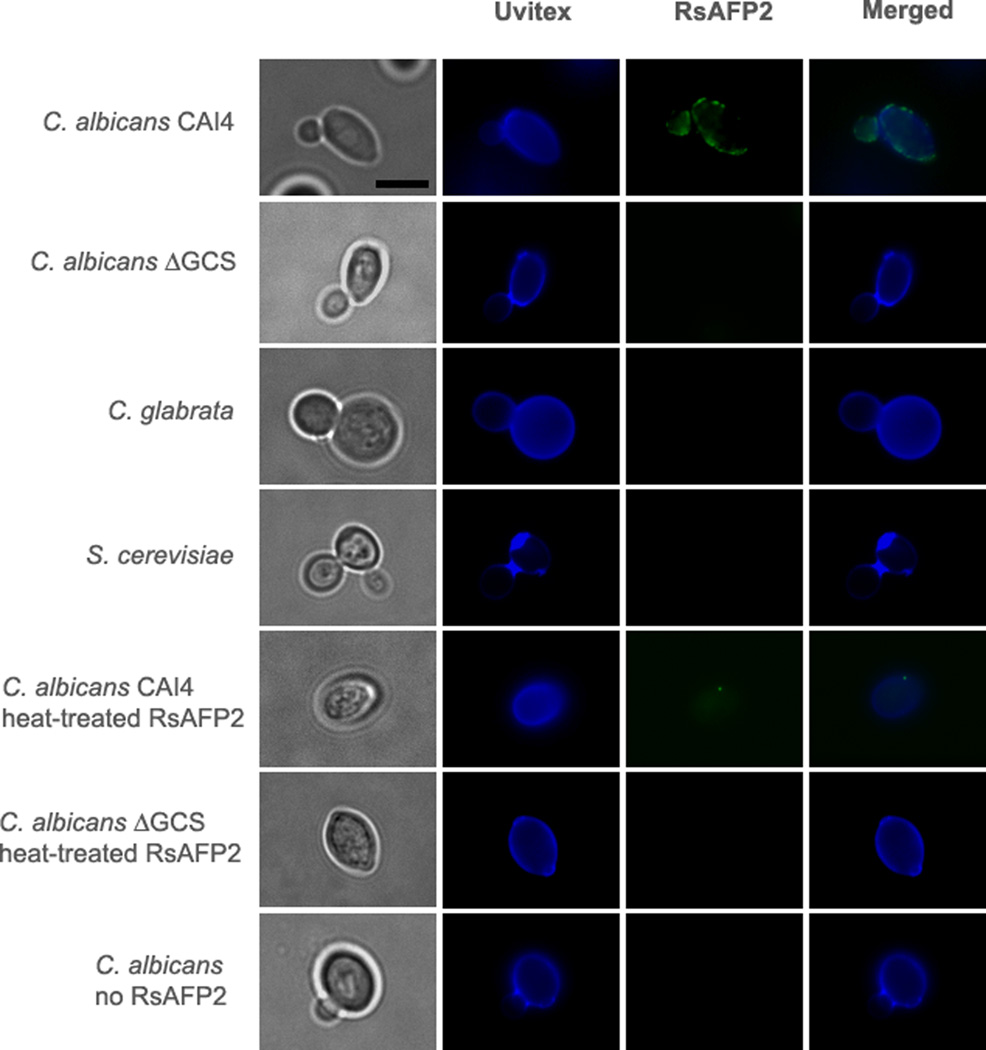
Second, using immunofluorescence microscopy we determined whether RsAFP2 interacts with the C. albicans cell wall (Fig. 3). C. albicans were incubated with 50 µg/ml RsAFP2, whereafter an anti-RsAFP2 serum was added. C. albicans Δgcs, C. glabrata and S. cerevisiae, which do not produce GlcCer and are RsAFP2-resistant, were used as negative controls (Leipelt et al., 2001; Tavares et al., 2008). We found that RsAFP2 interacts with the cell wall of C. albicans CAI4 and induces cell death in a process that requires the presence of GlcCer, as RsAFP2 was not observed to be associated with cell walls of RsAFP2-resistant C. glabrata and S. cerevisiae cells. In case of incubation of C. albicans Δgcs cells with RsAFP2, only a minor fraction of RsAFP2 was found to be associated to the cell surface as compared to C. albicans CAI4 cells. FACS analysis of C. albicans CAI4 or Δgcs cells upon incubation with 50 µg/ml RsAFP2 showed that 44.4% of the CAI4 cells reacted positive for cell surface RsAFP2, whereas only 14.2% of Δgcs cells reacted RsAFP2 positive. As incubation of C. albicans CAI4 cells with 25–50 µg/ml RsAFP2 for 5–24 h typically resuls in 45% to 65% survival as compared to the control treatment (water), the percentage of RsAFP2-reactive cells (i.e. 44.4%) is in the same range as the percentage of RsAFP2-killed cells. Incubation of C. albicans CAI4 with heat-inactivated RsAFP2 did not result in any cell surface-associated defensin.
Fig. 3. Localization of RsAFP2 at the fungal cell surface.
Epifluorescence microscopy followed by deconvolution images of C. albicans (CAI4 and Δgcs strains), C. glabrata and S. cerevisiae treated with 50 µg/ml (native or heat-inactivated) RsAFP2 for 3 h and further incubated with Uvitex B2 (blue) and anti-RsAFP2 antibodies in combination with a FITC-labeled goat anti-rabbit IgG (green). Scale bars = 5 µm.
Consistent with a predominant localization of RsAFP2 in the outer cell surface layers, we found that 43.0 ± 0.1% of GlcCer, the RsAFP2 target sphingolipid, is located in the C. albicans cell wall of strain 78, for strain CAI4 this value is 41.0 ± 0.1%. Next, internalization of RsAFP2by C. albicans cells was analyzed by quantitative HPLC analysis (François et al., 2009; Bink et al., 2010) of intracellular RsAFP2 as well as the fraction of RsAFP2 in the supernatant of cell cultures of C. albicans CAI4 and the Δgcs strain upon RsAFP2 treatment. Treatment of C. albicans CAI4 cells with 30 µg/ml RsAFP2 resulted in a very minor intracellular uptake of the plant defensin as only 0.74 ± 0.49% of the total amount of added RsAFP2 could be retrieved intracellularly. More than 95% RsAFP2 could be detected in the supernatant. In case of RsAFP2-treated Δgcs cells, 1.43 ± 0.19% RsAFP2 could be detected intracellularly. These data indicate that RsAFP2 does not need to be taken up intracellularly in C. albicans to exert its antifungal activity, which is consistent with the above presented microscopic data.
Our data of the genetic CaFT screen pointed to an important role for β-1,3-glucan synthesis in RsAFP2 tolerance. Therefore, the effect of RsAFP2 on the amount and structure of cell wall glucan was investigated. C. albicans CAI4 cells were treated with 0 or 50 µg/ml RsAFP2, after which polysaccharides in isolated cell walls were hydrolyzed with sulphuric acid followed by HPLC analysis of the resulting monosaccharides. These analyses showed that the relative amounts of glucan (and mannan) in the cell wall were unaltered upon RsAFP2 treatment for 2.5h or 16h. We measured 48±2% glucose and 24±1% mannan after 2.5h treatment with RsAFP2, and 53±4% glucose and 27±1% mannan for the mock incubation. After 16h treatment, 51±4% glucose and 27±2% mannan was present in cell wall of cells treated with RsAFP2 versus 46±2% glucose and 24±1% in corresponding control cell walls.
RsAFP2 activates the cell wall integrity (CWI) pathway via Mkc1p activation
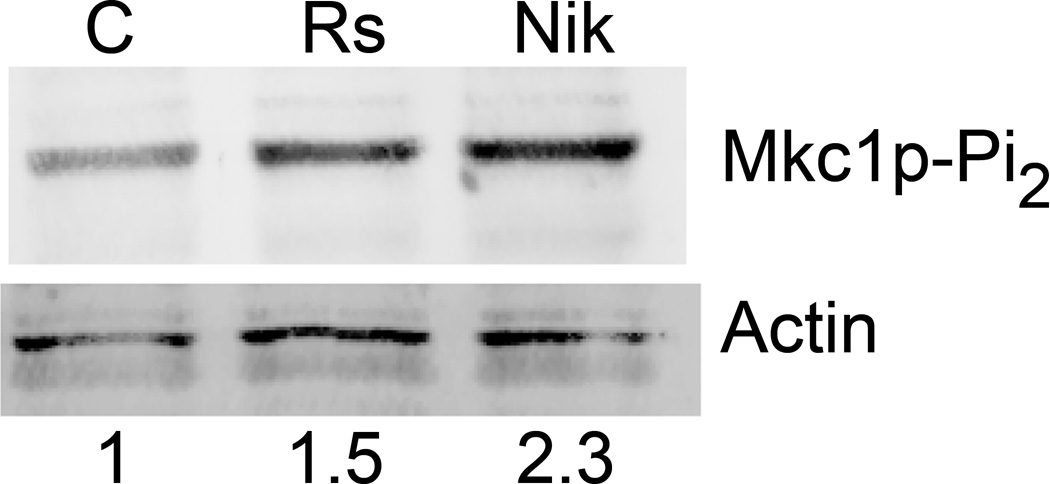
As we found that RsAFP2 interacts with the C. albicans cell wall and that various C. albicans heterozygotes for genes involved in the CWI are RsAFP2 hypersensitive, we investigated a putative activation of the CWI pathway by RsAFP2. To this end, we assessed the activation of Mkc1p as downstream interacting partner of Pkc1p by the determination of the degree of Mkc1p phosphorylation using anti-dually phosphorylated p42/44 MAPK rabbit monoclonal antibodies on immunoblots of cell extracts (Kumamoto, 2005). Exponentially grown C. albicans cells were treated with 50 µg/ml RsAFP2, 10 µg/ml Nikkomycin Z (positive control), a chitin synthase inhibitor that causes cell wall stress and activation of Mkc1p (Kumamoto, 2005), or water for one hour. Dually phosphorylated Mkc1p in cell extracts was detected by immunoblotting with antibody specific for the dually phosphorylated form of p42/44 Map kinases. A 1.5-fold increase in dually phosphorylated Mkc1p in response to RsAFP2 and a 2.3-fold increase in response to Nikkomycin Z were observed. These data point to the activation of the CWI pathway by RsAFP2, governing RsAFP2 tolerance.
RsAFP2 induces septin mislocalization and impairs the yeast-to-hypha transition in C. albicans
Septin localization upon RsAFP2 treatment was studied by inserting a SEP7-GFP tagged allele in C. albicans CAI4 (Blankenship et al., 2010), which was visualised using fluorescence microscopy. Thirty-five percent of the RsAFP2-treated cells showed abnormal septin localization (Fig. 5A; Fig. S1): 13.6% showed diffuse septin bands at the neck; 5.9% were characterized by septin spots in the daughter cell; and 3.1% had side-by-side necks. Moreover, 1.4% of the cells were characterized by septin that was localized around the periphery of the bud, and 3.2% of the cells had medium-sized buds with an additional bud at the distal end, both with septins at the mother-daughter interface. Septin localization within 7.7% of the RsAFP2-treated cells was otherwise abnormal. The GFP-tagged Sep7 septin also allowed us to determine neck sizes of budding C. albicans cells (Fig. 5B). Upon treatment of the yeast cells with 50 µg/ml RsAFP2 for 2h, we observed a 1.5-fold increase of the average neck size (2.72 µm, n=244, with a ratio of bud neck/mother cell diameter of 0.4) as compared to untreated cells (1.72 µm, n=99, p<0.001, bud neck/mother cell ratio of 0.32). Thus, RsAFP2 induced a general mislocalization of the septin ring. The RsAFP2-induced septin localization defects do not appear to be similar to the earlier reported ectopic septin localization caused by caspofungin (Blankenship et al., 2010). However, C. albicans cells treated with low levels of caspofungin for longer periods of time actually look similar to RsAFP2-treated cells (data not shown). These data indicate that the observed phenotypic differences between RsAFP2 and caspofungin may be due to dosage effects.
Fig. 5. RsAFP2 affects bud neck size and septin localization.
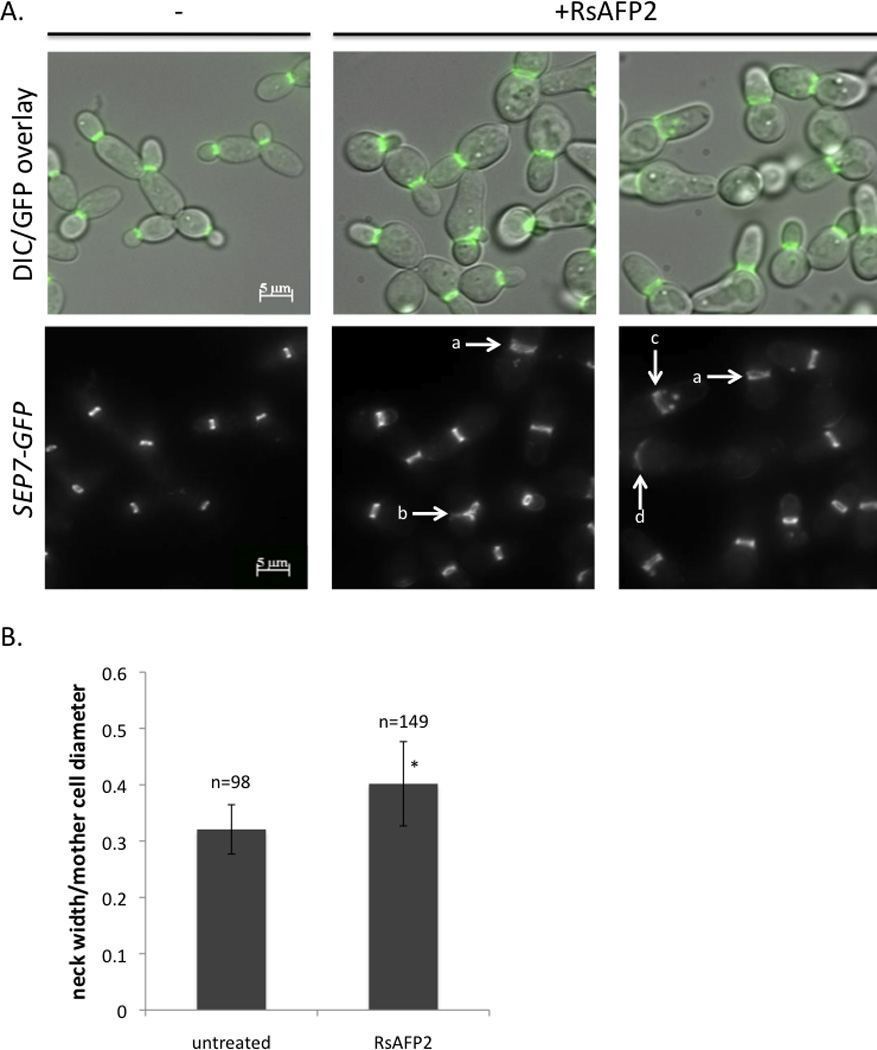
A, A SEP7-GFP-tagged strain (JRB247) was imaged after treatment with 50 µg/ml RsAFP2 in YPD/PDB for 2 h, and compared to an untreated control. The top panel represents the overlay of DIC (Differential Interference Contrast) and fluorescence microscopy images. Abnormal septin localization is indicated by arrows, including diffuse septin localization at the neck (a), side-by-side necks (b), septin spots in the daughter cell (c), and otherwise abnormal localization (d). B, The bud neck width/mother cell diameter ratio was measured in cells treated with 50 µg/ml RsAFP2 and untreated (−) cells. Values are statistically significant (p=3.68 × 10−22).
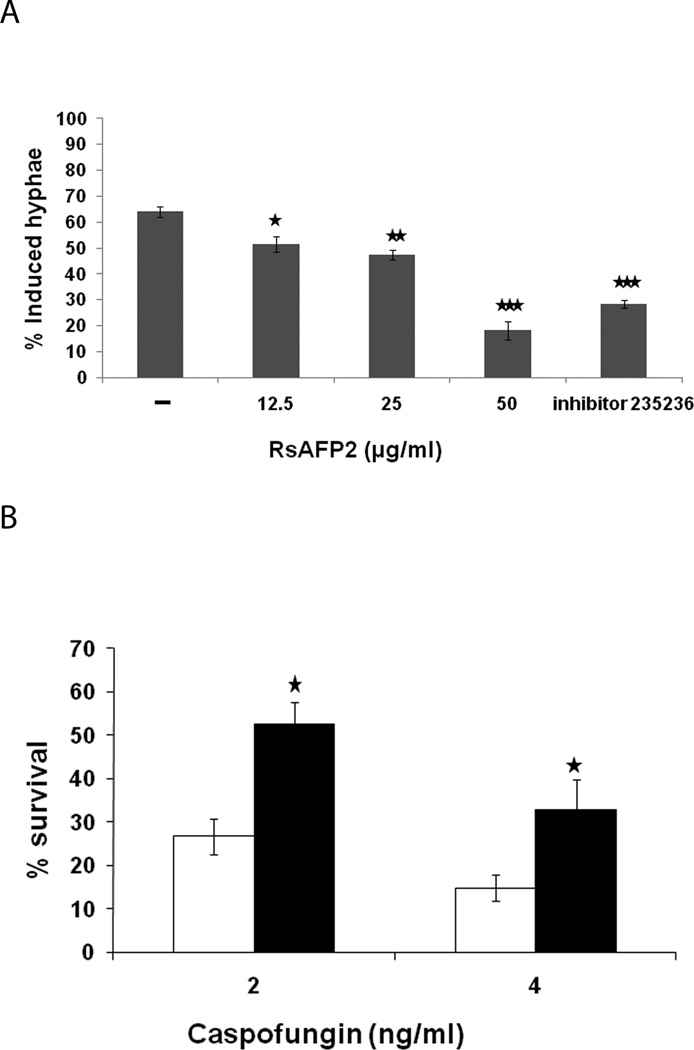
Recently, it was shown that abnormal septin localization may be associated with inhibition of the yeast-to-hypha transition in C. albicans (Toenjes et al., 2009). As Gin4, a S/T kinase that is involved in hyphal growth, was also identified in the RsAFP2 CaFT assay (Fig. 1A), we assessed whether RsAFP2 affects the yeast-to-hypha transition of C. albicans in fetal calf serum (FCS)-containing medium. C. albicans CAI4 cells were incubated with different concentrations of RsAFP2 in YPD in the presence of 10% FCS and the fraction of cells developing hyphae was determined using solid phase cytometry (Nailis et al., 2009). The applied RsAFP2 doses in YPD + 10% FCS had no effect on growth of the C. albicans cultures as assessed by Bioscreen analysis (data not shown). As a positive control, we used inhibitor 235236, which has been shown to block yeast-to-hypha transition in C. albicans (Toenjes et al., 2005). Treatment of C. albicans cultures with RsAFP2 reduced the fraction of hyphae in a dose-dependent manner (Fig. 6A). In the presence of water (mock treatment), 64.1 ± 2.1% of the C. albicans cell population showed hyphal growth in YPD supplemented with 10% FCS upon incubation for 4 h. The fraction of cells forming hyphae dropped to 18.2 ± 3.4% in the presence of 50 µg/ml (10 µM) RsAFP2, and to 28.5 ± 1.5% in the presence of 10 µg/ml (30 µM) inhibitor 235236. Therefore, we conclude that RsAFP2 impairs the yeast-to-hypha transition in C. albicans. In a curing experiment, septin localization returned to normal upon removal of RsAFP2. The average bud neck/cell diameter ratio for treated cells was 0.42±0.01, and for RsAFP2-washed out cells this was 0.31±0.01. Similarly, no significant differences in the fraction of hyphae were observed between RsAFP2- and water-treated C. albicans cell populations upon curing (39.0 ± 2.6% versus 44.3 ± 3.5%). This indicates that the RsAFP2-induced septin mislocalization and the block of the yeast-to-hypha transition is transient. As C. albicans cells that are trapped in the yeast form have been reported to be less susceptible to caspofungin (Wheeler et al., 2008), we studied a possible antagonism between RsAFP2 and caspofungin. Co-incubation of C. albicans cells with a mild RsAFP2 dose (5 µg/ml, resulting in 100% viability of the yeast culture) and caspofungin resulted in significantly increased survival rates of C. albicans cells, as compared to treatment with caspofungin alone (Fig. 6B), supporting the idea that the two antifungals have antagonistic effects. The applied RsAFP2 doses in PDB/YPD had no effect on growth of the C. albicans cultures as assessed by Bioscreen analysis (data not shown). Whereas loss of core septins leads to increased caspofungin sensitivity (Blankenship et al., 2010), RsAFP2-treatment results in increased caspofungin tolerance. We hypothesize that septin dispersal is part of the natural defense/tolerance against cell wall stress. Hence, loss of a core septin (as in septin mutant cells), may obstruct this protection, resulting in hypersensitivity to caspofungin. Upon co-incubation of cells with mild RsAFP2 doses, however, the core septins are still present and, according to the above hypothesis, the cell can deal with the cell wall stress imposed by caspofungin.
Fig. 6. RsAFP2 impairs the yeast-to hyphae transition in C. albicans.
A, Overnight grown C. albicans CAI4 cultures in YPD (2×108 cells/ml) were diluted in YPD/10%FCS (8×104 cells/ml) and incubated with different concentrations of RsAFP2 or 10 µg/ml inhibitor 235236. Using solid phase cytometry, the percentage of living yeast cells displaying hyphal morphology were determined. Data are means of triplicate measurements of one representative experiment out of three. B, Percentage survival (as determined by counting colony forming units) of caspofungin-treated cells in the absence (open bars) or presence of 5 µg/ml RsAFP2 (sublethal dose; black bars), normalized to that of the mock treated cells. 5 µg/ml RsAFP2 alone resulted in > 80% survival. Data represent mean ± SEM. *p<0.05; **p<0.01; ***p<0.001.
To further elucidate the role for Gin4 in governing tolerance to RsAFP2 in C. albicans, expression of GIN4 in RsAFP2-treated C. albicans cultures was assessed. A slight but significant induction of GIN4 expression (1.4-fold, standard deviation 0.2-fold, p= 0.05) was observed in RsAFP2-treated cells compared to untreated cells.
RsAFP2 induces ceramide accumulation in C. albicans membranes
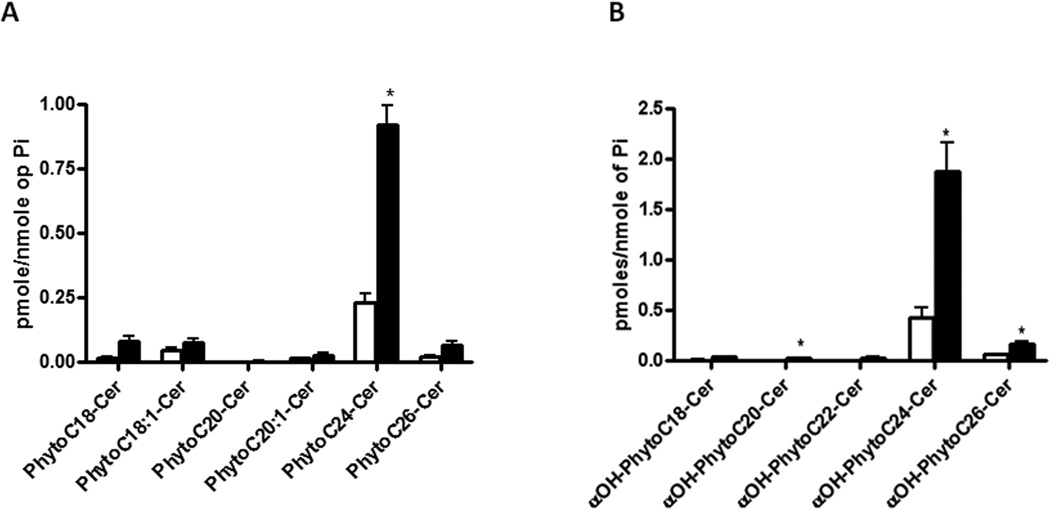
As accumulation of ceramides, the sphingolipid backbones, following aureobasidin treatment has been shown to induce septin mislocalization in membranes of the Basidiomycete Ustilago maydis (Cánovas and Pérez-Martín, 2009), we determined the effects of RsAFP2 on sphingolipid species in C. albicans CAI4. Sphingolipid metabolites, such as α-hydroxy-phytoceramides, dihydro- and phytoceramides, dihydro- and phytosphingosine, as well as the corresponding sphingoid base phosphates were analyzed in the total cell membrane fraction of C. albicans treated with RsAFP2 by mass spectrometry analysis. Treatment of C. albicans with RsAFP2 led to a significant increase in the most abundant ceramides, namely C-24phytoceramides and (α-hydroxy)C-24phytoceramide (Fig. 7). Levels of the other ceramide species or sphingolipid metabolites in C. albicans membranes were not significantly altered upon RsAFP2 treatment.
Fig. 7. RsAFP2 induces accumulation of ceramide in C. albicans membranes.
Levels of A, phyto- and B, α-hydroxy-phyto-ceramides in C. albicans CAI4 following treatment with water (open bars) or 25 µg/ml RsAFP2 (black bars). *p<0.05.
Discussion
To uncover tolerance mechanisms of C. albicans against the plant defensin RsAFP2 and gain more insight in its mode of action, we screened a collection of heterozygous C. albicans mutants for altered RsAFP2 sensitivity. Most of the altered RsAFP2-tolerance genes belong to a limited set of functional classes and define specific areas of cellular biology. Most prominent were genes involved in cell wall integrity and hyphal growth/septin ring formation (WSC1, ROM2, BIG1, DCW1, PGA62, RHO1, CDC24, GIN4). These processes are functionally connected; an intact septin ring is required for normal cell wall synthesis, and a normal cell wall structure is required for the formation of a septin ring. Moreover, septins are important for cell survival and are mislocalized upon cell wall stress (Blankenship et al., 2010). Other groups of RsAFP2-tolerance genes were involved in protein and vesicle transport, and in the regulation of transcription. The latter probably represents general stress tolerance mechanisms, as was described previously (Thevissen et al., 2007a; Parsons et al., 2004; Thorpe et al., 2004). More specifically, several RsAFP2-tolerance genes encode components of small GTPase signalling transduction pathways. The small GTPase Rho1 is activated by cell wall alterations, which can be sensed through the mechanosensor Wsc1 in the plasma membrane, via its GTP/GDP exchange factor (GEF) Rom2. The small GTPase Cdc42 is activated by various internal and external signals including calcium influx via its GEF Cdc24. RsAFP2 induces calcium influx in susceptible fungi (Thevissen et al., 1996). Once activated, Cdc42 regulates the organization of the actin cytoskeleton and the septins, and interacts with components of the exocytic machinery (Park et al., 1997). Both Cdc42 and Rho1 pathways have been shown to interact with each other, regulating exocytosis, septin organization, and cell wall synthesis (Drees et al., 2001).
To validate the obtained genetic data, we have focused on the effects of RsAFP2 on cell wall morphology and integrity, and on septin localization in C. albicans. We demonstrated that RsAFP2 interacts primarily with the cell wall of C. albicans in a GlcCer-dependent way, and that RsAFP2 does not need to be taken up intracellularly in C. albicans to exert its antifungal activity. The extracellular action of RsAFP2 is in contrast to the intracellular accumulation of other antifungals, like miconazole and BAR0329, a fungicidal piperazine-carboxamidine (François et al., 2009; Bink et al., 2010). Using the quantitative HPLC technique, we previously demonstrated a significant intracellular accumulation of miconazole (97.4 ± 1.5% of total amount miconazole added) and BAR0329 (38.6 ± 5.6% of total amount BAR0329 added) in yeast cells. The reason for the primary interaction of RsAFP2 with the C. albicans cell wall could be attributed to the presence of GlcCer in its cell wall. This observation is in line with a previous report describing the presence of GlcCer ligands in the cell wall of the fungal pathogen Cryptococcus neoformans (Rodrigues et al., 2000).
RsAFP2 is the first example of an antifungal plant defensin that acts via the extracellular side of fungal cells. Previously, it has been shown that a plant defensin from flowers of Nicotiana alata, NaD1, is taken up and localized to the cytoplasm of susceptible fungi (van der Weerden et al., 2008). Very recently, NaD1 was shown to permeabilize fungal cells via a novel mechanism, which required the presence of the fungal cell wall (van der Weerden et al., 2010). In the latter study, the authors hypothesized that an as yet unidentified NaD1-receptor may be located in the proteinaceous layer of the cell wall. An intracellular accumulation was also demonstrated for a plant defensin from Pisum sativum (PsD1). This defensin localizes to the nucleus of Neurospora crassa where it interacts with the cell cycle control protein Cyclin F (Lobo et al., 2007). Consistent with our observation that the plant defensin RsAFP2 interacts with the fungal cell wall, recent papers reported the interaction between antibacterial human, invertebrate and fungal defensins and the bacterial cell wall precursor Lipid II (Schmitt et al., 2010; Schneider et al., 2010; Sass et al., 2010), suggesting similarities in the mode of antifungal or antibacterial action of defensins from different kingdoms.
We further demonstrated that RsAFP2 activates the MAPK cell wall integrity pathway, a signal transduction pathway known to be activated by cell wall stress or membrane perturbing conditions in C. albicans (Kumamoto, 2005). These data are consistent with a previous report on the hypersensitivity of a mgv1-deletion mutant of Fusarium graminearum to RsAFP2 and other defensins like MsDef1 from Medicago sativa (Ramamoorthy et al., 2007). Mgv1 is a MAP kinase that is homologous to the S. cerevisiae Slt2 kinase, which is involved in regulating the maintenance of cell wall integrity (Hou et al., 2002) and is activated upon treatment of the fungus with MsDef1 (Ramamoorthy et al., 2007).
Consistent with the reported link between cell wall integrity and septin localization (Blankenship et al., 2010), we observed septin mislocalization in C. albicans following RsAFP2 treatment. Septins are an evolutionarily conserved family of filament-forming proteins that act in cell division, polarity determination, vesicle trafficking, and cytoskeletal dynamics (Sudbery, 2001). Except for ER and cell wall stress (Babour et al., 2010; Blankenship et al., 2010), no other stress has been shown to affect septin mislocalization, indicating that septin dispersal is not a general response to stress, nor is it a common hallmark of dying cells. Hence, the observed effect of RsAFP2 on septin mislocalization seems a unique feature of this antimicrobial compound, also reflected by its CaFT.
As RsAFP2 treatment results in septin mislocalization and septins are required for polarized growth of filamentous fungi (Boyce et al., 2005), this observation can also explain the previously observed hyperbranching of the filamentous fungus Fusarium culmorum, induced by RsAFP2 (Terras et al., 1992). It has been shown recently that abnormal septin localization may be associated with inhibition of the yeast-to-hypha transition in C. albicans (Toenjes et al., 2009), and RsAFP2 blocks this transition in C. albicans. As the S/T kinase Gin4, which phosphorylates the septin Cdc11 during hyphal growth, is part of the tolerance mechanism of C. albicans against RsAFP2, this may be a response to the blocking of the yeast-to-hypha transition induced by RsAFP2. Moreover, a lethal accumulation of ceramides in fungal membranes can induce septin mislocalization (Cánovas and Pérez-Martín, 2009). Through sphingolipidomics analysis, we demonstrated that RsAFP2 induces accumulation of long chain C24-phytoceramides.
It remains to be elucidated which enzymes are responsible for the RsAFP2-induced ceramide accumulation in membranes of susceptible fungi and which signaling pathway is regulating this process. The ceramide accumulation might result from de novo ceramide synthesis, through activation of a ceramide synthase enzyme, and/or via breakdown of glucosylceramides, the target of RsAFP2. However, as GlcCer in C. albicans are mainly composed of C18 fatty acids, in the latter case the question remains how C18-ceramides are converted to long chain C24-ceramides. Consistent with the first hypothesis, we found the orf19.7354 (orthologue of S. cerevisiae LAC1) heterozygous C. albicans deletion mutant to be RsAFP2 resistant (Fig. 1). Lac1 is a ceramide synthase component, involved in the synthesis of ceramide from C24/26(acyl)-coenzyme A and dihydrosphingosine or phytosphingosine.
Supplementary Material
Fig. 4. RsAFP2 activates the cell wall integrity pathway via Mkc1p.
C. albicans cells were treated with 50 µg/ml RsAFP2 (Rs), 10 µg/ml Nikkomycin (Nik) or water (control, C) for one hour. Protein was extracted and probed as described in experimental methods. Top panel, dually phosphorylated Mkc1p; bottom panel, actin. Numbers at bottom indicate amounts of dually phosphorylated Mkc1p normalized to actin levels and expressed relative to the water control. One representative experiment out of two is shown.
Acknowledgements
This work was supported by FWO-Vlaanderen (to B.C., T.C. & K.T. and krediet aan navorsers 1.5.141.09N). We are grateful for the postdoctoral fellowship to K.T. from K.U. Leuven Industrial Research Fund, and for the doctoral fellowships to A.B. and G.G. from FWO-Vlaanderen and IWT-Vlaanderen, respectively. L.N., S.R. and P.T. were supported by the Brazilian agencies CNPq and FAPERJ; J.B. was supported by NIH grant 7R01AI057804. We thank Carmen Berbegal and Sergi Ferrer (ENOLAB, University of Valencia, spain) for the help and use of equipment for analysis of cell wall sugars. Carol A. Kumamoto and Talya R. Davis are supported by grant R01AI081794 from the National Institute of Allergy and Infectious Diseases (to C.A.K.).
References
- Aerts AM, Carmona-Gutierrez D, Lefevre S, Govaert G, François IE, Madeo F, et al. The antifungal plant defensin RsAFP2 from radish induces apoptosis in a metacaspase independent way in Candida albicans. FEBS Lett. 2009;583:2513–2516. doi: 10.1016/j.febslet.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Aerts AM, François IE, Cammue BP, Thevissen K. The mode of antifungal action of plant, insect and human defensins. Cell Mol Life Sci. 2008;65:2069–2079. doi: 10.1007/s00018-008-8035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerts AM, François IE, Meert EM, Li QT, Cammue BP, Thevissen K. The antifungal activity of RsAFP2, a plant defensin from Raphanus sativus involves the induction of reactive oxygen species in Candida albicans. J Mol Microbiol Biotechnol. 2007;13:243–247. doi: 10.1159/000104753. [DOI] [PubMed] [Google Scholar]
- Babour A, Bicknell AA, Tourtellotte J, Niwa M. A surveillance pathway monitors the fitness of the endoplasmic reticulum to control its inheritance. Cell. 2010;142:256–269. doi: 10.1016/j.cell.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto-Bergter E, Pinto MR, Rodrigues ML. Structure and biological functions of fungal cerebrosides. An Acad Bras Cienc. 2004;76:67–84. doi: 10.1590/s0001-37652004000100007. [DOI] [PubMed] [Google Scholar]
- Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Bink A, Govaert G, François IE, Pellens K, Meerpoel L, Borgers M, et al. A fungicidal piperazine-1-carboxamidine induces mitochondrial fission-dependent apoptosis in yeast. FEMS Yeast Res. 2010;10:812–818. doi: 10.1111/j.1567-1364.2010.00663.x. [DOI] [PubMed] [Google Scholar]
- Blankenship JR, Fanning S, Hamaker JJ, Mitchell AP. An extensive circuitry for cell wall regulation in Candida albicans. PLoS Pathog. 2010;6:e1000752. doi: 10.1371/journal.ppat.1000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce KJ, Chang H, D'Souza CA, Kronstad JW. An Ustilago maydis septin is required for filamentous growth in culture and for full symptom development on maize. Eukaryot Cell. 2005;4:2044–2056. doi: 10.1128/EC.4.12.2044-2056.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cánovas D, Pérez-Martín J. Sphingolipid biosynthesis is required for polar growth in the dimorphic phytopathogen Ustilago maydis. Fungal Genet Biol. 2009;46:190–200. doi: 10.1016/j.fgb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Cowart LA, Obeid LM. Yeast sphingolipids: recent developments in understanding biosynthesis, regulation, and function. Biochim Biophys Acta. 2007;1771:421–431. doi: 10.1016/j.bbalip.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot PWJ, De Boer AD, Cunningham J, Dekker HL, De Jong L, Hellingwerf KJ, De Koster C, Klis FM. Proteomic analysis of Candida albicans cell walls reveals covalently bound carbohydrate-active enzymes and adhesins. Eukaryot Cell. 2004;3:955–965. doi: 10.1128/EC.3.4.955-965.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees BL, Sundin B, Brazeau E, Caviston JP, Chen GC, Guo W, et al. A protein interaction map for cell polarity development. J Cell Biol. 2001;154:549–571. doi: 10.1083/jcb.200104057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferket KK, Levery SB, Park C, Cammue BP, Thevissen K. Isolation and characterization of Neurospora crassa mutants resistant to antifungal plant defensins. Fungal Genet Biol. 2003;40:176–185. doi: 10.1016/s1087-1845(03)00085-9. [DOI] [PubMed] [Google Scholar]
- Folch K, Lees M, Soane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Fontaine T, Magnin T, Melhert A, Lamont D, Latge JP, Ferguson MA. Structures of the glycosylphosphatidylinositol membrane anchors from Aspergillus fumigatus membrane proteins. Glycobiology. 2003;13:169–177. doi: 10.1093/glycob/cwg004. [DOI] [PubMed] [Google Scholar]
- Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François IE, Bink A, Vandercappellen J, Ayscough KR, Toulmay A, Schneiter R, et al. Membrane rafts are involved in intracellular miconazole accumulation in yeast cells. J Biol Chem. 2009;284:32680–32685. doi: 10.1074/jbc.M109.014571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François IE, De Bolle MF, Dwyer G, Goderis IJ, Wouters PF, Verhaert PD, et al. Transgenic expression in Arabidopsis of a polyprotein construct leading to production of two different antimicrobial proteins. Plant Physiol. 2002;128:1346–1358. doi: 10.1104/pp.010794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen AJ, Cunha MM, Batista EJ, Seabra SH, De Souza W, Rozental S. Effects of tricyclazole (5-methyl-1,2,4-triazol(3,4) benzothiazole), a specific DHN-melanin inhibitor, on the morphology of Fonsecaea pedrosoi conidia and sclerotic cells. Microsc Res Tech. 2006;69:729–737. doi: 10.1002/jemt.20344. [DOI] [PubMed] [Google Scholar]
- Funato K, Riezman H. Vesicular and nonvesicular transport of ceramide from ER to the Golgi apparatus in yeast. J Cell Biol. 2001;155:949–959. doi: 10.1083/jcb.200105033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herath K, Harris G, Jayasuriya H, Zink D, Smith S, Vicente F, et al. Isolation, structure and biological activity of phomafungin, a cyclic lipodepsipeptide from a widespread tropical Phoma sp. Bioorg Med Chem. 2009;17:1361–1369. doi: 10.1016/j.bmc.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Hou Z, Xue C, Peng Y, Katan T, Kistler HC, Xu JR. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol Plant Microbe Interact. 2002;15:1119–1127. doi: 10.1094/MPMI.2002.15.11.1119. [DOI] [PubMed] [Google Scholar]
- Kumamoto CA. A contact-activated kinase signals Candida albicans invasive growth and biofilm development. Proc Natl Acad Sci U S A. 2005;102:5576–5581. doi: 10.1073/pnas.0407097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipelt M, Warnecke D, Zähringer U, Ott C, Müller F, Hube B, Heinz E. Glucosylceramide synthases, a gene family responsible for the biosynthesis of glucosphingolipids in animals, plants, and fungi. J Biol Chem. 2001;276:33621–33629. doi: 10.1074/jbc.M104952200. [DOI] [PubMed] [Google Scholar]
- Lobo DS, Pereira IB, Fragel-Madeira L, Medeiros LN, Cabral LM, Faria J, et al. Antifungal Pisum sativum defensin 1 interacts with Neurospora crassa cyclin F related to the cell cycle. Biochemistry. 2007;46:987–996. doi: 10.1021/bi061441j. [DOI] [PubMed] [Google Scholar]
- Mandala SM, Frommer BR, Thornton RA, Kurtz MB, Young NM, Cabello, et al. Inhibition of serine palmitoyl-transferase activity by lipoxamycin. J Antibiot (Tokyo) 1994;47:376–379. doi: 10.7164/antibiotics.47.376. [DOI] [PubMed] [Google Scholar]
- Mavor AL, Thewes S, Hube B. Systemic fungal infections caused by Candida species: epidemiology, infection process and virulence attributes. Curr Drug Targets. 2005;6:863–874. doi: 10.2174/138945005774912735. [DOI] [PubMed] [Google Scholar]
- Nailis H, Coenye T, Van Nieuwerburgh F, Deforce D, Nelis HJ. Development and evaluation of different normalization strategies for gene expression studies in Candida albicans biofilms by real-time PCR. BMC Mol Biol. 2006;7:25. doi: 10.1186/1471-2199-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nailis H, Vandenbroucke R, Tilleman K, Deforce D, Nelis H, Coenye T. Monitoring ALS1 and ALS3 gene expression during in vitro Candida albicans biofilm formation under continuous flow conditions. Mycopathologia. 2009;167:9–17. doi: 10.1007/s11046-008-9148-6. [DOI] [PubMed] [Google Scholar]
- Noble SM, French S, Kohn LA, Chen V, Johnson AD. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet. 2010;42:590–598. doi: 10.1038/ng.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini G, De Groot PWJ, Coste AT, Karababa M, Klis FM, de Koster CG, Sanglard D. The CRH family coding for cell wall glycosylphosphatidylinositol proteins with a predicted transglycosidase domain affects cell wall organization and virulence of Candida albicans. J Biol Chem. 2006;281:40399–40411. doi: 10.1074/jbc.M606361200. [DOI] [PubMed] [Google Scholar]
- Park C, Bennion B, François IE, Ferket KK, Cammue BP, Thevissen K, Levery SB. Neutral glycolipids of the filamentous fungus Neurospora crassa: altered expression in plant defensin-resistant mutants. J Lipid Res. 2005;46:759–768. doi: 10.1194/jlr.M400457-JLR200. [DOI] [PubMed] [Google Scholar]
- Park HO, Bi E, Pringle JR, Herskowitz I. Two active states of the Ras-related Bud1/Rsr1 protein bind to different effectors to determine yeast cell polarity. Proc Natl Acad Sci U S A. 1997;94:4463–4468. doi: 10.1073/pnas.94.9.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons AB, Brost RL, Ding H, Li Z, Zhang C, Sheikh B, et al. Integration of chemical-genetic and genetic interaction data links bioactive compounds to cellular target pathways. Nat Biotechnol. 2004;22:62–69. doi: 10.1038/nbt919. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy V, Zhao X, Snyder AK, Xu JR, Shah DM. Two mitogen-activated protein kinase signalling cascades mediate basal resistance to antifungal plant defensins in Fusarium graminearum. Cell Microbiol. 2007;9:1491–1506. doi: 10.1111/j.1462-5822.2006.00887.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues ML, Travassos LR, Miranda KR, Franzen AJ, Rozental S, de Souza W, et al. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect Immun. 2000;68:7049–7060. doi: 10.1128/iai.68.12.7049-7060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass V, Schneider T, Wilmes M, Körner C, Tossi A, Novikova N, et al. Human beta-defensin 3 inhibits cell wall biosynthesis in Staphylococci. Infect Immun. 2010;78:2793–2800. doi: 10.1128/IAI.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt P, Wilmes M, Pugnière M, Aumelas A, Bachère E, Sahl HG, et al. Insight into invertebrate defensin mechanism of action: oyster defensins inhibit peptidoglycan biosynthesis by binding to lipid II. J Biol Chem. 2010;285:29208–29216. doi: 10.1074/jbc.M110.143388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Kruse T, Wimmer R, Wiedemann I, Sass V, Pag U, et al. Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science. 2010;328:1168–1172. doi: 10.1126/science.1185723. [DOI] [PubMed] [Google Scholar]
- Stone BA, Clarke AE. Chemistry and biology of (1,3)-β-glucan. Melbourne, Australia: La Trobe University Press; 1992. [Google Scholar]
- Sudbery PE. The germ tubes of Candida albicans hyphae and pseudohyphae show different patterns of septin ring localization. Mol Microbiol. 2001;41:19–31. doi: 10.1046/j.1365-2958.2001.02459.x. [DOI] [PubMed] [Google Scholar]
- Tavares PM, Thevissen K, Cammue BP, François IE, Barreto-Bergter E, Taborda CP, et al. In vitro activity of the antifungal plant defensin RsAFP2 against Candida isolates and its in vivo efficacy in prophylactic murine models of candidiasis. Antimicrob Agents Chemother. 2008;52:4522–4525. doi: 10.1128/AAC.00448-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terras FR, Schoofs HM, De Bolle MF, Van Leuven F, Rees SB, Vanderleyden J, et al. Analysis of two novel classes of plant antifungal proteins from radish (Raphanus sativus L.) seeds. J Biol Chem. 1992;267:15301–15309. [PubMed] [Google Scholar]
- Thevissen K, Ayscough KR, Aerts AM, Du W, De Brucker K, Meert EM, et al. Miconazole induces changes in actin cytoskeleton prior to reactive oxygen species induction in yeast. J Biol Chem. 2007a;282:21592–21597. doi: 10.1074/jbc.M608505200. [DOI] [PubMed] [Google Scholar]
- Thevissen K, Ghazi A, De Samblanx GW, Brownlee C, Osborn RW, Broekaert WF. Fungal membrane responses induced by plant defensins and thionins. J Biol Chem. 1996;271:15018–15025. doi: 10.1074/jbc.271.25.15018. [DOI] [PubMed] [Google Scholar]
- Thevissen K, Kristensen HH, Thomma BP, Cammue BP, François IE. Therapeutic potential of antifungal plant and insect defensins. Drug Discov Today. 2007b;12:966–971. doi: 10.1016/j.drudis.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Thevissen K, Terras FR, Broekaert WF. Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl Environ Microbiol. 1999;65:5451–5458. doi: 10.1128/aem.65.12.5451-5458.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevissen K, Warnecke DC, François IE, Leipelt M, Heinz E, Ott C, et al. Defensins from insects and plants interact with fungal glucosylceramides. J Biol Chem. 2004;279:3900–3905. doi: 10.1074/jbc.M311165200. [DOI] [PubMed] [Google Scholar]
- Thorpe GW, Fong CS, Alic N, Higgins VJ, Dawes IW. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: oxidative-stress-response genes. Proc Natl Acad Sci U S A. 2004;101:6564–6569. doi: 10.1073/pnas.0305888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toenjes KA, Munsee SM, Ibrahim AS, Jeffrey R, Edwards JE, Jr, Johnson DI. Small-molecule inhibitors of the budded-to-hyphal-form transition in the pathogenic yeast Candida albicans. Antimicrob Agents Chemother. 2005;49:963–972. doi: 10.1128/AAC.49.3.963-972.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toenjes KA, Stark BC, Brooks KM, Johnson DI. Inhibitors of cellular signalling are cytotoxic or block the budded-to-hyphal transition in the pathogenic yeast Candida albicans. J Med Microbiol. 2009;58(Pt 6):779–790. doi: 10.1099/jmm.0.006841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weerden NL, Hancock RE, Anderson MA. Permeabilization of fungal hyphae by the plant defensin NaD1 occurs through a cell wall dependent process. J Biol Chem. 2010 doi: 10.1074/jbc.M110.134882. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weerden NL, Lay FT, Anderson MA. The plant defensin, NaD1, enters the cytoplasm of Fusarium oxysporum hyphae. J Biol Chem. 2008;283:14445–14452. doi: 10.1074/jbc.M709867200. [DOI] [PubMed] [Google Scholar]
- Wheeler RT, Kombe D, Agarwala SD, Fink GR. Dynamic, morphotype-specific Candida albicans β-glucan exposure during infection and drug treatment. PLoS Pathog. 2008;4:e1000227. doi: 10.1371/journal.ppat.1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Jiang B, Ketela T, Lemieux S, Veillette K, Martel N, et al. Genome-wide fitness test and mechanism-of-action studies of inhibitory compounds in Candida albicans. PLoS Pathog. 2007;3:e92. doi: 10.1371/journal.ppat.0030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.