Abstract
Rickettsioses and bartonelloses are arthropod-borne diseases of mammals with widespread geographical distributions. Yet their occurrence in specific regions, their association with different vectors and hosts and the infection rate of arthropod-vectors with these agents remain poorly studied in South-east Asia. We conducted entomological field surveys in the Lao PDR (Laos) and Borneo, Malaysia by surveying fleas, ticks, and lice from domestic dogs and collected additional samples from domestic cows and pigs in Laos. Rickettsia felis was detected by real-time PCR with similar overall flea infection rate in Laos (76.6%, 69/90) and Borneo (74.4%, 268/360). Both of the encountered flea vectors Ctenocephalides orientis and Ctenocephalides felis felis were infected with R. felis. The degrees of similarity of partial gltA and ompA genes with recognized species indicate the rickettsia detected in two Boophilus spp. ticks collected from a cow in Laos may be a new species. Isolation and further characterization will be necessary to specify it as a new species. Bartonella clarridgeiae was detected in 3/90 (3.3%) and 2/360 (0.6%) of examined fleas from Laos and Borneo, respectively. Two fleas collected in Laos and one flea collected in Borneo were co-infected with both R. felis and B. clarridgeiae. Further investigations are needed in order to isolate these agents and to determine their epidemiology and aetiological role in unknown fever in patients from these areas.
Keywords: Siphonaptera, Anoplura, Acarina, Rickettsia, Bartonella, Laos, Borneo Island, Zoonotic disease transmission, Spotted fever
Since the beginning of the 20th century, ticks (Acarina), lice (Anoplura and Mallophaga) and fleas (Siphonaptera) have been implicated as vectors, reservoirs, and/or amplifiers of agents of human zoonoses, including rickettsioses and bartonelloses [14]. These diseases have been poorly investigated in South-east Asia, including the Lao PDR (Laos) [2,16] and Malaysian Borneo [10]. Among hospitalized patients in Vientiane, Laos, acute rickettsial infection was identified as the cause of fever in115 (27%) of 427 adults [16] and cause of jaundice or hepatitis in 29 (7.3%) from 392 patients admitted in Mahosot Hospital [27]. The organisms identified by serological analysis were Orientia tsutsugamushi, Rickettsia typhi, and spotted fever group Rickettsia (SFGR) (R. helvetica, R. felis, R. conorii subsp. indica, and Rickettsia “AT1”) [16,27]. In addition, Bartonella clarridgeiae and Rickettsia felis were detected in fleas collected in Phu Khao Khoay, near Vientiane [30]. A clinical case of murine typhus (R. typhi) was reported in a traveller from Brunei [9] and R. felis and R. typhi have been detected from flea species associated with small mammals in these area [3]. A recent study on flea-host associations, for example, compiled no more than 15 fleas species described to date to occur on small terrestrial mammals on Borneo Island, highlighting the lack of our understanding of the species diversity of possible vector and their role in transmitting diseases [32]. As part of our recent efforts to better understand the diversity, occurrence and distribution of ectoparasites of medical importance in South-East Asia, we collected ectoparasites from domestic dogs and some additional samples from domestic cows and pigs in Laos. We investigated the collected arthropods for Rickettsia and Bartonella species in order to examine which species of these disease-causing groups are present in northern Laos and Sabah, East Malaysia, on Borneo Island.
1. Materials and methods
1.1. Study sites and sampling
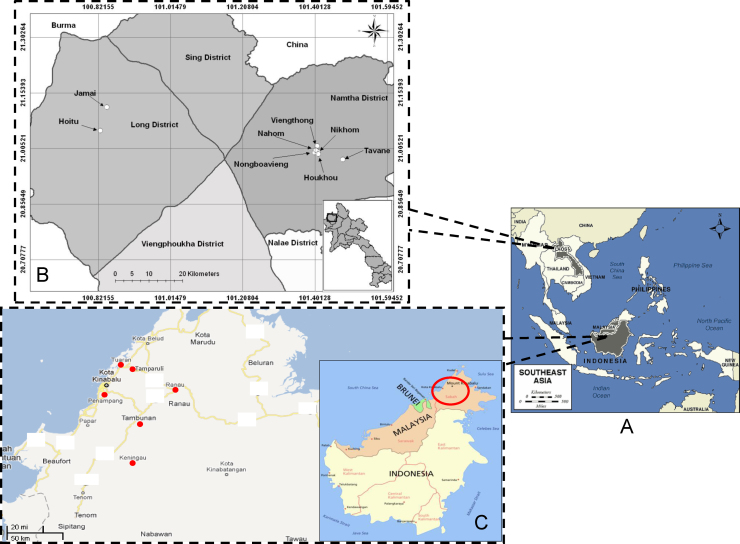
In Luang Namtha Province (20°55′N, 101°07′E), northwest Laos, ectoparasites were sampled in eight villages, including Tavane, Nikhom, Viengthong, Jamai, Hoitu, Houkhou, Nongbauvieng and Nahom (Fig. 1A and B).
Fig. 1.
(A) Location of Laos and Borneo Island in South-East Asia. (B) The sample sites where fleas, ticks and lice have been collected in Luang Namtha Province, Laos. (C) The sample sites where fleas have been collected in the state of Sabah from Malaysia, Borneo Island.
On Borneo (6°02′N, 116°7′E), in the state of Sabah, Malaysia, arthopods were collected near the capital town Kota Kinabalu and in the districts of Keningau, Penampang, Tambunan, Tamparuli, Tuaran, and Ranau which are near the Crocker Range National Park and Kinabalu National Park (Fig. 1C). We particularly focused on these sites as this study was part of our initial efforts to investigate possible interactions of wildlife and domestic animals and the potential of zoonotic diseases transmission near protected forests.
1.2. Collections and morphological identification of ectoparasites
In Luang Namtha Province, northwest Laos, fleas, ticks and lice were collected from domestic dogs, pigs and cows. In Borneo Island, fleas were only collected from domestic dogs. People encountered while travelling through the study area were asked for access to their dogs. We sampled only one dog per household of those consenting; usually, the sampled dog was chosen by the house owner based on availability and tameness. We brushed the dorsal fur of dogs from the neck to the tail for ten minutes with a flea comb (Trixie, Tarp, Germany, art. no. 23762). All ectoparasites were transferred with forceps to a tube containing 70% ethanol for later counting and identification and transported to France. All ectoparasites were morphologically identified to the species level by using morphological criteria within standard taxonomic keys [4,28].
1.3. Molecular analysis
DNAs of ectoparasites were extracted using the BioRobot MDx Workstation (Qiagen, Courtaboeuf, France) with a customized extraction protocol following the manufacturer's instructions. For DNA extraction, a negative control (one non-infected tick from laboratory colony) was used for each 15 samples. DNA was stored at 4 °C until use for further analysis.
Real-time quantitative (q)PCR was performed according to the manufacturer's protocol using a 7900HT Fast Real-Time PCR system (Applied Biosystems, Foster City, CA). Positive samples were considered when cycle thresholds were Ct ≤ 35. All samples were screened using SFGR-specific qPCR targeting gltA gene [25] and Bartonella genus-specific real-time PCR with a 21-bp probe targeting the intergenic spacer (ITS) [30]. Positive fleas for Rickettsia DNA were tested subsequently with primers and probe targeting a chromosomal gene specific of R. felis bioB, as described previously [30]. Positive ticks for Rickettsia DNA were tested by regular PCR targeting gltA and OmpA genes [23]. Bartonella positive samples were tested by regular PCR using primers amplifying ITS at fragment [15]. The Bartonella and Rickettsia DNA sequencing reagents were obtained with BigDye Terminator Cycle Sequencing Ready Reaction Kit (ABI PRISM, PE Applied Biosystems, Foster City, CA). The resulting sequences were edited and assembled using Chromas Pro 1.34 (Technelysium Pty. Ltd., Tewantin, Australia). The sequences were then analyzed by Basic Local Alignment Search Tool (BLAST) sequencing and further compared with other sequences available in the GenBank. R. montanensis, R. felis, and B. elizabethae DNA were included as positive control. Two negative controls were used for each test: sterile water and DNA extracted from non-infected ticks taken from a colony at the Unité des Rickettsies, Marseille.
We used Fisher's Exact Test for categorical data as implemented in the software package R [17].
2. Results
2.1. Collection and morphological identification of the ectoparasites
In Laos, a total of 90 fleas were collected from ten dogs in five villages, including Tavane, Nikhom, Viengthong, Jamai and Hoitu. These fleas were morphologically identified as Xenopsylla cheopis (n = 2), Pulex irritans (n = 3), Ctenocephalides felis felis, the cat flea (n = 19), and Ctenocephalides orientis, the Asian cat flea (n = 66).
Ninety-three ticks were collected, comprising 39 (41.9%) adults and 54 (58.1%) nymphs. Of the 39 adult ticks, 36 (92.3%) were identified as Rhipicephalus sanguineus collected from ten dogs, 2 (5.1%) Boophilus spp. collected from a one domestic cow and 1 (2.6%) Dermacentor spp. collected from one domestic pig. All nymphs (n = 54) were identified as Rhipicephalus spp. and were collected from seven dogs (Table 1). These ticks were collected in four villages, including Viengthong, Houkhou, Nongbauvieng and Nahom. Forty lice were collected on 12 pigs in Tavan region. All lice were identified as Haematopinus suis, based on their morphological characters.
Table 1.
The frequency of Rickettsia and Bartonella species in various flea and tick species sampled in Lao PDR and Sabah (Malaysia), Borneo Island.
| Animal species (number of individuals sampled) | Number (percentage) of positive pathogen detection/number of vertebrate host individuals with positives |
|||
|---|---|---|---|---|
| Rickettsia spp. | Rickettsia felis | Bartonella clarridgeiae | ||
| Luang Namtha Province, Laos | ||||
| Fleas species | Host species | |||
| Ctenocephalides orientis (n = 66) | Dog (n = 7) | 59 (89.4%)/n = 7 | 59 (89.4%)/n = 7 | 3 (4.5%)/n = 2 |
| Ctenocephalides felis felis (n = 19) | Dog (n = 3) | 10 (52.6%)/n = 2 | 10 (52.6%)/n = 2 | –/n = 0 |
| Pulex irritans (n = 3) | Dog (n = 2) | –/n = 0 | –/n = 0 | –/n = 0 |
| Xenopsylla cheopis (n = 2) | Dog (n = 1) | –/n = 0 | –/n = 0 | -–/n = 0 |
| Total | Dog (n = 10) | 69 (76.6%)/n = 7 | 69 (76.6%)/n = 7 | 3 (3.3%)b/n = 2 |
| Ticks species | Host species | |||
| Rhipicephalus sanguineus (n = 39) | Dog (n = 10) | –/n = 0 | –/n = 0 | –/n = 0 |
| Rhipicephalus spp. (n = 54) | Dog (n = 7) | –/n = 0 | –/n = 0 | –/n = 0 |
| Dermacentor spp. (n = 1) | Pig (n = 1) | –/n = 0 | –/n = 0 | –/n = 0 |
| Boophilus spp. (n = 2) | Cow (n = 1) | 2(100%)a/n = 1 | –/n = 0 | –/n = 0 |
| Total | Dog (n = 10); Pig (n = 1), Cow (n = 1) | 2 (2.1%)/n = 1 | – | – |
| Lice species | Host species | |||
| Haematopinus suis (n = 40) | Pig (n = 12) | –/n = 0 | –/n = 0 | –/n = 0 |
| Sabah (Malaysia), Borneo Island | ||||
| Fleas species | ||||
| Ctenocephalides orientis and Ctenocephalides felis felis (n = 360) | Dogs (n = 90) | –/n = 0 | 268 (74.4%)/n = 87 | 2 (0.6%)c/n = 2 |
Rickettsia sequences: sequence analysis of ompA gene [99.83% similarity with Rickettsia sp. FUJ98 (GenBank accession no. AF169629) and 95.29% similarity with Rickettsia heilongjiangensis 054 (GB no. CP002912)]; gltA gene [100% similarity with Rickettsia sp. LON-13 (GB no. AB516964) and 99.54% similarity with R. heilongjiangensis (GB no. AB473994)].
Bartonella sequences: sequence analysis of the intergenic spacer ITS showed 100% identity with B. clarridgeiae (GB no. EU589237).
Bartonella sequences: sequence analysis of the intergenic spacer ITS showed 99.27% identity with B. clarridgeiae (GB no. FN645454).
In Sabah, 1968 fleas were collected from 212 dogs. They were identified as Ctenocephalides orientis (75%, 106 out of 142 identified specimens), and Ctenocephalides felis felis (25%, 36 out of 142 identified specimens). The mean (±SD) number of fleas collected per individual dog was 9.3 ± 10.4 (max: 67 fleas on one dog, no fleas were found on 17 individuals). Not all fleas collected from Borneo Island were tested, we limited our molecular analysis to a maximum of five fleas per sampled dog and a total of 360 samples.
2.2. Rickettsia detection
Rickettsial DNA was detected in 69 of 90 (76.6%) fleas collected from Laos. All fleas positive for rickettsial DNA were positive by R. felis-specific qPCR. The frequency of R. felis was significantly higher in C. f. orientis (59/66; 89.4%) than in C. f. felis (10/19; 52.6%) (Fisher ‘s test odds ratio 0.14, p = 0.001). No R. felis DNA was detected in X. cheopis and P. irritans fleas. R. felis DNA was detected in fleas from five out of eight villages for our study (Table 1). Flea infection rates in these five villages ranged from 55% to 100% with significantly lower infection rate in Viengthong than other villages (Fisher's test p = 0.03; Table 2). Rickettsial DNA was detected in two Boophilus spp. ticks (2.1%; 2/93) collected on a cow in Houkhou. Sequence analysis of the ompA gene showed 99.83% (613/614) similarity with Rickettsia sp. FUJ98 (GenBank accession no. AF169629) and 95.29% similarity (588/617) with Rickettsia heilongjiangensis 054 (GenBank accession no. CP002912). Sequence analysis of gltA gene showed 100% (662/662) similarity with Rickettsia sp. LON-13 (GenBank accession no. AB516964) and 99.54% similarity (659/662) with R. heilongjiangensis (GenBank accession no. AB473994). No rickettsial DNA was detected in Haematopinus suis lice.
Table 2.
Distribution of Rickettsia and Bartonella spp. in vectors (fleas, ticks) sampled at the different sites of Luang Namtha Province from Laos and in different districts of Sabah (Malaysia), Borneo Island.
| Regions | Positive for Rickettsia in qPCR/number collected/% |
Probe R. felis bioB gene (fleas) | Positive for Bartonella (qPCR)/number collected (%) |
||
|---|---|---|---|---|---|
| Fleas | Ticks | Fleas | Ticks | ||
| Luang Namtha Province, Laos | |||||
| Tavane | 36/42 (85.7%) | – | 36 | 1/42 (4.7%) | – |
| Nikhom | 15/23 (65.2%) | – | 15 | 0/23 | – |
| Viengthong | 11/20 (55%) | 0/22 | 11 | 0/20 | 0/22 |
| Jamai | 5/5 (100%) | – | 5 | 0/5 | – |
| Hoitu | 2/2 (100%) | – | 2 | 2/2 (100%) | – |
| Houkhou | – | 2/8 (25%) | – | – | 0/8 |
| Nongbauvieng | – | 0/39 | – | – | 0/39 |
| Nahom | – | 0/24 | – | – | 0/24 |
| Total (%) | 69/90 (76.6%) | 2/93 (2.1%) | 69 | 3/90 (3.3%) | 0/93 |
| Sabah, (Malaysia), Borneo Island | |||||
| Keningau | 81/105 (77%) | – | 81 | – | – |
| Kota Kinabalu | 15/17 (88%) | – | 15 | – | – |
| Penampang | 15/20 /75%) | – | 15 | – | – |
| Tambunan | 100/158 (63%) | – | 100 | 1/158 (0.6%) | – |
| Tamparuli | 31/34 (91%) | – | 31 | 1/34 (3%) | – |
| Tuaran | 26/26 (100%) | – | 26 | – | – |
| Ranau | 0/0 | – | 0 | – | – |
| Total (%) | 268/360 (74.5%) | – | 268 | 2/360 (0.6%) | – |
In Borneo Island, Rickettsial DNA was detected in 268 of 360 samples (74.4%) individually tested from 90 dogs. All fleas positive for rickettsial DNA were positive by R. felis-specific qPCR. Notably, R. felis appeared to be ubiquitously present throughout the study area in Borneo as we were not able to find rickettsial DNA in only 3 of the 90 dogs for which the fleas collected on them were screened. The overall infection rates of fleas with rickettsial DNA in Laos (76.6%) and Borneo (74.4%) were similar (Fisher's exact test, odds ratio = 1.13, p = 0.79).
2.3. Bartonella detection
In ectoparasites from Laos, Bartonella DNA was detected in 3 out of 90 (3.3%) fleas, including only C. f. orientis fleas collected from dogs in Tavan and Hoitu (Table 1). Sequences obtained after PCR amplification and sequencing of partial ITS showed 100% identity with B. clarridgeiae (GenBank accession no. EU589237). Moreover, two fleas collected in Hoitu were co-infected by B. clarridgeiae and R. felis. No Bartonella DNA was detected in any ticks or lice.
Bartonella DNA was detected in 2 (2/360, 0.6%) fleas collected from dogs from Borneo. Sequences obtained after PCR amplification and sequencing of partial ITS showed 99.29% (702/707) identity with B. clarridgeiae (GenBank accession no. FN645454). Moreover, one flea was co-infected by B. clarridgeiae and R. felis.
3. Discussion
These findings provide the first evidence of Rickettisa felis in Malaysian Borneo and confirm the presence of this agent among ectoparasites in several provinces in Laos with high overall rates of infection of similar magnitude in Laos and Malaysian Borneo. Recently, R. felis has been detected in East Kalimantan (Indonesian Borneo) in pools of Xenopsylla cheopis collected from the shrew, Suncus murinus and the rat Rattus norvegicus [3]. These findings suggest that flea-borne spotted fever is likely to be a human disease in these areas and urges that R. felis should be included in the differential diagnosis of fever. In 1986, a serological survey in Sabah, East Malaysia showed that 16.5% of 412 human forest dwellers presented SFGR antibody [29]. Differences in flea infection rates among villages in Laos suggests that geographical heterogeneity in human disease risk is likely to occur, although our relatively small sample size for Laos did not allow a comprehensive spatial analysis and the prediction of flea abundance and infections rates under different environmental conditions as would be desirable. Moreover, flea abundance on dogs in Borneo differed with environmental conditions, mostly affected by housing conditions (K. Wells, unpublished data). We found some weak spatial structure in the prevalence of R. felis in fleas collected in Borneo (exploratory analysis with a variogram, results not shown), and we emphasize the need for further interdisciplinary studies that investigate pathogen spread under different environmental conditions likely to affect host and vector species.
R. felis has been associated with various species of fleas and human cases have been reported from North and South America, Europe, Asia, and North Africa [13]. Recently, a high prevalence of R. felis in dogs in Southeast Queensland, had suggested that dogs may act as an important reservoir host for R. felis and as a potential source of human rickettsial infection [7]. The animal hosts from which the infected ectoparasites were recovered represent a large range of different mammalian host species [18]. SE Asia rainforest are hotspots of mammalian species diversity and due to rapid economic growth, further factors that may be relevant to disease emergence such as housing conditions for companion animals, shifts in the abundance and community composition of wildlife species, land cover and exposure of domestic animals to wildlife and vice versa are rapidly changing [24,26,31]. Considerable research efforts are therefore needed to examine the epidemiology of R. felis and its relationship to particular regional environmental conditions. In 2007, the first evidence of R. felis was reported on fleas collected in dogs from Phu Khao Khoay area (∼100 km NE of Vientiane) from Laos [30]. In addition, R. felis infection was diagnosed in patient with unknown fever in Vientiane [16]. Recently, two studies conducted in Senegal and Kenya, have highlighted the importance of R. felis infection in patients with unexplained fever in sub-Saharan Africa [13,19,25].
In our study, the degrees of similarity of partial gltA and ompA genes with recognized species indicate the Rickettsia detected in ticks from Laos may be a new species. This Boophilus spp. tick Rickettsia is a species of the SFGR because its sequence exhibits > 92.7% homology with the 20 known Rickettsia species from this group and possess the ompA gene [6]. The closest validated Rickettsia species to our presumably new species is R. heilongjiangensis, the agent of far-eastern tick-borne disease, which occurs in eastern Russia and northern China [14]. However, only fragments of gltA and ompA genes have been sequenced in our study. To better describe this Rickettsia in detail, it will need to be isolated and the five genes fully specific to Rickettsia (rrs, gltA, ompA, ompB, and geneD) characterized [6]. Finally, other Rickettsia species transmitted by tick-vector circulate in Borneo, as tick typhus was detected by serology among people in Sarawak, East Malaysia [21].
In addition, we report the first detection of Bartonella clarridgeiae in fleas collected in Borneo and confirm its presence in Laos. B. clarridgeiae has been suggested as a minor causative agent of cat scratch disease (CSD) in humans [8]. The most frequent agent of CSD is B. henselae with its principal reservoir being domestic cats [1]. B. clarridgeiae has been detected on fleas from Europe, Asia, North America [22], Africa, New Zealand and very recently from New Caledonia [11]. The prevalence of B. clarridgeiae in cat fleas in Europe may be as great as 17% [20]. Nearby Laos, B. clarridgeiae has been identified on the Thai-Myanmar (Burma) border and in China [15,22] and it has been reported on fleas collected in dogs in Phu Khao Khoay in Laos [30]. A high prevalence of Bartonella spp. (25.5%), including B. elizabethae, B. tribocorum, B. phoceensis and two new Bartonella species, was reported in rodents collected in four Lao provinces [2]. To the best of our knowledge, no patients with Bartonella infection have been reported from Laos or Borneo. The infection rates in our study are relatively low, but given the ubiquitous presence of the vectors, we believe that it is possible that some fevers in these areas may be caused by Bartonella spp. A high Bartonella seroprevalence in rural Thailand has been reported recently in febrile (10%) and non-febrile patients (19%) [5]. Fatal human Bartonella endocarditis confirmed by serology, by qPCR, cell culture, and by immunohistochemical staining has recently been reported in Thailand [12].
In conclusion, our data show a high proportion of fleas being infected with R. felis and further report the presence of B. clarridgeiae in fleas from both Laos and Borneo. Infections due to these pathogens are likely to be underestimated and misdiagnosed, and should be considered in the differential diagnosis of unknown fever. Beside the importance of considering the pathogens in medical diagnosis, we highlight that more interdisciplinary research is necessary to perform epidemiological studies in the future that investigate further the species diversity of pathogens, their prevalence and also the role of components involved in disease emergence and transmission such as vectors, vertebrate hosts and the underlying environment.
Conflict of interests
The authors declared that they have no competing interests.
Acknowledgements
We thank Sabah Parks for the permit to conduct field work and all kind of support. We thank all the mammal owners for allowing us to examine their pets and stock animals in Laos and Borneo. Field work in Borneo has been funded by the “Landesoffensive zur Entwicklung wissenschaftlich-ökonomischer Exzellenz” (LOWE) of the state of Hesse in Germany through the Biodiversity and Climate Research Centre (Bik-F). The fieldwork in Laos was funded by the Wellcome Trust of Great Britain, as part of the Wellcome Trust-Mahosot Hospital-Oxford Tropical Medicine Research Collaboration, and the SFE Medical Project. We are very grateful to the Provincial Health Department of Luang Namtha, the Directors of Mahosot Hospital, the Director and staff of the Microbiology Laboratory, Mahosot Hospital and the Ministry of Health of the Government of the Lao PDR. We thank Koukeo Phommasone for his help for the Lao map.
Footnotes
Presented at the 6th International Meeting on Rickettsia and Rickettsial Diseases at Heraklion, Crete, Greece on June 5–7, 2011.
References
- 1.Angelakis E., Edouard S., La S.B., Raoult D. Bartonella henselae in skin biopsy specimens of patients with cat-scratch disease. Emerg Infect Dis. 2010;16(12):1963–1965. doi: 10.3201/eid1612.100647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelakis E., Khamphoukeo K., Grice D., Newton P.N., Roux V., Aplin K. Molecular detection of Bartonella species in rodents from the Lao PDR. Clin Microbiol Infect. 2009;15(Suppl 2):95–97. doi: 10.1111/j.1469-0691.2008.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbara K.A., Farzeli A., Ibrahim I.N., Antonjaya U., Yunianto A., Winoto I. Rickettsial infections of fleas collected from small mammals on four islands in Indonesia. J Med Entomol. 2010;47(6):1173–1178. doi: 10.1603/me10064. [DOI] [PubMed] [Google Scholar]
- 4.Beaucournu JC, Launay F. Les puces (Siphonaptera) de France et du bassin méditerranéen occidental. Paris: Fédération Française des Sociétés de Sciences Naturelles; 1990. 548 pp [in French].
- 5.Bhengsri S., Baggett H.C., Peruski L.F., Morway C., Bai Y., Fisk T.L. Bartonella seroprevalence in rural Thailand. Southeast Asian J Trop Med Public Health. 2011;42(3):687–692. [PubMed] [Google Scholar]
- 6.Fournier P.E., Dumler J.S., Greub G., Zhang J., Wu Y., Raoult D. Gene sequence-based criteria for identification of new rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol. 2003;41(12):5456–5465. doi: 10.1128/JCM.41.12.5456-5465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hii S.F., Kopp S.R., Abdad M.Y., Thompson M.F., O’Leary C.A., Rees R.L. Molecular evidence supports the role of dogs as potential reservoirs for Rickettsia felis. Vector Borne Zoonotic Dis. 2011;11(8):1007–1012. doi: 10.1089/vbz.2010.0270. [DOI] [PubMed] [Google Scholar]
- 8.Kordick D.L., Hilyard E.J., Hadfield T.L., Wilson K.H., Steigerwalt A.G., Brenner D.J. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease) J Clin Microbiol. 1997;35(7):1813–1818. doi: 10.1128/jcm.35.7.1813-1818.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kueh Y.K., Ti T.Y., Heptonstall J. Murine typhus infection complicated by dengue haemorrhagic fever. Ann Acad Med Singapore. 1988;17(4):595–599. [PubMed] [Google Scholar]
- 10.Marchette N.J. Rickettsioses (tick typhus Q-fever, urban typhus) in Malaya. J Med Entomol. 1966;2(4):339–371. doi: 10.1093/jmedent/2.4.339. [DOI] [PubMed] [Google Scholar]
- 11.Mediannikov O., Cabre O., Qu F., Socolovschi C., Davoust B., Marie J.L. Rickettsia felis and Bartonella clarridgeiae in fleas from New Caledonia. Vector Borne Zoonotic Dis. 2011;11(February (2)):181–183. doi: 10.1089/vbz.2009.0199. [DOI] [PubMed] [Google Scholar]
- 12.Pachirat O., Kosoy M., Bai Y., Prathani S., Puapairoj A., Zeidner N. The first reported case of Bartonella endocarditis in Thailand. Infectious Disease Reports. 2011;3(e8):44–45. doi: 10.4081/idr.2011.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parola P. Rickettsia felis: from a rare disease in the USA to a common cause of fever in sub-Saharan Africa. Clin Microbiol Infect. 2011;17(7):996–1000. doi: 10.1111/j.1469-0691.2011.03516.x. [DOI] [PubMed] [Google Scholar]
- 14.Parola P., Paddock C.D., Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18(4):719–756. doi: 10.1128/CMR.18.4.719-756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parola P., Sanogo O.Y., Lerdthusnee K., Zeaiter Z., Chauvancy G., Gonzalez J.P. Identification of Rickettsia spp. and Bartonella spp. in from the Thai-Myanmar border. Ann N Y Acad Sci. 2003;990:173–181. doi: 10.1111/j.1749-6632.2003.tb07359.x. [DOI] [PubMed] [Google Scholar]
- 16.Phongmany S., Rolain J.M., Phetsouvanh R., Blacksell S.D., Soukkhaseum V., Rasachack B. Rickettsial infections and fever, Vientiane, Laos. Emerg Infect Dis. 2006;12(2):256–262. doi: 10.3201/eid1202.050900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R Development Core Team. R: A language and environement for statistical computing [computer program]. Vienna, Austria. R Foundation for Statistical Computing 2010. http://cran.r-project.org/
- 18.Reif K.E., Macaluso K.R. Ecology of Rickettsia felis: a review. J Med Entomol. 2009;46(4):723–736. doi: 10.1603/033.046.0402. [DOI] [PubMed] [Google Scholar]
- 19.Richards A.L., Jiang J., Omulo S., Dare R., Abdirahman K., Ali A. Human Infection with Rickettsia felis. Kenya. Emerg Infect Dis. 2010;16(7):1081–1086. doi: 10.3201/eid1607.091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolain J.M., Franc M., Davoust B., Raoult D. Molecular detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in cat fleas, France. Emerg Infect Dis. 2003;9(3):338–342. doi: 10.3201/eid0903.020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sagin D.D., Ismail G., Nasian L.M., Jok J.J., Pang E.K. Rickettsial infection in five remote Orang Ulu villages in upper Rejang River, Sarawak, Malaysia. Southeast Asian J Trop Med Public Health. 2000;31(4):733–735. [PubMed] [Google Scholar]
- 22.Saisongkorh W., Rolain J.M., Suputtamongkol Y., Raoult D. Emerging Bartonella in humans and animals in Asia and Australia. J Med Assoc Thai. 2009;92(5):707–731. [PubMed] [Google Scholar]
- 23.Sarih M., Socolovschi C., Boudebouch N., Hassar M., Raoult D., Parola P. Spotted fever group rickettsiae in ticks, Morocco. Emerg Infect Dis. 2008;14(7):1067–1073. doi: 10.3201/eid1407.070096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schipper J., Chanson J.S., Chiozza F., Cox N.A., Hoffmann M., Katariya V. The status of the world's land and marine mammals: diversity, threat, and knowledge. Science. 2008;322(5899):225–230. doi: 10.1126/science.1165115. [DOI] [PubMed] [Google Scholar]
- 25.Socolovsch C., Mediannikov O., Sokhna C., Diatta G., Bassene H., Trape J. Rickettsia felis-associated uneruptive fever, Senegal. Emerg Infect Dis. 2010;16(7):1140–1142. doi: 10.3201/eid1607.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sodhi N.S., Koh L.P., Clements R., Wanger T.C., Hill K., Amer K.C. Conserving Southeast Asian forest biodiversity in human-modified landscapes. Biol Conserv. 2007;143:2375–2384. [Google Scholar]
- 27.Syhavong B., Rasachack B., Smythe L., Rolain J.M., Roque-Afonso A.M., Jenjaroen K. The infective causes of hepatitis and jaundice amongst hospitalised patients in Vientiane, Laos. Trans R Soc Trop Med Hyg. 2010;104(7):475–483. doi: 10.1016/j.trstmh.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanskul P., Stark H.E., Inlao I. A checklist of ticks of Thailand (Acari: Metastigmata: Ixodoidea) J Med Entomol. 1983;20(3):330–341. doi: 10.1093/jmedent/20.3.330. [DOI] [PubMed] [Google Scholar]
- 29.Taylor A.C., Hii J., Kelly D.J., Davis D.R., Lewis G.E., Jr. A serological survey of scrub, tick, and endemic typhus in Sabah, East Malaysia. Southeast Asian J Trop Med Public Health. 1986;17(4):613–619. [PubMed] [Google Scholar]
- 30.Varagnol M., Parola P., Jouan R., Beaucournu J.C., Rolain J.M., Raoult D. First detection of Rickettsia felis and Bartonella clarridgeiae in fleas from Laos. Clin Microbiol Infect. 2009;15(Suppl 2):334–335. doi: 10.1111/j.1469-0691.2008.02272.x. [DOI] [PubMed] [Google Scholar]
- 31.Wells K., Kalko E., Lakim M.B., Pfeiffer M. Effects of rain forest logging on species richness and assemblage composition of small mammals in southeast asia. J Biogeograph. 2007;34:1087–1099. [Google Scholar]
- 32.Wells K., Lakim M.B., Beaucournu J.C. Host specificity and niche partitioning in flea-small mammal networks in Bornean rainforests. Med Vet Entomol. 2011;25(3):311–319. doi: 10.1111/j.1365-2915.2010.00940.x. [DOI] [PubMed] [Google Scholar]