Abstract
Background
Insufficient clearance of apoptotic cells leads to increased inflammation and exaggerated organ injury. The opsonizing protein, milk fat globule EGF-factor 8 (MFG-E8), upregulates apoptotic cell clearance. The purpose of this study was to determine the degree of apoptotic cell clearance, and if inflammation, organ injury and survival are improved following treatment with recombinant human MFG-E8 (rhMFG-E8) after hemorrhagic shock.
Methods
Male mice underwent a pressure-controlled (25±5 mmHg) model of hemorrhagic shock for 90 min. They were resuscitated with normal saline with or without rhMFG-E8 over 30 min. At 3.5 h post-resuscitation, blood and tissue were collected. MFG-E8 levels in the plasma, lungs and spleen were measured. Apoptotic cell clearance was measured by cleaved caspase-3 levels and TUNEL staining. Neutrophil infiltration was assessed using myeloperoxidase activity in the lungs and spleen. Plasma and tissue levels of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) were measured by ELISA. Finally, a 7-day survival study was also conducted.
Results
MFG-E8 levels in the plasma, lungs and spleen significantly decreased by 33%, 44%, and 55%, respectively, at 3.5 h following hemorrhage and resuscitation. Treatment with rhMFG-E8 significantly improved apoptosis, by reducing TUNEL+ cells after treatment and restoring cleaved caspase-3 expression back to baseline. Neutrophil infiltration was blunted by 29% and 41% in the lungs and spleen, respectively. Cytokine expression was also reduced significantly, by 64–73% in plasma, 24–58% in the lungs and 49–76% in the spleen. Finally, animals demonstrated a superior survival rate over 7 days after treatment with rhMFG-E8.
Conclusion
The administration of rhMFG-E8 is a potent treatment in animals following hemorrhagic shock.
INTRODUCTION
Hemorrhage following trauma is a major cause of civilian and military deaths worldwide1. Due to the nature of the injury and the lack of therapeutic options, these patients are at a higher risk for multiple organ failure and death2. This relationship between hemorrhage and outcomes has been recognized for over 30 years3. Current standard of care utilizes a multi-disciplinary team-based approach focusing on source control, volume replacement, whole blood/component therapy, and prompt surgical intervention4. Despite our best efforts, mortality rates approach 50% for severe hemorrhage5.
One of the difficulties in improving outcomes after hemorrhagic shock is that the overwhelming number of protocols, guidelines, and preferences encountered from surgeon to surgeon prevent the usage of a universal approach. In addition to the variance, there is no therapeutic agent proven to reduce injury and increase survival4. Hemorrhagic shock induces a surge of inflammatory markers, including cytokines interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), which have been shown to increase mortality6. Furthermore, a prolonged and severe hemorrhagic state lends itself to regions of organ hypoxia and cell-death, thereby creating a pool of apoptotic cells7. If these apoptotic cells are not cleared, they will likely undergo a secondary necrosis and spill harmful toxins, worsening hemorrhagic shock8.
Milk fat globule-EGF factor 8 (MFG-E8) was originally identified as a component of milk, and is secreted from the mammary epithelia during lactation9. MFG-E8 is released by activated macrophages and immature dendritic cells in response to systemic injury or stress10. It has been shown to be a “bridging” molecule between professional phagocytes and apoptotic cells, by binding to specific regions on both cell populations11. We have previously demonstrated that the administration of recombinant murine MFG-E8 (rmMFG-E8) successfully attenuated multiple markers of organ injury during severe sepsis, as well as increasing apoptotic cell clearance and lessening acute lung injury after intestinal ischemia and reperfusion12–16. Recently, we used our recombinant human analogue of MFG-E8 (rhMFG-E8), and confirmed that it also provides multiple beneficial effects in severe sepsis17,18. Our goal in this study was to evaluate rhMFG-E8 as a therapeutic option during hemorrhagic shock, by demonstrating an attenuation of organ specific markers, cytokines, and increasing apoptotic cell clearance and survival.
MATERIALS AND METHODS
Experimental animals
Male C57/BL6 mice (20–25g), purchased from Taconic (Germantown, NY), were used for this study. The Institutional Animal Care and Use Committee (IACUC) of The Feinstein Institute approved all procedures and protocols for medical research. Animal experimentation was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources).
Animal Model of Hemorrhagic Shock and Administration of rhMFG-E8
A model of hemorrhagic shock was conducted as previously described19. Animals were kept on a 12-hour light-dark cycle with free access to food and water in a temperature-controlled room. The mice were fasted overnight but allowed water ad libitum before surgery. Animals were randomly assigned to either a Sham, Vehicle or rhMFG-E8 treatment group (n=9–11 animals/group). Vascular access was obtained via the femoral vessels. A length of PE-10 polyethylene tubing (Becton Dickinson and Company, Sparks, MD) was heparinized and introduced into the femoral arteries, after dissection from the femoral nerves. A digital blood pressure analyzer (Micro-Med, Louisville, KY) was used to monitor pressure continuously through a left femoral artery cannula. Hemorrhagic shock was induced by withdrawing 0.5ml of blood volume from each mouse through the right femoral arterial cannula over a 5 minute period, until the mean arterial pressure (MAP) stabilized at 25±5 mmHg. The MAP was maintained for 90 minutes by the withdrawal and administration of blood as needed. MAP was recorded at 5 minute intervals. Resuscitation was done immediately following hemorrhage, by infusing 1ml (2X of their shed blood volume) of normal saline over 30 minutes via the right femoral artery. The treatment group was given rhMFG-E8 (0.02 ug/g mouse body weight [BW]) mixed in 1ml of normal saline 95 minutes after the initiation of hemorrhage. Animals that received rhMFG-E8 did not receive additional fluid during treatment. Blood, plasma, and tissue were collected after 3.5 hours after resuscitation and stored at −80°C for later analysis. Sham animals were anesthitized, but not cannulated.
Source of rhMFG-E8
Human MGF-E8 is a 387-amino acid (aa) precursor that contains a 23-aa signal sequence and a 364-aa mature region (SwissProt # Q08431). The mature region of 1095 basepair fragment of human MFG-E8 cDNA generated by polymerase chain reaction was cloned into a bacterial expression vector. The Ex-M0438-B01 expression clone containing the 6×His-human MFG-E8 (R24-R387, i.e., the mature region) was purchased from GeneCopoeia, Rockville, MD, expressed and purified in our lab. The recombinant protein used in the study is greater than 99% pure, identified as human MFG-E8 with 95% confidence, and was rendered endotoxin free using Triton-X-114 treatment18.
MFG-E8 Levels
2μl of murine plasma (1:10 dilution) and 25μg of protein from the lungs and spleen were fractionated on a Bis-Tris gel and transferred to a 0.45-μm nitrocellulose membrane. Blots were blocked with 5% BSA in Tris-buffered saline containing 0.1% Tween 20 and incubated with hamster anti-mouse MFG-E8 antibody (1:4000) (clone 2422, MBL, Nagoya, Japan). After incubation with HRP-labeled goat-anti hamster IgG (Santa Cruz, CA) in 5% BSA-TBST, blots were washed with TBST and bands were detected using a chemiluminescent peroxidase substrate (ECLplus, Amersham, Little Chalfont, Buckinghamshire, UK) and exposed on a radiographic film. The serum level of MFG-E8 (Optical Density/mm2) were determined using a Bio-Rad Laboratories Imaging System (Hercules, CA). For the lungs and spleen, a MFG-E8/β-actin ratio was used.
Measurement of Neutrophil Activity
Neutrophil accumulation in the lungs and spleen was estimated using the myeloperoxidase (MPO) activity assay as described previously20. Briefly, 100 mg of tissue were suspended in 1 mL of 0.5% hexadecyltrimethylammonium bromide in 50 mmol/L phosphate buffer (pH 6.0), and sonicated for 90 seconds on ice. Homogenates were cleared by centrifuging at 13,400g for 10 min at 4°C, and the protein concentration of supernatants was determined by using Bio-Rad DC Protein Assay Kit (Bio-Rad, Hercules, CA, USA). The reaction was carried out in a 96-well plate by adding 290μL of 50 mmol/L phosphate buffer with 3μL substrate solution (containing 20 mg/mL o-dianisidine hydro chloride), and 3μL H2O2 (20 mmol/L). Sample (10μL) was added to each well to start the reaction. Plates were read spectrophotometrically at 460nm for 3 minutes on a CERES UV 900C microplate reader (Bio-Tek Inc., Winooski, VT, USA). MPO activity (1 unit defined as change in absorbance of 1 per min) was expressed as units per gram of tissue.
Measurement of Cytokines IL-1β, IL-6 and TNF-α
The pulmonary and splenic tissues were crushed and lysed in an ice-cold lysis buffer (1% Triton X-100 in TBS with protease inhibitors, pH 7.5) and sonicated for 30 seconds on ice. The tissue lysis solution was centrifuged at 12,000 rpm for 10 minutes. Serum was taken directly for measurement. The protein concentrations was measured using a Bio-Rad DC Protein Assay Kit (Bio-Rad, Hercules, CA). Interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in the plasma, lungs and spleen were quantified with the use of specific mouse enzyme linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions (BD Biosciences Pharmingen, San Diego, CA).
Measurement of Cleaved Caspase-3
Cleaved caspase-3 in the lungs and spleen were measured using a Western blot analysis as described above. Antibodies against cleaved caspase-3 were obtained from Cell Signaling Technology. The band densities were normalized by using a cleaved caspase-3/β-actin ratio.
Determination of Pulmonary and Splenic Cell Apoptosis
The terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) assay was carried out using an in situ cell death detection kit (Roche Diagnostics, Indianapolis, IN) in paraffin sections. The lungs and spleen were collected and fixed in 10% neutral buffered formalin and then processed for paraffin embedding and section; 6μm paraffin sections were dewaxed and rehydrated. Tissue sections were incubated with proteinase K (20μg/mL in 10 mM Tris-HCl, pH 7.4–8.0) for 20 minutes at room temperature and rinsed with 50mM Tris-buffered saline (TBS). The slides were then reacted with a mixture of label and enzyme solutions, supplied in the kit, for 60 minutes at 37°C in the dark. After washing with TBS for 3 times, the slides were mounted with Vectashield medium (Vector Labs, Burlingame, CA). A Nikon Eclipse Ti-S microscope with a fluorescent attachment was used to evaluate the slides. TUNEL+ cells showed green fluorescence, and propidium iodide (PI) staining showed red fluorescence on the nuclei. The section that reacted with the label solution alone served as the negative control.
Survival Study
The survival rate was assessed in a separate group of animals, which were assigned randomly into 2 groups: Vehicle (n=14) or rhMFG-E8 treatment (n=12). Sham animals were not included as they are expected to have a 100% survival rate. Normal saline (Vehicle) or rhMFG-E8 (0.02ug/g mouse BW) was administered via the right femoral artery over 30 minutes as described above. Following resuscitation, the femoral arteries were ligated and the incision was closed in layers. The rats were returned to their cages for recovery, and allowed food and water ad libitum throughout the 7 days. The survival rate was recorded over 7 days, and any surviving animals were sacrificed on Day 7.
Statistical Analysis
Data was expressed as means ± SEM and compared by Student’s t-test for two group analysis, or one-way analysis of variance (ANOVA) and Student-Newman-Keuls (SNK) test for multiple group analyses. The survival rate was estimated by the Kaplan-Meier method and compared by the log-rank test. Differences in values were considered significant if P < 0.05.
RESULTS
Suppression of MFG-E8 Following Hemorrhagic Shock
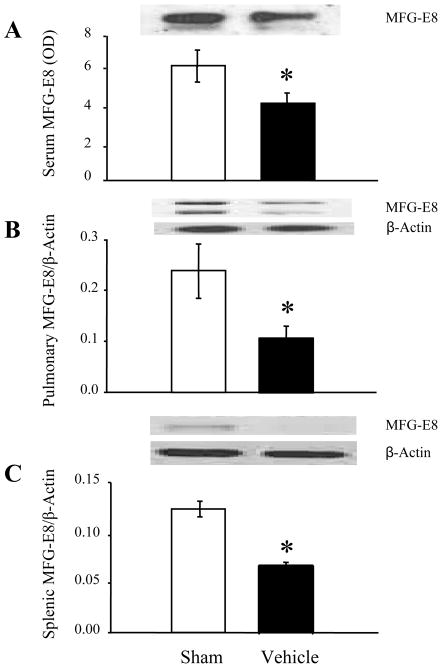
Previous work done in our lab has demonstrated that MFG-E8 levels in the plasma, lungs and spleen are significantly decreased after intestinal ischemia-reperfusion injury21. To confirm our findings in a model of hemorrhagic shock, we measured MFG-E8 levels at 4 hours post hemorrhagic shock. The two bands that are present in the Western blots from the lungs are most likely due to high glycosylation at the post translational modification, which in turn is expressed as two isoforms of MFG-E822. This modification may not be present in either the serum or spleen, or the level of glycosylation may be undetectable. Ultimately, MFG-E8 levels decreased significantly, by an average of 33% in plasma (Fig 1A), 55% (Fig 1B) in the lungs and 44% in the spleen (Fig 1C) after hemorrhagic shock. This reduction provides justification for the use of rhMFG-E8 as a treatment.
Figure 1. Changes in MFG-E8 levels in mice following hemorrhagic shock.
MFG-E8 expression in the plasma (A), lungs (B) and spleen(C) were measured following 90 minutes of hemorrhagic shock in either Sham or Vehicle (Normal Saline) animals (n=9–11/group). Representative blots are also presented. Data are presented as means ± SEM and compared using Student’s t-test: *P < 0.05 vs. Sham group.
Administration of rhMFG-E8 Suppresses Neutrophil Infiltration
MPO is used as a surrogate marker of neutrophil infiltration and activity. As shown in Table 1, MPO activity in the lungs were dramatically increased by 778% after hemorrhage. Treatment with rhMFG-E8 significantly reduced MPO levels by 29% (P<0.05). Mirroring the effects in the lungs, MPO levels in the spleen were similarly significantly decreased by 41% after infusion of rhMFG-E8, following an increase of 171% in Vehicle treated animals.
Table 1.
Alterations in MPO and cytokine levels following hemorrhagic shock
| Sham | Vehicle | rhMFG-E8 | ||
|---|---|---|---|---|
| Serum | IL-1β (pg/ml) | 1.6 ± 0.64 | 92.0 ± 18.87* | 25.3 ± 11.09*# |
| IL-6 (pg/ml) | 16.0 ± 2.26 | 2761.9 ± 585.11* | 995.1 ± 318.58*# | |
| TNF-α (pg/ml) | 2.2 ± 0.08 | 45.9 ± 9.58* | 13.0 ± 5.42*# | |
| Lungs | MPO (U/g) | 7.1 ± 0.92 | 62.7 ± 8.56* | 44.4 ± 3.36*# |
| IL-1β (ng/mg prot) | 2.0 ± 0.20 | 8.1 ± 1.94* | 4.2 ± 0.52*# | |
| IL-6 (pg/mg prot) | 344.5 ± 41.50 | 495.4 ± 60.70* | 337.7 ± 27.36# | |
| TNF-α (pg/mg prot) | 39.2 ± 3.25 | 52.4 ± 5.04* | 337.7 ± 1.56# | |
| Spleen | MPO (U/g) | 9.6 ± 0.49 | 26.1 ± 2.26* | 15.3 ± 3.15*# |
| IL-1β (ng/mg prot) | 0.3 ± 0.03 | 1.4 ± 0.24* | 0.6 ± 0.10*# | |
| IL-6 (ng/mg prot) | 0.1 ± 0.02 | 2.2 ± 0.90* | 0.5 ± 0.20*# | |
| TNF-α (pg/mg protein) | 69.2 ± 3.90 | 113.5 ± 16.24* | 45.4 ± 7.64# |
Serum, pulmonary, and splenic tissues from Sham, Vehicle (normal saline) and Treatment (rhMFG-E8) mice were measured for myeloperoxidase (MPO), interleukins 1β (IL-1β) and 6 (IL-6), and tumor necrosis factor-α (TNF-α) using commercially available assay kits. Pulmonary and splenic levels of MPO were measured using a Bio-Tek plate reader, and expressed in units per gram tissue (units/g). Data are presented as means ± SEM (n=9–11/group) and compared by Student’s t-test for two group analysis, and by one-way analysis of variance (ANOVA) and Student-Newman-Keuls methods for multiple group analysis.
P<0.05 vs. Sham group,
P<0.05 vs. Vehicle group.
rhMFG-E8 Suppresses the Inflammatory Response After Hemorrhagic Shock
In order to corroborate that rhMFG-E8 treatment can reduce pro-inflammatory cytokines after hemorrhagic shock, we measured three major cytokines: IL-1β, IL-6 and TNF-α. We found that plasma levels increased dramatically by 57, 173, and 21-fold in IL-1β, IL-6 and TNF-α, respectively, after hemorrhagic shock. Administration of rhMFG-E8 blunted the cytokine surge significantly by 73%, 64% and 72%, respectively (Table 1; P<0.05). We also explored cytokine levels in the pulmonary and splenic tissues. As expected, pro-inflammatory cytokine levels were elevated in pulmonary tissues by 425%, 43% and 34% for IL-1β, IL-6, and TNF-α, respectively. Splenic tissues also demonstrated an increase in cytokines, with an immense rise in IL-1β and IL-6 by 298% and 1838%, respectively. TNF-α was also significantly increased in Vehicle treated animals, by 64%, as compared to Sham (Table 1). In the pulmonary tissues, levels of IL-1β, IL-6, and TNF-α were significantly decreased by 58%, 32% and 24%, respectively (Table 1). One important observation to note is that treatment with rhMFG-E8 restored IL-6 and TNF-α levels in the lungs to their baseline levels. When we examined splenic tissues, we similarly noted a significant reduction in the cytokine levels, by 49%, 76% and 60% in IL-1β, IL-6, and TNF-α, respectively (Table 1). As with the pulmonary tissue, TNF-α was restored to their baseline level in the spleen. The administration of rhMFG-E8 had a profound effect in significantly reducing, and at times returning to baseline, both serum and tissue levels of the three cytokines we measured.
Administration of rhMFG-E8 Attenuates Apoptosis
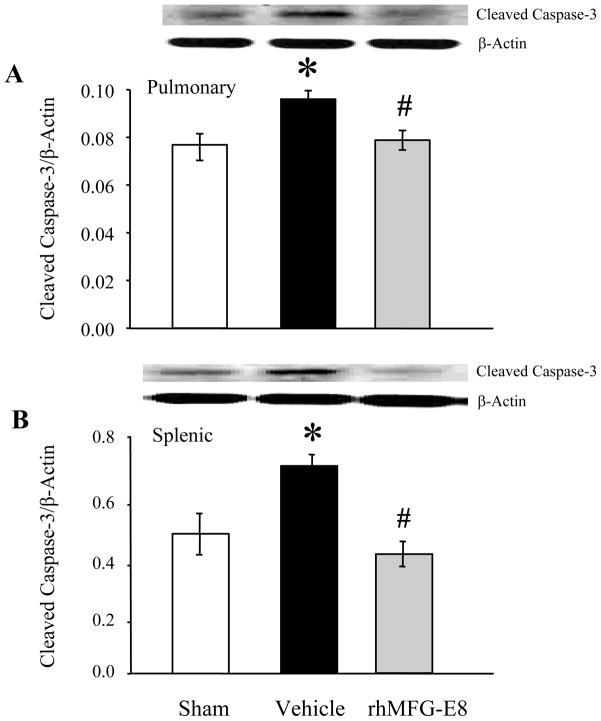
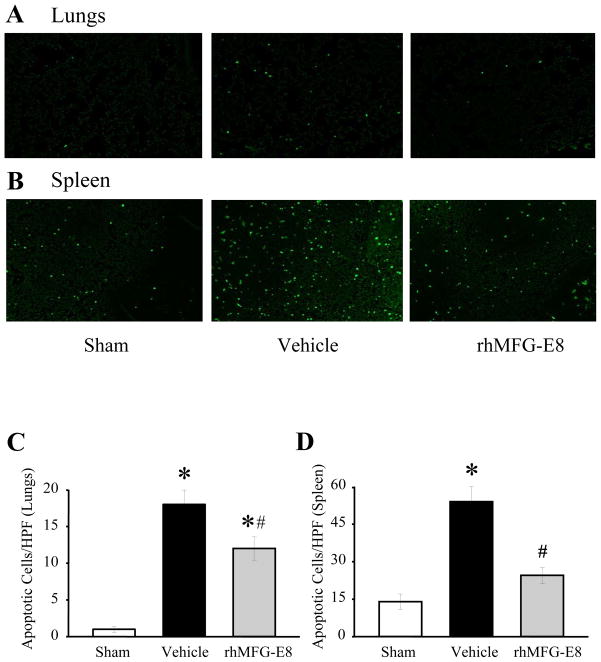
To evaluate the degree of apoptosis during hemorrhagic shock, we used cleaved caspase-3 as a marker. As shown in Figures 2A and 2B, cleaved caspase-3 levels in both pulmonary and splenic tissues were markedly increased by 48% and 24%, respectively, in Vehicle treated animals. By treating the animals with rhMFG-E8, we were able to significantly downregulate the expression of cleaved caspase-3 by 18% and 42% in the lungs and spleen, respectively (P<0.05). Treatment with rhMFG-E8 was extremely effective so that the levels of cleaved caspase-3 in both tissues actually returned to their baseline. We also chose to use TUNEL staining as another indicator for tissue apoptosis (Figs. 4A-B). Following hemorrhage, the number of TUNEL+ cells in Vehicle treated animals were markedly increased in both the pulmonary and splenic tissues, indicating an increase in apoptosis. However, following treatment with rhMFG-E8, there was a significant decrease in the number of TUNEL+ cells, as measured by the number of TUNEL+ cells per high powered field, in both the lungs and spleen (Figures 4C-D; P<0.05). What’s impressive is that the number of TUNEL+ cells in the spleen returned to their pre-hemorrhage levels. Therefore, rhMFG-E8 was instrumental in amplifying the clearance of apoptotic cells after hemorrhagic shock.
Figure 2. Alterations in cleaved caspase-3 levels following treatment with rhMFG-E8 after hemorrhagic shock.
Pulmonary (A) and splenic (B) tissues were evaluated for apoptosis using cleaved caspase-3 levels following treatment with rhMFG-E8. Representative blots are also shown. Data are presented as means ± SEM (n =9–11/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method: *P < 0.05 vs. Sham group; # P < 0.05 vs. Vehicle group.
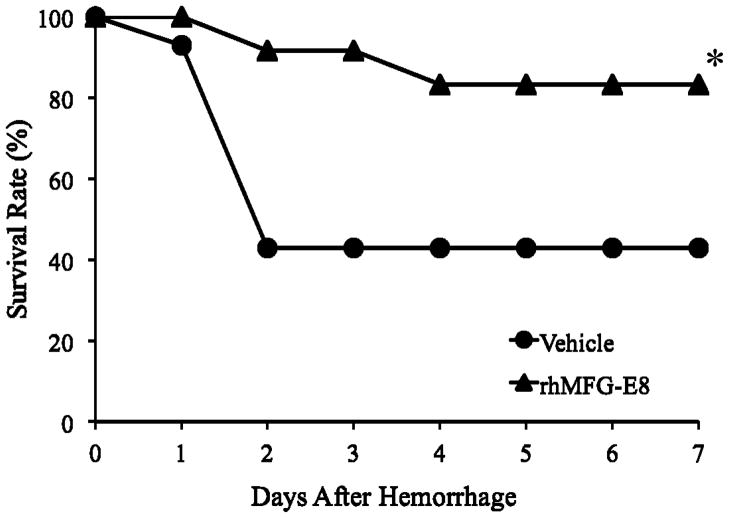
Figure 4. Alterations in the survival rate during 7 days after hemorrhagic shock and treatment with rhMFG-E8 or Vehicle (Normal Saline).
There were 12 animals in rhMFG-E8 group and 14 animals in the Vehicle group. The survival rate was estimated by the Kaplan-Meier method and compared by using the log-rank test. *P < 0.05 vs. Vehicle group.
Treatment with rhMFG-E8 Improves Survival
In a separate group of animals, we examined the effects of rhMFG-E8 treatment on animal survival following hemorrhagic shock. At the conclusion of day 7, there was an impressive improvement in the survival rate in rhMFG-E8 treated animals as compared to the Vehicle group (83% vs. 43%, respectively; P<0.05) (Fig 4). What is an important observation is that animals infused with rhMFG-E8 were protected considerably from mortality until day 3 (92% vs. 43%), whereas those animals treated with normal saline alone (Vehicle) had a greater than 50% mortality rate by day 2.
DISCUSSION
Shock, in its most basic form, is a state of inadequate perfusion. Excessive and prolonged hemorrhage is a common cause of shock, and subsequent organ failure23. Mortality and morbidity following hemorrhagic shock still remains high, approaching 50% without appropriate treatment5. Studies have looked at various methods, including vasoactive compounds, damage control surgery, blockade of biologic markers, and blood component therapies to improve outcomes24–26. Despite the vast amount of work done in elucidating the pathophysiology of hemorrhagic shock, no single therapeutic intervention that has been shown to be effective against hemorrhagic shock.
MFG-E8 is a secretory molecule, mainly produced by the spleen14, that was first described more than 20 years ago9,27. In a mouse model of sepsis, MFG-E8 is downregulated, and that downregulation results in harmful effects12,14. MFG-E8 acts as a “bridging” molecule between a phagocyte and apoptotic cell. Once both cells have detected and adhered to MFG-E8, the phagocyte engulfs both the apoptotic cell as well as the bound opsonin MFG-E822. Therefore, during times of stress and inflammation as seen during severe hemorrhage, circulating levels of MFG-E8 are rapidly consumed secondary to the increasing numbers of apoptotic cells. The serum levels of MFG-E8 can be thought of as the “rate-limiting” step in the clearance of apoptotic cells, and the exogenous administration of rhMFG-E8 is needed for swift and continuous removal of these cells. To our knowledge, there are no other opsonins that are similar to MFG-E8 than can be given in addition. Our lab has has done extensive work with MFG-E8 as a treatment option during sepsis (cecal ligation and puncture and acute alcohol ingestion model), intestinal inflammation, and intestinal ischemia and reperfusion injury12,21,28,29. In these studies, we used a recombinant murine form of MFG-E8 (rmMFG-E8). Recently, we have successfully demonstrated multiple beneficial effects in a cecal ligation and puncture model of sepsis using a recombinant human form of MFG-E8 (rhMFG-E8) 17. Although other investigators have attempted to pinpoint MFG-E8’s mechanism of action, by looking at Akt, osteopontin, CD14, TLR4, MAPK and NF-κB pathways12,29–31, it is important to note that the exact mechanism by which MFG-E8 functions is unclear.
In order to bring MFG-E8 one step closer to clinical applications, it was important to test the human form as a treatment so as to avoid a potential immunogenic response in humans. We did not anticipate any problems with rhMFG-E8 in our hemorrhagic shock model for two reasons: 1) The human variant is structurally similar to the murine variant, except for a single EGF-like domain and a lack of a proline/threonine rich domain, and 2) we have successfully used rhMFG-E8 in a rodent model of sepsis32,33. Recently, we tested the safety of rhMFG-E8 by infusing Sham animals with increasing doses of rhMFG-E8; we did not observe any negative effects17. Our chosen dose of 0.02 ug/g mouse body weight per animal was based on our previous studies. Animals were sacrificed at 3.5 hours after resuscitation. Although this time point may be too early to accurately determine end organ injury, we chose it in order to measure the acute markers of inflammation, such as cytokines, at their peak. If we were to sacrifice animals at a later time point, we may have missed this crest. To address later organ dysfunction and injury, we conducted a survival study so as to gauge the overall extent and severity of organ damage. Looking at our results, we have shown multiple favorable effects in our model of hemorrhagic shock. First, we confirmed that MFG-E8 levels in the serum, lungs and spleen were suppressed following hemorrhagic shock. Next, we measured MPO levels in pulmonary and splenic tissues and observed an increase in MPO levels following hemorrhage. The one-time infusion of rhMFG-E8 was sufficient to significantly depress MPO activity in these tissues. Clinically, MPO levels are used as a measurement of treatment efficacy, as well as a biomarker34. Therefore, the modest reduction we saw supports the premise that rhMFG-E8 increases apoptotic cell clearance, and therefore reduces total neutrophil migration and activation.
Important markers of morbidity and mortality in shock are systemic and tissue cytokine levels35. Our rhMFG-E8 proved to not only significantly depress serum, pulmonary and splenic levels of IL-1β, IL-6 and TNF-α, but impressively restored pulmonary levels of IL-6, TNF-α, and splenic TNF-α to their baselines. We chose our markers of injury and inflammation so as to reflect the systemwide damage, and subsequent attenuation by rhMFG-E8, in our model of severe hemorrhage. Specifically, we chose to use IL-6 as it has been shown to be a reliable early indicator of morbidity and mortality in a variety of clinical conditions36,37. If rhMFG-E8 is to be developed as a candidate for future clinical use, it is important to demonstrate that it is effective in lessening organ specific and systemic markers of injury. Since MFG-E8 works by increasing the uptake and clearance of apoptotic cells, we used cleaved caspase-3 and a TUNEL assay to quantify apoptosis. Cleaved caspase-3 was brought back to pre-hemorrhage levels following rhMFG-E8 treatment in the lungs and spleen, and our TUNEL data shows that rhMFG-E8 was able to clear apoptotic cells significantly in both the lungs and spleen. Other groups have demonstrated that the reduction in apoptotic cells is beneficial in models of sepsis and inflammation38. Our results are in agreement with the other groups, especially as seen with the improvement in overall survival and reduction in circulating cytokine levels. MFG-E8’s role and function in the clearance of apoptotic cells has been well described and detailed14,22,39. We have confirmed that treatment with rhMFG-E8 significantly enhances apoptotic cell clearance following our model of severe hemorrhage. With the increased phagocytosis and subsequent removal of apoptotic cells, secondary systemic inflammation and organ specific damage is reduced due to the diminished levels of toxins from necrotic cells. This is the link between how enhanced clearance of apoptotic cells can reduce overall injury and inflammation seen in a severe hemorrhage model. Finally, we used a separate group of animals to test rhMFG-E8’s ability to increase overall survival. If successful, this would provide a strong argument for developing rhMFG-E8 into a clinical therapeutic. Animals that were given a one-time dose of rhMFG-E8 demonstrated a statistically superior 7-day survival rate as compared to non-treated animals (83% vs. 43%) after hemorrhagic shock. What is important to note is that rhMFG-E8 animals did not experience a pronounced drop in survival until day 4, as compared to non-treated animals which experienced a greater than 50% mortality rate on day 2. This difference would allow for other interventions, potentially life-saving ones, to be performed in the interim.
We have clearly shown that rhMFG-E8 can reduce inflammation and increase survival in a model of hemorrhagic shock. However, there are some limitations. First, we have not examined other doses. The most effective dose may in fact be higher than the one chosen. Second, we infused rhMFG-E8 immediately following hemorrhage. We have not looked at various time-points for infusion. Third, we chose a one-time application, when in fact the best method to achieve a maximal benefit may be through multiple doses or a continuous infusion.
Our current study reinforces previous data on MFG-E8 which demonstrates a multitude of advantageous effects, from reducing multiple marker of organ specific injury and dampening systemic cytokine release to improving survival13,14,29,40. In summary, we have successfully established that rhMFG-E8 is an effective treatment against hemorrhagic shock. We have shown a decrease in neutrophil activity, as well as a down-regulation of the cytokine profile. Furthermore, rhMFG-E8 convincingly amplifies apoptotic cell clearance in various organs. Finally, there was a dramatic improvement in survival. Our study shows that rhMFG-E8 is a strong candidate for further clinical development.
Figure 3. TUNEL staining in pulmonary and splenic tissues after treatment with rhMFG-E8 following hemorrhagic.
shock: Pulmonary (A) and splenic (B) tissues from Sham, Vehicle, and rhMFG-E8 treated mice were evaluated for apoptosis by TUNEL staining. Representative photographs are shown. Pulmonary and splenic tissues depicting the number of TUNEL+ cells per high-powered field (40X) are also shown (C–D). Data are presented as means ± SEM (n =9–11/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method: *P < 0.05 vs. Sham group; #P < 0.05 vs. Vehicle group.
Acknowledgments
Financial Support: Supported in part by NIH grants R01 HL076179 & R01 GM057468 (PW).
Footnotes
AUTHOR CONTRIBUTIONS
Fangming Zhang: Animal work, data collection analysis and interpretation, manuscript writing.
Kavin Shah: Data analysis and interpretation, manuscript writing and editing, literature search, composition of figures and tables.
Lei Qi: Data collection and analysis.
Rongqian Wu: Study concept and design, manuscript, figures and table editing, literature search.
Rafael Barrera: Study concept and design, manuscript editing.
Jeffrey Nicastro: Study concept and design, manuscript editing.
Gene F Coppa: Study concept and design, manuscript editing.
Ping Wang: Study concept and design, data analysis and interpretation, manuscript writing and editing, literature search, figures and table editing.
Contributor Information
Fangming Zhang, Email: fzhang1@nshs.edu.
Kavin Shah, Email: kshah@nshs.edu.
Lei Qi, Email: docqilei@gmail.com.
Rongqian Wu, Email: rwu@nshs.edu.
Rafael Barrera, Email: rbarrera@lij.edu.
Jeffrey Nicastro, Email: jnicastro1@nshs.edu.
Gene F Coppa, Email: gcoppa@nshs.edu.
Ping Wang, Email: pwang@nshs.edu.
References
- 1.Cothren CC, Moore EE, Hedegaard HB, Meng K. Epidemiology of urban trauma deaths: a comprehensive reassessment 10 years later. World J Surg. 2007;31:1507–11. doi: 10.1007/s00268-007-9087-2. [DOI] [PubMed] [Google Scholar]
- 2.Moore EE, Knudson MM, Jurkovich GJ, Fildes JJ, Meredith JW. Emergency traumatologist or trauma and acute care surgeon: decision time. J Am Coll Surg. 2009;209:394–5. doi: 10.1016/j.jamcollsurg.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Neuman TS, Bockman MA, Moody P, et al. An autopsy study of traumatic deaths; San Diego County, 1979. Am J Surg. 1982;144:722–7. doi: 10.1016/0002-9610(82)90558-x. [DOI] [PubMed] [Google Scholar]
- 4.Rossaint R, Bouillon B, Cerny V, et al. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010;14:R52. doi: 10.1186/cc8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frith D, Goslings JC, Gaarder C, et al. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost. 2010;8:1919–25. doi: 10.1111/j.1538-7836.2010.03945.x. [DOI] [PubMed] [Google Scholar]
- 6.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 7.Zacharias N, Sailhamer EA, Li Y, et al. Histone deacetylase inhibitors prevent apoptosis following lethal hemorrhagic shock in rodent kidney cells. Resuscitation. 2011;82:105–9. doi: 10.1016/j.resuscitation.2010.09.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–16. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stubbs JD, Lekutis C, Singer KL, et al. cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor-like domains linked to factor VIII-like sequences. Proc Natl Acad Sci U S A. 1990;87:8417–21. doi: 10.1073/pnas.87.21.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–7. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 11.Oshima K, Aoki N, Negi M, Kishi M, Kitajima K, Matsuda T. Lactation-dependent expression of an mRNA splice variant with an exon for a multiply O-glycosylated domain of mouse milk fat globule glycoprotein MFG-E8. Biochem Biophys Res Commun. 1999;254:522–8. doi: 10.1006/bbrc.1998.0107. [DOI] [PubMed] [Google Scholar]
- 12.Komura H, Miksa M, Wu R, Goyert SM, Wang P. Milk fat globule epidermal growth factor-factor VIII is down-regulated in sepsis via the lipopolysaccharide-CD14 pathway. J Immunol. 2009;182:581–7. doi: 10.4049/jimmunol.182.1.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miksa M, Wu R, Dong W, Das P, Yang D, Wang P. Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis. Shock. 2006;25:586–93. doi: 10.1097/01.shk.0000209533.22941.d0. [DOI] [PubMed] [Google Scholar]
- 14.Miksa M, Wu R, Dong W, et al. Immature dendritic cell-derived exosomes rescue septic animals via milk fat globule epidermal growth factor-factor VIII [corrected] J Immunol. 2009;183:5983–90. doi: 10.4049/jimmunol.0802994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu R, Dong W, Zhou M, Cui X, Simms HH, Wang P. A novel approach to maintaining cardiovascular stability after hemorrhagic shock: beneficial effects of adrenomedullin and its binding protein. Surgery. 2005;137:200–8. doi: 10.1016/j.surg.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Wu R, Higuchi S, Dong W, et al. Reversing established sepsis in rats with human vasoactive hormone adrenomedullin and its binding protein. Mol Med. 2009;15:28–33. doi: 10.2119/molmed.2008.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah KG, Wu R, Jacob A, et al. Recombinant human milk fat globule-EGF factor 8 produces dose-dependent benefits in sepsis. Intensive Care Med. 2011 doi: 10.1007/s00134-011-2353-7. In Press. [DOI] [PubMed] [Google Scholar]
- 18.Qiang X, Li J, Wu R, et al. Expression and characterization fo recombinant human milk fat globule-EGF factor VIII. International Journal of Molecular Medicine. 2011 doi: 10.3892/ijmm.2011.782. In Press. [DOI] [PubMed] [Google Scholar]
- 19.Kohut LK, Darwiche SS, Brumfield JM, Frank AM, Billiar TR. Fixed volume or fixed pressure: a murine model of hemorrhagic shock. J Vis Exp. 2011 doi: 10.3791/2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah KG, Wu R, Jacob A, et al. Human ghrelin ameliorates organ injury and improves survival after radiation injury combined with severe sepsis. Mol Med. 2009;15:407–14. doi: 10.2119/molmed.2009.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui T, Miksa M, Wu R, et al. Milk fat globule epidermal growth factor 8 attenuates acute lung injury in mice after intestinal ischemia and reperfusion. Am J Respir Crit Care Med. 2010;181:238–46. doi: 10.1164/rccm.200804-625OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aziz M, Jacob A, Matsuda A, Wang P. Review: milk fat globule-EGF factor 8 expression, function and plausible signal transduction in resolving inflammation. Apoptosis. 2011 doi: 10.1007/s10495-011-0630-0. [DOI] [PubMed] [Google Scholar]
- 23.Smail N, Messiah A, Edouard A, et al. Role of systemic inflammatory response syndrome and infection in the occurrence of early multiple organ dysfunction syndrome following severe trauma. Intensive Care Med. 1995;21:813–6. doi: 10.1007/BF01700964. [DOI] [PubMed] [Google Scholar]
- 24.Hollenberg SM. Vasoactive drugs in circulatory shock. Am J Respir Crit Care Med. 2011;183:847–55. doi: 10.1164/rccm.201006-0972CI. [DOI] [PubMed] [Google Scholar]
- 25.Duchesne JC, McSwain NE, Jr, Cotton BA, et al. Damage control resuscitation: the new face of damage control. J Trauma. 2010;69:976–90. doi: 10.1097/TA.0b013e3181f2abc9. [DOI] [PubMed] [Google Scholar]
- 26.Sharar SR, Mihelcic DD, Han KT, Harlan JM, Winn RK. Ischemia reperfusion injury in the rabbit ear is reduced by both immediate and delayed CD18 leukocyte adherence blockade. J Immunol. 1994;153:2234–8. [PubMed] [Google Scholar]
- 27.Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004;14:277–87. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- 28.Wu R, Chaung WW, Zhou M, et al. Milk fat globule EGF factor 8 attenuates sepsis-induced apoptosis and organ injury in alcohol-intoxicated rats. Alcohol Clin Exp Res. 2010;34:1625–33. doi: 10.1111/j.1530-0277.2010.01248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aziz MM, Ishihara S, Mishima Y, et al. MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent alphavbeta3 integrin signaling. J Immunol. 2009;182:7222–32. doi: 10.4049/jimmunol.0803711. [DOI] [PubMed] [Google Scholar]
- 30.Jinushi M, Nakazaki Y, Carrasco DR, et al. Milk fat globule EGF-8 promotes melanoma progression through coordinated Akt and twist signaling in the tumor microenvironment. Cancer Res. 2008;68:8889–98. doi: 10.1158/0008-5472.CAN-08-2147. [DOI] [PubMed] [Google Scholar]
- 31.Miksa M, Amin D, Wu R, et al. Maturation-induced down-regulation of MFG-E8 impairs apoptotic cell clearance and enhances endotoxin response. Int J Mol Med. 2008;22:743–8. [PMC free article] [PubMed] [Google Scholar]
- 32.Hu CY, Wu CS, Tsai HF, Chang SK, Tsai WI, Hsu PN. Genetic polymorphism in milk fat globule-EGF factor 8 (MFG-E8) is associated with systemic lupus erythematosus in human. Lupus. 2009;18:676–81. doi: 10.1177/0961203309103027. [DOI] [PubMed] [Google Scholar]
- 33.Larocca D, Peterson JA, Urrea R, Kuniyoshi J, Bistrain AM, Ceriani RL. A Mr 46,000 human milk fat globule protein that is highly expressed in human breast tumors contains factor VIII-like domains. Cancer Res. 1991;51:4994–8. [PubMed] [Google Scholar]
- 34.Loria V, Dato I, Graziani F, Biasucci LM. Myeloperoxidase: a new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediators Inflamm. 2008;2008:135625. doi: 10.1155/2008/135625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulloa L, Tracey KJ. The “cytokine profile”: a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Frink M, van Griensven M, Kobbe P, et al. IL-6 predicts organ dysfunction and mortality in patients with multiple injuries. Scand J Trauma Resusc Emerg Med. 2009;17:49. doi: 10.1186/1757-7241-17-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pecoits-Filho R, Barany P, Lindholm B, Heimburger O, Stenvinkel P. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant. 2002;17:1684–8. doi: 10.1093/ndt/17.9.1684. [DOI] [PubMed] [Google Scholar]
- 38.Parrino J, Hotchkiss RS, Bray M. Prevention of immune cell apoptosis as potential therapeutic strategy for severe infections. Emerg Infect Dis. 2007;13:191–8. doi: 10.3201/eid1302.060963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuda A, Jacob A, Wu R, et al. Milk fat globule-EGF factor VIII in sepsis and ischemia-reperfusion injury. Mol Med. 2011;17:126–33. doi: 10.2119/molmed.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jinushi M, Sato M, Kanamoto A, et al. Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. J Exp Med. 2009;206:1317–26. doi: 10.1084/jem.20082614. [DOI] [PMC free article] [PubMed] [Google Scholar]