Abstract
Objectives
To compare the effects of relaxation practice and other exercise on a multisystem measure of physiologic dysregulation in a national sample of older Taiwanese.
Design
The study was a cross-sectional survey.
Settings/location
The study was conducted in Taiwan.
Subjects
A population-based sample of 1036 adults aged 53 and older completed an in-home interview and in-hospital physical examination. The final model is based on 934 respondents with complete data.
Outcome measures
The outcome measures were overall dysregulation, based on 26 biomarkers, and subscores for cardiovascular/metabolic risk factors and inflammatory markers.
Results
After adjustment for age and sex, overall dysregulation is 0.35 of a standard deviation (SD) lower for practitioners of relaxation techniques compared with nonpractitioners. The effect of exercise is smaller: 0.19 SD difference between those who exercise regularly and those who do not exercise. Similar effects of relaxation practice and regular exercise were found on inflammation, but smaller effects for cardiovascular/metabolic risk factors. In the presence of controls for sociodemographic characteristics, medication use, and a wide range of self-reported and interviewer-assessed health indicators, the effect of relaxation practice is attenuated but remains sizable (-0.19 of a SD for overall dysregulation); regular exercise has a comparable effect (-0.16 of a SD). The effects are similar for the inflammation subscore, but not significant for cardiovascular/metabolic risk factors after adjusting for health status.
Conclusions
The physiologic benefits of relaxation practice that have been demonstrated in small experimental studies are also evident in the general population of older Taiwanese who practice these techniques in everyday life. Relaxation practice is associated with lower levels of physiologic dysregulation, particularly with respect to inflammation. Among this sample of older adults, the effect appears to be at least as large as that for exercise. Older people with limited ability to engage in vigorous exercise may especially welcome such information.
Introduction
Avariety of mind–body practices such as meditation, qigong, t'ai chi, and yoga engage the parasympathetic nervous system to create a feeling of relaxation. Previous studies suggest that these techniques–referred to collectively as “relaxation practice”–can result in an improved physiologic profile. In longitudinal analyses, relaxation practice has been shown to reduce levels of blood pressure1–5; resting heart rate6; total cholesterol4; glucose3,7,8; inflammatory markers such as interleukin-6, C-reactive protein, and tumor necrosis factor α9,10; and stress hormones (i.e., cortisol, epinephrine, and norepinephrine).4,11–13
Allostatic load theory posits that cumulative exposure to stressors can result in dysregulation of multiple interrelated physiologic systems.14,15 Because relaxation practice may reduce reactivity to stressors,16 one might expect it to protect against physiologic dysregulation. Although prior research has examined the effects of relaxation practice on individual biomarkers, no one has explored how such practices affect overall physiologic regulation across multiple systems.
Previous studies of the physiologic benefits of relaxation practice are generally based on very small numbers of practitioners (n ≤25) and selective samples—often clinical populations; none is based on a representative sample of a broad-based population. Virtually all are experimental studies in which participants are offered training in a specific technique and encouraged to practice it regularly over a relatively short period of time, typically 3 months or less. Randomized, experimental/control studies are valuable for demonstrating that the effect is due to the intervention rather than to the characteristics of the individuals. Nonetheless, it is important to determine whether the demonstrated benefits carry over to those in the general population who incorporate relaxation practice into their everyday lives.
In this study, the link between relaxation practice and physiologic regulation in a large, nationally representative sample of older persons in Taiwan is examined. The effect of relaxation practice with other types of exercise is compared. A substantial number of the respondents report practicing a relaxation technique (n=140, 14%); nearly half of these practitioners began more than 5 years before the study, and the majority practice each day. The data include 26 markers of physiologic regulation across multiple systems. In addition to sociodemographic confounders, ther was control for a wide range of self-reported and performance-based health indicators in order to address potential reverse causality (i.e., healthy people may be more likely to practice relaxation techniques).
Materials and Methods
Data
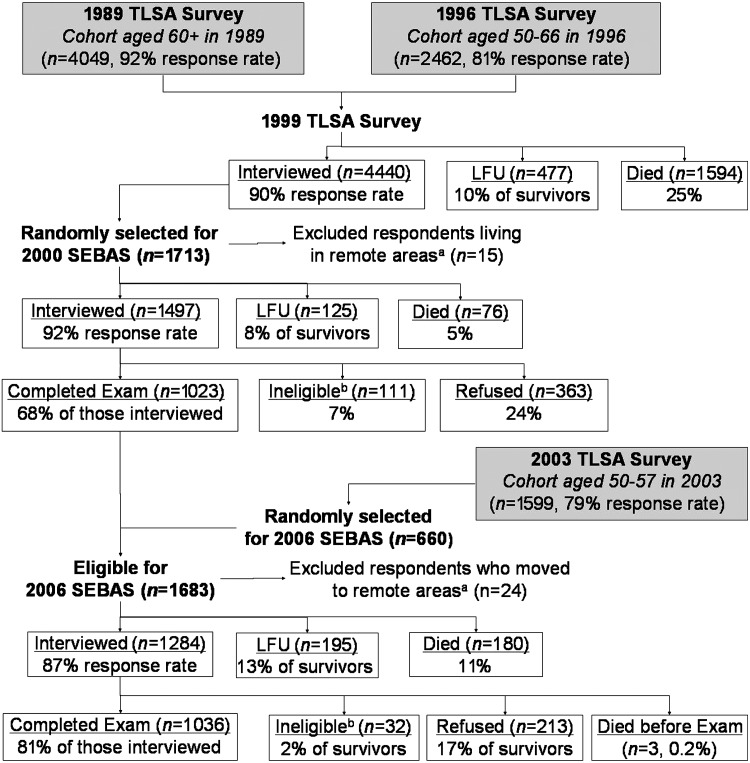
The 2006 wave of the Social Environment and Biomarkers of Aging Study comprised a nationally representative sample of Taiwanese aged 53 and older; persons aged 77 and older and urban residents were oversampled. It was based on a random sample of participants in the ongoing Taiwan Longitudinal Study on Aging, which began in 1989; younger refresher cohorts were added in 1996 and 2003. The 2006 sampling frame included (1) an older cohort (aged 60+) of respondents from the 1999 survey who completed the 2000 medical examination and (2) a younger cohort (aged 53–60) of respondents first interviewed in 2003. Figure 1 gives details regarding response rates and attrition.
FIG. 1.
Attrition among sample selected for Social Environment and Biomarkers of Aging Study (SEBAS), 2000–2006. aA few respondents living in remote areas were excluded from the subsample because they lived too far from the hospitals contracted to do the physical examination portion of the study. bSome respondents were not asked to participate in the hospital examination due to their health condition (i.e., living in an institution, seriously ill, catheter or diaper, kidney dialysis, other health condition that precludes blood drawing). TLSA, Taiwan Longitudinal Study on Aging (sometimes referred to as the Survey of Health and Living Status of the Near Elderly and Elderly in Taiwan); LFU, lost to follow-up.
Among the 1,284 respondents who completed the 2006 interview, 1036 (81%) participated in the physical examination; 3 died before the examination, 32 were not eligible because of a health condition, and 213 declined. Participation in the examination was lower among both the youngest (aged 53–59) and the oldest (80+) respondents, less-educated respondents, and those with limitations in activities of daily living, but, in the presence of controls for age, participation did not differ significantly by self-reported health status.17
The in-home interview included a series of performance-based assessments conducted by trained interviewers. Several weeks after the household interview, participants collected a 12-hour overnight urine sample (7 pm to 7 am), fasted overnight, and visited a nearby hospital the following morning for a physical examination that included collection of a blood specimen and measurements of blood pressure, height, weight, waist, and hip circumference. Compliance was high: 88% fasted overnight, provided an acceptable urine sample, and reported that they collected all of their urine during the collection period. Blood and urine specimens were analyzed at Union Clinical Laboratories in Taipei. Additional details about the study are provided elsewhere.18
Measures
Markers of physiologic dysregulation
A summary measure of physiologic dysregulation is based on 26 biologic markers that have been linked with all cause mortality, including 10 standard cardiovascular/metabolic risk factors, 8 inflammatory markers, and 8 other markers. Systolic and diastolic blood pressure were calculated as the average of the second and third readings, taken at least 20 minutes after arrival at the hospital using a mercury sphygmomanometer with the respondent in a seated position. Measurements of cortisol, epinephrine, and norepinephrine were obtained from the overnight urine specimen, which provided integrated values of basal operating levels during a period when most participants were resting; values were standardized for diuresis by dividing by the level of urinary creatinine. See Table 121 for details regarding assay methods for blood and urine specimens.
Table 1.
Levels Defining High-Risk Quintile for Markers of Physiologic Dysregulation
| |
|
|
|
Levels defined as high risk |
|
|---|---|---|---|---|---|
| Markers | Assay method | Lower DL | CV (%) | Low | High |
| Cardiovascular/metabolic | |||||
| Systolic blood pressure (mm Hg) | ≥147 | ||||
| Diastolic blood pressure (mm Hg) | ≤60 | ≥87 | |||
| Total cholesterol (mmol/L) | Cholesterol oxidase | 0.13 | 1.4 | ≤3.99 | ≥6.45 |
| HDL cholesterol (mmol/L) | Direct surfactant/dextran sulfate | 0.13 | 3.1 | ≤0.96 | |
| Triglycerides (mmol/L) | GPO method | 0.11 | 1.6 | ≥1.76 | |
| Glycosylated hemoglobin (%) | HPLC (Tosoh HCL-723) | 1.0 | 1.1 | ≥6.6 | |
| Fasting glucose (mmol/L) | Glucose oxidase | 0.17 | 1.7 | ≤4.77 | ≥7.88 |
| Body–mass index | ≤20.6 | ≥29.2 | |||
| Waist–hip ratio | ≥0.94 | ||||
| Resting pulse rate (beats/min) | ≥79 | ||||
| Inflammation | |||||
| White blood cell count (x 109/L) | Direct current | 0.02 | 2.3 | ≥7.4 | |
| Neutrophils (%) | Calculated from WBC, EO, BASO, LYMPH, MONO | 3.3 | ≥66.5 | ||
| Interleukin-6 (pg/mL) | ELISA (R&D Systems) | 0.7 | 12.1 | ≥4.31 | |
| C-reactive protein (nmol/L) | Immunoturbidimetry (Roche Cobas Integra 800) | 0.68 | 2.5 | ≥2.67 | |
| Fibrinogen (μmol/L) | Coagulation method (Sysmex CA-1500; reagent: Dade Behring Co.) | 2.35 | 2.5 | ≥11.34 | |
| Soluble ICAM-1 (ng/mL) | ELISA (R&D Systems) | 0.35 | 16.9 | ≥321.3 | |
| Soluble E-selectin (ng/mL) | ELISA (R&D Systems) | 0.1 | 14.3 | ≥55.6 | |
| sIL-6R (ng/mL) | ELISA (R&D Systems) | 0.008 | 10.5 | ≥52.44 | |
| Other markers | |||||
| DHEA-S (μmol/L) | ECLIA (Roche Hitachi Elecsys 2010) | 0.003 | 4.2 | ≤1.18 | |
| Cortisol (mol/mol creatinine) | a | ≥8.52 | |||
| Epinephrine (mol/mol creatinine) | a | ≤1.04 | ≥4.40 | ||
| Norepinephrine (mol/mol creatinine) | a | ≥23.5 | |||
| IGF-1 (nmol/L) | ELISA (Diagnostic System Laboratories) | 2.95 | 7.7 | ≤12.58 | |
| Creatinine clearance (mL/sec) | b | ≤0.8 | |||
| Albumin (g/L) | Bromcresol green (BCG) | 10 | 1.8 | ≤42 | |
| Homocysteine (μmol/L) | FPIA (Abbott IMx) | 0.068 | 41.4 | ≥14.57 | |
Conversion to traditional units: To convert HDL and total cholesterol to mg/dL, divide by 0.0259; triglycerides to mg/dL, divide by 0.0113; glucose to mg/dL, divide by 0.0555; white blood cell count to 103/μL, multiply by 1.0; C-reactive protein to mg/dL, divide by 9.524; fibrinogen to mg/dL, divide by 0.0294; DHEA-S to μg/dL, divide by 0.027; cortisol to μg per g creatinine, divide by 0.312; epinephrine to μg per g creatinine, divide by 0.617; norepinephrine to μg per g creatinine, divide by 0.669; IGF-1 to ng/mL, divide by 0.131; creatinine clearance to mL/min, multiply by 60; albumin to g/dL, divide by 10; homocysteine to mg/L, divide by 7.397.
Assays of urinary cortisol (DL=11.04 nmol/L [4 μg/L]; CV=8.6), epinephrine (DL=10.9 nmol/L (2 μg/L); CV=4.1), and norepinephrine (DL=11.8 nmol/L [2 μg/L]; CV=3.8) were done by HPLC. Urinary creatinine was assayed using the alkaline picrate method (DL=884 μmol/L [10 mg/dL]; CV=1.0).
Estimated based on serum creatinine (SCr), sex, age, and weight using the Cockcroft-Gault Formula.21 SCr was assayed using the alkaline picrate method (DL=0.1 mg/dL; CV=3.3%).
DL, detection limit; CV, coefficient of variation; HDL, high-density lipoprotein; GPO, glycerophosphate oxidase; HPLC, high-pressure liquid chromatography; WBC, white blood cells; EO, eosinophils; BASO, basophils; LYMPH, lymphocytes; MONO, monocytes; ELISA, enzyme-linked immunosorbent assay; ICAM-1, intercellular adhesion molecule-1; sIL6-R, soluble interleukin-6 receptor; DHEA-S, dehydroepiandrosterone sulfate; ECLIA, electrochemiluminescence immunoassay; IGF-1, insulinlike growth factor-1; FPIA, fluorescence polarization immunoassay.
The measure of physiologic dysregulation in this study is a realization of allostatic load with construction similar to previous measures.22–24 For each marker, the high-risk quintile based on the distribution for all respondents is identified (Table 1). If prior evidence suggests that mortality is associated with both high and low values of a given marker, then high risk is defined to include the top and bottom deciles. The summary measure counts the number of biomarkers falling in the high-risk quintile (potential range: 0–26). Similarly, two subscores are created using the 10 markers that represent standard cardiovascular/metabolic risk factors and the 8 markers that reflect inflammation. Because the eight remaining markers relate to a variety of functions and systems, they are not considered as a single group.
Relaxation practice
Respondents were asked whether they “engage in activities that help clear the mind and bring a feeling of calm [English translation]” such as qigong, t'ai chi, meditation, yoga, or other activities similar to qigong (e.g., waitan kung, xianggong, falun kung, yuanji dance). Although some of these practices are also a form of exercise, the survey questions attempted to distinguish between relaxation techniques—where the focus is not so much on the physical activity itself, but rather on calming both mind and body—and other types of exercise. Respondents who report any of the activities listed above are counted as practicing a relaxation technique (14%). In response to this question, some respondents reported other activities in a write-in category: 7% reported an aerobic activity (i.e., walking, mountaineering, swimming, biking, or playing ball games) and 7% reported some other activity (e.g., gardening, calligraphy, reading, and watching television). These activities were not coded as relaxation practice.
Exercise
Following the questions regarding relaxation practice, respondents were asked if they “exercise regularly, apart from the activities we just talked about?” If yes, they were asked about the frequency and duration of such exercise. Respondents were classified as engaging in regular exercise if they reported exercising at least 3 times a week for 30 minutes or more. Those who exercise less frequently or for a shorter duration were categorized as low/moderate exercisers. Thus, for the purposes of this study, the term “exercise” is used to refer to activity other than relaxation practice. Respondents who reported aerobic activity in response to the earlier question about relaxation are counted as exercisers.*
Control variables
A wide range of potential confounders are included that could affect both the physiologic profile and the likelihood of practicing a relaxation technique. Sociodemographic variables include sex, age, urban residence, ethnicity, marital status, educational attainment, income of the respondent and spouse, and employment status. Indicators of medication use include three dummy variables for antihypertensive, lipid-lowering, and diabetic medication. Self-reported measures of health comprise limitations related to activities of daily living and physical mobility, depressive symptoms, and various chronic conditions such as diabetes and heart disease. Interviewer-administered health assessments comprise cognitive function and four physical tests: grip strength (maximum of three trials on each hand), lung function (peak flow, maximum of three trials), timed walk, and timed chair stands (see Table 225,26 for details).
Table 2.
Descriptive Statistics for Covariates According to Relaxation Practice (n=1036)
| Variable | Nonpractitioners (n=896)a | Practitioners (n=140)a |
|---|---|---|
| Sociodemographic characteristics | ||
| Female, no. (%) | 398 (44.4) | 82 (58.6) |
| Age, mean (SD), y | 66.7 (10.1) | 63.8 (9.2) |
| Urban resident, no. (%) | 505 (56.4) | 99 (70.7) |
| Mainlander, no. (%) | 110 (12.3) | 21 (15.0) |
| Married, no. (%) | 667 (74.4) | 111 (79.3) |
| Education, mean (SD), y | 6.5 (4.8) | 8.9 (4.6) |
| Income in 2003, mean (SD), thousands NT$b | 482.2 (790.2) | 672.3 (885.3) |
| Not working/housework/ no formal job, no. (%) | 309 (34.5) | 50 (35.7) |
| Employed, no. (%) | 257 (28.7) | 45 (32.1) |
| Retired, no. (%) | 330 (36.8) | 45 (32.1) |
| Self-reported measures of health | ||
| Takes antihypertensive medication, no. (%) | 278 (31.1) | 38 (27.1) |
| Takes lipid-lowering medication, no. (%) | 63 (7.0) | 3 (2.1) |
| Takes diabetic medication, no. (%) | 138 (15.4) | 10 (7.1) |
| Any ADL limitations, no. (%)c | 74 (8.3) | 5 (3.6) |
| Mobility limitations index, mean (SD)d | 0.5 (1.3) | 0.0 (1.1) |
| CES-D, mean (SD)b,e | 5.0 (5.7) | 3.3 (4.4) |
| Ever had diabetes, no. (%) | 171 (18.0) | 14 (10.0) |
| Other current health conditions: | ||
| Heart disease, no. (%) | 150 (16.7) | 23 (16.4) |
| Lower respiratory disease, no. (%) | 61 (6.8) | 5 (3.6) |
| Arthritis, no. (%) | 137 (15.3) | 21 (15.0) |
| Gastric ulcer or stomach ailment, no. (%) | 130 (14.5) | 14 (10.0) |
| Liver or gallbladder disease, no. (%) | 69 (7.7) | 14 (10.0) |
| Cataracts, no. (%) | 160 (17.9) | 25 (17.9) |
| Kidney disease, no. (%) | 49 (5.5) | 6 (4.3) |
| Gout, no. (%) | 76 (8.5) | 9 (6.4) |
| Spinal/vertebral spur, no. (%) | 88 (9.8) | 14 (10.0) |
| Interviewer-administered health assessments | ||
| Cognitive function, mean (SD) b,f | 16.4 (3.6) | 17.9 (2.3) |
| Unable to perform grip strength test, no. (%)g | 29 (5.2) | 2 (2.9) |
| Grip strength, mean (SD), kgh | 27.7 (10.6) | 27.8 (10.2) |
| Unable to perform peak flow test, no. (%)b,g | 25 (2.8) | 2 (1.4) |
| Peak flow, mean (SD), L/minh | 331.8 (140.2) | 361.2 (132.6) |
| Unable to perform timed walk, no. (%)g,i | 33 (3.7) | 1 (0.7) |
| Normal walking speed, mean (SD), m/sec h,i | 0.84 (0.29) | 0.95 (0.26) |
| Unable to complete chair-stand test, no. (%)g,j | 80 (9.0) | 4 (2.9) |
| Chair-stand speed, mean (SD), stands/sech,j | 0.51 (0.19) | 0.56 (0.18) |
| Exercise | ||
| None, no. (%) | 436 (48.7) | 53 (37.9) |
| Low/moderate, no. (%) | 194 (21.7) | 23 (16.4) |
| Regular, no. (%) | 265 (29.6) | 64 (45.7) |
Unless otherwise specified, missing comprise fewer than 1% of the sample. Statistics are based on cases with valid data.
Missing for 1%-4% of the sample.
Includes respondents who report having any difficulty performing six ADLs without assistance: bathing; dressing/undressing; eating; getting out of bed/standing up/sitting in a chair; moving around the house; and using the toilet.
Based on self-reported difficulty performing 8 physical tasks without assistance: standing for 15 minutes, squatting, reaching over one's head, grasping with fingers, lifting/carrying 11–12 kg, running 20-30 m, walking 200–300 m, and climbing two or three flights of stairs. Each item is coded as follows: 0=no difficulty, 1=some difficulty, 2=great difficulty, 3=unable. On the basis of the validation and recommendations of Long and Pavalko,25 the index was constructed by summing the 8 items, adding a constant (0.5), and taking the logarithm of the result (observed range: −0.7 to 3.2).
Depressive symptoms are measured by a 10-item short form of the full CES-D, coded according to standard practice based on both the number and severity of symptoms (potential range: 0–30; observed range: 0–27).
Score is based on the number of cognitive tasks completed correctly, including basic orientation questions, a series of four subtractions, and immediate memory recall (potential range: 0–24; observed range: 0–23).
The respondent was coded as unable to perform the test if: s/he met the exclusion criteria;19 s/he attempted but was unable to perform the test or stopped because of discomfort, weakness, or frailty; or the respondent/interviewer felt it would be unsafe. The test was coded as missing if the respondent refused, was unable to understand or cooperate with the instructions, the equipment failed, or in the case of the chair-stand test, no suitable chair was available.
Among those able to perform the test.
Respondents were asked to walk 3 m (n=9 walked 2–2.5 m because of space limitations) at their normal speed, using a walking aid if needed. Walking speed was calculated as the recorded distance divided by the completion time for the faster of two trials.
The respondent was asked to stand up and sit down again 5 times in a row as quickly as possible without stopping; an armless, straight-back chair with a hard seat was used. For those able to complete five stands, the completion time was recorded. To adjust for differences in chair height, we regressed the completion time (ci) for individual i on chair height (hi) controlling for the respondent's age and height, separately for men and women.26 The adjusted chair stand completion time is calculated as 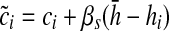 , where βs is the coefficient for chair height (hi) from the sex specific model and
, where βs is the coefficient for chair height (hi) from the sex specific model and 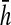 is the mean chair height among the entire sample. Chair-stand speed is computed as 5 divided by the adjusted time
is the mean chair height among the entire sample. Chair-stand speed is computed as 5 divided by the adjusted time 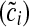 .
.
ADL, Activities of Daily Living; CES-D, Center for Epidemiologic Studies Depression scale; NT$, New Taiwan dollar; SD, standard deviation.
Analytic Strategy
The analyses are based on the sample of subjects who completed the 2006 examination (n=1036). The analyses begin with bivariate analyses that examine how practitioners of relaxation techniques differ from nonpractitioners in terms of sociodemographic characteristics, health status, and physiologic dysregulation.
Next, linear regression is used to model the relationship between relaxation practice and physiologic dysregulation. Outcome variables include the overall summary measure as well as the two subscores for cardiovascular/metabolic risk factors and for inflammation. Prior to fitting the models, each outcome is standardized to have a mean of 0 and SD of 1 so that the magnitude of the coefficients can be compared across outcomes. To account for the clustered sampling design, all models include a random effect for township.
In the first model, the effects of relaxation practice and exercise are investigated, adjusting only for sex, age, and an interaction between sex and age. The measure of overall dysregulation is missing for 8% (n=82) of the sample and 1 person is missing exercise, leaving an analysis sample of 953 for the model of overall dysregulation. For the subscores, the analysis sample is somewhat larger because there are fewer missing values.
The second model adds the remaining sociodemographic variables with the exception of income. Because income was not significant in preliminary models and because a sizeable number of respondents were missing these data, the income variable was dropped from the analysis.
In the third model, measures of medication use and health status were added. Four (4) variables–mobility limitations index, depressive symptoms, cognitive function, and walking speed–were tested in preliminary models, but were not significant for any of the outcomes. Thus, they were omitted from the final model. For this model, another 2% of the sample was lost because of missing data.
Results
There are several important sociodemographic differences between practitioners of relaxation techniques and nonpractitioners. Compared with their counterparts, practitioners are more likely to be female and live in urban areas and, on average, they are younger, better educated, and have higher income (Table 2). Ethnicity, marital status, and employment status are similar between the 2 groups.
Those who practice relaxation appear healthier than nonpractitioners in a variety of ways. This is true not only for self reported measures of medication use, physical functioning, psychologic well-being, diabetes, and most other health conditions, but also for interviewer-administered assessments of cognitive function, lung capacity, and physical mobility (Table 2).
Relaxation practice is also associated with lower levels of physiologic dyregulation (Table 3). Overall, the mean score is 3.8 among practitioners compared with 5.4 for nonpractitioners. That is, practitioners exhibit high risk levels for 1.6 fewer markers (out of 26) than their counterparts. The inflammatory markers account for 44% of that difference, although they represent less than one third (8/26) of all the markers included in the overall measure.
Table 3.
Physiologic Dysregulation According to Relaxation Practice
| Physiologic dysregulation | Nonpractitioner | Practitioner | Difference (95% CI)a |
|---|---|---|---|
| (n=817) | (n=135) | ||
| Overall, mean (SD)b | 5.4 (3.1) | 3.8 (2.6) | 1.6 (1.1, 2.1) |
| (n=876) | (n=139) | ||
| Cardiovascular/metabolic, mean (SD)c | 2.1 (1.6) | 1.6 (1.3) | 0.5 (0.3, 0.8) |
| (n=880) | (n=139) | ||
| Inflammation, mean (SD)d | 1.7 (1.6) | 1.0 (1.3) | 0.7 (0.5, 0.9) |
Two-group mean comparison t-test (two tailed), allowing for unequal variance (Satterthwaite's approximation).
Based on 26 markers (observed range: 0–16).
Based on 10 markers (observed range: 0–8).
Based on 8 markers (observed range: 0–7).
CI, confidence interval; SD, standard deviation.
Compared with relaxation practice, exercise appears to have a smaller effect. The average overall dysregulation score is 4.7 among respondents who exercise regularly versus 5.5 for those who do not exercise (Table 4). Again, the inflammatory markers contribute a disproportionate share of the difference.
Table 4.
Physiologic Dysregulation According to Exercise
| |
|
|
|
Difference, low/moderate – none |
Difference, regular – none |
|---|---|---|---|---|---|
| Physiologic dysregulation | None | Low/moderate | Regular | (95% CI)a | (95% CI)a |
| (n=432) | (n=209) | (n=312) | |||
| Overall, mean (SD)b | 5.5 (3.3) | 5.1 (3.0) | 4.7 (2.9) | 0.4 (-0.1, 0.9) | 0.8 (0.4, 1.2) |
| (n=473) | (n=216) | (n=325) | |||
| Cardiovascular/metabolic, mean (SD)c | 2.1 (1.6) | 1.9 (1.5) | 1.9 (1.6) | 0.2 (0.0, 0.5) | 0.2 (-0.1, 0.4) |
| (n=480) | (n=216) | (n=322) | |||
| Inflammation, mean (SD)d | 1.7 (1.7) | 1.6 (1.6) | 1.3 (1.4) | 0.1 (-0.1, 0.4) | 0.4 (0.2, 0.6) |
Two-group mean comparison t-test (two tailed), allowing for unequal variance (Satterthwaite's approximation).
Based on 26 markers (observed range: 0–16).
Based on 10 markers (observed range: 0–8).
Based on 8 markers (observed range: 0–7).
CI, confidence interval; SD, standard deviation.
Table 5 shows the estimated effects of relaxation practice and exercise on physiologic dysregulation after controlling for potential confounders. Adjusting for age and sex (Model 1), we find that overall dysregulation is 0.35 of a SD lower for practitioners of relaxation techniques compared with nonpractitioners. The effect of exercise is smaller: 0.19 SD difference between those who exercise regularly and those who do not exercise. We find similar effects of relaxation practice and regular exercise on inflammation, but smaller effects for cardiovascular/metabolic risk factors.
Table 5.
Coefficients (95% Confidence Interval) from Linear Models Predicting Physiologic Dysregulationa
| Overall dysregulation | Cardiovascular/metabolic | Inflammation | |
|---|---|---|---|
| Model 1: age and sex only | (n=953) | (n=1014) | (n=1018) |
| No exercise | [reference] | [reference] | [reference] |
| Low/moderate exercise | −0.10 (−0.25, 0.05) | −0.11 (−0.27, −0.05) | −0.07 (−0.23, 0.08) |
| Regular exercise | −0.19 (−0.32, −0.05) | −0.06 (−0.20, 0.08) | −0.21 (−0.35, −0.07) |
| Practices a relaxation technique | −0.35 (−0.52, −0.18) | −0.24 (−0.42, −0.06) | −0.32 (−0.50, −0.15) |
| R2 (for overall model) | 0.16 | 0.04 | 0.07 |
| Model 2: add sociodemographic controlsb | (n=953) | (n=1014) | (n=1018) |
| No exercise | [reference] | [reference] | [reference] |
| Low/moderate exercise | −0.07 (−0.22, 0.08) | −0.10 (−0.26, 0.06) | −0.04 (−0.19, 0.12) |
| Regular exercise | −0.19 (−0.33, −0.06) | −0.09 (−0.23, 0.05) | −0.21 (−0.35, −0.07) |
| Practices a relaxation technique | −0.29 (−0.46, −0.12) | −0.20 (−0.38, −0.02) | −0.26 (−0.44, −0.08) |
| R2 (for overall model) | 0.20 | 0.07 | 0.10 |
| Model 3: add measures of health statusc | (n=934) | (n=991) | (n=995) |
| No exercise | [reference] | [reference] | [reference] |
| Low/moderate exercise | 0.01 (−0.16, 0.13) | −0.08 (−0.20, 0.09) | 0.02 (−0.14, 0.17) |
| Regular exercise | −0.16 (−0.29, −0.03) | −0.05 (−0.19, 0.08) | −0.15 (−0.29, −0.01) |
| Practices a relaxation technique | −0.19 (−0.35, −0.03) | −0.06 (−0.23, 0.10) | −0.20 (−0.37, −0.02) |
| R2 (for overall model) | 0.33 | 0.25 | 0.16 |
Measures of physiologic dysregulation were standardized (mean=0, SD=1) prior to fitting the models so that coefficients can be compared across outcome. All models control for sex, age, age squared, sex*age, and sex*age squared in order to allow for sex differences in the age pattern. To account for the clustered sampling design, a random effect for township is included.
Includes urban, Mainlander, married, education, and employment status (working, retired).
Includes all the measures of health status listed in Table 2 except: mobility limitations, Center for Epidemiologic Studies Depression scale, cognitive function, and walking speed. Because these four variables were not significant in any of the models, they were omitted from the final model. For performance-based physical assessments, those who were unable to perform the test were identified with a dummy variable and the test score was coded to the minimum observed value.
SD, standard deviation.
With the addition of other sociodemographic variables (Model 2), the magnitude of the coefficient for relaxation practice is slightly attenuated, but there is no change in the coefficient for regular exercise. Thus, some of the difference in physiologic profile by relaxation practice results from differences in the sociodemographic characteristics of the two groups rather than from relaxation practice itself. Nonetheless, the effect of relaxation practice remains substantial: −0.29 SD for overall dysregulation.
In Model 3, controls for medication use and a wide range of health status indicators were added. Although the coefficient for relaxation practice is diminished, the effect remains sizeable (about one fifth of a SD) for overall dysregulation as well as for inflammation. Similar, albeit somewhat smaller, effects for regular exercise were found.
This final model represents an overly stringent test because part of the benefits of relaxation practice and exercise may be reflected in health status. People who practice relaxation techniques appear to be healthier than those who do not (Table 2). By controlling for health status, the authors hope to account for pre-existing differences between the 2 groups that could have led to selective use of relaxation practices. Nonetheless, it is possible that these respondents are healthier (and thus, present better physiologic profiles) because of their relaxation practice. Thus, controlling for differences in health status is likely to result in an underestimate of the effect of relaxation practice.
Discussion
The results of this study show that the physiologic benefits of relaxation practice that have been demonstrated in small experimental studies are also evident in the general population among those who practice these techniques in everyday life. It was found that relaxation practice is associated with lower levels of physiologic dysregulation, particularly with respect to inflammation. Among this sample of older adults, the effect appears to be at least as large as that for exercise. Auxiliary analyses (not shown) indicate that the results are robust to the inclusion of a control for smoking status.
In an observational study, where practitioners are self-selected, the possibility must be considered that practitioners exhibit a better physiologic profile not only because of relaxation practice, but also because healthier individuals are more willing or able to engage in relaxation practice. On the other hand, people may adopt relaxation practice in response to stress-related illness. By including extensive controls for health status, our final model in this study imposes a very stringent test. Theoretically, it would be expected that the effect of relaxation practice on physiologic parameters would ultimately lead to better health. Thus, to the extent that differences in health status result from relaxation practice, the benefit is likely to be underestimated. Remarkably, a sizeable effect is found of relaxation practice on physiologic dysregulation even after controlling for a wide range of self-reported and interviewer-assessed health indicators.
Longitudinal data may allow one to determine the temporal sequence. Although the authors have information about health status and physiologic dysregulation in 2000 for the older cohort, the questions about relaxation practice were asked only in 2006. Therefore, practitioners can be identified retrospectively, but this group does not include those who practiced such techniques in the past but discontinued prior to 2006. Using the available longitudinal data, it was found that the increase in physiologic dysregulation between 2000 and 2006 is smaller for those practicing a relaxation technique in 2006 relative to nonpractitioners. A significant difference persists even after controlling for sociodemographic characteristics, medication use in 2000, and self-reported measures of health status in 2000.
Other limitations of this study include possible misreporting, missing data, and limited statistical power. First, this study relies on self-reports of the frequency and duration of exercise and relaxation practice. Measurement error for these variables is likely to result in attenuation bias, and, thus, underestimation of the effect of these practices. Second, as much as 10% of the sample is excluded from some analyses because of missing data. In auxiliary analyses (not shown), multiple imputation was used to reestimate Model 3 (Table 5) for the full sample (n=1036). Whereas the coefficients for relaxation practice remained similar, the effect of regular exercise was slightly larger for models of overall dysregulation and inflammation: comparable with the effects of relaxation practice. Finally, despite the substantial number of practitioners in this sample, the study lacks sufficient power to (1) examine the effects on individual biomarkers; (2) evaluate the effects of frequency and duration of relaxation practice; and (3) determine whether the effects vary by type of practice.
Conclusions
Most people are well aware of the purported health benefits of aerobic activity, yet knowledge of the benefits of relaxation practice appears to be less pervasive. Older people with limited ability to engage in vigorous exercise may especially welcome such information. This study's finding that the effect of relaxation practices is as strong as that of exercise may stem from the older age of this sample. The nature of exercise at these ages may be less intense, and thus, the effect observed may be weaker than corresponding effects at younger ages. In any case, the potential benefits of behavioral factors such as relaxation practice should not be underestimated. If it came in a pill, everyone would take it.
Footnotes
Some respondents (n=60) reported aerobic activity in response to the earlier question about relaxation, but did not report exercise in the subsequent question. If the only activity reported for relaxation was aerobic, then exercise was classified as “low/moderate” or “regular” based on follow-up questions regarding the frequency and duration of those activities. A few respondents (n=9) reported both aerobic and nonaerobic activities for the purposes of relaxation; they were coded as low/moderate exercisers because the frequency and duration of the aerobic exercise could not be determined.
Acknowledgments
Funding for the Taiwan Longitudinal Study on Aging (TLSA) came from the Taiwan Department of Health, the Taiwan National Health Research Institute (grant number DD01-86IX-GR601S), and the Taiwan Provincial Government. The Social Environment and Biomarkers of Aging Study (SEBAS) was funded by the Demography and Epidemiology Unit of the Behavioral and Social Research Program of the National Institute on Aging (grant numbers R01 AG16790, R01 AG16661). The Bureau of Health Promotion (Department of Health, Taiwan) provided additional financial support for SEBAS 2000. This work also received support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number R24HD047879).
We gratefully acknowledge the hard work and dedication of the staff at the Center for Population and Health Survey Research, Bureau of Health Promotion, Taiwan Department of Health. We also thank iSTAT Healthcare consulting; Min-Long Lai and Susana Ong at Union Clinical Laboratory in Taipei; and the contracted hospitals, city/county Bureaus of Health and the health stations of the sampled townships for their assistance.
Disclosure Statement
No competing financial interests exist.
References
- 1.Koh KB. Lee Y. Beyn KM, et al. Counter-stress effects of relaxation on proinflammatory and anti-inflammatory cytokines. Brain Behav Immun. 2008;22:1130–1137. doi: 10.1016/j.bbi.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JW. Liu C. Kryscio RJ. Blood pressure response to transcendental meditation: A meta-analysis. Am J Hypertens. 2008;21:310–316. doi: 10.1038/ajh.2007.65. [DOI] [PubMed] [Google Scholar]
- 3.Paul-Labrador M. Polk D. Dwyer JH, et al. Effects of a randomized controlled trial of transcendental meditation on components of the metabolic syndrome in subjects with coronary heart disease. Arch Intern Med. 2006;166:1218–1224. doi: 10.1001/archinte.166.11.1218. [DOI] [PubMed] [Google Scholar]
- 4.Lee MS. Lee MS. Kim HJ, et al. Qigong reduced blood pressure and catecholamine levels of patients with essential hypertension. Int J Neurosci. 2003;113:1691–1701. doi: 10.1080/00207450390245306. [DOI] [PubMed] [Google Scholar]
- 5.Taylor-Piliae RE. Haskell WL. Froelicher ES. Hemodynamic responses to a community-based Tai Chi exercise intervention in ethnic Chinese adults with cardiovascular disease risk factors. Eur J Cardiovasc Nurs. 2006;5:165–174. doi: 10.1016/j.ejcnurse.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Lee MS. Lee MS. Kim HJ, et al. Effects of qigong on blood pressure, high-density lipoprotein cholesterol and other lipid levels in essential hypertension patients. Int J Neurosci. 2004;114:777–786. doi: 10.1080/00207450490441028. [DOI] [PubMed] [Google Scholar]
- 7.Singh S. Malhotra V. Singh KP, et al. Role of yoga in modifying certain cardiovascular functions in type 2 diabetic patients. J Assoc Physicians India. 2004;52:203–206. [PubMed] [Google Scholar]
- 8.Amita S. Prabhakar S. Manoj I, et al. Effect of yoga-nidra on blood glucose level in diabetic patients. Indian J Physiol Pharmacol. 2009;53:97–101. [PubMed] [Google Scholar]
- 9.Oh B. Butow P. Mullan B, et al. Medical Qigong for cancer patients: Pilot study of impact on quality of life, side effects of treatment and inflammation. Am J Chin Med. 2008;36:459–472. doi: 10.1142/S0192415X08005904. [DOI] [PubMed] [Google Scholar]
- 10.Pullen PR. Nagamia SH. Mehta PK, et al. Effects of yoga on inflammation and exercise capacity in patients with chronic heart failure. J Card Fail. 2008;14:407–413. doi: 10.1016/j.cardfail.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 11.MacLean CR. Walton KG. Wenneberg SR, et al. Effects of the Transcendental Meditation program on adaptive mechanisms: Changes in hormone levels and responses to stress after 4 months of practice. Psychoneuroendocrinology. 1997;22:277–295. doi: 10.1016/s0306-4530(97)00003-6. [DOI] [PubMed] [Google Scholar]
- 12.Curiati JA. Bocchi E. Freire JO, et al. Meditation reduces sympathetic activation and improves the quality of life in elderly patients with optimally treated heart failure: A prospective randomized study. J Altern Complement Med. 2005;11:465–472. doi: 10.1089/acm.2005.11.465. [DOI] [PubMed] [Google Scholar]
- 13.Vadiraja HS. Raghavendra RM. Nagarathna R, et al. Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: A randomized controlled trial. Integr Cancer Ther. 2009;8:37–46. doi: 10.1177/1534735409331456. [DOI] [PubMed] [Google Scholar]
- 14.McEwen BS. Sex, stress and the hippocampus: Allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 15.McEwen BS. Stellar E. Stress and the individual: Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 16.Esch T. Fricchione GL. Stefano GB. The therapeutic use of the relaxation response in stress-related diseases. Med Sci Monit. 2003;9:RA23–RA34. [PubMed] [Google Scholar]
- 17.Goldman N. Glei DA. Lin YH, et al. The serotonin transporter polymorphism (5-HTTLPR): Allelic variation and links with depressive symptoms. Depress Anxiety. 2010;27:260–269. doi: 10.1002/da.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang M. Glei D. Goldman N, et al. The Taiwan Biomarker Project. In: Weinstein M, editor; Vaupel JW, editor; Wachter KW, editor. Biosocial Surveys. Committee on Advances in Collecting and Utilizing Biological Indicators and Genetic Information in Social Science Surveys, Committee on Population, Division of Behavioral and Social Sciences and Education. Washington, DC: The National Academies Press; 2007. pp. 3-1–3-16. [Google Scholar]
- 19.Glei DA. Goldman N. Lin YH. Weinstein M. Age-related changes in biomarkers: Longitudinal data from a population-based sample. Research on Aging. 2011;33(3):312–326. doi: 10.1177/0164027511399105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang MC. Lin HS. Chuang YL, et al. Ann Arbor, MI: Inter-University Consortium for Political and Social Research; Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan, 2000 and 2006: Main documentation for SEBAS longitudinal public use data (released 2012). ICPSR03792-v5. [DOI] [Google Scholar]
- 21.Cockcroft DW. Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Glei DA. Goldman N. Chuang YL, et al. Do chronic stressors lead to physiological dysregulation? Testing the theory of allostatic load. Psychosom Med. 2007;69:769–776. doi: 10.1097/PSY.0b013e318157cba6. [DOI] [PubMed] [Google Scholar]
- 23.Seeman TE. Singer BH. Rowe JW, et al. Price of adaptation: Allostatic load and its health consequences. MacArthur Studies of Successful Aging. Arch Intern Med. 1997;157:2259–2268. [PubMed] [Google Scholar]
- 24.Seplaki CL. Goldman N. Glei D, et al. A comparative analysis of measurement approaches for physiological dysregulation in an older population. Exp Gerontol. 2005;40:438–449. doi: 10.1016/j.exger.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Long JS. Pavalko E. Comparing alternative measures of functional limitation. Med Care. 2004;42:19–27. doi: 10.1097/01.mlr.0000102293.37107.c5. [DOI] [PubMed] [Google Scholar]
- 26.Cornman JC. Glei D. Rodríguez G, et al. Demographic and socioeconomic status differences in perceptions of difficulty with mobility in late life. J Gerontol B Psychol Sci Soc Sci. 2011;66B:237–248. doi: 10.1093/geronb/gbq087. [DOI] [PMC free article] [PubMed] [Google Scholar]