Abstract
Objective
Positron emission tomography imaging studies in cocaine abusers have shown that decreased dopamine release in the striatum following an amphetamine challenge is associated with higher relapse rates. One possible mechanism that might lead to lower amphetamine-induced dopamine release is that fewer dopamine storage vesicles are available in the pre-synaptic terminals for release. Consistent with this hypothesis, postmortem studies have reported a reduction in the VMAT2, the membrane protein that regulates the size of the vesicular dopamine pool, in cocaine abusers relative to healthy subjects. In this study, we used PET and the VMAT2 radioligand [11C]-(+)-dihydrotetrabenazine (DTBZ) to assess the in vivo VMAT2 availability in a group of 12 recently abstinent cocaine abusers and matched healthy controls to confirm the postmortem findings.
Methods
[11C]DTBZ binding potential (BPND) was measured in subjects with kinetic analysis using the arterial input function and the simplified reference-tissue method if arterial input was unavailable.
Results
[11C]DTBZ BPND was significantly reduced by 10% in the limbic striatum, 16.3% in the associative striatum, and 13.4% in the sensori-motor striatum in cocaine abusers compared to controls.
Conclusions
The results of this in vivo PET study confirm previous in vitro reports of lower VMAT2 availability in the striatum of cocaine abusers. It also suggests a compensatory downregulation of the dopamine storage vesicles in response to chronic cocaine abuse and/or a loss of dopaminergic terminals. Further research is necessary to understand the clinical relevance of this observation as to whether it is related to relapse and outcome in abstinent cocaine abusers.
Keywords: [11C]DTBZ, cocaine abusers, VMAT2, PET
INTRODUCTION
Positron emission tomography (PET) imaging studies have demonstrated lower baseline dopamine levels and decreased stimulant (methylphenidate or d-amphetamine)-induced dopamine release in the striatum of chronic cocaine abusers relative to matched healthy controls (1–4). These studies also suggest that lower dopamine release in the ventral striatum in abstinent cocaine abusers is associated with relapse back to cocaine (2, 4). A possible mechanism that leads to lower stimulant-induced dopamine release in cocaine abuse may be related to the loss of dopaminergic terminals from chronic cocaine abuse. As the dopamine transporter is located exclusively in dopaminergic terminals (5), previous studies focused on the differences in dopamine transporter availability to measure dopaminergic terminals in cocaine abusers and controls. Unfortunately, the results of these postmortem and imaging studies that evaluated the dopamine transporter in chronic cocaine abusers were mixed and inconclusive (6). It is likely that the variation in the time since last use of cocaine (i.e., abstinence period) in different patient cohorts led to these discrepant findings (6). This issue posed a major problem to the reliable assessment of the available dopamine transporter in cocaine abusers because the dopamine transporter is highly vulnerable to regulation by drugs such as cocaine that alter synaptic dopamine concentrations (7, 8). This led to the investigation of other markers of the dopaminergic terminals such as the vesicular monoamine transporter, type 2 (VMAT2) in cocaine abusers.
VMAT2 is active in the pre-synaptic vesicular membranes (9) and is involved in the transport of the various monoamines such as serotonin, norepinephrine and dopamine from the cytoplasm to their storage vesicles. While VMAT2 is not specific to the vesicle of one particular monoaminergic terminal, VMAT2 in the striatum has been reported to largely (>95%) represent storage vesicles in the dopaminergic terminals (10). Four postmortem studies have contrasted the VMAT2 density in cocaine abusers and matched healthy controls with radiolabeled derivatives of dihydrotetrabenazine (DTBZ) as they are selective and specific for VMAT2 (11). Three out of these four studies reported a statistically significant decrease (range −12% to −22%) in [3H]DTBZ binding in the striatum of cocaine abusers relative to controls (12–15). As lower VMAT2 binding in cocaine abusers was associated with higher dopamine transporter binding in one of these studies (13), Little and colleagues hypothesized that individuals who had experienced greater exposure to cocaine (as evidenced by a compensatory upregulation of dopamine transporter) lost more dopamine terminals (as evidenced by lower VMAT2) due to toxicity. Lower VMAT2 binding in cocaine abusers, which results from a reduction in the number of dopamine storage vesicles and/or loss of pre-synaptic dopamine terminals is important because it informs the mechanisms that lead to the blunting of stimulant-induced dopamine release in cocaine dependence. As no in vivo studies have confirmed the lower VMAT2 binding reported in the in vitro literature, we studied the impact of chronic cocaine exposure on striatal VMAT2 binding potential (BPND) using PET and [11C]DTBZ in a group of 12 chronic cocaine abusers and 12 healthy controls subjects matched for age, gender, race, and nicotine smoking status.
MATERIALS AND METHODS
Human Subjects
Twenty-four subjects were enrolled in this study who were either cocaine dependent (n=12) or healthy comparison (n=12) subjects. The study was conducted following the approvals of the University of Pittsburgh Institutional Review Board and Radioactive Drug Research Committee. All subjects provided written informed consent. Cocaine abusers were recruited through flyers displayed at local community centers, buses, and addiction medicine clinics. Study criteria for cocaine abusers were [1] males or females between 18 and 60 years old, of all ethnic and racial origins; [2] fulfill DSM-IV criteria for cocaine dependence as assessed by SCID; [3] a positive urine screen for cocaine; [4] no DSM IV Axis I disorder other than cocaine abuse or dependence including abuse or dependence to alcohol or other drugs (nicotine dependence was allowed); [5] no current (as confirmed by urine drug screen at screening) use of opiates, cannabis, sedative-hypnotics, amphetamines, MDMA, and PCP [6] not currently on any prescription or over the counter medications; [7] no current or past severe medical or neurological illnesses (including glaucoma, seizure disorders, a focal finding on MRI such as stroke or tumor) as assessed by a complete medical assessment; [8] not currently pregnant; [9] no history of significant radioactivity exposure (nuclear medicine studies or occupational exposure); [10] no metallic objects in the body that are contraindicated for MRI. All eligible cocaine dependent subjects completed a minimum of twelve days of outpatient abstinence monitored with witnessed urine toxicology (all subjects underwent urine drug screens for cocaine and other recreational drugs 3 times/week for two consecutive weeks). Following the twelve-day outpatient abstinence period subjects were admitted to an inpatient research unit for two days prior to the PET scan. This abstinence monitoring protocol ensured that all subjects were abstinence for a minimum of two weeks prior to the PET scan. Healthy control subjects with no past or present neurological or psychiatric illnesses including substance abuse (confirmed by urine drug screen both at screening and the day of the PET scan) underwent the PET scan as outpatients.
Analysis of PET data
Prior to PET imaging, a spoiled gradient recalled sequence (SPGR) magnetic resonance imaging (MRI) scan was obtained using a GE Signa 1.5 Tesla scanner for determination of regions of interest.
[11C]-(+)-α-dihydrotetrabenazine (DTBZ) was synthesized using the methodology reported previously by Kilbourn, et al. (11). PET imaging sessions were conducted with the ECAT EXACT HR+ camera. Following a 10 minute transmission scan, [11C]DTBZ was injected intravenously over a 45 second period and emission data were collected in the 3D mode for 60 minutes. The scanning duration was based on previous [11C]DTBZ studies in humans that showed time stable (< 5% difference in distribution volume, VT in both the regions of interest and reference) outcome measures at 60 minute with kinetic analysis (16).
Arterial blood samples were collected for derivation of a metabolite corrected arterial input function (30 total samples with 20 over initial 2 minutes; see Figure 1). Following centrifugation of the samples (2 min at 12500 g), plasma was collected and activity measured in 200 μl aliquots on a gamma counter. A subset of samples at 2, 15, 30, 45 and 60 minutes were further processed for determination of the fraction of radioactivity corresponding to the parent compound. Metabolite analysis to determine the percentage of unchanged [11C]DTBZ in the plasma was performed using a solid-phase extraction procedure described previously (16, 17)
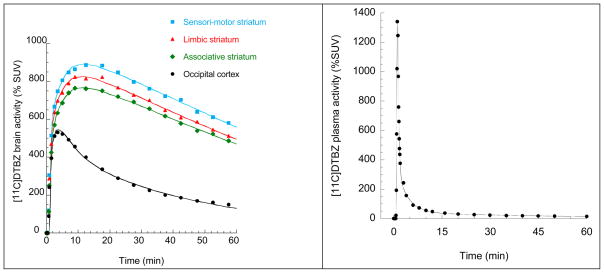
Figure 1.
(Left Panel) [11C]DTBZ brain time activity curves in occipital cortex and functional subdivisions of the striatum. The lines fitted to the data points in the occipital cortex and functional subdivisions of the striatum are from the two-tissue compartment model. (Right Panel) [11C]DTBZ plasma time-activity curve in which data (black circles) shown are measured activities corrected for metabolites, and the line is a three-exponential fit.
All region drawing and image analysis was performed blind to the subject diagnosis with MEDx (Sensor Systems, Inc., Sterling, Virginia) and SPM2 (Statistical parametric mapping). Regions of interest were drawn on the MRI and transferred to the co-registered PET scan. The primary region of interest, the striatum was divided into five anatomical and three functional subdivisions using published criteria (18). The three functional subdivisions of the striatum include the limbic striatum (which, included the ventral striatum), the associative striatum (which, included the precommissural caudate, precommissural putamen and postcommissural caudate) and sensori-motor striatum (which, included the postcommissural putamen). The occipital cortex was used as a reference region (16, 17). Correction for head movement and co-registration of the PET data to the MR were done using methods described in (19)
In this section we describe outcome variables using the consensus nomenclature for in vivo imaging of reversibly binding radioligands (20). Baseline VMAT2 availability was estimated using the PET outcome measure BPND, i.e., binding potential relative to non-displaceable uptake. The lack of arterial input function and plasma free fraction measurements in all subjects precluded us from evaluating two other related PET outcome measurements binding potential relative to total plasma (BPP) and free plasma concentration (BPF) in this dataset. As the concentration of VMAT2 is negligible in the occipital cortex (16, 17), such that only free and nonspecifically bound radiotracer is considered to contribute to VT in the occipital cortex (VT OCC), VT OCC was assumed to be equal to the non-displaceable distribution volume (VND).
If arterial input function was available (n=10 subjects/group), [11C]DTBZ regional distribution volumes (VT, mL of plasma/g of tissue) were derived with kinetic analysis. For this kinetic analysis, a two-tissue compartment (see Figure 1) was used to define both the regions of interest and reference region as previously described in (21). Following, which [11C]DTBZ BPND was derived as
| Eq. 1 |
where fND is the free fraction of radiotracer in brain expressed relative to the non-displaceable concentration, Bavail is the density of VMAT2 available to bind to [11C]DTBZ in vivo and KD is the equilibrium disassociation constant of [11C]DTBZ. If arterial input function was unavailable because of inability to place an arterial line (n =2 subjects/group), [11C]DTBZ BPND was derived using the simplified reference-tissue method as described in (22). Finally, to ensure there was no bias in combining the data from subjects for whom an arterial input function was available and for those for whom the input function was not obtained, all of the available data (n=12/group) were also analyzed without the arterial input function using the simplified reference-tissue method.
Statistical analysis
Group demographic and baseline scan parameter (such as injected dose, mass, plasma clearance, VND) comparisons were performed with unpaired t tests. Group differences in [11C]DTBZ BPND were analyzed with a multivariate analysis of variance (MANOVA) with [11C]DTBZ BPND in the regions of interest (i.e., five anatomical subdivisions) as the dependent measure and diagnostic group as the fixed factor. This primary analysis was followed by contrasts in the individual striatal subdivisions with two tailed unpaired t tests. A false discovery rate (FDR) correction with α = 0.05 was applied to correct for multiple comparisons in the five anatomical subdivisions of the striatum (23). Correlations between VMAT2 availability and clinical variables such as duration and amount of money spent on cocaine abuse in addicts were performed using Pearson Product moment correlation coefficient. A probability value of 0.05 was selected as significance level for all analyses.
RESULTS
Twelve cocaine dependent subjects (4 women and 8 men, mean age 43 ± 8 years) and 12 healthy comparison subjects (4 women and 8 men, mean age 41 ± 8 years) were enrolled in this study. Subjects were matched on both ethnicity (cocaine abusers: 8 African American and 4 Caucasian; healthy controls: 5 African American and 7 Caucasian) and smoking status (7 smokers/group) as best as possible. The cocaine abusers reported smoking crack cocaine on average of 18 ± 7 years and were spending $560 ± 480 weekly.
Scan parameters
Critical PET scan parameters are listed in Table 1. [11C]DTBZ injected dose, specific activity at time of injection, and injected mass did not differ between the groups. No significant between-group differences were observed in the clearance rate of [11C]DTBZ from the plasma compartment, or in [11C]DTBZ occipital cortex distribution volume, VND measure (data available from n=10 subjects/group, in whom arterial line placement was successful).
Table 1.
Scan parameters
| Parameter | Controls | Cocaine abusers | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| Mean | SD | Mean | SD | ||
| Injected dose (mCi) | 10.1 | 1.0 | 10.3 | 0.8 | 0.17 |
| SA (Ci/mmoles) | 2660 | 1340 | 3270 | 1672 | 0.88 |
| Injected Mass (ug) | 1.5 | 1.0 | 1.2 | 0.5 | 0.64 |
| Clearance (L/h)† | 136.9 | 34.9 | 147.6 | 29.9 | 0.36 |
| Occipital VT (mL cm−3)† | 5.30 | 0.44 | 5.56 | 0.66 | 0.32 |
Values are mean and standard deviation (SD), n = 12 per group (unless noted as different)
p-values are from two-tailed, unpaired t tests
n= 10/group
Regional volumes
No significant between-group differences were found in the regions of interest or reference region volumes (Table 2), suggesting lack of measurable volumetric changes in the human striatum after chronic cocaine abuse.
Table 2.
Regional Volumes (denoted in mm3)
| Regions | Controls | Cocaine abusers | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| Mean | SD | Mean | SD | ||
| Occipital cortex | 51835 | 10165 | 52377 | 7615 | 0.88 |
| Ventral striatum | 1826 | 336 | 1708 | 302 | 0.38 |
| Associative striatum | 7723 | 815 | 7606 | 828 | 0.73 |
| Precommissural dorsal caudate | 3436 | 515 | 3577 | 542 | 0.52 |
| Postcommissural caudate | 2024 | 292 | 1886 | 498 | 0.42 |
| Precommissural anterior putamen | 2262 | 407 | 2143 | 441 | 0.50 |
| Postcommissural putamen | 4848 | 1015 | 4844 | 518 | 0.99 |
| Whole striatum | 14396 | 1699 | 14158 | 851 | 0.67 |
Values are mean and standard deviation (SD), n = 12 per group
p-values are from two-tailed, unpaired t tests
Measurement of VMAT2 availability
A multivariate ANOVA performed on the BPND data, which was derived with kinetic analysis in n=10 subjects/group and simplified reference-tissue method analysis in n=2 subjects/group demonstrated that cocaine abusers had significantly lower [11C]DTBZ BPND in the striatal subdivisions relative to healthy controls (effect of diagnosis: F=4.98, df =5, p =0.005).
A multivariate ANOVA performed on the BPND data, which was derived using the simplified reference-tissue method analysis in n=12 subjects/group also demonstrated that cocaine abusers had significantly lower [11C]DTBZ BPND in the striatal subdivisions relative to healthy controls (effect of diagnosis: F=4.52, df =5, p =0.008).
[11C]DTBZ BPND in the striatum and its subdivisions derived using kinetic analysis/simplified reference-tissue method and simplified reference-tissue methods are shown in Tables 3 and 4, respectively. Also included in these tables are the p values from the unpaired t tests that were used to contrast the individual striatal subdivisions. The p-values of the five anatomical subdivisions of the striatum in Tables 3 and 4 remained significant (< 0.05) after applying the false discovery rate correction for multiple comparisons.
Table 3.
Regional [11C]DTBZ binding potential (BPND) in healthy controls and chronic cocaine abusers †
| Functional subdivision | Anatomical subdivision | Controls | Cocaine abusers | Difference | p-value | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | SD | Mean | SD | Mean | |||
| Limbic striatum | Ventral striatum | 2.16 | 0.30 | 1.94 | 0.15 | −10.0% | 0.0399 |
| Associative striatum | 2.34 | 0.20 | 2.03 | 0.16 | −13.4% | 0.0004 | |
| Precommissural dorsal caudate | 2.48 | 0.26 | 2.08 | 0.25 | −16.3% | 0.0007 | |
| Postcommissural caudate | 1.84 | 0.22 | 1.55 | 0.30 | −15.8% | 0.0137 | |
| Precommissural anterior putamen | 2.61 | 0.18 | 2.34 | 0.16 | −10.2% | 0.0011 | |
| Sensori-motor striatum | Postcommissural putamen | 3.03 | 0.24 | 2.68 | 0.22 | −11.5% | 0.0014 |
| Whole striatum | 2.55 | 0.20 | 2.25 | 0.16 | −11.7% | 0.0006 | |
Values are mean and standard deviation (SD), n = 12 per group
Associative striatum values are a weighted average of Precommissural dorsal caudate, Postcommissural caudate, and Precommissural anterior putamen; Whole striatum values are a weighted average of the five anatomical subdivisions
p-values are from two-tailed, unpaired t tests
BPND data as derived using two-tissue compartment kinetic analysis in n=10 subjects/group and using simplified reference-tissue method in n=2 subjects/group who did not receive an arterial line.
Table 4.
Regional [11C]DTBZ binding potential (BPND) in healthy controls and chronic cocaine abusers as derived using the simplified reference-tissue method
| Functional subdivision | Anatomical subdivision | Controls | Cocaine abusers | Difference | p-value | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | SD | Mean | SD | ||||
| Limbic striatum | Ventral striatum | 2.00 | 0.27 | 1.80 | 0.15 | −10.0% | 0.0407 |
| Associative striatum | 2.14 | 0.20 | 1.84 | 0.20 | −13.9% | 0.0012 | |
| Precommissural dorsal caudate | 2.25 | 0.24 | 1.87 | 0.26 | −16.6% | 0.0016 | |
| Postcommissural caudate | 1.66 | 0.20 | 1.39 | 0.29 | −16.1% | 0.0156 | |
| Precommissural anterior putamen | 2.42 | 0.18 | 2.15 | 0.16 | −11.1% | 0.0007 | |
| Sensori-motor striatum | Postcommissural putamen | 2.74 | 0.21 | 2.44 | 0.20 | −11.3% | 0.0013 |
| Whole striatum | 2.32 | 0.19 | 2.04 | 0.17 | −12.2% | 0.0010 | |
Values are mean and standard deviation (SD), n = 12 per group
Associative striatum values are a weighted average of Precommissural dorsal caudate, Postcommissural caudate, and Precommissural anterior putamen; Whole striatum values are a weighted average of the five anatomical subdivisions
p-values are from two-tailed, unpaired t tests
Age-corrected correlation analyses revealed no significant associations between VMAT2 availability in the striatum and the duration in years (r = − 0.42, p = 0.20), or amount in dollars (r = 0.26, p = 0.43) of cocaine use. No significant associations were noted when the same correlations were performed using VMAT2 availability in the functional or anatomical subdivisions of the striatum.
DISCUSSION
In this human imaging study, we investigated VMAT2 availability in a group of subjects who regularly abused cocaine for nearly two decades and confirmed lower VMAT2 availability (10 to 16%) relative to matched healthy controls. The results of this study are in agreement with three out of four studies that have previously evaluated this issue in postmortem brain tissue and reported a comparable decrease in VMAT2 (12 to 22%) after chronic cocaine abuse. They are also in agreement with one (24), but not another [11C]DTBZ PET study that has evaluated this issue in methamphetamine abusers (25). The study by Boileau et al (25), which reported a 10–22% increase in VMAT2 binding in the subdivisions of the striatum in methamphetamine abusers is inconsistent with both the results of a previous study by Johannson et al (24) in methamphetamine abusers and this study in cocaine abusers showing a decrease in VMAT2. A possible reason for the paradoxical increase in VMAT2 in methamphetamine dependence reported by Boileau et al is related to the scanning of a relatively large proportion of the methamphetamine abusers shortly after the cessation of drug use (8/14 methamphetamine dependent subjects tested positive for methamphetamine and/or cocaine on the day of the PET scan) as opposed to a longer period of abstinence. Thus, it is likely that the evaluation of VMAT2 binding in their study was influenced by methamphetamine-induced transient alterations in dopamine concentration as demonstrated by these authors in follow-up investigations (26, 27). In our study, and the study by Johansson et al (24), the interaction between cocaine (or methamphetamine)-induced transient alterations in dopamine and VMAT2 were less of an issue as all subjects were abstinent from drugs for a minimum of 14-days prior to the PET scan. Nevertheless, as shown in Figure 2, data from this study indicates an overlap in [11C] DTBZ BPND between the cocaine abusers and controls, which may be due to various demographic and clinical factors such as social status, the duration and amount of cocaine abused, or genetic polymorphisms—all of which needs to be investigated in a larger cohort.
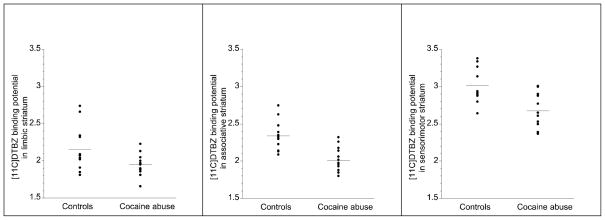
Figure 2.
Distribution of [11C]DTBZ BPND in limbic, associative and sensori-motor striatum in healthy controls and patients with cocaine dependence (The horizontal lines in each graph represent the mean values). Cocaine abusers displayed lower VMAT2 availability compared to controls in all three subdivisions of the striatum (limbic striatum, p =0.0399; associative striatum, p =0.0004; sensori-motor striatum, p = 0.0014)
It is not possible to ascertain from this study whether the lower VMAT2 in cocaine addicts reflects a compensatory downregulation of pre-synaptic dopamine storage vesicles or a loss of dopaminergic terminals or a combination of both. A downregulation of vesicular dopamine stores may suggest a compensatory mechanism that counteracts the repeated release of dopamine into the synapse that is caused by chronic cocaine abuse. Alternatively, the loss of dopamine terminals suggests a more permanent cocaine-induced neurotoxicity that leads to reduced dopamine release. Future studies in cocaine addicts are necessary to determine whether there is recovery of [11C]DTBZ binding potential to the levels observed in healthy controls with more prolonged duration of abstinence such as six to twelve months. Such a recovery would indicate a compensatory mechanism as opposed to neurotoxicity of chronic cocaine abuse in humans.
The blunting of stimulant-induced dopamine release in cocaine abusers is one of the most robust and replicated findings in the addiction imaging literature (1, 2, 4, 28). As more recent studies in the laboratory and clinical settings also suggest that this phenomenon is associated with relapse back to cocaine in addicts it is critical to understand the mechanisms that contribute to lower dopamine release in advancing therapeutics for addictive disorders (2, 4). Two sites have been identified and discussed in the literature with respect to the mechanism of action of amphetamines (and to a lesser extent other stimulants such as methylphenidate): vesicular depletion, the process by which amphetamine displaces dopamine from secretory vesicles into the neuronal cytoplasm (29) and reverse transport, the process by which cytoplasmic dopamine is released into the extracellular space by outward transport by the dopamine transporter (30). The relative importance of these two processes, vesicular depletion and reverse transport with respect to the amount of dopamine released following an acute amphetamine challenge has been debated extensively in the literature(31–33). Nevertheless, pre-clinical studies clearly show the number (Bmax) of dopamine storage vesicles and dopamine transporters impact the amount of dopamine released following an acute amphetamine challenge (34, 35). Based on these data we hypothesized that a decrease in available dopamine storage vesicles and/or dopamine transporters following chronic cocaine leads to blunting of amphetamine-induced dopamine release in cocaine dependence. In a review of the literature on this topic we found little evidence to support a decrease in dopamine transporter availability in cocaine abusers (6). Seven of the ten postmortem studies that contrasted the dopamine transporter binding in cocaine abusers and controls supported an increase, two studies no change and one study a decrease (reviewed in Table IV in 6). The in vivo PET studies that had evaluated this issue were also split --with one suggesting a modest upregulation of dopamine transporter during early withdrawal (< 96 hours) (36) and the other suggesting no change following three to twelve weeks of abstinence (37). In contrast to the data on the dopamine transporter, the in vitro literature and this PET study support decreased VMAT availability in cocaine abusers. As VMAT2 both directly regulates the size of the vesicular dopamine pool and indirectly influences the amount of dopamine that is available in the cytosol for carrier-mediated dopamine release (reverse transport of dopamine transporter), it is likely that lower VMAT2 leads to reduced stimulant-induced dopamine release in cocaine addicts. Future studies should attempt to understand the clinical relevance of lower VMAT2 as to whether this is related to relapse and outcome in abstinent cocaine abusers.
Finally, in contrast to the postmortem and imaging data in cocaine abusers, no such lowering in [3H]DTBZ binding is evidenced in chronic cocaine treated rodents (38, 39). This discrepancy in VMAT2 binding between the chronic cocaine human and rodent data may explain the paradoxical findings observed with regards to acute amphetamine challenge across species. In human cocaine abusers, decreased dopamine release is observed, whereas studies in rodents repeatedly exposed to cocaine demonstrate increased dopamine release following an acute amphetamine challenge (termed sensitization, reviewed in 6). These data suggest that perhaps chronic and repeated exposure to cocaine leads to loss or death of dopaminergic nerve terminals in humans, but not in rodents, which are typically exposed to cocaine for a relatively shorter duration of time (weeks in rodents compared to decades in humans) in the laboratory. Future investigations need to investigate whether relatively modest reductions in presynaptic dopamine nerve terminals (measured as 10 to 20% reduction in VMAT2 binding) lead to profound reductions (60 to 90%) in stimulant-induced dopamine release as observed in cocaine abusing humans relative to healthy controls (2).
In conclusion, we found that repeated exposure to cocaine is associated with lower VMAT2 availability in the striatum in cocaine abusers. This reduction in striatal VMAT2 that leads to lower vesicular dopamine in the pre-synaptic terminals may be one of the several mechanisms that contributes to the reduced dopamine release in the brain’s reward circuit and thereby drives relapse in cocaine addicts. Further research is necessary to understand the clinical significance of this finding in a much larger sample of cocaine dependent subjects.
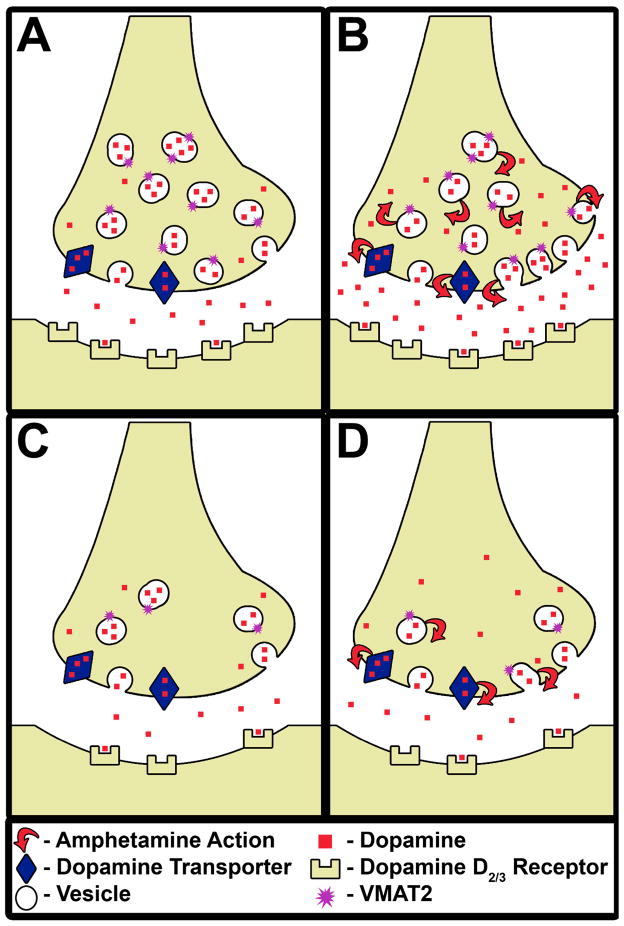
Figure 3.
Illustrates the mechanisms that contribute to less (or blunted) amphetamine-induced dopamine release in cocaine abusers (Panels C and D) relative to healthy controls (Panels A and B). Cocaine abusers (Panel C) have [1] fewer vesicular monoamine transporters [2] comparable number of dopamine transporters and [3] fewer dopamine D2/3 receptors relative to healthy controls (Panel A) at baseline. Following, an acute amphetamine challenge (the red arrows show the mechanisms by which amphetamine releases dopamine via reverse transport at the dopamine transporter and depletion of the vesicular dopamine stores) less dopamine is released in cocaine abusers (Panel D) relative to healthy controls (Panel B), which may be due to a reduction in the number of pre-synaptic dopamine storage vesicles (chronic cocaine-induced adaptation) and/or terminals (chronic cocaine-induced toxicity). It is possible that either or both these possibilities is reflected as lower VMAT2 binding in cocaine abusers relative to healthy controls as observed in this study.
Table 5.
Glossary table for technical terms used in the manuscript
| Terms | Definition |
|---|---|
| dopaminergic terminal | pre-synaptic axons that contains dopaminergic vesicles |
| vesicular membrane | membranous vesicles that encapsulate and store neurotransmitters within the cytoplasm of pre-synaptic axons |
| vesicular dopamine | dopamine that is sequestered in a vesicular membrane |
| vesicular monoamine transporter, type 2 (VMAT2) availability | VMAT2 that is available for binding to [11C]DTBZ |
| dopamine transporter availability | dopamine transporter that is available for binding to its radiotracer |
| arterial input function | concentration of radiotracer in blood as a function of time |
| plasma free fraction | the fraction of the radioligand that is not bound to plasma proteins at equilibrium |
| kinetic analysis | an analysis that derives information about neuroreceptor PET outcome measures by estimating the rate constants governing transfer between the plasma and brain compartments (requires arterial blood sampling). |
| reference tissue method | an analysis that derives information about neuroreceptor PET outcome measures when blood sampling is not possible |
Acknowledgments
The project described was supported by Award Number R03DA024704 from the National Institute On Drug Abuse (NIDA) under the American Reinvestment and Recovery Act of 2009 (ARRA) and CTSA-UL1 RR024153 from the National Center for Research Resources (NCRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Drug Abuse or the National Institutes of Health.
Footnotes
DISCLOSURES
The authors report no competing interests
References
- 1.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–3. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 2.Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164(4):622–9. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- 3.Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R, Slifstein M, Van Heertum R, Kleber HD. Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am J Psychiatry. 2009;166(10):1170–7. doi: 10.1176/appi.ajp.2009.08121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez D, Carpenter KM, Liu F, MS, Broft A, Friedman AC, Kumar D, Van Huertum R, Kleber H, Nunes E. Imaging dopamine transmision in cocaine dependence: response to treatment linked to neurochemistry. Am J Psychiatry. 2011 doi: 10.1176/appi.ajp.2010.10050748. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996;16(2):436–47. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62(11):851–69. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- 7.Vander Borght T, Kilbourn M, Desmond T, Kuhl D, Frey K. The vesicular monoamine transporter is not regulated by dopaminergic drug treatments. Eur J Pharmacol. 1995;294(2–3):577–83. doi: 10.1016/0014-2999(95)00594-3. [DOI] [PubMed] [Google Scholar]
- 8.Kilbourn MR, Frey KA, Vander Borght T, Sherman PS. Effects of dopaminergic drug treatments on in vivo radioligand binding to brain vesicular monoamine transporters. Nucl Med Biol. 1996;23(4):467–71. doi: 10.1016/0969-8051(96)00023-6. [DOI] [PubMed] [Google Scholar]
- 9.Henry JP, Scherman D. Radioligands of the vesicular monoamine transporter and their use as markers of monoamine storage vesicles. Biochem Pharmacol. 1989;38(15):2395–404. doi: 10.1016/0006-2952(89)90082-8. [DOI] [PubMed] [Google Scholar]
- 10.Kish SJ, Robitaille Y, el-Awar M, Clark B, Schut L, Ball MJ, Young LT, Currier R, Shannak K. Striatal monoamine neurotransmitters and metabolites in dominantly inherited olivopontocerebellar atrophy. Neurology. 1992;42(8):1573–7. doi: 10.1212/wnl.42.8.1573. [DOI] [PubMed] [Google Scholar]
- 11.Kilbourn M, Lee L, Vander Borght T, Jewett D, Frey K. Binding of alpha-dihydrotetrabenazine to the vesicular monoamine transporter is stereospecific. Eur J Pharmacol. 1995;278(3):249–52. doi: 10.1016/0014-2999(95)00162-e. [DOI] [PubMed] [Google Scholar]
- 12.Little KY, Zhang L, Desmond T, Frey KA, Dalack GW, Cassin BJ. Striatal dopaminergic abnormalities in human cocaine users. Am J Psychiatry. 1999;156(2):238–45. doi: 10.1176/ajp.156.2.238. [DOI] [PubMed] [Google Scholar]
- 13.Little KY, Krolewski DM, Zhang L, Cassin BJ. Loss of striatal vesicular monoamine transporter protein (VMAT2) in human cocaine users. Am J Psychiatry. 2003;160(1):47–55. doi: 10.1176/appi.ajp.160.1.47. [DOI] [PubMed] [Google Scholar]
- 14.Wilson JM, Levey AI, Bergeron C, Kalasinsky K, Ang L, Peretti F, Adams VI, Smialek J, Anderson WR, Shannak K, Deck J, Niznik HB, Kish SJ. Striatal dopamine, dopamine transporter, and vesicular monoamine transporter in chronic cocaine users. Ann Neurol. 1996;40(3):428–39. doi: 10.1002/ana.410400312. [DOI] [PubMed] [Google Scholar]
- 15.Staley JK, Talbot JZ, Ciliax BJ, Miller GW, Levey AI, Kung MP, Kung HF, Mash DC. Radioligand binding and immunoautoradiographic evidence for a lack of toxicity to dopaminergic nerve terminals in human cocaine overdose victims. Brain Res. 1997;747(2):219–29. doi: 10.1016/s0006-8993(96)01196-1. [DOI] [PubMed] [Google Scholar]
- 16.Koeppe RA, Frey KA, Kume A, Albin R, Kilbourn MR, Kuhl DE. Equilibrium versus compartmental analysis for assessment of the vesicular monoamine transporter using (+)-alpha-[C-11]dihydrotetrabenazine (DTBZ) and positron emission tomography. J Cereb Blood Flow Metab. 1997;17(9):919–31. doi: 10.1097/00004647-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Chan GL, Holden JE, Stoessl AJ, Samii A, Doudet DJ, Dobko T, Morrison KS, Adam M, Schulzer M, Calne DB, Ruth TJ. Reproducibility studies with 11C-DTBZ, a monoamine vesicular transporter inhibitor in healthy human subjects. J Nucl Med. 1999;40(2):283–9. [PubMed] [Google Scholar]
- 18.Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23(3):285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 19.Narendran R, Mason NS, Laymon C, Lopresti B, Velasquez N, May M, Kendro S, Martinez D, Mathis C, Frankle G. A comparative evaluation of the dopamine D2/3 agonist radiotracer [11C]NPA and antagonist [11C]raclopride to measure amphetamine-induced dopamine release in the human striatum. Journal of Pharmacology and Experimental Therapeutics. 2010;63(7):574–84. doi: 10.1124/jpet.109.163501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–9. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 21.Koeppe RA, Frey KA, Vander Borght TM, Karlamangla A, Jewett DM, Lee LC, Kilbourn MR, Kuhl DE. Kinetic evaluation of [11C]dihydrotetrabenazine by dynamic PET: measurement of vesicular monoamine transporter. J Cereb Blood Flow Metab. 1996;16(6):1288–99. doi: 10.1097/00004647-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 22.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4(3 Pt 1):153–8. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;(57):289–300. [Google Scholar]
- 24.Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, Schuster CR. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology. 2006;185(3):327–38. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- 25.Boileau I, Rusjan P, Houle S, Wilkins D, Tong J, Selby P, Guttman M, Saint-Cyr JA, Wilson AA, Kish SJ. Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: Is VMAT2 a stable dopamine neuron biomarker? J Neurosci. 2008;28(39):9850–6. doi: 10.1523/JNEUROSCI.3008-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong J, Wilson AA, Boileau I, Houle S, Kish SJ. Dopamine modulating drugs influence striatal (+)-[11C]DTBZ binding in rats: VMAT2 binding is sensitive to changes in vesicular dopamine concentration. Synapse. 2008;62(11):873–6. doi: 10.1002/syn.20573. [DOI] [PubMed] [Google Scholar]
- 27.Boileau I, Houle S, Rusjan PM, Furukawa Y, Wilkins D, Tong J, Selby P, Wilson AA, Kish SJ. Influence of a low dose of amphetamine on vesicular monoamine transporter binding: a PET (+)[11C]DTBZ study in humans. Synapse. 2010;64(6):417–20. doi: 10.1002/syn.20743. [DOI] [PubMed] [Google Scholar]
- 28.Malison RT, Mechanic KY, Klummp H, Baldwin R, Kosten TR, Seibyl JP. Reduced amphetamine-stimulated dopamine release in cocaine addicts as measured by [123I]IBZM SPECT. J Nucl Med. 1999;40(5 Suppl):110P. [Google Scholar]
- 29.Sulzer D, Rayport S. Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action. Neuron. 1990;5(6):797–808. doi: 10.1016/0896-6273(90)90339-h. [DOI] [PubMed] [Google Scholar]
- 30.Liang NY, Rutledge CO. Evidence for carrier-mediated efflux of dopamine from corpus striatum. Biochem Pharmacol. 1982;31(15):2479–84. doi: 10.1016/0006-2952(82)90057-0. [DOI] [PubMed] [Google Scholar]
- 31.Sulzer D, Pothos E, Sung HM, Maidment NT, Hoebel BG, Rayport S. Weak base model of amphetamine action. Ann N Y Acad Sci. 1992;654:525–8. doi: 10.1111/j.1749-6632.1992.tb26020.x. [DOI] [PubMed] [Google Scholar]
- 32.Sulzer D, Maidment NT, Rayport S. Amphetamine and other weak bases act to promote reverse transport of dopamine in ventral midbrain neurons. J Neurochem. 1993;60(2):527–35. doi: 10.1111/j.1471-4159.1993.tb03181.x. [DOI] [PubMed] [Google Scholar]
- 33.Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–77. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- 34.Patel J, Mooslehner KA, Chan PM, Emson PC, Stamford JA. Presynaptic control of striatal dopamine neurotransmission in adult vesicular monoamine transporter 2 (VMAT2) mutant mice. J Neurochem. 2003;85(4):898–910. doi: 10.1046/j.1471-4159.2003.01732.x. [DOI] [PubMed] [Google Scholar]
- 35.Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18(6):1979–86. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malison RT, Best SE, van Dyck CH, McCance EF, Wallace EA, Laruelle M, Baldwin RM, Seibyl JP, Price LH, Kosten TR, Innis RB. Elevated striatal dopamine transporters during acute cocaine abstinence as measured by [123I] beta-CIT SPECT. Am J Psychiatry. 1998;155(6):832–4. doi: 10.1176/ajp.155.6.832. [DOI] [PubMed] [Google Scholar]
- 37.Volkow ND, Fowler JS, Logan J, Wang GJ, Hitzerman R, MacGregor R, Dewey SL, Wolf AP. Decreased binding of 11-C cocaine in the brain of cocaine addicts. J Nucl Med. 1992;33:888. [Google Scholar]
- 38.Wilson JM, Kish SJ. The vesicular monoamine transporter, in contrast to the dopamine transporter, is not altered by chronic cocaine self-administration in the rat. J Neurosci. 1996;16(10):3507–10. doi: 10.1523/JNEUROSCI.16-10-03507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boulay D, Duterte-Boucher D, Leroux-Nicollet I, Naudon L, Costentin J. Locomotor sensitization and decrease in [3H]mazindol binding to the dopamine transporter in the nucleus accumbens are delayed after chronic treatments by GBR12783 or cocaine. J Pharmacol Exp Ther. 1996;278(1):330–7. [PubMed] [Google Scholar]